Mechanical Properties of Differently Nanostructured and High-Pressure Compressed Hydroxyapatite-Based Materials for Bone Tissue Regeneration
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Hydroxyapatite (HAp) Nanoparticles
2.3. Characterization of HAp Nanoparticles
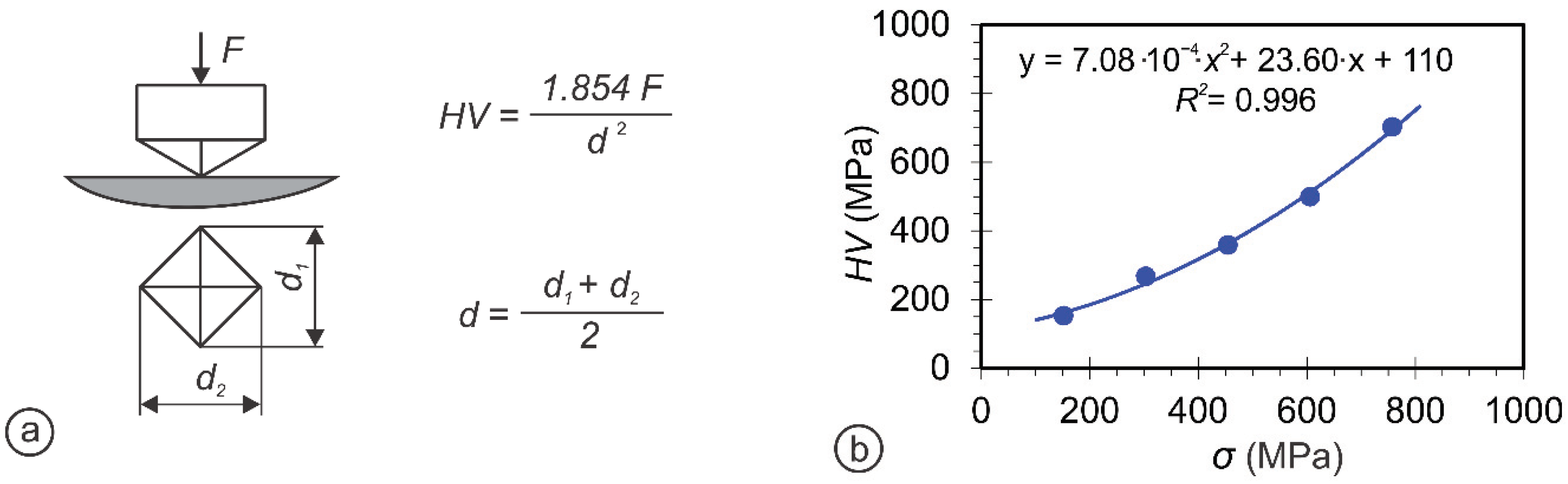
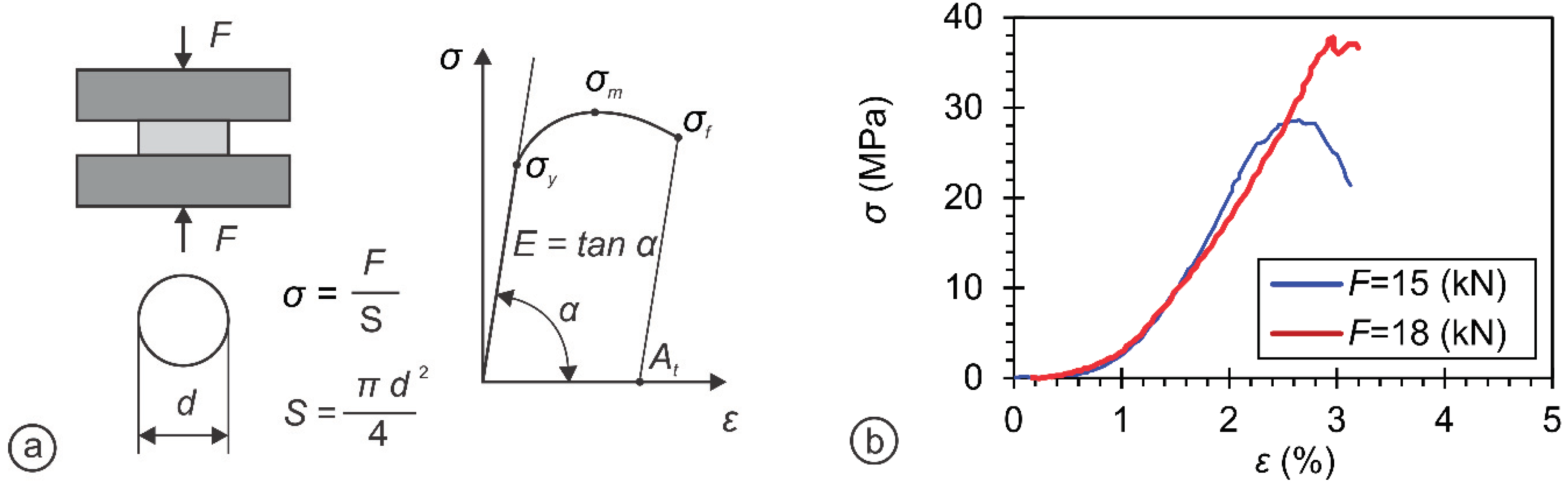
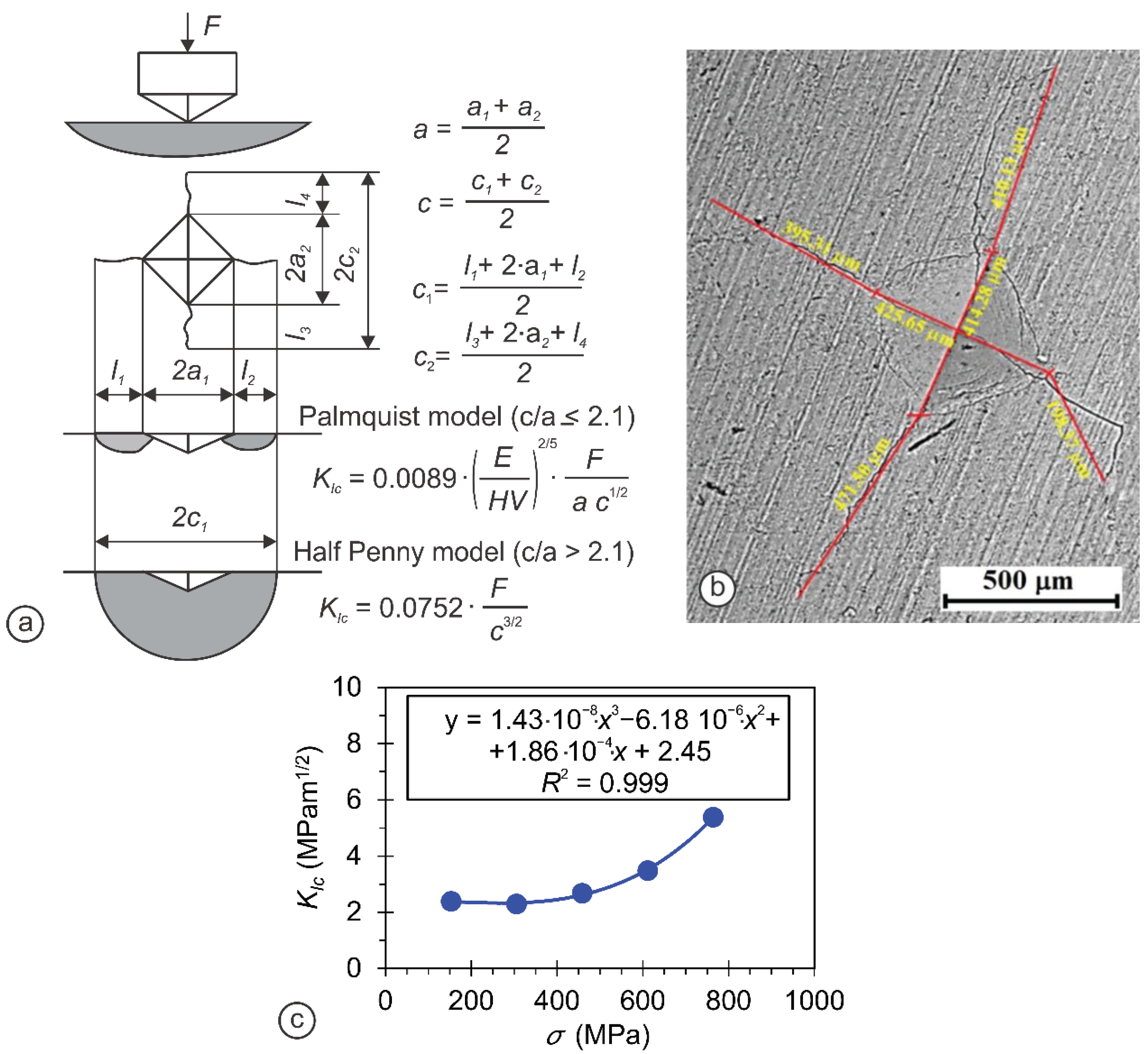
2.4. Preparation and Mechanical Testing of Pellet Materials from HAp Nanoparticles
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Synthesized HAp
3.2. Mechanical Properties of Hap-Based Nanomaterials
3.2.1. Optimization of the Compression Load for Materials Preparation
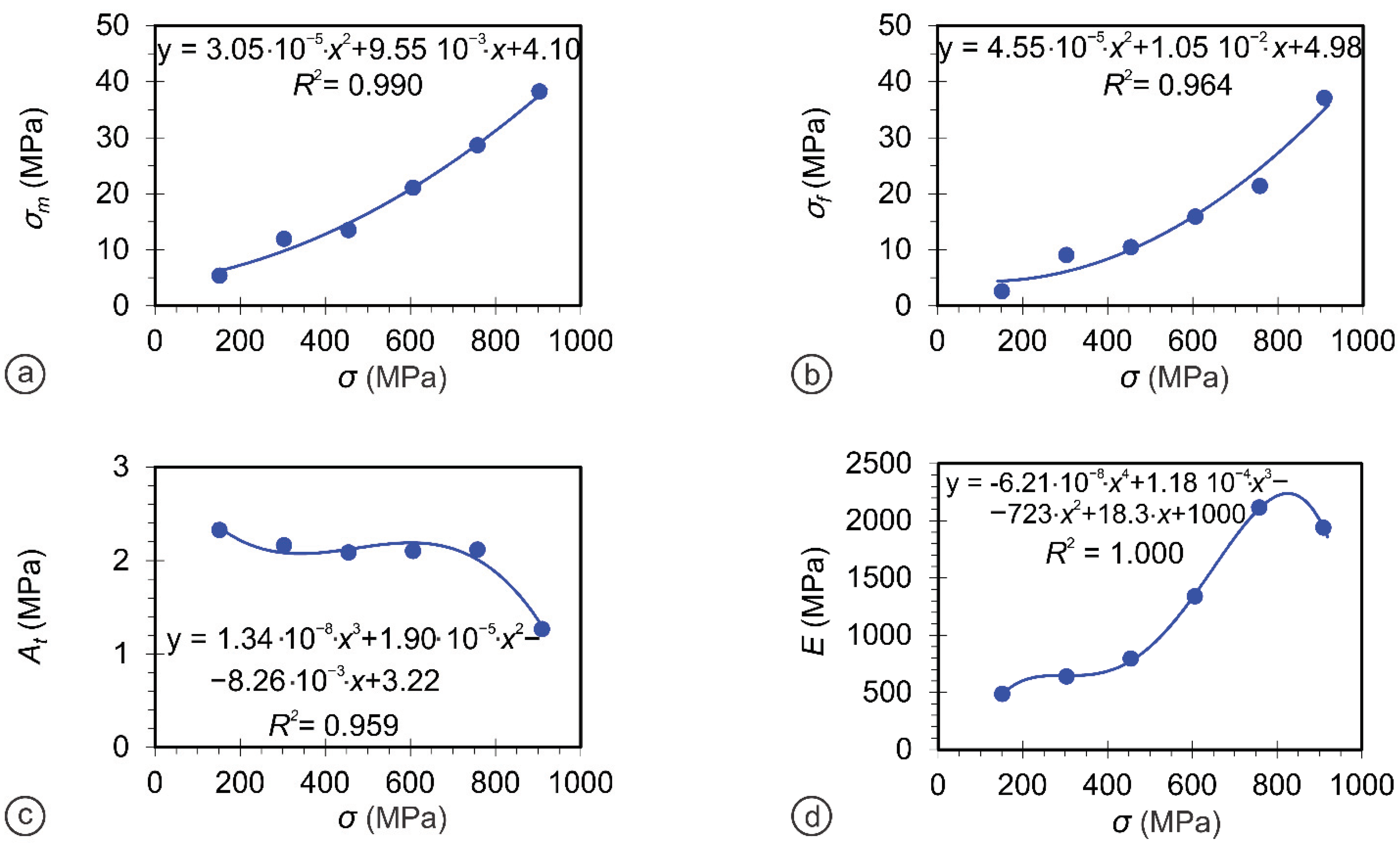
3.2.2. Comparing the Mechanical Properties of Differently Nanostructured Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammadi, M.; Tulliani, J.-M.; Montanaro, L.; Palmero, P. Gelcasting and sintering of hydroxyapatite materials: Effect of particle size and Ca/P ratio on microstructural, mechanical and biological properties. J. Eur. Ceram. Soc. 2021, 41, 7301–7310. [Google Scholar] [CrossRef]
- Hench, L.L. An Introduction to Bioceramics, 2nd ed.; Imperial College Press: London, UK, 2013. [Google Scholar] [CrossRef]
- Koski, C.; Bose, S. Effects of amylose content on the mechanical properties of starch-hydroxyapatite 3D printed bone scaffolds. Addit. Manuf. 2019, 30, 100817. [Google Scholar] [CrossRef]
- Lara-Ochoa, S.; Ortega-Lara, W.; Guerrero-Beltrán, C.E. Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications. Pharmaceutics 2021, 13, 1642. [Google Scholar] [CrossRef] [PubMed]
- Machado, T.R.; Leite, I.; Inada, N.; Li, M.; da Silva, J.; Andrés, J.; Beltrán-Mir, H.; Cordoncillo, E.; Longo, E. Designing biocompatible and multicolor fluorescent hydroxyapatite nanoparticles for cell-imaging applications. Mater. Today Chem. 2019, 14, 100211. [Google Scholar] [CrossRef]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef]
- Mondal, S.; Pal, U. 3D hydroxyapatite scaffold for bone regeneration and local drug delivery applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101131. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Abudhahir, K.M.; Selvamurugan, N.; Vimalraj, S.; Murugesan, R.; Srinivasan, N.; Moorthi, A. Formulation and biological actions of nano-bioglass ceramic particles doped with Calcarea phosphorica for bone tissue engineering. Mater. Sci. Eng. C 2018, 83, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Bhattacharjee, B.N.; Kumar, D.; Rai, S.B.; Parkash, O. Effect of a chelating agent at different pH on the spectroscopic and structural properties of microwave derived hydroxyapatite nanoparticles: A bone mimetic material. New J. Chem. 2016, 40, 5432–5441. [Google Scholar] [CrossRef]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 4, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, J.L. Biomechanical Properties of Bone. In Bone Densitometry and Osteoporosis; Genant, H.K., Guglielmi, G., Jergas, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 143–161. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.; Epari, D.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Fabrication, Properties and Applications of Dense Hydroxyapatite: A Review. J. Funct. Biomater. 2015, 6, 1099–1140. [Google Scholar] [CrossRef] [Green Version]
- Lett, J.A.; Sagadevan, S.; Fatimah, I.; Hoque, E.; Lokanathan, Y.; Léonard, E.; Alshahateet, S.F.; Schirhagl, R.; Oh, W.C. Recent advances in natural polymer-based hydroxyapatite scaffolds: Properties and applications. Eur. Polym. J. 2021, 148, 110360. [Google Scholar] [CrossRef]
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 922. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Samani, S.; Shokrgozar, M.A.; Kundu, S.C.; Reis, R.L.; Fatahi, Y.; Kaplan, D.L. Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 2018, 36, 68–91. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite Based Materials for Bone Tissue Engineering: A Brief and Comprehensive Introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Harrison, C.; Hatton, P.; Gentile, P.; Miller, C. Nanoscale Strontium-Substituted Hydroxyapatite Pastes and Gels for Bone Tissue Regeneration. Nanomaterials 2021, 11, 1611. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lim, J.; Teoh, S.-H. Review: Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2013, 31, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, W.; Schnitzler, V.; Tancret, F.; Bouler, J.-M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef]
- Hendra, H.; Dadan, R.; Djuansjah, J.R.P. Metals for Biomedical Applications. In Biomedical Engineering: From Theory to Applications; BoD: Norderstedt, Germany, 2011. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate-based bioceramics and its clinical applications. In Clinical Applications of Biomaterials. State-of-the-Art Progress, Trends and Novel Approaches; Springer: Berlin/Heidelberg, Germany, 2017; pp. 123–226. [Google Scholar] [CrossRef]
- Wan, C.; Chen, B. Synthesis and characterization of biomimetic hydroxyapatite/sepiolite nanocomposites. Nanoscale 2011, 3, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Cunniffe, G.M.; O’Brien, F.J.; Partap, S.; Levingstone, T.J.; Stanton, K.T.; Dickson, G.R. The synthesis and characterization of nanophase hydroxyapatite using a novel dispersant-aided precipitation method. J. Biomed. Mater. Res. Part A 2010, 95, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Deng, Y.; Feng, P.; Mao, Z.; Li, P.; Yang, B.; Deng, J.; Cao, Y.; Shuai, C.; Peng, S. Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration. Int. J. Mol. Sci. 2014, 15, 4714–4732. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Barabás, R.; Czikó, M.; Dékány, I.; Bizo, L.; Bogya, E.S. Comparative study of particle size analysis of hydroxyapatite-based nanomaterials. Chem. Pap. 2013, 67, 1414–1423. [Google Scholar] [CrossRef]
- El Briak-BenAbdeslam, H.; Mochales, C.; Ginebra, M.P.; Nurit, J.; Planell, J.A.; Boudeville, P. Dry mechanochemical synthesis of hydroxyapatites from dicalcium phosphate dihydrate and calcium oxide: A kinetic study. J. Biomed. Mater. Res. 2003, 67, 927–937. [Google Scholar] [CrossRef]
- Pang, Y.; Bao, X. Influence of temperature, ripening time and calcination on the morphology and crystallinity of hydroxyapatite nanoparticles. J. Eur. Ceram. Soc. 2003, 23, 1697–1704. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.-H.; Zhou, P.-L.; Yang, S.; Yu, X.-B.; Yang, L.-Z. Controllable synthesis of hydroxyapatite nanocrystals via a dendrimer-assisted hydrothermal process. Mater. Res. Bull. 2007, 42, 1611–1618. [Google Scholar] [CrossRef]
- Jevtić, M.; Mitrić, M.; Škapin, S.; Jančar, B.; Ignjatovic, N.; Uskokovic, D. Crystal Structure of Hydroxyapatite Nanorods Synthesized by Sonochemical Homogeneous Precipitation. Cryst. Growth Des. 2008, 8, 2217–2222. [Google Scholar] [CrossRef]
- Rouhani, P.; Taghavinia, N.; Rouhani, S. Rapid growth of hydroxyapatite nanoparticles using ultrasonic irradiation. Ultrason. Sonochem. 2010, 17, 853–856. [Google Scholar] [CrossRef]
- Giardina, M.A.; Fanovich, M.A. Synthesis of nanocrystalline hydroxyapatite from Ca(OH)2 and H3PO4 assisted by ultrasonic irradiation. Ceram. Int. 2010, 36, 1961–1969. [Google Scholar] [CrossRef]
- Kruzic, J.; Kim, D.K.; Koester, K.; Ritchie, R. Indentation techniques for evaluating the fracture toughness of biomaterials and hard tissues. J. Mech. Behav. Biomed. Mater. 2009, 2, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, C. Bulk Properties of Biomaterials and Testing Techniques. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Oxford, UK, 2019; pp. 53–64. [Google Scholar] [CrossRef]
- Ingole, V.H.; Hussein, K.H.; Kashale, A.A.; Gattu, K.; Dhanayat, S.S.; Vinchurkar, A.; Chang, J.-Y.; Ghule, A.V. Invitro Bioactivity and Osteogenic Activity Study of Solid State Synthesized Nano-Hydroxyapatite using Recycled Eggshell Bio-waste. Chemistry 2016, 1, 3901–3908. [Google Scholar] [CrossRef]
- Ingole, V.H.; Hussein, K.H.; Kashale, A.A.; Ghule, K.; Vuherer, T.; Kokol, V.; Chang, J.-Y.; Ling, Y.-C.; Vinchurkar, A.; Dhakal, H.; et al. Ultrasound-assisted green economic synthesis of hydroxyapatite nanoparticles using eggshell biowaste and study of mechanical and biological properties for orthopedic applications. J. Biomed. Mater. Res. Part A 2017, 105, 2935–2947. [Google Scholar] [CrossRef] [Green Version]
- Ćorić, D.; Renjo, M.M.; Ćurković, L. Vickers indentation fracture toughness of Y-TZP dental ceramics. Int. J. Refract. Met. Hard Mater. 2017, 64, 14–19. [Google Scholar] [CrossRef]
- Sakharova, N.; Fernandes, J.V.; Antunes, J.; Oliveira, M. Comparison between Berkovich, Vickers and conical indentation tests: A three-dimensional numerical simulation study. Int. J. Solids Struct. 2009, 46, 1095–1104. [Google Scholar] [CrossRef] [Green Version]
- Moradkhani, A.; Baharvandi, H.; Tajdari, M.; Latifi, H.; Martikainen, J. Determination of fracture toughness using the area of micro-crack tracks left in brittle materials by Vickers indentation test. J. Adv. Ceram. 2013, 2, 87–102. [Google Scholar] [CrossRef]
- Saranya, S.; Justin, S.J.S.; Solomon, R.V.; Wilson, P. l-arginine directed and ultrasonically aided growth of nanocrystalline hydroxyapatite particles with tunable morphology. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 270–279. [Google Scholar] [CrossRef]
- Al Nasim, M.N.; Li, Y.; Wen, M.; Wen, C. A review of high-strength nanolaminates and evaluation of their properties. J. Mater. Sci. Technol. 2020, 50, 215–244. [Google Scholar] [CrossRef]
- Anstis, G.; Chantikul, P.; Lawn, B.; Marshall, D. A Critical Evaluation of Indentation Techniques for Measuring Fracture Toughness: I, Direct Crack Measurements. J. Am. Ceram. Soc. 1981, 64, 533–538. [Google Scholar] [CrossRef]
- Kannan, S.; Lemos, A.F.; Ferreira, J.M.F. Synthesis and Mechanical Performance of Biological-like Hydroxyapatites. Chem. Mater. 2006, 18, 2181–2186. [Google Scholar] [CrossRef]
- Liu, J.; Ye, X.; Wang, H.; Zhu, M.; Wang, B.; Yan, H. The influence of pH and temperature on the morphology of hydroxyapatite synthesized by hydrothermal method. Ceram. Int. 2003, 29, 629–633. [Google Scholar] [CrossRef]
- Poinern, G.E.; Brundavanam, R.K.; Mondinos, N.; Jiang, Z.-T. Synthesis and characterisation of nanohydroxyapatite using an ultrasound assisted method. Ultrason. Sonochemistry 2009, 16, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Rusu, V.M.; Ng, C.-H.; Wilke, M.; Tiersch, B.; Fratzl, P.; Peter, M.G. Size-controlled hydroxyapatite nanoparticles as self-organized organic–inorganic composite materials. Biomaterials 2005, 26, 5414–5426. [Google Scholar] [CrossRef]
- Morales, J.G.; Clemente, R.R.; Armas, B.; Combescure, C.; Berjoan, R.; Cubo, J.; Martínez, E.; Carmona, J.G.; Garelik, S.; Murtra, J.; et al. Controlled Nucleation and Growth of Thin Hydroxyapatite Layers on Titanium Implants by Using Induction Heating Technique. Langmuir 2004, 20, 5174–5178. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Wei, K.; Zhang, S.; Wang, X. Surfactant-assisted synthesis of hydroxyapatite particles. Mater. Lett. 2006, 60, 3227–3231. [Google Scholar] [CrossRef]
- Kaygili, O.; Tatar, C.; Yakuphanoglu, F. Structural and dielectrical properties of Mg3–Ca3(PO4)2 bioceramics obtained from hydroxyapatite by sol–gel method. Ceram. Int. 2012, 38, 5713–5722. [Google Scholar] [CrossRef]
- Tsai, W.; Yang, J.; Lai, C.; Cheng, Y.; Lin, C.; Yeh, C. Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour. Technol. 2006, 97, 488–493. [Google Scholar] [CrossRef]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [Green Version]
- Muralithran, G.; Ramesh, S. The effects of sintering temperature on the properties of hydroxyapatite. Ceram. Int. 2000, 26, 221–230. [Google Scholar] [CrossRef]
- Forero-Sossa, P.; Olvera-Alvarez, I.; Salazar-Martinez, J.; Espinosa-Arbelaez, D.; Segura-Giraldo, B.; Giraldo-Betancur, A. Biogenic hydroxyapatite powders: Effects of source and processing methodologies on physicochemical properties and bioactive response. Mater. Charact. 2021, 173, 110950. [Google Scholar] [CrossRef]
- Ma, D.; Wang, J.; Sun, L. Methodology for measuring fracture toughness of ceramic materials by instrumented indentation test with vickers indenter. J. Am. Ceram. Soc. 2017, 16, 695–2308. [Google Scholar] [CrossRef]
- Huang, J.; Best, S.M. Chapter 1—Ceramic biomaterials for tissue engineering. In Tissue Engineering Using Ceramics and Polymers, 3rd ed.; Boccaccini, A.R., Ma, P.X., Liverani, L., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 3–40. [Google Scholar]
- Arjunan, A.; Baroutaji, A.; Praveen, A.S.; Robinson, J.; Wang, C. Classification of Biomaterial Functionality. In Encyclopedia of Smart Materials; Olabi, A.-G., Ed.; Elsevier: Oxford, UK, 2021; pp. 86–102. [Google Scholar] [CrossRef]
- Ramesh, S.; Tan, C.; Tolouei, R.; Amiriyan, M.; Purbolaksono, J.; Sopyan, I.; Teng, W. Sintering behavior of hydroxyapatite prepared from different routes. Mater. Des. 2012, 34, 148–154. [Google Scholar] [CrossRef]
- Prokopiev, O.; Sevostianov, I. Dependence of the mechanical properties of sintered hydroxyapatite on the sintering temperature. Mater. Sci. Eng. A 2006, 431, 218–227. [Google Scholar] [CrossRef]
- Khamkongkaeo, A.; Boonchuduang, T.; Klysubun, W.; Amonpattaratkit, P.; Chunate, H.-T.; Tuchinda, N.; Pimsawat, A.; Daengsakul, S.; Suksangrat, P.; Sailuam, W.; et al. Sintering behavior and mechanical properties of hydroxyapatite ceramics prepared from Nile Tilapia (Oreochromis niloticus) bone and commercial powder for biomedical applications. Ceram. Int. 2021, 47, 34575–34584. [Google Scholar] [CrossRef]
- Partheniadis, I.; Papanikolaou, T.; Noisternig, M.F.; Griesser, U.J.; Kantiranis, N.; Nikolakakis, I. Structure reinforcement of porous hydroxyapatite pellets using sodium carbonate as sintering aid: Microstructure, secondary phases and mechanical properties. Adv. Powder Technol. 2019, 30, 1642–1654. [Google Scholar] [CrossRef]
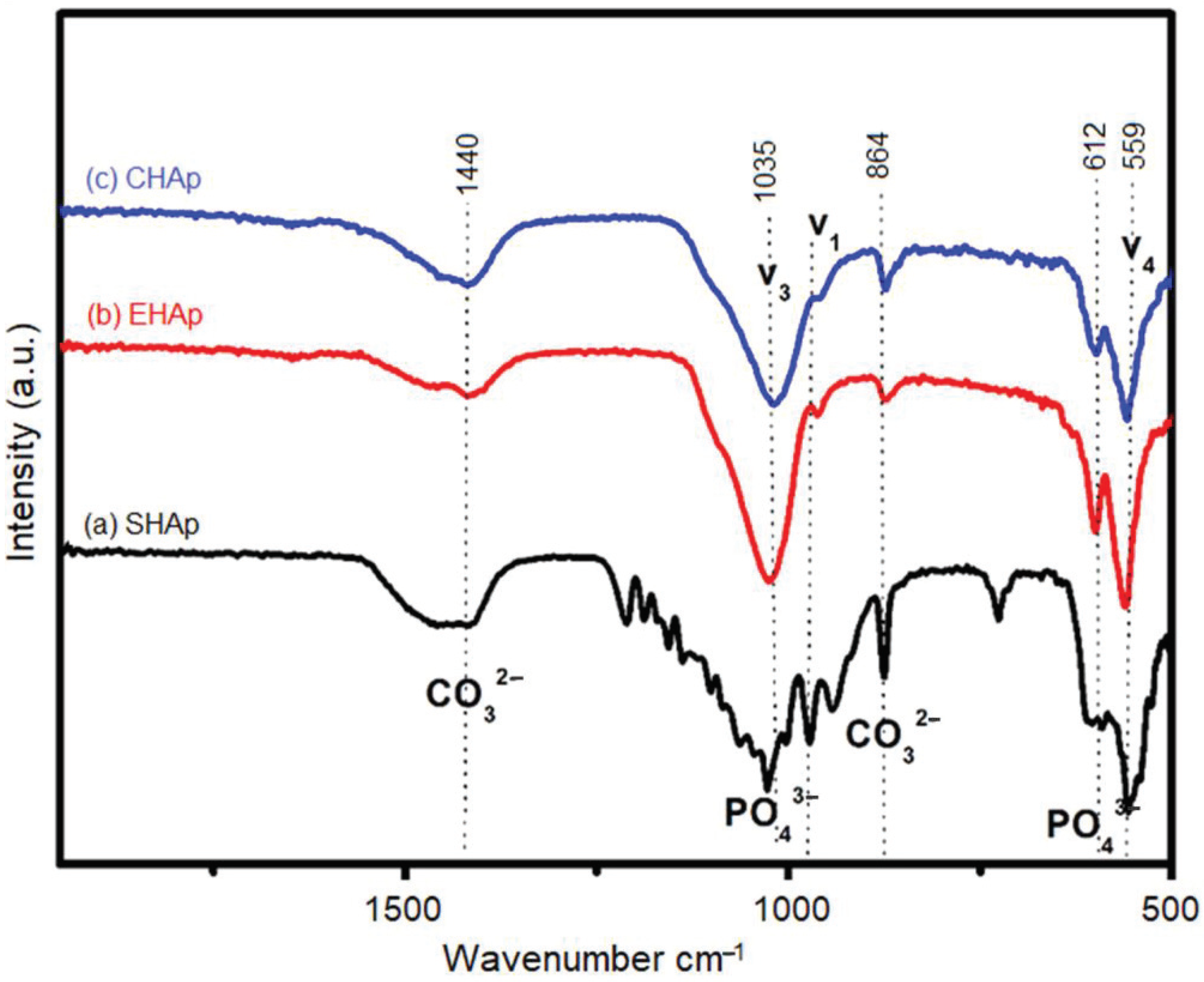

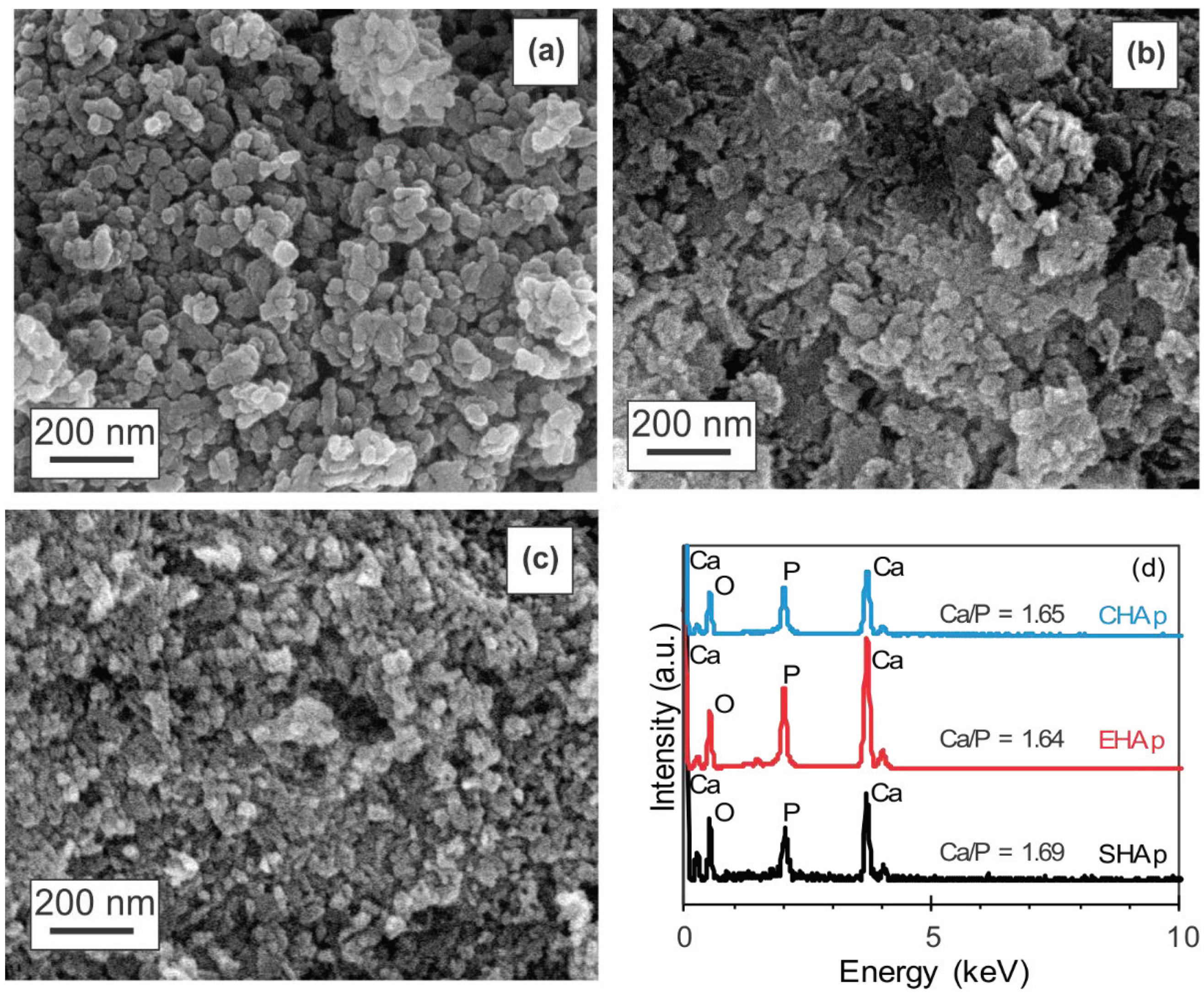
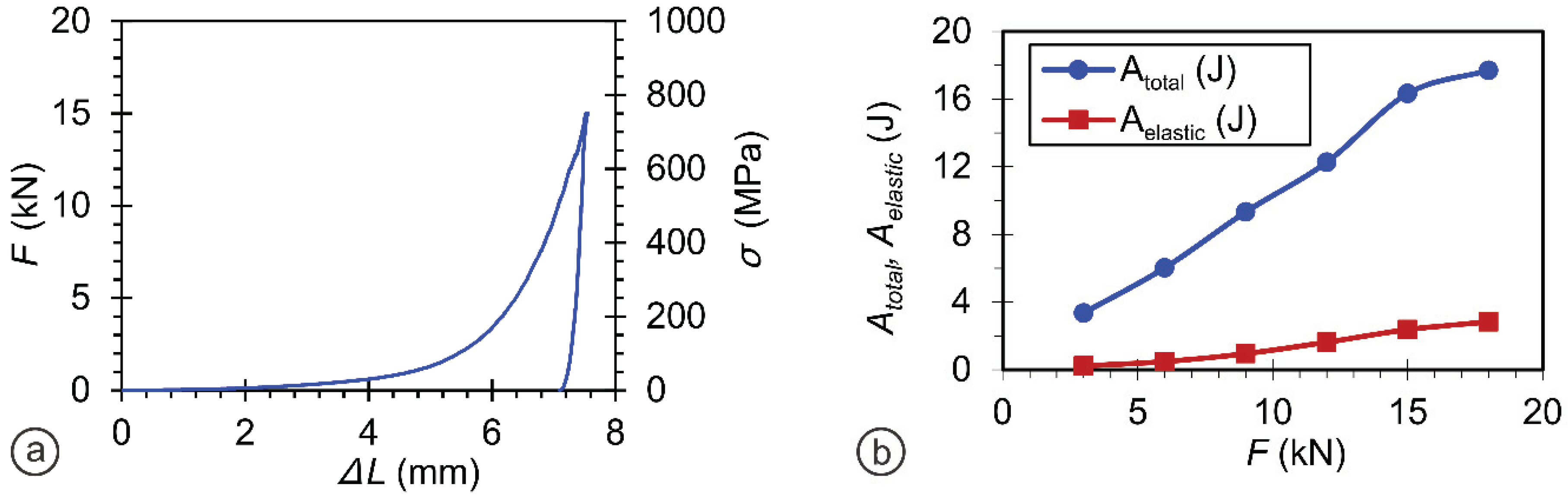
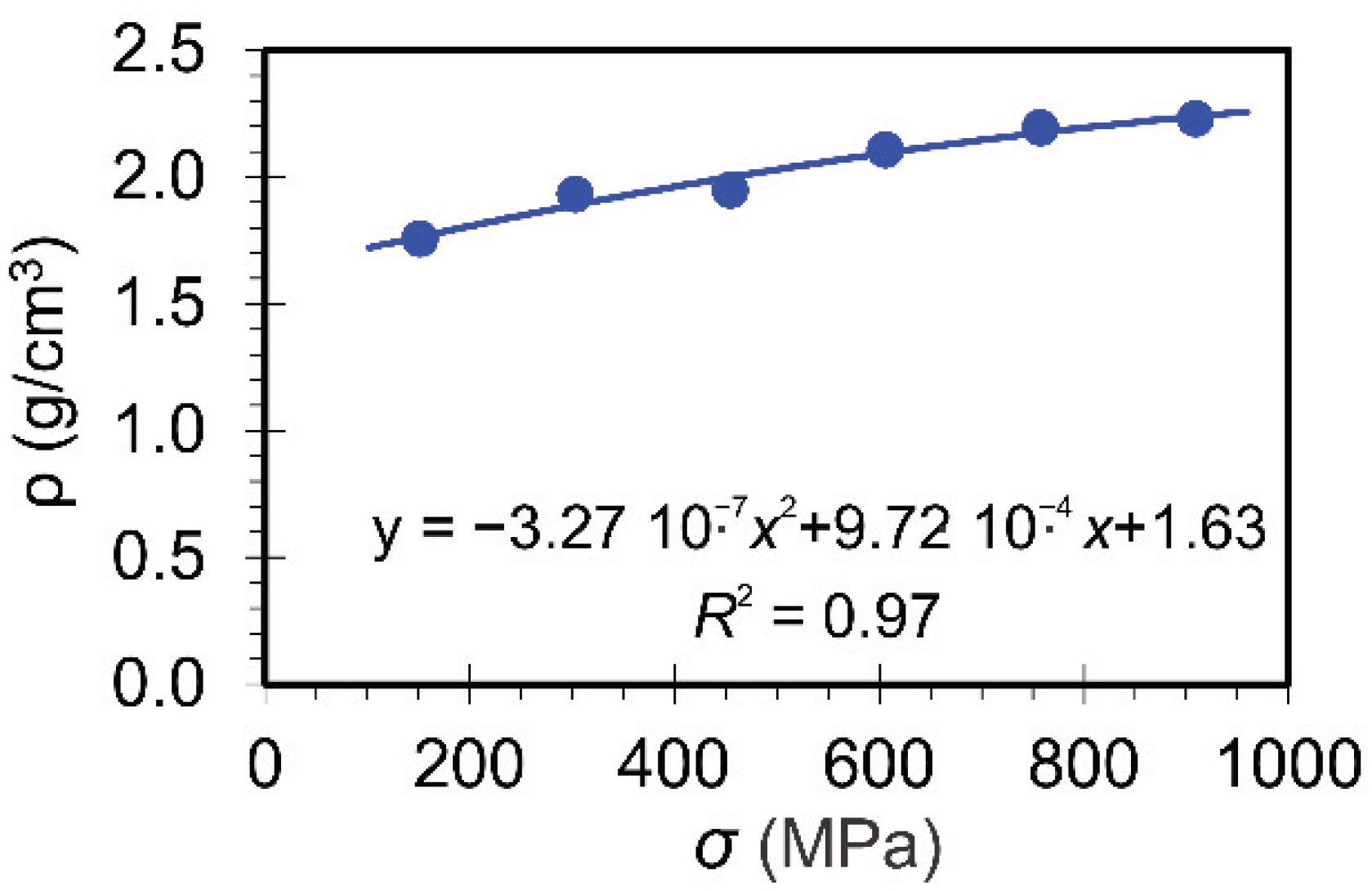

| Sample | Crystal Structure | a (Ǻ) | c (Ǻ) | Crystallite Size (nm) |
|---|---|---|---|---|
| SHAp | Hexagonal | 11.00 | 7.00 | 10.30 |
| EHAp | Hexagonal | 10.33 | 7.11 | 7.90 |
| CHAp | Hexagonal | 9.3 | 7.10 | 5.64 |
| Samples | Preparation | Hardness (GPA) | Fracture Toughness (MPa m1/2) | Compression Strength (MPa) | References |
|---|---|---|---|---|---|
| HAp | Sintering at 1250 °C | 5.8 | - | [62] | |
| HAp | Sintering at 1300 °C | 5.47 | 0.75 | - | [60] |
| HAp | Sintering at 1150 °C | - | - | 4.39 | [63] |
| SHAp | Uniaxial high-pressure compression at an ambient temperature | 7.02 | 5.40 | 28.40 | This study |
| EHAp | 7.12 | 2.27 | 54.20 | This study | |
| CHAp | 6.39 | 6.57 | 47.70 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingole, V.H.; Ghule, S.S.; Vuherer, T.; Kokol, V.; Ghule, A.V. Mechanical Properties of Differently Nanostructured and High-Pressure Compressed Hydroxyapatite-Based Materials for Bone Tissue Regeneration. Minerals 2021, 11, 1390. https://doi.org/10.3390/min11121390
Ingole VH, Ghule SS, Vuherer T, Kokol V, Ghule AV. Mechanical Properties of Differently Nanostructured and High-Pressure Compressed Hydroxyapatite-Based Materials for Bone Tissue Regeneration. Minerals. 2021; 11(12):1390. https://doi.org/10.3390/min11121390
Chicago/Turabian StyleIngole, Vijay H., Shubham S. Ghule, Tomaž Vuherer, Vanja Kokol, and Anil V. Ghule. 2021. "Mechanical Properties of Differently Nanostructured and High-Pressure Compressed Hydroxyapatite-Based Materials for Bone Tissue Regeneration" Minerals 11, no. 12: 1390. https://doi.org/10.3390/min11121390
APA StyleIngole, V. H., Ghule, S. S., Vuherer, T., Kokol, V., & Ghule, A. V. (2021). Mechanical Properties of Differently Nanostructured and High-Pressure Compressed Hydroxyapatite-Based Materials for Bone Tissue Regeneration. Minerals, 11(12), 1390. https://doi.org/10.3390/min11121390








