1. Introduction
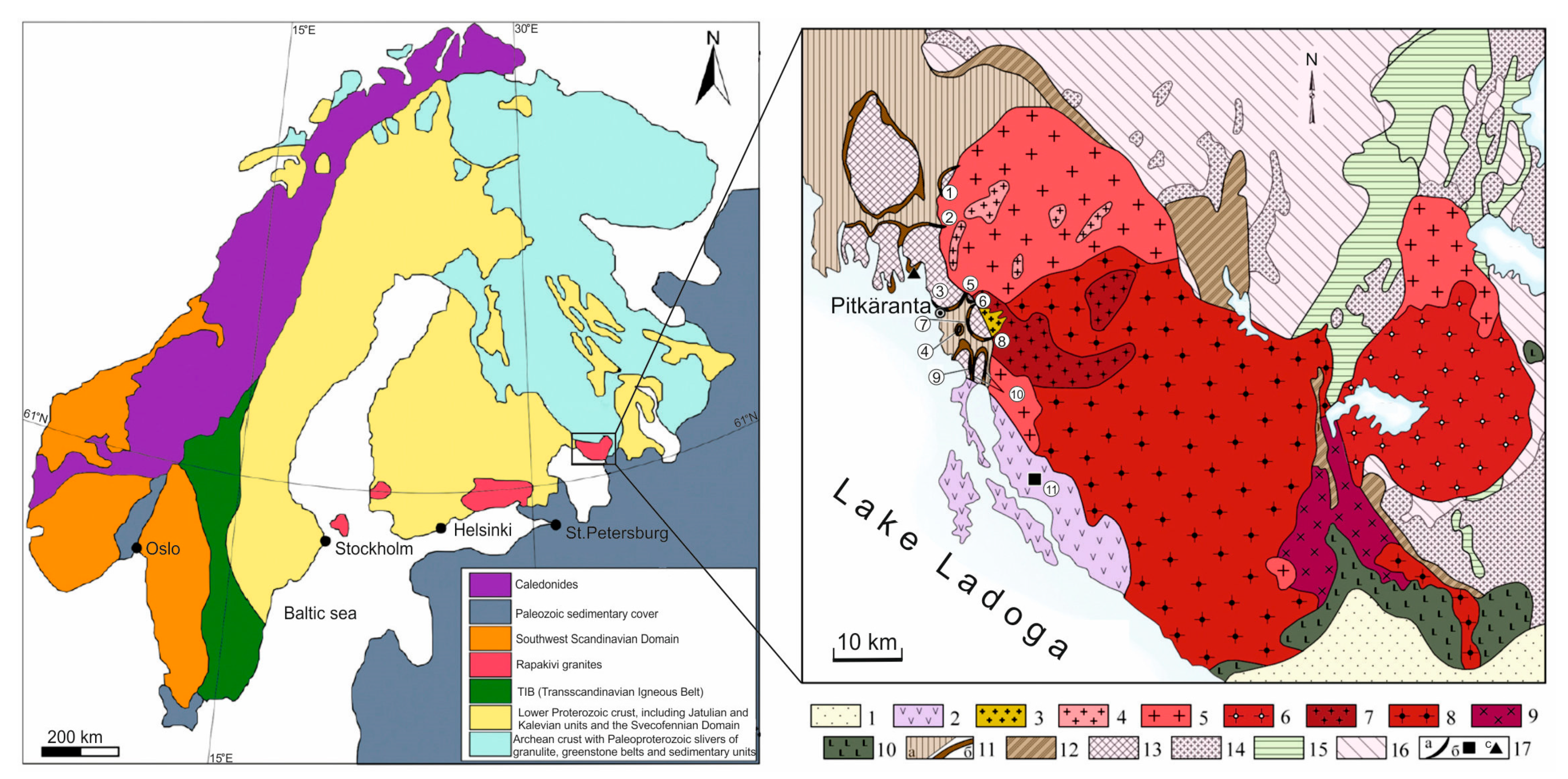
The Pitkäranta Mining District (PMD) is located on the northeastern shore of Lake Ladoga, NW Russia (
Figure 1). It extends for ~40 km from north to south at the western exocontact of the Salmi anorthosite-rapakivi granite batholiths (SARGB). Copper ore was first reported from the Pitkäranta area in the late 18th century, and the discovery of the Pitkäranta deposit, followed by its mining, dates back to 1810 [
1]. There were over 50 active Fe, Cu, Sn, Ag mines in the PMD in 1832–1904 [
2]. Tin was discovered in PMD skarns in 1834 [
3].
In the ~200-year period of the mining and study of skarn ore rocks of the PMD which preceded our investigation 1.1 Mt of ore (~500 t of tin, ~7000 t of copper, ~60,000 t of iron, and ~11 t of silver containing about 16 kg of gold) were produced, three new minerals: berborite [
6], fluorvesuvianite [
7] and hydroxylchondrodite [
8] were discovered and over 50 ore minerals: magnetite, chalcopyrite, sphalerite, pyrite, cassiterite, stokesite, eakerite, scheelite, native bismuth, arsenopyrite, löllingite, mushketovite, wickmanite, schoenfliesite, fluoborite, native gold, electrum, native silver, helvite, ludwigite, suanite, etc. [
2,
9,
10,
11]. In recent past few years some rare minerals, e.g., roquesite, were discovered in PMD ores [
12,
13,
14,
15].
The rapakivi granites of the SARGB are A-type granites, as indicated by their geochemical characteristics. Rapakivi plutons in the Fennoscandian Shield were occasionally described as metallogenically barren in previous literature [
16,
17] although their genetic relation to Fe-Cu-Sn-Zn mineralization at PMD has been established much earlier [
2,
18]. The ore-forming potential of rapakivi granites on a global scale was firstly evaluated by the end of the 20th century, with several economic deposits of various types considered to be genetically associated with them [
5], including unusually large tin deposits in Brazil [
19,
20]. Revealing indium (roquesite) mineralization in greisens from Vyborg Batholith rapakivi granites [
21,
22], in the skarns of the PMD [
12,
23], and economic concentrations of In, Bi, Be and Cd and other rare metals in PMD ores [
13], provided another argument in favor of the metallogenic potential of rapakivi granites. Using up-to-date analytical methods, it has become possible to infer some formation characteristics of roquesite, bismuth sulfosalts, and helvite-group minerals during multi-stage skarn and ore formation related to the multiple discontinuous additions of hydrothermal solutions generated by SARGB granitoid formed by six magmatic pulses [
5].
However, mineralogical description of PMD skarn ores, presented in many publications [
10,
11,
12,
13,
14,
15,
24,
25,
26], covers mainly Sn-, Cu-, Zn-, and Fe-bearing minerals with few exceptions. In the present paper, the minerals of rare-metals (In, Bi, Be, Te, Se) and new metallogenic prospects of the PMD are described. Peculiarities of rare metal mineralization in propylitized skarns of deposits with Fe-Sn-Zn-Cu ores and in aposkarn greisens (greisenized skarns ) of deposits with Fe-Sn-Zn-Be ores are considered. The features of the distribution of roquesite in skarn ores are shown.
2. Geological and Metallogenetic Background
The modern geological structure of the PMD as the eastern flank of the Ladoga Structure is the result of its long (>1 Ga) evolution, which comprised continental and ocean margin rift formation with the opening of the Svecofennian ocean followed by the convergent interaction of the newly-formed oceanic crust with the Archean craton [
23,
27]. Correspondingly, the formation of structural-mineralogical complexes in the province was subdivided into several stages terminated by the closing of the ocean followed by the formation of an accretionary-collisional orogen, post-collisional uplift, cratonization, and Mesoproterozoic intraplate discrete magmatism [
23].
Archean basement rocks (gneiss, granite-gneiss, amphibolite; 2.7–2.66 Ga) have preserved only as so-called «rimmed granite-gneiss domes» after P. Eskola [
28] rimmed by the volcanic-sedimentary rocks of the Pitkäranta suite (Ludicovian, 2.1–1.92 Ga) overlain by Ladoga turbidites (Kalevian, 1.92–1.8 Ga) (
Figure 1 and
Figure 2). The Pitkäranta suite section is dominated by ortho- and para-amphibolites, amphibole, mica and graphite schists, locally quartzites and carbonate rocks (dominantly dolomites or their calcitized varieties) transformed to marble, carbonate-silicate rock, and skarn. They have traced on the margins of gneissose granite domes as 200–500 m thick, locally discontinuous zones (
Figure 1 and
Figure 2). The carbonate rocks of the suite are represented by two horizons totaling 40–120 m in thickness.
Ludicovian and Kalevian rocks in the PMD were metamorphosed under amphibolite-facies conditions and are cut by Svecofennian (syn- and post-orogenic) granites and pegmatites as well as the SARGB of Riphean age (1530–1547 Ma) [
5,
30,
31]. In the southern part of the province, all the above structural-mineralogical complexes are overlain by Jotnian volcanic-sedimentary rocks (Salmi suite, 1.48 Ga) (
Figure 1 and
Figure 2).
The SARGB (S = ~5000 km
2) is confined to a conjugate zone between the Mesoproterozoic Svecofennian accretionary-collisional orogen and the Archean Karelian Craton (
Figure 1). The batholith intruded along an overthrust zone between these tectonic structures. It consists of the Salmi massif proper located in the folded region and its satellite, the Ulälega massif located in craton rocks (
Figure 1). The batholith cross-cuts Archean granitoids and greenstone belts as well as Lower Proterozoic (Jatulian, Ludicovian, and Kalevian) supracrustal rock complexes. The batholith was produced by six discrete intrusion pulses of mafic and felsic magma throughout ~17 Ma (1546.7 ± 1.7–1529.9 ± 0.6) [
5,
30,
31]. In the southwestern portion, the batholith is cut by subvolcanic basic rock bodies—which are comagmatic to the Salmi basalts dated at 1457–1459 Ma [
32], and in the northeastern portion, it is cut by moderately medium-basic alkaline rock dikes of the Enäjoki complex. Their age is unknown.
Geophysical data show that the SARGB is a subhorizontal lamina-like body varying in thickness from 2 km in its northwestern portion to 10 km in its southeastern portion [
4,
33]. The rocks are succeeded consistently in the same direction with an increase in their basicity and age (
Figure 1).
Batholith granitoids are tentatively subdivided into three groups [
5,
26,
34] distinguished earlier [
26,
34] as intrusion phases dominated by the two former groups: (1) biotite-amphibole granites, (2) biotite granites and (3) topaz-bearing Li-F granites. The latter form small stock-like bodies and dikes, whose apical and endocontact zones typically display striated textures (
Figure 3) and stockscheiders. The dark-colored minerals (biotite-lepidomelane, protolithionite, ferrohastingsite, ferrohedenbergite, fayalite) of all three groups of granitoids are characterized solely by high iron concentration [Fe/(Fe + Mg)]—0.8–1.0. Accessory minerals are commonly represented by fluorite, zircon, apatite, ilmenite, magnetite, anatase, allanite, uraninite, and thorite. Granites of the second and third groups also contain monazite, bastnäsite, topaz, cassiterite, rutile, ilmenorutile, columbite, pyrochlore, and thorianite.
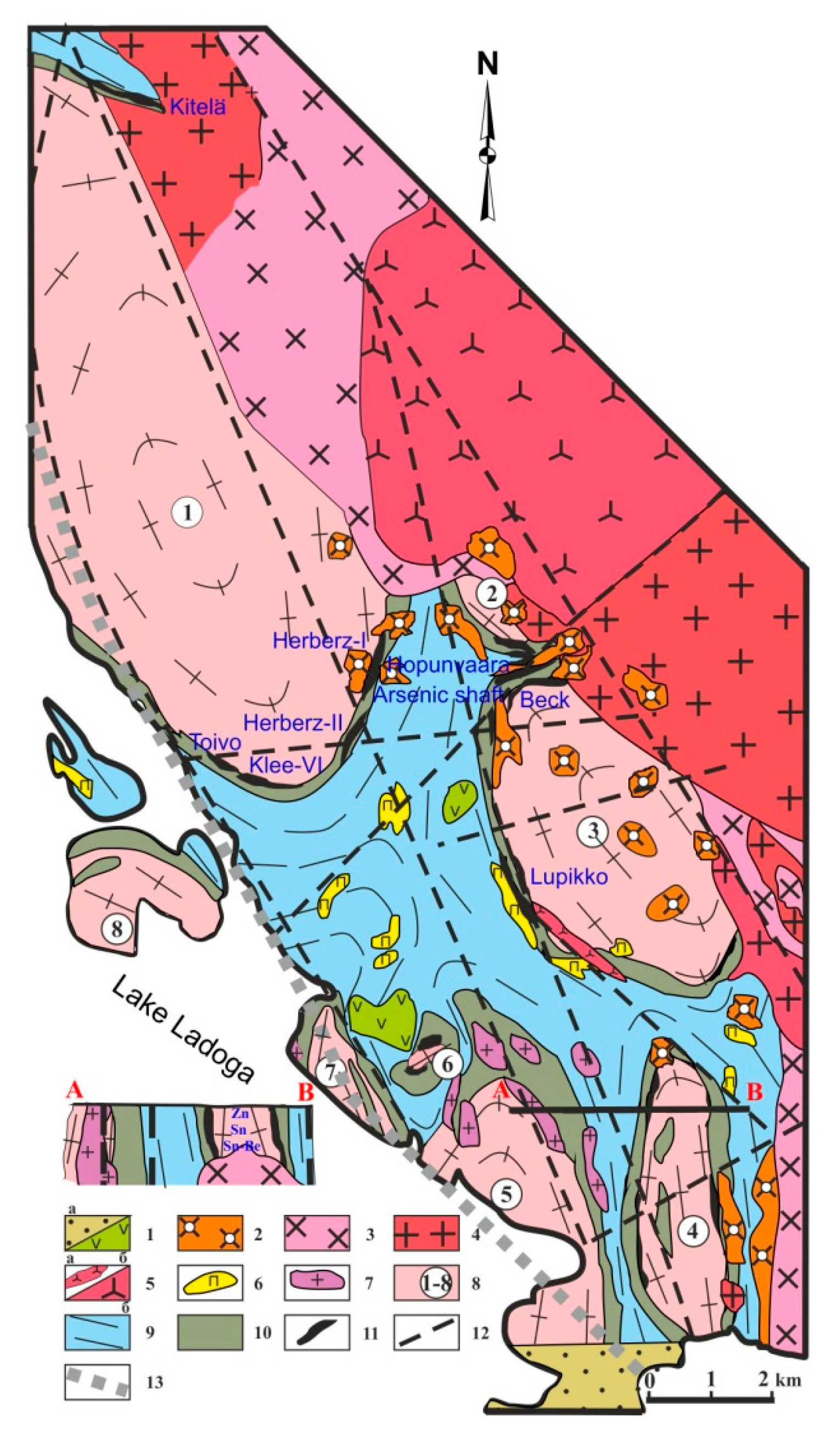
Skarn deposits and occurrences in the PMD are located at the western gently plunging exocontact of the SARGB in a zone composed of the most differentiated rapakivi granites (
Figure 2). The geological map of the province was compiled by A.E. Tornebom [
16]. More details were added by O. Trustedt [
2], who completed the map, which has mainly remained unchanged to the present day. The above geologists proposed a skarn model of PMD ore formation, where the carbonate rock horizons were affected by hydrothermal fluids supplied from the SARGB. The model was supported later by most researchers and was further updated [
5,
24,
25,
26,
34,
35,
36,
37].
In these deposits, the following generalized zoning of ore-bearing metasomatic rocks from the top of the massif is observed: altered skarns—Fe-Zn-Sn-Cu; aposkarn greisens—Sn-Zn-Be-fluorite (±Cu); low-temperature aposkarn metasomatic rocks—Sn, Pb, Zn (±Cu). This zoning, affected by many factors, is either disturbed locally or does not manifest itself at all but generally persists for the PMD [
29,
34,
38,
39]. In this case, all aposkarn metasomatic rocks are not ubiquitous in the skarns but evolve in them along linearly elongated zones up to several meters in thickness or less commonly form shapeless and columnar bodies with veined ramifications up to 4–5 m in thickness. Aposkarn greisens are spatially associated with Li-F-granite bodies and feldspathic or quartz-feldspathic metasomatic rocks and fluorite-quartz-mica greisens evolving after gneissose granites and granites.
Geochronological data (Sm-Nd mineral isochrones for tin-bearing skarns from the Kitteli deposit) suggest conditionally two stages of ore formation in skarns: 1558 ± 30 Ma and 1546 ± 28 Ma [
5,
29].
The skarn ore bodies formed after Pitkäranta carbonate rocks rimming remobilized Archean gneissose granite domes (
Figure 2). The skarns are propylitized almost everywhere and are greisenized at some places. Depending on the degree of greisen alteration, the skarns display Sn-Cu-base metal mineralization with magnetite (Pitkäranta dome—Old Ore Field, Kittelä; Heposelkä and Kulismajoki domes)—poor or no greisenization and Be-Sn-base metal with fluorite and magnetite (Pitkäranta dome—New Ore Field; Lupikko, Uksa, and Ristiniemi domes)—greisenization is strong. Sn-Cu-base metal deposits, bigger than Be-Sn-base metal deposits, occur in the exocontact zone of the massif, whose top plunges steeply (Kittelä) or are confined to the steep bend of the top, where a gentle bend passes into subvertical (Old Ore Field, Heposelka). The top of granites in such zones is at a depth of 1 km [
26,
33]. Be-Sn-base metal deposits are commonly located above Li-F granite domes unexposed by erosion and are spatially associated with the dikes of these granites and stockscheiders.
The ore bodies display irregular lenticular and sheet-like shapes caused by the morphology of skarn bodies that often exhibit complex structures resulting from the heterogeneous composition and structure of original carbonate units and the overlapping of ore-bearing metasomatic rocks formed at the various stages of mineral formation. The types of ore-bearing metasomatic rocks recognized in the deposits of the PMD are [
26,
29,
34,
35,
36]: magnesian skarns formed at magmatic and postmagmatic stages, calcareous apomagnesian skarns, aposkarn greisens (fluorite-vesuvianite-magnetite, fluorite-mica and magnetite-fluorite-mica), and propylites, feldspathic, and quartz-carbonate metasomatic rocks.
Magnesian skarns commonly occur only as relics among the lime skarns that replace them. They are best-preserved at the Kulismajoki occurrence at the margin of the Kulismajoki gneissose granite dome (
Figure 1) [
34]. Concomitant Fe and Zn mineralization is associated with magnesian skarns. Calcareous apomagnesian skarns (the dominant type of metasomatic rocks at PMD deposits) commonly host Fe-Zn and Sn-mineralization. In addition to cassiterite, considerable quantities of tin in these skarns are concentrated in the isomorphic form in garnets [
4,
14,
26,
29,
34], where tin concentrations are as high as 2.7% [
14]. Hopunvaara calcareous apomagnesian skarns, subjected to greisen transformations, and converted to fluorite-phlogopite-vesuvianite-magnetite metasomatic rocks, display a rhythmically striated structure (
Figure 4).
Associated with aposkarn greisens is beryllium mineralization; the bulk of Be (up to 0.85%) is concentrated in vesuvianite [
5]. Greisens often exhibit tungsten (scheelite) and Mo mineralization. Some molybdenite crystals are 5–6 cm across. Gradual transitions of aposkarn greisens to propylites are occasionally observed.
Original carbonate units are thin and display a heterogeneous composition and structure. Therefore, persistent ore bodies are mainly confined to steeply dipping skarn bodies. The central highest-grade portions of the ore bodies occur practically at the same hypsometric level for most deposits of the province. Confined to this surface are Sn- and Be-Sn ore columns mainly oriented sub horizontally in the carbonate bed plane. This is probably due to the ore deposition pattern in zones where steeply dipping ore-feeding channels are intersected by lit-par-lit ore-controlling dislocations in carbonate horizons [
5]. The most significant tin deposits are Kitelä, Uuksa, and Hopunvaara [
40,
41], and the most significant base metal deposit is Hopunlampi.
Geochronological data on PMD ore-bearing skarns [
29,
42,
43] support the 19th and early 20th geological concepts [
2,
16], according to which they are genetically related to SARGB granites. The results of Pb-isotope systematics of ores and magmatic rocks from the PMD [
30,
37,
44] are indicative of a common source of ore matter in the skarn deposits and rapakivi granites of the SARGB. However, molybdenite mineralization in skarns seems to be of polygenic-polychronic origin. The age of molybdenite from Li-F granites (1575 Ma, Re-Os) [
45] is similar to the lower age boundary (1547 Ma) of Salmi pluton granite intrusion and much older than molybdenites from Old Ore Field and Kitelä skarns (1827–1763 Ma). This age seems to be consistent with the timing of the formation of Svecofennian granites and pegmatites occurring in the province. This seems to partly corroborate the correctness of M. Saksela’s [
25] three-stage model for the formation of PMD ores: stage I with pegmatites and stages II and III with rapakivi granites.
3. Materials and Methods
Rare-metal mineralization was studied using samples taken by the author in 1978–1980 and 2008–2016 from ore-bearing skarn exposures, prospecting workings, mine dumps, and drill cores (not preserved). Polished sections for Kitelä, stored at the Regional Geological Archives, Republic of Karelia, were also analyzed. A total of over 200 samples from the Old and New Ore Fields of the Pitkäranta deposit, the Hopunvaara and Lupikko Ore Fields, the Kitelä deposit as well as the Kulismajoki, Heposelka and Avtodor occurrences were analyzed. Specimens for ICP MS analysis were prepared from all samples and polished sections were made. The polished sections were studied by optical microscopy (an Axiolab-pol-u optical microscope equipped with a digital photographic camera and a computer) at the Institute of Geology, Karelian Research Centre, Russian Academy of Sciences.
The minerals were all analyzed at the Centre for Team Use, Karelian Research Centre, RAS, Petrozavodsk, Russia. The minerals’ composition was analyzed in polished thin sections with a VEGA II LSH Scanning Electron Microscope (Tescan, Brno, Czech Republic) at the Centre for Collective Usage Karelian Research Centre (Petrozavosdk, Russia). The instrument was equipped with an energy dispersive spectrometry (EDS) Energy 350 system and an SDD X-Act3 detector (Oxford Inca Energy, Oxford, UK). Operating conditions were: 20 kV accelerating voltage, 5 nA probe current, 1 µs EDS process time, 105 cnts/s, 30 s counting time. The spectral lines for each element are CuKα, FeKα, ZnKα, MnKα, SKα, FKα, InLα, AgLα, AuLα, TeLα, SeLα, SnLα, AsLα, BiMα, PbMα, WMα. The following standards were used: CaCO3, CaF2, FeS2, PbTe, HgTe, TlSbSe2, InAs, NaCl, Cu, Co, Ni, Zn, Mn, As, Se, Ag, Au, Sn, Te, W, Bi. SEM-EDS quantitative data and determination of the analysis accuracy were acquired and processed using the Microanalysis Suite Issue 12, INCA Suite version 4.01; standard deviation (S) for In—0.6–1.8%, Pb—1.3–4.4%, Bi—0.8–4.2%, Te—0.4–2.0%, Se—0.4–1.3%, Cu—0.7–2.3%, Zn—1.0–2.4%, Ag—0.3-2.3%, S—0.4–0.7%.
Trace elements in sphalerite and chalcopyrite were identified by the LA-ICP-MS method on an X-SERIES-2 quadrupole mass-spectrometer (Thermo Scientific, Waltham, MA, USA) equipped with a UP-266 Macro Laser Ablation attachment (New Wave Research, MODEL UP266 MACRO AT, Fermont, CA, USA) in the IG KSC RAS (analyst A.S.Paramonov) following the method [
46]. The Nd:Yag laser operates with a wavelength of 266 nm and energy output of 0.133 mJ (scan speed 70 μm/sec, impulse frequency 10 Hz). All measurements were carried out with identical parameters. Standard NIST 612 was used for calibration procedures. The measured trace element concentration values are characterized following parameters of relative standard deviation (RSD): for transition metals (Co, Mn, Cr, V, Cu, Zn) <15%, for As <20%, for In, Cd <25%.
Trace, rare earth, and transition element concentrations (V, Cr, Co, Ni, Rb, Sr, Y, Zr, Nb, Ba, Hf, Ta, Pb, Th, and U; V, Cr, Co, Ni, Rb, Ta, and Pb concentrations were measured for some samples) were determined by mass spectrometry with inductively coupled plasma (ICP-MS). Element abundances were measured on a Thermo Scientific X-Series 2 ICP-MS quadrupole mass spectrometer (Thermo Scientific, Waltham, MA, USA) after [
46], at the Analytical Center of the Institute of Geology, Karelian Research Center, Russian Academy of Sciences (KarRC RAS, Petrozavodsk, Karelia, Russia). Analyses were performed on 100 mg subsamples, which were decomposed by the acid breakdown in an open system. Samples were decomposed with high purity acids (70% HClO
4, 65 % HNO
3, 40 % HF), additionally purified by distillation in a PTFE/PFA SubboilingEcoIR acid distillatory manufactured by the High-Purity Standards Company (North Charleston, SC, USA), and deionized water. ICP-MS calibration was performed using a 68-(ICP-MS-68A-A) and 13-element (ICP-MS-68A-B) standard solutions manufactured by the High-Purity Standards Company. The study samples, reference samples, and blank samples were measured in groups of 15–20 with the measurements of calibration blocks to detect instrument drift in the interim. The SGD-2A, S-1412, and BHVO2 standard rock samples were used as certified reference samples. The measured concentration values are characterized by low relative standard deviation (RSD) values; the percentages are lower than 7% for most elements and 7–12%, for Co, Ni, Y, and Ta.
5. Discussion
Examination of skarns PMD reveals the following paragenetic sequence of hydrothermal ore mineralization (
Figure 15) which can be recognized based on mineralogical and structural relationships.
The magnesian-skarn stage is marked by the formation of a small number of ore minerals: magnetite, boron minerals, sphalerite, chalcopyrite. Magnetite, Sn-bearing garnet, cassiterite, sphalerite, and chalcopyrite crystallized during the formation of calcareous skarns. The stages of aposcarn greisens and propylitized skarns are marked by the richest ore mineralization. Be-containing vesuvian, helvine, danalite, chrysoberyl, and roquesite are the earliest rare metal minerals of these stages.
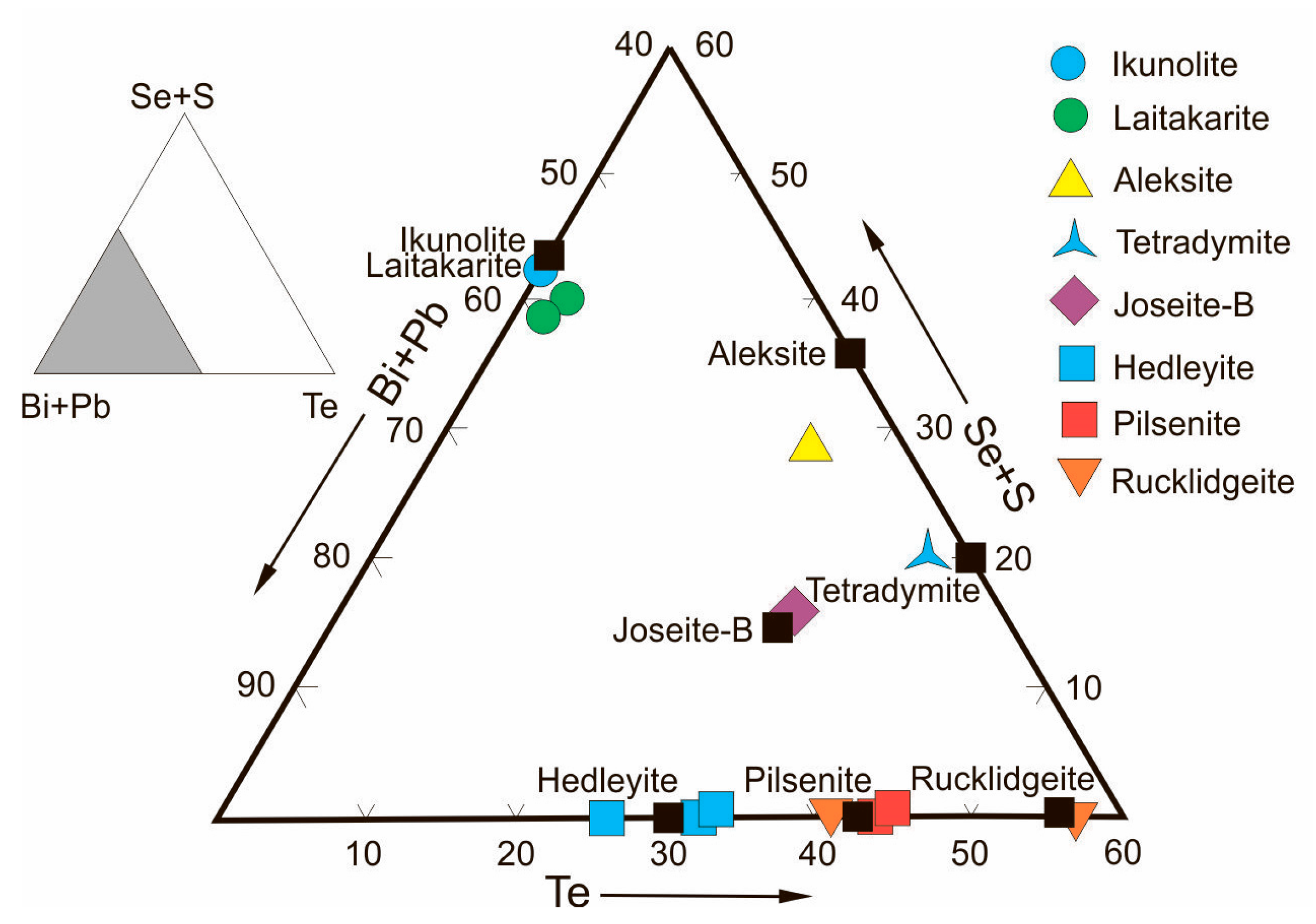
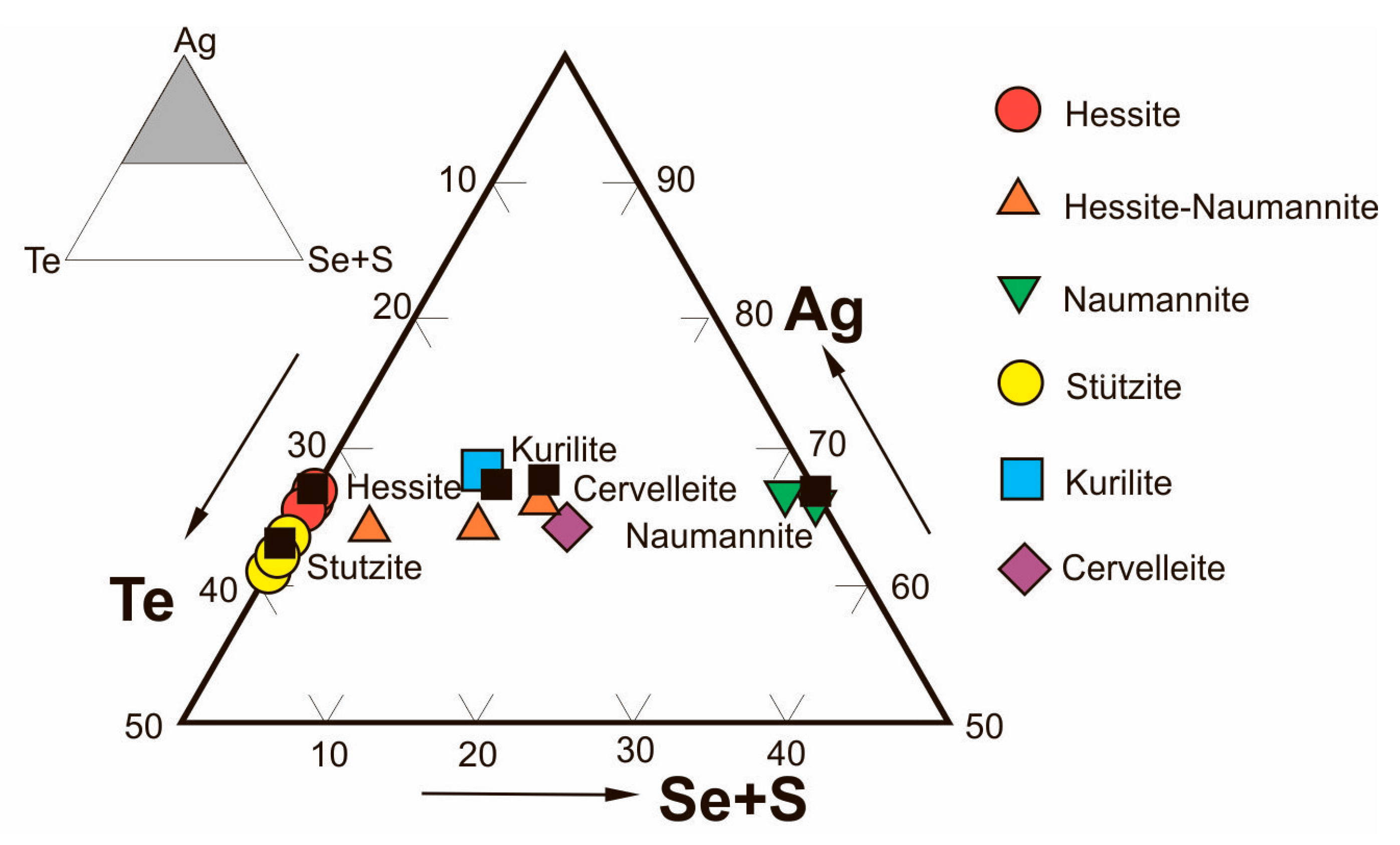
Minerals of Bi, Te, Se, Ag, and Au were formed mainly at the stage of propylitized skarns. Most of them are contained in skarns in trace amounts (
Figure 15) and, as a result, their paragenetic ratios cannot be determined at this stage of research and this is to be done in the framework of future research. Some conclusions in this aspect can be drawn when comparing the rare metal mineralization in PMD skarns and other regions [
54,
55,
56,
57,
58].
Rare-metal (In, Bi, Te, Se, Be) mineralization in the PMD is consistent in species diversity with that of skarn deposits, in which the cessation of ore formation was paralleled by the formation of low-temperature metasomatic rocks (propylites, serpentinites, etc.) with the well-defined ore-geochemical association Au-Bi-Pb-Sb-Ag-Te-As [
54,
55,
56,
57] and deposits with aposkarn metasomatic rocks such as fluorite-enriched greisens with Be [
58]. This seems to be due to the long (17 million years) discrete (6 pulses) formation of the SARGB. Therefore, the physic-chemical parameters of mineralization at its exocontact are highly varied. This probably explains the crystallization of bismuth sulphosalts of nonstoichiometric composition, which is also noted in skarn deposits of other regions, for example, at the Stan Terg deposit in Kosovo (Romania) [
54].
The cobaltite thermometer [
59] shows that the Co-Ni-Fe sulphoarsenide formation temperature varies considerably: Lupikko—420–500 °C, Hopunvaara—300–480 °C, Herberz-1 ~300 °C. The temperatures shown by the arsenopyrite thermometer are [
60]: Herbertz-2—340–450 °C, Hopunvaara, Arsenic Shaft—380–430 °C; Lupikko—340–480 °C. Widespread “chalcopyrite dissease” in sphalerite from PMD ore suggests temperatures of no less than 334 °C [
61] and up to 500 °C [
62]. Star-like sphalerite aggregates, often identified in Pitkäranta ores, suggest sphalerite crystallization at a temperature of >500 °C as a modification of α-chalcopyrite characterized by the higher solubility of ZnS in it [
63]. Relics of löllingite in arsenopyrite from Arsenic Shaft ores indicate a temperature of 400–500 °C and log fS
2 of about –10 [
64]. A temperature of 350–380 °C for electrum-sphalerite paragenesis in Lupikko ores, calculated with a corresponding thermometer [
65] was found to be unusually high. The formation temperatures of beryllium mineralization, estimated from the homogenization temperature of fluid inclusions in phenakite, which replaces helvite, are estimated at 330–340 °C [
66]. The chlorite thermometer [
67] shows that the temperature of hydrothermal-metasomatic alterations at a propylitik stage for PMD deposits varies from 230 to 290 °C. Curiously, the lowest temperatures were yielded by high-Fe chlorites similar to thuringite-bavalite most commonly found to be associated with gold and silver minerals. The common presence of Au-Ag-Bi-Te-S low-temperature mineral association (32 minerals) in the metasomatic rocks studied suggests that ore formation took place also at temperatures much lower than those estimated by geothermometry methods (<200 °C).
The great diversity of rare-metal ore mineralization (native metals, oxides and hydroxides, carbonates, tellurides, sulfides, sulfosalts, borates, and silicates) in PMD ores are also indicative of marked variations in log fO2 and log fS2 upon its formation. Apparently, bismuth sulfosalts, tellurides, and roquesite crystallized from solutions with a high Bi-Te-Se content under various temperature conditions and possibly during the decay of previously formed minerals of solid solutions containing Bi, Ag, Te, Se, In, Zn, and Sn.
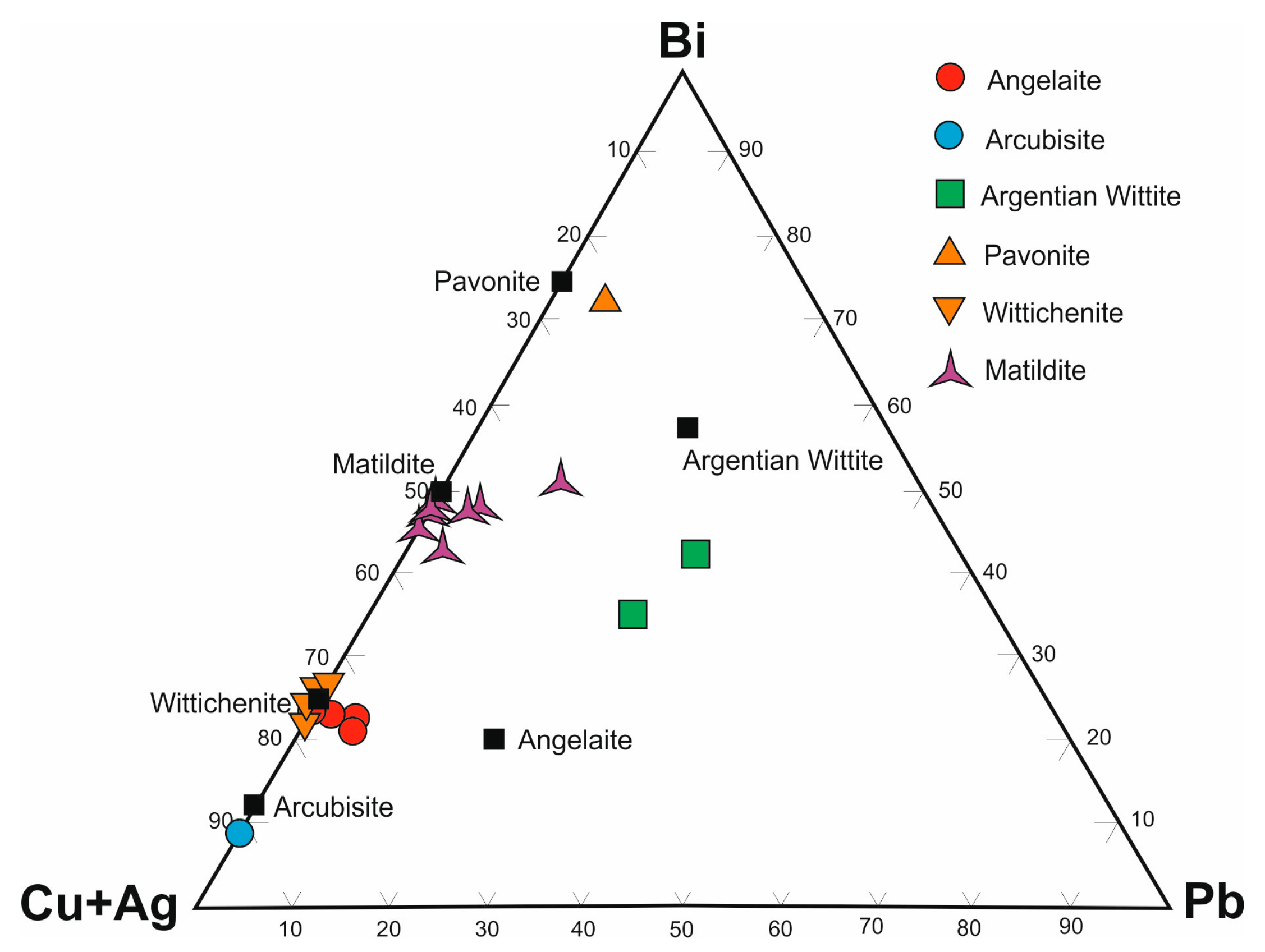
Bismuth sulfosalts in PMD ores occur as Ag-bearing phases (matildite, angelaite, pavonite, arcubisite, argentian wittite), copper sulfosalts (wittichenite, emplectite), bismuthine-aikinite series sulfosalts (aikinite, gladite, pekoite), and nickel sulfosalt (parkerite). They are associated with native bismuth, bismuthinite, bismite, bismuthite, and zavaritskite. Most mineral associations of bismuth sulfosalts form over a wide temperature range of 200 to 400 °C [
68,
69,
70,
71,
72]. According to A.A. Godovikov [
68] emplectite can be used for them as a maximum geothermometer, because at a temperature of 360–383 °C it is decomposed to wittichenite and cuprobismuthite, and the wittichenite formation temperature is not higher than 350–400 °C [
68]. When associated with chalcopyrite, it is stable at a temperature below 395 °C, and when associated with emplectite, at less than 318 °C [
73].
Matildite, commonly occurring as subhexagonal and xenomorphic grains, seems to be one of low-temperature Ag sulfobismuthides in PMD skarns. The ores contain no thinly tabular and reticulate matildite aggregates typomorphic upon the decomposition of solid α-AgBiS
2↔PbS solution at temperatures below 230–175 °C [
74] and upon the inversion of α-AgBiS
2→ß-AgBiS
2 at a temperature of 174–182 °C [
75]. This suggests its crystallization directly from hydrothermal solutions, possibly together with galena, at a temperature below 215–220 °C, a boundary temperature for α→ß inversion of matildite [
68]. There is another interpretation of matildite aggregates: they could have produced by the decomposition of chalcopyrite containing isomorphic Ag and Bi impurities at a temperature below 220 °C [
76,
77] and the average sulfidation conditions of solutions after [
78]. Kitelä ores occasionally contain schapbachite—a high-temperature cubic phase of matildite containing up to 20 % Pb (
Table 6), which, according to the results of studies [
79], is indicative of its formation temperature above 200 °C.
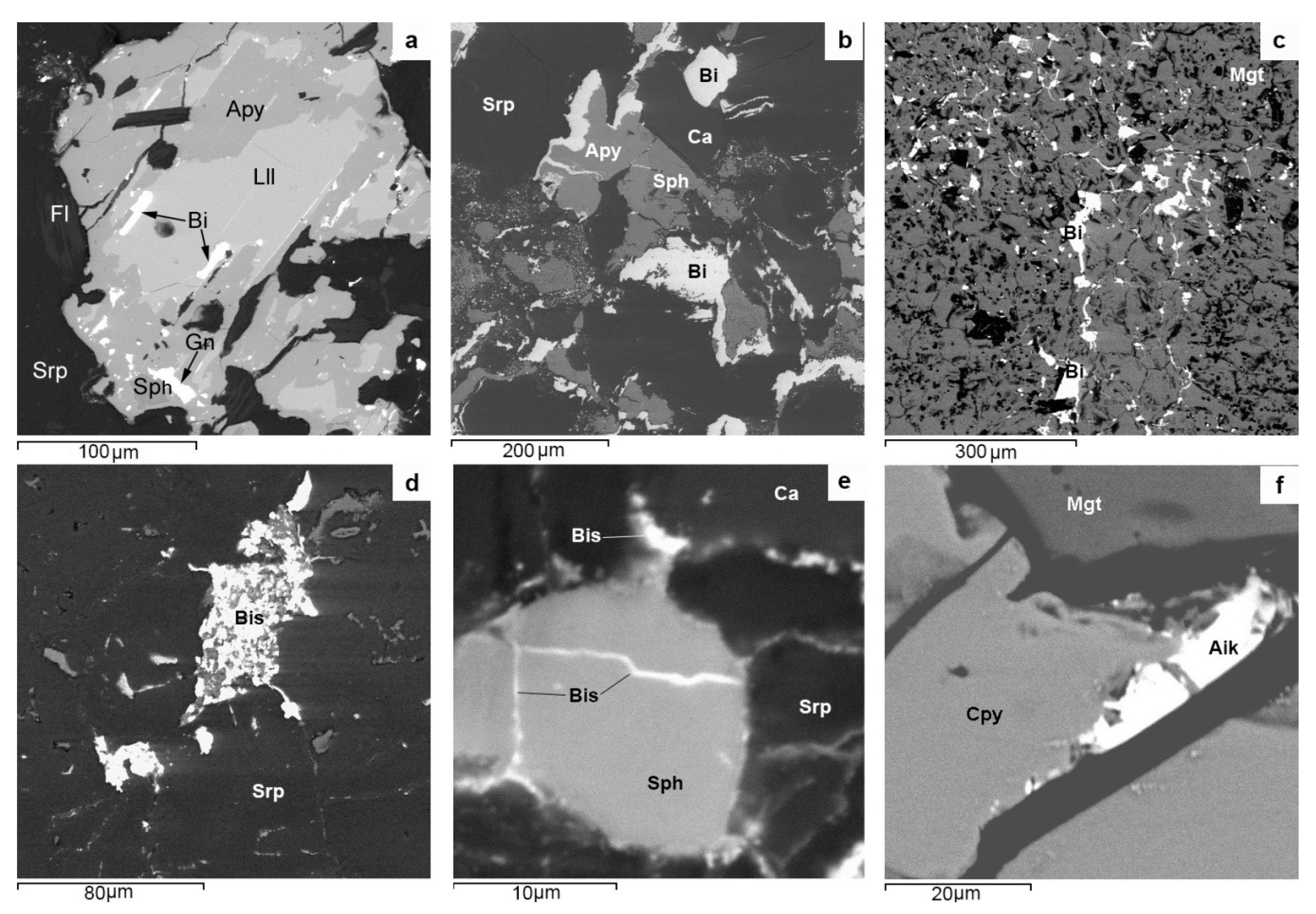
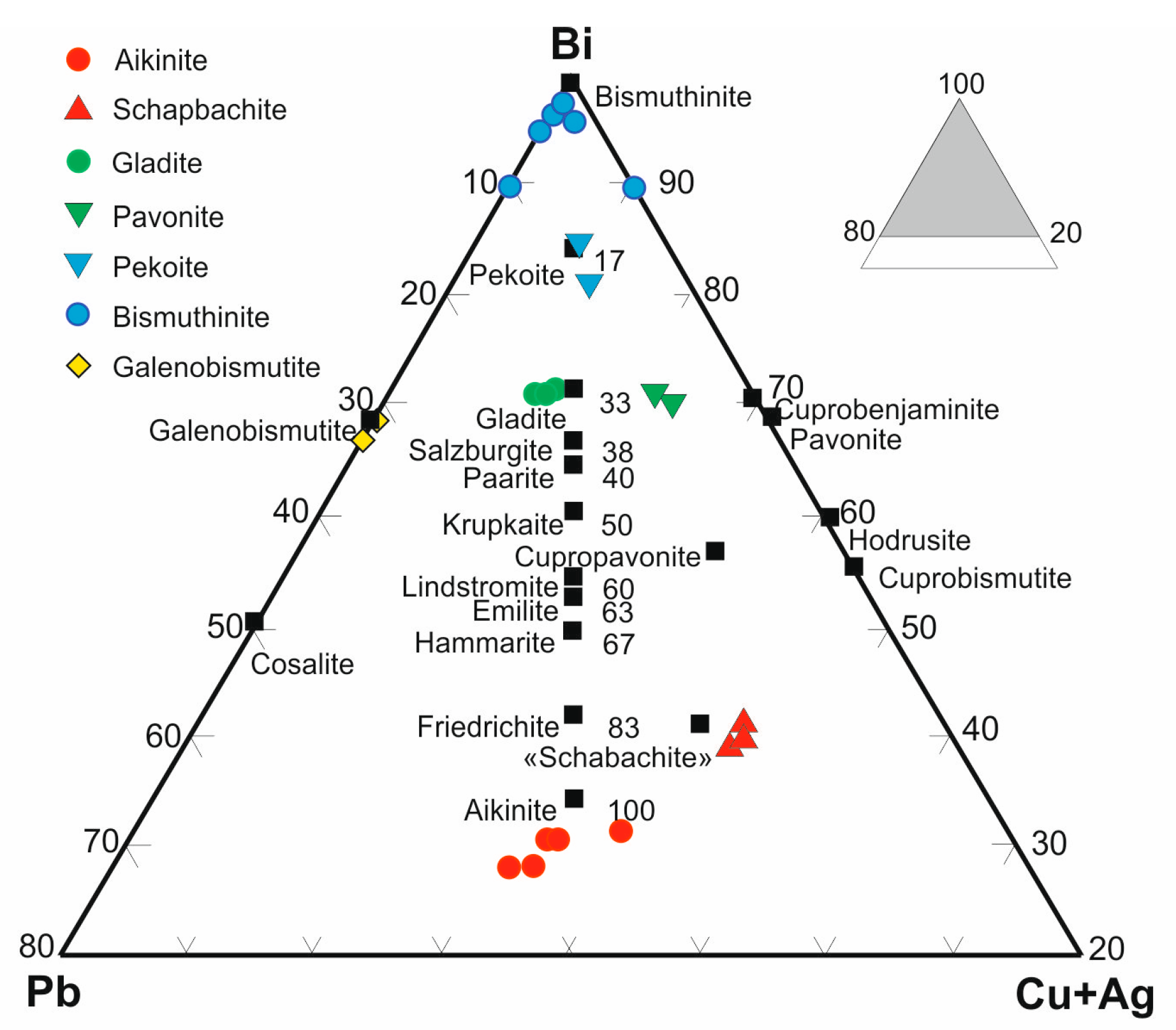
Bismuthine-aikinite series sulfosalts in PMD ores are only represented by its end-members: aikinite, gladite, pekoite, and bismuthinite (
Figure 16). The latter is most common. Correspondingly, their aikanitic number [
80]—N
Aik = [2Pb/(Pb + Bi)] varies in two ranges: 101–120 for aikinite and 20–30 for gladite and pekoite. N
Aik values for aikinite exceeding 100 seems to be due to analytical errors caused by the micron size of aikinite aggregates and trapping of impurities. Aikinite is present in propylitized skarns with Fe-Cu-Zn-Sn mineralization (Kitelä), while other minerals of this series occur in greisenized skarns with Fe-Zn-As-Sn-Be ores (Lupikko, Herberz-1, Arsenic Shaft). The absence of intermediate mineral phases of bismuthinite-aikinite series in Pitkäranta ores seems to be due to the discrete manifestation of ore processes caused by the multiple pulse-like supplies of hydrothermal solutions generated in each of six magmatic impulses that gave rise to the SARGB. In this case, the normal evolution of ore processes was disturbed at each stage, and the fluids generated by lithium-fluorine granites were responsible for a gap in the mineral formation of sulfosalts of bismuthinite-aikinitic series with the small-scale separation of only its extreme members (gladite and pekoite) in aposkarn greisens.
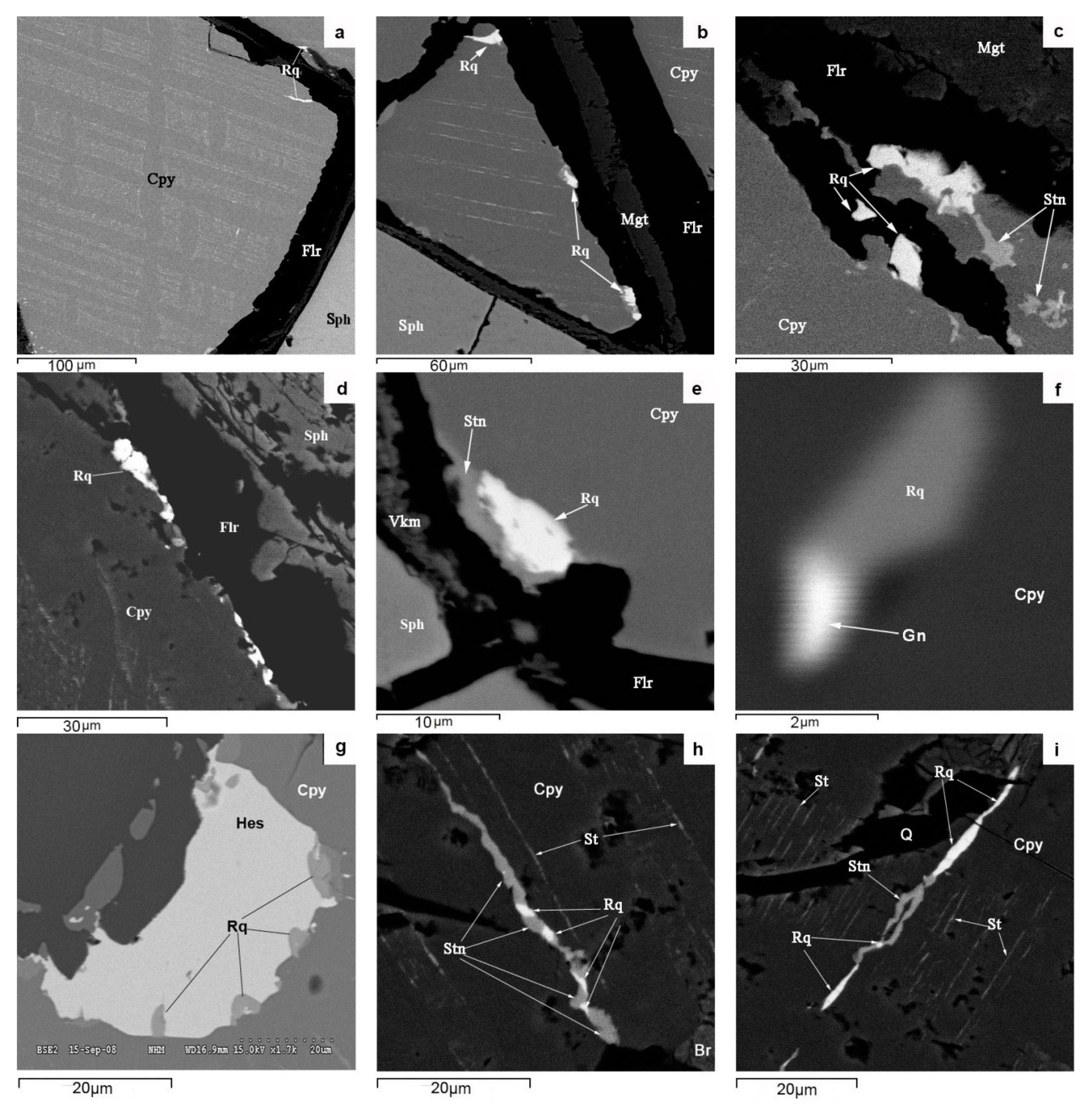
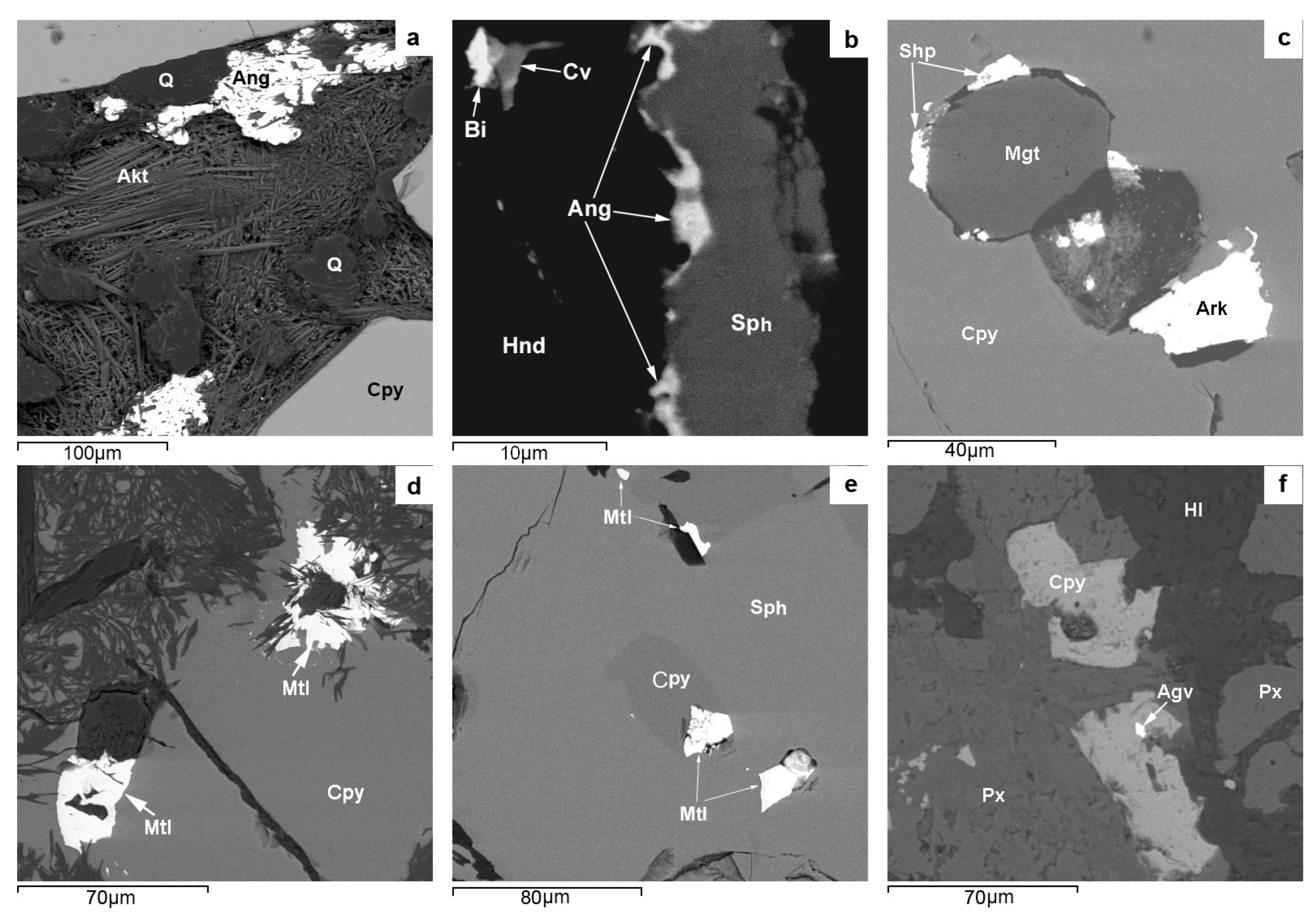
The morphological diversity (single grains, micro inclusions, streaks, rims with micron-sized streaky ramifications, films, intergrowths, and drop-like units) of native bismuth aggregates (
Figure 6a,c) suggests that bismuth formed later than the major ore minerals of PMD skarns and that it was repeatedly remobilized and converted to molten condition. It occurs as micron-sized inclusions in both relatively high-temperature minerals, such as löllingite, pyrrhotite, and magnetite, and in low-temperature minerals, e.g., bornite, fluorite, and serpentine. It forms intergrowths with a variety of ore minerals: sphalerite, covellite, laitakarite, bismuthinite, altaite, and acanthite. These relationships of native bismuth with various minerals seem to be due to multiple fluid-thermal effects on the skarn ores of the SARGB pluton, which was forming discretely during six magmatic pulses which covered a period of 17 million years [
5]. In this case, bismuth could collect, concentrate, and localize Au following the Liquid Bismuth Collector Model [
81,
82,
83,
84]. Single analyses show that PMD ores contain >1 ppm of gold and that there are electrum inclusions in native bismuth. Native gold and kurilite, an Au-bearing mineral, associated with bismuth are occasionally encountered.
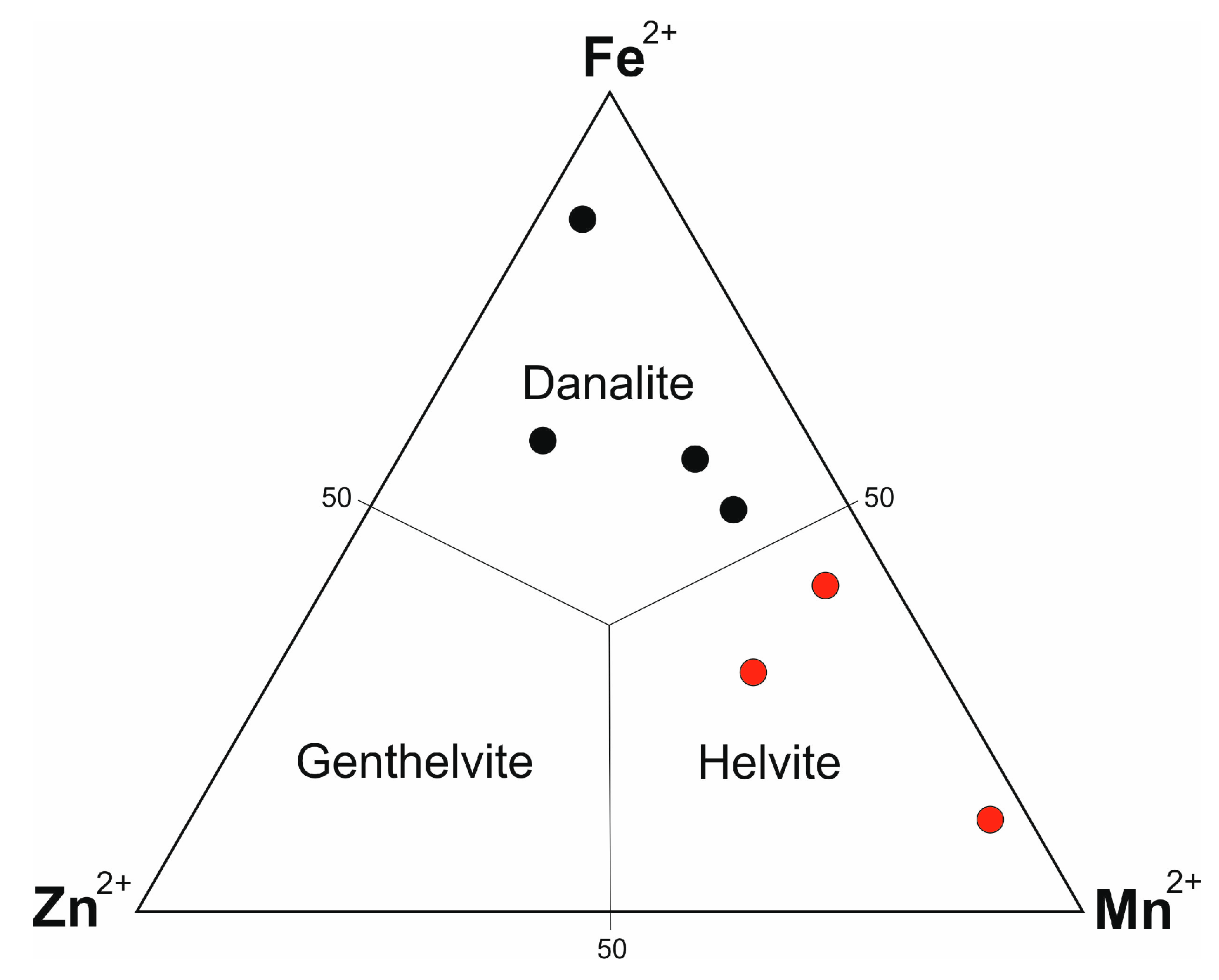
Helvite-group minerals in the PMD were found only in aposkarn greisens. The bizarre composition of this group of minerals (Mn, Fe, Zn)
4(Be
3Si
3O
12)S, in which the orthosilicate and sulfide of the metal are combined is responsible for the high sensitivity of these minerals to variations in the fugacity of O
2 and S
2 and the activity of phenacite [
85]. Therefore, the conditions conducive to the formation of helvite-group minerals are limited by the fairly narrow parameters of log fO
2 and log fS
2 values and depend on mineral associations [
58]. Such ore-forming conditions in Pitkäranta skarns could exist only at the final stages of their hydrothermal-metasomatic alterations triggered by lithium-fluorine granites. Roquesite mineralization seems to have taken place under similar conditions.
All indium minerals, including roquesite, occur very seldom in nature, although elevated In concentrations have been revealed in base metal ores at many deposits. Therefore, conditions limiting the formation of indium minerals, including roquesite, are widely discussed in the modern geological literature [
21,
22,
86,
87].
Roquesite and In distribution in the ores and sphalerite (a major In concentrator mineral) of PMD deposits exhibit a certain pattern of its own. ICP MS and LA-ICP-MS analyses show that the highest average In concentrations in the ore (~500 ppm) and in sphalerite (~3200 ppm) are characteristic of roquesite-free propylitized skarns with Fe-Zn-Sn mineralization at the Kulismajoki occurrence, which is far from Li-F-granites (
Figure 1). Roquesite occurs mainly in the greisen alteration zone of Cu-Zn-Sn skarns with abundant arsenopyrite-löllingite mineralization and an average In concentration of ~300 ppm in the ore and ~500 ppm in sphalerite. Such skarn alteration areas are spatially confined to Li-F granite units at sites where the top of the Salmi massif plunges gently with sharp bends (Hopunvaara, Arsenic Shaft). The biggest numerous roquesite aggregates, consisting dominantly of micron-sized streaks with stannoidite in chalcopyrite, which has undergone the decay of solid solution with separation of stannite, and grains at the sphalerite-chalcopyrite boundary (
Figure 5) suggest that its formation was contributed to by indium released upon the decay of In-bearing solid sphalerite—(Cu
1+ In
3+) ↔ (Zn
2+, Fe
2+) and chalcopyrite—In
3+ ↔ Fe
3+ and 2Fe
3+ ↔ (Fe
2+, Zn
2+) Sn
4+ solutions. The existence of a wide range of such solid solutions has been shown by Z. Johan [
88], N. Cook et al. [
21], and E. Ohta [
51]. It is most probable that solid In solutions in sphalerite and chalcopyrite decayed to form roquesite at a lithium-fluorine granite intrusion stage, which completed the formation of the multi-phase SARGB. Roquesite grains commonly adjoin micron-sized fluorite veinlets filling fractures in chalcopyrite and sphalerite or contacts between them (
Figure 5). The formation temperature of roquesite associated with chalcopyrite, sphalerite, stannine, and stannoidite, shown by a stannine-sphalerite geothermometer [
49,
50], varies from 270 to 320 °C. However, scarce finds of roquesite in intergrowths with hessite (
Figure 5g) and galena (
Figure 5f) suggest its late formation under lower-temperature conditions at low Zn concentration in fluids.
Geochronological data [
5,
29] show that there were probably two stages of Fe-Cu-Zn-Sn mineralization in PMD skarns: 1558 ± 30 Ma and 1546 ± 28 Ma. The age of aposkarn greisens (with roquesite mineralization, as became clear later), estimated by the Sm-Nd isochrones method for minerals, is 1492 ± 25 Ma [
29], i.e., they formed with a minimum time of 13 Ma separating it from the crystallization of the latest rapakivi granites (1530 ± 0.6 Ma). Consequently, roquesite mineralization associated with the SARGB, like that in the Vyborg rapakivi massif [
15,
21], took place upon the formation of greisens and was a small-scale event, while economic indium resources of ~2400 t [
13,
14,
23] in the PMD are concentrated in Sn-Cu-Zn skarn ores, in which sphalerite is a major In concentrator mineral (maximum In concentration is 1.5%, average In concentration from 174 analyses is 2001 ppm).
6. Conclusions
Rare-metal mineralization in the ores of skarn deposits in the PMD is mainly represented by In, Bi, Be, Te, and Se. It is consistent in the diversity of mineral varieties to that of skarn deposits, in which the cessation of ore formation was paralleled by the formation of low-temperature metasomatic rocks (propylites, serpentinites, etc.) with the well-defined ore-geochemical association Au-Bi-Ag-Te-As, and deposits with aposkarn metasomatic rocks such as fluorite-enriched greisens with Be minerals and roquesite.
The distribution pattern of rare-metal mineralization in PMD skarn ores, its composition, and relationships with other minerals was controlled by the discrete manifestation pattern of ore processes affected by the multiple pulse-like supply of hydrothermal solutions generated in each of six magmatic impulses that gave rise to the SARGB. The normal evolution of ore processes was disrupted at each of these stages. As a result, fluids associated with lithium-fluorine granites were responsible for the final mineralization pattern known at present.
The absence of solid solution decomposition structures in sulphobismuthides from PMD ores indicates that they mainly crystallized from hydrothermal solutions at low temperatures.
The morphological features of native bismuth and its association with Ag- and Au-bearing sulphobismuthides, and in a few cases with electrum, could indicate the development of an ore process, which is consistent to some extent with the Liquid Bismuth Collector Model.
Roquesite in PMD ores formed upon the greisen alteration of skarns and was contributed to by indium released upon the decay of In-bearing solid sphalerite—(Cu1+ In3+) ↔ (Zn2+, Fe2+) and chalcopyrite—In3+ ↔ Fe3+ и 2Fe3+ ↔ (Fe2+, Zn2+) Sn4+ solutions. Sphalerite with an average In concentration of 2001 ppm, rather than roquesite, is a major In-concentrating mineral. Predicted In resources in PMD sphalerite ores are estimated at 2400 t.