Abstract
The presence of drugs in aquatic bodies is a prevailing issue, and their removal by adsorption is an effective treatment. Among the adsorbents, those based clay minerals have been proposed. Bentonite is a clay mineral that is widely studied as an adsorbent due to its unique physicochemical properties, such as cation exchange capacity (CEC), intercalation, and adsorption. The properties of bentonites can be improved through chemical modifications, such as the incorporation of organic and/or inorganic compounds. These modifications allow for the efficient removal of different contaminants, including pharmaceutical compounds. In this work, raw sodium bentonite (Na+-Bent) and vanadyl bentonites were prepared using 100 (BentV1), 300 (BentV3), and 500% (BentV5) of the cationic exchange capacity of the Na+-Bent and further used for amiloride removal from aqueous solution. Analysis of X-ray fluorescence and Na+ in solution after interaction indicated that the principal mechanism of interaction between bentonite and ions was the ion exchange between sodium of the matrix and vanadyl in solution. Infrared spectroscopy suggested the contribution of coordination of the interlayer water with the vanadyl ions and hydrogen bonding between vanadyl and structural OH. X-ray diffraction analysis indicated that vanadyl ions were incorporated onto Na+-Bent. Amiloride adsorption was better at pH 5.8, using a solid dosage of 75 mg of Na+-Bent, 25 mg of BentV1 and BentV5, and 50 mg of BentV3. The adsorption occurred briefly until 20 min, and maximum removal values were 457.08, 374.64, 102.56, and 25.63 mg·g−1 for Na+-Bent, BentV1, BentV3, and BentV5, respectively. At lower drug concentrations (48.78 and 91.24 mg·g−1 for Na+-Bent and BentV3), the best performance was obtained for the BentV3 sample.
1. Introduction
The occurrence of drugs on the aquatic medium in concentrations of μg·L−1 or ng·L−1 can result in damage to animal and plant lives [1,2] and long-term water contamination due to low biodegradability [3,4,5,6,7]. Therefore, new technologies for drug removal that are frequently detected in aquatic ecosystems are a prevailing issue [7,8,9,10,11,12]. Among the treatments proposed for environmental remediation, adsorption is a promising alternative considering its relatively simple operation. The development of new and efficient adsorbents, including those based on clay minerals, has been described in recent years [13,14]. Clay minerals are natural or synthetic phyllosilicates, and their properties are adequate for adsorption, such as cation exchange capacity [15,16], expansion, high surface specific area [17], and presence of acidic and basic sites on the surface. These properties are important for their interaction with organic pollutants, especially drugs [18]. Among clay minerals, bentonite (Bent) has been used in environmental remediation processes [19,20,21]. The term bentonite is a rock with at least 50% smectite, particularly montmorillonite [22]. Montmorillonite (Mt) is a 2:1 phyllosilicate with layers in nanometers (≈1 nm) and a net negative charge in the lamella in the 0.2–0.6 range. The negative charge occurs through the exchange of trivalent cations (Al3+) for divalent (Mg2+) in the octahedral layer [23,24], and its neutralization occurs by exchangeable hydrated cations that are present in the interlamellar region, especially Na+ and Ca2+. The surface properties of bentonites can also be changed through chemical modification processes [25], including ion exchange with organic and inorganic cations [26], organic and inorganic anion bonds at the surface edge, intercalation of organic compounds [27], acid activation [28], and pillarization [29]. All modifications enable new properties and new adsorption sites to bentonites and can expand their applications in environmental remediation [30,31,32]. Ion exchange reactions in bentonites by inorganic [33] or organocations [34] have been widely explored for drug removal [25,35]. It is known that the nature of the interlayer cation plays a vital role in drug interactions [36,37,38,39]. However, although the use of sodium form of Mt (Na-Mt) is reported for drug removal [40,41], the low sedimentation and high swelling capacity of sodium bentonites can limit their application in wastewater treatment operations, especially in column adsorption processes [40,42]. Therefore, the nature of the interlayer cation and type of the drug are important aspects for the performance of Mt. Additionally, the type of interaction between organic species and Mt in adsorption is mainly influenced by the drug speciation, which is pH-dependent [36,43,44,45]. Amiloride (N-Amidino-3,5-diamino-6-chloropyrazinecarboxamide hydrochloride hydrate, Figure 1) is an oral diuretic drug that promotes potassium retention by the organism and the reduction of sodium and chlorine by excretion and hypertension. It is often used as a booster for other diuretics and to combat congestive heart failure since it has a weaker action than thiazide diuretics [46]. Studies have shown that amiloride does not undergo metabolic transformation and approximately half of the oral dose can be excreted through urine. In addition, the remainder of the unmetabolized drug can be recovered in the feces [47,48]. This can cause a major environmental problem, considering that the wastewater from sewage treatment plants is the most common source of these contaminants, which are released by the population through excreta or final disposal, and collected in sewage networks that do not have treatment plants designed to remove them [49,50]. Moreover, amiloride is photosensitive and undergoes a rather complex photodegradation process involving many different photoproducts [51]. Amiloride mineralization has been proposed as an alternative for drug removal at concentrations of 10 mg·L−1. Its photodegradation was evaluated using catalysts based on ZnO:g-C3N4 heterostructures with different amounts of g-C3N4 (15%, 50%, and 85%) [52]. Depending on the water pH during photodegradation, simultaneous protonation can occur, resulting in more chemical species that can be photodegraded and, consequently, producing more photoproducts [53]. Therefore, amiloride removal by adsorption on inorganic matrices containing photoactive species may result in the possibility of photodegradation of the drug later, as reported in the literature for other drugs, including ciprofloxacin [54], tetracycline [55], amoxicillin, diclofenac sodium, and paracetamol [30]. Clay minerals have also been described for drug photodegradation [56]. However, amiloride adsorption by clay minerals has been poorly reported in the literature. For example, a modified chitosan magnesium phyllosilicate was used for amiloride removal at pH 5.8, resulting in a maximum adsorption capacity of 55.74 mg·g−1 at 1500 mg·L−1 after 24 h [57].
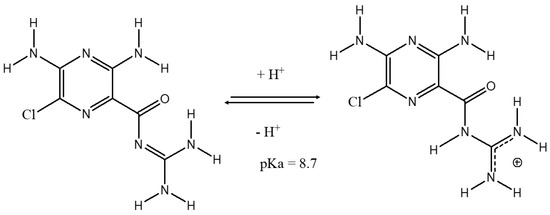
Figure 1.
Planar molecular structure of the amiloride.
In this context, the present study aimed to evaluate the use of vanadyl-exchanged bentonites at different concentrations as adsorbents for amiloride hydrochloride at pH 5.8, which is the natural pH of the species in an aqueous solution. The influence of the amount of vanadyl ions (VO2+) incorporated into the clay mineral was evaluated and compared to sodium bentonite. Vanadyl derivatives proved to be more active and selective in oxidation processes [58]. Vanadyl ion was adsorbed on hydrated hectorite and the study indicated that hydrolysis of VOH was promoted at low levels of adsorption [59]. The hydrolyzed product was adsorbed on the clay surfaces, with a ligand environment that was partially aqueous and partially hydroxide in nature [59]. Chiral bis(oxazoline) ligands are used to promote the enantioselective vanadium-catalyzed oxidation of sulfides with alkyl hydroperoxides. Several bis(oxazoline)-VO complexes have been prepared and supported by cation exchange in Laponite [60]. Vanadium oxides in clay minerals have been also reported as potential catalysts for oxidation reactions [61,62]. In a recent study, vanadium species were incorporated into a free aluminum layered silicate, followed by heat treatment to form V2O5. Vanadium oxide was anchored on the silicate surface, and the optical and magnetic properties of the hybrid materials suggested its potential application as a catalyst [63]. Therefore, the preparation of vanadyl exchange bentonites is described for the first time, and their performance for amiloride removal was evaluated as a function of the vanadyl content in the silicate matrix.
2. Materials and Methods
2.1. Chemicals and Materials
A sodium bentonite sample was obtained from the Bentonise Bentonita Company, Campina Grande, Brazil. The CEC was 88 cmol(+)/kg, as measured by the ammonium-exchange determination [64,65]. The chemical composition of the bentonite indicated the following constituents: SiO2 (52.98%), Al2O3 (18.35%), Fe2O3 (3.96%), Na2O (2.56%), MgO (2.47%), TiO2 (0.18%), K2O (0.22%), and CaO (0.01%) [61]. Vanadyl sulfate hydrate (VOSO4 H2O, 97% purity) was supplied by Alfa Aesar (Haverhill, MA, USA), and amiloride hydrochloride hydrate (C6H8ClN7O·HCl·xH2O, purity 98%, CAS 2016-88-8) from Sigma Aldrich (St. Louis, MO, USA) was used in the adsorption tests. All the chemicals were used without prior treatment. Distilled water was used as the solvent in all the procedures.
2.2. Quartz Removal
Initially, quartz removal of raw bentonite was performed using the centrifugation/decantation method [65]. A sample of approximately 100 g was washed with 500 mL of distilled water, centrifuged, dried at 50 °C for 24 h, and sieved through a 200 mesh. Therefore, sodium bentonite (Na+-Bent) was used in the adsorbent preparation and adsorption tests.
2.3. Preparation of the Vanadyl Exchanged Bentonites
Na+-Bent (10.0 g) was reacted with 100 mL vanadyl sulfate solutions at concentrations of 100%, 300%, and 500% of the CEC of the clay. At this condition, salt amounts of 1.76, 5.29, and 8.81 g in vanadyl sulfate/Na+-Bent dispersions were maintained at 200 rpm and 30 °C for 144 h in a thermostabilized bath. The vanadyl sulfate solution in the reaction vessel was repositioned every 48 h to control the volume of the solvent. The solids were separated by centrifugation at 7500 rpm for 5 min and washed with distilled water. Finally, the prepared bentonites were dried at 60 °C for 72 h and named as BentV1, BentV3, and BentV5 for the solids prepared with vanadyl sulfate at 100%, 300%, and 500% of clay CEC, respectively.
2.4. Adsorption of Amirolide
Both Na+-Bent and exchanged bentonites were used in the adsorption tests. The influence of the adsorbent dosage was determined for 25, 50, 75, and 100 mg of the solid dispersed in 50 mL of 50 mg·L−1 amiloride solution at pH 5.8. At pH 5.8, the cationic form of amiloride is predominant in the solution, and its biological activation occurs at the same value [66]. The dispersions were maintained in orbital agitation at 30 °C in a TE-420 model Tecnal incubator for 24 h. After each determination, the adsorbent samples were recovered by centrifugation, and the solids were dried at 50 °C for further characterization. Amirolide concentration in the solution was monitored before and after adsorption at 286 nm by UV-VIS spectrometry using a Shimadzu spectrometer UV-2550 (Shimadzu, Kyoto, Japan) model. The amount of adsorbed amiloride was calculated using Equation (1):
where Ci and Ce are the initial and equilibrium amiloride concentrations, respectively; Vc is the volume of the drug solution; and m is the mass of the solid used in each test. The influence of the time in the adsorption was performed at 10–120 min intervals at pH 5.8, and the optimal adsorbent dosage and the same procedure were adopted. The equilibrium isotherms were evaluated for 10–100 mg·L−1 and 10–500 mg·L−1 initial amiloride concentrations at the optimal time obtained in the kinetic tests at pH 5.8.
2.5. Characterizations
XRD diffractometry was performed at room temperature in a Shimadzu XD3A model, with a scan rate of 0.03 s−1, using CuKα (λ) as the source radiation (λ = 0.15406 nm), between 2θ of 2.5 to 80° and a fixed power source (30 kV and 30 mA)). The average crystallite size was calculated based on the Scherrer equation [67], defined as:
where D is the crystallite size; K is a constant whose value depends on the shape of the particle (which is equal to 0.89 for spherical particles); λ is the wavelength of the electromagnetic radiation (λCu = 1.5406 Å); θ is half the angle of diffraction; and β is the full width at half maximum (FWHM) of the peak crystallite size on the 002 plane, whose contributions due to instrumental enlargement and strain were discounted.
Fourier transform infrared spectroscopy (FTIR) was performed using a Bomem MB-series FT spectrometer, Quebec, Canada. The samples were prepared using KBr pellets at a concentration of 2%. The FTIR spectra (4000–400 cm−1) were recorded with a resolution of 4 cm−1 and 32 accumulations.
X-ray fluorescence (XRF) was performed using a Shimadzu model EDX-7000 (Shimadzu, Kyoto, Japan) under a vacuum, with a 10 mm collimator, scanning from sodium to uranium.
Inductively coupled plasma optical emission spectrometry (ICP OES) was performed using a Spectro, Arcos model (SPECTRO, Kleve, Germany) and used to quantify sodium in the solution.
The zeta potential (ζ) was monitored using a Zetasizer Nano Zs (Malvern Panalytical, Malvern, UK) for isoelectric titration through pH titration. Solutions of 0.100 mol·L−1 NaOH or 0.500 mol·L−1 HNO3 were used to adjust the pH.
Scanning electron microscopy (SEM) images were obtained using a TESCAN VEGA3 SEM (TESCAN, Brno, The Czech Republic), corresponding to a tungsten thermionic emission system, suitable for high and low vacuum operations and equipped with an energy dispersive X-ray spectrometer (EDS), manufactured by Oxford Instruments (Abingdon, UK).
2.6. Kinetic and Equilibrium Models
Experimental adsorption data used in the kinetic and equilibrium models are described in Supplementary Materials Figure S1. For kinetics, experimental data were adjusted to fit the three kinetic models (as shown in Equations (1)–(3) in Supplementary Material SI1): Pseudo-first-order [68], pseudo-second-order [69], and simplified Elovich equation [70], assuming αβt >> 1.
The equilibrium isotherms were analyzed using the Langmuir [71], Freundlich [72], and Temkin [73] models (following Equations (4)–(6) in Supplementary Material SI1).
Standard deviation (SD) was used to verify which equation models were best suited to describe the experimental data [74]. Equation (3) was used for SD determination in the following form:
where qi,exp and qi,model are the experimental adsorbed amiloride amount and theoretical amount obtained by the kinetic or equilibrium models; np is the number of performed experiments; and p is the number of parameters of the fitted model.
3. Results
3.1. Characterizations of the Sodium and Vanadyl Exchanged Bentonites before and after Amiloride Adsorption
3.1.1. X-ray Fluorescence (XRF)
The presence of vanadyl after the exchange process was monitored using X-ray fluorescence (Table 1). The analysis revealed that part of the vanadyl was incorporated into the clay samples with vanadyl concentrations of 75.6, 153.8, and 175.6 cmol(+) kg−1, corresponding to 86, 175, and 199% of CEC for BentV1, BentV3, and BentV5, respectively. The use of vanadyl in high proportions also resulted in better incorporation of the ions in the clay sample. However, a significant amount of vanadyl remained in the solution for the three conditions, as indicated by X-ray fluorescence analysis. After interaction with amiloride, the amounts of vanadyl in solids with maximum amounts of adsorbed drug were 64.0, 134.2, and 145.0 cmol(+) kg−1, suggesting that vanadyl cations can be exchanged with the cationic drug during the adsorption process.

Table 1.
Chemical composition by XRF for bentonites before and after interaction with amiloride.
Sobrenadant analysis of the solution after the interaction showed the presence of sodium (Table 1). Sodium amounts were 76.7, 70.8, and 70.8 cmol(+) kg−1, corresponding to 87% of CEC for BentV1 and 80% of CEC for both BentV3 and BentV5. Results indicated that ion exchanged between vanadyl and sodium was a predominant process of interaction considering that the amount of Na+ in all cases is close to CEC of the raw bentonite sample (88 cmol(+) kg−1). Moreover, it suggested that vanadyl can be incorporated on external sites of the bentonite or (vanadyl/sulphate) ionic pair intercalated as observed for the interaction between ammonium salts and bentonite at concentrations higher than the CEC of the clay sample [58].
3.1.2. X-ray Diffractometry
The XRD patterns of the samples are presented in Figure 2, with the phases indexed by the ICDD 00-060-0318 and 01-070-8055 files. Sodium bentonite XRD indicated that Mt is the principal constituent. The characteristic Mt reflection was observed at 2θ = 7.24°, representing the basal spacing d(001) of 1.22 nm, typical of sodium Mt [75]. Other reflections were also observed at 2θ = 19.8, 28.5, 34.9, and 61.8°, which were indexed to the Mt phase in concordance with the 00-060-0318ICDD file. The reflection at 61.9° was associated with the (060) plane of dioctahedral phyllosilicate [23]. The impurity of quartz in the raw sample was associated with reflection at 26.5°, in agreement with the 01-070-8055 ICDD file. The structure of the bentonite changed after modification. The interlayer distances (calculated using Bragg’s law) and the thickness of the bentonite (calculated using the Debye-Scherer equation) were different post-modification. The data for the samples are presented in Table 2. After reaction with vanadyl, the (001) reflection changed for lower 2θ values, indicating an increase in the basal spacing, which are 1.34, 1.37, and 1 nm for BentV1, BentV3, and BentV5, respectively. The thickness of the bentonite (0.96 nm) indicated that values of the free spacing to accommodate the amiloride molecules were 0.38, 0.41, and 0.04 nm for BentV1, BentV3, and BentV5, respectively. Therefore, the XRD data suggested that samples with higher amounts of vanadyl can present more difficulty in accommodating amiloride molecules. Values of D indicated a decrease in the crystallite size in the 001 direction after vanadyl exchange and amiloride adsorption without a regular relationship between the concentration of vanadyl and amiloride, except for BentV1Amil, where the D value was higher than BentV1.
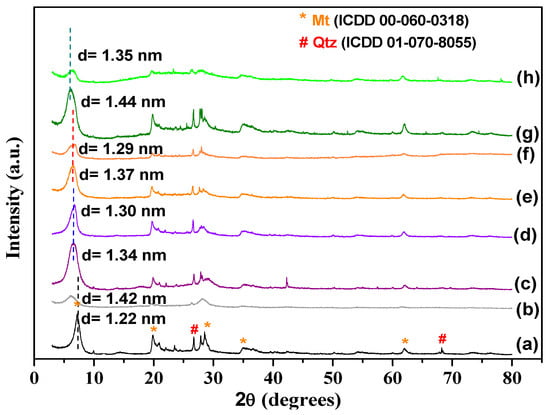
Figure 2.
XRD patterns of (a) Na+-Bent, (b) Na+-Bent/Ami, (c,d) BentV1, (e,f) BentV3, (g,h) BentV5, before and after amiloride adsorption at 30 °C, pH 5.8.

Table 2.
Parameters calculated according to the XRD results.
In the sample that interacted with amiloride, it was observed that d(001) increased from 1.22 to 1.42 nm for Na+-Bent, which is an indication of the amiloride intercalation in the interlayer spacing of the Mt. For the vanadyl exchanged bentonites, d(001) reduced for all samples. The d(001) values changed from 1.34 to 1.30 nm, 1.37 to 1.29 nm, and 1.44 to 1.35 nm in BentV1, BentV3, and BentV5, respectively. Broader reflections were observed in all samples after the interaction with amiloride, especially Na+-Bent, BentV3, and BentV5, suggesting a reduction in the crystallinity of the solids after the drug interaction [76]. The XRD results suggested that amiloride was adsorbed on the surface of the vanadyl exchanged bentonites, while amiloride intercalation was predominant for Na+-Bent. It is inferred that the entrance of vanadyl in the interlayer of the precursor sample decreased the free exchange sites, and the drug was preferentially adsorbed on the surface of the vanadyl bentonites.
3.1.3. Zeta Potential
According to the zeta potential (ζ) measurements of the solids before and after cation exchange (Figure 3), the surface charge was always negative in aqueous media, as expected for particles with negative structural charges. The solid particles were submitted to a process involving only cationic exchange, not compromising the bentonite structure, resulting in the substitution of Al3+ in the octahedral layer by Mg2+ or Fe2+ and Si4+ in the tetrahedral layer by Fe3+ or Al3+.
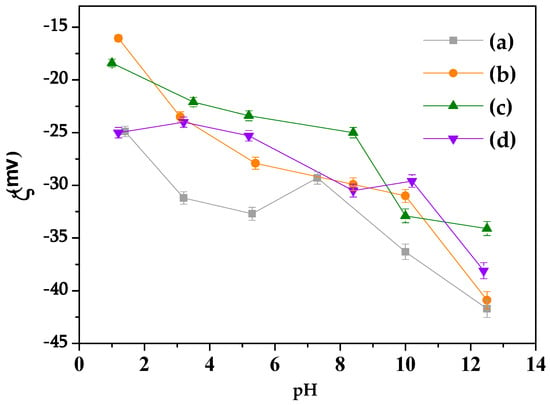
Figure 3.
Zeta potential measurements (ξ) of the solids. (a) Na+-Bent, (b) BentV1, (c) BentV3, and (d) BentV5.
3.1.4. Spectroscopy
FTIR spectroscopy was useful for the monitoring of alterations in the OH absorptions and presence of the amiloride in the solids (Figure 4).
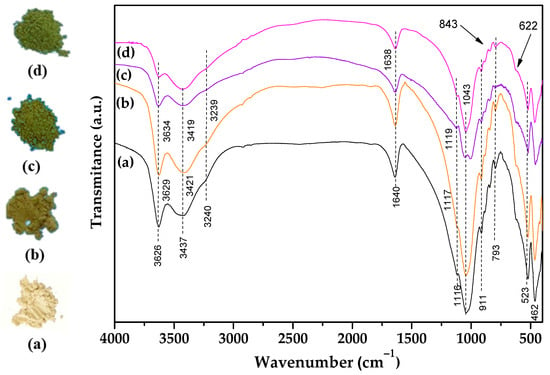
Figure 4.
Solid images and FTIR spectra for (a) Na+-Bent, (b) BentV1, (c) BentV3, and (d) BentV5.
The FTIR spectra of the sodium and vanadyl bentonites showed characteristic bands of the inorganic matrix. Vibrations related to structural OH stretching (Al–OH and Mg–OH) shifted from 3626 to 3634 cm−1 as vanadyl ions were incorporated in the clay sample, which suggested that structural OH groups are also involved in the interaction [77]. The bands at 3437 cm−1 related to OH stretching in water and Si-OH were unchanged [77], and OH deformation in water occurred at 1635 cm−1 without alteration in their position [77]. Bands at 1117 and 1043 cm−1 are assigned to Si–O–Si and Si–O–Al antisymmetric and symmetric stretching, respectively. These latter bands changed to higher wavenumbers after vanadyl was exchanged and reached 1126 and 1060 cm−1. The shift for higher wavenumbers indicates stronger bonding, which may be associated with the coordination of the interlayer water with the vanadyl ions, considering that vanadyl species form stable complexes with water molecules in five coordination [78]. The formation of interlayer complexes can result in weak interactions between water and the Si–O–Si and Si–O–Al surfaces. The bands at 911 and 835/842 cm−1 were assigned to OH bending in Al2OH and AlMgOH of the octahedral layer of clay minerals, thus reflecting the partial substitution of Al3+ by Mg2+ [76]. At 462 cm−1, the bands were attributed to Si–O and Al–O–Si bending [79]. Vanadyl ions were assigned to the bands between 843 and 622 cm−1, which are attributed to the V-O antisymmetric and symmetrical stretching [80], while the band at 622 cm−1 probably overlapped with the clay mineral vibrations. FTIR spectra of the amiloride-adsorbed samples (Figure 5) showed the presence of the drug on solids. All of the absorption of the inorganic part was present. The broad and intense band at 3420 cm−1 is attributed to the N-H symmetrical stretching of the primary amine substituted in the pyrazine ring [81]. Two other broad bands were observed at 3339 and 3177 cm−1, which were assigned to N-H asymmetric and symmetric stretching of guanidine, respectively [81,82]. Other characteristic bands were observed at 1676 cm−1, corresponding to the C=O stretching of the disubstituted amide; 1642 cm−1, attributed to the NH2 deformation of the guanidine; 1543 cm−1, associated with stretching of the tetrasubstituted pyrazine ring; 1383 cm−1, attributed to the C-N stretching of the carboxamide; 1065 and 1250 cm−1, attributed to the ortho-substituted chlorine and the C-N group in the pyrazine ring, respectively [81,82]. The bands between 841/845 and 622 cm−1, assigned to V-O antisymmetric and symmetric stretching [80], were unchanged after amiloride.

Figure 5.
FTIR spectra for (a) Na+Bent, (b) BentV1, (c) BentV3, (d) BentV5 after the interaction with amiloride at 30 °C pH 5.8 and (e) free amiloride hydrochloride.
3.1.5. SEM and EDX Analysis
The morphologies of the samples before and after amiloride adsorption were examined by SEM, as shown in Figure 6i and Supplementary Materials Figure S1i, respectively. Raw Bent presented a typical flake morphology, which remained unchanged after vanadyl exchange, independent of the specific composition. For the amiloride-loaded samples, the morphology was the same, indicating that solids were stable under the conditions used (Figure S1i).
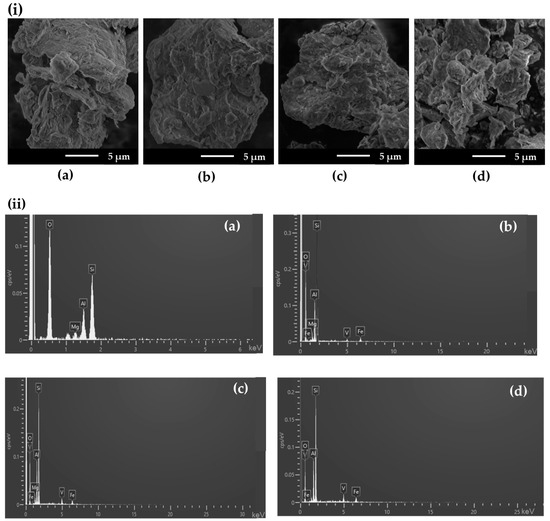
Figure 6.
(i) SEM images and (ii) EDX spectra for (a) Na+-Bent, (b) BentV1, (c) BentV3, and (d) BentV5.
EDX spectra and elemental mapping of the samples are presented in Figure 6ii and Figure S1ii, respectively. For samples before adsorption, EDX spectra showed the presence of the main elements in raw Bent (Si, Al, Mg, O) and vanadyl bentonites (Si, Al, Mg, O, V). Furthermore, EDX vanadium mapping (Figure S2) indicated that the element was uniformly distributed in the samples before and after amiloride adsorption.
3.2. Mechanism of Amiloride Clay Interaction
Based on characterization, the interaction between the drug and the clay mineral surface is mostly electrostatic, resulting in a cation exchange process with hydrated vanadium cations [61]. The drug can also interact by hydrogen bonding with the nitrogen and oxygen in its interlayer water molecules and the terminal OH of the phyllosilicate structure [76]. However, considering the stability formed by the vanadyl ion coordinated by water molecules, [78] the coordination by amiloride in the vanadyl ions is unlikely, as indicated by the infrared spectra. A preferable coordination by the water molecules in relation to the drug is also possible considering the pKa values of the species involved. In fact, vanadyl ions have pKa 5.38 [78] and are stronger acids than water (pKa = 15.9) and amiloride (pKa = 8.4) [51]. Therefore, water has a higher pKa among the species and, consequently, has a stronger conjugate base, favoring coordination with the vanadium oxidation in an acid-base reaction between the vanadyl ion and ligands. Therefore, the drug molecules were probably inserted in the interlayer region parallel to the phyllosilicate sheets, considering the size of the molecule and d(001) value (Figure 7). Although bentonite is composed of expandable phyllosilicate, its basal spacing does not increase after amiloride adsorption on the modified samples. Similar results were also reported for the adsorption of thiabendazole [37] and amitriptyline [45] by bentonites.
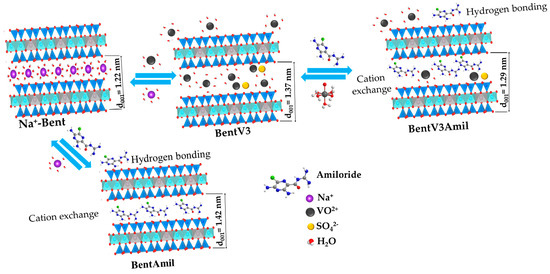
Figure 7.
Proposed scheme for amiloride/vanadyl-bentonites.
3.3. Adsorption
3.3.1. Adsorbent Dosage
The influence of the adsorbent dosage on amiloride adsorption by bentonites was evaluated for masses between 25 to 100 mg (see Figure S3). Thus, the adsorbent concentrations in the suspension were 0.5, 1.0, 1.5, and 2 mg·L−1. The results showed that the maximum removal of 89.8% was obtained with 75 mg of Na+-Bent, while the performance of the exchanged vanadyl bentonites improved by 96.3, 94.7, and 86.5% at 50 mg adsorbent dosage for BentV1, BentV3, and BentV5, respectively.
3.3.2. Influence of Time
The effect of time on amiloride adsorption (Figure S4) was monitored for 120 min. Fast adsorption of the drug was obtained at 20 min for Na+-Bent, BentV1, and BentV5 and 60 min for BentV3. Maximum amounts of the adsorbed drug were 80.7, 36.1, 91.5, and 20.6 mg·g−1 for Bent, BentV1, BentV3, and BentV5, respectively. The results indicated that the presence of vanadyl on bentonite drastically affected its performance, possibly due to the blocking of the adsorption sites. The experimental adsorption data were fitted to kinetic models (Figure S4), and the results were (Table 3) better adjusted for the pseudo-second-order model for all of the investigated systems, as shown by the R2 and SD values. The results obtained from characterization of the amiloride/vanadyl-bentonites hybrids indicated that chemisorption contributed to the interaction mechanism.

Table 3.
Kinetic parameters obtained from the pseudo-first-order, pseudo-second-order, and Elovich equations for nonlinear fitting of amiloride adsorption on vanadyl-bentonites (experimental conditions: 30 °C, pH 5.8, and 50 mg·L−1 amiloride solution).
3.3.3. Equilibrium Isotherms
The influence of concentration on amiloride adsorption was determined to be between 10 and 500 mg·L−1 at pH 5.8 and 30 °C for 150 min. The isotherms for vanadyl bentonites showed an increase in qe, achieving constant concentrations (Ci) of 100 mg·L−1 for Na+-Bent and BentV1, 50 mg·L−1 for BentV3, and 30 mg·L−1 for BentV5 (Figure 6). Maximum amounts (qmax) of adsorbed drug by Na+-Bent, BentV1, BentV3, and BentV5 were 457.08, 374.64, 102.56, and 25.63 mg·g−1, respectively (Figure 8). There are no studies on amiloride adsorption in species exchanged with vanadyl in the literature. However, amiloride adsorption for release purposes has been found for talc phyllosilicate in its natural form and talc phyllosilicate hybridized with chitosan [57]. In that study, the modified material presented better amiloride adsorption performance, which had maximum adsorption of 55.74 mg·g−1 in the hybrid and 49.53 mg·g−1 in the raw-talc, both in contact with an adsorbate solution with a concentration of 1500 mg·L−1 [57]. The obtained adsorption capacity was also higher than the one observed for chitosan (4.71 mg·g−1) [57].
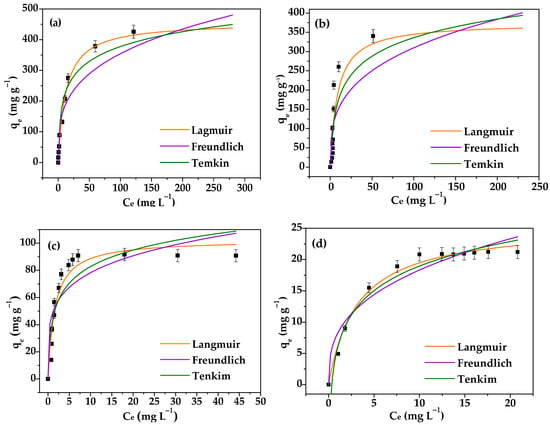
Figure 8.
Equilibrium isotherms and their fitting to the Langmuir, Freundlich, and Temkin models for amiloride adsorption on (a) Na+-Bent, (b) BentV1, (c) BentV3, and (d) BentV5 at 30 °C and pH 5.8.
The adsorption parameters were evaluated according to the Langmuir, Freundlich, and Temkin adsorption models (Table 4). From the R2 values, the experimental data were better fitted by the Langmuir model for all of the studied solids, indicating that chemisorption may be the predominant mechanism involved in amiloride adsorption. The Langmuir model is based on homogeneous adsorption sites.

Table 4.
Adsorption parameters of amiloride on vanadyl-bentonites at 30 °C and pH 5.8 according to the Langmuir, Freundlich, and Temkin models.
As observed in the kinetic study, the adsorption was lower by the vanadyl-exchanged bentonites. However, Na+-Bent presented a better performance in comparison to the other solids. Equilibrium occurred at higher drug concentration, while at lower amiloride concentration (20–70 mg·L−1), the performance of the adsorbent followed the order BentV3 > Na+-Bent > BentV1 > BentV5. The adsorbed capacities were 15.75 and 37.14 mg·g−1 at 20 mg·L−1, 26 and 56.6 mg·g−1 at 30 mg·L−1, and 48.8 and 91.2 mg·g−1 at 50 mg·L−1 for Bent and BentV3, respectively. The maximum performance occurred for BentV3 at 50 mg·L−1, while Na+-Bent increased its performance at an initial drug concentration of 70 mg·L−1. Therefore, Na+-Bent was less efficient at lower amiloride concentrations, which are typical drug occurrence conditions in aquatic bodies [1,19,83].
4. Conclusions
The incorporation of vanadyl in sodium bentonite was performed at different proportions of the CEC. However, partial incorporation of the initial vanadyl was observed.
The difference in the proportion of VO2+ ions resulted in different behaviors in the amiloride adsorption, and the material prepared at 100% CEC showed better performance, with maximum adsorption of 343.46 mg·g−1 at 500 mg·L−1 initial amiloride concentration in an equilibrium time of 20 min.
At lower amiloride concentrations, BentV3 showed better performance than all of the solids, including Na+-Bent at 20–70 mg·L−1. The adsorption of Na+-Bent was higher than the exchanged bentonites at concentrations above 100 mg·L−1.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/min11121327/s1, Figure S1: (i) SEM images and (ii) EDX spectra for (a) Na+-Bent/Amil, (b) BentV1/Amil, (c) BentV3/Amil and (d) BentV5/Amil. Figure S2: Vanadium mapping (i) before and (ii) after interaction with amiloride, of the (a) BentV1, (b) BentV3 and (c) BentV5. Figure S3: Effect of the adsorbent dosage (m) in the amiloride for amiloride adsorption on (a) Na+-Bent, (b) BentV1, (c) BentV3 and (d) BentV5 at 30 °C and pH 5.8. Figure S4: Effect of the time and their fitting to the pseudo-first-order, pseudo-second-order and Elovich models for amiloride adsorption on (a) Na+-Bent, (b) BentV1, (c) BentV3 and (d) BentV5 at 30 °C and pH 5.8. Supplementary SI1: Kinetic and equilibrium models.
Author Contributions
Conceptualization, M.G.F.; Funding acquisition, J.O., R.R.P.-G. and E.C.S.-F.; Investigation, L.O. and E.C.S.-F.; Methodology, L.O. and R.R.P.-G.; Project administration, M.G.F.; Supervision, J.O. and M.G.F.; Writing—original draft, L.O.; Writing—review & editing, J.O., R.R.P.-G., E.C.S.-F. and M.G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Brazilian National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES). MG Fonseca (CNPq grant number 310921/2017-1), JA Osajima (CNPq grant number 307256/2018-9), and LS Oliveira (CAPES grant number 888824400252019-01).
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sodré, F.F.; Locatelli, M.A.F.; Jardim, W.F. Occurrence of emerging contaminants in Brazilian drinking waters: A sewage-to-tap issue. Water Air Soil Pollut. 2010, 206, 57–67. [Google Scholar] [CrossRef]
- De Andrade, J.R.; Oliveira, M.F.; Da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Lonappan, L.; Kaur, S.; Kumar, R.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Romita, R.; Agostiniano, A.; Nuzzo, S.; Cosma, P. Commercial bentonite clay as low-cost and recyclable ‘natural’ adsorbent for the Carbendazim removal/recover from water: Overview on the adsorption process and preliminary photodegradation considerations. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602. in press. [Google Scholar] [CrossRef]
- Quadra, G.R.; Oliveira de Souza, H.; dos Santos Costa, R.; dos Santos Fernandez, M.A. Do pharmaceuticals reach and affect the aquatic ecosystems in Brazil? A critical review of current studies in a developing country. Environ. Sci. Pollut. Res. 2017, 24, 1200–1218. [Google Scholar] [CrossRef] [PubMed]
- Świacka, K.; Szaniawska, A.; Caban, M. Evaluation of bioconcentration and metabolism of diclofenac in mussels Mytilus trossulus—laboratory study. Mar. Pollut. Bull. J. 2019, 141, 249–255. [Google Scholar] [CrossRef]
- De Jesus Silva Chaves, M.; Barbosa, S.C.; Mallinowski, M.M.; Volpato, D.; Castro, I.B.; Franco, T.C.R.S.; Primel, E.G. Pharmaceuticals and personal care products in a Brazilian wetland of international importance: Occurrence and environmental risk assessment. Sci. Total Environ. 2020, 734, 139–374. [Google Scholar]
- Veras, T.B.; Luiz Ribeiro de Paiva, A.; Duarte, M.M.M.B.; Napoleão, D.C.; da Silva Pereira Cabral, J.J. Analysis of the presence of anti-inflammatories drugs in surface water: A case study in Beberibe river—PE, Brazil. Chemosphere 2019, 222, 961–969. [Google Scholar] [CrossRef]
- Pivetta, R.C.; Rodrigues-Silva, C.; Ribeiro, A.R.; Rath, S. Tracking the occurrence of psychotropic pharmaceuticals in Brazilian wastewater treatment plants and surface water, with assessment of environmental risks. Sci. Total Environ. 2020, 727, 138–661. [Google Scholar] [CrossRef]
- Arsand, J.B.; Roff, R.B.; Jank, L.; Bussamara, R.; Dallegrave, A.; Bento, F.M.; Kmetzsch, L.; Falção, D.A.; Peralba, M.C.R.; Gomes, A.A.; et al. Presence of antibiotic resistance genes and its association with antibiotic occurrence in Dilúvio River in southern Brazil. Sci. Total Environ. 2020, 738, 139–781. [Google Scholar] [CrossRef]
- Aus der Beek, T.; Weber, F.A.; Bergman, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Trigueiro, P.; Pereira, F.A.R.; Guillermin, D.; Rigaud, B.; Balme, S.; Janot, J.; Ieda, M.G.; Fonseca, M.G.; Walter, P.; Jaber, M. Dyes and Pigments When anthraquinone dyes meet pillared montmorillonite: Stability or fading upon exposure to light? Dyes Pigm. 2018, 159, 384–394. [Google Scholar] [CrossRef]
- Starón, P.; Chwastowski, J.; Banach, M. Sorption behavior of methylene blue from aqueous solution by raphia fibers. Int. J. Environ. Sci. Technol. 2019, 16, 8449–8460. [Google Scholar] [CrossRef] [Green Version]
- Sophia, C.A.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef] [PubMed]
- França, D.B.; Trigueiro, P.; Filho, E.C.S.; Fonseca, M.G.; Jaber, M. Monitoring diclofenac adsorption by organophilic alkylpyridinium bentonites. Chemosphere 2020, 242, 125019. [Google Scholar] [CrossRef] [PubMed]
- Yotsuji, K.; Tachi, Y.; Sakuma, H.; Kawamura, K. Effect of interlayer cations on montmorillonite swelling: Comparison between molecular dynamic simulations and experiments. Appl. Clay Sci. 2021, 204, 106034. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. Introduction to Pure Clay Minerals. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 211–222. [Google Scholar]
- Wypych, F.; Satyanarayana, K.G. Clay Surfaces: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Maged, A.; Iqbal, J.; Kharbish, S.; Ismael, I.S.; Bhatnagar, A. Tuning tetracycline removal from aqueous solution onto activated 2:1 layered clay mineral: Characterization, sorption and mechanistic studies. J. Hazard. Mater. 2020, 384, 121–320. [Google Scholar] [CrossRef] [PubMed]
- Xing Zha, S.; Zhou, Y.; Jin, X.; Chen, Z. The removal of amoxicillin from wastewater using organobentonite. J. Environ. Manage. 2013, 129, 569–576. [Google Scholar]
- Wu, Q.; Que, Z.; Li, Z.; Chen, S.; Zhang, W.; Yin, K. Photodegradation of ciprofloxacin adsorbed in the intracrystalline space of montmorillonite. J. Hazard. Mater. 2018, 359, 414–420. [Google Scholar] [CrossRef]
- Bergaya, F.; Jaber, M. Clay for nanocomposites. In Rubber-Clay Nanocomposites, 1st ed.; Galimberti, M., Ed.; IntechOpen: London, UK, 2011; pp. 1–44. [Google Scholar]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Structure and mineralogy of clay minerals. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 21–81. [Google Scholar]
- He, H.; Ma, L.; Zhu, J.; Frost, R.L.; Theng, B.K.G.; Bergaya, F. Synthesis of organoclays: A critical review and some unresolved issues. Appl. Clay Sci. 2014, 100, 22–28. [Google Scholar] [CrossRef]
- Parolo, M.E.; Avena, M.J.; Pettinari, G.R.; Baschini, M.T. Influence of Ca2+ on tetracycline adsorption on montmorillonite. J. Colloid Interface Sci. 2012, 368, 420–426. [Google Scholar] [CrossRef]
- Wu, L.; Liao, L.; Lv, G. Influence of interlayer cations on organic intercalation of montmorillonite. J. Colloid Interface Sci. 2015, 454, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, S.; Ahmad, Z.; Ishaq, M.; Sarwar, M.I. Aromatic-aliphatic polyamide/montmorillonite clay nanocomposite materials: Synthesis, nanostructure and properties. Mater. Sci. Eng. A 2009, 525, 30–36. [Google Scholar] [CrossRef]
- Komadel, P.; Madejová, J. Acid Activation of Clay Minerals. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 385–409. [Google Scholar]
- Fatimah, I.; Wijaya, K.; Narsito. Microwave assisted preparation of TiO2/Al-pillared saponite for photocatalytic phenol photo-oxidation in aqueous solution. Arab. J. Chem. 2015, 8, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, M.; Saini, V.K.; Suthar, S. Ti-pillared montmorillonite clay for adsorptive removal of amoxicillin, imipramine, diclofenac-sodium, and paracetamol from water. J. Hazard. Mater. 2020, 399, 122832. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.; Boussafir, M.; Fougère, L.; Destandau, E.; Sugahara, Y.; Guégan, R. Use of a clay mineral and its nonionic and cationic organoclay derivatives for the removal of pharmaceuticals from rural wastewater effluents. Chemosphere 2020, 259, 127–480. [Google Scholar] [CrossRef]
- Maged, A.; Kharbish, S.; Ismael, I.S.; Bhatnagar, A. Characterization of activated bentonite clay mineral and the mechanisms underlying its sorption for ciprofloxacin from aqueous solution. Environ. Sci. Pollut. Res. 2020, 32, 980–997. [Google Scholar] [CrossRef]
- Rakić, V.; Rajić, N.; Daković, A.; Auroux, A. The adsorption of salicylic acid, acetylsalicylic acid and atenolol from aqueous solutions onto natural zeolites and clays: Clinoptilolite, bentonite and kaolin. Microporous Mesoporous Mater. 2013, 166, 185–194. [Google Scholar] [CrossRef]
- Nourmoradi, H.; Daneshfar, A.; Mazloomi, S.; Bagheri, J.; Barati, S. Removal of Penicillin G from aqueous solutions by a cationic surfactant modified montmorillonite. MethodsX 2019, 6, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.; Guégan, R.; Thiebault, T.; Le Milbeau, C.; Muller, F.; Teixeira, V.; Giovanela, M.; Boussafir, M. Adsorption of diclofenac onto organoclays: Effects of surfactant and environmental (pH and temperature) conditions. J. Hazard. Mater. 2017, 323, 558–566. [Google Scholar] [CrossRef]
- Bizi, M.; El Bachra, F.E. Evaluation of the ciprofloxacin adsorption capacity of common industrial minerals and application to tap water treatment. Powder Technol. 2020, 362, 323–333. [Google Scholar] [CrossRef]
- Cavalcanti, G.R.S.; Fonseca, M.G.; da Silva Filho, E.C.; Jaber, M. Thiabendazole/bentonites hybrids as controlled release systems. Colloids Surf. B Biointerfaces 2019, 176, 249–255. [Google Scholar] [CrossRef]
- Parolo, M.E.; Avena, M.J.; Savini, M.C.; Baschini, M.T.; Nicotra, V. Adsorption and circular dichroism of tetracycline on sodium and calcium-montmorillonites. Colloids Surf. A Physicochem. Eng. Asp. 2013, 417, 57–64. [Google Scholar] [CrossRef]
- Rivagli, E.; Pastorello, A.; Sturini, M.; Maraschi, F.; Speltini, A.; Zampori, L.; Setti, M.; Malavasi, L.; Profumo, A. Clay minerals for adsorption of veterinary FQs: Behavior and modeling. J. Environ. Chem. Eng. 2014, 2, 738–744. [Google Scholar] [CrossRef]
- Mahouachi, L.; Rastogi, T.; Palm, W.U.; Ghorbel-Abid, I.; Ben Hassen Chehimi, D.; Kümmerer, K. Natural clay as a sorbent to remove pharmaceutical micropollutants from wastewater. Chemosphere 2020, 258, 127–213. [Google Scholar] [CrossRef] [PubMed]
- Thiebault, T.; Boussafir, M.; Guégan, R.; Le Milbeau, C.; Le Forestier, L. Clayey-sand filter for the removal of pharmaceuticals from wastewater effluent: Percolation experiments. Environ. Sci. Water Res. Technol. 2016, 2, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, D. Efficient use of hybrid materials in the remediation of aquatic environment contaminated with micro-pollutant diclofenac sodium. Chem. Eng. J. 2015, 263, 364–373. [Google Scholar]
- Silva, D.T.C.; Arruda, I.E.S.; França, L.M.; França, D.B.; Fonseca, M.G.; Soares, M.F.L.R.; Iborra, C.V.; Soares-Sobrinho, J.L. Tamoxifen/montmorillonite system—Effect of the experimental conditions. Appl. Clay Sci. 2019, 180, 105–142. [Google Scholar] [CrossRef]
- Del Mar Orta, M.; Martín, J.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Adsorption of propranolol onto montmorillonite: Kinetic, isotherm and pH studies. Appl. Clay Sci. 2019, 173, 107–114. [Google Scholar] [CrossRef]
- Chang, P.-H.; Jiang, W.-T.; Li, Z.; Kuo, C.-Y.; Jean, J.-H.; Chen, W.-R.; Lv, G. Mechanism of amitriptyline adsorption on Ca-montmorillonite (SAz-2). J. Hazard. Mater. 2014, 277, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, K.; Jonassen, T.E.N.; Christensen, S.; Shirley, D.G. Amiloride inhibits proximal tubular reabsorption in conscious euvolemic rats. Eur. J. Pharmacol. 2002, 437, 85–90. [Google Scholar] [CrossRef]
- Sun, Q.; Sever, P. Amiloride: A review. J. Renin-Angiotensin-Aldosterone Syst. 2020, 21, 1470320320975893. [Google Scholar] [CrossRef]
- Vidt, D.G. Mechanism of Action, P harmaco kinetics, Adverse Effects, and Therapeutic Uses of Amiloride Hydrochloride, A New Potassium-Sparing Diuretic. Pharmacotherapy 1981, 1, 179–187. [Google Scholar] [CrossRef]
- Brack, W.; Altenburger, R.; Schüürmann, G.; Krauss, M.; López Herráez, D.; van Gils, J.; Slobodnik, J.; Munthe, J.; Manfred Gawlik, B.; van Wezel, A.; et al. Umbuzeiro, The SOLUTIONS project: Challenges and responses for present and future emerging pollutants in land and water resources management. Sci. Total Environ. 2015, 503–504, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef] [Green Version]
- Calza, P.; Massolino, C.; Medana, C.; Baiocchi, C. Study of the photolytic and photocatalytic transformation of amiloride in water. J. Pharm. Biomed. Anal. 2008, 48, 315–320. [Google Scholar] [CrossRef]
- Martins, N.D.J.; Gomes, I.S.C.; da Silva, G.T.S.T.; Torres, J.A.; Avansi, W., Jr.; Ribeiro, C.; Malagutti, A.R.; Mourão, H.A.J.L. Facile preparation of ZnO:g-C3N4 heterostructures and their application in amiloride photodegradation and CO2 photoreduction. J. Alloys Compd. 2020, 856, 156798. [Google Scholar] [CrossRef]
- De Luca, M.; Ioele, G.; Mas, S.; Tauler, R.; Ragno, G. A study of pH-dependent photodegradation of amiloride by a multivariate curve resolution approach to combined kinetic and acid–base titration UV data. Analyst 2015, 137, 5428–5435. [Google Scholar] [CrossRef]
- Wang, C.J.; Li, Z.; Jiang, W.T. Adsorption of ciprofloxacin on 2:1 dioctahedral clay minerals. Appl. Clay Sci. 2011, 53, 723–728. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Z.; Wang, B.; An, H.; Chen, Z.; Cui, H. Adsorption and photocatalytic degradation of tetracycline hydrochloride using a palygorskite-supported Cu2O-TiO2 composite. Appl. Clay Sci. 2016, 119, 311–320. [Google Scholar] [CrossRef]
- Gil, A.; Santamaría, L.; Korili, S.A.; Vicente, M.A.; Barbosa, L.V.; Souza, S.D.; Marçal, L.; Faria, E.H.; Ciuffi, K.J. A review of organic-inorganic hybrid clay based adsorbents for contaminants removal: Synthesis, perspectives and applications. J. Environ. Chem. Eng. 2021, 9, 105808. [Google Scholar] [CrossRef]
- Lima, L.C.B.; Coelho, C.C.; Silva, F.C.; Meneguin, A.B.; Barud, H.S.; Bezerra, R.D.S.; Viseras, C.; Osajima, J.A.; Silva-Filho, E.C. Hybrid Systems Based on Talc and Chitosan for Controlled Drug Release. Materials 2019, 12, 3634. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Pérez, M.O. Supported, bulk and bulk-supported vanadium oxide catalysts: A short review with an historical perspective. Catal. Today 2017, 285, 226–233. [Google Scholar] [CrossRef]
- Mcbride, M.B. Mobility and reactions of VO2+ on hydrated smectite surfaces I. Clays Clay Miner. 1979, 27, 91–96. [Google Scholar] [CrossRef]
- Zid, T.B.; Fadhli, M.; Khedher, I.; Fraile, J.M. New bis(oxazoline)–vanadyl complexes, supported by electrostatic interaction in Laponite clay, asheterogeneous catalysts forasymmetric oxidation of methyl phenyl sulfide. Microporous Mesoporous Mater. 2016, 239, 167–172. [Google Scholar]
- Gao, X.; Xu, J. A new application of clay-supported vanadium oxide catalyst to selective hydroxylation of benzene to phenol. Appl. Clay Sci. 2006, 33, 1–6. [Google Scholar] [CrossRef]
- Soriano, M.D.; Cecilia, J.A.; Natoli, A.; Jiménez-Jiménez, J.; López Nieto, J.M.; Rodríguez-Castellón, E. Vanadium oxide supported on porous clay heterostructure for the partial oxidation of hydrogen sulphide to sulfur. Catal. Today 2015, 254, 36–42. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Zheng, J.; Hu, T.; Meng, C. Synthesis, structure, optical and magnetic properties of interlamellar decoration of magadiite using vanadium oxide species. Microporous Mesoporous Mater. 2017, 244, 264–277. [Google Scholar] [CrossRef]
- Dohrmann, R. Cation exchange capacity methodology I: An efficient model for the detection of incorrect cation exchange capacity and exchangeable cation results. Appl. Clay Sci. 2006, 34, 31–37. [Google Scholar] [CrossRef]
- Brito, D.F.; Silva-Filho, E.C.; Fonseca, M.G.; Jaber, M. Organophilic bentonites obtained by microwave heating as adsorbents for anionic dyes. J. Environ. Chem. Eng. 2018, 6, 7080–7090. [Google Scholar] [CrossRef] [Green Version]
- Amilwide, D.J. Amiloride: Transport a molecular probe of sodium in tissues and cells. Am. J. Physiol. 1982, 242, 131–145. [Google Scholar]
- Cullity, B.D.; Stock, S.R. Elements of X ray Diffraction, 3rd ed.; AW Publish: New York, NY, USA, 1967. [Google Scholar]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster kungliga svenska vetenskapsakdemiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils1. Soil Sci. Soc. Am. J. 1984, 44, 265–268. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Temkin, M.J.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Physicochim. USSR 1940, 12, 217–222. [Google Scholar]
- Lima, É.C.; Adebayo, M.A.; Machado, F.M. Kinetic and Equilibrium Models of Adsorption. In Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications, 1st ed.; Bergmann, C.P., Machado, F.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 33–69. [Google Scholar]
- Bergaya, F.; Lagaly, G. General introduction: Clays, clay minerals, and clay science. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–18. [Google Scholar]
- Lagaly, G.; Ogawa, M.; Dékány, I. Clay mineral–organic interactions. In Handbook of Clay Science, 2nd ed.; Bergaya, F., Lagaly, G., Eds.; Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 435–505. [Google Scholar]
- Günister, E.; Pestreli, Ü.D.C.H.; Atici, O.; Güngör, N. Synthesis and characterization of chitosan-MMT biocomposite systems. Carbohydr. Polym. 2007, 67, 358–365. [Google Scholar] [CrossRef]
- Jiang, Z.; Klyukin, K.; Alexandrov, V. Structure, hydrolysis and diffusion of aqueous vanadium ions from Car-Parrinello molecular dynamics. J. Chem. Phys. 2016, 145, 114303. [Google Scholar] [CrossRef] [Green Version]
- Madejová, J.; Bujdák, J.; Janek, M.; Komadel, P. Comparative FT-IR study of structural modifications during acid treatment of dioctahedral smectites and hectorite. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1998, 54, 1397–1406. [Google Scholar] [CrossRef]
- Zhen, Q.; Li, L.; Li, R.; Lu, F.; Li, Z.; Wang, Y. Morphology controllable preparation and infrared emissivity of vanadium pentoxide. Infrared Phys. Technol. 2015, 71, 303–306. [Google Scholar] [CrossRef]
- Mazzo, D.J.; Obetz, C.L.; Shuster, J.E. Analytical Profiles of Drugs Substances, 1st ed.; Florey, K., Brittain, H., Eds.; Elsevier: Amsterdam, Netherlands, 1986; pp. 237–301. [Google Scholar]
- Lin-Vien, D. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules, 1st ed.; Academic Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Bagnati, E.Z.R.; Natangelo, F.F.M.; Fanelli, D.C.R. Environmental Loads and Detection of Pharmaceuticals in Italy. Pharm. Environ. 2001, 3, 19–27. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).