Paleogeographic Characteristics of the Mengyejing Formation in the Simao Basin during Its Depositional Period and Its Indication of Potash Mineralization: A Case Study of MZK-3 Well
Abstract
1. Introduction
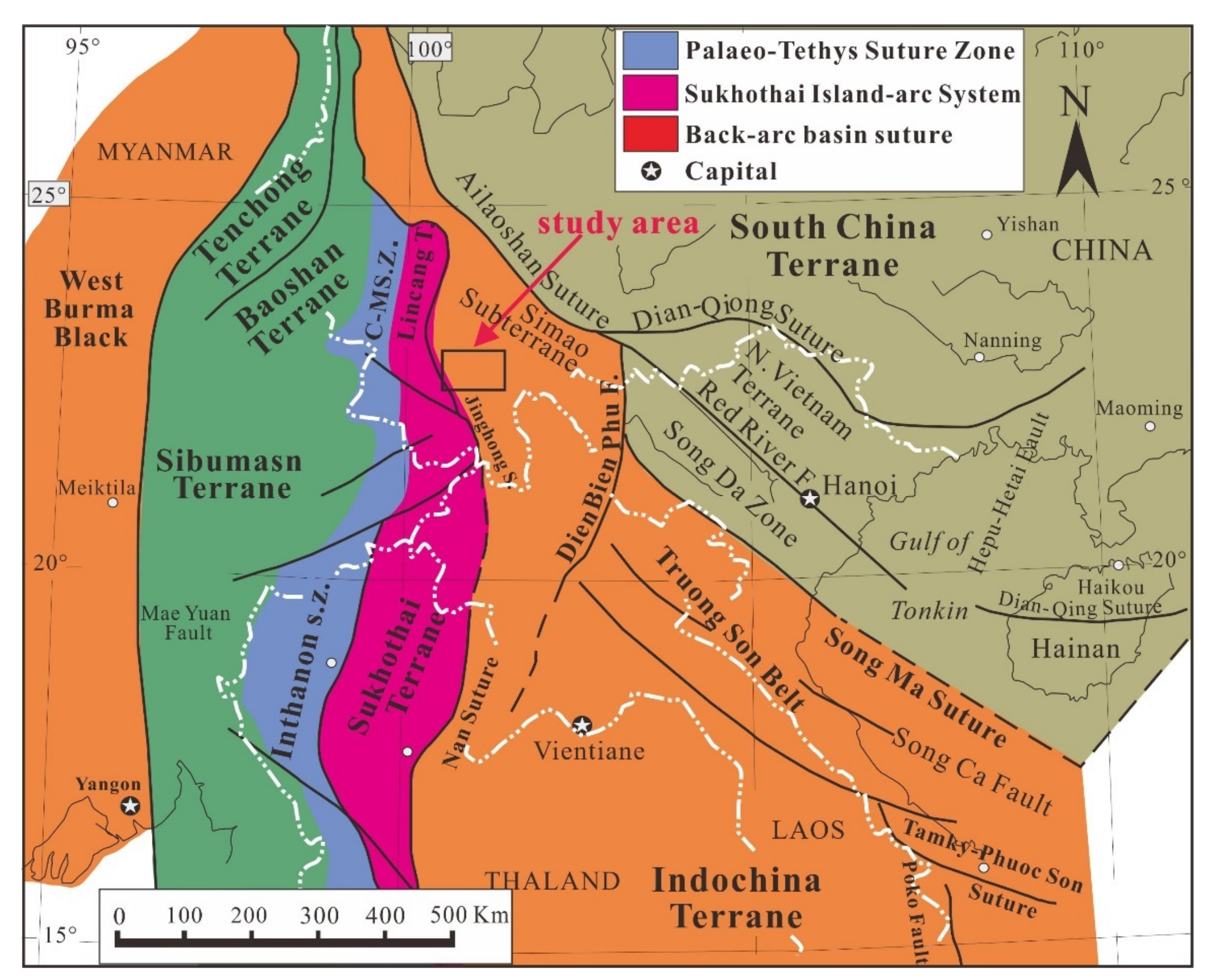
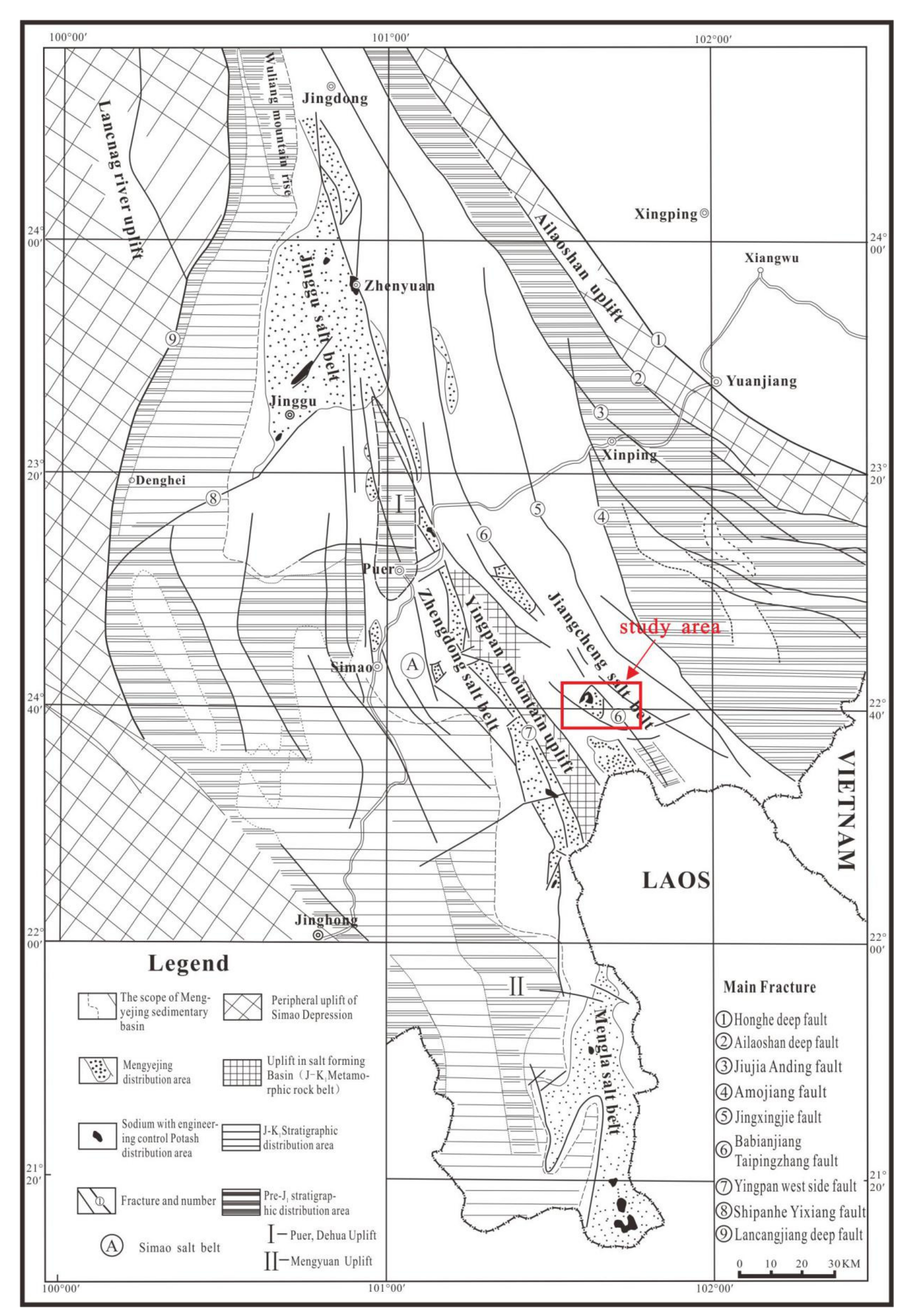
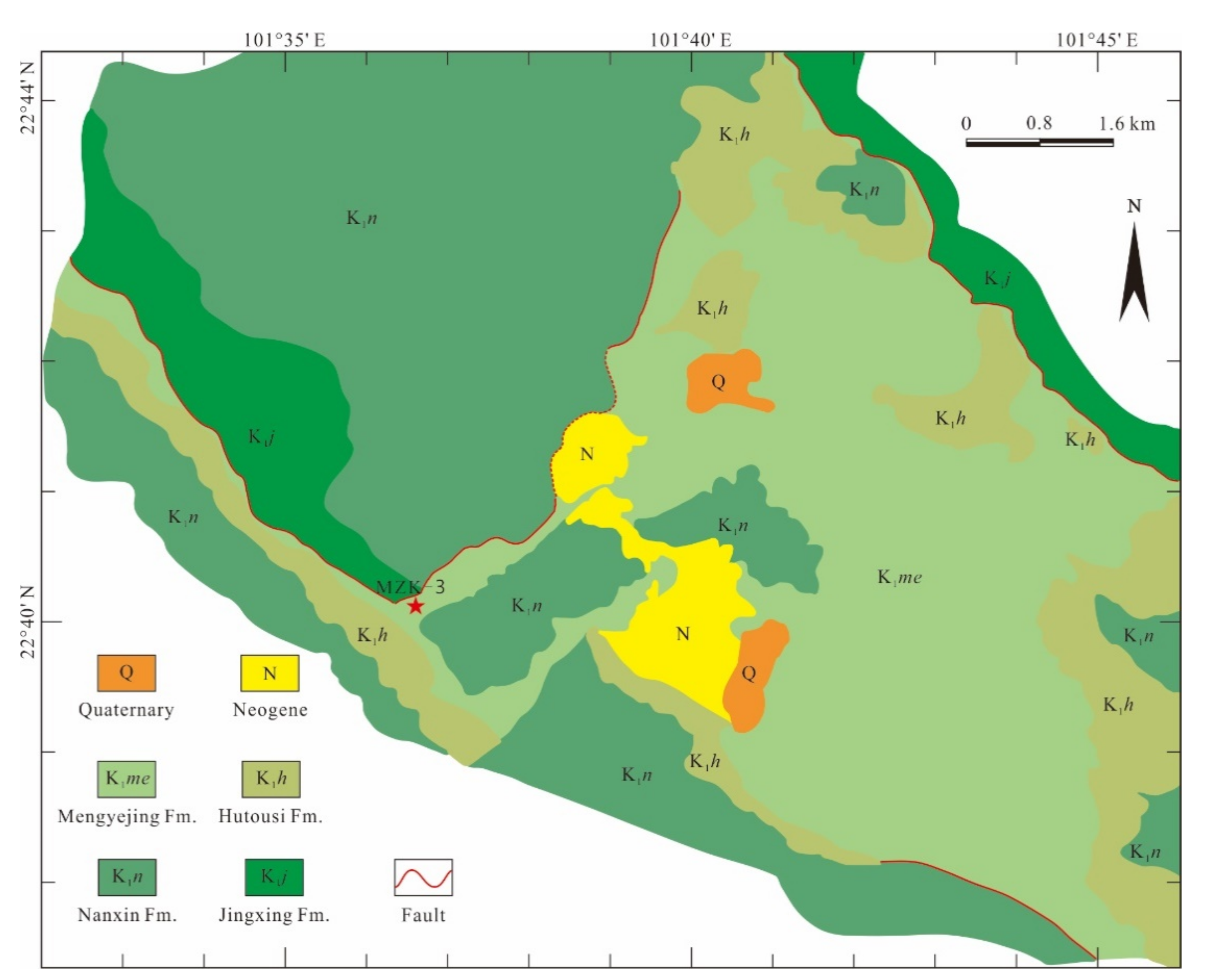
2. Geological Setting
3. Samples and Analytical Methods
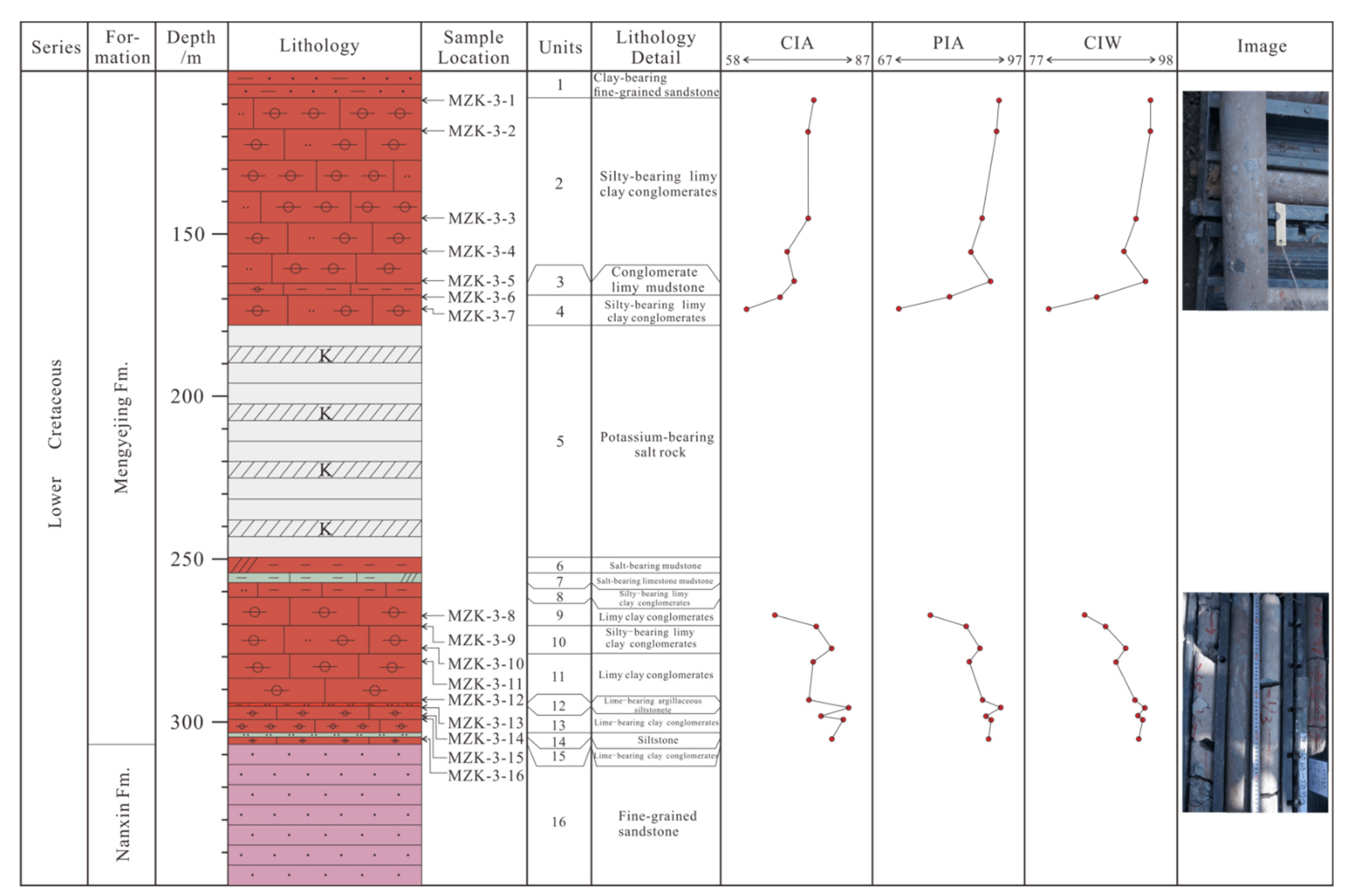
3.1. Samples
3.2. Methods
3.2.1. Boron
3.2.2. X-ray Fluorescent Spectrometry
3.2.3. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
3.2.4. Clay Mineral Analysis
4. Results
4.1. Major Elements
4.2. Trace Elements
| Samples | Depth [m] | Lithology | Major Element Concentrations (wt%) | CIA | PIA | CIW | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | Fe2 | MgO | CaO | Na2O | K2O | ||||||
| MZK-3-1 | 109.2 | C. C. | 13.7 | 4.9 | 3.8 | 7.9 | 0.1 | 4.1 | 74 | 97 | 98 |
| MZK-3-2 | 118.3 | C. C. | 13.7 | 4.6 | 4.3 | 5.1 | 0.1 | 4.3 | 73 | 97 | 98 |
| MZK-3-3 | 146.9 | C. C. | 14.6 | 6.2 | 6.4 | 7.6 | 0.3 | 4.0 | 73 | 91 | 94 |
| MZK-3-4 | 156.1 | C. C. | 14.0 | 5.9 | 7.8 | 8.3 | 0.4 | 3.9 | 72 | 88 | 91 |
| MZK-3-5 | 164.9 | C. C. | 14.1 | 5.0 | 6.2 | 6.2 | 0.2 | 4.4 | 72 | 93 | 96 |
| MZK-3-6 | 169.0 | C. C. | 14.5 | 5.3 | 6.6 | 4.3 | 0.7 | 3.6 | 70 | 82 | 86 |
| MZK-3-7 | 173.1 | C. C. | 12.9 | 4.7 | 3.7 | 8.4 | 1.1 | 4.7 | 60 | 68 | 78 |
| Salt | / | / | / | / | / | / | / | / | / | / | |
| MZK-3-8 | 267.4 | C. C. | 12.6 | 4.8 | 13.1 | 0.6 | 0.9 | 3.5 | 66 | 77 | 83 |
| MZK-3-9 | 270.5 | C. C. | 14.4 | 4.3 | 11.7 | 4.0 | 0.6 | 1.9 | 78 | 86 | 88 |
| MZK-3-10 | 277.3 | C. C. | 13.8 | 3.5 | 11.2 | 7.3 | 0.4 | 1.8 | 81 | 90 | 91 |
| MZK-3-11 | 281.4 | C. C. | 9.0 | 3.8 | 2.7 | 7.3 | 0.3 | 1.9 | 75 | 88 | 90 |
| MZK-3-12 | 293.1 | C. C. | 13.5 | 7.4 | 3.6 | 7.2 | 0.3 | 3.4 | 74 | 91 | 93 |
| MZK-3-13 | 295.6 | C. C. | 16.3 | 5.9 | 2.0 | 2.8 | 0.2 | 1.7 | 87 | 96 | 96 |
| MZK-3-14 | 298.1 | C. C. | 10.2 | 4.0 | 3.7 | 10.3 | 0.2 | 2.0 | 78 | 92 | 94 |
| MZK-3-15 | 299.1 | C. C. | 17.0 | 6.5 | 2.4 | 3.0 | 0.3 | 2.0 | 84 | 94 | 95 |
| MZK-3-16 | 305.2 | C. C. | 16.5 | 6.1 | 2.5 | 4.7 | 0.3 | 2.3 | 83 | 93 | 94 |
| Numbers | Depth [m] | Lithology | Trace Element Concentrations [μg/g] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Ba | Co | Cr | Cu | Ga | Mn | Ni | Rb | Sr | V | Zn | Zr | |||
| MZK-3-1 | 109.2 | C. C. | 86.7 | 371.1 | 12.8 | 74.0 | 13.0 | 15.0 | 765.5 | 29.3 | 113.0 | 73.1 | 92.4 | 41.5 | 189.1 |
| MZK-3-2 | 118.3 | C. C. | 140.8 | 357.5 | 13.6 | 70.1 | 63.6 | 15.4 | 680.0 | 28.0 | 114.9 | 82.3 | 84.9 | 44.6 | 189.2 |
| MZK-3-3 | 146.9 | C. C. | 69.0 | 278.1 | 16.8 | 73.3 | 14.1 | 14.3 | 609.7 | 34.6 | 122.4 | 92.5 | 94.7 | 102.3 | 135.1 |
| MZK-3-4 | 156.1 | C. C. | 67.3 | 239.1 | 19.1 | 67.0 | 20.9 | 16.5 | 703.2 | 35.4 | 121.6 | 99.8 | 93.3 | 78.1 | 117.9 |
| MZK-3-5 | 164.9 | C. C. | 72.6 | 248.5 | 9.5 | 65.3 | 7.5 | 16.8 | 643.7 | 30.4 | 117.4 | 45.4 | 90.9 | 57.7 | 177.2 |
| MZK-3-6 | 169.0 | C. C. | 195.9 | 224.9 | 11.2 | 69.4 | 4.7 | 16.2 | 284.1 | 35.5 | 106.9 | 144.8 | 95.7 | 70.6 | 193.5 |
| MZK-3-7 | 173.1 | C. C. | 72.9 | 271.5 | 12.2 | 64.0 | 14.2 | 15.8 | 513.4 | 30.8 | 104.0 | 63.4 | 78.2 | 57.2 | 158.2 |
| Salt | / | / | / | / | / | / | / | / | / | / | / | / | / | / | / |
| MZK-3-8 | 267.4 | C. C. | 105.3 | 196.0 | 12.2 | 73.0 | 5.6 | 14.9 | 410.7 | 33.3 | 92.7 | 30.9 | 85.9 | 41.2 | 149.9 |
| MZK-3-9 | 270.5 | C. C. | 235.6 | 200.4 | 9.1 | 71.2 | 13.2 | 16.4 | 243.4 | 46.2 | 61.7 | 91.4 | 89.3 | 43.8 | 184.0 |
| MZK-3-10 | 277.3 | C. C. | 253.3 | 205.1 | 15.2 | 73.6 | 22.6 | 16.2 | 378.0 | 36.5 | 60.0 | 1102.9 | 105.2 | 45.9 | 166.6 |
| MZK-3-11 | 281.4 | C. C. | 81.6 | 5216.6 | 8.2 | 68.6 | 12.3 | 10.6 | 752.0 | 20.3 | 71.4 | 131.2 | 91.5 | 36.4 | 262.6 |
| MZK-3-12 | 293.1 | C. C. | 144.6 | 256.2 | 28.8 | 73.6 | 7.4 | 14.3 | 515.2 | 63.6 | 101.1 | 76.9 | 97.5 | 86.1 | 174.6 |
| MZK-3-13 | 295.6 | C. C. | 88.0 | 340.1 | 15.3 | 95.8 | 38.6 | 18.4 | 701.3 | 38.3 | 85.1 | 284.0 | 117.8 | 38.9 | 229.1 |
| MZK-3-14 | 298.1 | C. C. | 43.2 | 489.9 | 13.9 | 64.3 | 17.1 | 11.6 | 858.6 | 22.7 | 71.8 | 61.7 | 78.7 | 46.2 | 224.0 |
| MZK-3-15 | 299.1 | C. C. | 100.4 | 334.4 | 17.4 | 99.8 | 51.9 | 19.6 | 924.0 | 45.2 | 96.1 | 254.8 | 128.2 | 46.9 | 172.9 |
| MZK-3-16 | 305.2 | C. C. | 90.3 | 871.9 | 16.0 | 95.5 | 24.6 | 18.4 | 933.9 | 39.4 | 105.8 | 240.3 | 124.4 | 46.1 | 189.3 |
| Post Archean Australian Shale (PAAS) | / | 650 | 23 | 110 | 50 | 20 | 850 | 55 | 160 | 200 | 150 | 85 | 210 | ||
| Chondrite | 6.2 | 5 | 1300 | 6600 | 250 | 12 | 7400 | 40,000 | 5 | 20 | 200 | 1300 | 37 | ||
4.3. Clay Mineral
| Samples | Depth [m] | Results/wt% | RML/S% | |||
|---|---|---|---|---|---|---|
| Illite | Kaolinite | Chlorite | I/S | C/S | ||
| MZK-3-2 | 118.31 | 73 | 3 | 8 | 16 | / |
| MZK-3-3 | 146.88 | 84 | 3 | 5 | 8 | / |
| MZK-3-4 | 156.08 | 80 | 1 | 6 | 13 | / |
| MZK-3-6 | 168.95 | 76 | 6 | 12 | 6 | / |
| MZK-3-7 | 173.05 | 74 | 5 | 9 | 12 | / |
| Salt | / | / | / | / | / | / |
| MZK-3-8 | 267.35 | 64 | 0 | 6 | 27 | 3 |
| MZK-3-9 | 270.5 | 32 | 15 | 26 | 11 | 16 |
| MZK-3-10 | 277.3 | 56 | 0 | 44 | 0 | / |
| MZK-3-11 | 281.43 | 78 | 12 | 6 | 4 | / |
| MZK-3-12 | 293.05 | 76 | 12 | / | 12 | / |
| MZK-3-13 | 298.05 | 59 | 32 | / | 9 | / |
| MZK-3-14 | 295.6 | 53 | 41 | / | 6 | / |
| MZK-3-15 | 299.05 | 43 | 52 | / | 5 | / |
| MZK-3-16 | 305.15 | 44 | 40 | / | 16 | / |
| Samples | Depth [m] | EF (Enrichment Factors) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ba | Co | Cr | Cu | Ga | Mn | Ni | Rb | Sr | V | Zn | Zr | ||
| MZK-3-1 | 109.2 | 0.8 | 0.8 | 0.9 | 0.4 | 1.0 | 1.2 | 0.7 | 1.0 | 0.5 | 0.9 | 0.7 | 1.2 |
| MZK-3-2 | 118.3 | 0.8 | 0.8 | 0.9 | 1.8 | 1.1 | 1.1 | 0.7 | 1.0 | 0.6 | 0.8 | 0.7 | 1.2 |
| MZK-3-3 | 146.9 | 0.6 | 0.9 | 0.9 | 0.4 | 0.9 | 0.9 | 0.8 | 1.0 | 0.6 | 0.8 | 1.6 | 0.8 |
| MZK-3-4 | 156.1 | 0.5 | 1.1 | 0.8 | 0.6 | 1.1 | 1.1 | 0.9 | 1.0 | 0.7 | 0.8 | 1.2 | 0.8 |
| MZK-3-5 | 164.9 | 0.5 | 0.5 | 0.8 | 0.2 | 1.1 | 1.0 | 0.7 | 1.0 | 0.3 | 0.8 | 0.9 | 1.1 |
| MZK-3-6 | 169.0 | 0.4 | 0.6 | 0.8 | 0.1 | 1.1 | 0.4 | 0.8 | 0.9 | 0.9 | 0.8 | 1.1 | 1.2 |
| MZK-3-7 | 173.1 | 0.6 | 0.8 | 0.9 | 0.4 | 1.2 | 0.9 | 0.8 | 1.0 | 0.5 | 0.8 | 1.0 | 1.1 |
| Salt | / | / | / | / | / | / | / | / | / | / | / | / | / |
| MZK-3-8 | 267.4 | 0.5 | 0.8 | 1.0 | 0.2 | 1.1 | 0.7 | 0.9 | 0.9 | 0.2 | 0.9 | 0.7 | 1.1 |
| MZK-3-9 | 270.5 | 0.4 | 0.5 | 0.9 | 0.3 | 1.1 | 0.4 | 1.1 | 0.5 | 0.6 | 0.8 | 0.7 | 1.2 |
| MZK-3-10 | 277.3 | 0.4 | 0.9 | 0.9 | 0.6 | 1.1 | 0.6 | 0.9 | 0.5 | 7.6 | 1.0 | 0.7 | 1.1 |
| MZK-3-11 | 281.4 | 16.8 | 0.7 | 1.3 | 0.5 | 1.1 | 1.9 | 0.8 | 0.9 | 1.4 | 1.3 | 0.9 | 2.6 |
| MZK-3-12 | 293.1 | 0.6 | 1.8 | 0.9 | 0.2 | 1.0 | 0.8 | 1.6 | 0.9 | 0.5 | 0.9 | 1.4 | 1.2 |
| MZK-3-13 | 295.6 | 0.6 | 0.8 | 1.0 | 0.9 | 1.1 | 1.0 | 0.8 | 0.6 | 1.6 | 0.9 | 0.5 | 1.3 |
| MZK-3-14 | 298.1 | 1.4 | 1.1 | 1.1 | 0.6 | 1.1 | 1.9 | 0.8 | 0.8 | 0.6 | 1.0 | 1.0 | 2.0 |
| MZK-3-15 | 299.1 | 0.6 | 0.8 | 1.0 | 1.2 | 1.1 | 1.2 | 0.9 | 0.7 | 1.4 | 0.9 | 0.6 | 0.9 |
| MZK-3-16 | 305.2 | 1.5 | 0.8 | 1.0 | 0.6 | 1.1 | 1.3 | 0.8 | 0.8 | 1.4 | 0.9 | 0.6 | 1.0 |
| Average | / | 1.7 | 0.9 | 0.9 | 0.6 | 1.1 | 1.03 | 0.9 | 0.8 | 1.2 | 0.9 | 0.9 | 1.2 |
5. Discussion
5.1. Paleoclimate of MYJ Fm. in Sedimentary Period
5.2. Redox Conditions
5.3. Paleosalinity
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miao, W.; Ma, H.; Zhang, X.; Shi, H.; Li, Y.; Rong, Z. Mineralogical and geochemical characteristics of detrital rocks in the Mengyejing Formation and evolution of the sedimentary environment of paleolake in Simao Basin, Yunnan province. Acta Geol. Sin. 2015, 89, 2096–2107. [Google Scholar]
- Miao, W.; Ma, H.; Zhang, X.; Zhang, Y.; Li, Y. Clay mineral characteristics of salt sequence in drill hole SHK4 of the Mengyejing potassium deposit of Jiangcheng, Lanping-Simao Basin, Yunnan province, and their sylvite-forming significance. Acta Geosci. Sin. 2013, 34, 537–546. [Google Scholar]
- Shi, H.; Ma, H.; Miao, W.; Li, Y.; Zhang, X. Characteristics and geological significances of rare earth and trace elements from Upper Cretaceous Mengyejing Formation of Simao Basin in Jiangcheng County, Yunnan province. Geochimica 2014, 43, 415–427. [Google Scholar]
- Metcalfe, I. Permian tectonic framework and palaeogeography of SE Asia. J. Asian Earth Sci. 2002, 20, 551–566. [Google Scholar] [CrossRef]
- Sone, M.; Metcalfe, I. Parallel Tethyan sutures in mainland Southeast Asia: New insights for Palaeo-Tethys closure and implications for the Indosinian orogeny. C. R. Geosci. 2008, 340, 166–179. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, J.; Li, W.; Li, H.; Cai, Z.; Yan, Z.; Ma, C. Pako-Tethys system and accretionary orogen in the Tibet Plateau. Acta Petrol. Sin. 2013, 29, 1847–1860. [Google Scholar]
- Huang, K.; Opdyke, N.D. Paleomagnetic results from cretaceous and Jurassic rocks of South and Southwest Yunnan—evidence for large clockwise rotations in the Indo-China and Shan Thai malay terranes. Earth Planet. Sci. Lett. 1993, 117, 507–524. [Google Scholar] [CrossRef]
- Yin, J.; Sun, Z.; Yang, Z.; Liang, Q. Cretaceous and Early Tertiary paleomagnetic results from the Langping Basin and its geological implications. Chin. J. Geophys. 1999, 42, 648. [Google Scholar]
- Cong, B.; Wu, G.; Zhang, Q.; Zhang, R.; Zhuo, M.; Zhao, D.; Zhang, W. Rocks of the Paleo Tethys tectonic belt in Western Yunnan, China tectonic evolution. Sci. China 1993, 23, 1201. [Google Scholar]
- Tong, Y.-B.; Yang, Z.; Zheng, L.-D.; Xu, Y.-L.; Wang, H.; Gao, L.; Hu, X.-Z. Internal crustal deformation in the northern part of Shan-Thai Block: New evidence from paleomagnetic results of Cretaceous and Paleogene redbeds. Tectonophysics 2013, 608, 1138–1158. [Google Scholar] [CrossRef]
- Qu, Y.H.; Yuan, P.Q.; Shuai, K.Y.; Zhang, Y.; Cai, K.Q.; Jia, S.Y.; Chen, C.D. Potashforming Rules and Prospect of the Lower Tertiary in the Lanping-Simao Basin, Yunnan; Geological Publishing House: Beijing, China, 1998; pp. 1–118. [Google Scholar]
- Chen, Y.; Liao, Z.; Wei, Z.; Li, M. Characteristics and tectonic evolution of the Lanping-Simao Mesozoic Basin. Pet. Geol. Exp. 2004, 26, 219–222, 228. [Google Scholar]
- Zongting, L.; Yuekun, C. Nature and evolution of Lanping-Simao Basin prototype. J. Tongji Univ. Nat. Sci. 2005, 33, 1527–1531. [Google Scholar]
- Tan, F. The Sedimentary Characteristics of Simao Triassic rear arc foreland basin, Yunnan province. Acta Sedimentol. Sin. 2002, 20, 560–567. [Google Scholar]
- Wu, N.P.; Jiang, S.Y.; Liao, Q.L.; Pan, J.Y.; Dai, B.Z. Lead and sulfur isotope geochemistry and the ore sources of the vein-type copper deposits in Lanping-Simao Basin, Yunnan province. Acta Petrol. Sin. 2003, 19, 799–807. [Google Scholar]
- Hongfu, Y.; Shunbao, W.; Yuansheng, D.; Yuanqiao, P. South China defined as part of Tethyan archipelagic ocean system. Earth Sci. J. China Univ. Geosci. 1999, 24, 1–12. [Google Scholar]
- Fuguang, Y.I.N.; Guitang, P.A.N.; Fang, W.A.N.; Xingzhen, L.I.; Fangguo, W. Tectonic facies along the Nujiang-Lancangjiang-Jinshajiang orogenic belt in Southwestern China. Sediment. Geol. Tethyan Geol. 2006, 26, 33–39. [Google Scholar]
- Zheng, M.; Zhang, Z.; Yin, H.; Tan, X.; Yu, C.; Shi, L.; Zhang, X.; Yang, J.; Jiao, J.; Wu, G. A new viewpoint concerning the Formation of the Mengyejing Potash Deposit in Jiangcheng, Yunnan. Acta Geosci. Sin. 2014, 35, 11–24. [Google Scholar]
- Yuan, Q.; Qin, Z.; Wei, H.; Sheng, S.; Shan, F. The ore-forming age and palaeoenvironment of the Mengyejing Formation in Jiangcheng, Yunnan province. Acta Geosci. Sin. 2013, 34, 631–637. [Google Scholar]
- Wang, L.; Liu, C.; Fei, M.; Shen, L.; Zhang, H.; Zhao, Y. First SHRIMP U–Pb zircon ages of the potash-bearing Mengyejing Formation, Simao Basin, southwestern Yunnan, China. Cretac. Res. 2015, 52, 238–250. [Google Scholar] [CrossRef]
- Cohen, K.M.; Finney, S.M.; Gibbard, P.L.; Episodes, F.J.J. The ICS International Chronostratigraphic Chart. Episodes 2013, 36, 199–204. [Google Scholar] [CrossRef]
- Zheng, Z.; Yin, H.; Zhang, Z.; Zheng, M.; Yang, J. Strontium isotope characteristics and the origin of salt deposits in Mengyejing, Yunnan province, SW China. J. Nanjing Univ. 2012, 48, 719–727. [Google Scholar]
- Brumsack, H.J. The inorganic geochemistry of Cretaceous black shales (DSDP Leg 41) in comparison to modern upwelling sediments from the Gulf of California. Geol. Soc. Lond. Spec. Publ. 1986, 21, 447–462. [Google Scholar] [CrossRef]
- Calvert, S.E.; Pedersen, T.F. Geochemistry of recent oxic and anoxic marine-sediments—implications for the geological record. Mar. Geol. 1993, 113, 67–88. [Google Scholar] [CrossRef]
- Morford, J.L.; Emerson, S. The geochemistry of redox sensitive trace metals in sediments. Geochim. Et Cosmochim. Acta 1999, 63, 1735–1750. [Google Scholar] [CrossRef]
- Piper, D.Z.; Perkins, R.B. A modern vs. Permian black shale—the hydrography, primary productivity, and water-column chemistry of deposition. Chem. Geol. 2004, 206, 177–197. [Google Scholar] [CrossRef]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the elements in some major units of the Earth’s Crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Fedo, C.M.; Nesbitt, H.W.; Young, G.M. Unraveling the effects of potassium metasomatism in sedimentary-rocks and paleosols, with implications for paleoweathering conditions and provenance. Geology 1995, 23, 921–924. [Google Scholar] [CrossRef]
- Harnois, L. The CIW index—A new chemical index of weathering. Sediment. Geol. 1988, 55, 319–322. [Google Scholar] [CrossRef]
- Mclennan, S.M. Weathering and Global Denudation. J. Geol. 1993, 101, 295–303. [Google Scholar] [CrossRef]
- Brownlow, H. Geochemistry; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1979; p. 498. [Google Scholar]
- Wu, C.; Liu, C.; Yi, H.; Xia, G.; Zhang, H.; Wang, L.; Li, G.; Wagreich, M. Mid-Cretaceous desert system in the Simao Basin, southwestern China, and its implications for sea-level change during a greenhouse climate. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 468, 529–544. [Google Scholar] [CrossRef]
- Alexandrine, N.N.; Simon, N.; Gabriel, N. The late Pleistocene—Holocene paleoclimate reconstruction in the Adamawa plateau (Central Cameroon) inferred from the geochemistry and mineralogy of the Lake Fonjak sediments. J. Afr. Earth Sci. 2019, 150, 23–36. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Gao, X.; Zhang, H. Provenance and paleogeography of the Late Cretaceous Mengyejing Formation, Simao Basin, southeastern Tibetan Plateau: Whole-rock geochemistry, U-Pb geochronology, and Hf isotopic constraints. Sediment. Geol. 2014, 304, 44–58. [Google Scholar] [CrossRef]
- Gingele, F.X.; De Deckker, P.; Hillenbrand, C.D. Late Quaternary fluctuations of the Leeuwin Current and palaeoclimates on the adjacent land masses: Clay mineral evidence. Aust. J. Earth Sci. 2001, 48, 867–874. [Google Scholar] [CrossRef]
- Vanderaveroet, P. Miocene to Pleistocene clay mineral sedimentation on the New Jersey shelf. Oceanol. Acta 2000, 23, 25–36. [Google Scholar] [CrossRef]
- Roy, D.K.; Roser, B.P. Climatic control on the composition of Carboniferous–Permian Gondwana sediments, Khalaspir basin, Bangladesh. Gondwana Res. 2013, 23, 1163–1171. [Google Scholar] [CrossRef]
- Beckmann, B.; Flogel, S.; Hofmann, P.; Schulz, M.; Wagner, T. Orbital forcing of Cretaceous river discharge in tropical Africa and ocean response. Nature 2005, 437, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Lerman, A. Lakes: Chemistry, Geology, Physics; Geological Press: Beijing, China, 1989; pp. 10–100. [Google Scholar]
- Cao, H.; Guo, W.; Shan, X.; Ma, L.; Sun, P. Paleolimnological environments and organic accumulation of the nenjiang formation in the Southeastern Songliao Basin, China. Oil Shale 2015, 32, 5–24. [Google Scholar] [CrossRef]
- Sarki Yandoka, B.M.; Abdullah, W.H.; Abubakar, M.B.; Hakimi, M.H.; Adegoke, A.K. Geochemical characterisation of Early Cretaceous lacustrine sediments of Bima Formation, Yola Sub-basin, Northern Benue Trough, NE Nigeria: Organic matter input, preservation, paleoenvironment and palaeoclimatic conditions. Mar. Pet. Geol. 2015, 61, 82–94. [Google Scholar] [CrossRef]
- Fang, X.; Song, C.; Yan, M.; Zan, J.; Liu, C.; Sha, J.; Zhang, W.; Zeng, Y.; Wu, S.; Zhang, D. Mesozoic litho- and magneto-stratigraphic evidence from the central Tibetan Plateau for megamonsoon evolution and potential evaporites. Gondwana Res. 2016, 37, 110–129. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, C.; Zhang, H.; Li, Z.; Ding, T.; Wang, M. The controls of paleotemperature on potassium salt precipitation in ancient salt lakes. Acta Petrol. Sin. 2015, 31, 2751–2756. [Google Scholar]
- Quinbyhunt, M.S.; Wilde, P. Thermodynamic zonation in the black shale facies based on iron-manganese vanadium content. Chem. Geol. 1994, 113, 297–317. [Google Scholar] [CrossRef]
- Algeo, T.J.; Maynard, J.B. Trace-element behavior and redox facies in core shales of Upper Pennsylvanian Kansas-type cyclothems. Chem. Geol. 2004, 206, 289–318. [Google Scholar] [CrossRef]
- Arthur, M.A.; Sageman, B.B. Marine black shales—Depositional mechanisms and environments of ancient-deposits. Annu. Rev. Earth Planet. Sci. 1994, 22, 499–551. [Google Scholar] [CrossRef]
- McManus, J.; Berelson, W.M.; Hammond, D.E.; Klinkhammer, G.P. Barium cycling in the North Pacific: Implications for the Utility of Ba as a paleoproductivity and paleoalkalinity proxy. Paleoceanography 1999, 14, 53–61. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- Dill, H. Metallogenesis of Early Paleozoic graptolite shales from the Graefenthal Horst (Northern Bavaria-Federal-Republic-of-Germany). Econ. Geol. 1986, 81, 889–903. [Google Scholar] [CrossRef]
- Hatch, J.R.; Leventhal, J.S. Relationship between inferred redox potential of the depositional environment and geochemistry of the Upper Pennsylvanian (Missourian) Stark Shale Member of the Dennis Limestone, Wabaunsee County, Kansas, USA. Chem. Geol. 1992, 99, 65–82. [Google Scholar] [CrossRef]
- Jones, B.; Manning, D.A.C. Comparison of geochemical indexes used for the interpretation of palaeoredox conditions in ancient mudstones. Chem. Geol. 1994, 111, 111–129. [Google Scholar] [CrossRef]
- Dill, H.; Teschner, M.; Wehner, H. Petrography, inorganic and organic geochemistry of Lower Permian Carbonaceous fan sequences (brandschiefer series)—federal-republic-of-germany—constraints to their paleogeography and assessment of teir source rock potential. Chem. Geol. 1988, 67, 307–325. [Google Scholar] [CrossRef]
- Guangzhi, L.I.; Bin, H.U.; Tianlong, D.; Ziyan, Y. Petroleum geological significance of microelements V and Ni. Nat. Gas Geosci. 2008, 19, 13–17. [Google Scholar]
- Huerta-Diaz, A.M.; Morse, J.W. Pyritization of trace metals in anoxic marine sediments. Geochim. Et Cosmochim. Acta 1992, 56, 2681–2702. [Google Scholar] [CrossRef]
- Liu, C.; Wu, C.; Wang, L.; Fang, X.; Zhao, Y.; Yan, M.; Zhang, Y.; Cao, Y.; Zhang, H.; Lu, F. Advance in the study of forming condition and prediction of potash deposits of marine basins in China’s Small Blocks: Review. Acta Geosci. Sin. 2016, 37, 581–606. [Google Scholar]
- Couch, E.L. Calculation of paleosalinities from boron and clay mineral data. Aapg Bull. 1971, 55, 1829–1837. [Google Scholar]
- Zheng, M.; Zhang, Z.; Zhang, Y.; Liu, X.; Yin, H. Potash Exploration characteristics in China:New understanding and research progress. Acta Geosci. Sin. 2012, 33, 280–294. [Google Scholar]
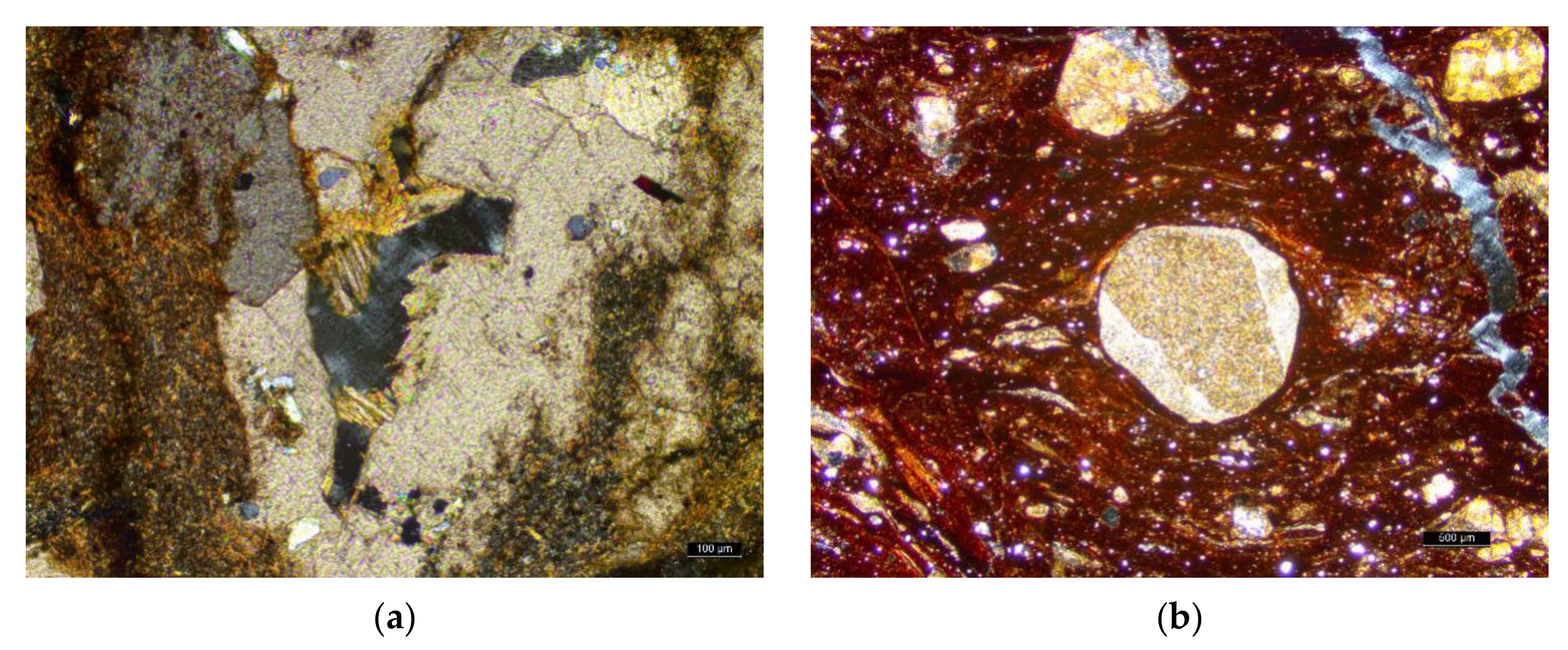
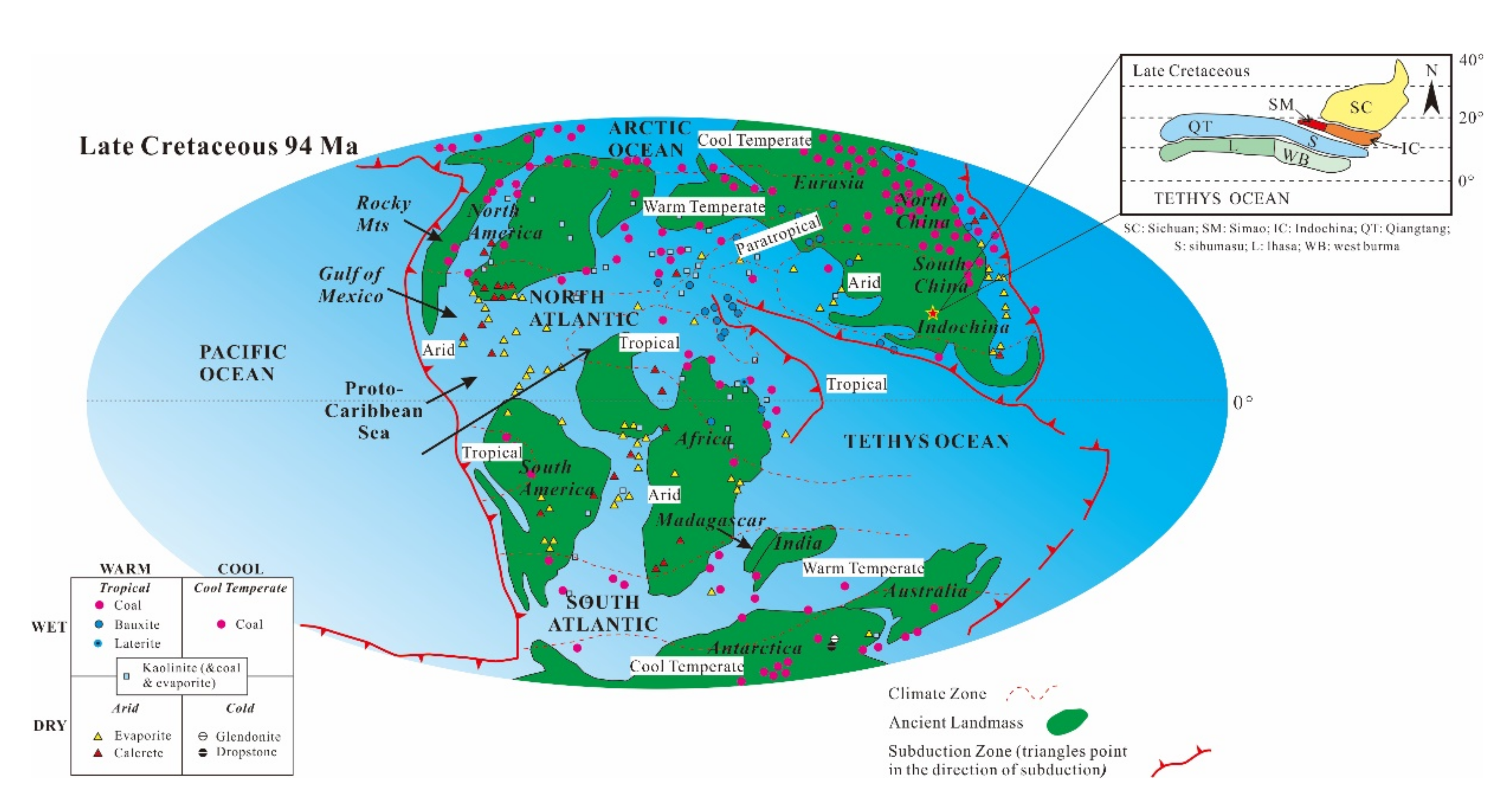
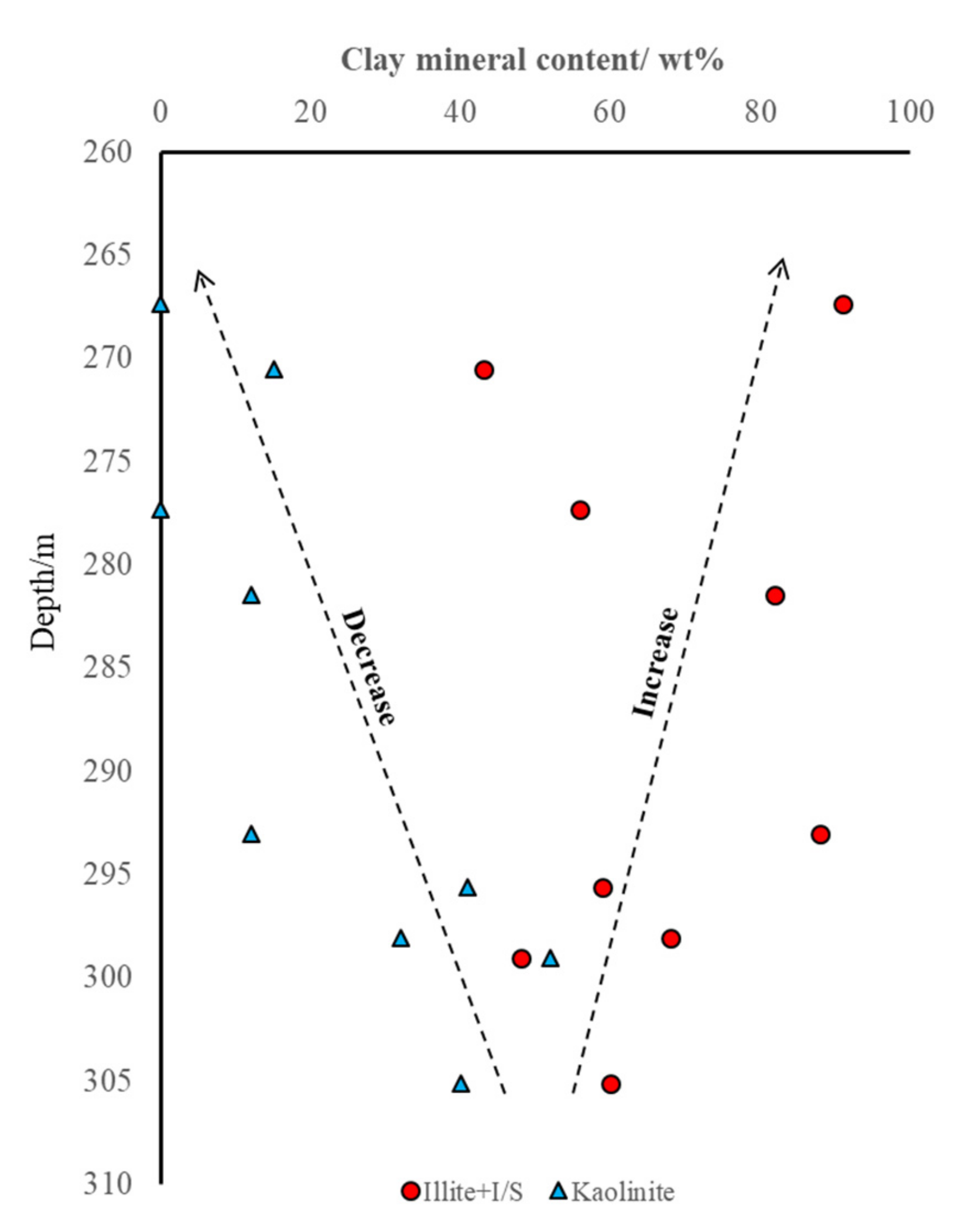
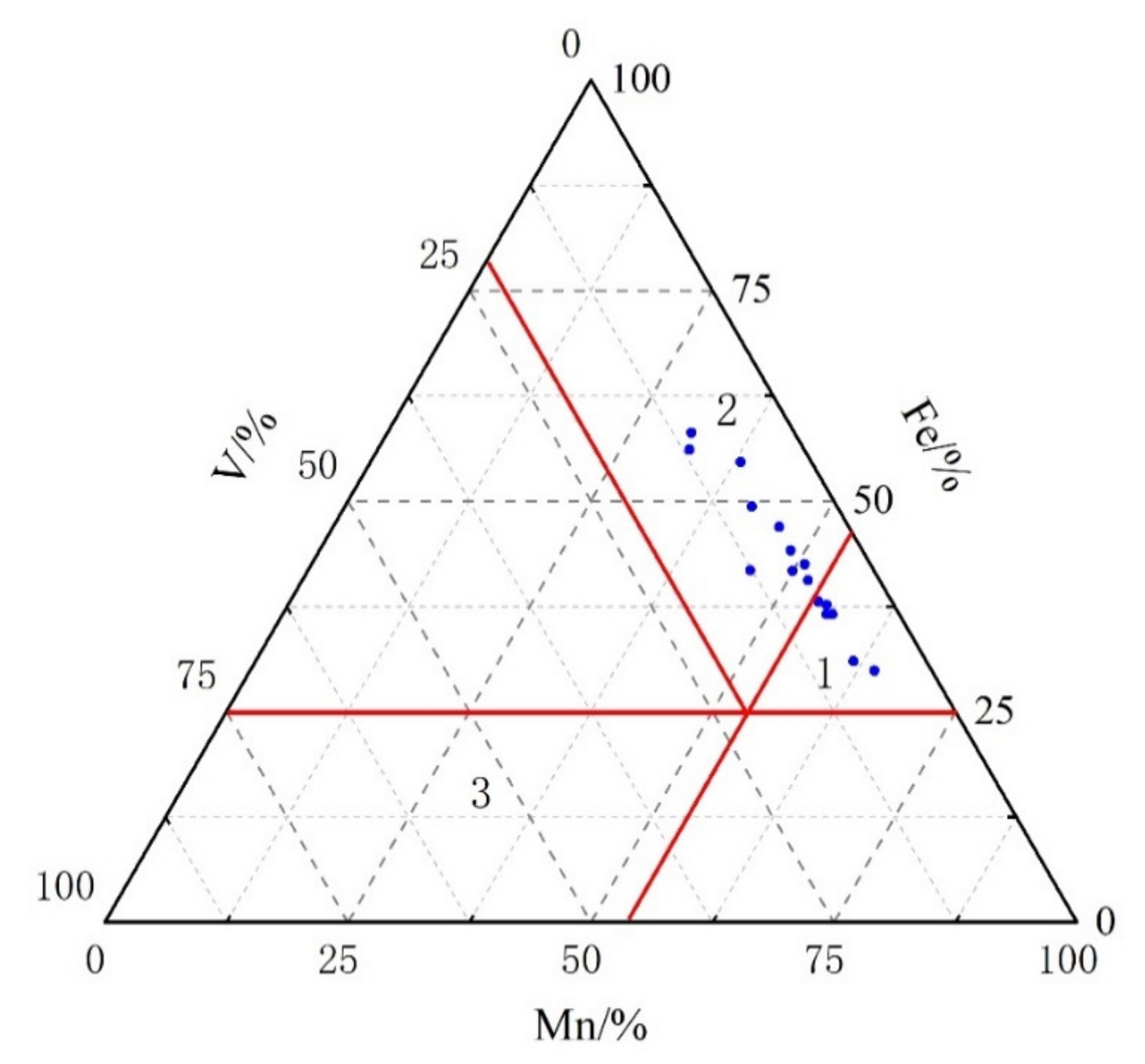
| Samples | Depth [m] | Ga/Rb | Sr/Cu | Numbers | Depth [m] | Ga/Rb | Sr/Cu |
|---|---|---|---|---|---|---|---|
| MZK-3-1 | 109.2 | 0.13 | 5.63 | MZK-3-9 | 270.5 | 0.27 | 6.94 |
| MZK-3-3 | 118.3 | 0.13 | 1.29 | MZK-3-10 | 277.3 | 0.27 | 48.89 |
| MZK-3-3 | 146.9 | 0.12 | 6.58 | MZK-3-11 | 281.4 | 0.15 | 10.68 |
| MZK-3-4 | 156.1 | 0.14 | 4.77 | MZK-3-12 | 293.1 | 0.14 | 10.40 |
| MZK-3-5 | 164.9 | 0.14 | 6.05 | MZK-3-13 | 295.6 | 0.22 | 7.35 |
| MZK-3-6 | 169.0 | 0.15 | 30.51 | MZK-3-14 | 298.1 | 0.16 | 3.60 |
| MZK-3-7 | 173.1 | 0.15 | 4.47 | MZK-3-15 | 299.1 | 0.20 | 4.91 |
| MZK-3-8 | 267.4 | 0.16 | 5.51 | MZK-3-16 | 305.2 | 0.17 | 9.76 |
| Samples | Depth [m] | B [ppm] | B Content of Kaolinite [ppm] | Paleosalinity (‰) |
|---|---|---|---|---|
| MZK-3-2 | 118.3 | 140.8 | 47.5 | 29 |
| MZK-3-3 | 146.9 | 69.0 | 20.3 | 12 |
| MZK-3-4 | 156.1 | 67.3 | 20.9 | 13 |
| MZK-3-6 | 169.0 | 195.9 | 63.1 | 38 |
| MZK-3-7 | 173.1 | 72.9 | 24.1 | 15 |
| Salt | / | / | / | / |
| MZK-3-8 | 267.4 | 105.3 | 40.5 | 25 |
| MZK-3-9 | 270.5 | 235.6 | 152.0 | 92 |
| MZK-3-10 | 277.3 | 253.3 | 113.1 | 69 |
| MZK-3-11 | 281.4 | 81.6 | 25.2 | 15 |
| MZK-3-12 | 293.1 | 144.6 | 45.6 | 28 |
| MZK-3-13 | 295.6 | 88.0 | 32.7 | 20 |
| MZK-3-14 | 298.1 | 43.2 | 17.0 | 10 |
| MZK-3-15 | 299.1 | 100.4 | 44.7 | 27 |
| MZK-3-16 | 305.2 | 90.3 | 41.5 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, P.; Miao, Z.; Zheng, M.; Zhang, X.; Ruan, Z.; Xu, Q. Paleogeographic Characteristics of the Mengyejing Formation in the Simao Basin during Its Depositional Period and Its Indication of Potash Mineralization: A Case Study of MZK-3 Well. Minerals 2021, 11, 338. https://doi.org/10.3390/min11040338
Lou P, Miao Z, Zheng M, Zhang X, Ruan Z, Xu Q. Paleogeographic Characteristics of the Mengyejing Formation in the Simao Basin during Its Depositional Period and Its Indication of Potash Mineralization: A Case Study of MZK-3 Well. Minerals. 2021; 11(4):338. https://doi.org/10.3390/min11040338
Chicago/Turabian StyleLou, Pengcheng, Zhongying Miao, Mianping Zheng, Xuefei Zhang, Zhuang Ruan, and Qihui Xu. 2021. "Paleogeographic Characteristics of the Mengyejing Formation in the Simao Basin during Its Depositional Period and Its Indication of Potash Mineralization: A Case Study of MZK-3 Well" Minerals 11, no. 4: 338. https://doi.org/10.3390/min11040338
APA StyleLou, P., Miao, Z., Zheng, M., Zhang, X., Ruan, Z., & Xu, Q. (2021). Paleogeographic Characteristics of the Mengyejing Formation in the Simao Basin during Its Depositional Period and Its Indication of Potash Mineralization: A Case Study of MZK-3 Well. Minerals, 11(4), 338. https://doi.org/10.3390/min11040338






