Disseminated Gold–Sulfide Mineralization in Metasomatites of the Khangalas Deposit, Yana–Kolyma Metallogenic Belt (Northeast Russia): Analysis of the Texture, Geochemistry, and S Isotopic Composition of Pyrite and Arsenopyrite
Abstract
1. Introduction
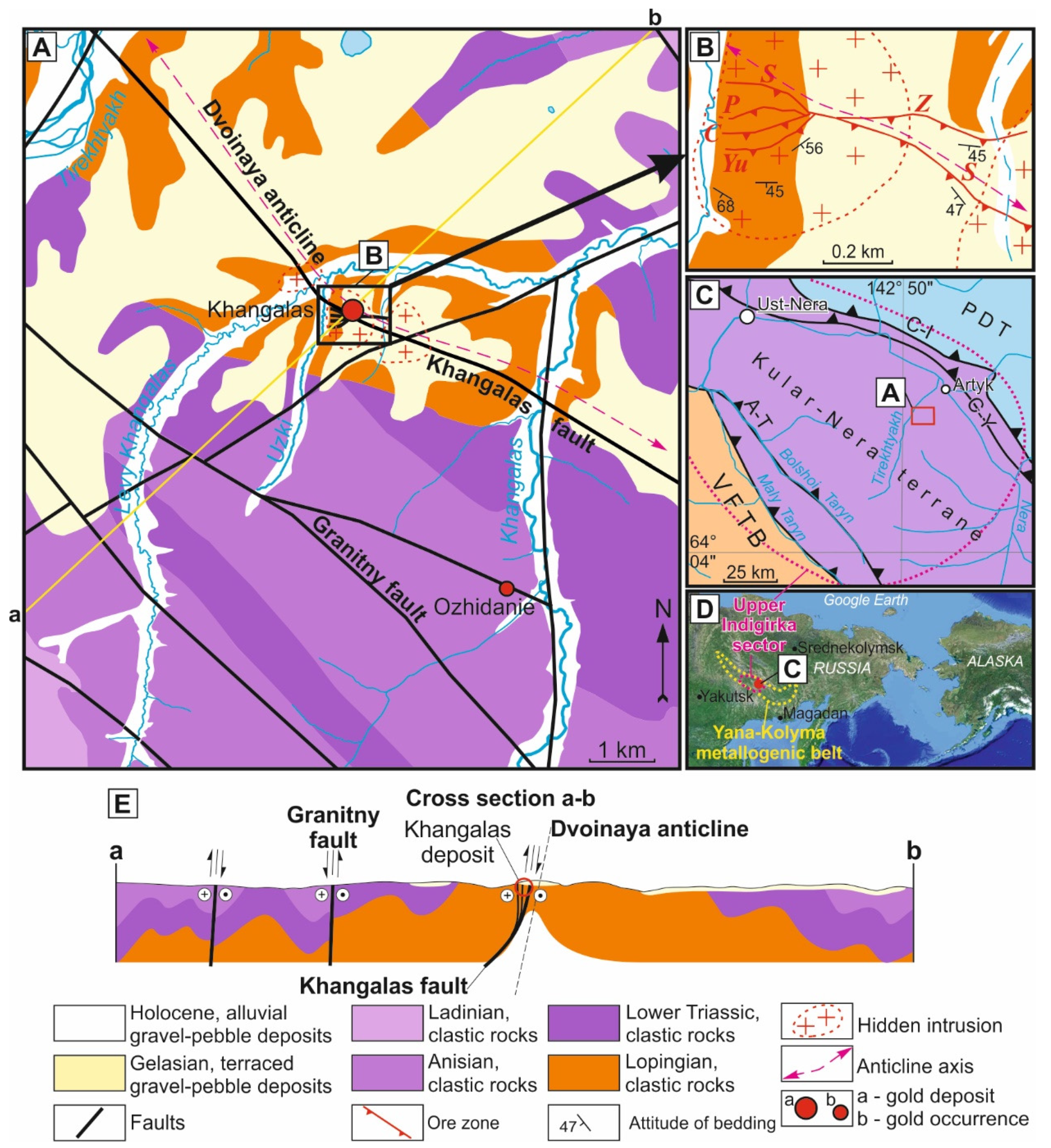
2. Regional Geology and Mineralization
2.1. Regional Tectonic Framework
2.2. Geology of the Khangalas Deposit
2.2.1. Structures and Host Rocks
2.2.2. Mineralization
3. Materials and Methods
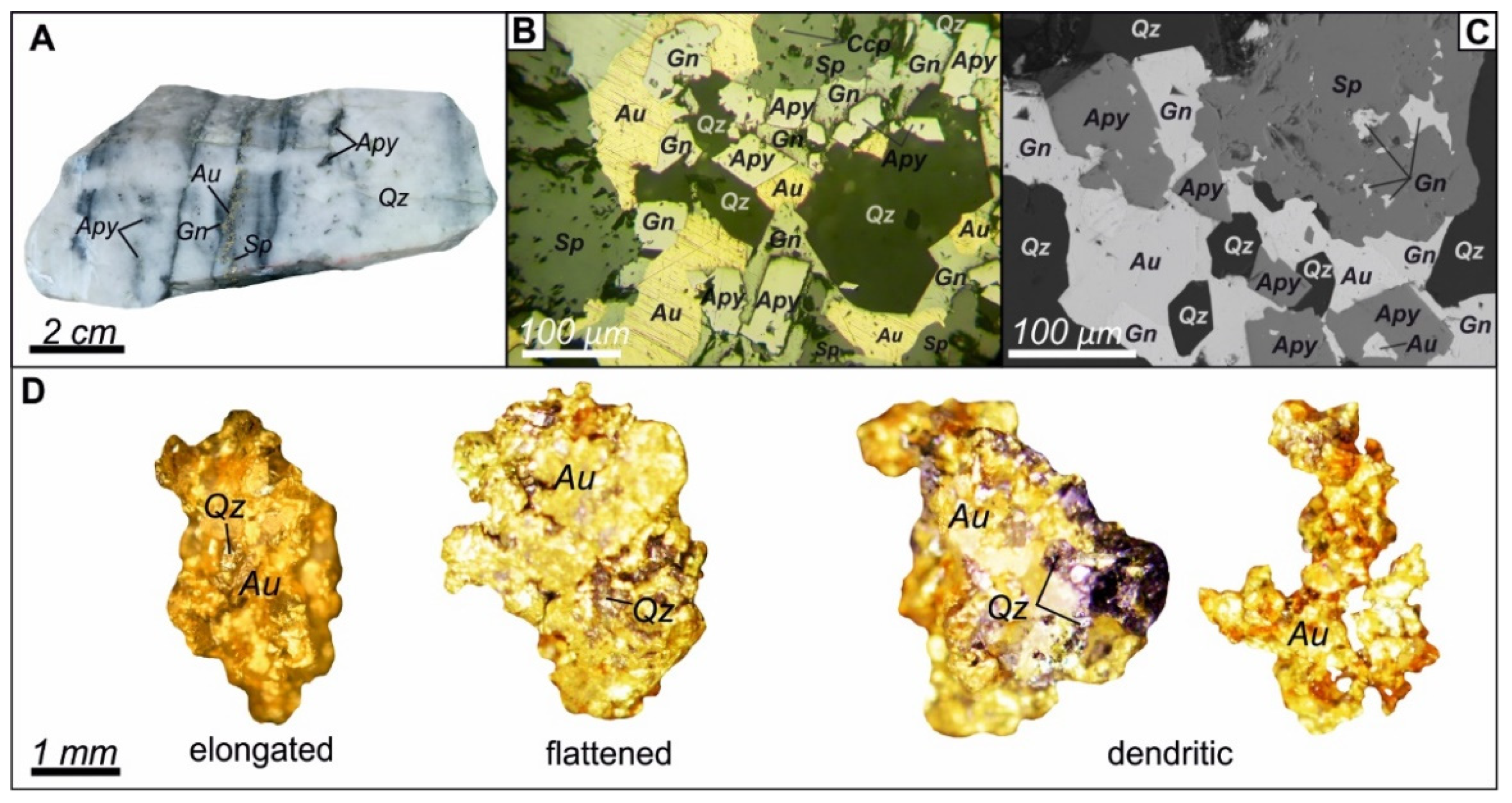
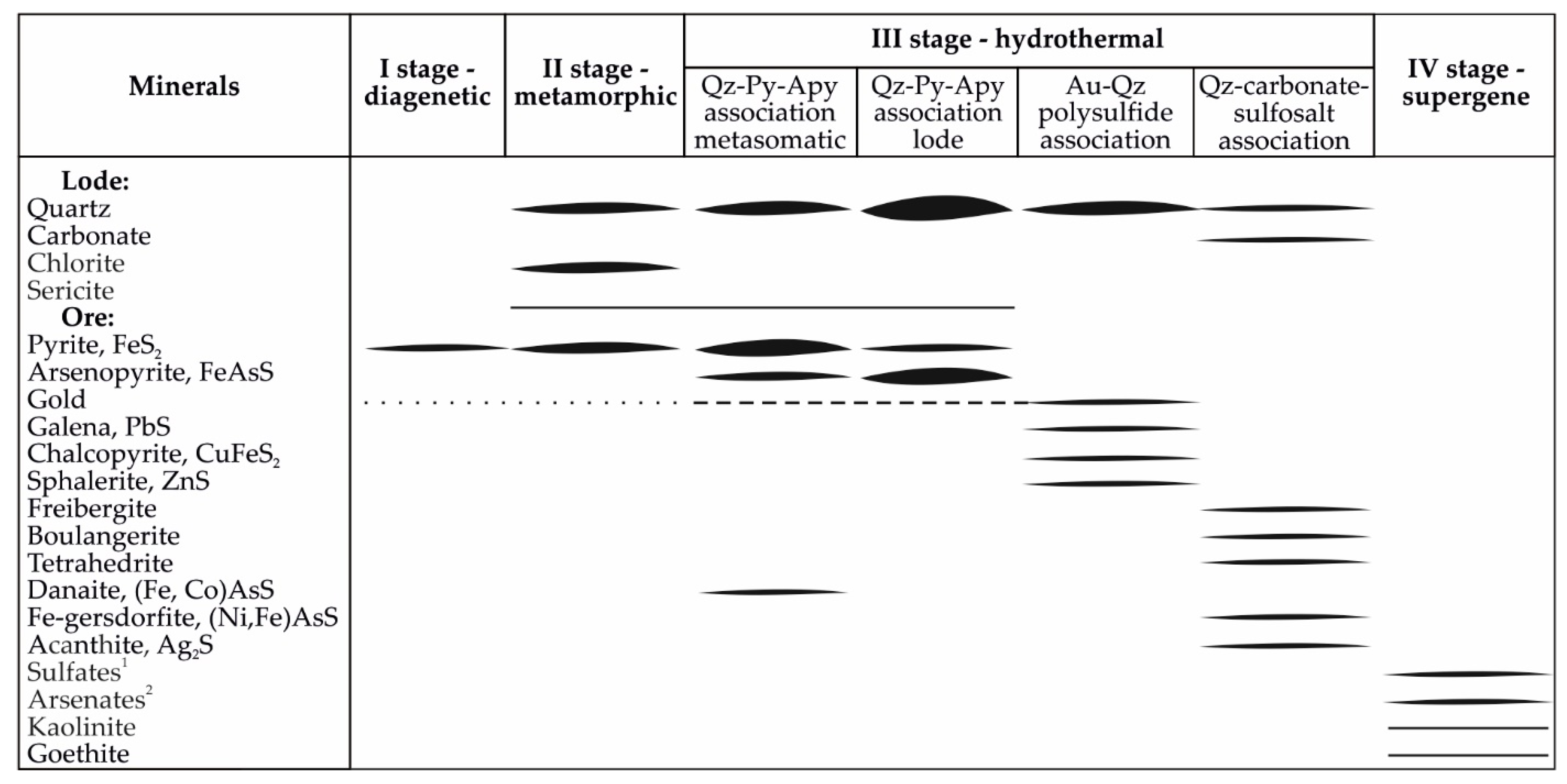
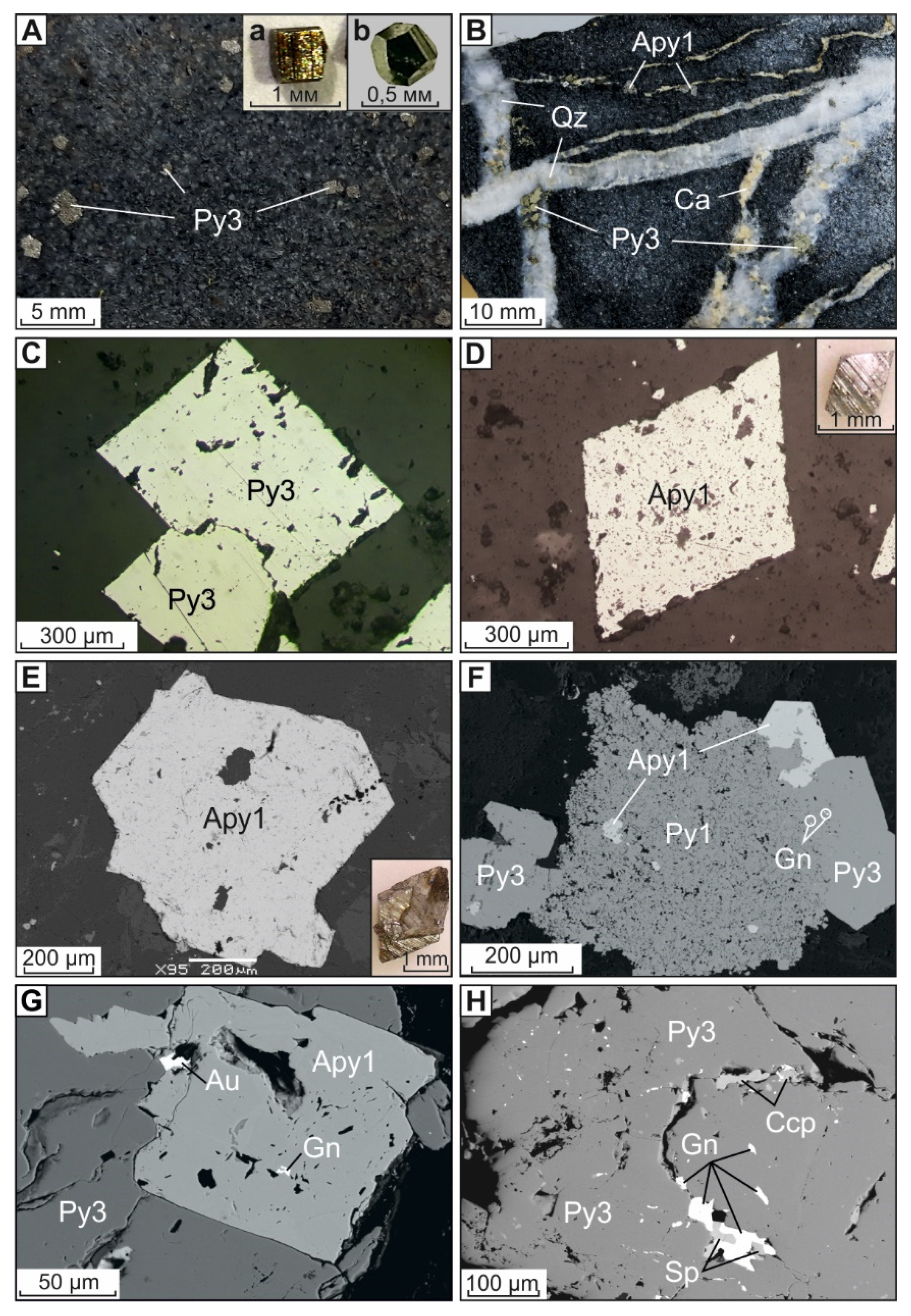
4. Results
4.1. Pyrite and Arsenopyrite Types and Textures
4.1.1. Diagenetic Pyrite (Py1)
4.1.2. Metamorphic Pyrite (Py2)
4.1.3. Metasomatic Pyrite (Py3) and Arsenopyrite (Apy1)
4.1.4. Vein Pyrite (Py4) and Arsenopyrite (Apy2)
4.2. Composition of Pyrite and Arsenopyrite
4.2.1. EPMA Results: Major and Minor Elements CAPS
4.2.2. Gold and Trace Element Concentrations in Py3 and Apy1 According to LA-IСP-MS Data
4.3. Gold Content of Sulfides from Proximal Metasomatites and in Veins
4.4. Sulfur Isotopic Composition of Sulfides
5. Discussion
5.1. Pyrite and Arsenopyrite Types and Textures
5.2. Composition of Pyrite and Arsenopyrite
5.3. Invisible Gold and Its Relationship with Other Elements in Py3 and Apy1 According to LA-ICP-MS Data
5.3.1. Invisible Gold in Py3
5.3.2. Invisible Gold in Apy1
5.4. Gold Content of Proximal Metasomatites and Their Sulfides
5.5. Sources of Metals
5.5.1. Pyrite Genesis as Evidenced by Co/Ni Ratio
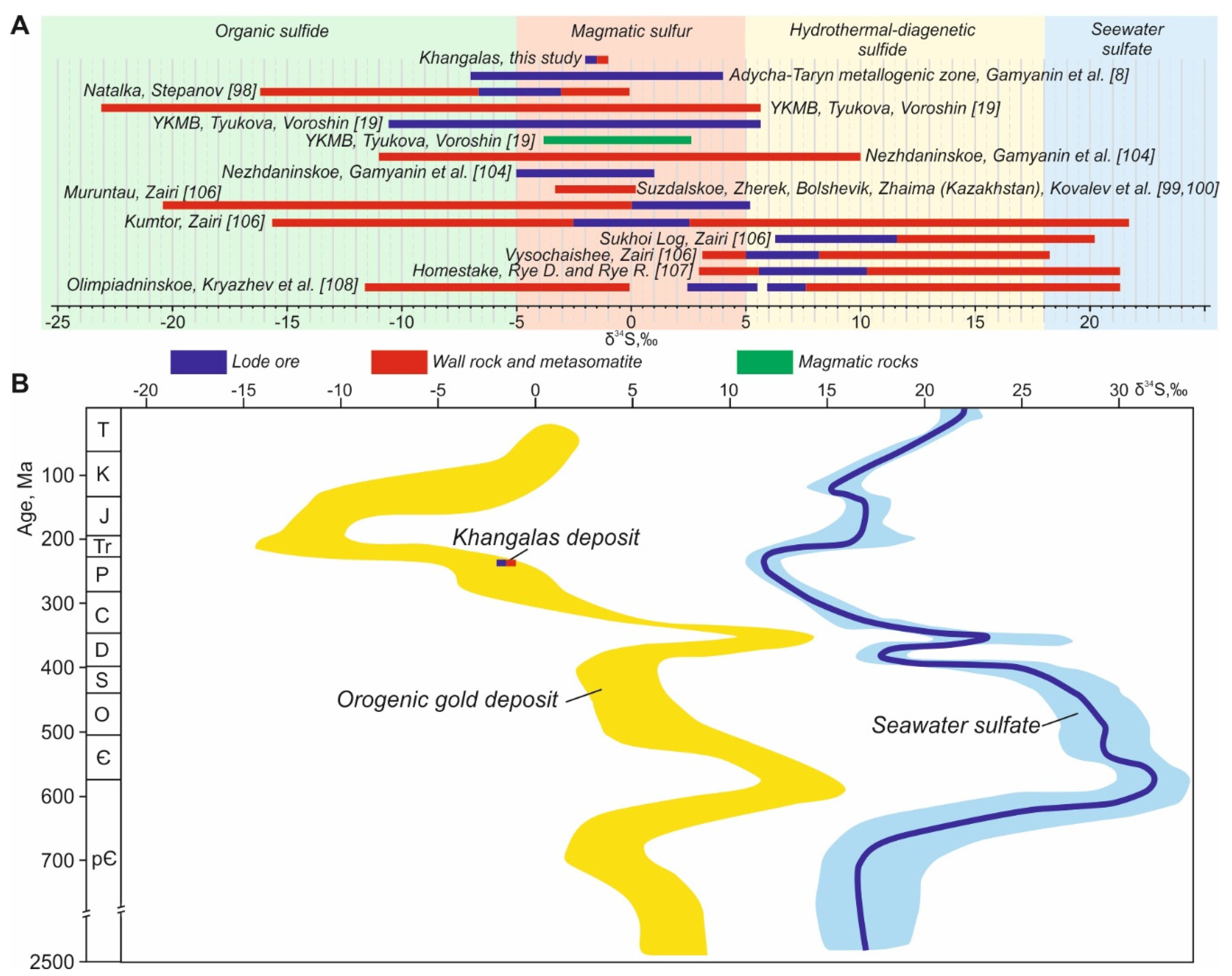
5.5.2. Origin of Hydrothermal Sulfides According to Stable Sulfur Isotopes
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goryachev, N.A.; Pirajno, F. Gold deposit and gold metallogeny of Far East Russia. Ore Geol. Rev. 2014, 59, 123–151. [Google Scholar] [CrossRef]
- Mikhailov, B.K.; Struzhkov, S.F.; Aristov, V.V.; Natalenko, M.V.; Tsymbalyuk, N.V.; Tyamisov, N.E.; Uzyunkoyan, А.А. Gold potensial of the Yana–Kolyma province. Ore Met. 2007, 5, 4–17. (In Russian) [Google Scholar]
- Kerrich, R.; Goldfarb, R.; Groves, D.; Garwin, S.; Jia, Y. The characteristics, origins, and geodynamic settings of supergiant gold metallogenic provinces. Sci. China Ser. D Earth Sci. 2000, 43, 68. [Google Scholar] [CrossRef]
- Goryachev, N.A. Geology of Mesozoic Gold–Quartz Vein Belts of Northeast Asia; NEISRI FEB RAS: Magadan, Russia, 1998. (In Russian) [Google Scholar]
- Gamyanin, G.N. Mineralogical and Genetic Aspects of Gold Mineralization of the Verkhoyansk–Kolyma Mesozoids; GEOS: Moscow, Russia, 2001. (In Russian) [Google Scholar]
- Fridovsky, V.Y. Structures of gold ore fields and deposits of Yana–Kolyma ore belt. In Metallogeny of Collisional Geodynamic Settings; Mezhelovsky, N.V., Gusev, G.S., Eds.; GEOS: Moscow, Russia, 2002; Volume 1, pp. 6–241. (In Russian) [Google Scholar]
- Amuzinsky, V.A. Metallogenic Epochs and Content of Ore Complexes of the Verkhoyansk Folded System; YSU Publishing House: Yakutsk, Russia, 2005. (In Russian) [Google Scholar]
- Gamyanin, G.N.; Fridovsky, V.Y.; Vikent’eva, O.V. Noble-metal mineralization of the Adycha–Taryn metallogenic zone: Geochemistry of stable isotopes, fluid regime, and ore formation conditions. Russ. Geol. Geophys. 2018, 59, 1271–1287. [Google Scholar] [CrossRef]
- Fridovsky, V.Y. Structural control of orogenic gold deposits of the Verkhoyansk–Kolyma folded region, northeast Russia. Ore Geol. Rev. 2018, 103, 38–55. [Google Scholar] [CrossRef]
- Goryachev, N.A.; Vikentyeva, O.V.; Bortnikov, N.S.; Prokofiev, V.Y.; Alpatov, V.A.; Golub, V.V. World-class Natalka gold deposit: REE distribution, fluid inclusions, stable oxygen isotopes and ore formation conditions (North-East of Russia). Gеol. Rudn. Mеst. 2008, 50, 414–444. (In Russian) [Google Scholar]
- Goryachev, N.A.; Sotskaya, O.T.; Mikhalitsyna, T.I.; Goryacheva, E.M.; Manshin, A.P. Estimation of Au-Pt-Pd-Ni in ores of typical deposits (Natalka, Degdekan) in the black shale strata of the Yana-Kolyma gold belt. Vestnik ONZ RAS 2012, 325–336. (In Russian) [Google Scholar]
- Goryachev, N.A.; Sotskaya, O.T.; Ignatiev, A.V.; Velivetskaya, T.I.; Goryacheva, E.M.; Semyshev, F.I.; Berdnikov, N.V.; Malinovsky, M.A.; Alshevsky, A.V. About sulfide mineralization of the zones of large faults of the Yana-Kolyma orogenic belt. Bull. Northeast Sci. Center FEB RAS 2020, 1, 11–29. (In Russian) [Google Scholar] [CrossRef]
- Sotskaya, O.T.; Goryachev, N.A. Micromineral forms of gold and silver in disseminated sulfide “black shale” ores (North-East of Russia). Geology and mineral resources of the North-East of Russia. In Proceedings of the All-Russian Conference, Yakutsk, Russia, 29–30 March 2012. (In Russian). [Google Scholar]
- Voroshin, S.V.; Tyukova, E.E.; Newberry, R.J.; Layer, P.W. Orogenic gold and rare metal deposits of the Upper Kolyma District, Northeastern Russia: Relation to igneous rocks, timing, and metal assemblages. Ore Geol. Rev. 2014, 62, 1–24. [Google Scholar] [CrossRef]
- Sidorova, N.V.; Aristov, V.V.; Grigorieva, A.V.; Sidorov, A.A. “Invisible” gold in pyrite and arsenopyrite from the Pavlik deposit (North-East of Russia). Dokl. Earth Sci. 2020, 495, 821–826. [Google Scholar] [CrossRef]
- Palyanova, G.A. Minerals of gold and silver in sulfide ores. Geol. Ore Depos. 2020, 62, 426–449. [Google Scholar] [CrossRef]
- Volkov, A.V.; Sidorov, A.A. Invisible gold. Bull. RAS 2017, 87, 40–49. (In Russian) [Google Scholar] [CrossRef]
- Tyukova, E.E.; Voroshin, S.V. Composition and Paragenesis of Arsenopyrite in Deposits and Host Rocks of the Upper Kolyma Region (to the Interpretation of the Genesis of Sulfide Associations); NEISRI FEB RAS: Magadan, Russia, 2007. (In Russian) [Google Scholar]
- Tyukova, E.E.; Voroshin, S.V. Isotopic composition of sulfur in sulfides from ores and host rocks of the Upper Kolyma region (Magadan region). Russ. J. Pac. Geol. 2008, 27, 29–43. (In Russian) [Google Scholar]
- Babkin, P.V.; Gelman, M.L.; Veldyaksov, F.F.; Pavlyuchenko, L.A. Prospects for identifying gold mineralization in sulfidization zones in the black shale strata of the North-East USSR. Kolyma 2001, 2, 14–22. (In Russian) [Google Scholar]
- Izmailov, L.I. Pyrrhotite Mineralization of Metal-Bearing Zones of the Kolyma River Basin; Science: Novosibirsk, Russia, 1976. (In Russian) [Google Scholar]
- Volkov, A.V.; Sidorov, A.A.; Goncharov, V.I.; Sidorov, V.A. Disseminated gold-sulfide deposits in the Russian Northeast. Geol. Ore Dep. 2002, 44, 159–174. [Google Scholar]
- Fridovsky, V.Y.; Yakovleva, K.Y.; Vernikovskaya, A.E.; Vernikovsky, V.A.; Matushkin, N.Y.; Kadilnikov, P.I.; Rodionov, N.V. Geodynamic Emplacement Setting of Late Jurassic Dikes of the Yana–Kolyma Gold Belt, NE Folded Framing of the Siberian Craton: Geochemical, Petrologic, and U–Pb Zircon Data. Minerals 2020, 10, 1000. [Google Scholar] [CrossRef]
- Fridovsky, V.Y.; Kudrin, M.V. Deformation structures of the Khangalassky ore cluster. Geology and mineral resources of the North-East of Russia. In Proceedings of the All-Russian scientific-practical Conference, Yakutsk, Russia, 31 March–2 April 2015; pp. 537–540. (In Russian). [Google Scholar]
- Fridovsky, V.Y.; Kudrin, M.V.; Polufuntikova, L.I. Multi-stage deformation of the Khangalas ore cluster (Verkhoyansk–Kolyma folded region, northeast Russia): Ore-controlling reverse thrust faults and post-mineral strike-slip faults. Minerals 2018, 8, 270. [Google Scholar] [CrossRef]
- Rozhkov, I.S.; Grinberg, G.A.; Gamyanin, G.A.; Kukhtinskiy, Y.G.; Solovyev, V.I. Late Mesozoic Magmatism and Gold Mineralization of the Upper Indigirsky Region; Science: Moscow, Russia, 1971. (In Russian) [Google Scholar]
- Amuzinsky, V.A.; Anisimova, G.S.; Zhdanov, Y.Y. Native Gold of Yakutia, Upper Indigirsky District; VO Nauka: Novosibirsk, Russia, 1992. (In Russian) [Google Scholar]
- Nesterov, N.V. Supergene Enrichment of Gold Deposits in Northeast Asia; Nauka: Novosibirsk, Russia, 1985. (In Russian) [Google Scholar]
- Kudrin, M.V.; Zayakina, N.V.; Vasilieva, T.I. Minerals of the oxidation zone of the Khangalas gold deposit (Eastern Yakutia). Geology and mineral resources of the North-East of Russia. In Proceedings of the VIII All-Russian Scientific-Practical Conference, Yakutsk, Russia, 18–20 April 2018; pp. 77–80. (In Russian). [Google Scholar]
- Kudrin, M.V.; Vasilieva, T.I.; Fridovsky, V.Y.; Zayakina, N.V.; Polufuntikova, L.I. Minerals of the weathering crust of the Khangalassky ore cluster (North-East of Russia). Geology and mineral resources of the North-East of Russia. In Proceedings of the IX All-Russian Scientific and Practical Conference, Yakutsk, Russia, 10–12 April 2019; pp. 53–56. (In Russian). [Google Scholar]
- Skryabin, A.I. Reconstruction of the Lateral Zoning of Gold Mineralization (Yano-Kolyma Belt); YSC SB RAS: Yakutsk, Russia, 2010. (In Russian) [Google Scholar]
- GeoInfoComLLC. Available online: http://mestor.geoinfocom.ru/publ/1-1-0-55 (accessed on 15 January 2021).
- Parfenov, L.M.; Kuzmin, M.I. Tectonics, Geodynamics and Metallogeny of the Territory of the Republic of Sakha (Yakutia); Parfenov, L.M., Kuzmin, M.I., Eds.; MAIK Nauka/Interperiodika: Moscow, Russia, 2001. (In Russian) [Google Scholar]
- Zaitsev, A.I.; Fridovsky, V.Y.; Vernikovskaya, A.E.; Kudrin, M.V.; Yakovleva, K.Y.; Kadilnikov, P.I. Rb–Sr isotopic study of basites of the dyke complex of the Taryn ore-magmatic system (Northeast Russia). Russ. J. Domes. Geol. 2018, 5, 50–61. (In Russian) [Google Scholar]
- Parfenov, L.M.; Berzin, N.A.; Khanchuk, A.I.; Badarch, G.; Belochenko, V.G.; Bulgatov, A.I.; Dril, C.I.; Kirillova, G.L.; Kuzmin, M.I.; Nokleberg, W.J.; et al. Model of orogenic belt formation in Central and Northeast Asia. Russ. J. Pac. Geol. 2003, 22, 7–41. (In Russian) [Google Scholar]
- Akinin, V.V.; Prokopiev, A.V.; Toro, J.; Miller, E.L.; Wooden, J.; Goryachev, N.A.; Alshevsky, A.V.; Bakharev, A.G.; Trunilina, V.A. U–PB SHRIMP ages of granitoids from the Main batholith belt (North East Asia). Dokl. Acad. Sci. 2009, 426, 216–221. [Google Scholar]
- Akinin, V.V.; Miller, E.L.; Toro, J.; Prokopiev, A.V.; Gottlieb, E.S.; Pearcey, S.; Trunilina, V.A. Episodicity and the dance of late mesozoic magmatism and deformation along the northern circum-pacific margin: NE Russia to the Сordillera. Earth-Sci. Rev. 2020, 208, 103272. [Google Scholar] [CrossRef]
- Akimov, G.Y. New age data on gold–quartz mineralization in the Verkhnyaya Indigirka area, Yakutia. Dokl. Acad. Sci. 2004, 398, 80–83. (In Russian) [Google Scholar]
- Whitney, D.L.; Ewans, B.W. Abbreviations of names of rock forming minerals. Am. Miner. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Kudrin, M.V.; Zayakina, N.V.; Fridovsky, V.Y.; Galenchikova, L.T. Hydrous ferric sulfate–Fe(SO4)(OH)·2H2O from the supergene zone of the Khangalas gold deposit, Eastern Yakutia, Russia. Notes Rus. Min. Soc. 2020, 149, 126–141. [Google Scholar]
- Zayakina, N.V.; Kudrin, M.V.; Fridovsky, V.Y. Unknown sulfate-arsenate-phosphate Al and Fe from the Khangalas deposit (Eastern Yakutia). Geology and Mineral Resources of the North-East of Russia. In Proceedings of the X All-Russian Scientific-Practical Conference with International Participation, Yakutsk, Russia, 8–10 April 2020; pp. 210–214. (In Russian). [Google Scholar]
- Nesterov, N.V. Secondary zoning of gold ore deposits in Yakutia. In Bulletin of the Tomsk Polytechnic University; TPU: Tomsk, Russia, 1970; pp. 242–247. (In Russian) [Google Scholar]
- Wilson, S.A.; Ridley, W.I.; Koenig, A.E. Development of sulphide calibration standards for the laser ablation inductively-coupled plasma mass spectrometry technique. J. Anal. At. Spectrom. 2002, 17, 406–409. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. Atomic Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Longerich, H.P.; Jackson, S.E.; Günther, D. Inter-laboratory note. Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. J. Anal. Atomic Spectrom. 1996, 11, 899–904. [Google Scholar] [CrossRef]
- Nadeev, A.; Mikhailov, D.; Chuvilin, E.; Koroteev, D.; Shako, V. Visualization of clay and frozen substances inside porous rocks using X-ray micro-computed tomography. J. Microsc. Anal.–Tomogr. Suppl. 2013, 27, 8–11. [Google Scholar]
- Mayorova, T.P.; Statsenko, E.O.; Trifonov, A.A.; Nesterenko, G.V. X-ray microtomography and field emission electron microscopy – new possibilities for studying highly dispersed gold ores. Bull. Inst. Geol. KSC UB RAS. 2013, 11, 34–38. (In Russian) [Google Scholar]
- Krupskaya, V.V.; Zakusin, S.V.; Zhukhlistov, A.P.; Dorzhieva, O.V.; Sudin, V.V.; Kryuchkova, L.Y.; Zubkov, A.A. Newly formed smectite as an indicator of transformations of the geological environment under the influence of highly reactive solutions accompanying liquid radioactive waste. J. Geoecol. Engin. Geol. Hydrogeol. Geocr. 2016, 5, 412–421. [Google Scholar]
- Jarzyna, J.A.; Krakowska, P.I.; Puskarczyk, E.; Wawrzyniak-Guz, K.; Bielecki, J.; Tkocz, K.; Tarasiuk, J.; Wro’nski, S.; Dohnalik, M. X-ray computed microtomography—A useful tool for petrophysical properties determination. Comput. Geosc. 2016, 20, 1155–1167. [Google Scholar] [CrossRef]
- Voitenko, V.N. Form of crystallization of native gold in arsenopyrite ores of the Bazovskoye deposit (according to X-ray microtomography data). In Practical Microtomography, Proceedings of the III All-Russian Scientific Conference; SPBGU: St. Petersburg, Russia, 2014; pp. 26–28. (In Russian) [Google Scholar]
- Naumov, E.A.; Kovalev, K.R.; Kalinin, Y.A.; Palyanova, G.A.; Voitenko, V.N. The use of high-resolution X-ray tomography methods in mineralogical research. In Scientific and Methodological Foundations of Forecasting, Prospecting, Evaluation of Diamond, Precious and Non-Ferrous Metal Deposits; TSINIGRI: Moscow, Russia, 2019; pp. 126–127. (In Russian) [Google Scholar]
- Korost, D.V.; Kalmykov, G.A.; Yapaskurt, V.O.; Ivanov, M.K. Application of computer microtomography to study the structure of terrigenous reservoirs. Geol. Oil Gas. 2010, 2, 36–42. (In Russian) [Google Scholar]
- Korost, D.V.; Ariskin, A.A.; Pshenitsyn, I.V.; Khomyak, A.N. X-ray computed tomography as a method for reconstructing the 3d characteristics of dispersed sulfides and spinel in plagiodunites of the Yoko-Dovyren intrusion. Petrol 2019, 27, 401–419. (In Russian) [Google Scholar] [CrossRef]
- Vorobiev, A.E.; Kozyrev, E.N.; Honore, C. Communication nanorelief Pyrite and Arsenopyrite with the values of adsorption of gold. In Science, Education, Culture and Information and Educational Activities-the Basis of Sustainable Development of Mountain Territories, Proceedings of the VIII International Scientific-Practical Conference; SKGMI: Vladikavkaz, Russia, 2015; pp. 264–272. (In Russian) [Google Scholar]
- Kovalchuk, E.V.; Tagirov, B.R.; Vikentyev, I.V.; Chareev, D.A.; Tyukova, E.E.; Nikolsky, M.S.; Bortnikov, N.S. “Invisible” Gold in Synthetic and Natural Arsenopyrite Crystals, Vorontsovka Deposit, Northern Urals. Geol. Ore Dep. 2019, 61, 447–468. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Essene, E.J.; Kelly, W.C. A re-examination of the arsenopyrite geothermometer; pressure considerations and applications to natural assemblages. Can. Mineral. 1985, 23, 517–534. [Google Scholar]
- Lentz, D.R. Sphalerite and arsenopyrite at the Brunswick No. 12 massive sulfide deposit, Bathurst camp, New Brunswick: Constraints on P-T evolution. Can. Mineral. 2002, 40, 19–31. [Google Scholar] [CrossRef]
- Lee, M.; Shin, D.; Yoo, B.; Im, H.; Pak, S.; Choi, S. LA-ICP-MS trace element analysis of arsenopyrite from the Samgwang gold deposit, South Korea, and its genetic implications. Ore Geol. Rev. 2019, 114, 103147. [Google Scholar] [CrossRef]
- Fleet, M.E.; Mumin, A.H. Gold-bearing arsenian pyrite and marcasite and arsenopyrite from Carlin Trend gold deposits and laboratory synthesis. Am. Mineral. 1997, 82, 182–193. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. Acta. 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Wang, C.; Shao, Y.; Huang, K.; Zhou, H.; Zhang, J.; Liu, Z.; Liu, Q. Ore-Forming Processes at the Xiajinbao Gold Deposit in Eastern Hebei Province: Constraints from EPMA and LA-ICPMS Analysis. Minerals 2018, 8, 388. [Google Scholar]
- Pshenichkin, A.Y. On the form of finding trace elements in pyrite. Expl. Prot. Min. Resour. 2010, 11, 46–49. (In Russian) [Google Scholar]
- Large, R.R.; Bull, S.W.; Maslennikov, V.V. A carbonaceous sedimentary source rock model for Carlin-type and orogenic gold deposits. Econ. Geol. 2011, 106, 331–358. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Large, R.R.; Maslennikova, S.P.; Arkhireeva, N.S. Typochemism of pyrite and pyrrhotite as a reflection of the evolution of gold-bearing carbonaceous deposits in marginal oceanic structures. Metall. Anc. Mod. Oceans 2013, 19, 32–35. [Google Scholar]
- Samusikov, V.P. Regularities of the concentration of isomorphic-impurity elements in minerals during hydrothermal ore formation. Russ. Geol. Geoph. 2010, 51, 338–352. [Google Scholar]
- Bortnikov, N.S.; Gamyanin, G.N.; Vikentieva, O.V.; Prokofiev, V.Y.; Alpatov, V.A.; Bakharev, A.G. Composition and origin of fluids in the hydrothermal system of the Nezhdaninsky gold ore deposit (Sakha-Yakutia, Russia). Geol. Ore Depos. 2007, 49, 99–145. [Google Scholar] [CrossRef]
- Aristov, V.V.; Kryazhev, S.G.; Ryzhov, O.B.; Volfson, A.A.; Prokofiev, V.Y.; Sidorova, N.V.; Sidorov, A.A. Sources of fluids and ore matter of gold and antimony mineralization of the Adychansk ore region (Eastern Yakutia). Pap. Acad. Sci. 2017, 476, 174–180. [Google Scholar]
- Large, R.R.; Maslennikov, V.V. Invisible Gold Paragenesis and Geochemistry in Pyrite from Orogenic and Sediment-Hosted Gold Deposits. Minerals 2020, 10, 339. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Maslennikova, S.P.; Large, R.R.; Danyushevsky, L.V. Study of trace element zonation in vent chimneys from the Silurian Yaman-Kasay volcanic-hosted massive sulfide deposit (southern Urals, Russia) using laser ablation–inductively coupled plasma mass spectrometry (LA-ICPMS). Econ. Geol. 2009, 104, 1111–1141. [Google Scholar] [CrossRef]
- Keith, M.; Smith, D.J.; Jenkin, G.R.; Holwell, D.A.; Dye, M.D. A review of Te and Se systematics in hydrothermal pyrite from precious metal deposits: Insights into ore-forming processes. Ore Geol. Rev. 2018, 96, 269–282. [Google Scholar] [CrossRef]
- Deditius, A.P.; Reich, M.; Kesler, S.E.; Utsunomiya, S.; Chryssoulis, S.L.; Walshe, J.; Ewing, R.C. The coupled geochemistry of Au and As in pyrite from hydrothermal ore deposits. Geochim. Cosmochim. Acta 2014, 140, 644–670. [Google Scholar] [CrossRef]
- Vikentiev, I.V. Invisible and microscopic gold in pyrite: Research methods and new data for pyrite ores of the Urals. Geol. Ore Depos. 2015, 57, 267–298. [Google Scholar]
- Voitkevich, G.V.; Miroshnikov, A.E.; Povarenykh, A.S.; Prokhorov, V.G. A Short Guide to Geochem., 2nd ed.; Nedra: Moscow, Russia, 1977. (In Russian) [Google Scholar]
- Belikova, G.I.; Salikhov, D.N.; Berdnikov, P.G. On the question of gold isomorphism in pyrite. Geol. Collect. GI USC RAS 2002, 3, 190–193. (In Russian) [Google Scholar]
- Chouinard, A.; Paquette, J.; Williams-Jones, A.E. Crystallographic controls on trace-element incorporation in auriferous pyrite from the Pascua epithermal high-sulfidation deposit, Chile-Argentina. Can. Miner. 2005, 43, 951–963. [Google Scholar] [CrossRef]
- Tauson, V.L.; Kravtsova, R.G.; Smagunov, N.V.; Spiridonov, A.M.; Grebenshchikova, V.I.; Budyak, A.E. Structurally and superficially bound gold in pyrite from deposits of different genetic types. Russ. Geol. Geophys. 2014, 55, 273–289. [Google Scholar] [CrossRef]
- Gao, F.; Du, Y.; Pang, Z.; Du, Y.; Xin, F.; Xie, J. LA-ICP-MS Trace-Element Analysis of Pyrite from the Huanxiangwa Gold Deposit, Xiong’ershan District, China: Implications for Ore Genesis. Minerals 2019, 9, 157. [Google Scholar] [CrossRef]
- Vaughan, J.P.; Kyin, A. Refractory gold ores in Archaean greenstones, Western Australia: Mineralogy, gold paragenesis, metallurgical characterization and classification. Miner. Magaz. 2004, 68, 255–277. [Google Scholar] [CrossRef]
- Moskvitina, L.V.; Moskvitin, S.G.; Anisimova, G.S. Research of Nanoscale Gold by Methods of Tunneling and Atomic-Powered Microscopy with Chemical and Ion-Plasma Etching in the Kuchus Deposit (Republic Sakha (Yakutia). In International Science and Technology Conference “Earth Science”-Section One, Proceedings of the Iop Conference Series: Earth and Environmental Science, Russky Island, Russia, 4–6 March 2019; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 272, pp. 1–7. [Google Scholar]
- Gregory, D.D.; Large, R.R.; Halpin, J.A.; Baturina, E.L.; Lyons, T.W.; Wu, S.; Danyushevsky, L.; Sack, P.J.; Chappaz, A.; Maslennikov, V.V.; et al. Trace element content of sedimentary pyrite in black shales. Econ. Geol. 2015, 110, 1389–1410. [Google Scholar] [CrossRef]
- Tomkins, A.G.; Mavrogenes, J.A. Redistribution of gold within arsenopyrite and lollingite during pro-and retrograde metamorphism: Application to timing of mineralization. Econ. Geol. 2001, 96, 525–534. [Google Scholar] [CrossRef]
- Morey, A.A.; Tomkins, A.G.; Bierlein, F.P.; Weinberg, R.F.; Davidson, G.J. Bimodal distribution of gold in pyrite and arsenopyrite: Examples from the Archean Boorara and Bardoc shear systems, Yilgarn craton, Western Australia. Econ. Geol. 2008, 103, 599–614. [Google Scholar] [CrossRef]
- Sung, Y.H.; Brugger, J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Nugus, M. Invisible gold in arsenian pyrite and arsenopyrite from a multistage Archaean gold deposit: Sunrise Dam, Eastern Goldfields Province, Western Australia. Miner. Dep. 2009, 44, 765. [Google Scholar] [CrossRef]
- Zaitsev, A.I.; Fridovsky, V.Y.; Yakovleva, K.Y.; Kudrin, M.V.; Vernikovskaya, A.E. Composition and age of the basitic dikes of the Nastenka site of the Malo–Tarynskoe orogenic gold deposit (Verkhoyansk–Kolyma folded region, Northeast Russia). In Proceedings of the 19th International Multidisciplinary Scientific GeoConference-SGEM, Albena, Bulgaria, 30 June–6 July 2019; pp. 99–108. [Google Scholar]
- Bortnikov, N.S.; Gamyanin, G.N.; Vikent’eva, O.V.; Prokof’ev, V.Y.; Prokop’ev, A.V. The Sarylakh and Sentachan gold–antimony deposits, Sakha-Yakutia: A case of combined mesothermal gold–quartz and epithermal stibnite ores. Geol. Ore Dep. 2010, 52, 339–372. (In Russian) [Google Scholar] [CrossRef]
- Aristov, V.V.; Prokofiev, V.Y.; Imamendinov, B.N.; Kryazhev, S.G.; Alekseev, V.Y.; Sidorov, A.A. Features of ore formation at the Drazhnoe gold-quartz deposit (Eastern Yakutia, Russia). Dokl. Earth Sci. 2015, 464, 879–884. [Google Scholar] [CrossRef]
- GV GOLD. Available online: https://www.gvgold.ru/ru/our-assets/taryn-business-unit/ (accessed on 25 January 2021).
- Bralia, A.; Sabatini, G.; Troja, F. A revaluation of the Co/Ni ratio in pyrite as geochemical tool in ore genesis problems. Miner. Depos. 1979, 14, 353–374. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Mao, J.W. Textural control on gold distribution in As-free pyrite from the Dongping, Huangtuliang and Hougou gold deposits, North China craton (Hebei Province, China). Chem. Geol. 2009, 264, 101–121. [Google Scholar] [CrossRef]
- Azovskova, O.B.; Utochkina, N.V.; Zubova, T.P. Geochemical features of pyrite and marcasite from weathering crusts and “ancient” loose deposits of the Aktai area (Northern Urals). In EZHEGODNIK-2013, Tr. IGG UrO RAN; URO RAN: Ufa, Russia, 2014; Volume 161, pp. 238–245. (In Russian) [Google Scholar]
- Shanks, W. Stable Isotope Geochemistry of Mineral Deposits; Elsevier Ltd.: Amsterdam, The Nederland, 2014. [Google Scholar]
- Goldfarb, R.J.; Groves, D.I. Orogenic gold: Common or evolving fluid and metal sources through time. Lithos 2015, 233, 2–26. [Google Scholar] [CrossRef]
- LaFlamme, C.; Sugiono, D.; Thébaud, N.; Caruso, S.; Fiorentini, M.L.; Selvaraja, V.; Jeon, H.; Voute, F.; Martin, L. Multiple sulfur isotopes monitor fluid evolution of an orogenic gold deposit. Geoch. Cosmoch. Acta 2018, 222, 436–446. [Google Scholar] [CrossRef]
- Kryazhev, S.G. Isotope-geochemical and genetic models of gold deposits in carbonaceous-terrigenous strata. Domest. Geol. 2017, 1, 28–38. (In Russian) [Google Scholar]
- Chang, Z.; Large, R.; Maslennikov, V. Sulfur isotopes in sediment-hosted orogenic gold deposits: Evidence for an early timing and a seawater sulfur source. Geology 2008, 36, 971–974. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Miller, L.D.; Leach, D.L.; Snee, L.W. Gold deposits in metamorphic rocks of Alaska. Econ. Geol. 1997, 9, 151–190. [Google Scholar]
- Goldfarb, R.J.; Leach, D.L.; Rose, S.C.; Landis, G.P. Fluid inclusion geochemistry of gold-bearing quartz veins of the Juneau Gold Belt, southeastern Alaska-implications for ore genesis. Econ. Geol. 1989, 6, 363–375. [Google Scholar]
- Xue, Y.; Campbell, I.H.; Ireland, T.R.; Holden, P.; Armstrong, R. No mass-independent sulfur isotope fractionation in auriferous fluids supports a magmatic origin for Archean gold deposits. Geology 2013, 41, 791–794. [Google Scholar] [CrossRef]
- Kovalev, K.R.; Kalinin, Y.A.; Naumov, E.A.; Kolesnikova, M.K.; Korolyuk, V.N. Gold content of arsenopyrite of gold-sulfide deposits of East Kazakhstan. Geol. Geoph. 2011, 52, 225–242. [Google Scholar]
- Kovalev, K.R.; Kuzmina, O.N.; Dyachkov, B.A.; Vladimirov, A.G.; Kalinin, Y.A.; Naumov, E.A.; Kirillov, M.V.; Annikova, I.Y. Gold-sulfide disseminated mineralization of the Zhaima deposit (East Kazakhstan). Geol. Ore Dep. 2016, 58, 116–133. [Google Scholar] [CrossRef]
- Mikhalitsyna, T.I.; Sotskaya, O.T. The role of black-shales in the formation of gold-ore deposits Natalka and Pavlik (Yano-Kolymsky orogene belt). Russ. Geol. Geoph. 2020, 61, 1648–1671. [Google Scholar]
- Eremin, R.A.; Voroshin, S.V.; Sidorov, V.A.; Shakhtyrov, V.G.; Pristavko, V.A.; Gashtold, V.V. Geology and genesis of the Natalka gold deposit, Northeast Russia. Inter. Geol. Rev. 1994, 36, 1113–1138. [Google Scholar] [CrossRef]
- Stepanov, V.A. Zoning of Gold-Quartz Mineralization in Central Kolyma (Magadan Region, Russia); Dalnauka: Vladivostok, Russia, 2001. (In Russian) [Google Scholar]
- Gamyanin, G.N.; Bortnikov, N.C.; Alpatov, V.V. The Nezhdaninskoe Gold Ore Deposit is A Unique Deposit in the North-East of Russia; GEOS: Moscow, Russia, 2000. (In Russian) [Google Scholar]
- Seltmann, R.; Goldfarb, R.; Zu, B.; Creaser, R.; Dolgopolova, A.; Shatov, V. Muruntau, Uzbekistan: The World’s Largest Epigenetic Gold Deposit. Soc. Econ. Geol. 2020, 23, 497–521. [Google Scholar]
- Zairi, N.M. Isotope-Geochemical Models of the Formation of Deposits of Gold-Carbon Formation. Ph.D. Thesis, TSINIGRI, Moscow, Russia, 1992. (In Russian). [Google Scholar]
- Rye, D.M.; Rye, R.O. Homestake Gold Mine, South Dakota: I. Stable Isotope Studies. Econ. Geol. 1974, 69, 293–317. [Google Scholar] [CrossRef]
- Kryazhev, S.G.; Glukhov, A.P.; Rusinova, O.V.; Kuznetsova, S.V. Isotope-geochemical regime of the formation of the gold-quartz deposit Sovetskoe. In Applied Geochemistry (Analytical Research), 4th ed.; IMGRE: Moscow, Russia, 2003; pp. 154–164. (In Russian) [Google Scholar]
- Ohmoto, H. Stable isotope geochemistry of ore deposits. In High Temperature Geological Processes (Review in Mineralogy); Mineralogical Society of America: Washington, DC, USA, 1986; pp. 491–559. [Google Scholar]
- Claypool, G.E.; Holser, W.T.; Kaplan, I.R.; Sakai, H.; Zak, I. The age curves of sulfur and oxygen isotopes in marine sulfate and their mutual interpretation. Chem. Geol. 1980, 28, 199–260. [Google Scholar] [CrossRef]



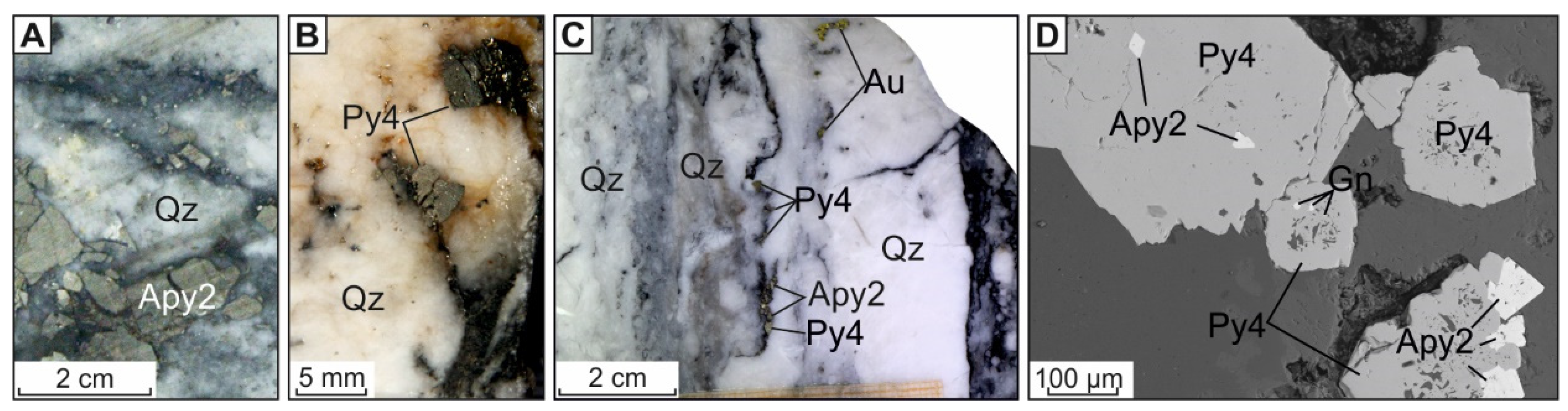



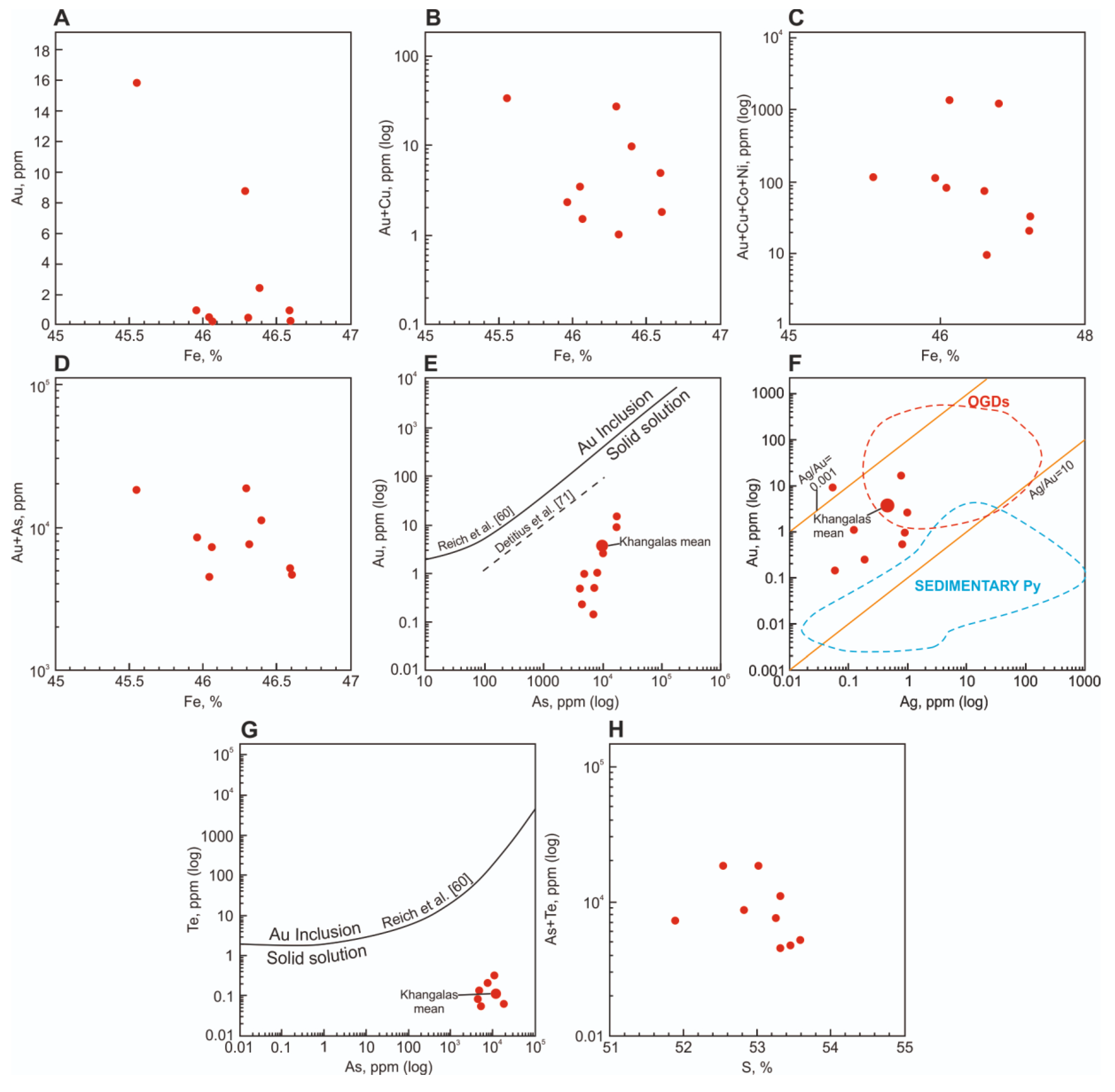
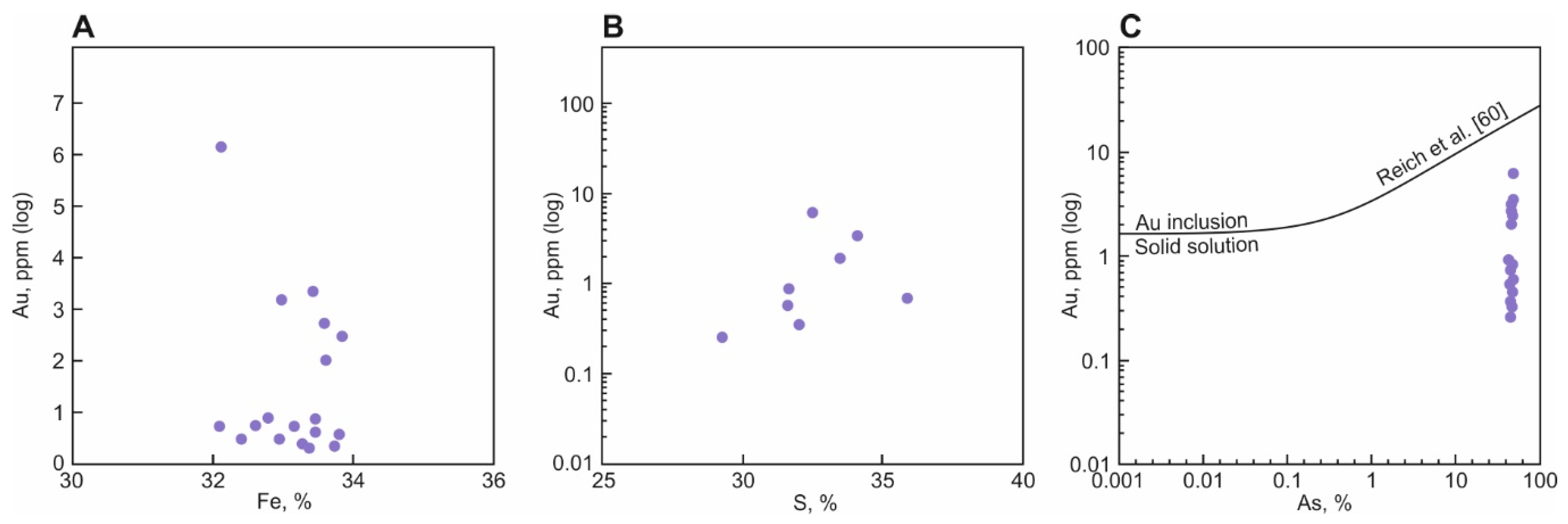
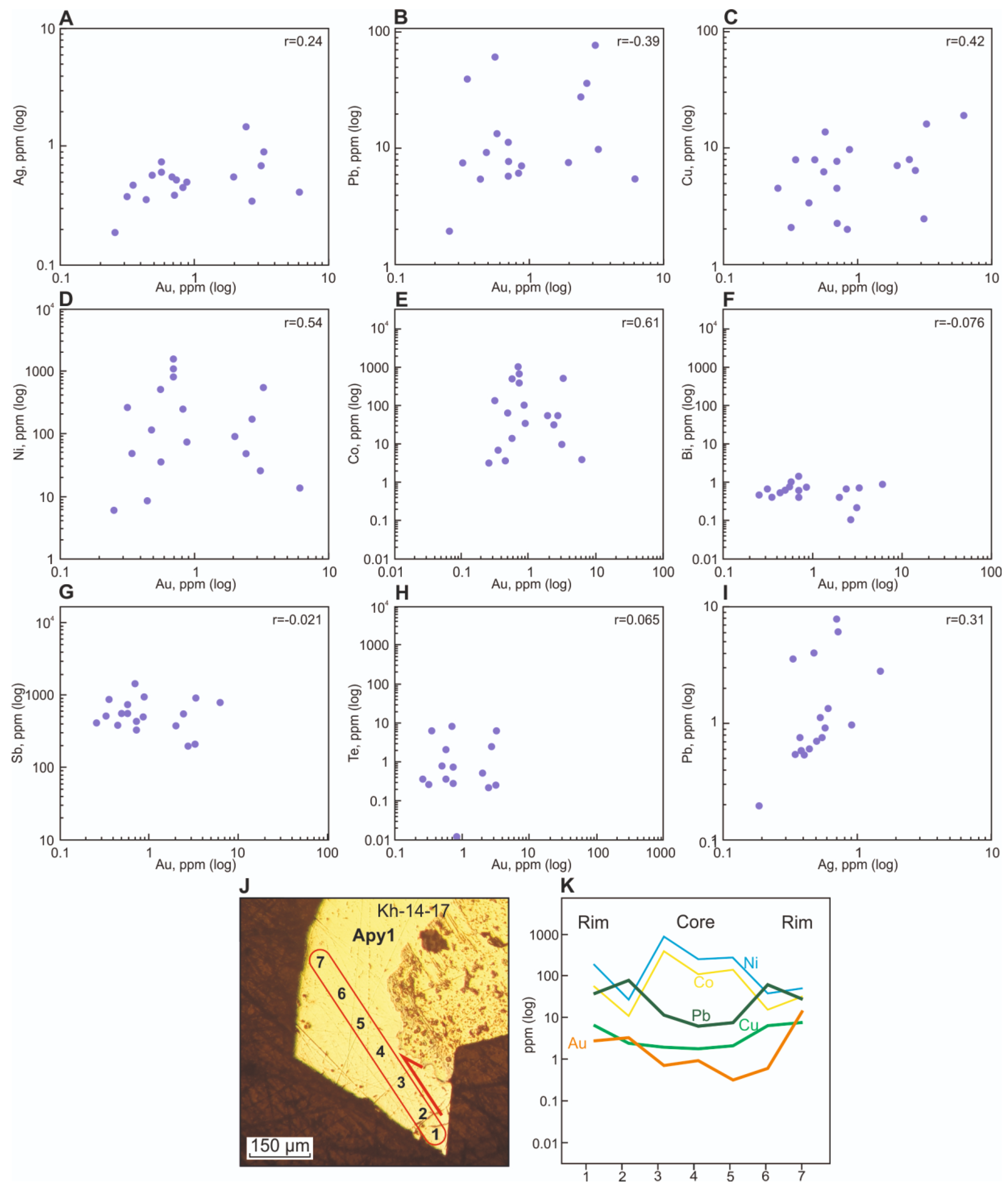
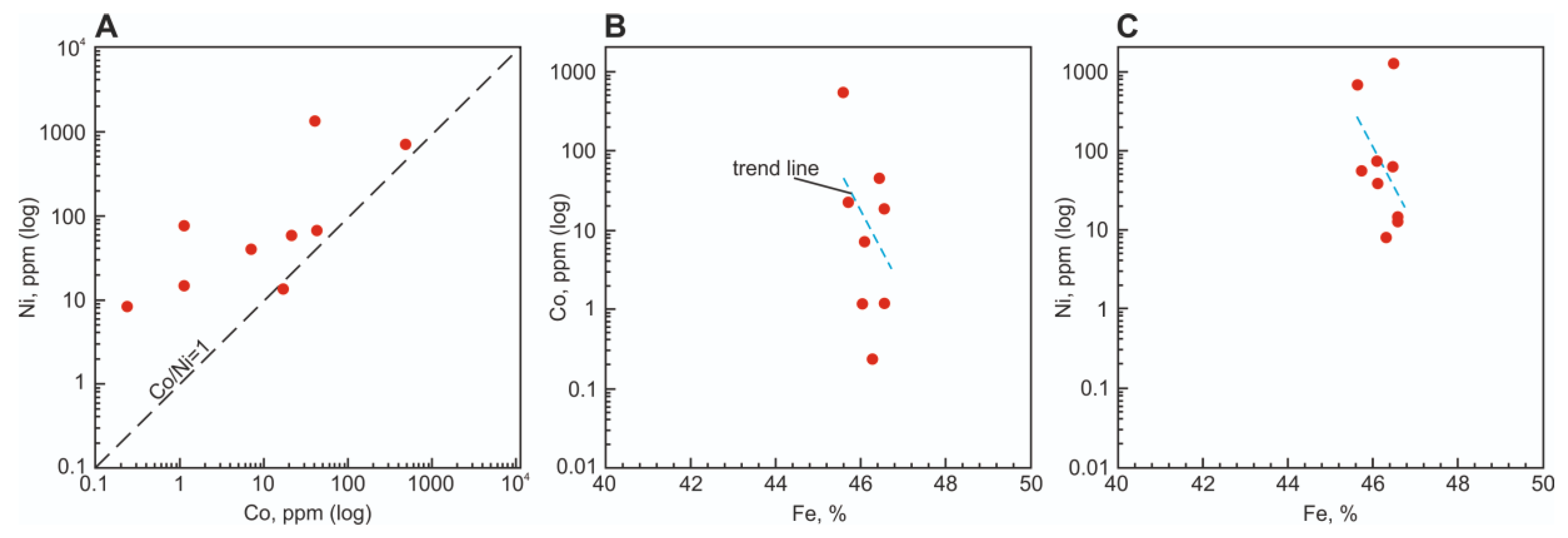
| No | Sample | Fe | S | As | Co | Ni | Cu | Sb | Pb |
|---|---|---|---|---|---|---|---|---|---|
| Diagenetic Py1 | |||||||||
| 1 | K-40-14; n = 11 | * | nd | ||||||
| 2 | K-55-14; n = 10 | nd | |||||||
| 3 | K-61-14; n = 5 | nd | |||||||
| 4 | K-23-14; n = 5 | ||||||||
| 5 | Kpr2-4-14; n = 4 | 0.01 | nd | ||||||
| 6 | K-4-14; n = 6 | 0.03 | nd | ||||||
| 7 | K-7-17; n = 19 | nd | |||||||
| 8 | KG-32-19, n = 1 | 46.80 | 53.66 | 0.18 | nd | 0.02 | nd | 0.06 | nd |
| 9 | KG-7-19, n = 1 | 46.50 | 52.31 | 0.01 | nd | 0.01 | nd | 0.05 | nd |
| Metamorphic Py2 | |||||||||
| 10 | K-40-14; n = 4 | nd | |||||||
| 11 | K-55-14; n = 3 | nd | |||||||
| 12 | K-4-14; n = 4 | nd | |||||||
| 13 | K-23-14; n = 7 | ||||||||
| 14 | KG-29-19; n = 4 | ||||||||
| Hydrothermal-metasomatic Py3 | |||||||||
| 15 | K-32-14; n = 16 | nd | |||||||
| 16 | K-51-14; n = 27 | nd | |||||||
| 17 | K-52-14; n = 24 | nd | |||||||
| 18 | K-55-14; n = 6 | nd | |||||||
| 19 | K-61-14; n = 17 | nd | |||||||
| 20 | K-9-17/1; n = 18 | nd | |||||||
| 21 | K-4-17; n = 13 | nd | |||||||
| 22 | K-14-17; n = 19 | ||||||||
| 23 | K-35-17; n = 24 | ||||||||
| 24 | KG-12-19; n = 25 | ||||||||
| 25 | KG-13-19; n = 39 | ||||||||
| 26 | KG-9-19; n = 5 | ||||||||
| 27 | KG-18-19; n = 26 | ||||||||
| 28 | KG-24-19; n = 21 | ||||||||
| 29 | KG-29-19; n = 20 | ||||||||
| 30 | KG-30-19/1; n = 21 | ||||||||
| 31 | Kpr-4-14; n = 9 | ||||||||
| 32 | KG-30-19/2; n = 9 | nd | |||||||
| 33 | K-7-17; n = 10 | nd | |||||||
| 34 | K-5-14/1; n = 27 | nd | |||||||
| Hydrothermal vein Py4 | |||||||||
| 35 | KG-1-19; n = 14 | nd | |||||||
| 36 | K-45-14; n = 9 | nd | |||||||
| Hydrothermal-metasomatic Apy1 | |||||||||
| 37 | K-32-14; n = 10 | nd | |||||||
| 38 | K-51-14; n = 4 | nd | |||||||
| 39 | K-52-14; n = 5 | nd | |||||||
| 40 | K-4-17; n = 5 | nd | |||||||
| 41 | KG-9-19; n = 5 | 0.01 | nd | ||||||
| 42 | KG-30-19/1; n = 5 | nd | nd | ||||||
| 43 | K-7-17; n = 15 | nd | nd | ||||||
| Hydrothermal vein Apy2 | |||||||||
| 44 | KG-11-19; n = 43 | nd | |||||||
| 45 | K-21-14; n = 24 | nd | |||||||
| Sample | Spot Position | As | Ti | V | Cr | Mn | Co | Ni | Cu | Zn | Ga | Ge | Se | Mo | Ag | Cd | In | Sn | Sb | Te | W | Tl | Pb | Bi | Au | Pd | Ba | Pt | Hg | Au/Ag |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K-4-17 | Asp10-6 | - | 218 | 1.23 | 6.3 | 1.04 | 521 | 565 | 15.9 | 15 | 0.036 | 1.99 | 323 | 1.53 | 0.9 | nd | 0.048 | 0.09 | 890 | 5.9 | 2.44 | 0.045 | 9.7 | 0.71 | 3.31 | 0.025 | 2.25 | 0.051 | 1.26 | 3.68 |
| K-4-17 | Asp11-11 | - | 2060 | 5.07 | 3.4 | 1.45 | 57.5 | 93 | 7 | 4.2 | 0.035 | 2.36 | 104 | 0.56 | 0.55 | 0.57 | 0.122 | 0.68 | 385 | 0.5 | 0.83 | 0.01 | 7.41 | 0.417 | 2 | 0.012 | 2.49 | 0.033 | 0.41 | 3.64 |
| K-4-17 | Asp12-14 | - | 37.5 | 0.204 | 2.6 | 1.16 | 524 | 534 | 14 | 3.7 | 0.055 | 1.8 | 268 | 21.6 | 0.6 | 0.16 | 0.119 | 0.18 | 721 | 1.97 | 0.129 | 0.11 | 13.5 | 0.99 | 0.574 | 0.003 | 0.29 | 0.006 | 0.3 | 0.96 |
| K-4-17 | Asp13-15 | - | 0.8 | 0.077 | 3.3 | 1.09 | 3.24 | 6.3 | 4.6 | 5.7 | 0.019 | 1.86 | 156 | 7.16 | 0.186 | 0.07 | 0.076 | 0.22 | 416 | 0.35 | 0.016 | 0.026 | 1.95 | 0.466 | 0.255 | bdl | 0.28 | bdl | 0.42 | 1.37 |
| K-4-17 | Asp14-15 | - | 8 | 0.132 | 2.3 | 0.3 | 7.2 | 50 | 7.9 | 3.3 | 0.085 | 1.95 | 66.9 | 7.72 | 0.47 | nd | 0.119 | bdl | 883 | 6.2 | 0.22 | 0.03 | 38.9 | 0.402 | 0.352 | 0.002 | 0.33 | 0.028 | 0.82 | 0.75 |
| K-4-17 | Asp15-25 | - | 0.97 | 0.194 | 2.9 | 1.71 | 4.22 | 13.6 | 19 | 10 | 0.013 | 2.01 | 187 | 1.38 | 0.41 | 0.043 | 0.133 | 0.13 | 778 | bdl | bdl | 0.012 | 5.49 | 0.89 | 6.13 | 0.001 | 0.08 | 0.057 | 0.98 | 14.95 |
| K-4-17 | Asp16-26 | - | 1.06 | 0.069 | 0.59 | 1.35 | 1070 | 1680 | 7.8 | 3.6 | 0.021 | 2.17 | 329 | 9.9 | 0.55 | 0.036 | 0.114 | 0.07 | 1414 | 8 | 0.19 | 0.043 | 7.83 | 1.42 | 0.7 | 0.017 | 0.34 | 0.01 | 1.95 | 1.27 |
| K-4-17 | Asp17-33 | - | 21.7 | 0.29 | 2.7 | 1.29 | 35.7 | 76 | 9.8 | 2.55 | 0.059 | 2.01 | 107 | 20 | 0.5 | nd | 0.097 | bdl | 926 | 0.01 | 0.082 | 0.019 | 7.12 | 0.77 | 0.88 | bdl | 0.11 | 0.018 | 0.63 | 1.76 |
| K-4-17 | LineA1-1 | - | 295 | 0.62 | 1.15 | nd | 65 | 122.1 | 8 | 2 | 0.014 | 3.82 | 126.2 | 9.22 | 0.57 | nd | 0.062 | 0.126 | 550 | 0.74 | 1.68 | 0.0239 | 9.18 | 0.641 | 0.488 | bdl | 3.1 | bdl | 0.51 | 0.86 |
| K-4-17 | LineA1-2 | - | 242 | 0.42 | 0.71 | nd | 3.7 | 8.9 | 3.44 | 0.69 | bdl | 4.19 | 98 | 3.05 | 0.35 | 0.1 | 0.06 | 0.3 | 378 | bdl | 1.17 | 0.0038 | 5.43 | 0.56 | 0.44 | bdl | 2.2 | bdl | 1.03 | 1.26 |
| K-4-17 | LineA1-3 | - | 32 | 0.111 | 0.27 | nd | 728 | 1182 | 4.57 | 1.63 | 0.0079 | 3.88 | 145.7 | 6.03 | 0.388 | nd | 0.059 | 0.1 | 429 | 0.7 | 0.27 | 0.0049 | 5.79 | 0.636 | 0.715 | bdl | 0.29 | 0.004 | 0.64 | 1.84 |
| K-4-17 | LineA2 | - | 3.1 | 0.089 | 89 | nd | 56.5 | 184 | 6.5 | 4.3 | 0.058 | 4.06 | 48.9 | 0.156 | 0.341 | nd | 0.035 | 0.12 | 193.8 | 2.34 | 0.035 | 0.044 | 36.3 | 0.113 | 2.7 | bdl | 4.2 | 0.012 | 0.43 | 7.92 |
| K-4-17 | LineA2 | - | 2.93 | 0.025 | 5.6 | nd | 10.32 | 26.1 | 2.46 | 2.47 | 0.0086 | 3.8 | 93.1 | 2.34 | 0.698 | nd | 0.062 | 0.091 | 208.8 | 0.24 | 0.026 | 0.0176 | 76 | 0.226 | 3.16 | bdl | 2.61 | 0.02 | 0.95 | 4.53 |
| K-4-17 | LineA2 | - | 399 | 0.945 | 1.89 | nd | 401 | 874 | 2.27 | 2.3 | 0.051 | 3.89 | 118.5 | 1.5 | 0.54 | nd | 0.0534 | 0.262 | 328.3 | 0.28 | 0.93 | 0.0297 | 11.4 | 0.454 | 0.715 | bdl | 2.82 | 0.033 | 0.72 | 1.32 |
| K-4-17 | LineA2 | - | 72 | 0.201 | 2.2 | nd | 109.7 | 250.8 | 2.05 | 1.69 | 0.022 | 4.09 | 71.2 | 8.68 | 0.448 | nd | 0.052 | 0.184 | 495 | bdl | 0.13 | 0.0178 | 6.16 | 0.712 | 0.833 | 0.016 | 0.54 | 0.008 | 0.46 | 1.86 |
| K-4-17 | LineA2 | - | 99 | 0.334 | 1.98 | nd | 140 | 276 | 2.07 | 2.28 | 0.0314 | 3.76 | 118.5 | 11.85 | 0.376 | 0.0031 | 0.0544 | 0.182 | 512.6 | 0.255 | 0.451 | 0.0087 | 7.46 | 0.659 | 0.319 | bdl | 1.08 | 0.011 | 0.57 | 0.85 |
| K-4-17 | LineA2 | - | 278 | 0.61 | 3.1 | nd | 14.84 | 37.5 | 6.3 | 2.29 | 0.0114 | 3.67 | 130.3 | 11.07 | 0.732 | nd | 0.051 | 0.218 | 550.6 | 0.35 | 0.76 | 0.0067 | 60.2 | 0.759 | 0.562 | 0.024 | 1.16 | 0.010 | 0.61 | 0.77 |
| K-4-17 | LineA2 | - | 7790 | 15 | 14.8 | nd | 32.6 | 50 | 7.91 | 2.08 | 0.16 | 3.92 | 112.9 | 11.18 | 1.49 | nd | 0.037 | 0.8 | 534.3 | 0.21 | 22.6 | 0.0071 | 27.4 | 0.693 | 2.43 | 0.011 | 30.5 | 0.017 | 0.25 | 1.63 |
| Minimum | 0.8 | 0.0 | 0.3 | 0.3 | 3.2 | 6.3 | 2.1 | 0.7 | 0.0079 | 1.8 | 48.9 | 0.2 | 0.2 | 0.0 | 0.04 | 0.07 | 193.8 | 0.01 | 0.02 | 0.004 | 2.0 | 0.1 | 0.3 | 0.001 | 0.08 | 0.004 | 0.25 | 0.75 | ||
| Maximum | 7790.0 | 15.0 | 89.0 | 1.7 | 1070.0 | 1680.0 | 19.0 | 15.0 | 0.16 | 4.2 | 329.0 | 21.6 | 1.5 | 0.6 | 0.13 | 0.80 | 1414.0 | 8.00 | 22.60 | 0.110 | 76.0 | 1.4 | 6.1 | 0.025 | 30.5 | 0.057 | 1.95 | 14.95 | ||
| Average | 642.3 | 1.4 | 8.0 | 1.2 | 210.3 | 335.0 | 7.3 | 3.9 | 0.040 | 3.1 | 144.7 | 7.5 | 0.6 | 0.1 | 0.08 | 0.23 | 588.5 | 1.87 | 1.88 | 0.026 | 18.7 | 0.6 | 1.5 | 0.012 | 3.04 | 0.021 | 0.72 | 2.84 | ||
| Std dev | 1846.3 | 3.6 | 20.5 | 0.4 | 309.8 | 471.4 | 4.8 | 3.5 | 0.038 | 1.0 | 82.0 | 6.3 | 0.3 | 0.2 | 0.0 | 0.2 | 302.5 | 2.5 | 5.2 | 0.025 | 21.0 | 0.3 | 1.6 | 0.009 | 6.97 | 0.017 | 0.41 | 3.52 | ||
| CV | 287% | 252% | 254% | 35% | 147% | 141% | 66% | 89% | 94% | 32% | 57% | 84% | 51% | 140% | 43% | 89% | 51% | 133% | 279% | 98% | 112% | 46% | 105% | 74% | 229% | 79% | 57% | |||
| K-4-17 | Py1-1 | 4890 | 2470 | 7.16 | 10.3 | 0.85 | 1.13 | 14.4 | 3.96 | 3.51 | 0.197 | 2.49 | 4.4 | 0.079 | 0.92 | nd | 0.005 | 0.23 | 10.19 | 0.056 | 9.71 | 0.0076 | 66 | 0.243 | 0.955 | 0.008 | 3.88 | 0.062 | bdl | 1.04 |
| K-4-17 | Py2-3 | 7110 | 0.7 | 0.028 | 0.38 | 0.57 | 0.233 | 8.2 | 0.54 | 3.62 | 0.055 | 2.67 | 6.2 | 0.21 | 0.0076 | nd | 0.0021 | 0.11 | 0.25 | 0.21 | 0.067 | 0.018 | 0.479 | 0.048 | 0.502 | bdl | 0.008 | 0.0073 | 0.02 | 66.05 |
| K-4-17 | Py3-4 | 4390 | 8.6 | 0.116 | 0.39 | 0.82 | 17.5 | 13.4 | 1.58 | 3.8 | 0.056 | 2.59 | 2.6 | 0.64 | 0.196 | nd | 0.021 | 0.059 | 2 | 0.13 | 0.055 | 0.0074 | 3.71 | 0.084 | 0.236 | 0.01 | 0.16 | 0.034 | 0.01 | 1.20 |
| K-9-17 | Py4-7 | 4220 | 0.78 | 0.058 | 0.52 | 0.41 | 1.15 | 74.9 | 3 | 4.2 | 0.018 | 2.46 | 7.2 | 0.059 | 0.85 | 0.024 | 0.0033 | 0.033 | 407 | 0.083 | 0.019 | 0.065 | 860 | 0.93 | 0.507 | 0.0036 | 0.008 | 0.029 | 0.2 | 0.60 |
| K-9-17 | Py5-8 | 17480 | 36.1 | 0.35 | 0.98 | 0.4 | 7.05 | 39 | 19 | 4.7 | 0.19 | 2.71 | 3.5 | 0.047 | 0.055 | 0.036 | 0.017 | 0.07 | 6.19 | bdl | 0.08 | 0.047 | 3.35 | 0.09 | 8.83 | 0.01 | 1.83 | 0.037 | 0.33 | 160.55 |
| K-9-17 | Py6-10 | 17260 | 2390 | 8.42 | 7.9 | 1.53 | 21.3 | 56.9 | 18 | 5.3 | 0.191 | 2.66 | 4.5 | 0.71 | 0.8 | 0.065 | 0.0105 | 0.31 | 40.1 | 0.06 | 8.66 | 0.089 | 26.7 | 0.446 | 15.85 | 0.013 | 4.68 | 0.064 | 0.36 | 19.81 |
| K-14-17 | Py7-11 | 10280 | 77 | 0.235 | 0.58 | 7.55 | 505 | 690 | 7.3 | 6.4 | 0.009 | 2.69 | 52.1 | 1.04 | 1.01 | 0.027 | 0.0012 | 0.07 | 8.42 | 0.31 | 0.112 | 0.03 | 23.3 | 0.457 | 2.5 | 0.0027 | 0.151 | 0.044 | 0.33 | 2.48 |
| K-14-17 | Py8-13 | 6820 | 79 | 0.105 | 1.09 | 0.74 | 41.6 | 1298 | 1.4 | 4.65 | 0.036 | 2.7 | 40.3 | 0.29 | 0.062 | nd | bdl | 0.1 | 1.05 | 0.21 | 0.096 | 0.0028 | 2.75 | 0.043 | 0.143 | 0.0028 | 0.05 | 0.013 | bdl | 2.31 |
| K-42-17 | Py9-18 | 8030 | 208 | 0.274 | 0.77 | 0.73 | 43.5 | 64.9 | 1.29 | 4.22 | 0.018 | 2.63 | 73.6 | bdl | 0.128 | nd | 0.0031 | 0.03 | 1 | bdl | 0.56 | bdl | 3.34 | 0.024 | 1.028 | 0.007 | 0.008 | 0.01 | bdl | 304.69 |
| Minimum | 4220.0 | 0.7 | 0.028 | 0.38 | 0.40 | 0.23 | 8.2 | 0.5 | 3.5 | 0.0 | 2.5 | 2.6 | 0.05 | 0.01 | 0.024 | 0.001 | 0.03 | 0.3 | 0.1 | 0.0 | 0.003 | 0.5 | 0.02 | 0.1 | 0.003 | 0.01 | 0.01 | 0.01 | 0.6 | |
| Maximum | 17480.0 | 2470.0 | 8.42 | 10.30 | 7.55 | 505.0 | 1298.0 | 19.0 | 6.4 | 0.2 | 2.7 | 73.6 | 1.04 | 1.01 | 0.065 | 0.021 | 0.31 | 407.0 | 0.3 | 9.7 | 0.089 | 860.0 | 0.93 | 39.0 | 0.013 | 4.68 | 0.06 | 0.36 | 304.7 | |
| Average | 8942.2 | 585.6 | 1.86 | 2.55 | 1.51 | 70.9 | 251.1 | 6.2 | 4.5 | 0.1 | 2.6 | 21.6 | 0.38 | 0.45 | 0.038 | 0.008 | 0.11 | 52.9 | 0.2 | 2.2 | 0.033 | 110.0 | 0.26 | 7.6 | 0.007 | 1.20 | 0.03 | 0.21 | 62.1 | |
| Std dev | 5147.9 | 1047.8 | 3.38 | 3.77 | 2.29 | 163.6 | 448.4 | 7.2 | 0.9 | 0.1 | 0.1 | 26.7 | 0.37 | 0.43 | 0.023 | 0.007 | 0.10 | 133.4 | 0.1 | 4.0 | 0.031 | 282.1 | 0.30 | 12.9 | 0.004 | 1.85 | 0.02 | 0.16 | 105.3 | |
| CV | 58% | 179% | 182% | 148% | 151% | 231% | 179% | 116% | 20% | 96% | 3% | 124% | 96% | 96% | 61% | 95% | 85% | 252% | 70% | 186% | 94% | 257% | 115% | 170% | 60% | 155% | 63% | 79% |
| Sample | Mineral/Rock | Content | Au/Ag | |
|---|---|---|---|---|
| Au, ppm | Ag, ppm | |||
| K-4-17 | Ру3 | 7.39 | 8.73 | 0.8 |
| K-9-17 | Ру3 | 21.4 | 5.64 | 3.8 |
| K-9-17 | Ру3 | 22.37 | 7.8 | 2.9 |
| K-14-17 | Ру3 | 3.54 | 1.31 | 2.7 |
| K-14-17 | Ру3 | 0.76 | 1.15 | 0.7 |
| KG-9-19 | Ру3 | 4.89 | 2.74 | 1.8 |
| KG-32-19 | Ру3 | 10.06 | 5.44 | 1.8 |
| KG-20-19 | Ру3 | 11.87 | 6.54 | 1.8 |
| K-13-18 | Ру3 | 3.67 | 6.95 | 0.5 |
| KG-8-19 | Ру3 | 39.32 | 17.38 | 2.3 |
| KG-30-19 | Ру3 | 12.36 | 1.13 | 10.9 |
| Average | 12.51 | 5.89 | ||
| Std dev | 11.32 | 4.71 | ||
| CV | 91% | 80% | ||
| K-4-17 | Ару1 | 12.3 | 0.43 | 28.6 |
| KG-26-19 | Ару1 | 16.44 | 11.83 | 1.4 |
| KG-29-19 | Ару1 | 23.8 | 7.2 | 3.3 |
| Average | 17.51 | 6.49 | ||
| Std dev | 5.82 | 5.73 | ||
| CV | 33% | 88% | ||
| KG-23-19 | Ру4 | 27.07 | 4.46 | 6.1 |
| K-5-17 | Ру4 | 9.42 | 3.47 | 2.7 |
| KG-34-19 | Ру4 | 51.42 | 11.13 | 4.6 |
| Average | 29.30 | 6.35 | ||
| Std dev | 21.09 | 4.17 | ||
| CV | 72% | 66% | ||
| KG-35-19 | Ару2 | 20.49 | 2.06 | 9.9 |
| K-4-17 | Sandstone with sulfides and quartz veinlets | 0.084 | 0.088 | 1.0 |
| K-9-17 | Sandstone with sulfides and quartz veinlets | 0.740 | 0.084 | 8.8 |
| K-14-17 | Sandstone with sulfides | 0.001 | 0.032 | 0.0 |
| K-25-17 | Sandstone with sulfides | 0.240 | 0.042 | 5.7 |
| K-27-17 | Sandstone with sulfides | 0.059 | 0.007 | 8.4 |
| K-28-17 | Sandstone with sulfides | 0.064 | 0.097 | 0.7 |
| K-40-17 | Sandstone with sulfides | 5.29 | 0.142 | 37.3 |
| K-41-17 | Siltstone with pyrite | 0.006 | 0.041 | 0.1 |
| Average | 0.81 | 0.07 | ||
| Std dev | 1.83 | 0.04 | ||
| CV | 225% | 66% | ||
| № | Sample | Generation | δ34SVCDT (‰) |
|---|---|---|---|
| 1 | K-4-17 | Apy1 | −1.2 |
| 2 | KG-9-19 | Apy1 | −1.4 |
| 3 | K-9-17 | Py3 | −0.6 |
| 4 | KG-32-19 | Py3 | −1.3 |
| 5 | KG-35-19 | Apy2 | −2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudrin, M.V.; Fridovsky, V.Y.; Polufuntikova, L.I.; Kryuchkova, L.Y. Disseminated Gold–Sulfide Mineralization in Metasomatites of the Khangalas Deposit, Yana–Kolyma Metallogenic Belt (Northeast Russia): Analysis of the Texture, Geochemistry, and S Isotopic Composition of Pyrite and Arsenopyrite. Minerals 2021, 11, 403. https://doi.org/10.3390/min11040403
Kudrin MV, Fridovsky VY, Polufuntikova LI, Kryuchkova LY. Disseminated Gold–Sulfide Mineralization in Metasomatites of the Khangalas Deposit, Yana–Kolyma Metallogenic Belt (Northeast Russia): Analysis of the Texture, Geochemistry, and S Isotopic Composition of Pyrite and Arsenopyrite. Minerals. 2021; 11(4):403. https://doi.org/10.3390/min11040403
Chicago/Turabian StyleKudrin, Maxim V., Valery Yu. Fridovsky, Lena I. Polufuntikova, and Lyudmila Yu. Kryuchkova. 2021. "Disseminated Gold–Sulfide Mineralization in Metasomatites of the Khangalas Deposit, Yana–Kolyma Metallogenic Belt (Northeast Russia): Analysis of the Texture, Geochemistry, and S Isotopic Composition of Pyrite and Arsenopyrite" Minerals 11, no. 4: 403. https://doi.org/10.3390/min11040403
APA StyleKudrin, M. V., Fridovsky, V. Y., Polufuntikova, L. I., & Kryuchkova, L. Y. (2021). Disseminated Gold–Sulfide Mineralization in Metasomatites of the Khangalas Deposit, Yana–Kolyma Metallogenic Belt (Northeast Russia): Analysis of the Texture, Geochemistry, and S Isotopic Composition of Pyrite and Arsenopyrite. Minerals, 11(4), 403. https://doi.org/10.3390/min11040403






