Preparation of a Highly Porous Clay-Based Absorbent for Hazard Spillage Mitigation via Two-Step Expansion of Vermiculite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of eVMT Absorbents
2.3. Material Characterizations
2.4. Absorptive Removal Performance against Liquid Hazards
2.5. Fabrication and Demonstration of a Prototype Absorbent Assembly
3. Results and Discussion
3.1. Expansion Behavior of Palabora Vermiculite
3.2. Characterization of Material Properties
3.3. Hazardous Liquid-Uptake Performances
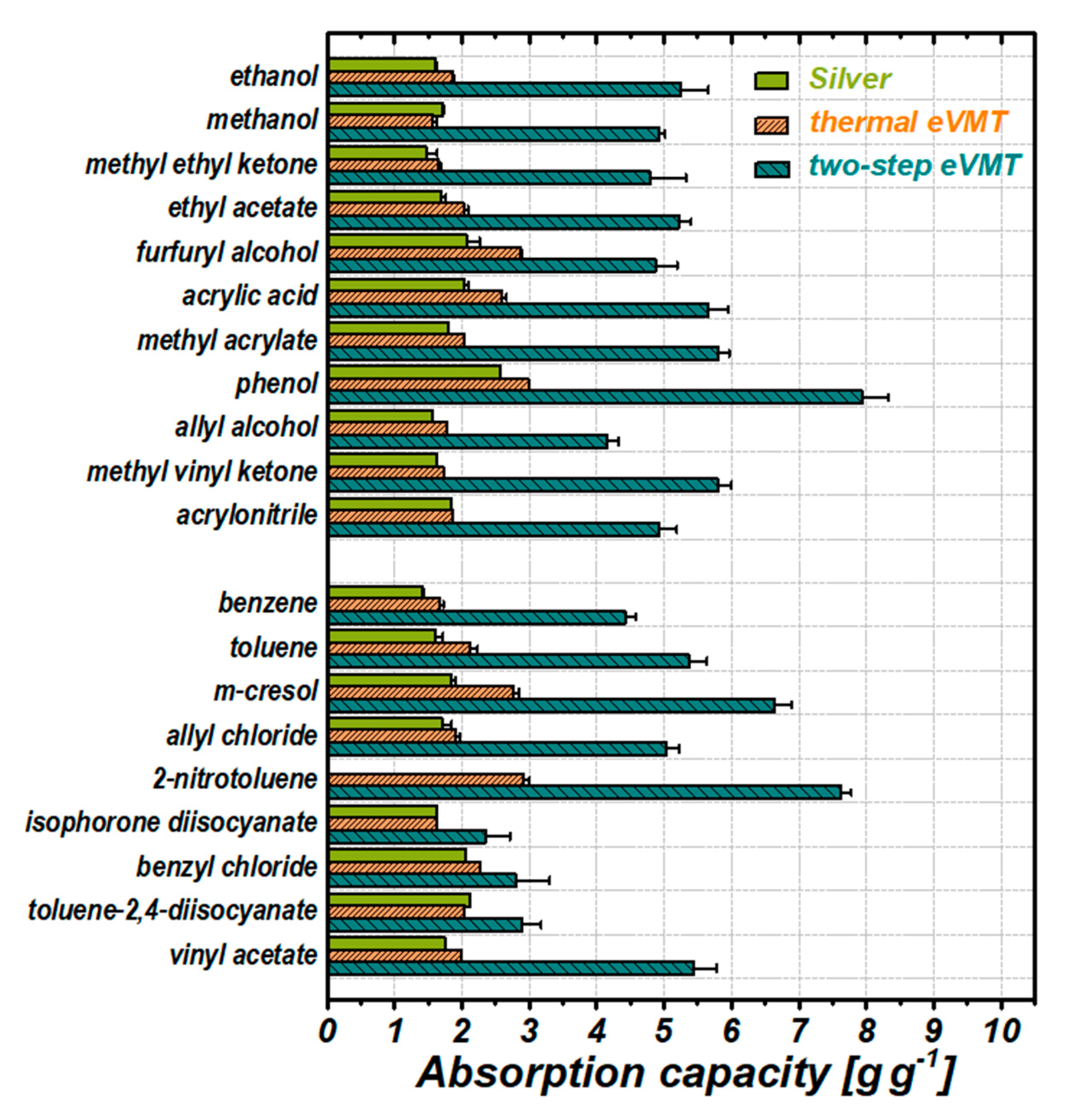
3.3.1. Absorption Capacity
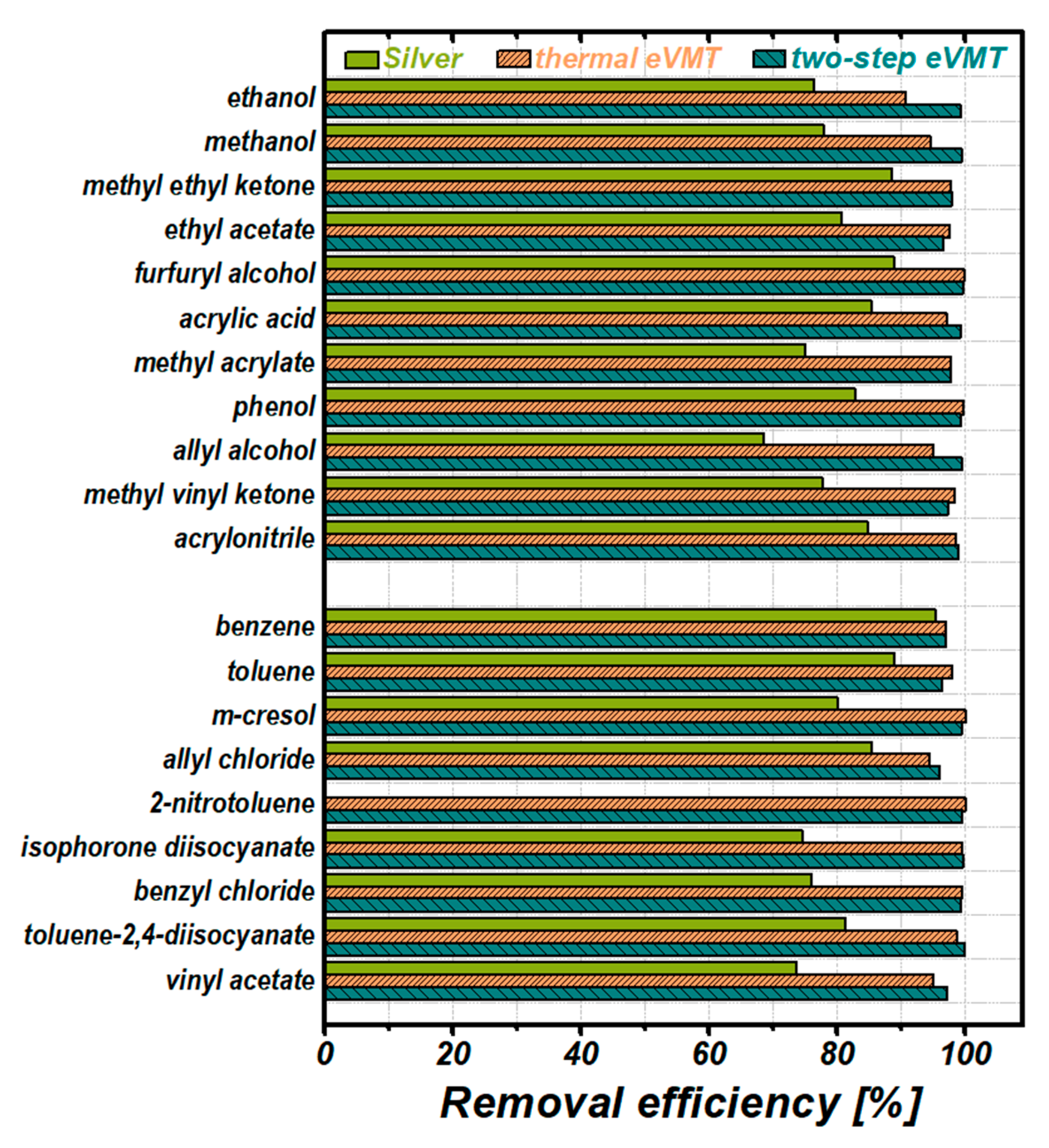
3.3.2. Removal Efficiency
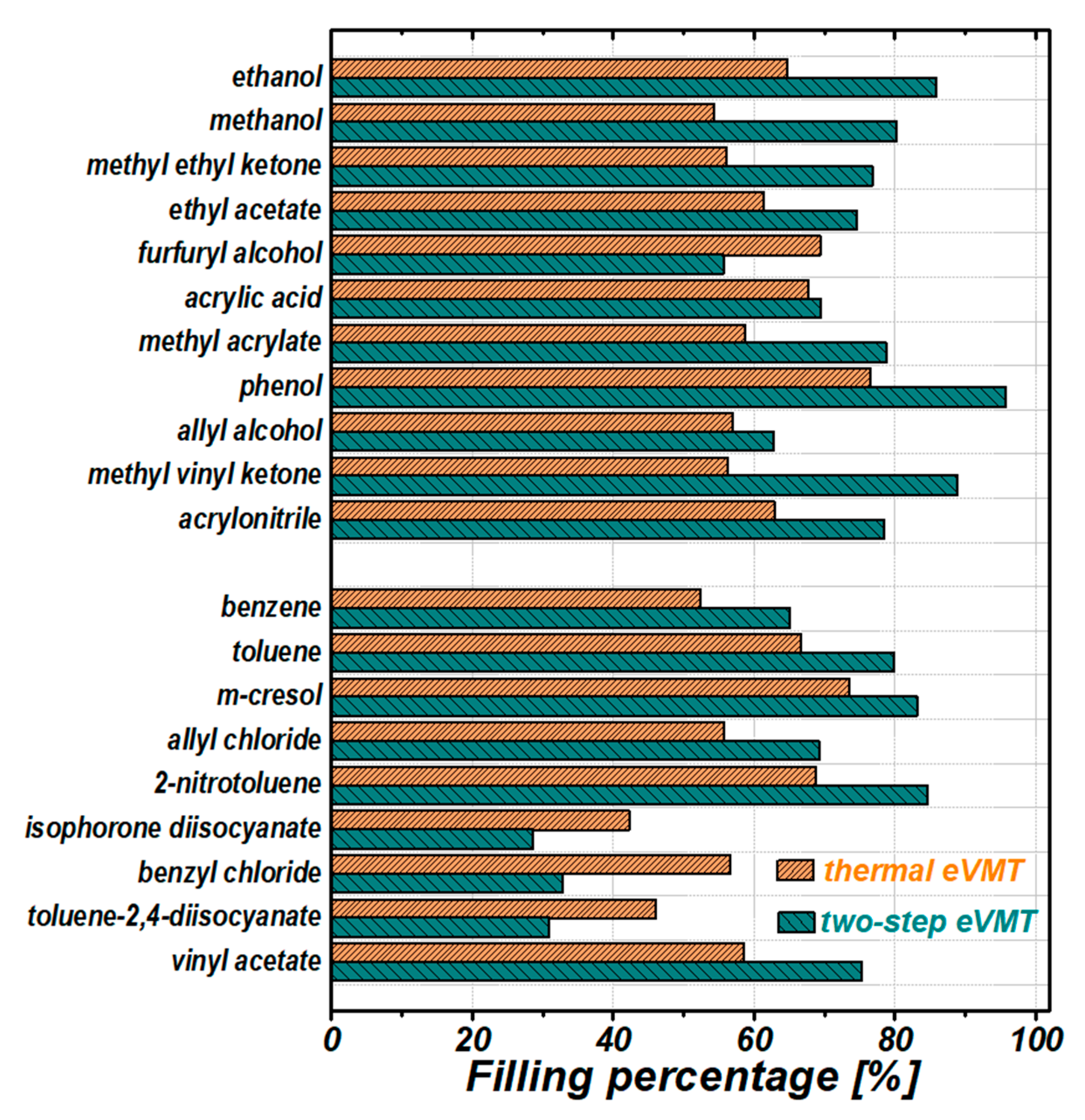
3.3.3. Analysis of the Absorption Performance
3.4. Hazard Mitigation Using the Prototype Absorbent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Ping, H.; Ma, Z.-H.; Pan, L.-G. Statistical analysis of sudden chemical leakage accidents reported in China between 2006 and 2011. Environ. Sci. Pollut. Res. 2014, 21, 5547–5553. [Google Scholar] [CrossRef]
- Oliveira, L.M.; Saleem, J.; Bazargan, A.; Duarte, J.L.d.S.; McKay, G.; Meili, L. Sorption as a rapidly response for oil spill accidents: A material and mechanistic approach. J. Hazard. Mater. 2020, 407, 124842. [Google Scholar] [CrossRef]
- Bandura, L.; Woszuk, A.; Kołodyńska, D.; Franus, W. Application of mineral sorbents for removal of petroleum substances: A review. Minerals 2017, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Hoang, A.T.; Nguyen, X.P.; Duong, X.Q.; Huynh, T.T. Sorbent-based devices for the removal of spilled oil from water: A review. Environ. Sci. Pollut. Res. 2021, 28, 28876–28910. [Google Scholar] [CrossRef]
- Melvold, R.; Gibson, S. A guidance manual for selection and use of sorbents for liquid hazardous substance releases. J. Hazard. Mater. 1988, 17, 329–335. [Google Scholar] [CrossRef]
- Bastani, D.; Safekordi, A.; Alihosseini, A.; Taghikhani, V. Study of oil sorption by expanded perlite at 298.15 K. Sep. Purif. Technol. 2006, 52, 295–300. [Google Scholar] [CrossRef]
- Cuong, N.D.; Hue, V.T.; Kim, Y.S. Thermally expanded vermiculite as a risk-free and general-purpose sorbent for hazardous chemical spillages. Clay Miner. 2019, 54, 235–243. [Google Scholar] [CrossRef]
- Radetic, M.; Ilic, V.; Radojevic, D.; Miladinovic, R.; Jocic, D.; Jovancic, P. Efficiency of recycled wool-based nonwoven material for the removal of oils from water. Chemosphere 2008, 70, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Xie, X.; Yin, K.; Zhou, Y.; Wan, S.; He, L.; Xu, F.; Banhart, F.; Sun, L.; Ruoff, R.S. Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv. Funct. Mater. 2012, 22, 4421–4425. [Google Scholar] [CrossRef]
- Ren, R.-P.; Li, W.; Lv, Y.-K. A robust, superhydrophobic graphene aerogel as a recyclable sorbent for oils and organic solvents at various temperatures. J. Colloid Interface Sci. 2017, 500, 63–68. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Li, C.; Liang, H.-W.; Zhang, Y.-N.; Wang, X.; Chen, J.-F.; Yu, S.-H. Carbon nanofiber aerogels for emergent cleanup of oil spillage and chemical leakage under harsh conditions. Sci. Rep. 2014, 4, 4079. [Google Scholar] [CrossRef]
- Davoodi, S.M.; Taheran, M.; Brar, S.K.; Galvez-Cloutier, R.; Martel, R. Hydrophobic dolomite sorbent for oil spill clean-ups: Kinetic modeling and isotherm study. Fuel 2019, 251, 57–72. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, M.; Liu, C.; Fu, L.; Lv, Y.; Feng, Y.; Huang, P.; Yang, F.; Song, P.; Liu, M. Lightweight, amphipathic and fire-resistant prGO/MXene spherical beads for rapid elimination of hazardous chemicals. J. Hazard. Mater. 2022, 423, 127069. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Jahanban-Esfahlan, R.; Tabibiazar, M.; Roufegarinejad, L.; Amarowicz, R. Recent advances in the use of walnut (Juglans regia L.) shell as a valuable plant-based bio-sorbent for the removal of hazardous materials. RSC Adv. 2020, 10, 7026–7047. [Google Scholar] [CrossRef] [Green Version]
- Ion, I.; Bogdan, D.; Mincu, M.M.; Ion, A.C. Modified exfoliated carbon nanoplatelets as sorbents for ammonium from natural mineral waters. Molecules 2021, 26, 3541. [Google Scholar] [CrossRef] [PubMed]
- Herrick, E.C.; Carstea, D.; Goldgraben, G. Sorbent Materials for Cleanup of Hazardous Spills; Environmental Protection Agency: Cincinnati, OH, USA, 1982; pp. 25–44.
- Duman, O.; Tunç, S.; Polat, T.G. Determination of adsorptive properties of expanded vermiculite for the removal of CI Basic Red 9 from aqueous solution: Kinetic, isotherm and thermodynamic studies. Appl. Clay Sci. 2015, 109, 22–32. [Google Scholar] [CrossRef]
- Marcos, C.; Rodríguez, I. Thermoexfoliated commercial vermiculites for Ni2+ removal. Appl. Clay Sci. 2016, 132–133, 685–693. [Google Scholar] [CrossRef]
- Da Silva, U.G.; Melo, M.A.D.F.; da Silva, A.F.; de Farias, R.F. Adsorption of crude oil on anhydrous and hydrophobized vermiculite. J. Colloid Interface Sci. 2003, 260, 302–304. [Google Scholar] [CrossRef]
- Purceno, A.D.; Barrioni, B.R.; Dias, A.; da Costa, G.M.; Lago, R.M.; Moura, F.C. Carbon nanostructures-modified expanded vermiculites produced by chemical vapor deposition from ethanol. Appl. Clay Sci. 2011, 54, 15–19. [Google Scholar] [CrossRef]
- Machado, L.C.R.; Lima, F.W.J.; Paniago, R.; Ardisson, J.D.; Sapag, K.; Lago, R.M. Polymer coated vermiculite–iron composites: Novel floatable magnetic adsorbents for water spilled contaminants. Appl. Clay Sci. 2006, 31, 207–215. [Google Scholar] [CrossRef]
- Marcos, C.; Arango, Y.C.; Rodriguez, I. X-ray diffraction studies of the thermal behaviour of commercial vermiculites. Appl. Clay Sci. 2009, 42, 368–378. [Google Scholar] [CrossRef]
- El Mouzdahir, Y.; Elmchaouri, A.; Mahboub, R.; Gil, A.; Korili, S. Synthesis of nano-layered vermiculite of low density by thermal treatment. Powder Technol. 2009, 189, 2–5. [Google Scholar] [CrossRef]
- Marcos, C.; Rodríguez, I. Expansion behaviour of commercial vermiculites at 1000 °C. Appl. Clay Sci. 2010, 48, 492–498. [Google Scholar] [CrossRef]
- Udoudo, O.; Folorunso, O.; Dodds, C.; Kingman, S.; Ure, A. Understanding the performance of a pilot vermiculite exfoliation system through process mineralogy. Miner. Eng. 2015, 82, 84–91. [Google Scholar] [CrossRef]
- Obut, A.; Girgin, I.; Yörükoǵlu, A. Microwave exfoliation of vermiculite and phlogopite. Clays Clay Miner. 2003, 51, 452–456. [Google Scholar] [CrossRef]
- Folorunso, O.; Dodds, C.; Dimitrakis, G.; Kingman, S. Continuous energy efficient exfoliation of vermiculite through microwave heating. Int. J. Miner. Process. 2012, 114–117, 69–79. [Google Scholar] [CrossRef]
- Obut, A.; Girgin, İ. Hydrogen peroxide exfoliation of vermiculite and phlogopite. Miner. Eng. 2002, 15, 683–687. [Google Scholar] [CrossRef]
- Üçgül, E.; Girgin, İ. Chemical exfoliation characteristics of Karakoc phlogopite in hydrogen peroxide solution. Turk. J. Chem. 2002, 26, 431–440. [Google Scholar]
- Marcos, C.; Rodríguez, I. Exfoliation of vermiculites with chemical treatment using hydrogen peroxide and thermal treatment using microwaves. Appl. Clay Sci. 2014, 87, 219–227. [Google Scholar] [CrossRef]
- Weiss, Z.; Valaskova, M.; Seidlerova, J.; Supova-Kristkova, M.; Sustai, O.; Matejka, V.; Capkova, P. Preparation of vermiculite nanoparticles using thermal hydrogen peroxide treatment. J. Nanosci. Nanotechnol. 2006, 6, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.C.; Bui, T.T.; Cho, Y.B.; Kim, Y.S. Highly hydrophobic polydimethylsiloxane-coated expanded vermiculite sorbents for selective oil removal from water. Nanomaterials 2021, 11, 367. [Google Scholar] [CrossRef]
- Justo, A.; Maqueda, C.; Pérez Rodriguez, J.L.; Morillo, E. Expansibility of some vermiculites. Appl. Clay Sci. 1989, 4, 509–519. [Google Scholar] [CrossRef]
- Muiambo, H.F.; Focke, W.W.; Atanasova, M.; Benhamida, A. Characterization of urea-modified Palabora vermiculite. Appl. Clay Sci. 2015, 105–106, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Wada, N.; Hines, D.; Whittingham, M. Hydration states and phase transitions in vermiculite intercalation compounds. Phys. Rev. B 1987, 36, 2844. [Google Scholar] [CrossRef] [PubMed]
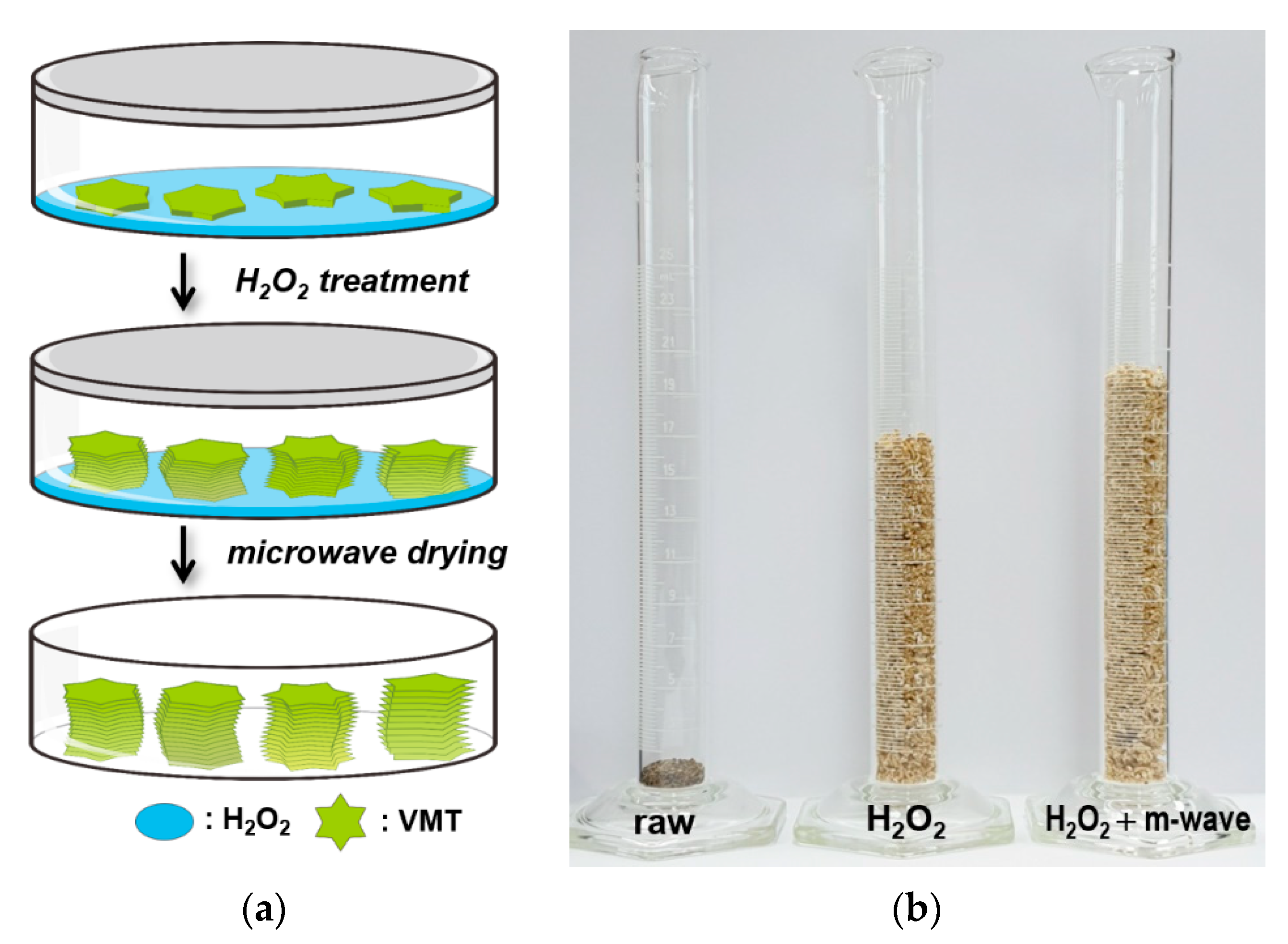

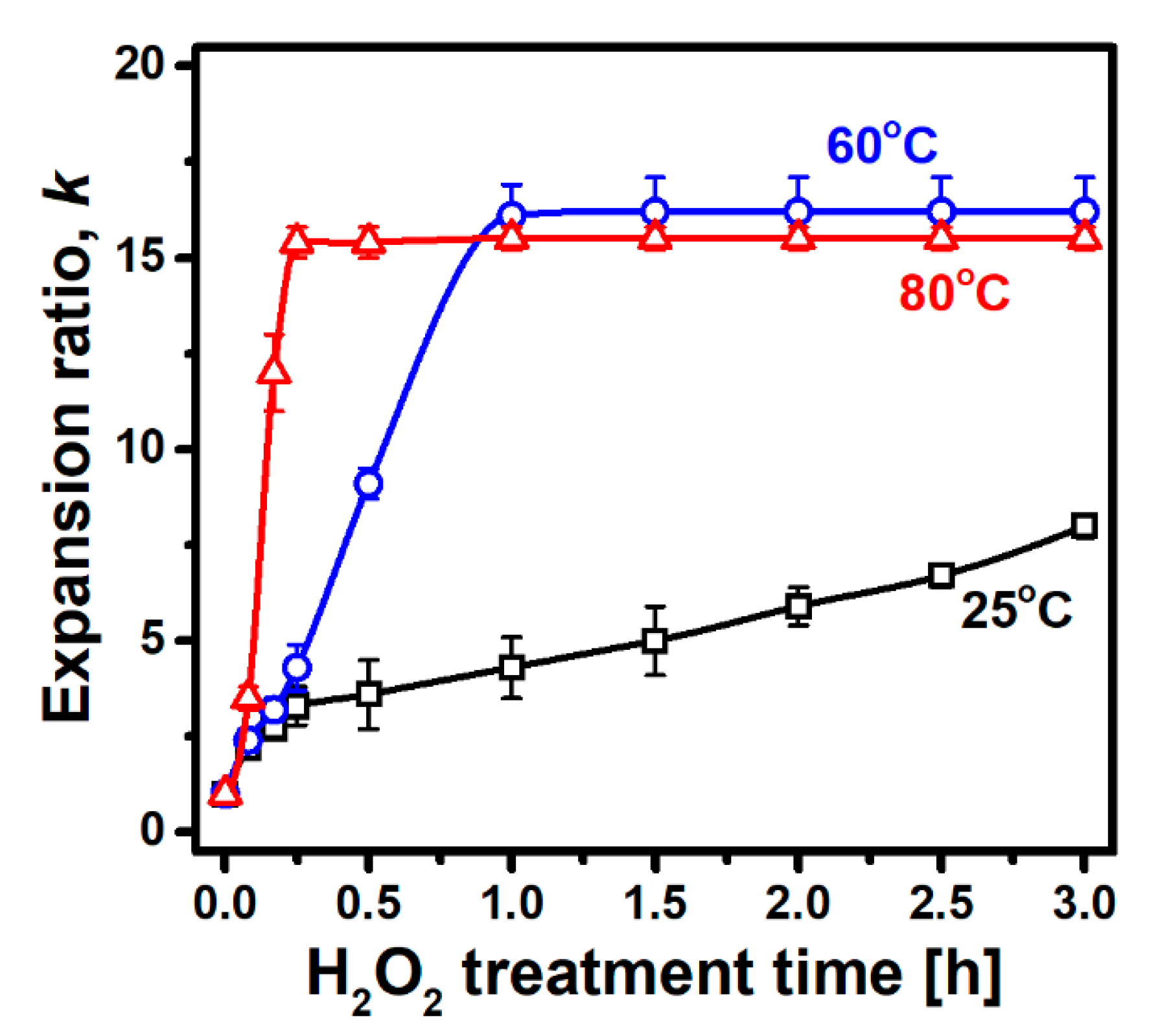
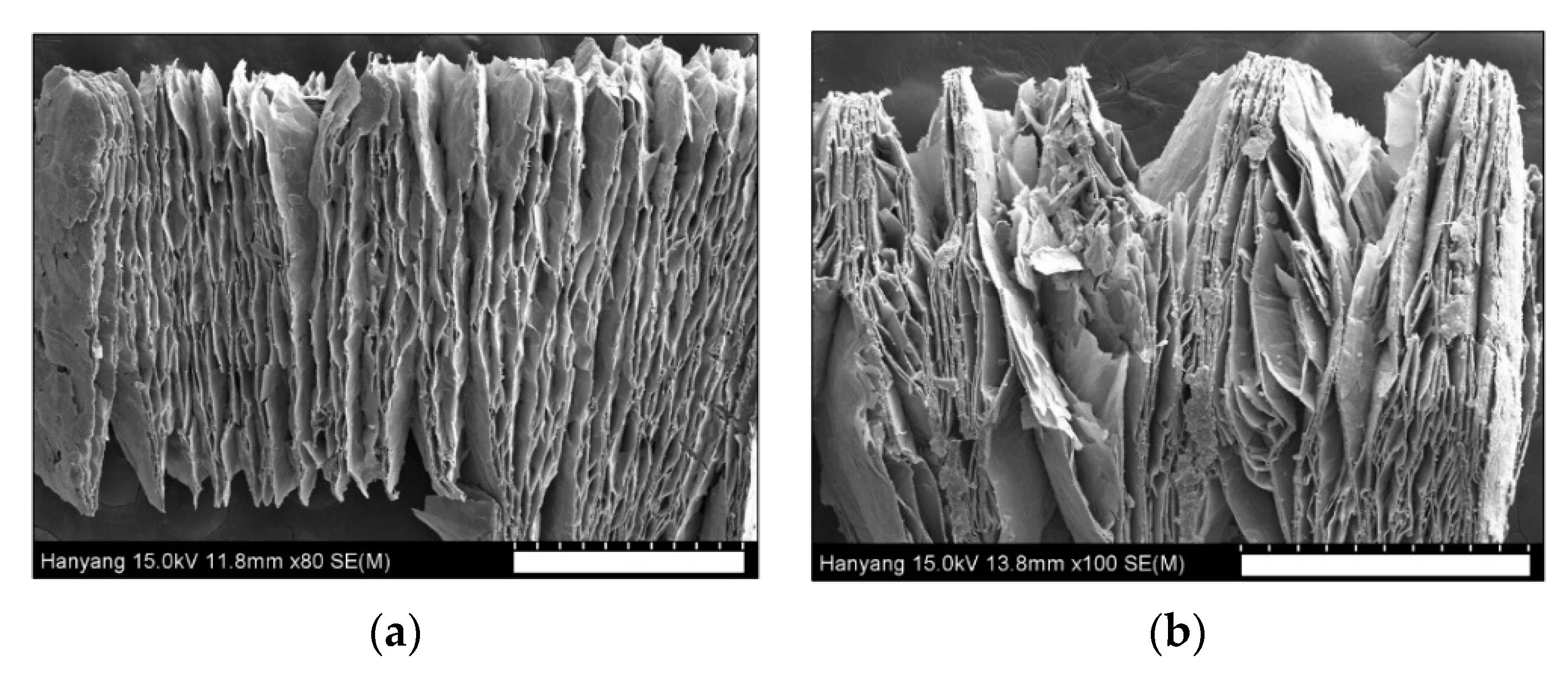
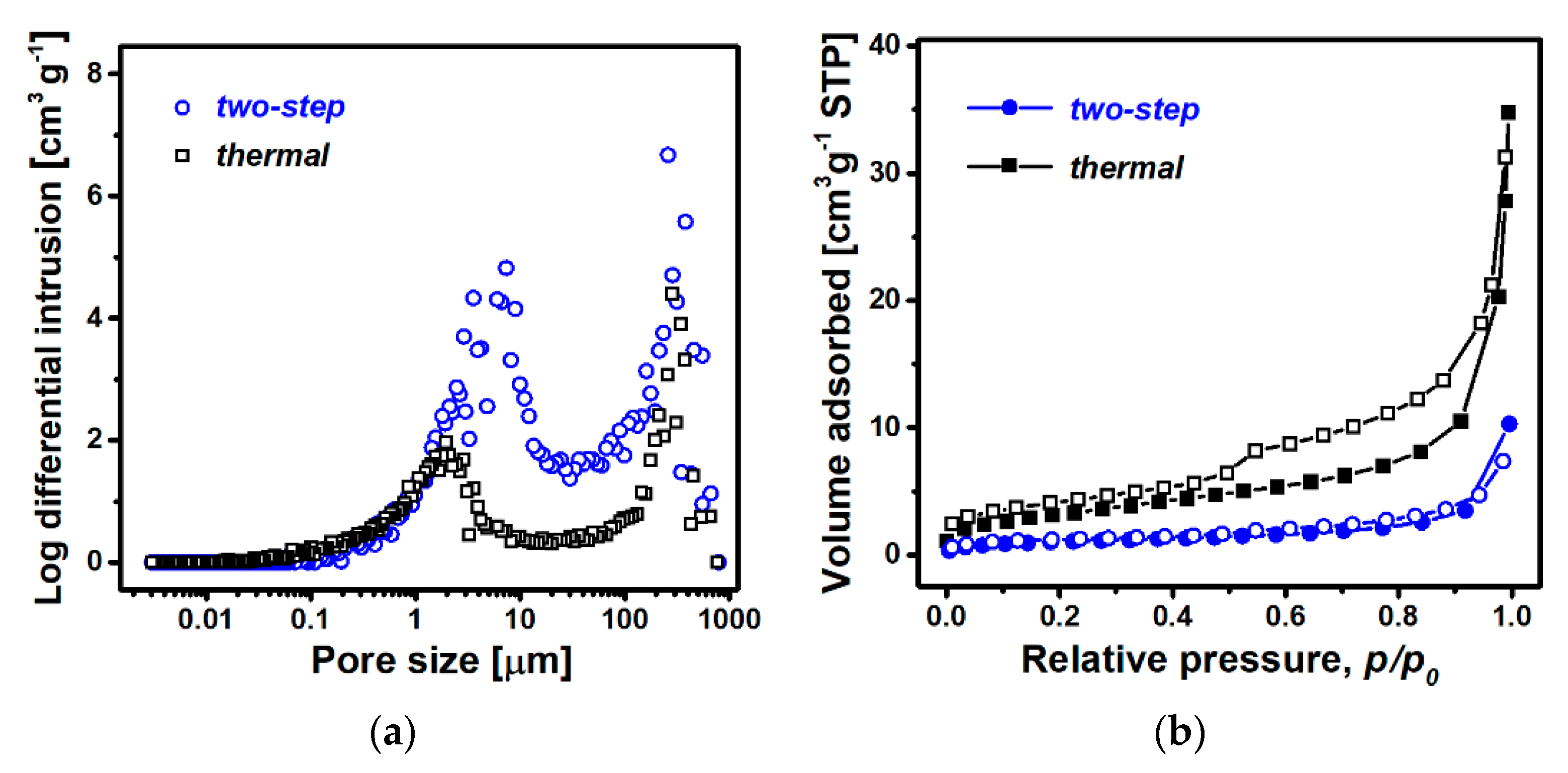
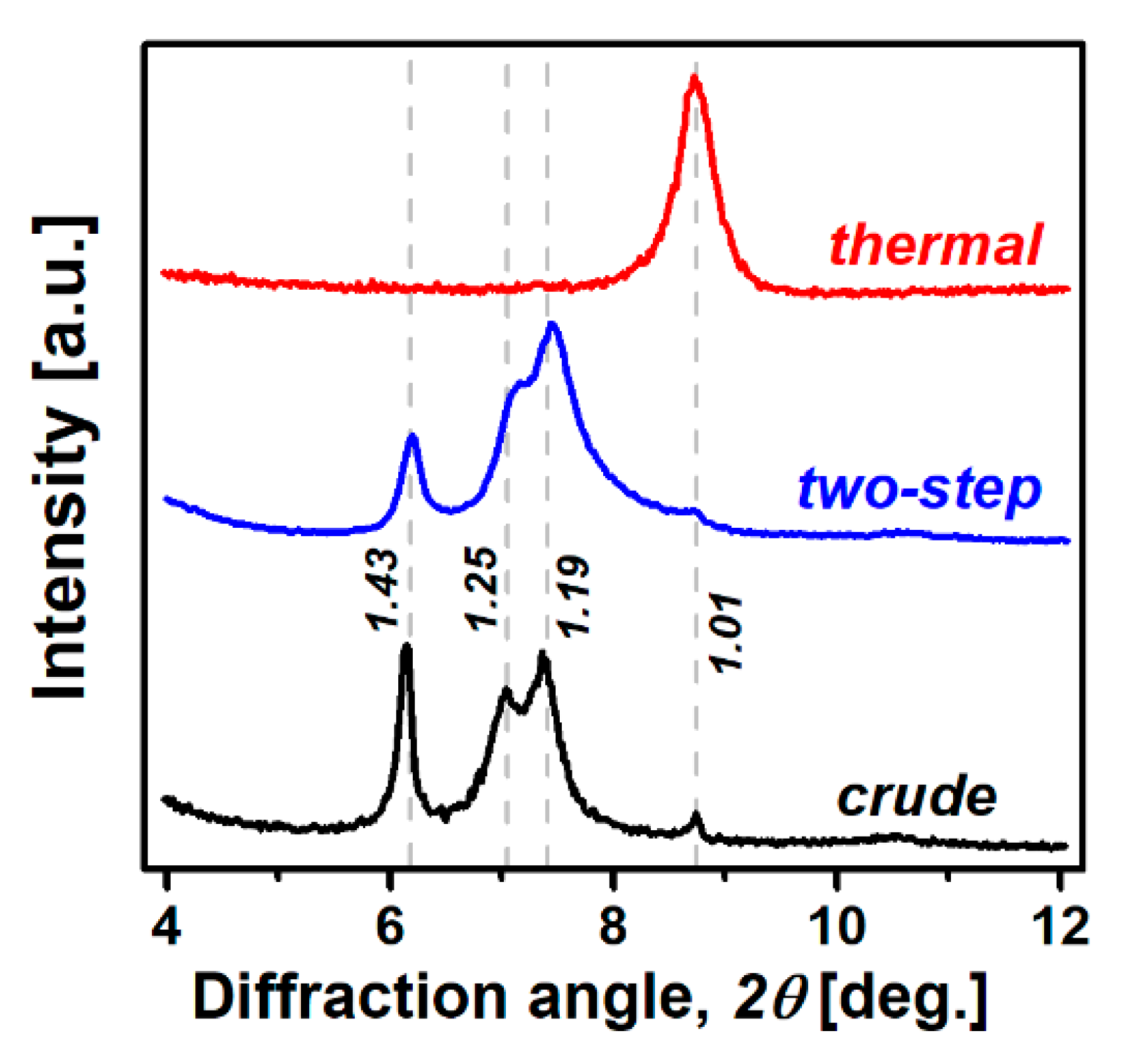
| Sample Preparation | Planar Size a [mm] | Thickness b [mm] | Apparent Density [g cm−3] | Expansion Ratio, k |
|---|---|---|---|---|
| Two-step method | 0.48 | 0.11 | 0.050 | 20 |
| Thermal heating | 0.84 | 0.15 | 0.151 | 6.6 |
| Commercial Silver | 1.32 | - | 0.167 | 6.0 b |
| SiO2 | MgO | Al2O3 | K2O | FeO | TiO2 | CaO | Na2O | MnO | NiO | Cr2O3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content [wt%] | 42.1 | 17.4 | 11.0 | 8.6 | 15.6 | 2.2 | 2.4 | 0.33 | 0.13 | 0.06 | 0.08 |
| Analysis Method | Properties | Two-Step | Heating |
|---|---|---|---|
| Hg porosimetry | Total pore volume [cm3 g−1] | 7.75 | 3.65 |
| Total pore area [m2 g−1] | 8.38 | 13.0 | |
| N2 sorption | Total pore volume [cm3 g−1] | 0.015 | 0.046 |
| BET surface area [m2 g−1] | 3.57 | 11.8 |
| Chemicals | Uptake Performance | No. of Mitigation Trial | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Ethanol | absorption capacity [g g−1] | 6.35 | 5.69 | 5.12 | 4.50 | 4.50 | 3.43 | 2.82 |
| removal efficiency a [%] | 18.3 | 33.3 | 50.0 | 63.3 | 78.3 | 88.3 | 97.0 | |
| Methanol | absorption capacity [g g−1] | 5.94 | 5.37 | 5.40 | 4.98 | 4.88 | 3.55 | 1.92 |
| removal efficiency a [%] | 16.7 | 33.3 | 50.0 | 66.7 | 83.3 | 91.7 | 98.3 | |
| Acrylonitrile | absorption capacity [g g−1] | 6.55 | 6.12 | 6.05 | 5.82 | 5.12 | 4.21 | 0.43 |
| removal efficiency a [%] | 16.7 | 33.3 | 50.0 | 66.7 | 83.3 | 98.3 | 99.6 | |
| Toluene | absorption capacity [g g−1] | 7.19 | 6.87 | 6.61 | 6.59 | 4.86 | 4.07 | |
| removal efficiency a [%] | 20.0 | 36.7 | 63.3 | 75.0 | 90.0 | 98.3 | ||
| Vinyl acetate | absorption capacity [g g−1] | 6.01 | 7.04 | 6.45 | 6.00 | 4.60 | 4.61 | 3.29 |
| removal efficiency a [%] | 16.7 | 33.3 | 50 | 66.7 | 78.3 | 91.7 | 99.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.C.; Bui, T.T.; Cho, Y.B.; Kim, Y.S. Preparation of a Highly Porous Clay-Based Absorbent for Hazard Spillage Mitigation via Two-Step Expansion of Vermiculite. Minerals 2021, 11, 1371. https://doi.org/10.3390/min11121371
Nguyen DC, Bui TT, Cho YB, Kim YS. Preparation of a Highly Porous Clay-Based Absorbent for Hazard Spillage Mitigation via Two-Step Expansion of Vermiculite. Minerals. 2021; 11(12):1371. https://doi.org/10.3390/min11121371
Chicago/Turabian StyleNguyen, Duc Cuong, Trung Tuyen Bui, Yeong Beom Cho, and Yong Shin Kim. 2021. "Preparation of a Highly Porous Clay-Based Absorbent for Hazard Spillage Mitigation via Two-Step Expansion of Vermiculite" Minerals 11, no. 12: 1371. https://doi.org/10.3390/min11121371
APA StyleNguyen, D. C., Bui, T. T., Cho, Y. B., & Kim, Y. S. (2021). Preparation of a Highly Porous Clay-Based Absorbent for Hazard Spillage Mitigation via Two-Step Expansion of Vermiculite. Minerals, 11(12), 1371. https://doi.org/10.3390/min11121371







