Abstract
Land reclamation is a common practice leading to the restoration of areas affected by industrial activity. Soil studies in reclaimed areas are very useful to determine the effectiveness of reclamation works. The goal of the study was to investigate soil properties, mineral composition, total concentrations of Zn, Pb, Cd and As and chemical forms of these elements in order to assess the success of land reclamation of the abandoned mine tailing disposal site of the “Trzebionka” Zn-Pb mine in Trzebinia, southern Poland. The disposal site was reclaimed by covering tailings with a layer of inert material with a thickness up to 25 cm. The topsoil of the studied soil profiles was comprised of sandy loamy/loamy materials and the subsoil was comprised of sandy tailing materials. The soils were characterized by a neutral or slightly alkaline reaction due to the high content of carbonates. The dominant mineral in the subsoil was dolomite. The studied soils were considerably contaminated with Zn, Pb, Cd and As. A high load of mobile Zn, Pb and Cd was typical of the subsoil material. The reclamation layer does not provide sufficient isolation of toxic tailings from the environment and there is still a high risk of element uptake by plants.
1. Introduction
For centuries, man has exploited zinc and lead deposits in many part of the world [1,2,3,4,5,6], including Poland, where Zn-Pb ore deposits occur mainly in the region of Silesian and Cracow Upland [7,8,9,10]. The activity of Zn-Pb mines have caused environmental pollution by the deposition of tailings (post-flotation wastes) on the land surface and the emission of dust containing trace elements such as Zn, Pb, Cd and As [11,12,13]. Tailings from the mines have been deposited in dams and on heaps of gangue containing significant amounts of exploited ore [14,15]. Disposal sites are a source of environmental pollution due to the weathering of tailings and leaching of hazardous trace elements which disrupt the life functions of plants [10,16], animals and soil microorganisms [17,18,19,20,21,22], and/or can cause the destruction of habitat near to the disposal sites [23,24,25,26,27].
Tailing dams and mine heaps are subject to land reclamation (e.g., by covering with a layer of inert material) in order to reduce the impact of disposal sites on the local environment. The introduction of vegetation or spontaneous plant succession on the surface of disposal sites initiates soil-forming processes. As a consequence, technogenic soils (Technosols) [28,29] begin to form. According to WRB classification [28], Technosols are soils containing ≥20% (by volume, weighted average) artefacts in the upper 100 cm from the soil surface or to continuous rock or technic hard material or a cemented or indurated layer. Artefacts (according to WRB) are solid or liquid substances that are, for example, (a) created or substantially modified by humans as part of an industrial or artisanal manufacturing process; or (b) brought to the surface by human activity from a depth where they were not influenced by surface processes, and deposited in an environment where they do not commonly occur, with properties substantially different from the environment where they are placed [28].
The abandoned “Trzebionka” mine and disposal site near the mine was extensively studied. Geological studies were conducted, focusing on the tectonic setting of Zn-Pb mineralization in the deposit [30] and observations on ore rhythmites [31]. The groundwater chemical composition was investigated [32,33,34,35,36]. An assessment of the state of the environment around tailing dams was also examined [37]. Moreover, a study was conducted on the problems and effects of mine tailing dam remediation [38]. Furthermore, monitoring of dust emissions from tailing dams was conducted [39].
The soils developed on Zn-Pb mine tailing disposal sites were also extensively studied. Most of the studies focused on soil biology and ecology. Research was conducted on the role of mycorrhizal fungi [40,41,42,43] in the reclamation of tailings. The studies showed that the roots of the grass species studied were colonized by arbuscular mycorrhizal fungi, and the values of all mycorrhization parameters increased with the age of the deposited tailings [44,45,46]. The species diversity of carabid beetles and ants [47] as well as the life history traits of ground beetles [48] were studied as indicators of ecological restoration success. It was investigated whether the reclaimed heaps provide the habitat for earthworms and what are their species diversity and biomass is [49].
High contamination of soils developed on Zn-Pb mine tailing disposal sites with trace elements is their common feature [1,10,50,51,52,53,54,55,56]. It was also confirmed that metals are taken up by plants introduced into Zn-Pb industrial wastes from the “Trzebionka” mine [57]. However, the behaviour and availability of trace elements occurring in these soils have not been recognized in detail. Moreover, sometimes reclamation works have not been conducted properly or finalized as intended; thus, the reduction of the impact of disposal sites on the environment expected after land reclamation can in fact be illusory. Therefore, there is a need for studies focusing on the assessment of the effectiveness of reclamation techniques (e.g., covering with inert materials) in terms of the restoration of former disposal sites where tailings from Zn and Pb mines were deposited.
The aim of the present study was to determine soil properties, mineral composition, total concentrations and forms of such elements as Zn, Pb, Cd and As in technogenic soils developed on the tailing disposal site of the abandoned “Trzebionka” Zn-Pb mine in Trzebinia town, southern Poland. The mine was an underground zinc and lead ore mine. Zinc and lead deposits occur in that area as a sulphide (mainly galena and sphalerite) mineralization in Middle Triassic (Muschelkalk) dolomitic formations [58,59,60,61,62]. The results were the basis for a discussion on (1) the degree of contamination of the studied soils by hazardous elements (Zn, Pb, Cd, As), (2) the mobility of studied elements in the Technosols, as well as (3) the issue of success and sustainability of land reclamation practises in terms of reduction of hazards related with the occurrence of highly toxic mine tailings on the land surface.
2. Materials and Methods
2.1. Study Area
The study area is located on the border of two municipalities of Trzebinia and Chrzanów towns in the western part of the Małopolskie Voivodship, southern Poland. The study was conducted on a disposal site where former tailing dams of the abandoned “Trzebionka” mine were located. Metal ores were enriched in the mine by the ore grinding and flotation process. The tailings were deposited in tanks which were in use from the 1960s until 2009. The tailings comprised a sandy material containing ground dolomite, remnants of Zn- and Pb-bearing minerals as well as trace contents of other metal-bearing minerals [34]. During the mine operation, the disposal site was formed by the embankment of tanks with the tailing material. After filling each tank on a given level, another (higher) level of new tanks was formed, and so on. In 2009, due to the exhaustion of ore resources, the “Trzebionka” mine was closed and the deposition of tailings was stopped. At present, the disposal site is drained. Its area is approximately 64 ha at the bottom and about 30 ha at the top with a slope inclination of 17 to 35% [63]. The slopes of the consecutive tank levels were successively reclaimed by covering with a thin layer of inert soil material (a sandy loamy/loamy material rich in soil organic matter) and afforestation mainly by Scots pine (Pinus sylvestris) and silver birch (Betula pendula) [64]. The upper part of the disposal site is at present a flat area (a plateau), which was reclaimed after cessation of the mine by covering tailings with a layer of inert sandy loamy material. A mixture of grass and legume species, for example, red clover (Trifolium pratense L.) and vetches (Vicia L.), was introduced there. Nowadays, the entire surface of the disposal site is covered with vegetation. The site dominates over the Trzebinia and Chrzanów area as it is approximately 70 m high and exceeds an altitude of 320 m a.s.l. The flat area in the upper part of the disposal site was transformed into agricultural land (pasture).
2.2. Materials
The analyses were carried out on sixteen soil samples from three soil profiles (Figure 1, Table 1) representing the upper part of the disposal site and its slopes. Profile TR1 was located on the top (a plateau) of the disposal site, profile TR2 in the upper part of the slope, and profile TR3 in the lower part of the slope. Profile TR1 was reclaimed by covering the tailings with sandy loamy material rich in soil organic matter (SOM) with a thickness of 25 cm. In profiles TR2 and TR3, soil material present in the A (humus) horizon was probably deposited during reclamation, washed away from the top of the landfill and partly originated due to the accumulation and humification of plant residues. All soils were bipartite formations: the subsoil (C horizons) was built of tailings and the topsoil (A horizons) was comprised of a material deposited on a disposal site during reclamation. Soil profiles were described during field works according to the guidelines for soil description [65]. The field studies were conducted in May 2015.

Figure 1.
Location of profiles TR1, TR2, TR3 on the mine tailing disposal site of the abandoned mine of Zn and Pb ores “Trzebionka” in Trzebinia (www.google.pl/maps, accessed on 12 April 2021).

Table 1.
Field description of the studied soil profiles.
The studied soils were classified as different variants of Spolic Technosols according to WRB [28] (Table 1) and Industriosols according to the Polish Soil Classification [66,67].
2.3. Laboratory Analyses
2.3.1. Soil Properties and Soil Classification
The analyses were carried out on samples taken from each horizon distinguished in soil profiles during field works. In the laboratory, living roots were removed from soil samples and then the samples were dried at room temperature and sieved through a 2 mm sieve in order to obtain fine earth (<2 mm) from soil samples. Fine earth was subject to laboratory analyses. Particle size distribution was determined by Bouyoucose–Casagrande method with Prószyński’s modification [68]. Soil textural classes were defined according to the U.S.D.A. classification [69]. Soil pH was measured using the potentiometric method in H2O and 1M KCl. The contents of carbonates were determined by means of the Scheibler volumetric method (reagent: 10% w/w HCl). Total organic carbon (TOC) content was measured on the TOC-5000A (Shimadzu, Kyoto, Japan) analyser after carbonate removal using 10% HCl. Contents of total nitrogen (TN) and total sulphur (TS) were determined using CHNS elemental analyser (the vario MACRO cube, Elementar, Langenselbold, Hesse, Germany). The soil profiles were classified according to the World Reference Base for Soil Resources [28] and Polish Soil Classification [66,67].
2.3.2. X-ray Diffraction Analyses
The mineral composition of soil samples was determined using powder X-ray diffraction method (XRD). The fine earth soil materials (<2 mm) were ground and powders were analysed with the use of Bruker AXS D5005 diffractometer equipped with the KRISTALLOFLEX® 760 X-ray generator, the vertical goniometer, 1 mm divergence slit, 2 mm anti-scatter slit, 0.6 detector slit, and a graphite diffracted-beam monochromator. CoKα radiation was used with the applied voltage of 40 kV and 35 mA current. Random mounts of the ground materials were scanned from 3 to 70° 2θ at a counting time of 2 s per 0.01° step. XRD analyses were performed in the Department of Soil Science, Institute of Agriculture, Warsaw University of Life Sciences—SGGW, Warszawa, Poland.
2.3.3. Total Concentrations of the Studied Elements in Soils
Total element concentrations (Zn, Pb, Cd, As) were determined by microwave sample mineralization (Milestone Ethos UP) using the following reagents: 6 mL HNO3, 2 mL HClO4, 5 mL HF, 1 mL H2O2, and 1 mL H3BO3 (the latter was added after digestion) [70]. The analysis of extracts was carried out using inductively coupled plasma—optical emission spectrometry (ICP-OES, Perkin Elmer Avio 200, Waltham, MA, USA). The instrument was equipped with a dual, backside-illuminated, cooled CCD detector, echelle monochromator (echelle grating: 79 line/mm, blaze angle: 63.8°), and glass baffled cyclonic spray chamber. Other parameters were as follows: plasma gas flow (12 L/min), auxiliary gas flow (0.2 L/min), nebuliser gas flow (0.7 L/min), RF power 1500 Watts, and pump flow rate 1 mL/min. Argon was used as the operating gas. The following wavelengths were used during analysis: 213.857 nm for Zn, 220.353 nm for Pb, 214.440 nm for Cd and 188.979 nm for As. Calibration curves were obtained based on the dilutions (with 2% HNO3 prepared from 65 % pure per analysis HNO3 Sigma-Aldrich and MiliQ H2O) of standard element solutions (Supelco®, Bellefonte, PA, USA). The analyses and blanks were conducted in duplicates.
2.3.4. Sequential Extraction Analysis
Operationally defined chemical forms of selected elements (Zn, Pb, Cd, As) were determined using the seven-stage sequential extraction procedure by Zeien and Brümmer [71], which allowed for chemical fractionation of elements into seven fractions (from F1 to F7) in order to assess their mobility. Operational fractions and reagents used in the analyses were shown in Table 2 and the details are available elsewhere [71]. Concentration of elements in soil extracts gained during fractionation studies were determined by means of atomic absorption spectroscopy (AAS) (Thermo Fisher iCE 3000, Waltham, MA, USA). Parameters used in the analysis were described in Table S1 (see Supplementary Materials). Soil extracts were analysed in duplicates. Blanks were run in duplicates with each set of fractions. Analytical-grade reagents were used in the analyses. An analytical quality control was verified by the analysis of certified reference material (SS-2 contaminated soil, ColdBlock Technologies Inc., Zimmerman Ave, Niagara Falls, ON, Canada) (Table S2, see Supplementary Materials) and single element calibration solution.

Table 2.
Seven-stage sequential extraction procedure and description of operational fraction according to the Zeien and Brümmer method [71].
3. Results
3.1. Soil Morphology and Properties
The studied profiles were poorly developed technogenic soils (Technosols). All profiles were bipartite and had a similar morphology (Figure 2). The subsurface C horizons in the subsoil were composed of greyish-yellowish tailings. The topsoil (A horizons) comprised a brown material deposited on tailings during reclamation works. The A horizon was considerably thicker at the plateau of the disposal site (25 cm in profile TR1) than in the profiles developed on the slope of the disposal site (10 cm in profile TR2 and TR3). The border between the reclamation material layer and deposited tailing layer was sharp or clear.
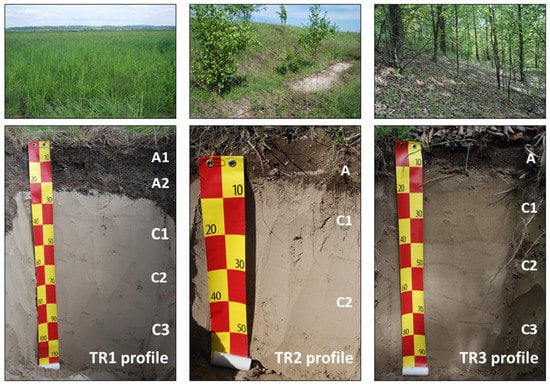
Figure 2.
Studied soil profiles and their surroundings.
The A horizons of the studied soils were loamy or sandy loamy materials and the tailings from C horizons had the texture of sands (Table 3). All the studied soils were characterized by a neutral or slightly alkaline reaction. This is due to the high content of carbonates. The soil pH in all profiles ranged from pH 6.3 to 8.5 (Table 3). The soil pH generally increased with profile depth. The highest concentration of carbonates was found in C horizons (up to 89.7%). The topsoils were characterized by low carbonate contents, up to a maximum of 26.4% in profile TR2 (which had an admixture of carbonate-bearing material from C horizons), and up to 1.8% in profile TR1 and TR3. The A horizons of all profiles contained up to 7.9% of TOC. The content of TN in these horizons was in the range 0.17 to 0.35%, whereas in C horizons it ranged from 0.01 to 0.02%. The A horizons contained from 0.06 to 0.13% of TS, while in C horizons contents of TS were in the range 0.13 to 0.22% (Table 3).

Table 3.
Selected physical and chemical properties of soils.
3.2. Mineral Composition of the Studied Soils
The mineral composition and distribution were similar in all studied soils. Quartz was a predominating mineral in topsoil A horizons of all profiles (Table 4; Figures S1–S3 in Supplementary Materials); furthermore, dolomite displayed considerable abundance in the topsoil of the profile TR2. The A horizons also contained feldspars (albite and orthoclase) and some clay minerals (most likely chlorite or some swelling clays). The predominating mineral in the subsoil (C horizons) of all soil profiles was dolomite (Table 4; Figures S1–S3 in Supplementary Materials). Quartz was a minor mineral. The C horizons also contained admixtures of calcite, smithsonite, pyrite and sphalerite.

Table 4.
Mineral composition of the studied soil samples based on powder XRD analyses. Minerals are arranged in a semiquantitative order based on the relation of peak intensities in the XRD patterns (the most abundant minerals are labelled as +++, and the least abundant ones as +; R—refractory minerals, nd—the minerals which were not detected using powder XRD analysis, -: not analysed).
3.3. Total Concentrations of the Studied Elements in Soils
Much higher concentrations of Zn, Pb, Cd and As were detected in the subsoil (C horizons) than the topsoil (A horizons) of the studied profiles (Table 5). The total concentrations of Zn ranged from 394 mg·kg−1 in A2 horizon (profile TR1) to 13,953 mg·kg−1 in C2 horizon (profile TR3). The lowest content of Pb was 166 mg·kg−1 in the topsoil of profile TR1, whereas the highest was in C3 horizon was 4493 mg·kg−1 in the same profile. The lowest concentration of Cd was recorded in the topsoil of profile TR1 (4 mg·kg−1), while the highest content was noted in the lowest horizon of the same profile (117 mg·kg−1). The total content of As amounted to between 11 mg·kg−1 in the topsoil of profile TR1 to 50 mg·kg−1 in C1 horizon of profile TR3.

Table 5.
Total concentrations of studied elements (in mg·kg−1).
3.4. Chemical Forms of Zn, Pb, Cd and As in the Studied Soils
The studied soils were characterized by the domination of Zn in F7 (elements in residual fraction), which comprised on average 40% of total concentration of that element in topsoil horizons and 35% in subsoil horizons of all studied soils (Figure 3). Zinc in F5 (elements bound to amorphous or poorly crystalline iron oxyhydroxides), constituted on average 22% of total concentration in topsoil horizons and 24% in the subsoil horizons. On average, Zn in F6 (elements bound to crystalline iron oxyhydroxides) constituted 16% of total Zn content in the topsoil and 14% in the subsoil of all profiles. F2 (specially adsorbed and near-surface occluded elements) constituted 7% (topsoil) and 13% (subsoil), F4 (elements bound to organic matter) constituted 10% (topsoil) and 4% (subsoil), F1 (exchangeable elements) constituted 2% (topsoil) and 5% (subsoil), and F3 (elements bound to manganese oxides) constituted 4% (both in the topsoil and subsoil horizons) of total concentration of Zn. Although the share of F1 (i.e., the most mobile form) of Zn was low, it should be emphasized that the concentrations of Zn expressed in mg·kg−1 are significant. For example, in the C1 horizon of profile TR3, the content of Zn in F1 reached up to 803 mg·kg−1 (the total concentration of Zn in that horizon was 13,648 mg·kg−1).
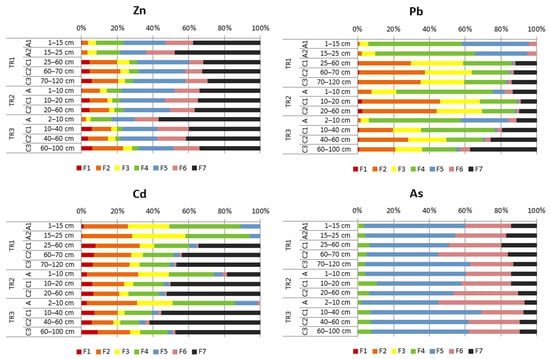
Figure 3.
Chemical forms of Zn, Pb, Cd and As in the studied soils. Explanation of chemical forms of elements: F1—exchangeable (i.e., unspecifically adsorbed) elements, as well as easily soluble elements in organometallic complexes; F2—specifically adsorbed and near-surface occluded elements, elements bound to CaCO3, as well as elements in organometallic complexes of low bond strength; F3—elements bound to manganese oxides; F4—elements bound to organic matter; F5—elements bound to amorphous or poorly crystalline iron oxyhydroxides; F6—elements bound to crystalline iron oxyhydroxides; F7—elements in residual fraction.
Lead in all profiles occurred mainly in F4 (on average 51% of total Pb concentration in the topsoil horizons and 24% in subsoil horizons) (Figure 3). In the topsoil, Pb in F2 constituted, on average, only 8% of total Pb, but in the subsoil it was 31%. Lead in F3 constituted 10% of total Pb concentration in topsoil horizons and 22% in subsoil horizons. On average, Pb in F5 was 18% of the total Pb in the topsoil and 1% in the subsoil, Pb in F7 (residuum) was 7% in the topsoil and 17% in the subsoil, Pb in F6 was 4% (topsoil) and 2% (subsoil), and Pb in F1 was 1% in subsoil in all studied soils.
All the studied soils were characterized by the domination of Cd in F7 (residuum), which constituted more that 60% in the subsoil (C2 horizon) of profile TR3 (Figure 3). In all profiles, the contribution of F2, F3, F4 and F5 was significantly higher in the A horizons than in the C horizons, while with F1 was the opposite proportion. The percentage of Cd in F6 was negligible.
The F5 was a predominating form of As in all studied soils and it contributed on average more than 50%, both in the subsoil and topsoil (Figure 3). In all profiles, the proportion of As in F6 was higher in the subsoil than in topsoil and constituted at least 20% of all profiles. On average 6% of total As occurred in F4 and up to 20% in F7. There was no As in F1, F2 and F3.
4. Discussion
4.1. Contamination of Soils Based on the Polish Standard
The concentrations of trace elements in studied soils were compared with the Polish legal requirements [72] for assessment of the pollution of the land surface. The regulation constitutes a soil quality standard in Poland. This regulation takes into account soils and sediments from a depth 0 to 25 cm and <25 cm below the land surface. As for the soils taken from a depth of 0 to 25 cm, Polish regulation divides lands into four groups (from I to IV). Group I includes built-up and urbanised areas; group II, arable lands, orchards and meadows; group III, forests and wooded and bushy areas; and group IV, industrial areas, communication areas, areas of production facilities, warehouses, areas of technical infrastructure and mining areas. Group II is subdivided into three subgroups (II-1, II-2, II-3) distinguished on the basis of soil properties (particle size distribution, pH, content of total organic carbon). The permissible metal contents for a depth <25 cm for all soil groups is dependent on the water permeability of soil (the border value is 1 × 10−7 m·s−1).
The studied soils were assigned to group IV as they were located in a former mining area. Soil materials taken from depths <25 cm of profileTR1 should be considered contaminated, as the total content of Zn, Pb, Cd and As has exceeded the permitted standards (Table 6). Acceptable values were exceeded by Zn, Pb and Cd throughout profile TR2 and in the subsoil of profile TR3 also by As. On the basis of the Polish regulation [72], only the topsoil (A horizons) of profile TR1 and TR3 could be considered as unpolluted. Due to the fact that profile TR1 is located on the top of the landfill, which is currently used for agricultural purposes, the general content of elements, at a depth of 0 to 25 cm, was also compared with permissible metal contents for group II. Soils are classified as: in horizon A1, mineral-organic with TOC content of 3.5 to 6% (subgroup 2) and in horizon A2, mineral-organic and organic with a total organic carbon content of more than 6% (subgroup 3). According to the Polish regulation [72], soil material from A1 horizon of profile TR1 should be considered contaminated with Zn and Cd, as concentrations of these metals exceeded the permitted standards (Table 7).

Table 6.
Exceeded content of trace elements in soils (group IV) according to the Polish regulation [72].

Table 7.
Exceeded content of trace elements in soils (group II) according to the Polish regulation [72].
4.2. Mobility of Selected Elements in the Studied Technosols
The object of the research is a disposal site containing mine tailing, which was originally deposited in ponds. It is a place susceptible to the intense weathering of phases containing toxic elements, and thus having a negative impact on the surrounding environment [73]. The studied soils are characterized by high concentrations of Zn, Pb, Cd and As (Table 5) with varying degrees of mobility (Figure 3). The danger caused by the presence of trace elements in the soil therefore, depends not only on the total concentration, but also on the availability and mobility of the elements. The mobility of trace elements depends mostly on the physical and chemical state of the element in the soil and the properties of the soil such as pH, content of SOM, redox conditions, as well as the content and type of constituents with the capacity for sorption [74,75].
In topsoil A horizons, which are characterized mainly by alkaline soil reaction (Table 3), the highest Zn content is concentrated mainly in residual fraction (F7) and is occluded in amorphous and crystalline Fe oxyhydroxides (Figure 3) limiting the release of Zn to the environment. Zinc in F7 is most likely bound in sulphides (sphalerite) or it is possible in a silicate form (e.g., hemimorphite); however, the latter mineral was not detected based on the methods used in the study. The most characteristic for alkaline conditions is Zn occluded in poorly crystalline Fe oxyhydroxides, of which colloidal forms catch Zn to a larger extent than the well-crystalline forms [76,77]. Zn in F4 occurs in markedly higher percentage in the topsoil, which suggests that Zn is bound to organic matter as organic complexes of high stability (Figure 3, Table 3). Zinc is known from its affinity with SOM, which in turn causes its concentration in topsoil typically rich in SOM [78,79]. Zinc in F2, which accounted for a larger proportion of the subsoil, can be related with the highest carbonate (dolomite and smithsonite) contents in the subsoil (Figure S1, Table 3 and Table 4). According to the studies conducted in the Olkusz zinc and lead ore district [80], it can be assumed that smithsonite (ZnCO3) is the main Zn-bearing mineral. Moreover, some Zn admixtures occur in dolomite [81]. A relatively high percentage of Zn in subsoil occurs in F1, which indicated high mobility of Zn. Zinc is one of the most mobile metals in the soil environment, which is influenced by its mobile nature [78,79].
The present study showed that Pb predominantly occurred in mobile (F1 and F2) and mid-mobile forms (F3 and F4) (Figure 3). This is consistent with the previous studies that the highest amounts of Pb are found in Mn oxides and it is less abundant in cerussite, bound with Fe oxides and dolomite [7]. A common feature of Pb is its affinity with SOM (i.e., the formations of Pb-organic complexes of high stability) [82]. Lead in F4, which is considered to be bound to organic matter, constitutes a significantly high share in the topsoil containing SOM (Table 3). It is mainly bound in the form of organometallic complexes, which minimizes toxic effects on the environment [82]. A higher percentage of Pb in F3, which is thought to be occluded in manganese oxides (and small proportion of organically bound elements), were located in the subsoil (Figure 3), where the highest manganese concentrations were also recorded (significantly higher than in the topsoil) (not shown in the article).
Cadmium was the most mobile element in the investigated soils (Figure 3). The highest share of F1, considered the most mobile metal form, was found in subsoil layers, where also very high absolute values of these cadmium forms were recorded (Figure S1, Table 3). Cadmium in soils formed on Zn-Pb mine tailing heaps is found as an admixture in carbonates, silicates and Zn-bearing aluminosilicates, as well as in exchangeable forms [7]. Cadmium has no relevant function in biological processes [83]. Both the topsoil and subsoil layers showed Cd contents and mobility high enough to suggest that the metal is most likely taken by plants in the disposal site (Figure S1). In spite of the fact that cadmium mobility increases under acidic conditions and the studied soils showed near-neutral and alkaline reaction (Table 3), which causes precipitation of this element in the form of poorly soluble carbonates and phosphates [84], high proportions of available forms were recorded in the studied soils (Figure S1). A significantly higher share of cadmium in forms associated with organic matter is found in the topsoil (Figure 2), which is related to the occurrence of SOM.
Arsenic in the studied soils occurred only in mid-mobile and immobile forms and was mostly bound to amorphous or poorly crystalline Fe oxyhydroxides (Figure 3). In previous studies, it was also found that As is not present in bioavailable forms, but predominated in F7, i.e., in the least mobile form [85]. The studied soils were characterised by a near-neutral or slightly alkaline reaction, which supports the precipitation of Fe oxyhydroxides that capture As. In this kind of environment, As is barely soluble and is not released to the soil solution [7,86,87].
4.3. The Major Environmental Problems Related with the Studied Soils and Disposal Site
One of the major problems related with zinc and lead ore mining is a generation of tailings during ore processing. Mines are forced to collect these tailings in disposal sites [3,54,63,88,89,90,91]. In the 20th century in Poland, not all disposal sites have been reclaimed (or reclamation was insufficient) due to the lack of legal regulations. Despite the decreasing amount of generated tailing, reclamation processes do not keep pace with their formation [92]. However, the disposal sites receiving tailings require reclamation in order to (1) limit the spread of tailing material particles and related trace elements due to wind erosion, and (2) limit the uptake of trace elements from tailings by plants. Blowing out the fine fractions from the surfaces of disposal sites is a threat to the local environment. This phenomenon intensifies in dry and windy periods. Different methods for limiting the spread of toxic tailing material particles were used in the case of the “Trzebionka” mine in Trzebinia. For example, water sprinkling of the disposal site surface was carried out in the area; some areas were also latexed [39]. Nevertheless, there were episodes of increased dust rise, which were related with strong winds and inadequate preservation of the ground, as well as construction activities during operation and closure of the disposal site [39].
There is no effective method of reclamation for the tailing disposal sites [93]. According to “phytosociological” classification [94], these tailing disposal sites belong to the group with the greatest difficulties in reclamation. Nevertheless, the disposal site in Trzebinia was reclaimed. The parts of the disposal site where no other technical works have been carried out (e.g., slopes of the tanks) have been subjected to technical (covering the surface with a thin layer of inert soil material) and biological (plant introduction) reclamation. It allowed for the stabilization of slopes of the disposal site. After the mine closure in 2009, the plateau in the upper part of the disposal site was levelled, covered with a 25-cm-thick layer of sandy loamy material, and grasses and legumes were introduced on the surface [63].
The results presented herein showed that reclamation works conducted on the surface of disposal site of the former “Trzebionka” mine were insufficient. This is evidenced by studies of soil properties which show that the investigated soils have positive and negative features. First of all, the positive feature of studied soils is a thick layer of inert soil material which involves, in particular, soils developed on the plateau of the disposal site (area represented by the profile TR1). A positive feature of that layer is the heavier (loamy or sandy loamy) texture in comparison with the sandy subsoils, so the former has a higher water retention capacity and does not dry out as much as the sand below it. Another positive feature of that layer is its high content of TOC and TN (Table 3). Low general contents of trace elements and their fewer mobile forms in topsoil show that the reclamation was partly successful. The topsoil permitted isolation of highly contaminated tailings from the environment to some degree. Predomination of dolomite (CaMg(CO3)2) in the mineral composition of soils is also an advantage, because it makes the soils rich in Ca and Mg.
The study showed some negative features of the soils on the studied disposal site. Firstly, the layer of inert soil material covering the disposal site is too thin to isolate tailings from the environment. This causes plant roots to reach the toxic tailings and can absorb harmful elements (e.g., Zn, Pb and Cd), which in turn contributes to the spread of toxic elements in the local trophic chains. The results presented herein showed that there is a great potential possibility of element (e.g., Zn, Pb or Cd) uptake due to their high concentration in the most mobile forms (Figure 3). The extent of element uptake by plants was not in the scope of the present paper; however, it will be studied in the future. Another disadvantage of using a thin layer of inert material is that toxic tailing material is often found on the surface of the disposal site as a consequence of erosion or activity of fauna. This can result in spreading tailing material particles on the surface of the disposal site and its vicinity.
The area where profile TR1 is located represents a plateau in the upper part of the disposal site, which is transformed into a pasture. If the total content of Zn and Cd for the II group of soils (i.e., soils used as arable lands, meadows and pastures) is compared according to the Polish legal requirements [72], these area should be considered contaminated, as the concentrations of Zn and Cd exceeded the permitted standards. Therefore, agricultural use of that area should be banned, because the area is highly toxic. There is a strong possibility that chemical pollutants occurring in soils will affect vegetation and animals, indirectly through the food chain and directly by inhaling contaminated dust.
Despite the previous reclamation and partial improvement of soil condition on the disposal site, it is recommended to proceed the reclamation works once again in order to cover the site with a thick layer of inert material and introduce a new plant cover with shallow-rooting plants (e.g., grasses or perennials). Then, the area should be monitored for about 30 years in order to control the quality of soils, water and plants.
5. Conclusions
Properties, mineralogy, concentrations of toxic trace elements (Zn, Pb, Cd and As) and their chemical forms in technogenic soils developed on the tailing disposal site of the abandoned Zn-Pb “Trzebionka” mine in southern Poland were studied in order to assess the success of previous reclamation works as well as highlight the major environmental issues related with trace element mobility, the degree of contamination of soils in the studied area and potential influence of the disposal site on the local environment.
The soils have a similar morphology: the subsoil is constituted of tailings and the topsoil is built of soil material deposited on the tailings during reclamation works. The subsoil is a carbonate-rich (69 to 89%) sandy material with pH 8.2 to 8.5 consisting mainly of dolomite with admixtures of other minerals (smithsonite, calcite, quartz, sphalerite and pyrite). The thickness of the topsoil ranges from 10 to 25 cm. The topsoil was a loamy material with a pHH2O from 6.9 to 8.1, content of carbonates and TOC up to 1.8 and 7.9%, respectively.
According to Polish regulation [72] for soils in industrial areas (group IV), the topsoil of some profiles can be considered unpolluted. However, if one takes into account soils used agriculturally (group II), the same soils should be considered contaminated, as the total content of Zn and Cd has exceeded the permitted standards. This shows that the agricultural use of the studied disposal site should be banned.
In all profiles, Zn dominated in forms considered immobile. Nevertheless, the most mobile forms of Zn, Pb and Cd reached very high absolute values (expressed in mg·kg−1), particularly in the subsoil of the studied profiles. Arsenic was present in mid-mobile and immobile forms in the studied soils.
The results show that the layer of inert soil material occurring in the topsoil of the studied profiles is too thin to isolate tailings efficiently from the environment. Only soils located on the plateau in the upper part of the disposal site exhibit some favourable physical and chemical properties in the topsoil (loamy texture, neutral reaction, high TOC and TN content). Nevertheless, all the studied soils contain large amounts of mobile Zn, Pb, Cd in the subsoil, which is in the range of rhizosphere and causes a threat of element uptake by plants. The reclamation works are not sufficient and should be conducted once again. New reclamation works should focus on (1) full isolation of toxic tailings from the environment by a thick soil layer or geomembrane and (2) covering the surface with plants with shallow root systems.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/min11060559/s1, Table S1. AAS operating conditions used for Thermo Fisher iCE 3000 apparatus. Table S2. Determination results of the certified reference material SS-2 contaminated soil by means of the AAS. Figure S1. Mineral composition of profile TR1. The d values (in italics) are in nm. Mineral symbols: A—albite, C—calcite, CM—clay minerals, Do—dolomite, Fs—feldspars (undivided), Or—orthoclase, Py—pyrite, Q—quartz, Sm—smithsonite, Sp—sphalerite. Figure S2. Mineral composition of profile TR2. The d values (in italics) are in nm. Mineral symbols: A—albite, C—calcite, CM—clay minerals, Do—dolomite, Fs—feldspars (undivided), Or—orthoclase, Q—quartz, Sm—smithsonite. Figure S3. Mineral composition of profile TR3. The d values (in italics) are in nm. Mineral symbols: A—albite, C—calcite, CM—clay minerals, Do—dolomite, Fs—feldspars (undivided), M—mica, Or—orthoclase, Q—quartz, Sm—smithsonite.
Author Contributions
Conceptualization, Ł.U., W.K. and M.T.; methodology, Ł.U., W.K., M.T. and A.P.; validation, Ł.U., W.K., M.T. and A.P.; formal analysis, M.T.; investigation, M.T., K.P.-P., Ł.U., W.K. and A.P.; writing—original draft preparation, M.T; writing—review and editing, Ł.U. and M.T.; visualization, M.T. and Ł.U.; supervision, Ł.U. and W.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education (subvention for the Institute of Agriculture, Warsaw University of Life Sciences—SGGW).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Romero-Baena, A.J.; González, I.; Galán, E. Soil pollution by mining activities in Andalusia (South Spain)—The role of Mineralogy and Geochemistry in three case studies. J. Soils Sediments 2018, 18, 2231–2247. [Google Scholar] [CrossRef]
- Yuan, M.; Xu, Z.P.; Nguyen, T.; Baumgartl, T.; Huang, L. Interaction of Humic Acid with Cu/Pb-Zn Tailings of Different Degrees of Weathering. Soil Sci. Soc. Am. J. 2017, 81, 712–722. [Google Scholar] [CrossRef]
- Pellegrini, S.; García, G.; Peñas-Castejon, J.M.; Vignozzi, N.; Costantini, E.A.C. Pedogenesis in mine tails affects macroporosity, hydrological properties, and pollutant flow. Catena 2016, 136, 3–16. [Google Scholar] [CrossRef]
- Iavazzo, P.; Adamo, P.; Boni, M.; Hillier, S.; Zampella, M. Mineralogy and chemical forms of lead and zinc in abandoned mine wastes and soils: An example from Morocco. J. Geochem. Explor. 2012, 113, 56–67. [Google Scholar] [CrossRef]
- Vaněk, A.; Borůvka, L.; Drábek, O.; Mihaljevič, M.; Komárek, M. Mobility of lead, zinc and cadmium in alluvial soils heavily polluted by smelting industry. Plant Soil Environ. 2005, 51, 316–321. [Google Scholar] [CrossRef]
- Bevandić, S.; Blannin, R.; Vander Auwera, J.; Delmelle, N.; Caterina, D.; Nguyen, F.; Muchez, P. Geochemical and Mineralogical Characterisation of Historic Zn–Pb Mine Waste, Plombières, East Belgium. Minerals 2021, 11, 28. [Google Scholar] [CrossRef]
- Jerzykowska, I.; Majzlan, J.; Michalik, M.; Göttlicher, J.; Steininger, R.; Błachowski, A.; Ruebenbauer, K. Mineralogy and speciation of Zn and As in Fe-oxide-clay aggregates in the mining waste at the MVT Zn-Pb deposits near Olkusz, Poland. Geochemistry 2014, 74, 393–406. [Google Scholar] [CrossRef]
- Pająk, M.; Błońska, E.; Szostak, M.; Gąsiorek, M.; Pietrzykowski, M.; Urban, O.; Derbis, P. Restoration of Vegetation in Relation to Soil Properties of Spoil Heap Heavily Contaminated with Heavy Metals. Water Air Soil Pollut. 2018, 229, 392. [Google Scholar] [CrossRef] [PubMed]
- Cabała, J. Metale Ciężkie w Środowisku Glebowym Olkuskiego Rejonu Eksploatacji rud Zn-Pb, 1st ed.; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2009; pp. 1–128. [Google Scholar]
- Kicińska, A.; Smreczak, B.; Jadczyszyn, J. Soil bioavailability of cadmium, lead, and zinc in the areas of Zn-Pb ore mining and processing (Bukowno, Olkusz). J. Ecol. Eng. 2019, 20, 84–92. [Google Scholar] [CrossRef]
- Ghorbel, M.; Munoz, M.; Solmon, F. Health hazard prospecting by modeling wind transfer of metal-bearing dust from mining waste dumps: Application to Jebel Ressas Pb-Zn-Cd abandoned mining site (Tunisia). Environ. Geochem. Health 2014, 36, 935–951. [Google Scholar] [CrossRef] [PubMed]
- Kon, L.C.; Durucan, S.; Korre, A. The development and application of a wind erosion model for the assessment of fugitive dust emissions from mine tailings dumps. Int. J. Min. Reclam. Environ. 2007, 21, 198–218. [Google Scholar] [CrossRef]
- Sánchez Bisquert, D.; Matías Peñas Castejón, J.; García Fernández, G. The impact of atmospheric dust deposition and trace elements levels on the villages surrounding the former mining areas in a semi-arid environment (SE Spain). Atmos. Environ. 2017, 152, 256–269. [Google Scholar] [CrossRef]
- Álvarez, E.; Fernández Marcos, M.L.; Vaamonde, C.; Fernández-Sanjurjo, M.J. Heavy metals in the dump of an abandoned mine in Galicia (NW Spain) and in the spontaneously occurring vegetation. Sci. Total Environ. 2003, 313, 185–197. [Google Scholar] [CrossRef]
- Wierzbicka, M.; Szarek-Łukaszewska, G.; Grodzińska, K. Highly toxic thallium in plants from the vicinity of Olkusz (Poland). Ecotoxicol. Environ. Saf. 2004, 59, 84–88. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Górecka, H.; Górecki, H. Bioavailability of heavy metals from polluted soils to plants. Sci. Total Environ. 2005, 337, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Pająk, M.; Błońska, E.; Frąc, M.; Oszust, K. Functional Diversity and Microbial Activity of Forest Soils that Are Heavily Contaminated by Lead and Zinc. Water Air Soil Pollut. 2016, 227, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kiepas, A.; Wrońska, H. Reakcje mikroorganizmów na długotrwałe i silne skażenie gleby metalami ciężkimi. In Obieg Pierwiastków w Przyrodzie, Monografia; Gworek, B., Mocek, A., Eds.; Instytut Ochrony Środowiska: Warsaw, Poland, 2001; Volume 1, pp. 365–371. [Google Scholar]
- Kelly, J.J.; Häggblom, M.M.; Tate, R.L. Effects of heavy metal contamination and remediation on soil microbial communities in the vicinity of a zinc smelter as indicated by analysis of microbial community phospholipid fatty acid profiles. Biol. Fertil. Soils 2003, 38, 65–71. [Google Scholar] [CrossRef]
- Niklińska, M.; Chodak, M.; Laskowski, R. Characterization of the forest humus microbial community in a heavy metal polluted area. Soil Biol. Biochem. 2005, 37, 2185–2194. [Google Scholar] [CrossRef]
- Yang, Y.G.; Jin, Z.S.; Bi, X.Y.; Li, F.L.; Sun, L.; Liu, J.; Fu, Z.Y. Atmospheric Deposition-Carried Pb, Zn, and Cd from a Zinc Smelter and Their Effect on Soil Microorganisms. Pedosphere 2009, 19, 422–433. [Google Scholar] [CrossRef]
- Wolińska, A.; Włodarczyk, K.; Kuźniar, A.; Marzec-Grządziel, A.; Grządziel, J.; Gałązka, A.; Uzarowicz, Ł. Soil Microbial Community Profiling and Bacterial Metabolic Activity of Technosols as an Effect of Soil Properties following Land Reclamation: A Case Study from the Abandoned Iron Sulphide and Uranium Mine in Rudki (South-Central Poland). Agronomy 2020, 10, 1795. [Google Scholar] [CrossRef]
- Csavina, J.; Taylor, M.P.; Félix, O.; Rine, K.P.; Eduardo Sáez, A.; Betterton, E.A. Size-resolved dust and aerosol contaminants associated with copper and lead smelting emissions: Implications for emission management and human health. Sci. Total Environ. 2014, 493, 750–756. [Google Scholar] [CrossRef]
- Entwistle, J.A.; Hursthouse, A.S.; Marinho Reis, P.A.; Stewart, A.G. Metalliferous Mine Dust: Human Health Impacts and the Potential Determinants of Disease in Mining Communities. Curr. Pollut. Rep. 2019, 5, 67–83. [Google Scholar] [CrossRef]
- Lee, J.S.; Chon, H.T.; Kim, K.W. Human risk assessment of As, Cd, Cu and Zn in the abandoned metal mine site. Environ. Geochem. Health 2005, 27, 185–191. [Google Scholar] [CrossRef]
- Taylor, M.P.; Mould, S.A.; Kristensen, L.J.; Rouillon, M. Environmental arsenic, cadmium and lead dust emissions from metal mine operations: Implications for environmental management, monitoring and human health. Environ. Res. 2014, 135, 296–303. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, B.; Fan, C.; Zhao, P.; Shen, S. Human health risk from soil heavy metal contamination under different land uses near Dabaoshan Mine, Southern China. Sci. Total Environ. 2012, 417–418, 45–54. [Google Scholar] [CrossRef] [PubMed]
- IUSS Working Group. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; WRB 2014, Update 2015; FAO: Rome, Italy, 2015; pp. 1–192. [Google Scholar]
- Kabała, C.; Greinert, A.; Charzyński, P.; Uzarowicz, Ł. Technogenic soils—Soils of the year 2020 in Poland. Concept, properties and classification of technogenic soils in Poland. Soil Sci. Annu. 2020, 71, 267–280. [Google Scholar] [CrossRef]
- Górecka, E.; Kibitlewski, S.; Kryza, A.; Szuwarzyński, M. Uwarunkowania tektoniczne mineralizacji Zn-Pb na przykładzie złoża Trzebionka. Przegląd Geol. 1991, 39, 158. [Google Scholar]
- Fontboté, L.; Amstutz, G.C. Observations on ore rhythmites of the Trzebionka mine, Upper Silesian-Cracow region, Poland. In Ore Genesis. Special Publication of the Society for Geology Applied to Mineral Deposits; Springer: Berlin/Heidelberg, Germany, 1982; Volume 2, pp. 83–91. [Google Scholar] [CrossRef]
- Kasprzak, A.; Motyka, J. Wpływ zatapiania kopalni “Trzebionka” na zmiany chemizmu wód podziemnych w utworach triasu. Przegląd Geol. 2015, 63, 805–809. [Google Scholar]
- Gajowiec, B.; Witkowski, A. Impact of lead/zinc ore mining on groundwater quality in Trzebionka mine (Southern Poland). Mine Water Environ. 1993, 12, 1–10. [Google Scholar] [CrossRef]
- Klojzy-Karczmarczyk, B.; Kryza, A.; Kurek, T.; Mazurek, J. Analysis of changes in water chemistry of the Triassic aquifer in the initial phase of the Trzebionka mine flooding. Biul. Państwowego Inst. Geol. 2013, 456, 281–285. [Google Scholar]
- Klojzy-Karczmarczyk, B.; Mazurek, J. Stan chemiczny wód powierzchniowych w rejonie składowiska odpadów poflotacyjnych kopalni rud Zn-Pb “Trzebionka” na etapie jego zamykania. Biul. Państwowego Inst. Geol. 2011, 445, 301–308. [Google Scholar]
- Czop, M.; Motyka, J.; Szuwarzyński, M. Environmental impact of the AMD buffering process on the groundwater quality in the Trzebionka zinc-lead mine vincity (Souuth Poland). In Proceedings of the IMWA Symposium 2007: Water in Mining Environments, Cagliari, Italy, 27–31 May 2007. [Google Scholar]
- Żelazny, S.; Włodarczyk, B. Ocena stanu środowiska wokół osadników, odpadów poflotacyjnych w ZG” Trzebionka”. Cuprum: Czasopismo Naukowo-Techniczne Górnictwa Rud 2003, 3, 97–108. [Google Scholar]
- Trafas, M. Problemy i efekty rekultywacji osadników odpadów poflotacyjnych rud Zn i Pb na przykładzie osadnika ZG Trzebionka. Zesz. Nauk. Akad. Górniczo Hutniczej Krakowie. Sozol. Sozotech. 1988, 26, 185–199. [Google Scholar]
- Klojzy-Karczmarczyk, B.; Kurek, T.; Mazurek, J. Emisja zanieczyszczeń pyłowych na etapie eksploatacji oraz zamykania składowiska odpadów poflotacyjnych kopalni rudy Zn-Pb “Trzebionka”. Rocznik Ochrona Środowiska 2012, 14, 844–855. [Google Scholar]
- Turnau, K.; Orlowska, E.; Ryszka, P.; Zubek, S.; Anielska, T.; Gawronski, S.; Jurkiewicz, A. Role of mycorrhizal fungi in phytoremediation and toxicity monitoring of heavy metal rich industrial wastes in Southern Poland. In Soil and Water Pollution Monitoring, Protection and Remediation; Twardowska, I., Allen, H.E., Häggblom, M.M., Stefaniak, S., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 69, pp. 533–551. [Google Scholar] [CrossRef]
- Ryszka, P.; Turnau, K. Arbuscular mycorrhiza of introduced and native grasses colonizing zinc wastes: Implications for restoration practices. Plant Soil 2007, 298, 219–229. [Google Scholar] [CrossRef]
- Orłowska, E.; Ryszka, P.; Jurkiewicz, A.; Turnau, K. Effectiveness of arbuscular mycorrhizal fungal (AMF) strains in colonisation of plants involved in phytostabilisation of zinc wastes. Geoderma 2005, 129, 92–98. [Google Scholar] [CrossRef]
- Słomka, A.; Kuta, E.; Szarek-Łukaszewska, G.; Godzik, B.; Kapusta, P.; Tylko, G.; Bothe, H. Violets of the section Melanium, their colonization by arbuscular mycorrhizal fungi and their occurrence on heavy metal heaps. J. Plant Physiol. 2011, 168, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Ogar, A.; Sobczyk, Ł.; Turnau, K. Effect of combined microbes on plant tolerance to Zn-Pb contaminations. Environ. Sci. Pollut. Res. 2015, 22, 19142–19156. [Google Scholar] [CrossRef]
- Turnau, K.; Ryszka, P.; Wojtczak, G. Metal tolerant mycorrhizal plants: A review from the perspective on industrial waste in temperate region. In Arbuscular Mycorrhizas: Physiology and Function. Soil Biology; Koltai, H., Kapulnik, Y., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 257–276. [Google Scholar] [CrossRef]
- Turnau, K.; Gawroński, S.; Ryszka, P.; Zook, D. Mycorrhizal-based phytostabilization of Zn-Pb tailings: Lessons from the Trzebionka mining works (Southern Poland). In Bio-Geo Interactions in Metal-Contaminated Soils; Kothe, E., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 31, pp. 327–348. [Google Scholar] [CrossRef]
- Kędzior, R.; Szwalec, A.; Mundała, P.; Skalski, T. Ground beetle (Coleoptera, Carabidae) life history traits as indicators of habitat recovering processes in postindustrial areas. Ecol. Eng. 2020, 142, 105615. [Google Scholar] [CrossRef]
- Okrutniak, M.; Grześ, I.M.; Bonczar, Z. Species Diversity of Carabid Beetles and Ants of Two Reclaimed Tailing Ponds. Polish J. Environ. Stud. 2018, 27, 2703–2710. [Google Scholar] [CrossRef]
- Okrutniak, M.; Grześ, I.M.; Bonczar, Z. Fauna dżdżownic składowisk kopalni cynku i ołowiu. Polish J. Sustain. Dev. 2015, 19. [Google Scholar] [CrossRef]
- Brunarska, I. Speciation and Mobility of Heavy Metals (Zn, Pb, As, Cd and Tl) in Flotation Tailing Pond from ZGH “Bolesław” in Bukowno, S Poland. In Proceedings of the 1st Geochemical conference on “Contemporary problems of geochemistry”, Kielce, Poland, 27–30 September 2010; pp. 1–3. [Google Scholar]
- Egli, M.; Berger, A.; Kündig, R.; Krebs, R.; de Castro Portes, R.; Berger, R.; Widmer, R. The long-term interaction of mine tailings with soils and the wider environment: Examples from Mont Chemin, Switzerland. J. Geochem. Explor. 2017, 182, 53–69. [Google Scholar] [CrossRef][Green Version]
- Gałuszka, A.; Migaszewski, Z.M.; Dołęgowska, S.; Michalik, A. Geochemical anomalies of trace elements in unremediated soils of Mt. Karczówka, a historic lead mining area in the city of Kielce, Poland. Sci. Total Environ. 2018, 639, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Helios-Rybicka, E.; Wójcik, R. Heavy metals (Zn, Pb, Cd, Tl) and As—their mobilisation in the wastes from the Zn-Pb mining and smelting industry at the Bukowno, Upper Silesia, Paland. In Proceedings of the Securing the Future, International Conference on Mining and Environment, Skelleftea, Sweden, 27 June–1 July 2005. [Google Scholar]
- Root, R.A.; Hayes, S.M.; Hammond, C.M.; Maier, R.M.; Chorover, J. Toxic metal(loid) speciation during weathering of iron sulfide mine tailings under semi-arid climate. Appl. Geochem. 2015, 62, 131–149. [Google Scholar] [CrossRef]
- Uzarowicz, Ł.; Charzyński, P.; Greinert, A.; Hulisz, P.; Kabała, C.; Kusza, G.; Kwasowski, W.; Pędziwiatr, A. Studies of technogenic soils in Poland: Past, present, and future perspectives. Soil Sci. Annu. 2020, 71, 281–299. [Google Scholar] [CrossRef]
- Strzyszcz, Z. Właściwości fizyczne, fizykochemiczne i chemiczne odpadów poflotacyjnych rud cynku i ołowiu w aspekcie ich biologicznej rekultywacji. Arch. Ochr. Środowiska 1980, 3, 19–50. [Google Scholar]
- Turnau, K.; Ostachowicz, B.; Wojtczak, G.; Anielska, T.; Sobczyk, Ł. Metal uptake by xerothermic plants introduced into Zn-Pb industrial wastes. Plant Soil 2010, 337, 299–311. [Google Scholar] [CrossRef]
- Kozlowski, A. Origin of Zn-Pb ores in the Olkusz and Chrzanow districts: A model based on fluid inclusions. Acta Geol. Pol. 1995, 45, 83–141. [Google Scholar]
- Sobczyński, P.; Szuwarzyński, M. Dolomites and ore horizons in the Lower Muschelkalk of the Trzebionka mine (Cracow-Silesian region). Ann. Soc. Geol. Pol. 1974, 44, 545–556. [Google Scholar]
- Szuwarzyński, M. Ore bodies in the Silesian-Cracow Zn-Pb ore district, Poland. Polish Geol. Inst. Spec. Pap. 1996, 154, 9–24. [Google Scholar]
- Szuwarzynski, M. The lead and zinc ore deposits in the vicinity of Chrzanow. Kwart. Geol. 1993, 37, 209–228. [Google Scholar]
- Wodzicki, A. Origin of the Cracovian-Silesian Zn-Pb deposits. Ann. Soc. Geol. Pol. 1987, 57, 3–36. [Google Scholar]
- Trafas, M. Changes in the properties of post-flotation wastes due to vegetation introduced during process of reclamation. Appl. Geochem. 1996, 11, 181–185. [Google Scholar] [CrossRef]
- Kurek, T.; Włodarczyk, B. Eksploatacja składowiska odpadów poflotacyjnych w ZG Trzebionka S.A. WUG: Bezpieczeństwo Pracy i Ochrona Środowiska w Górnictwie 2009, 1, 17–22. [Google Scholar]
- Jahn, R.; Blume, H.; Asio, V.; Spaargaren, O.; Schad, P. Guidelines for Soil Description, 4th ed.; FAO: Rome, Italy, 2006; pp. 1–97. [Google Scholar]
- Polish Soil Classification (Systematyka Gleb Polski). Soil Science Society of Poland, Commission on Soil Genesis, Classification and Cartography; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu, Polskie Towarzystwo Gleboznawcze Wrocław: Warszawa, Poland, 2019; pp. 1–235. [Google Scholar]
- Kabała, C.; Charzyński, P.; Chodorowski, J.; Drewnik, M.; Glina, B.; Greinert, A.; Hulisz, P.; Jankowski, M.; Jonczak, J.; Łabaz, B.; et al. Polish soil classification, 6th edition—Principles, classification scheme and correlations. Soil Sci. Annu. 2019, 70, 71–97. [Google Scholar] [CrossRef]
- Bednarek, R.; Dziadowiec, H.; Pokojska, U.; Prusinkiewicz, Z. Badania Ekologiczno-Gleboznawcze; PWN: Warszawa, Poland, 2014; p. 344. [Google Scholar]
- Ditzler, C.; Scheffe, K.; Monger, H.C. Soil Science Division Staff Soil Survey Manual; USDA Handbook: Washington, DC, USA, 2017; p. 18. [Google Scholar]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; pp. 1–437. [Google Scholar]
- Zeien, H.; Brümmer, G.W. Chemische Extraktion zur bestimmung von schwermetallbindungsformen in böden. Mitteilungen Dtsch. Bodenkundlichen Gesellschaft 1989, 39, 505–510. [Google Scholar]
- Regulation of the Minister of the Environment of September 1, 2016 on How to Assess the Pollution of the Land Surface. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160001395 (accessed on 23 February 2020).
- Babbou-Abdelmalek, C.; Sebei, A.; Chaabani, F. Incurred environmental risks and potential contamination sources in an abandoned mine site. Afr. J. Environ. Sci. Technol. 2011, 5, 894–915. [Google Scholar] [CrossRef]
- Adamo, P.; Zampella, M. Chemical Speciation to Assess Potentially Toxic Metals’ (PTMs’) Bioavailability and Geochemical Forms in Polluted Soils. In Environmental Geochemistry; Elsevier: Amsterdam, The Netherlands, 2008; pp. 175–212. [Google Scholar] [CrossRef]
- González, I.; Galán, E.; Romero, A. Assessing soil quality in areas affected by sulfide mining. Application to soils in the Iberian Pyrite Belt (SW Spain). Minerals 2011, 1, 73–108. [Google Scholar] [CrossRef]
- Uzarowicz, Ł. Microscopic and microchemical study of iron sulphide weathering in a chronosequence of technogenic and natural soils. Geoderma 2013, 198, 137–150. [Google Scholar] [CrossRef]
- Ettler, V. Soil contamination near non-ferrous metal smelters: A review. Appl. Geochem. 2016, 64, 56–74. [Google Scholar] [CrossRef]
- Alloway, B.J. Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability. In Environmental Pollution, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 22, pp. 11–50. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2015; pp. 1–468. [Google Scholar] [CrossRef]
- Swęd, M.K.; Duczmal-Czernikiewicz, A. Geochemical and mineralogical characteristics of calamines from the Olkusz zinc and lead ore district (South Poland). Geol. Q. 2019, 63, 699–710. [Google Scholar] [CrossRef]
- Boni, M.; Mondillo, N.; Balassone, G.; Federico, N.; Mezzocannone, V. Zincian dolomite: A peculiar dedolomitization case? Geology 2011, 39, 183–186. [Google Scholar] [CrossRef]
- Rożek, D.; Nadłonek, W.; Cabała, J. Forms of heavy metals (Zn, Pb, Cd) occurring in rhizospheres from the areas of former and contemporary Zn-Pb ore mining. Min. Sci. 2015, 22, 125–138. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. Int. Sch. Res. Not. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Evanko, C.R.; Dzombak, D.A. Remediation of Metals-Contaminated Soils and Groundwater; Technology Evaluation Report; Ground-Water Remediation Technologies Analysis Center: Pittsburgh, PA, USA, 1997; pp. 5–13. [Google Scholar]
- Brunarska, I.; Szarek-Łukaszewska, G. Mineral composition and weathering processes in soils on oxidized zinc ore mine waste. In Buckler Mustard (Biscutella laevigata L.) an Extraordinary Plant on Ordinary Mine Heaps Near Olkusz; W. Szafer Institute of Botany, Polish Academy of Sciences: Cracow, Poland, 2020; pp. 53–77. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010; pp. 9–37. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Heavy metal sorption by clay minerals and oxides of iron and manganese. Mineral. Pol. 1980, 11, 3–12. [Google Scholar]
- Strzyszcz, Z. Bezglebowa metoda rekultywacji terenów poprzemysłowych w woj. śląskim—Osiągnięcia i zagrożenia. Rocz. Gleb. 2004, 55, 405–418. [Google Scholar]
- Cappuyns, V.; Swennen, R.; Vandamme, A.; Niclaes, M. Environmental impact of the former Pb-Zn mining and smelting in East Belgium. J. Geochem. Explor. 2006, 88, 6–9. [Google Scholar] [CrossRef]
- Trafas, M.; Eckes, T. Glebotwórcze aspekty oceny utworów sztucznych na przykładzie odpadów po flotacji rud cynku i ołowiu. Geomat. Environ. Eng. 2007, 1, 97–110. [Google Scholar]
- Aleksander-Kwaterczak, U.; Ciszewski, D. Metal Mobility in Afforested Sites of an Abandoned Zn-Pb Ore Mining Area. Appl. Sci. 2020, 10, 6041. [Google Scholar] [CrossRef]
- Strzyszcz, Z.; Łukasik, A. Zasady stosowania różnorodnych odpadów do rekultywacji biologicznej terenów poprzemysłowych na Śląsku. Gospod. Surowcami Miner. 2008, 24, 41–49. [Google Scholar]
- Pietrzykowski, M.; Piechnik, L. Rekultywacja osadników po flotacji rud cynkowo-ołowiowych. Aura 2006, 10, 30–31. [Google Scholar]
- Krzaklewski, W. Forest Reclamation and Biological Post Industrial Land Management; AR: Cracow, Poland, 1988; pp. 15–35. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).