Fractions of Ni, Pb, Cr, and Their Impact on Enzyme Activities of Arable Land Cultivated by the Simplified Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Soil Samples
2.2. Physicochemical Analysis
2.3. Enzyme Activities
2.4. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Studied Soils
3.2. Total Metal Content and Fractions
3.3. Impact of Metal Fractions on Enzyme Activities
4. Discussion
4.1. Distribution of Heavy Metals in Chemical Fractions
4.2. Correlations between Soil Characteristics and Heavy Metal Fractions
4.3. Effect of Heavy Metal Fractions on Soil Enzyme Activities
4.4. Impact of Sampling Date on Enzyme Activities and Fractional Composition of Heavy Metals
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pogorilyi, R.; Melnyk, I.; Zub, Y.; Seisenbaeva, G.; Kessler, V. Enzyme immobilization on a nanoadsorbent for improved stability against heavy metal poisoning. Colloids Surf. B 2016, 144, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Cho, H.S.; Moon, Y.C.; Ban, S.J.; Kim, J.Y. Cadmium and lead uptake capacity of energy crops and distribution of metals within the plant structures. KSCE J. Civ. Eng. 2013, 17, 44–50. [Google Scholar] [CrossRef]
- Wydro, U.; Jabłońska-Trypuć, A.; Hawrylik, E.; Butarewicz, A.; Rodziewicz, J.; Janczukowicz, W.; Wołejko, E. Heavy Metals Behavior in Soil/Plant System after Sewage Sludge Application. Energies 2021, 14, 1584. [Google Scholar] [CrossRef]
- Puga, A.P.; Abreu, C.A.; Melo, L.; Paz-Ferreiro, J.; Beesley, L. Cadmium, lead, and zinc mobility and plant uptake in a mine soil amended with sugarcane straw biochar. Environ. Sci. Pollut. Res. 2015, 22, 17606–17614. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Lin, S.; Zhao, J.; Zhang, W.; Lin, K.; Lu, Q.; Zhou, B. Toxic responses of microorganisms to nickel exposure in farmland soil in the presence of earthworm (Eisenia fetida). Chemosphere 2018, 192, 43–50. [Google Scholar] [CrossRef]
- Shang, H.; Yang, Q.; Wei, S.; Wang, J. The Effects of Mercury and Lead on Microbial Biomass of Paddy Soil from Southwest of China. Procedia Environ. Sci. 2012, 12, 468–473. [Google Scholar] [CrossRef][Green Version]
- Pushkar, B.; Sevak, P.; Parab, S.; Nilkanth, N. Chromium pollution and its bioremediation mechanisms in bacteria: A review. J. Environ. Manag. 2021, 287, 112279. [Google Scholar] [CrossRef]
- Ul Hassan, Z.; Ali, S.; Farid, M.; Rizwan, M.; Shahid, M.J. Effect of chromium (Cr) on the microbial and enzymatic activities in the soil: A Review. In Biodiversity Conservation in Changing Climate; Khan, A.A., Abid, M., Omar, A.M., Zaidi, N.H., Maheshwari, R.K., Eds.; Lenin Media Private Limited: Delhi, India, 2016; ISBN 978-93-85995-03-3. [Google Scholar]
- Oijagbe, I.J.; Abubakar, B.Y.; Edogbanya, P.R.O.; Suleiman, M.O.; Olorunmola, J.B. Effects of heavy metals on soil microbial biomass carbon. MOJ Biol. Med. 2019, 4, 30–32. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; ISBN 0-8493-1575-1. [Google Scholar]
- Wahsha, M.; Nadimi-Goki, M.; Fornasier, F.; Al-Jawasreh, R.; Hussein, E.I.; Bini, C. Microbial enzymes as an early warning management tool for monitoring mining site soils. Catena 2017, 148, 40–45. [Google Scholar] [CrossRef]
- Madejón, E.; Burgos, P.; López, R.; Cabrera, F.; López-Núñez, R. Soil enzymatic response to addition of heavy metals with organic residues. Biol. Fertil. Soils 2001, 34, 144–150. [Google Scholar] [CrossRef]
- Dec, D. Assessment of the microbiological activity in agricultural and urban soils. Soil Sci. Annu. 2014, 65, 156–160. [Google Scholar] [CrossRef][Green Version]
- Panich-Pat, T.; Wongchawalit, J.; Keawsringam, T. Lead Accumulation and Isolation of Rhizobacteria from Maize Grown in Contaminated Soil. Pol. J. Environ. Stud. 2015, 24, 2017–2020. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, M.; Xiao, R.; Chen, J.; Yu, F. Impact of salinity and Pb on enzyme activities of a saline soil from the Yellow River delta: A microcosm study. Phys. Chem. Earth 2017, 97, 77–87. [Google Scholar] [CrossRef]
- Donderski, W.; Swiontek Brzezinska, M. The influence of heavy metals on the activity of chitinases produced by planktonic, benthic and epiphytic bacteria. Pol. J. Environ. Stud. 2005, 14, 851–859. [Google Scholar]
- Dindar, E.; Şağban, F.O.T.; Başkaya, H.S. Variations of soil enzyme activities in petroleum-hydrocarbon contaminated soil. Int. Biodeterior. Biodegrad. 2015, 105, 268–275. [Google Scholar] [CrossRef]
- Hagmann, D.F.; Goodey, N.M.; Mathieu, C.; Evans, J.; Aronson, M.F.; Gallagher, F.; Krumins, J.A. Effect of metal contamination on microbial enzymatic activity in soil. Soil Biol. Biochem. 2015, 91, 291–297. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, M.; Cao, Y.; Wang, J.; Tang, M.; Ban, Y. Comparisons of Soil Properties, Enzyme Activities and Microbial Communities in Heavy Metal Contaminated Bulk and Rhizosphere Soils of Robinia pseudoacacia L. in the Northern Foot of Qinling Mountain. Forests 2017, 8, 430. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, N.S.; Singh, D.K. Impact of heavy metal contamination and seasonal variations on enzyme’s activity of Yamuna river soil in Delhi and NCR. Appl. Water Sci. 2020, 10, 83. [Google Scholar] [CrossRef]
- Institute of Meteorology and Water Management—National Research Institute. The Data Has Been Processed. 2015. Available online: https://danepubliczne.imgw.pl/data/dane_pomiarowo_obserwacyjne/dane_meteorologiczne/miesieczne/opad/2015/ (accessed on 31 May 2021).
- Polish Committee for Standardization. Soil Quality—Determination of Organic Carbon by Dichromate (VI) Oxidation in the Presence of Sulphuric Acid; PN-ISO-14235:2003—Polish Version; Polish Committee for Standardization: Warsaw, Poland, 2003. [Google Scholar]
- Polish Committee for Standardization. Soil Quality—Determination of pH; PN-ISO-10390:1997—Polish Version; Polish Committee for Standardization: Warsaw, Poland, 1997. [Google Scholar]
- Polish Committee for Standardization. Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method; PN-ISO-11261:2002—Polish Version; Polish Committee for Standardization: Warsaw, Poland, 2002. [Google Scholar]
- Polish Committee for Standardization. Soils and Mineral Deposits—Soil Sampling and Determination of Granulometric Composition; PN-R-04032:1998—Polish Version; Polish Committee for Standardization: Warsaw, Poland, 1998. [Google Scholar]
- Polish Committee for Standardization. Soils and Mineral Deposits—Division into Fractions and Granulometric Groups; PN-R-04033:1998—Polish Version; Polish Committee for Standardization: Warsaw, Poland, 1998. [Google Scholar]
- Polish Committee for Standardization. Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia; PN-ISO-11466:2002—Polish Version; Polish Committee for Standardization: Warsaw, Poland, 2002. [Google Scholar]
- Leśniewska, B.; Świerad, E.; Łukowski, A.; Wiater, J.; Godlewska-Żyłkiewicz, B. Ultrasound assisted extraction for determi-nation of mobile fractions of copper in soil. Rocz. Państwowego Zakładu Hig. 2014, 65, 67–74. [Google Scholar]
- Thalmann, A. Zur Methodik der Bestimmung der Dehydrogenase Aktivität in Boden Mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenylphosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Macura, J.; Vágnerová, K. Kolorimetrická metoda stanoveni aktivity proteolityckych enzymuv pude. Rostl. Vyrob. 1969, 15, 173–180. [Google Scholar]
- Hoffmann, G.; Teicher, K. Ein kolorimetrisches Verfahren zur Bestimmung der Ureaseaktivität in Böden. J. Plant Nutr. Soil Sci. 1961, 95, 55–63. [Google Scholar] [CrossRef]
- Łukowski, A.; Dec, D. Influence of Zn, Cd, and Cu fractions on enzymatic activity of arable soils. Environ. Monit. Assess. 2018, 190, 278. [Google Scholar] [CrossRef]
- Khadhar, S.; Sdiri, A.; Chekirben, A.; Azouzi, R.; Charef, A. Integration of sequential extraction, chemical analysis and statistical tools for the availability risk assessment of heavy metals in sludge amended soils. Environ. Pollut. 2020, 263, 114543. [Google Scholar] [CrossRef]
- Martínez-Toledo, Á.; Montes-Rocha, A.; González-Mille, D.J.; Espinosa-Reyes, G.; Torres-Dosal, A.; Mejia-Saavedra, J.J.; Ilizaliturri-Hernández, C.A. Evaluation of enzyme activities in long-term polluted soils with mine tailing deposits of San Luis Potosí, México. J. Soils Sediments 2017, 17, 364–375. [Google Scholar] [CrossRef]
- Salam, A.; Shaheen, S.M.; Bashir, S.; Khan, I.; Wang, J.; Rinklebe, J.; Rehman, F.U.; Hu, H. Rice straw- and rapeseed residue-derived biochars affect the geochemical fractions and phytoavailability of Cu and Pb to maize in a contaminated soil under different moisture content. J. Environ. Manag. 2019, 237, 5–14. [Google Scholar] [CrossRef]
- Ure, A.M.; Quevauviller, P.; Muntau, H.; Griepink, B. Speciation of Heavy Metals in Soils and Sediments. An Account of the Improvement and Harmonization of Extraction Techniques Undertaken Under the Auspices of the BCR of the Commission of the European Communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Rodgers, K.J.; Hursthouse, A.; Cuthbert, S. The Potential of Sequential Extraction in the Characterisation and Management of Wastes from Steel Processing: A Prospective Review. Int. J. Environ. Res. Public Health 2015, 12, 11724–11755. [Google Scholar] [CrossRef] [PubMed]
- Zeiner, M.; Cindrić, I.J.; Mikelić, I.L.; Medunić, G.; Kampić, Š.; Tomašić, N.; Stingeder, G. The determination of the extractability of selected elements from agricultural soil. Environ. Monit. Assess. 2012, 185, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lee, L.; Dayan, S.; Grinshtein, M.; Shaw, R. Speciation of heavy metals in garden soils: Evidences from selective and sequential chemical leaching. J. Soils Sediments 2011, 11, 628–638. [Google Scholar] [CrossRef]
- Sungur, A.; Soylak, M.; Özcan, H. Chemical fractionation, mobility and environmental impacts of heavy metals in greenhouse soils from Çanakkale, Turkey. Environ. Earth Sci. 2016, 75, 334–344. [Google Scholar] [CrossRef]
- Gattullo, C.E.; Allegretta, I.; Porfido, C.; Rascio, I.; Spagnuolo, M.; Terzano, R. Assessing chromium pollution and natural stabilization processes in agricultural soils by bulk and micro X-ray analyses. Environ. Sci. Pollut. Res. 2020, 27, 22967–22979. [Google Scholar] [CrossRef]
- Bilguun, U.; Namkhainyambuu, D.; Purevsuren, B.; Soyol-Erdene, T.-O.; Tuuguu, E.; Daichaa, D. Sources, Enrichment, and Geochemical Fractions of Soil Trace Metals in Ulaanbaatar, Mongolia. Arch. Environ. Contam. Toxicol. 2020, 79, 219–232. [Google Scholar] [CrossRef]
- Zong, Y.; Xiao, Q.; Lu, S. Distribution, bioavailability, and leachability of heavy metals in soil particle size fractions of urban soils (northeastern China). Environ. Sci. Pollut. Res. 2016, 23, 14600–14607. [Google Scholar]
- Boughattas, I.; Hattab, S.; Alphonse, V.; Livet, A.; Giusti-Miller, S.; Boussetta, H.; Banni, M.; Bousserrhine, N. Use of earthworms Eisenia andrei on the bioremediation of contaminated area in north of Tunisia and microbial soil enzymes as bioindicator of change on heavy metals speciation. J. Soils Sediments 2019, 19, 296–309. [Google Scholar] [CrossRef]
- Qu, J.; Ren, G.; Chen, B.; Fan, J.; Yong, E. Effects of lead and zinc mining contamination on bacterial community diversity and enzyme activities of vicinal cropland. Environ. Monit. Assess. 2011, 182, 597–606. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Kurcharski, J.; Lajszner, W. The effects of copper on soil biochemical properties and its interaction with other heavy metals. Pol. J. Environ. Stud. 2006, 15, 927–934. [Google Scholar]
- Miśkowiec, P.; Olech, Z. Searching for the Correlation Between the Activity of Urease and the Content of Nickel in the Soil Samples: The Role of Metal Speciation. J. Soil Sci. Plant Nutr. 2020, 20, 1904–1911. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Alam Cheema, S.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment—A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef]
- Kotroczó, Z.; Veres, Z.; Fekete, I.; Krakomperger, Z.; Tóth, J.A.; Lajtha, K.; Tóthmérész, B. Soil enzyme activity in response to long-term organic matter manipulation. Soil Biol. Biochem. 2014, 70, 237–243. [Google Scholar] [CrossRef]
- Antil, R.S.; Mahata, M.K.; Narwal, R.P. Effect of substrate concentration, soil moisture, and organic materials on urease activity of soil contaminated with lead. Arch. Agron. Soil Sci. 2006, 52, 61–68. [Google Scholar] [CrossRef]
- Varga, B.; Janda, T.; László, E.; Veisz, O. Influence of abiotic stresses on the antioxidant enzyme activity of cereals. Acta Physiol. Plant. 2011, 34, 849–858. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Y.; Zhang, J.; Chen, Y.; Yang, L.; Li, H.; Wang, L. Factors influencing soil enzyme activity in China’s forest ecosystems. Plant Ecol. 2017, 219, 31–44. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Y.; Xu, Z.; Gu, X.; Xu, J.; Tao, Y.; He, H.; Wang, A.; Liu, Y.; Niu, L. Soil Microbial Community and Enzyme Activity Responses to Herbaceous Plant Expansion in the Changbai Mountains Tundra, China. Chin. Geogr. Sci. 2019, 29, 985–1000. [Google Scholar] [CrossRef]
- Łukowski, A.; Wiater, J. Influence of mineral fertilization on lead, cadmium and chromium fraction contens in soil. Pol. J. Environ. Stud. 2011, 20, 951–960. [Google Scholar]

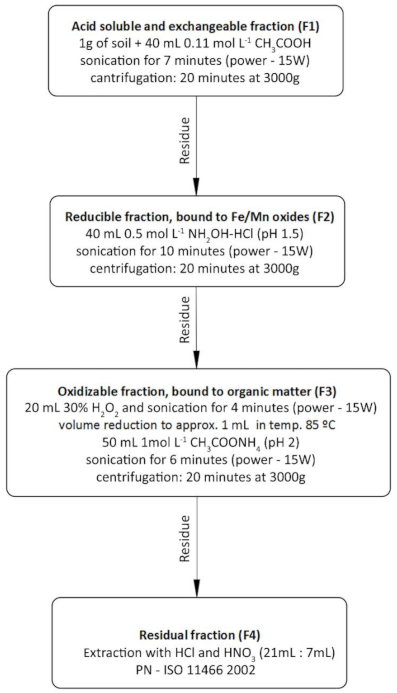
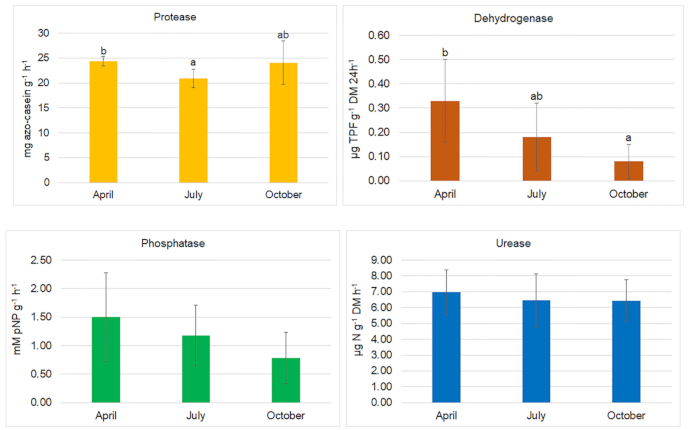
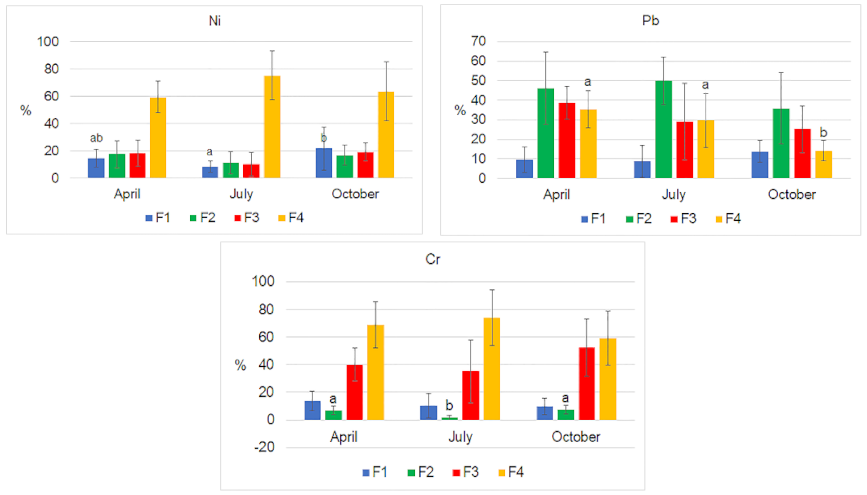
| Month | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Rainfall Summary, mm | 64.2 | 18.9 | 52.0 | 36.1 | 49.3 | 32.6 | 70.1 | 12.9 | 84.1 | 14.9 | 118.4 | 54.5 |
| Average Temperature, °C | − 0.7 | − 1.0 | 4.2 | 6.8 | 11.3 | 15.4 | 17.6 | 20.3 | 14.0 | 6.1 | 4.2 | 2.6 |
| Sampling Site | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Protease mg azo-casein g−1·h−1 | 24.26 ± 2.07 | 22.27 ± 1.91 | 21.04 ± 2.05 | 24.27 ± 0.72 | 22.40 ± 1.64 | 21.08 ± 1.91 | 26.68 ± 5.56 | 22.43 ± 1.55 | 24.39 ± 1.78 |
| Phosphatase mM pNP g−1·h−1 | 0.89 ± 0.24 | 1.66 ± 0.17 | 0.96 ± 0.29 | 0.93 ± 0.21 | 1.66 ± 0.88 | 0.72 ± 0.27 | 1.41 ± 0.80 | 1.07 ± 0.61 | 0.38 ± 0.05 |
| Dehydrogenase μg TPF g−1 DM 24h−1 | 0.11 ± 0.05 | 0.11 ± 0.01 | 0.22 ± 0.12 | 0.34 ± 0.18 | 0.23 ± 0.14 | 0.18 ± 0.11 | 0.19 ± 0.15 | 0.16 ± 0.15 | 0.04 ± 0.02 |
| Urease µgN g−1 DM h−1 | 7.36 ± 0.83 | 8.43 ± 0.41 | 6.38 ± 0.92 | 5.09 ± 1.28 | 5.65 ± 0.52 | 6.61 ± 1.76 | 7.43 ± 1.23 | 6.40 ± 0.68 | 6.18 ± 1.12 |
| pH | 6.5 ± 0.3 | 5.7 ± 0.7 | 4.9 ± 0.7 | 6.5 ± 0.9 | 5.7 ± 0.7 | 5.2 ± 0.9 | 5.7 ± 0.1 | 5.9 ± 1.2 | 5.1 ± 1.8 |
| Organic C, % | 1.87 ± 1.68 | 2.05 ± 0.35 | 2.02 ± 0.21 | 2.37 ± 1.52 | 2.25 ± 0.30 | 1.19 ± 0.33 | 3.36 ± 2.68 | 1.48 ± 0.35 | 1.12 ± 0.27 |
| N, % | 0.31 ± 0.02 | 0.19 ± 0.01 | 0.27 ± 0.03 | 0.32 ± 0.13 | 0.31 ± 0.15 | 0.22 ± 0.05 | 0.23 ± 0.04 | 0.28 ± 0.07 | 0.21 ± 0.02 |
| Sand, % | 20.9 ± 14.5 | 21.9 ± 1.1 | 22.4 ± 6.6 | 27.2 ± 9.1 | 23.4 ± 0.7 | 39.0 ± 11.6 | 22.1 ± 1.8 | 36.2 ± 24.2 | 56.1 ± 8.3 |
| Silt, % | 25.0 ± 9.0 | 15.6 ± 4.9 | 24.6 ± 4.7 | 29.9 ± 1.9 | 22.7 ± 5.2 | 32.0 ± 4.6 | 30.1 ± 3.9 | 31.5 ± 2.4 | 16.5 ± 1.3 |
| Clay, % | 27.6 ± 4.1 | 27.4 ± 2.0 | 30.7 ± 5.2 | 7.6 ± 0.9 | 31.4 ± 3.5 | 8.2 ± 1.5 | 29.6 ± 3.8 | 7.3 ± 2.1 | 8.3 ± 1.4 |
| Ni, mg kg−1 | 13.0 ± 6.2 | 21.9 ± 2.2 | 17.1 ± 4.0 | 9.5 ± 2.6 | 13.0 ± 7.7 | 10.2 ± 6.0 | 10.5 ± 3.7 | 8.1 ± 3.1 | 10.3 ± 9.4 |
| Pb, mg kg−1 | 11.7 ± 4.7 | 16.6 ± 2.0 | 13.8 ± 2.5 | 12.2 ± 5.2 | 15.8 ± 9.2 | 10.1 ± 1.8 | 13.8 ± 6.0 | 11.0 ± 0.4 | 7.1 ± 1.9 |
| Cr, mg kg−1 | 28.8 ± 13.7 | 45.6 ± 3.8 | 35.3 ± 10.0 | 22.0 ± 5.7 | 27.8 ± 13.1 | 24.9 ± 14.4 | 19.1 ± 3.5 | 21.5 ± 2.9 | 12.6 ± 3.1 |
| pH | Organic C | N | Sand | Silt | Clay | |
|---|---|---|---|---|---|---|
| Protease | 0.253 | 0.139 | 0.001 | −0.124 | 0.047 | −0.030 |
| Phosphatase | −0.204 | 0.035 | −0.044 | −0.306 | −0.149 | 0.200 |
| Dehydrogenase | −0.240 | −0.299 | 0.290 | −0.061 | 0.038 | −0.154 |
| Urease | 0.070 | 0.022 | −0.283 | −0.341 | −0.168 | 0.266 |
| Ni | Pb | Cr | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F1 | F2 | F3 | F4 | F1 | F2 | F3 | F4 | |
| pH | 0.080 | 0.037 | −0.070 | −0.022 | 0.326 | −0.135 | 0.002 | −0.142 | −0.115 | −0.266 | 0.120 | −0.149 |
| Organic C | 0.299 | 0.099 | 0.054 | −0.277 | 0.293 | −0.264 | 0.117 | −0.328 | −0.009 | 0.197 | 0.524 * | −0.301 |
| Sand | 0.667 * | −0.050 | 0.082 | −0.209 | 0.628 * | −0.312 | −0.184 | −0.254 | 0.398 * | 0.244 | 0.224 | −0.499 * |
| Silt | 0.271 | 0.312 | 0.331 | −0.079 | 0.184 | 0.249 | −0.121 | −0.482 * | 0.100 | 0.276 | 0.129 | −0.390 * |
| Clay | −0.497 * | −0.282 | −0.124 | 0.209 | −0.487 * | 0.228 | −0.098 | 0.044 | −0.352 | −0.272 | −0.177 | 0.298 |
| Ni | Pb | Cr | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Sampling in April | ||||||||||||
| F1 | F2 | F3 | F4 | F1 | F2 | F3 | F4 | F1 | F2 | F3 | F4 | |
| pH | −0.420 | −0.289 | −0.201 | −0.096 | −0.291 | −0.394 | 0.124 | −0.100 | −0.237 | −0.261 | −0.278 | 0.037 |
| Organic C | −0.177 | −0.001 | 0.297 | −0.806 * | −0.091 | −0.043 | 0.832 * | −0.640 | 0.330 | 0.292 | 0.783 * | −0.261 |
| Protease | −0.291 | −0.263 | 0.103 | 0.058 | −0.325 | −0.101 | −0.011 | −0.171 | −0.107 | −0.137 | −0.093 | 0.002 |
| Phosphatase | −0.033 | 0.087 | −0.050 | −0.650 | −0.047 | 0.047 | 0.491 | −0.505 | 0.354 | 0.181 | 0.508 | −0.379 |
| Dehydrogenase | 0.806 * | 0.441 | 0.527 | 0.130 | 0.570 | 0.742 * | −0.416 | −0.008 | 0.464 | 0.595 | 0.166 | −0.473 |
| Urease | −0.641 | −0.165 | −0.057 | 0.124 | −0.235 | −0.843 * | 0.419 | 0.234 | −0.271 | −0.356 | −0.134 | 0.438 |
| Soil Sampling in July | ||||||||||||
| pH | 0.611 | 0.515 | 0.177 | −0.305 | 0.600 | 0.206 | 0.453 | 0.275 | 0.521 | 0.214 | 0.448 | −0.139 |
| Organic C | −0.498 | −0.330 | −0.478 | −0.331 | 0.220 | −0.501 | 0.558 | −0.191 | 0.628 | −0.263 | 0.405 | −0.003 |
| Protease | 0.637 | 0.653 | 0.263 | −0.360 | 0.490 | 0.260 | 0.210 | 0.266 | 0.218 | 0.121 | 0.182 | −0.024 |
| Phosphatase | −0.688 * | −0.378 | −0.602 | −0.284 | 0.355 | −0.387 | 0.316 | −0.310 | 0.422 | −0.435 | 0.258 | −0.201 |
| Dehydrogenase | 0.564 | 0.532 | 0.346 | −0.094 | 0.040 | 0.146 | −0.007 | 0.080 | 0.195 | 0.057 | −0.083 | 0.015 |
| Urease | −0.202 | −0.427 | 0.290 | 0.707 * | −0.262 | 0.324 | −0.374 | −0.453 | −0.462 | −0.053 | −0.093 | −0.574 |
| Soil Sampling in October | ||||||||||||
| pH | 0.047 | 0.113 | −0.107 | −0.022 | 0.433 | −0.056 | −0.087 | 0.118 | −0.502 | −0.543 | −0.116 | −0.195 |
| Organic C | 0.342 | 0.247 | −0.101 | −0.191 | 0.517 | −0.238 | 0.038 | −0.098 | −0.324 | 0.142 | 0.545 | −0.348 |
| Protease | 0.003 | 0.022 | −0.152 | 0.366 | 0.417 | −0.038 | −0.120 | 0.567 | −0.183 | 0.036 | 0.261 | 0.146 |
| Phosphatase | −0.731 * | 0.058 | −0.423 | 0.242 | −0.702 * | 0.207 | −0.233 | 0.134 | −0.464 | −0.632 | −0.473 | 0.708 * |
| Dehydrogenase | −0.356 | −0.248 | 0.392 | −0.772 * | −0.266 | 0.544 | 0.518 | −0.241 | −0.279 | −0.291 | 0.053 | −0.382 |
| Urease | −0.437 | −0.118 | −0.360 | −0.471 | −0.089 | 0.522 | 0.029 | 0.282 | −0.497 | −0.622 | −0.282 | 0.103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łukowski, A.; Dec, D. Fractions of Ni, Pb, Cr, and Their Impact on Enzyme Activities of Arable Land Cultivated by the Simplified Method. Minerals 2021, 11, 584. https://doi.org/10.3390/min11060584
Łukowski A, Dec D. Fractions of Ni, Pb, Cr, and Their Impact on Enzyme Activities of Arable Land Cultivated by the Simplified Method. Minerals. 2021; 11(6):584. https://doi.org/10.3390/min11060584
Chicago/Turabian StyleŁukowski, Adam, and Dorota Dec. 2021. "Fractions of Ni, Pb, Cr, and Their Impact on Enzyme Activities of Arable Land Cultivated by the Simplified Method" Minerals 11, no. 6: 584. https://doi.org/10.3390/min11060584
APA StyleŁukowski, A., & Dec, D. (2021). Fractions of Ni, Pb, Cr, and Their Impact on Enzyme Activities of Arable Land Cultivated by the Simplified Method. Minerals, 11(6), 584. https://doi.org/10.3390/min11060584







