Effect of Lattice Substitution on Adsorption of Hexavalent Chromium by Montmorillonite, Nontronite, and Beidellite
Abstract
:1. Introduction
2. Materials, Methods and Models
2.1. Minerals
2.2. Adsorption Experiments
2.3. Analyses
2.4. Computational Methods
3. Results and Discussion
3.1. The Remaining Cr(VI) Content in the Solution
3.2. The Adsorption of Cr(VI) on Mineral Surfaces
3.3. Mulliken Populations of Crystal Structure and Adsorption Bond
3.4. Electron Localization Function Interprets the Nature of Bonding
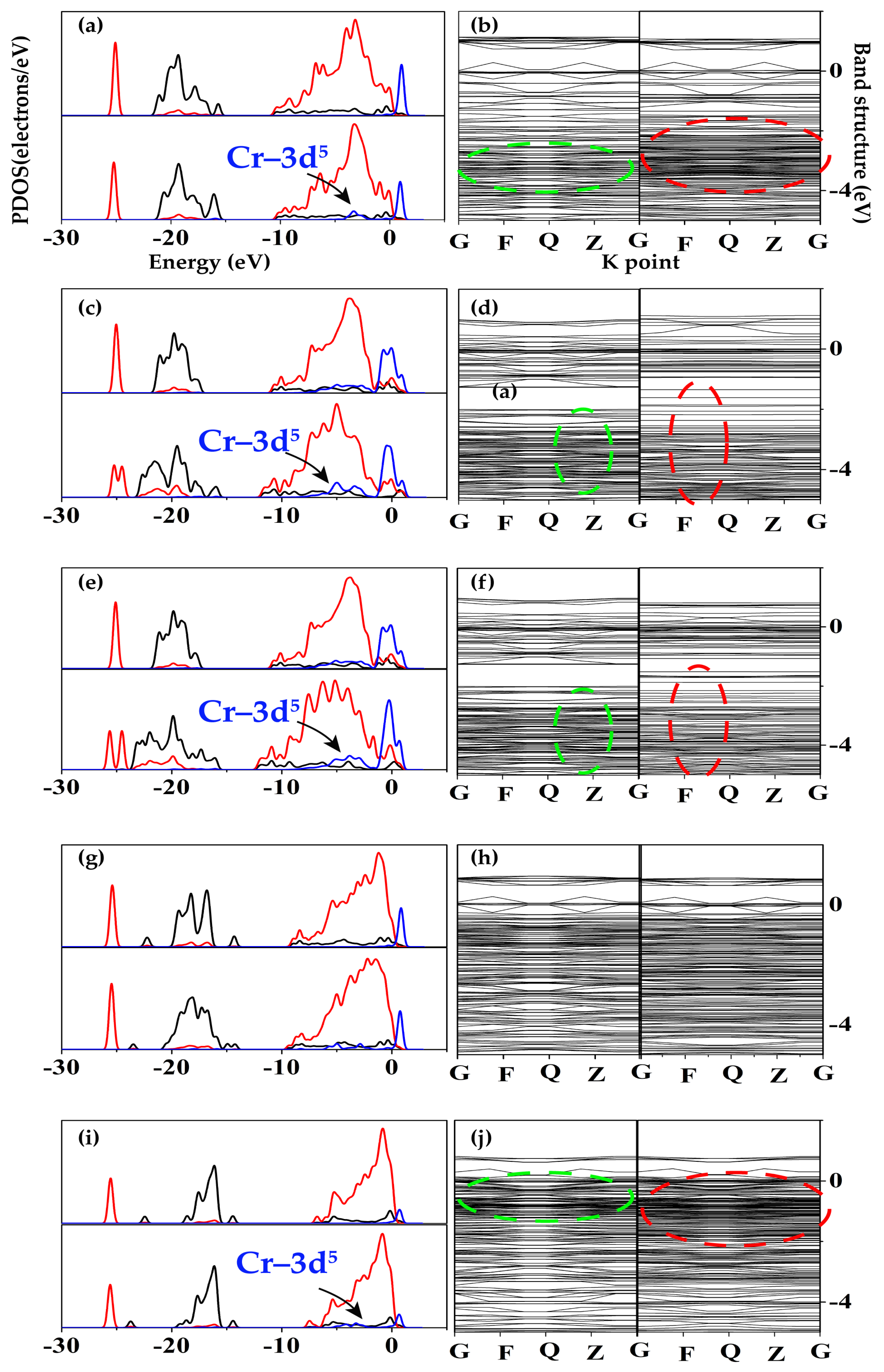
3.5. Density of States Reveal the Formation of Adsorption Bond
3.6. Band Structures Reveal the Strength of Adsorption Bond and the Distinctive Valence Electrons
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilbur, S.; Abadin, H.; Fay, M.; Yu, D.; Tencza, B.; Ingerman, L.; Klotzbach, J.; Jmaes, S. Toxicological Profile for Chromium; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2012. [Google Scholar]
- Zeng, Z.; Luo, W.; Wang, Z.; Yi, F. Water Pollution and Its Causes in the Tuojiang River Basin, China: An Artificial Neural Network Analysis. Sustainability 2021, 13, 792. [Google Scholar] [CrossRef]
- Zeng, Z.; Luo, W.; Yi, F.; Huang, F.; Wang, C.; Zhang, Y.; Cheng, Q.; Wang, Z. Horizontal Distribution of Cadmium in Urban Constructed Wetlands: A Case Study. Sustainability 2021, 13, 5381. [Google Scholar] [CrossRef]
- Dai, R.; Liu, J.; Yu, C.; Sun, R.; Lan, Y.; Mao, J. A comparative study of oxidation of Cr(lll) in aqueous ions, complex ions and insoluble compounds by manganese-bearing mineral(birnessite). Chemosphere 2009, 76, 536–541. [Google Scholar] [CrossRef]
- Aldrich, M.; Gardea-Torresdey, J.; Peralta-Videa, J.; Parsons, J. Uptake and reduction of Cr(VI) to Cr(III) by mesquite (Prosopis spp.): Chromate-plant interaction in hydroponics andsolid media studied using XAS. Environ. Sci. Technol. 2003, 37, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- DesMarias, T.; Costa, M. Mechanisms of chromium-induced toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Kaya, A.; Ören, A. Adsorption of zinc from aqueous solutions to bentonite. J. Hazard. Mater. 2005, 125, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Andini, S.; Cioffi, R.; Montagnaro, F.; Pisciotta, F.; Santoro, L. Simultaneous adsorption of chlorophenol and heavy metal ions on organophilic bentonite. Appl. Clay Sci. 2006, 31, 126–133. [Google Scholar] [CrossRef]
- Donat, R.; Akdogan, A.; Erdem, E.; Cetisli, H. Thermodynamics of Pb2+ and Ni2+ adsorption onto natural bentonite from aqueous solutions. J. Colloid Interface Sci. 2005, 286, 43–52. [Google Scholar] [CrossRef]
- Anna, B.; Kleopas, M.; Constantine, S.; Anestis, F.; Maria, B. Adsorption of Cd(II), Cu(II), Ni(II) and Pb(II) onto natural bentonite: Study in mono- and multi-metal systems. Environ. Earth Sci. 2015, 73, 5435–5444. [Google Scholar] [CrossRef]
- Tohdee, K.; Kaewsichan, L.; Asadullah. Enhancement of adsorption efficiency of heavy metal Cu(II) and Zn(II) onto cationic surfactant modified bentonite. J. Environ. Chem. Eng. 2018, 6, 2821–2828. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, S. Adsorption of copper and nickel on Na-bentonite. Process. Saf. Environ. Prot. 2010, 88, 62–66. [Google Scholar] [CrossRef]
- Rathnayake, S.; Martens, W.; Xi, Y.; Frost, R.; Ayoko, G. Remediation of Cr (VI) by inorganic-organic clay. J. Colloid Interface Sci. 2017, 490, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Luo, H. Adsorption of hexavalent chromium onto montmorillonite modified with hydroxyaluminum and cetyltrimethylammonium bromide. Appl. Surf. Sci. 2010, 257, 769–775. [Google Scholar] [CrossRef]
- Castro-Castro, J.; Macías-Quiroga, I.; Giraldo-Gómez, G.; Sanabria-González, N. Adsorption of Cr(VI) in Aqueous Solution Using a Surfactant-Modified Bentonite. Sci. World J. 2020, 2020, 3628163. [Google Scholar] [CrossRef]
- Abollino, O.; Acetob, M.; Malandrinoa, M.; Sarzaninia, C.; Mentastia, E. Adsorption of heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Res. 2003, 37, 1619–1627. [Google Scholar] [CrossRef]
- Singh, R.; Dong, H.; Zeng, Q.; Zhang, L.; Rengasamy, K. Hexavalent chromium removal by chitosan modified-bioreduced nontronite. Geochim. Cosmochim. Acta 2017, 210, 25–41. [Google Scholar] [CrossRef]
- Kwak, S.; Yoo, J.; Baek, K. Synergistic and inhibitory reduction of Cr(VI) by montmorillonite, citric acid, and Mn(II). J. Soils Sediments 2018, 18, 205–210. [Google Scholar] [CrossRef]
- Öncel, M. Adsorption of copper (II) from aqueous solution by Beidellite. Environ. Geol. 2008, 55, 1767–1775. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Gupta, S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Shu, Z.; Liu, L.; Qiu, G.; Yang, X.; Zhang, M.; Tan, W.; Liu, C.; Wu, F. Photochemical formation process of schwertmannite on montmorillonite and corresponding Cr (VI) adsorption capacity. ACS Earth Space Chem. 2019, 3, 718–727. [Google Scholar] [CrossRef]
- Handy, N.C.; Lee, A. The adiabatic approximation. Chem. Phys. Lett. 1996, 252, 425–430. [Google Scholar] [CrossRef]
- Echenique, P.; Alonso, J.L. A mathematical and computational review of Hartree-Fock SCF methods in Quantum Chemistry. Mol. Phys. 2007, 105, 3057–3098. [Google Scholar] [CrossRef] [Green Version]
- Kolmanovich, V.; Reznik, I. Decompositions of electron density and the shell problem in Hohenberg-Kohn functional theory. Solid State Commun. 1984, 50, 117–120. [Google Scholar] [CrossRef]
- Nagy, Á. Kohn-Sham equations for multiplets. Phys. Rev. A 1998, 57, 1672–1677. [Google Scholar] [CrossRef]
- Cottenier, S. Density Functional Theory and the Family of (L) APW-Methods: A Step-by-Step Introduction; Instituut Voor Kernen Stralingsfysica: Leuven, Belgium, 2002. [Google Scholar]
- Jackson, K.; Pederson, M.R. Accurate forces in a local-orbital approach to the local-density approximation. Phys. Rev. B 1990, 42, 3276–3281. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Yue, W. Erratum: Accurate and simple density functional for the electronic exchange energy: Generalized gradient approximation. Phys. Rev. B 1989, 40, 3399. [Google Scholar] [CrossRef]
- Kohn, W. Electronic structure of matter-wave functions and density funcionals. Riv. Morden Phys. 1999, 71, 1253–1266. [Google Scholar]
- Ceperley, D.; Berni, J. Ground state of the electron gas by a stochastic method. Phys. Rev. Lett. 1980, 45, 566–569. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.; Yue, W. Accurate and simple density functional for the electronic exchange energy: Generalized gradient approximation. Phys. Rev. B 1986, 33, 8800–8802. [Google Scholar] [CrossRef]
- Kirkpatrick, S.; Gelatt, C.; Vecchi, M. Optimization by simulated annealing. Science 1983, 220, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Segall, M.; Pickard, C.; Hasnip, P.; Probert, M.; Refson, K.; Payne, M. First principles methods using CASTEP. Z. Krist.-Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Delley, B. DMol3 DFT studies: From molecules and molecular environments to surfaces and solids. Comput. Mater. Sci. 2000, 17, 122–126. [Google Scholar] [CrossRef]
- Rani, P.; Dubey, G.; Jindal, V. DFT study of optical properties of pure and doped graphene. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 62, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Ossiander, M.; Riemensberger, J.; Neppl, S.; Mittermair, M.; Schaffer, M.; Duensing, A.; Wagner, M.; Heider, R.; Wurzer, M.; Gerl, M.; et al. Absolute timing of the photoelectric effect. Nature 2018, 561, 374–377. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Soleimani, M.; Siahpoosh, Z. Ghezeljeh nanoclay as a new natural adsorbent for the removal of copper and mercury ions: Equilibrium, kinetics and thermodynamics studies. Chin. J. Chem. Eng. 2015, 23, 1819–1833. [Google Scholar] [CrossRef]
- Viani, A.; Gualtieri, A.; Artioli, G. The nature of disorder in montmorillonite by simulation of X-ray powder patterns. Am. Mineral. 2002, 87, 966–975. [Google Scholar] [CrossRef]
- Manceau, A.; Chateigner, D.; Gates, W. Polarized EXAFS, distance-valence least-squares modeling (DVLS), and quantitative texture analysis approaches to the structural refinement of Garfield nontronite. Phys. Chem. Miner. 1998, 25, 347–365. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Bian, L.; Dong, F.; Li, W.; Song, M.; Nie, J.; Liu, X.; Huo, T.; Zhang, H.; Xu, B.; et al. DFT and 2D-CA methods unravelling the mechanism of interfacial interaction between amino acids and Ca-montmorillonite. Appl. Clay Sci. 2019, 183, 105356. [Google Scholar] [CrossRef]
- Bian, L.; Dong, F.; Song, M.X.; Dong, H.; Li, W.; Duan, T.; Xu, J.; Zhang, X. DFT and two-dimensional correlation analysis methods for evaluating the Pu3+–Pu4+ electronic transition of plutonium-doped zircon. J. Hazard. Mater. 2015, 294, 47–56. [Google Scholar] [CrossRef]
- Bian, L.; Song, M.; Dong, F.; Duan, T.; Xu, J.; Li, W.; Zhang, X. DFT and two-dimensional correlation analysis for evaluating the oxygen defect mechanism of low-density 4f (or 5f) elements interacting with Ca-Mt. RSC Adv. 2015, 5, 28601–28610. [Google Scholar] [CrossRef]
- Bian, L.; Xu, J.; Song, M.; Dong, F.; Dong, H.; Shi, F.; Zhang, X.; Duan, T. First principles simulation of temperature dependent electronic transition of FM-AFM phase BFO. J. Mol. Modeling 2015, 21, 91. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Zhang, Y.; Zang, Y.Z.; Liu, C.; Wang, W.; Han, R.; Ji, N.; Zhang, S.; Liu, Q. Promotion of A-Site Ag-Doped Perovskites for the Catalytic Oxidation of Soot: Synergistic Catalytic Effect of Dual Active Sites. ACS Catal. 2021, 11, 14224–14236. [Google Scholar] [CrossRef]
- Liu, X.; Yang, S.; Gu, P.; Liu, S.; Yang, G. Adsorption and removal of metal ions by smectites nanoparticles: Mechanistic aspects, and impacts of charge location and edge structure. Appl. Clay Sci. 2021, 201, 105957. [Google Scholar] [CrossRef]
- Garfinkel-Shweky, D.; Yariv, S. Metachromasy in clay dye systems: The adsorption of acridine orange by Na-beidellite. Clay Miner. 1999, 34, 459–467. [Google Scholar] [CrossRef]
- Greathouse, J.; Cygan, R. Water structure and aqueous uranyl (VI) adsorption equilibria onto external surfaces of beidellite, montmorillonite, and pyrophyllite: Results from molecular simulations. Environ. Sci. Technol. 2006, 40, 3865–3871. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Min, F.; Liu, L.; Chen, J. The adsorption of CaOH+ on (001) basal and (010) edge surface of Na-montmorillonite: A DFT study. Surf. Interface Anal. 2007, 49, 267–277. [Google Scholar] [CrossRef]
- Chen, J.; Min, F.; Liu, L.; Liu, C. Mechanism research on surface hydration of kaolinite, insights from DFT and MD simulations. Appl. Surf. Sci. 2019, 476, 6–15. [Google Scholar] [CrossRef]
- Cygan, R.; Liang, J.; Kalinichev, G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Segall, M.; Shah, R.; Pickard, C.; Payne, M. Population analysis of plane-wave electronic structure calculations. Phys. Rev. B 1996, 54, 16317–16320. [Google Scholar] [CrossRef] [Green Version]
- Segall, M.; Pickard, C.; Shah, R.; Payne, M. Population analysis in plane wave electronic structure calculations. Mol. Phys. 2010, 89, 571–577. [Google Scholar] [CrossRef]
- Peng, C.; Zhong, Y.; Wang, G.; Min, F.; Qin, L. Atomic-level insights into the adsorption of rare earth Y (OH) 3-nn+ (n = 1–3) ions on kaolinite surface. Appl. Surf. Sci. 2019, 469, 357–367. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Q.; Zhuang, L.; Zhao, Z. Adsorption of Ca(II) and K(I) on the kaolinite surface: A DFT study with an experimental verification. Mol. Phys. 2020, 119, 9. [Google Scholar] [CrossRef]
- Levitt, M.; Raleigh, D.; Creuzet, F.; Griffin, R. Theory and simulations of homonuclear spin pair systems in rotating solids. J. Chem. Phys. 1990, 92, 6347–6364. [Google Scholar] [CrossRef]
- Miroslav, K.; Wagner, F.; Grin, Y. Electron localization function for transition-metal compounds. Theor. Chem. Acc. 2002, 108, 150–156. [Google Scholar] [CrossRef]
- Sproul, G. Electronegativity and bond type: Predicting bond type. J. Chem. Educ. 2001, 78, 387. [Google Scholar] [CrossRef]
- Thomas, U.; Erastova, V.; Greenwell, H. Ion adsorption at clay-mineral surfaces: The Hofmeister series for hydrated smectite minerals. Clays Clay Miner. 2016, 64, 472–487. [Google Scholar] [CrossRef] [Green Version]
- Pecini, E.; Marcelo, J. Measuring the isoelectric point of the edges of clay mineral particles: The case of montmorillonite. Langmuir 2013, 29, 14926–14934. [Google Scholar] [CrossRef]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay mineral adsorbents for heavy metal removal from wastewater: A review. Environ. Chem. Lett. 2019, 17, 629–654. [Google Scholar] [CrossRef]
- Li, Y.; Gao, B.; Wu, T.; Sun, D.; Li, X.; Wang, B.; Lu, F. Hexavalent chromium removal from aqueous solution by adsorption on aluminum magnesium mixed hydroxide. Water Res. 2009, 43, 3067–3075. [Google Scholar] [CrossRef]
- Bharti, V.; Balomajumder, C. Magnetic magnesium ferrite–doped multi-walled carbon nanotubes: An advanced treatment of chromium-containing wastewater. Environ. Sci. Pollut. Res. 2020, 27, 13844–13854. [Google Scholar] [CrossRef]
- Ebrahim, S.; Ahmed, M.; Saad, E.; Salem, S.; Ahmed, A.; Mahmoud, H.; Fayez, M.; Hosam, A.; Essa, M.; Amr, F. The Catalytic Activity of Biosynthesized Magnesium Oxide Nanoparticles (MgO-NPs) for Inhibiting the Growth of Pathogenic Microbes, Tanning Effluent Treatment, and Chromium Ion Removal. Catalysts 2021, 11, 821. [Google Scholar] [CrossRef]
- Matsukevich, I.; Lipai, Y.; Romanovski, V. Cu/MgO and Ni/MgO composite nanoparticles for fast, high-efficiency adsorption of aqueous lead (II) and chromium (III) ions. J. Mater. Sci. 2021, 56, 5031–5040. [Google Scholar] [CrossRef]
- Alshammari, M.; Ahmed, I.; Alsharari, J.; Alsohaimi, I.; Al-Muaikel, N.; Alraddadi, T.; Hasanin, T. Adsorption of Cr (VI) using α-Fe2O3 coated hydroxy magnesium silicate (HMS): Isotherm, thermodynamic and kinetic study. Int. J. Environ. Anal. Chem. 2021, 1–17. [Google Scholar] [CrossRef]
- Abdollah, D.; Hafez, G.; Parviz, D.; Amir, K.; Sye, H.; Ali, P.; Alireza, B. An investigation and comparison of removing heavy metals (lead and chromium) from aqueous solutions using magnesium oxide nanoparticles. Pol. J. Environ. Stud. 2016, 25, 557–562. [Google Scholar]
- Zeng, Z.; Luo, W.; Yi, F.; Wang, Z. Cadmium Uptake, In Vivo Metastasis and Subcellular Environmental Response of Five Wetland Plants Using DFT Method. Sustainability 2021, 13, 7872. [Google Scholar] [CrossRef]
- Alessio, S.; Curcio, G.; Limonti, C. Hexavalent chromium reduction by zero-valent magnesium particles in column systems. J. Environ. Manag. 2021, 293, 112905. [Google Scholar] [CrossRef]
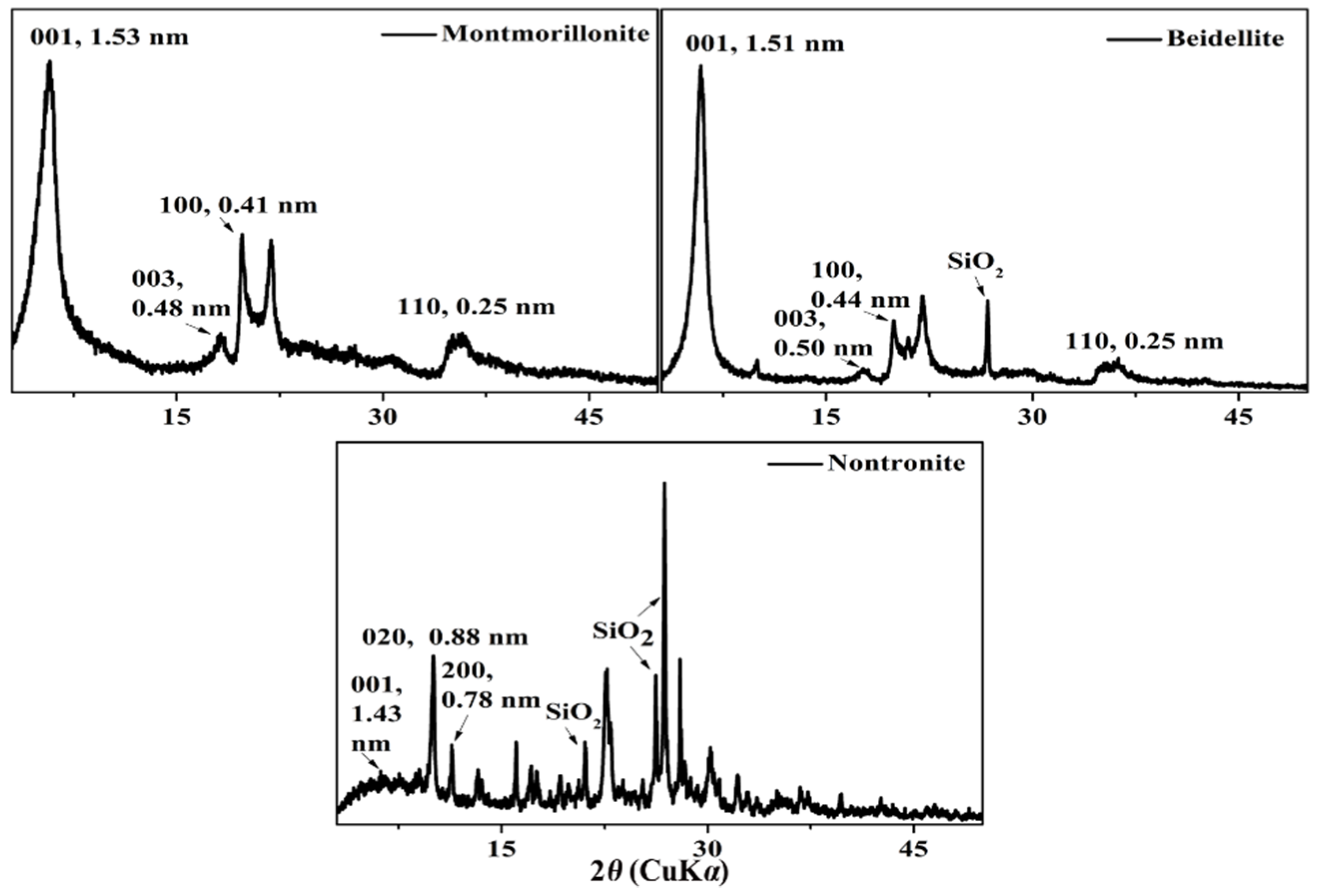
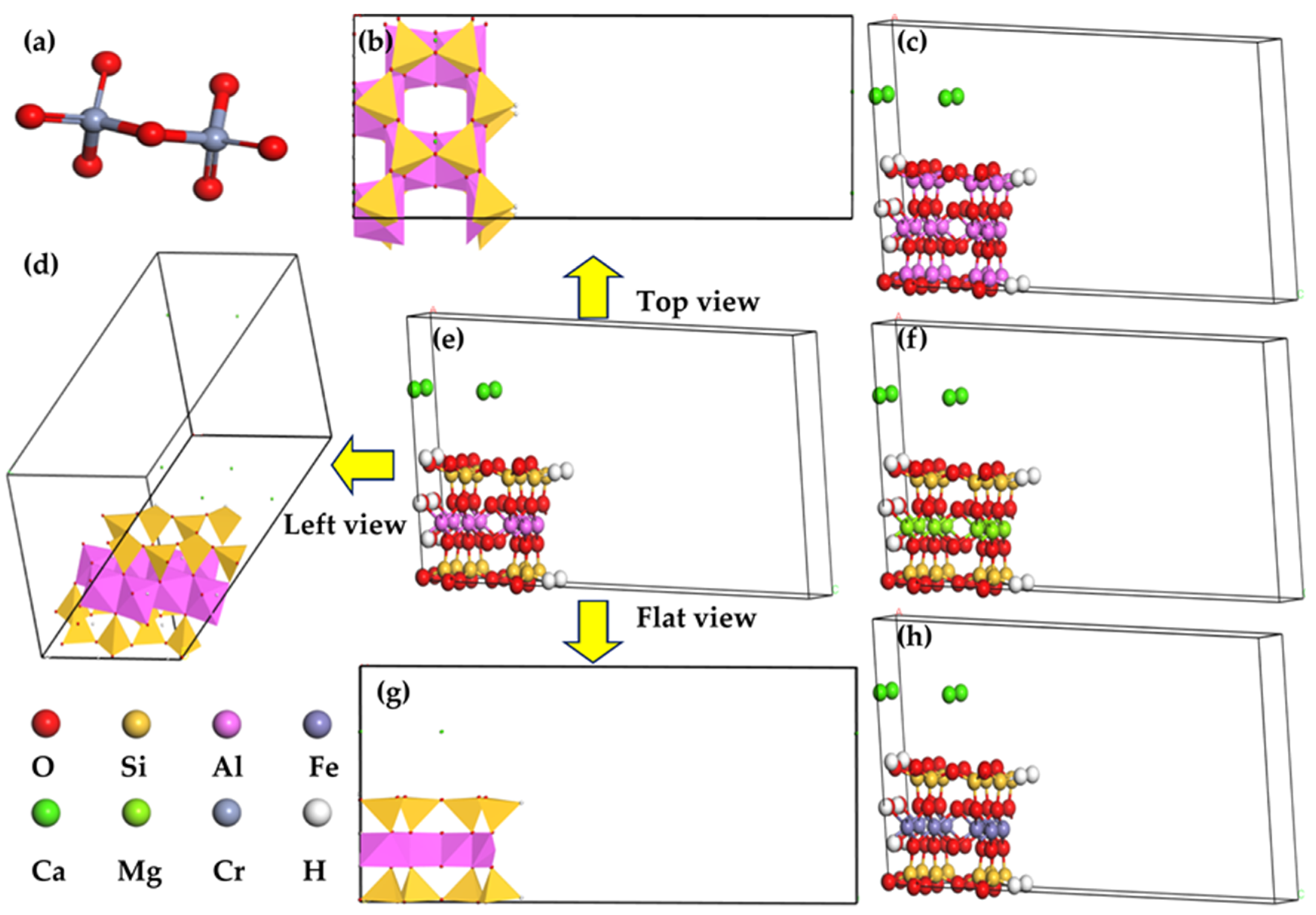

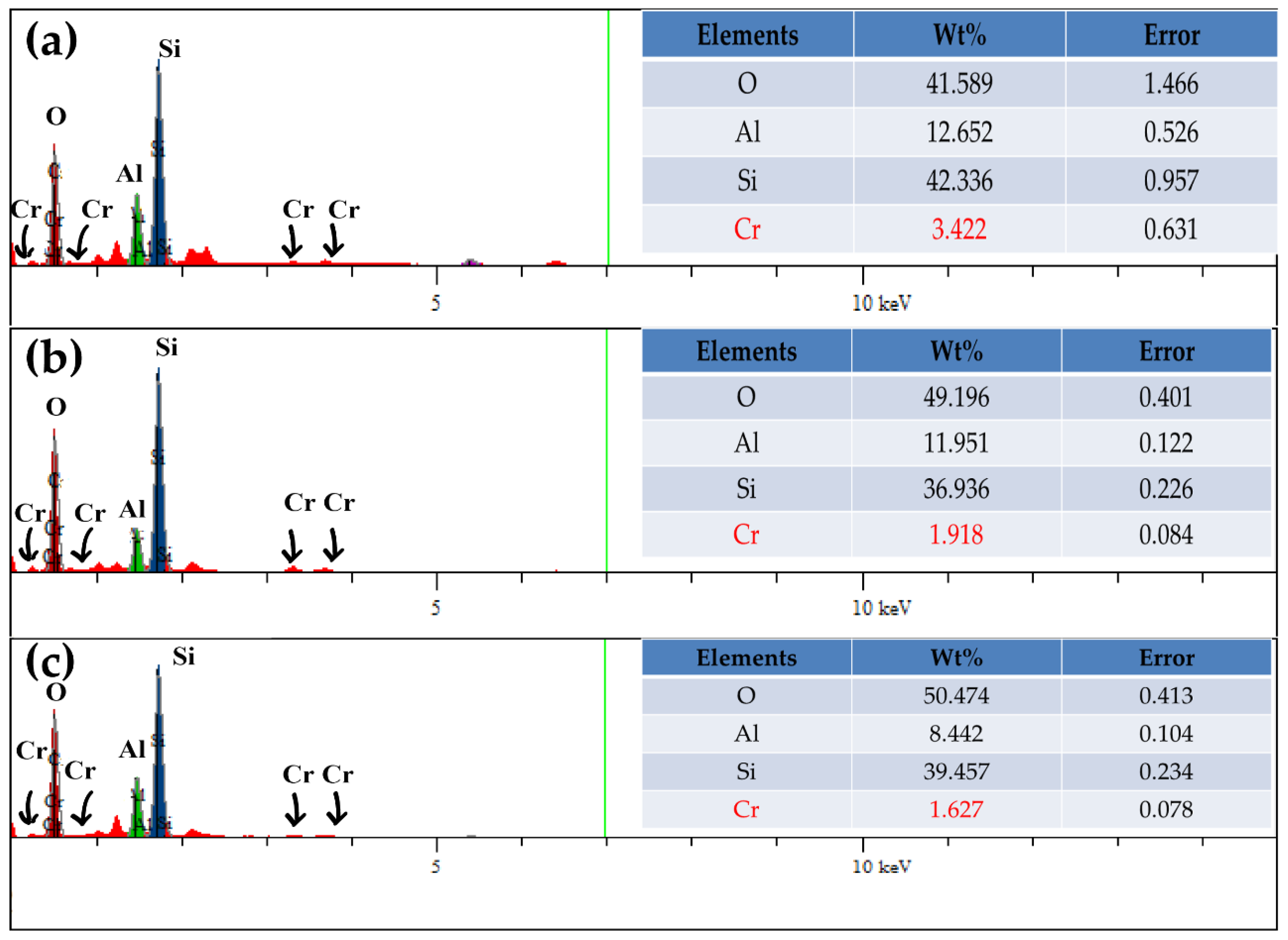

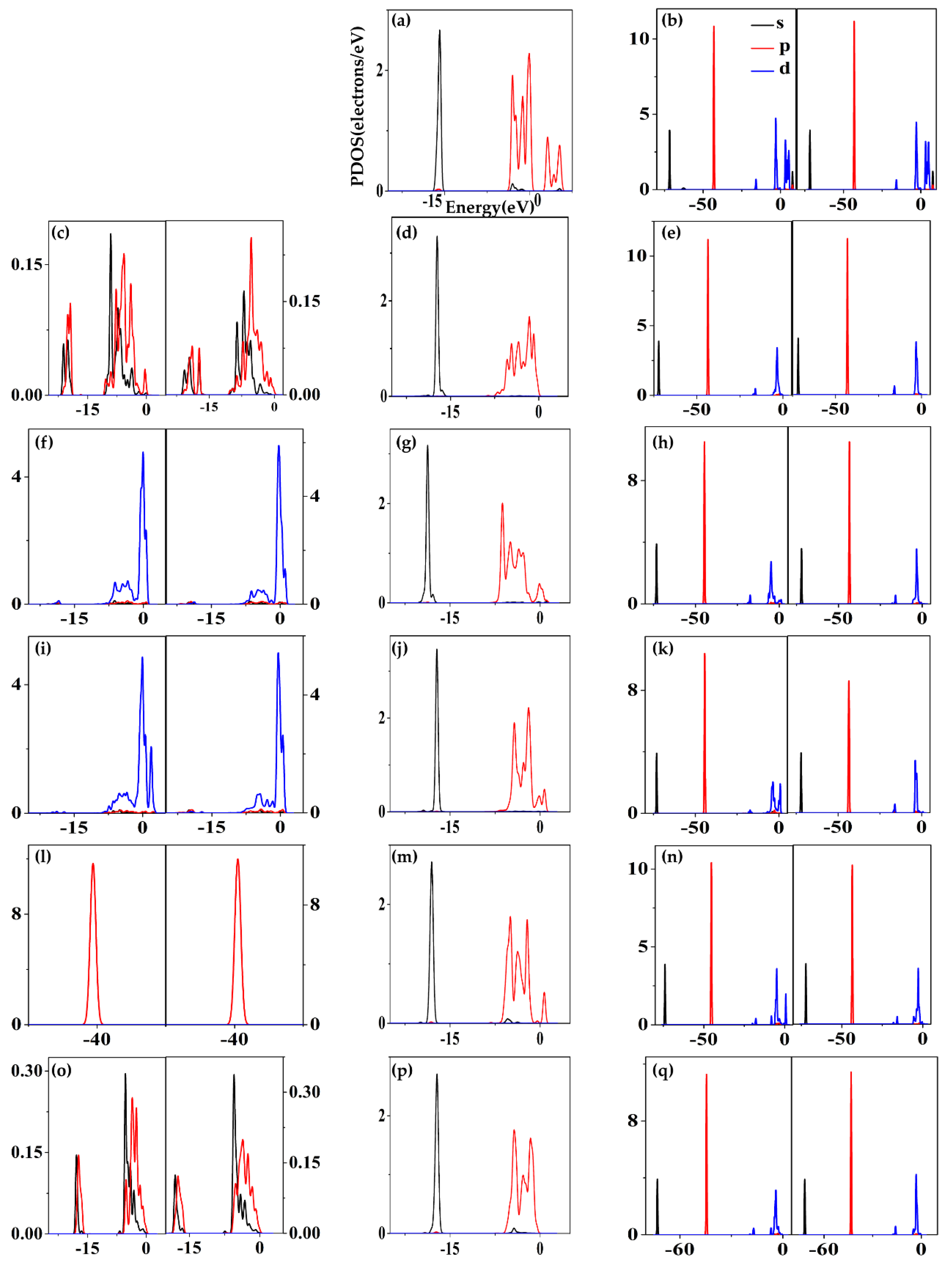
| Components | Montmorillonite | Nontronite | Beidellite |
|---|---|---|---|
| SiO2 | 50.19 | 59.54 | 59.22 |
| Al2O3 | 22.42 | 11.18 | 11.78 |
| Fe2O3 | 3.16 | 1.68 | 0.89 |
| MgO2 | 1.94 | 1.06 | 3.76 |
| Cl | 0.74 | \ | <0.01 |
| SO3 | 0.59 | \ | 0.02 |
| TiO2 | 0.36 | 0.26 | \ |
| CaO | 0.18 | 2.75 | 3.06 |
| K2O | 0.14 | 2.11 | 0.43 |
| Na2O | 0.12 | 1.06 | 0.53 |
| Minerals | Residual Concentration (mg/L) | Initial Concentration (mg/L) |
|---|---|---|
| Montmorillonite | 18 | 30 |
| Nontronite | 21 | 30 |
| Beidellite | 22 | 30 |
| Minerals | O | AlO | Si | Fe | Mg | AlT |
|---|---|---|---|---|---|---|
| Montmorillonite | −1.16~−1.20/−1.12 [41]/ −1.00~−1.06 [51]/−1.05 [52,53] | 1.98/2.01 [41]/1.58 [53] | 1.88~2.27/2.10 [53] | \ | \ | \ |
| Nontronite–Fe(II) | −0.90~−0.96/−1.16 [41] | \ | 1.83~2.22 | 1.08/1.03 [41]/1.58 [53] | \ | \ |
| Nontronite–Fe(III) | −0.90~−0.96/−1.16 [41] | \ | 1.86~2.22 | 1.09/1.03 [41]/1.58 [53] | \ | \ |
| Montmorillonite–Mg | −1.26~−1.29/−1.12 [41] | \ | 1.79~2.18 | \ | 1.69/2.40 [41]/1.36 [53] | \ |
| Beidellite | −1.18~−1.20 | 1.84/2.01 [41] | \ | \ | \ | 1.66~1.88/1.58 [53] |
| Substance | O(mineral) | Al(mineral) | Si(mineral) | Mg | Fe | O(Cr2O72−) | Cr−1 | Cr−2 |
|---|---|---|---|---|---|---|---|---|
| Cr2O72 | \ | \ | \ | \ | \ | −0.32~−0.34 | 1.29 | 1.29 |
| Montmorillonite | −1.15~−1.18 | 1.87 | 1.85~2.26 | \ | \ | −0.43~−0.83 | 1.33 | 1.27 |
| Nontronite–Fe(II) | −0.85~−0.92 | \ | 1.79~2.21 | \ | 1.12 | −0.39~−0.63 | 1.30 | 1.24 |
| Nontronite–Fe(III) | −0.84~−0.91 | \ | 1.81~2.24 | \ | 1.14 | −0.42~−0.59 | 1.30 | 1.25 |
| Montmorillonite–Mg | −1.14~−1.27 | \ | 1.75~2.13 | 1.65 | \ | −0.32~−0.71 | 1.31 | 1.23 |
| Beidellite | −1.18~−1.19 | 1.79/1.65~1.86 a | \ | \ | \ | −0.33~−0.79 | 1.35 | 1.28 |
| Minerals | Original Mineral | Adsorption System | Cr2O72− | ||
|---|---|---|---|---|---|
| X–O | Y–O | X–O | Y–O | X–O | |
| Montmorillonite | 0.19~0.39 | 0.47~0.63 | 0.18~0.48 | 0.49~0.62 | 0.48 |
| Nontronite–Fe(II) | 0.17~0.41 | 0.53~0.60 | 0.17~0.52 | 0.48~0.60 | 0.52 |
| Nontronite–Fe(III) | 0.17~0.41 | 0.50~0.82 | 0.17~0.42 | 0.48~0.60 | 0.50 |
| Montmorillonite–Mg | −0.24~−1.52 | 0.47~0.67 | −0.31~−1.67 | 0.39~0.78 | −0.47 |
| Beidellite | 0.08~0.44 | 0.08~0.44 | 0.40~0.62 | 0.38~0.61 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, W.; Zeng, Z.; Bian, L. Effect of Lattice Substitution on Adsorption of Hexavalent Chromium by Montmorillonite, Nontronite, and Beidellite. Minerals 2021, 11, 1407. https://doi.org/10.3390/min11121407
Luo W, Zeng Z, Bian L. Effect of Lattice Substitution on Adsorption of Hexavalent Chromium by Montmorillonite, Nontronite, and Beidellite. Minerals. 2021; 11(12):1407. https://doi.org/10.3390/min11121407
Chicago/Turabian StyleLuo, Weige, Zheng Zeng, and Liang Bian. 2021. "Effect of Lattice Substitution on Adsorption of Hexavalent Chromium by Montmorillonite, Nontronite, and Beidellite" Minerals 11, no. 12: 1407. https://doi.org/10.3390/min11121407
APA StyleLuo, W., Zeng, Z., & Bian, L. (2021). Effect of Lattice Substitution on Adsorption of Hexavalent Chromium by Montmorillonite, Nontronite, and Beidellite. Minerals, 11(12), 1407. https://doi.org/10.3390/min11121407







