Relationship between Chamosite Alteration and Fe-Plugging in Sandstone Pores during Acid In Situ Leaching of Uranium
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussions
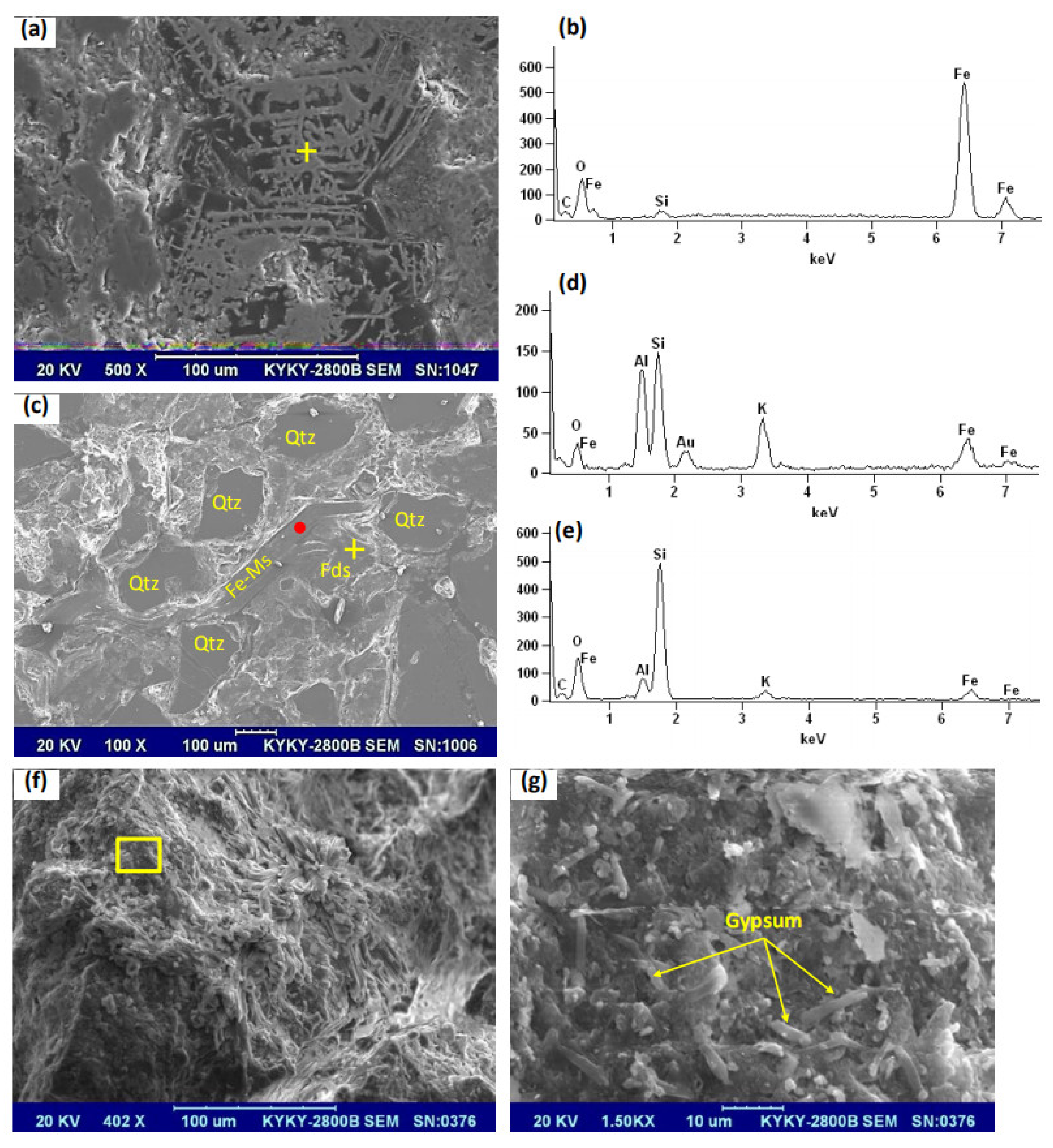
4.1. Identification of Plugging Materials
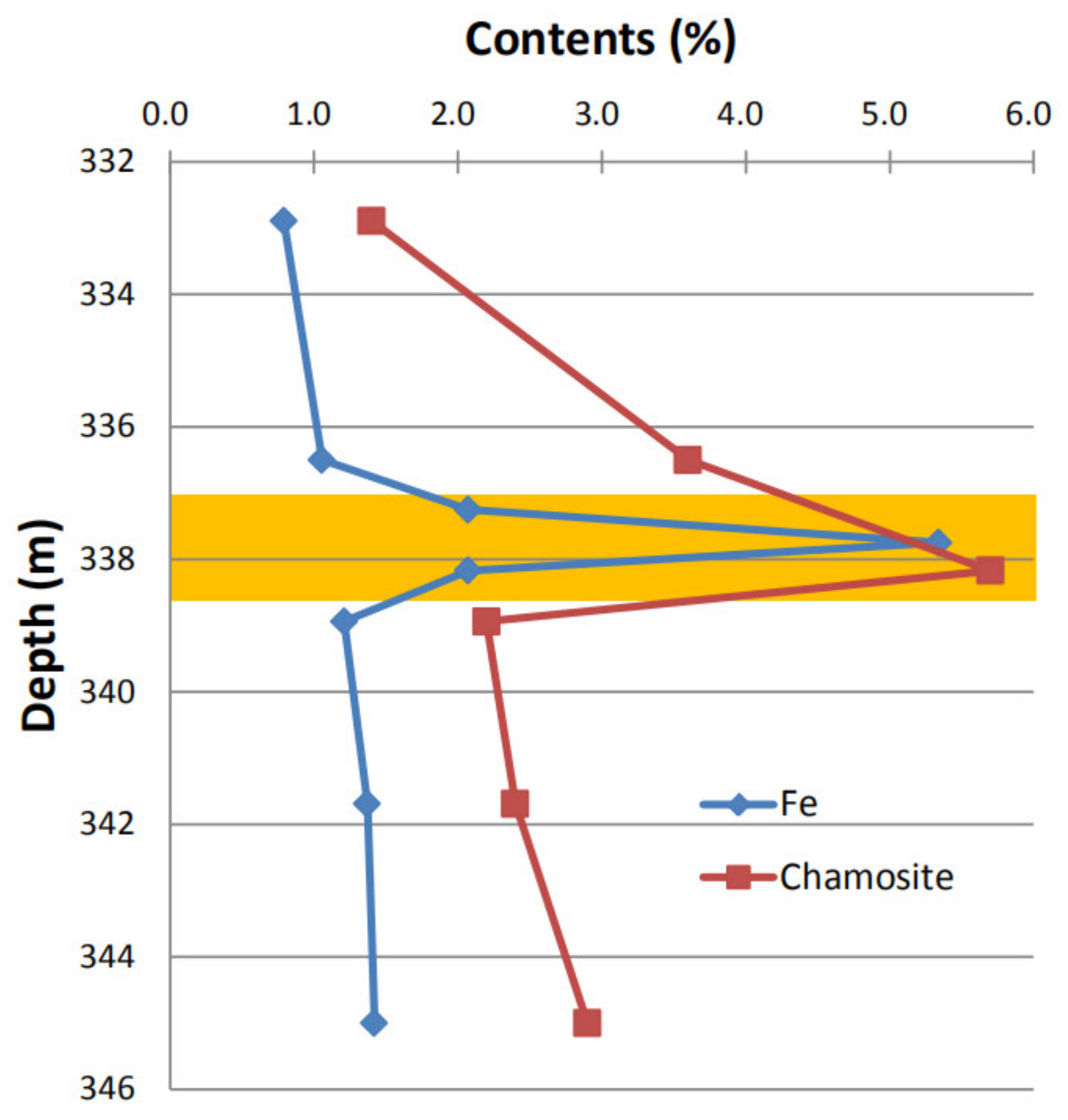
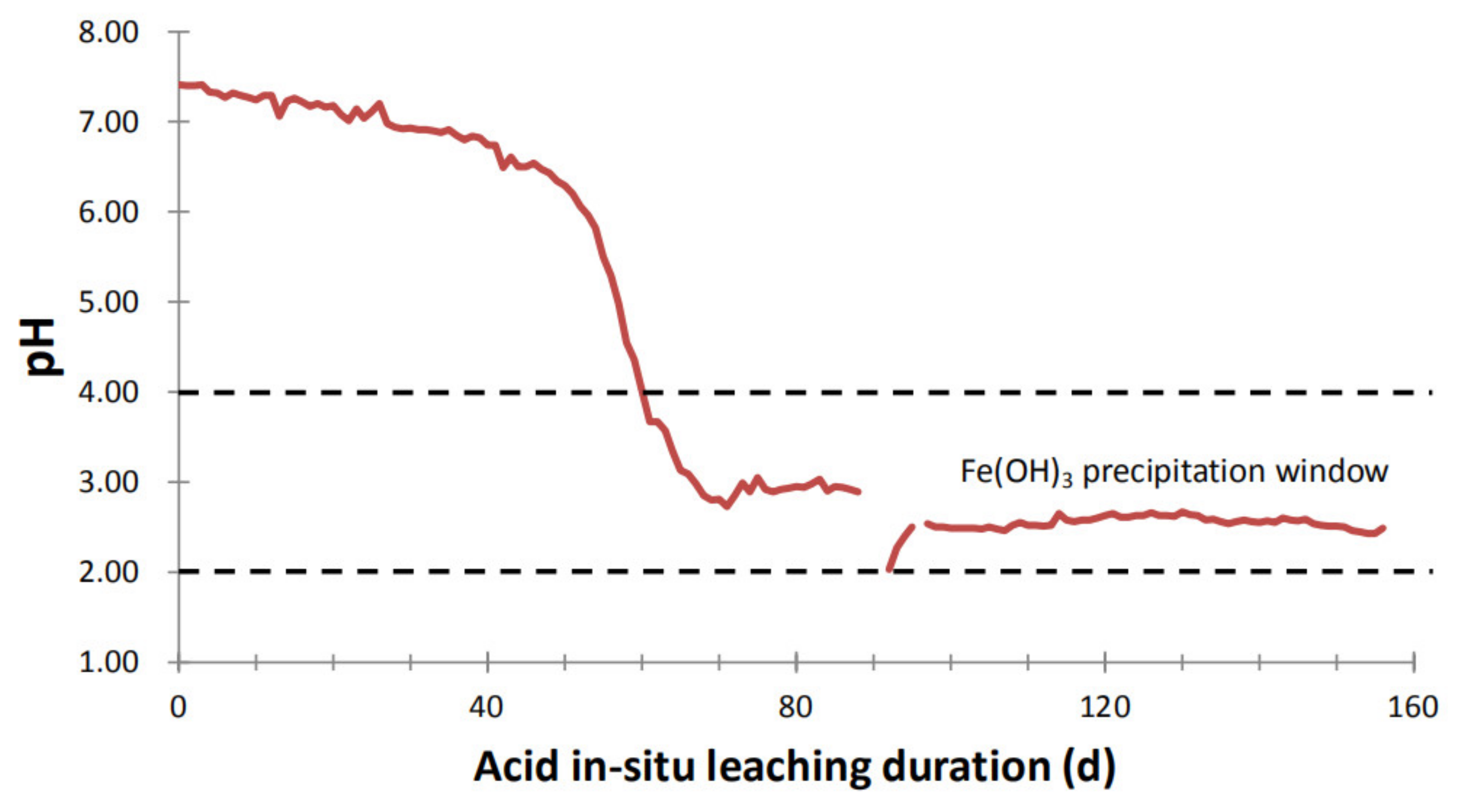
4.2. Source of Iron and the Plugging Mechanism
4.3. Implication for Antiplugging
5. Conclusions
- The pore-plugging in the course of acid ISL of sandstone uranium deposits in Yili Basin, NW China, is caused by precipitation of Fe(OH)3 and a slight of gypsum (CaSO4·2H2O).
- Alteration/dissolution of chamosite and to a less extent of Fe-bearing muscovite and feldspar provides Fe for Fe(OH)3 precipitation. Hydrolysis of ferric ions in acidic environment with pH of underground solutions at 2.0–4.0 generates Fe(OH)3 precipitates plugging the sandstone in the way of reducing the size and connectivity of pores.
- Our study suggests that the main and most simple measure to avoid such Fe(OH)3 plugging would be to sustain pH values of the solutions throughout the leaching passage below 2.0.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ilankoon, I.M.S.K.; Tang, Y.; Ghorbani, Y.; Northey, S.; Yellishetty, M.; Deng, X.; McBride, D. The current state and future directions of percolation leaching in the Chinese mining industry: Challenges and opportunities. Miner. Eng. 2018, 125, 206–222. [Google Scholar] [CrossRef]
- Seredkin, M.; Zabolotsky, A.; Jeffress, G. In situ recovery, an alternative to conventional methods of mining: Exploration, resource estimation, environmental issues, project evaluation and economics. Ore Geol. Rev. 2016, 79, 500–514. [Google Scholar] [CrossRef]
- Abzalov, M.Z. Sandstone-hosted uranium deposits amenable for exploitation by in situ leaching technologies. Appl. Earth Sci. 2012, 121, 55–64. [Google Scholar] [CrossRef]
- Zammit, C.M.; Brugger, J.; Southam, G.; Reith, F. In situ recovery of uranium—The microbial influence. Hydrometallurgy 2014, 150, 236–244. [Google Scholar] [CrossRef]
- Saunders, J.A.; Pivetz, B.E.; Voorhies, N.; Wilkin, R.T. Potential aquifer vulnerability in regions down-gradient from uranium in situ recovery (ISR) sites. J. Environ. Manag. 2016, 183, 67–83. [Google Scholar] [CrossRef]
- Nuclear Energy Agency and International Atomic Energy Agency. Uranium 2020: Resources, Production and Demand; Nuclear Energy Agency: Paris, France, 2020. [Google Scholar]
- Mudd, G.M. Critical review of acid in situ leach uranium mining 1. USA and Australia. Environ. Geol. 2001, 4, 390–403. [Google Scholar] [CrossRef]
- Mudd, G.M. Critical review of acid in situ leach uranium mining 2. Soviet block and Asia. Environ. Geol. 2001, 41, 404–416. [Google Scholar] [CrossRef]
- Que, W.; Tan, Y.; Zeng, Y.; Wang, S. Geochemical Kinetics and Mass Transport of In-Situ Uranium Leaching; China Nuclear Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Dangelmayr, M.A.; Reimus, P.W.; Wasserman, N.L.; Punsal, J.J.; Johnson, R.H.; Clay, J.T.; Stone, J.J. Laboratory column experiments and transport modeling to evaluate retardation of uranium in an aquifer downgradient of a uranium in-situ recovery site. Appl. Geochem. 2017, 80, 1–13. [Google Scholar] [CrossRef]
- Sun, Z.X.; Liu, J.H.; Shi, W.J.; Jiao, X.R. Mechanism of Chemical Plugging during the In-Situ Leach Mining Process in the Shihongtan Uranium Deposit, Xinjiang, China Water-Rock Interaction; ISTP: Guanajuato, Mexico, 2010; pp. 559–563. [Google Scholar]
- Pownceby, M.I.; Johnson, C. Geometallurgy of Australian uranium deposits. Ore Geol. Rev. 2014, 56, 25–44. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, J.; Xu, Y.; Zhang, C. Mineral alteration and pore-plugging caused by acid in situ leaching: A case study of the Wuyier uranium deposit, Xinjiang, NW China. Arab. J. Geosci. 2018, 11, 707. [Google Scholar] [CrossRef]
- Ben Simon, R.; Thiry, M.; Schmitt, J.-M.; Lagneau, V.; Langlais, V.; Bélières, M. Kinetic reactive transport modelling of column tests for uranium In Situ Recovery (ISR) mining. Appl. Geochem. 2014, 51, 116–129. [Google Scholar] [CrossRef]
- Sun, Z.X.; Gao, B.; Liu, J.H.; Shi, W.J. Geochemical modelling of fluid-rock interaction during acid ISL uranium mining in SHT Uranium Deposit, NW-China. Geochim. Cosmochim. Acta 2009, 73, A1294. [Google Scholar]
- Hou, B.; Keeling, J.; Li, Z. Paleovalley-related uranium deposits in Australia and China: A review of geological and exploration models and methods. Ore Geol. Rev. 2017, 88, 201–234. [Google Scholar] [CrossRef]
- Min, M.; Chen, J.; Wang, J.; Wei, G.; Fayek, M. Mineral paragenesis and textures associated with sandstone-hosted roll-front uranium deposits, NW China. Ore Geol. Rev. 2005, 26, 51–69. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, G.; Xu, L.; Liu, J.; Sun, Z.; Shi, W. Uranium recovery from sandstone-type uranium deposit by acid in-situ leaching—An example from the Kujieertai. Hydrometallurgy 2020, 191, 105209. [Google Scholar] [CrossRef]
- Li, P.; Liu, G.; Duan, B.; Feng, G.; Shao, Y.; Zhou, J. Analysis on the problems of high acid consumption and low uranium concentration leachates during in-situ leaching for a deposit in Xinjiang. Uranium Min. Metall. 2018, 37, 26–31, (In Chinese with English abstract). [Google Scholar]
- Dai, S.; Yang, J.; Ward, C.R.; Hower, J.C.; Liu, H.; Garrison, T.M.; French, D.; O’Keefe, J.M.K. Geochemical and mineralogical evidence for a coal-hosted uranium deposit in the Yili Basin, Xinjiang, northwestern China. Ore Geol. Rev. 2015, 70, 1–30. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, F.-J.; Xia, F.; Zhang, C.-Y.; Feng, Z.-B.; Ullah, R.; Zhang, P.-F. Provenance constraints on the Xishanyao Formation, southern Yili Basin, northwest China: Evidence from petrology, geochemistry, and detrital zircon U-Pb geochronology. Can. J. Earth Sci. 2018, 55, 1020–1035. [Google Scholar] [CrossRef]
- Yue, S.; Wang, G. Relationship between the hydrogeochemical environment and sandstone-type uranium mineralization in the Ili basin, China. Appl. Geochem. 2011, 26, 133–139. [Google Scholar] [CrossRef]
- Song, H.; Ni, S.; Chi, G.; Zhang, C.; Hou, M.; Liu, H.; Wang, G.; Yan, W. Systematic variations of H-O-C isotopes in different alteration zones of sandstone-hosted uranium deposits in the southern margin of the Yili Basin (Xinjiang, China): A review and implications for the ore-forming mechanisms. Ore Geol. Rev. 2019, 107, 615–628. [Google Scholar] [CrossRef]
- SY/T 5163-2010. Analysis Method for Clay Minerals and Ordinary Non-Clay Minerals in Sedimentary Rocks by the X-ray Diffraction; Petroleum Industrial Publishing House: Beijing, China, 2010. (In Chinese) [Google Scholar]
- Min, M.; Luo, X.-Z.; Mao, S.-L.; Wang, Z.-Q.; Wang, R.C.; Qin, L.-F.; Tan, X.-L. An excellent fossil wood cell texture with primary uranium minerals at a sandstone-hosted roll-type uranium deposit, NW China. Ore Geol. Rev. 2001, 17, 233–239. [Google Scholar] [CrossRef]
- Niu, Y.; Yao, Y.; Wen, Z.; Xu, G. Simulation and Control of In-Situ Uranium Leaching; Beijing Research Institute of Chemical Engineering and Metallurgy: Beijing, China, 2016. (In Chinese) [Google Scholar]
- Chandra, A.P.; Gerson, A.R. The mechanisms of pyrite oxidation and leaching: A fundamental perspective. Surf. Sci. Rep. 2010, 65, 293–315. [Google Scholar] [CrossRef]
- Wen, Z.; Yao, Y.; Niu, Y.; Xu, G.; Xie, T.; He, K.; Zhang, C. Formation of precipitation and its effect on uranium in-situ leaching process by acid. Uranium Min. Metall. 2015, 34, 171–177, (In Chinese with English abstract). [Google Scholar]
- Yuan, G.; Cao, Y.; Gluyas, J.; Jia, Z. Reactive transport modeling of coupled feldspar dissolution and secondary mineral precipitation and its implication for diagenetic interaction in sandstones. Geochim. Cosmochim. Acta 2017, 207, 232–255. [Google Scholar] [CrossRef]
- Baumgartner, J.; Faivre, D. Iron solubility, colloids and their impact on iron (oxyhydr)oxide formation from solution. Earth-Sci. Rev. 2015, 150, 520–530. [Google Scholar] [CrossRef]
- Rosenberg, Y.O.; Reznik, I.J.; Zmora-Nahum, S.; Ganor, J. The effect of pH on the formation of a gypsum scale in the presence of a phosphonate antiscalant. Desalination 2012, 284, 207–220. [Google Scholar] [CrossRef]
- Shukla, J.; Mohandas, V.P.; Kumar, A. Effect of pH on the Solubility of CaSO4·2H2O in Aqueous NaCl Solutions and Physicochemical Solution Properties at 35 °C. J. Chem. Eng. Data 2008, 53, 2797–2800. [Google Scholar] [CrossRef]
- Tan, K.; Li, C.; Liu, J.; Qu, H.; Xia, L.; Hu, Y.; Li, Y. A novel method using a complex surfactant for in-situ leaching of low permeable sandstone uranium deposits. Hydrometallurgy 2014, 150, 99–106. [Google Scholar] [CrossRef]
- Espenscheid, W.F.; Heilweil, I.J.; Yan, T.Y. Calcite Control in an In Situ Leach Operation. US Patent US 4,103,963, 1 August 1978. [Google Scholar]
- Yan, T.Y. Calcite Removal from Carbonated Water—Uranium in Situ Leaching System. Min. Metall. Explor. 1984, 1, 130–135. [Google Scholar] [CrossRef]
- Su, X.; Wang, H.; Liu, N. CO2+O2 In-Situ Uranium Leaching; China Nuclear Energy Press: Beijing, China, 2016. (In Chinese) [Google Scholar]

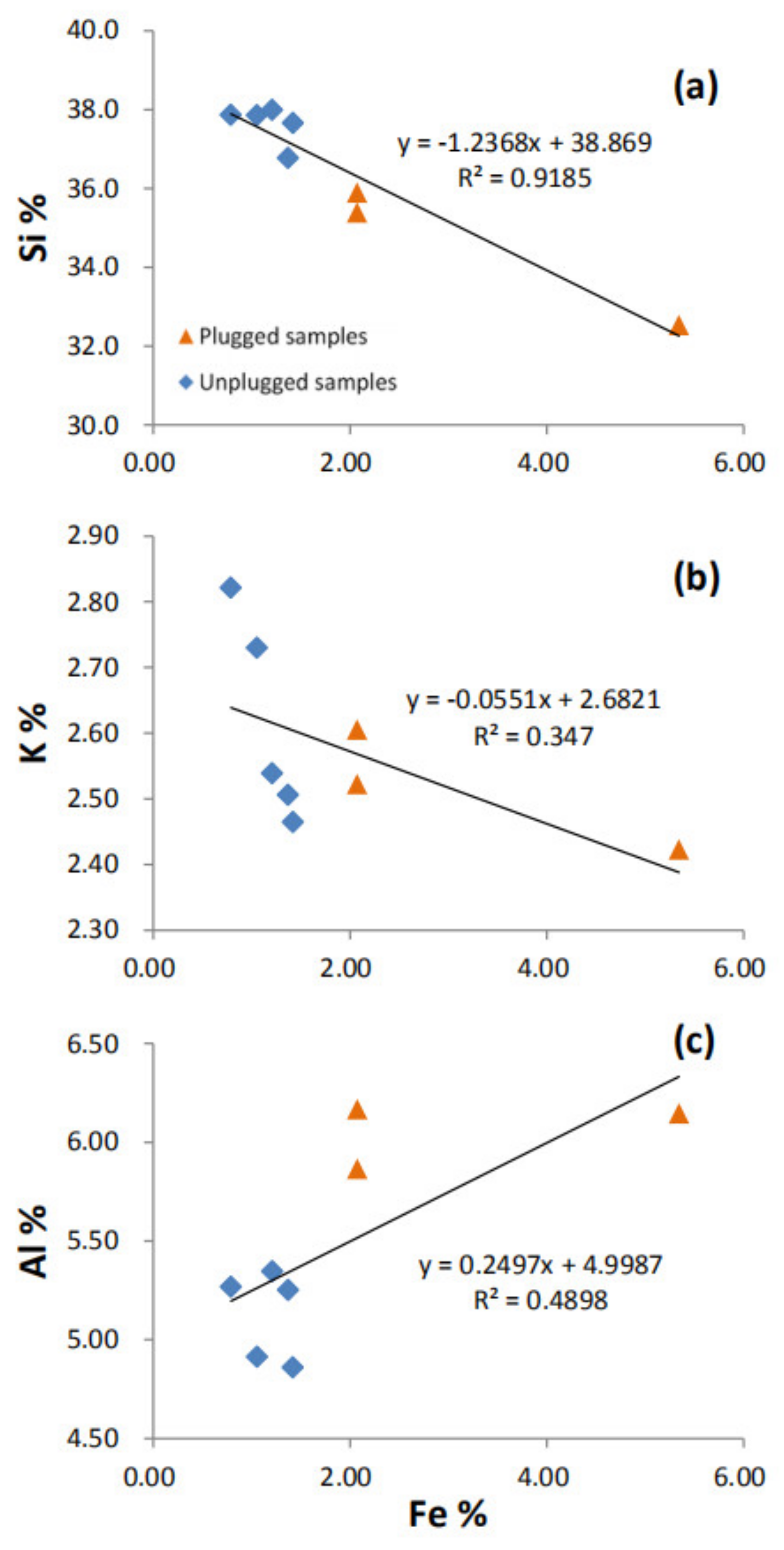


| Sample Location | Samples | Si | Ti | Al | Fe | Mn | Mg | Ca | Na | K | P | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unplugged area | UP-1 | 37.9 | 0.13 | 5.27 | 0.79 | 0.01 | 0.21 | 0.22 | 0.80 | 2.82 | 0.03 | 0.18 |
| UP-2 | 37.9 | 0.13 | 4.91 | 1.06 | 0.01 | 0.28 | 0.31 | 0.64 | 2.73 | 0.02 | 0.20 | |
| Plugged samples | P-3 | 35.4 | 0.24 | 6.17 | 2.07 | 0.02 | 0.45 | 0.29 | 0.69 | 2.52 | 0.03 | 0.54 |
| P-4 | 32.5 | 0.26 | 6.15 | 5.34 | 0.02 | 0.68 | 0.29 | 0.55 | 2.42 | 0.03 | 0.44 | |
| P-5 | 35.9 | 0.21 | 5.87 | 2.07 | 0.02 | 0.43 | 0.25 | 0.65 | 2.61 | 0.03 | 0.22 | |
| Unplugged area | UP-6 | 38.0 | 0.14 | 5.35 | 1.21 | 0.01 | 0.32 | 0.22 | 0.68 | 2.54 | 0.02 | 0.18 |
| UP-7 | 36.8 | 0.13 | 5.25 | 1.37 | 0.01 | 0.30 | 0.24 | 0.65 | 2.51 | 0.02 | 0.19 | |
| UP-8 | 37.7 | 0.12 | 4.86 | 1.42 | 0.01 | 0.27 | 0.21 | 0.61 | 2.46 | 0.02 | 0.23 |
| Sample Location | Samples/Minerals | Quartz | Albite | Microcline | Muscovite | Kaolinite | Chamosite |
|---|---|---|---|---|---|---|---|
| Unplugged | UP-1 | 50.9 | 16.5 | 15.7 | 9.1 | 6.4 | 1.4 |
| UP-2 | 49 | 13.9 | 14.5 | 9.6 | 9.4 | 3.6 | |
| Plugged | P-5 | 44.6 | 13.5 | 16.0 | 10.4 | 9.7 | 5.7 |
| Unplugged | UP-6 | 52.4 | 8.9 | 18.9 | 7.5 | 10.1 | 2.2 |
| UP-7 | 49.9 | 13.5 | 20.5 | 5.2 | 8.5 | 2.4 | |
| UP-8 | 55.2 | 8.3 | 16.3 | 8.1 | 9.3 | 2.9 |
| Elements | Si | Al | Fe | Ca | Mg | K | Na | S | Ti | Mn | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 5.96 | 4.48 | 28.38 | 5.79 | 0.63 | 0.57 | 0.15 | 9.85 | 0.12 | 0.03 | 1.44 |
| Elements/Contents (%) | O | Mg | Al | Si | K | Ti | Fe |
|---|---|---|---|---|---|---|---|
| Fe-plugging materials | 36.8 | - | - | 1.37 | - | - | 61.8 |
| 33.7 | - | 0.69 | 3.41 | - | - | 62.2 | |
| 28.5 | - | 0.14 | 1.79 | - | - | 69.6 | |
| 32.0 | - | 0.36 | 2.44 | - | - | 65.2 | |
| 25.3 | - | 1.04 | 2.69 | - | - | 71.0 | |
| 29.3 | - | - | 1.70 | - | - | 69.0 | |
| 32.8 | - | 0.67 | 3.07 | - | - | 63.4 | |
| 38.0 | - | 1.67 | 4.40 | - | - | 56.0 | |
| 30.1 | - | 0.4 | 2.32 | - | - | 67.2 | |
| Feldspar | 39.3 | - | 12.7 | 30.0 | 4.79 | - | 13.2 |
| 56.4 | - | 3.92 | 31.1 | 2.30 | - | 6.23 | |
| 53.7 | - | 13.3 | 20.6 | 6.27 | - | 6.15 | |
| 57.5 | - | 12.1 | 19.5 | 5.85 | - | 4.98 | |
| Muscovite | 43.0 | - | 17.7 | 25.4 | 10.4 | - | 3.52 |
| 55.1 | - | 8.88 | 16.6 | 4.78 | - | 14.6 | |
| 42.6 | - | 15.1 | 18.7 | 16.0 | - | 7.62 | |
| 47.9 | 1.12 | 13.2 | 21.0 | 10.2 | 2.05 | 4.51 | |
| 37.8 | - | 18.8 | 23.0 | 10.2 | - | 10.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Li, P. Relationship between Chamosite Alteration and Fe-Plugging in Sandstone Pores during Acid In Situ Leaching of Uranium. Minerals 2021, 11, 497. https://doi.org/10.3390/min11050497
Zhao L, Li P. Relationship between Chamosite Alteration and Fe-Plugging in Sandstone Pores during Acid In Situ Leaching of Uranium. Minerals. 2021; 11(5):497. https://doi.org/10.3390/min11050497
Chicago/Turabian StyleZhao, Lixin, and Po Li. 2021. "Relationship between Chamosite Alteration and Fe-Plugging in Sandstone Pores during Acid In Situ Leaching of Uranium" Minerals 11, no. 5: 497. https://doi.org/10.3390/min11050497
APA StyleZhao, L., & Li, P. (2021). Relationship between Chamosite Alteration and Fe-Plugging in Sandstone Pores during Acid In Situ Leaching of Uranium. Minerals, 11(5), 497. https://doi.org/10.3390/min11050497





