Iron Silicides in Fulgurites
Abstract
1. An Overview on Fulgurites and Prebiotic Chemistry
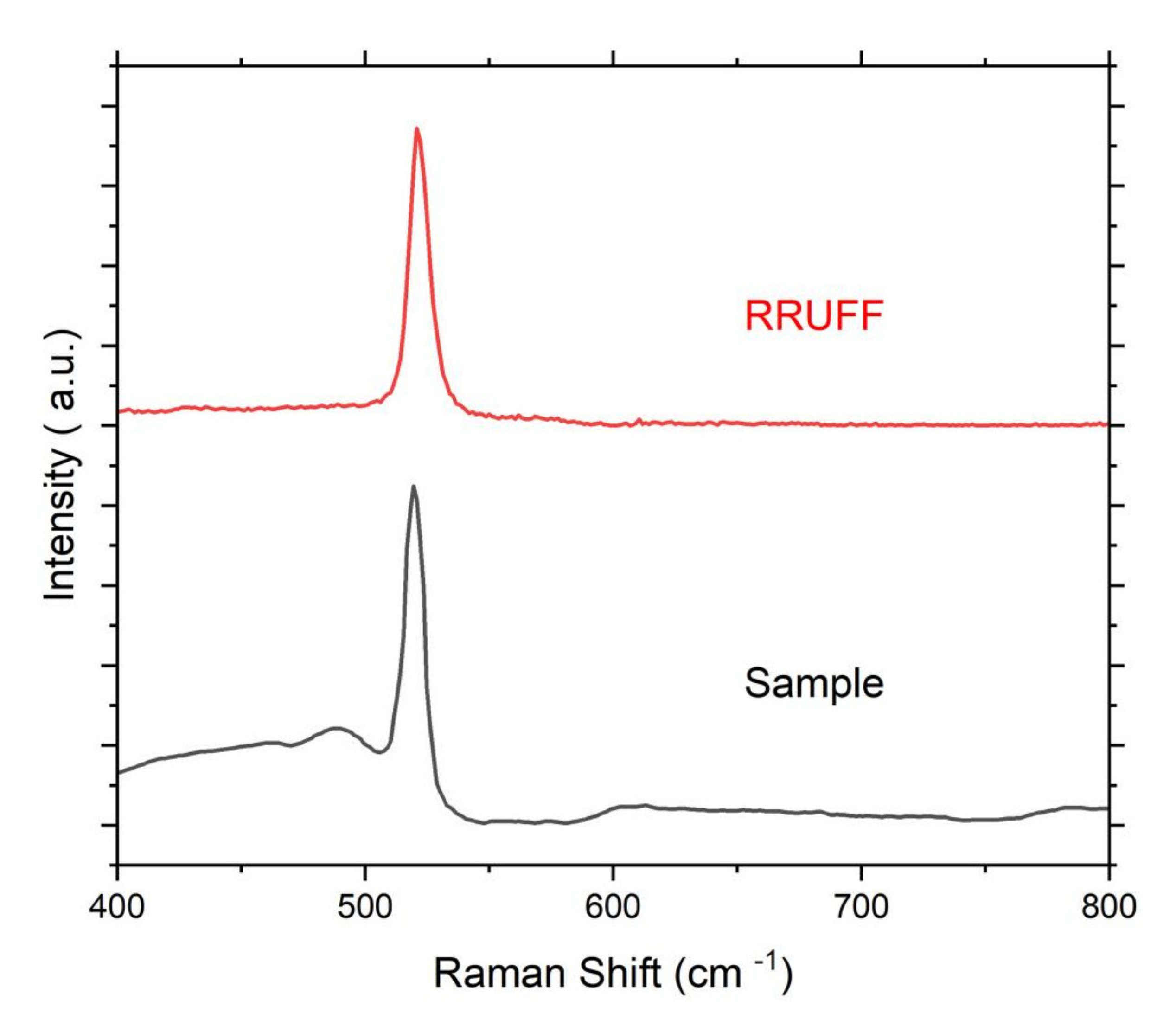
2. The Occurrences of Iron Silicides in Fulgurites
3. The Formation of Iron Silicide in Fulgurites
4. Comparison between Iron Silicides in Fulgurites and in Other Rocks
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pasek, M.A.; Pasek, V.D. The forensics of fulgurite formation. Miner. Petrol. 2018, 112, 185–198. [Google Scholar] [CrossRef]
- Essene, E.J.; Fisher, D.C. Lightning strike fusion: Extreme reduction and metal-silicate liquid immiscibility. Science 1986, 234, 189–193. [Google Scholar] [CrossRef]
- Pasek, M.A.; Block, K.; Pasek, V. Fulgurite morphology: A classification scheme and clues to formation. Contrib. Miner. Petrol. 2012, 164, 477–492. [Google Scholar] [CrossRef]
- Martin Crespo, T.; Lozano Fernandez, R.P.; Gonzalez Laguna, R. The fulgurite of Torre de Moncorvo (Portugal): Description and analysis of the glass. Eur. J. Mineral. 2009, 21, 783–794. [Google Scholar] [CrossRef]
- Feng, T.; Lang, C.; Pasek, M.A. The origin of blue coloration in a fulgurite from Marquette, Michigan. Lithos 2019, 342, 288–294. [Google Scholar] [CrossRef]
- Rakov, V.A.; Uman, M.A. Lightning: Physics and Effects, 1st ed.; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- O’Keefe, J.A. Natural glass. J. Non-Cryst. Solids 1984, 67, 1–17. [Google Scholar] [CrossRef]
- McCloy, J.S. Frontiers in natural and un-natural glasses: An interdisciplinary dialogue and review. J. Non-Cryst. Solids X 2019, 4, 100035. [Google Scholar] [CrossRef]
- Grapes, R. Pyrometamorphism, 2nd ed.; Springer Science & Business Media: Berlin, Germany, 2010. [Google Scholar]
- Ende, M.; Schorr, S.; Kloess, G.; Franz, A.; Tovar, M. Shocked quartz in Sahara fulgurite. Eur. J. Mineral. 2012, 24, 499–507. [Google Scholar] [CrossRef]
- Chen, J.; Elmi, C.; Goldsby, D.; Gieré, R. Generation of shock lamellae and melting in rocks by lightning-induced shock waves and electrical heating. Geophys. Res. Lett. 2017, 44, 8757–8768. [Google Scholar] [CrossRef]
- Borucki, W.J.; Bar-Nun, A.; Scarf, F.L.; Cook II, A.F.; Hunt, G.E. Lightning activity on Jupiter. Icarus 1982, 52, 492–502. [Google Scholar] [CrossRef]
- Uman, M.A.; Beasley, W.H.; Tiller, J.A.; Lin, Y.T.; Krider, E.P.; Weidmann, C.D.; Krehbiel, P.R.; Brook, M.; Few, A.A., Jr.; Bohannon, J.L.; et al. An unusual lightning flash at Kennedy Space Center. Science 1978, 201, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Uman, M.A. The Art and Science of Lightning Protection, 1st ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Pasek, M.A.; Hurst, M. A fossilized energy distribution of lightning. Sci. Rep. 2016, 6, 30586. [Google Scholar] [CrossRef] [PubMed]
- Gieré, R.; Wimmenauer, W.; Müller-Sigmund, H.; Wirth, R.; Lumpkin, G.R.; Smith, K.L. Lightning-induced shock lamellae in quartz. Am. Mineral. 2015, 100, 1645–1648. [Google Scholar] [CrossRef]
- Carter, E.A.; Pasek, M.A.; Smith, T.; Kee, T.P.; Hines, P.; Edwards, H.G. Rapid Raman mapping of a fulgurite. Anal. Bioanal. Chem. 2010, 397, 2647–2658. [Google Scholar] [CrossRef] [PubMed]
- Kenny, G.G.; Pasek, M.A. The response of zircon to the extreme pressures and temperatures of a lightning strike. Sci. Rep. 2021, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.; Block, K. Lightning-induced reduction of phosphorus oxidation state. Nat. Geosci. 2009, 2, 553–556. [Google Scholar] [CrossRef]
- Hess, B.L.; Piazolo, S.; Harvey, J. Lightning strikes as a major facilitator of prebiotic phosphorus reduction on early Earth. Nat. Commun. 2021, 12, 1535. [Google Scholar] [CrossRef]
- Plyashkevich, A.A.; Minyuk, P.S.; Subbotnikova, T.V.; Alshevsky, A.V. Newly formed minerals of the Fe–P–S system in Kolyma fulgurite. Dokl. Earth Sci. 2016, 467, 380–383. [Google Scholar] [CrossRef]
- Sheffer, A.A. Chemical Reduction of Silicates by Meteorite Impacts and Lightning Strikes. Ph.D. Thesis, University of Arizona, Tucson, AZ, USA, 2007. [Google Scholar]
- Sheffer, A.A.; Dyar, M.D. 57Fe Mössbauer spectroscopy of fulgurites: Implications for chemical reduction. In Proceedings of the 35th Lunar and Planetary Science Conference, Woodlands, TX, USA, 15–19 March 2004; p. 1372. [Google Scholar]
- Sheffer, A.A.; Melosh, H.J.; Jarnot, B.M.; Lauretta, D.S. Reduction of silicates at high temperature: Fulgurites and thermodynamic modeling. In Proceedings of the 34th Lunar and Planetary Science Conference, Woodlands, TX, USA, 17–21 March 2003; p. 1467. [Google Scholar]
- Cardona, M.R.; Castro, K.F.; García, P.P.C.; Hernandez, L.E.O. Mineralogical study of binary iron silicides (Fe–Si system) in a fulgurite from Hidalgo, Mexico. Bol Minerol. 2006, 17, 69–76. [Google Scholar]
- Miller, S.L.; Urey, H.C. Organic compound synthesis on the primitive earth. Science 1959, 130, 234–251. [Google Scholar] [CrossRef]
- Navarro-Gonzalez, R.; McKay, C.P.; Mvondo, D.N. A possible nitrogen crisis for Archaean life due to reduced nitrogen fixation by lightning. Nature 2001, 412, 61–64. [Google Scholar] [CrossRef]
- Ballhaus, C.; Wirth, R.; Fonseca, R.O.C.; Blanchard, H.; Pröll, W.; Bragagni, A.; Nagel, T.; Schreiber, A.; Dittrich, S.; Thome, V.; et al. Ultra-high pressure and ultra-reduced minerals in ophiolites may form by lightning strikes. Geochem. Perspect. Lett. 2017, 5, 42–46. [Google Scholar] [CrossRef]
- Hiltl, M.; Bauer, F.; Ernstson, K.; Mayer, W.; Neumair, A.; Rappenglück, M.A. SEM and TEM analyses of minerals xifengite, gupeiite, Fe2Si (hapkeite?), titanium carbide (TiC) and cubic moissanite (SiC) from the subsoil in the alpine foreland: Are they cosmochemical? In Proceedings of the 42nd Lunar and Planetary Science Conference, Woodlands, TX, USA, 7–11 March 2011; p. 1391, No. 1608. [Google Scholar]
- Keil, K.; Berkley, J.L.; Fuchs, L.H. Suessite, Fe3Si: A new mineral in the North Haig ureilite. Am. Mineral. 1982, 67, 126–131. [Google Scholar]
- Anand, M.; Taylor, L.A.; Nazarov, M.A.; Shu, J.; Mao, H.K.; Hemley, R.J. Space weathering on airless planetary bodies: Clues from the lunar mineral hapkeite. Proc. Natl. Acad. Sci. USA 2004, 101, 6847–6851. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Bai, W.; Li, G.; Xiong, M.; Yang, J.; Ma, Z.; Rong, H. Naquite, FeSi, a new mineral species from Luobusha, Tibet, western China. Acta Geol. Sin.-Engl. Ed. 2012, 86, 533–538. [Google Scholar]
- Li, G.; Bai, W.; Shi, N.; Fang, Q.; Xiong, M.; Yang, J.; Ma, Z.; Rong, H. Linzhiite, FeSi2, a redefined and revalidated new mineral species from Luobusha, Tibet, China. Eur. J. Mineral. 2012, 24, 1047–1052. [Google Scholar] [CrossRef]
- Bai, W.; Shi, N.; Fang, Q.; Li, G.; Xiong, M.; Yang, J.; Rong, H. Luobusaite: A new mineral. Acta Geol. Sin.-Engl. Ed. 2006, 80, 656–659. [Google Scholar]
- Roberts, S.E.; Sheffer, A.A.; McCanta, M.C.; Dyar, M.D.; Sklute, E.C. Oxidation state of iron in fulgurites and Trinitite: Implications for redox changes during abrupt high-temperature and pressure events. Geochim. Cosmochim. Acta 2019, 266, 332–350. [Google Scholar] [CrossRef]
- Block, K.M. Fulgurite classification, petrology, and implications for planetary processes. Master’s Thesis, University of Arizona, Tucson, AZ, USA, 2011. [Google Scholar]
- Reyes-Salas, A.M.; Macías-Romo, C.; Ortega-Gutiérrez, F.; Reyes-Salas, E.O.; Flores-Gutiérrez, D.; Alba-Aldave, L.; Girón-García, P.; Robles-Camacho, J.; García-Martínez, J.L.; Cervantes-de la Cruz, K.E. Petrographic, geochemical and mineralogical study of the San Jose de Lourdes fulgurite, Zacatecas, Mexico. Rev. Mex. Cienc. Geol. 2017, 34, 170–181. [Google Scholar] [CrossRef][Green Version]
- Stefano, C.J.; Hackney, S.A.; Kampf, A.R. The occurrence of iron silicides in a fulgurite: Implications for fulgurite genesis. Can. Mineral. 2020, 58, 115–123. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; Ma, M.; Jing, Q.; Li, G.; Sun, L.; Wang, W. Morphologies of Fe-66.7 at.% Si alloy solidified in a drop tube. Sci. China G Phys. Astron. 2005, 48, 658–666. [Google Scholar] [CrossRef]
- Walter, M. An exogenic fulgurite occurrence in Oswego, Oswego County, New York. Rocks Miner. 2011, 86, 264–270. [Google Scholar] [CrossRef]
- Parnell, J.; Thackrey, S.; Muirhead, D.K.; Wright, A.J. Transient high-temperature processing of silicates in fulgurites as analogues for meteorite and impact melts. In Proceedings of the 39th Lunar and Planetary Science Conference, Woodlands, TX, USA, 7–11 March 2008; No. 1391. p. 1286. [Google Scholar]
- Feng, T.; Gull, M.; Omran, A.; Abbott-Lyon, H.; Pasek, M.A. Evolution of Ephemeral Phosphate Minerals on Planetary Environments. ACS Earth Space Chem. 2021, 5, 1647–1656. [Google Scholar] [CrossRef]
- Elmi, C.; Chen, J.; Goldsby, D.; Gieré, R. Mineralogical and compositional features of rock fulgurites: A record of lightning effects on granite. Am. Mineral. 2017, 102, 1470–1481. [Google Scholar] [CrossRef]
- Sponholz, B.; Baumhauer, R.; Felix-Henningsen, P. Fulgurites in the southern Central Sahara, Republic of Niger and their palaeoenvironmental significance. Holocene 1993, 3, 97–104. [Google Scholar] [CrossRef]
- Hultgren, R.; Desai, P.D.; Hawkins, D.T.; Gleiser, M.; Kelley, K.K. Selected Values of the Thermodynamic Properties of the Binary Alloys, 1st ed.; American Society for Metals: Metal Parks, OH, USA, 1973; p. 878. [Google Scholar]
- Rietmeijer, F.J.; Nakamura, T.; Tsuchiyama, A.; Uesugi, K.; Nakano, T.; Leroux, H. Origin and formation of iron silicide phases in the aerogel of the Stardust mission. Meteorit. Planet. Sci. 2008, 43, 121–134. [Google Scholar] [CrossRef]
- Nazarov, M.A.; Demidova, S.I.; Anosova, M.O.; Kostitsyn, Y.A.; Ntaflos, T.; Brandstaetter, F. Native silicon and iron silicides in the Dhofar 280 lunar meteorite. Petrology 2012, 20, 506–519. [Google Scholar] [CrossRef]
- Nazarov, M.A.; Shornikov, S.I.; Demidova, S.I. Origin of native silicon and iron silicides in the Dhofar 280 lunar meteorite. Petrology 2015, 23, 168–175. [Google Scholar] [CrossRef]
- Ross, A.J.; Downes, H.; Herrin, J.S.; Mittlefehldt, D.W.; Humayun, M.; Smith, C. The origin of iron silicides in ureilite meteorites. Geochemistry 2019, 79, 125539. [Google Scholar] [CrossRef]
- Spicuzza, M.J.; Valley, J.W.; Fournelle, J.; Huberty, J.M.; Treiman, A. Native silicon and Fe-silicides from the Apollo 16 lunar regolith: Extreme reduction, metal-silicate immiscibility, and shock melting. In Proceedings of the 42nd Lunar and Planetary Science Conference, Woodlands, TX, USA, 7–11 March 2011; p. 2231, No. 1608. [Google Scholar]
- Kubaschewski, O. Iron—Binary Phase Diagrams, 1st ed.; Springer: Dusseldorf, Germany, 1982; pp. 136–139. [Google Scholar]
- Wasserman, A.A.; Melosh, H.J.; Lauretta, D.S. Fulgurites: A look at transient high temperature processes in silicates. In Proceedings of the 33rd Lunar and Planetary Science Conference, Woodlands, TX, USA, 11–15 March 2002; p. 1308. [Google Scholar]
- Schaller, J.; Weiske, A.; Berger, F. Thunderbolt in biogeochemistry: Galvanic effects of lightning as another source for metal remobilization. Sci. Rep. 2013, 3, 3122. [Google Scholar] [CrossRef] [PubMed]
- Rowan, L.R.; Ahrens, T.J. Observations of impact-induced molten metal-silicate partitioning. Earth Planet. Sci. Lett. 1994, 122, 71–88. [Google Scholar] [CrossRef]
- Millot, M.; Dubrovinskaia, N.; Černok, A.; Blaha, S.; Dubrovinsky, L.; Braun, D.G.; Celliers, G.W.; Collins, J.H.; Jeanloz, R. Shock compression of stishovite and melting of silica at planetary interior conditions. Science 2015, 347, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Herrin, J.S.; Mittlefehldt, D.W.; Jones, J.H. Petrogenesis of Fe, Si-metals in Brecciated Ureilites. In Proceedings of the 71st Annual Meeting of the Meteoritical Society, Matsue, Japan, 28 July–1 August 2008; p. 5327. [Google Scholar]
- Smith, C.L.; Ross, A.J.; Downes, H. Iron Silicides in Polymict Ureilites—Recording the Complex History of the Ureilite Parent Body? In Proceedings of the 73rd Annual Meeting of the Meteoritical Society, New York, NY, USA, 26–30 July 2010; p. 5221. [Google Scholar]
- Grossman, L.; Olsen, E.; Lattimer, J.M. Silicon in carbonaceous chondrite metal: Relic of high-temperature condensation. Science 1979, 20, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Lauretta, D.S.; Buseck, P.R.; Zega, T.J. Opaque minerals in the matrix of the Bishunpur (LL3. 1) chondrite: Constraints on the chondrule formation environment. Geochim. Cosmochim. Acta 2001, 65, 1337–1353. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Grossman, L. Chemical condensation sequences in supernova ejecta. Moon Planets 1978, 19, 169–184. [Google Scholar] [CrossRef]
- Ishimaru, S.; Arai, S.; Shukuno, H. Metal-saturated peridotite in the mantle wedge inferred from metal-bearing peridotite xenoliths from Avacha volcano, Kamchatka. Earth Planet. Sci. Lett. 2009, 284, 352–360. [Google Scholar] [CrossRef]
- Shiryaev, A.A.; Griffin, W.L.; Stoyanov, E.; Kagi, H. Natural silicon carbide from different geological settings: Polytypes, trace elements, inclusions. In Proceedings of the 9th International Kimberlite Conference: Extended Abstracts, Frankfurt, Germany, 15 October 2008. No. 9IKC-A-00075. [Google Scholar]
- Wang, C.; Li, X.; Liu, Z.; Li, Y.; Jansa, L.; Dai, J.; Wei, Y. Revision of the Cretaceous–Paleogene stratigraphic framework, facies architecture and provenance of the Xigaze forearc basin along the Yarlung Zangbo suture zone. Gondwana Res. 2012, 22, 415–433. [Google Scholar] [CrossRef]
- Lyakhovich, V.V. Origin of accessory moissanite. Int. Geol. Rev. 1980, 22, 961–970. [Google Scholar] [CrossRef]
- Mathez, E.A.; Fogel, R.A.; Hutcheon, I.D.; Marshintsev, V.K. Carbon isotopic composition and origin of SiC from kimberlites of Yakutia, Russia. Geochim. Cosmochim. Acta 1995, 59, 781–791. [Google Scholar] [CrossRef]
- Di Pierro, S.; Gnos, E.; Grobety, B.H.; Armbruster, T.; Bernasconi, S.M.; Ulmer, P. Rock-forming moissanite (natural α-silicon carbide). Am. Mineral. 2003, 88, 1817–1821. [Google Scholar] [CrossRef]
- Shiryaev, A.A.; Griffin, W.L.; Stoyanov, E. Moissanite (SiC) from kimberlites: Polytypes, trace elements, inclusions and speculations on origin. Lithos 2011, 122, 152–164. [Google Scholar] [CrossRef]
- Zhang, F.; Oganov, A.R. Iron silicides at pressures of the Earth’s inner core. Geophys. Res. Lett. 2010, 37, 1–4. [Google Scholar] [CrossRef]
- Mills, D.B.; Ward, L.M.; Jones, C.; Sweeten, B.; Forth, M.; Treusch, A.H.; Canfield, D.E. Oxygen requirements of the earliest animals. Proc. Natl. Acad. Sci. USA 2014, 111, 4168–4172. [Google Scholar] [CrossRef] [PubMed]
- Hazen, R.M.; Papineau, D.; Bleeker, W.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A.; Yang, H. Mineral evolution. Am. Mineral. 2008, 93, 1693–1720. [Google Scholar] [CrossRef]
- Hazen, R.M.; Morrison, S.M. An evolutionary system of mineralogy. Part I: Stellar mineralogy (>13 to 4.6 Ga). Am. Mineral. 2020, 105, 627–651. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.A.; Lauretta, D.S. Aqueous corrosion of phosphide minerals from iron meteorites: A highly reactive source of prebiotic phosphorus on the surface of the early Earth. Astrobiology 2005, 5, 515–535. [Google Scholar] [CrossRef] [PubMed]
- Bindi, L.; Steinhardt, P.J.; Yao, N.; Lu, P.J. Natural quasicrystals. Science 2009, 324, 1306–1309. [Google Scholar] [CrossRef]
- Bindi, L.; Steinhardt, P.J.; Yao, N.; Lu, P.J. Icosahedrite, Al63Cu24Fe13, the first natural quasicrystal. Am. Mineral. 2011, 96, 928–931. [Google Scholar] [CrossRef]
- Bindi, L.; Kolb, W.; Eby, G.N.; Asimow, P.D.; Wallace, T.C.; Steinhardt, P.J. Accidental synthesis of a previously unknown quasicrystal in the first atomic bomb test. Proc. Natl. Acad. Sci. USA 2021, 118, e2101350118. [Google Scholar] [CrossRef] [PubMed]



| Fulgurite Name | Fulgurite Catalog (Pasek et al. [3]) | Fe-Si Minerals Reported | Reference |
|---|---|---|---|
| Winans Lake Fulgurite, MI | II | FeSi, Fe3Si7, FeTiSi2 | [2] |
| West Virginia Fulgurite, WV | IV | FeSi, FeSi2, FeTiSi2 | [22,35,36] |
| Farmington Fulgurite, CT | II | FeTiSi2, FeSi2, Fe2Al3Si3, Fe10Al27Si23 | [22] |
| York Fulgurite, PA | II, exo | Fe3Si, Fe2Si, Fe5Si3, Fe7Si3, Fe8Si3 | [3] |
| El Rosario Fulgurite, Mexico | II | FeSi2, FeSi, (Fe, Ti)Si2 | [25] |
| Zacatecas Fulgurite, Mexico | II | Fe5Si, Fe3Si, Fe2Si, FeSi | [37] |
| Houghton Lake Fulgurite, MI | II | FeSi, FeSi2, Fe5Si3 | [38] |
| Sahara Fulgurite, Sahara Desert | I | FeSi | [41] |
| Oswego Fulgurite, NY | II, exo | Al4Si3Fe | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, T.; Abbatiello, J.; Omran, A.; Mehta, C.; Pasek, M.A. Iron Silicides in Fulgurites. Minerals 2021, 11, 1394. https://doi.org/10.3390/min11121394
Feng T, Abbatiello J, Omran A, Mehta C, Pasek MA. Iron Silicides in Fulgurites. Minerals. 2021; 11(12):1394. https://doi.org/10.3390/min11121394
Chicago/Turabian StyleFeng, Tian, Joshua Abbatiello, Arthur Omran, Christopher Mehta, and Matthew A. Pasek. 2021. "Iron Silicides in Fulgurites" Minerals 11, no. 12: 1394. https://doi.org/10.3390/min11121394
APA StyleFeng, T., Abbatiello, J., Omran, A., Mehta, C., & Pasek, M. A. (2021). Iron Silicides in Fulgurites. Minerals, 11(12), 1394. https://doi.org/10.3390/min11121394







