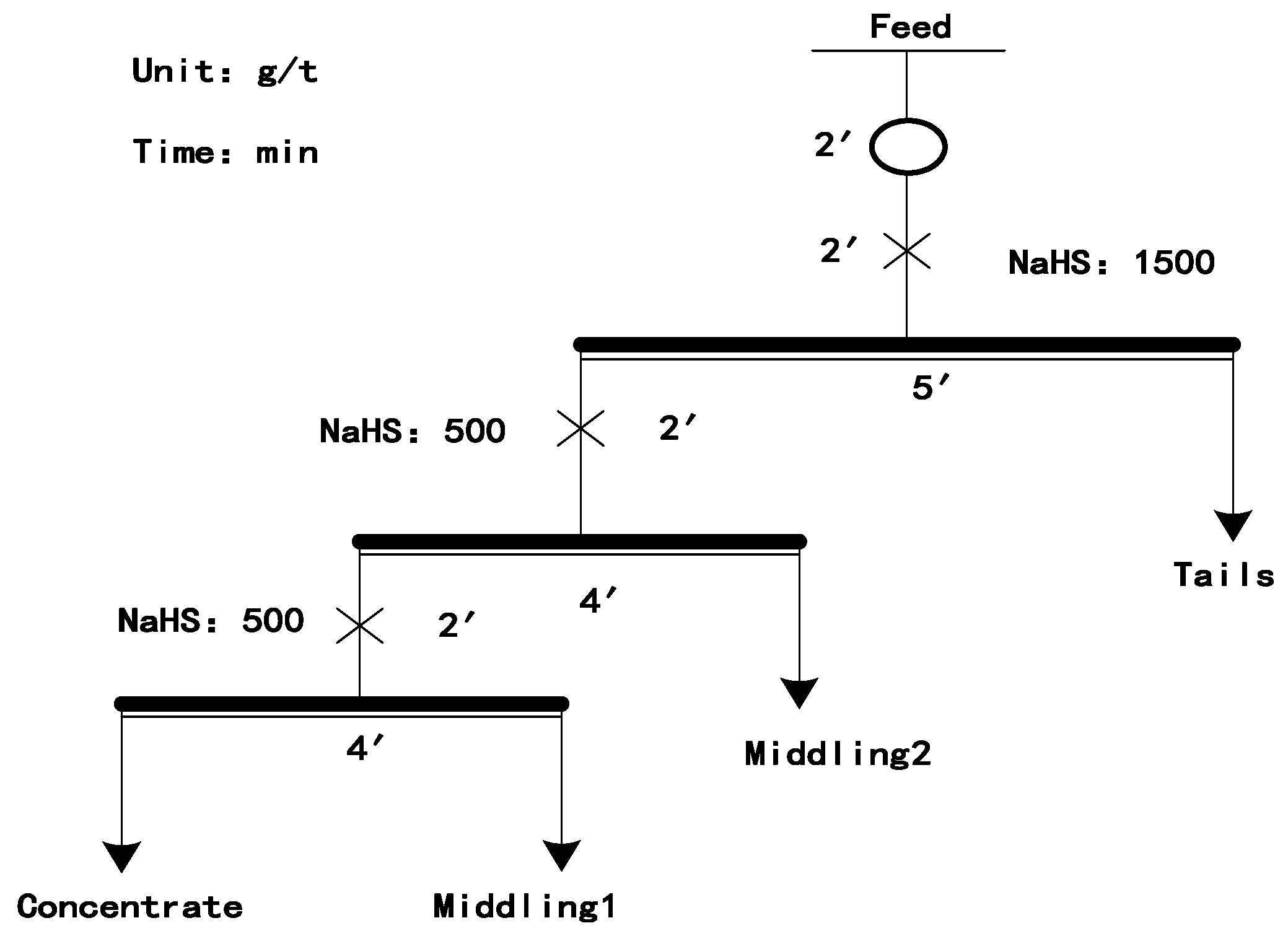4.1. Water Quality Analysis
Flotation is a water-intensive process, in order to minimize the use of freshwater, most mining operations in the world use recycled water, underground water, saline water, or seawater, which contain various inorganic and organic species. The content of these process water from different sites at Wushan plant are routinely measured on a daily basis.
Table 6 gives representative analysis data of water quality at Wushan. It is seen that the water recycled to flotation circuit contain quite amount of Ca (more than 300 mg/L), Fe (1~3 mg/L), Cu (5~6 mg/L). Calcium might partially come from the flotation units where alkaline pH has to be maintained using cost-effective lime (CaO or Ca(OH)
2) in order to depress pyrite. Iron is a result of inorganic flocculant added at water treatment plant, supplied by municipal sewage. Acid mine drainage occurs, especially at open pit and tailings dam where iron and copper can be increasingly present. Wushan is located at a high latitude and in a cold region, making the use of sewage water and drainage water necessary in winter and summer, respectively. In general, the water quality at the plant is poor, which may negatively affect the flotation at Cu-Mo separation circuit.
4.2. Activation of Silicate Minerals Flotation
It is obvious from process mineralogical data that silicate minerals are mostly liberated with minor interlocking with other sulfides. The access of silicate particles into the molybdenum concentrate could be due to probable flotation processes such as entrainment, slime coating, and most importantly, activation, which are not related to interlocking and degree of liberation. Fine particle entrainment in the froth is a common phenomenon in a lot of flotation situations. However, it does not necessarily imply that they are majorly responsible for the recovery of silicate minerals at Wushan operation, since their mass fraction of −600 mesh is only 16.24% (
Table 2). Overall, the surface properties should be responsible for their inadvertent activation in both bulk flotation circuit and Cu-Mo separation flotation circuit.
It is necessary to review the published research where a number of competing factors can be contributing to the flotation behavior of silicate minerals, especially their activation and depression mechanisms. Calcium ions can form colloidal hydroxides, carbonates, and sulfates, depressing the flotation of sulfide minerals using xanthate as collector at a relatively lower pH [
14]. The flotation of molybdenite can also be negatively affected by significantly high concentration of Ca
2+ and Mg
2+ ions that existing in sea water [
15,
16]. Hirajima et al. [
17] and Wisnu-Suyantara et al. [
18] reported the depressing effect of Ca
2+ ions on the flotation of molybdenite at pH > 9, which is possibly due to the adsorption of CaCO
3 precipitates on the molybdenite surface. A similar detrimental effect of Ca
2+ on molybdenite floatability was reported by Lucay et al. [
19] in the presence of sulfate ions. In contrast, the flotation of quartz can usually be activated by calcium ions when sodium oleate is used as collector [
8,
9,
10]. Moreover, Fe(III) and Cu(II) ions preferably activate the flotation of feldspar and quartz when NaOL is used as collector around pH 7 [
20]. These cations adsorb onto mineral surfaces, leading to an expected increase in NaOL collector adsorption with a subsequent enhancement of flotation recoveries. It has also been reported that quartz can be activated by Cu(II) and Ni(II) and floated with xanthate in the pH 7~10 [
21]. In this region, copper hydroxide and nickel hydroxide are stable species precipitating on the surface of silicate minerals, promoting xanthate adsorption. These results indicate the complexity of metal cations effect on the floatability of different minerals in a Cu-Mo ore.
Wushan’s bulk flotation circuit involves a combination of collectors which cannot be disclosed. The flotation collectors of copper sulfide minerals becomes more and more novel nowadays, with the main focus being on the application of non-thiol collectors [
22]. In this way, the molybdenite concentrate can easily be cleaned to yield a high Mo recovery with a low consumption of NaHS during Cu-Mo separation circuit. The adsorbed molecules can then be easily removed by NaHS from the chalcopyrite surface uncontaminated with thiol collectors, but this may not be easy for Ca
2+ activated silicate minerals because the Ca
2+ ions are not significantly affected by hydrosulfide ions. Thus, the activated silicate minerals retain their floatability when tranferring from bulk flotation circuit to Cu-Mo separation flotation circuit. The recovery of silicate minerals are generally lower than the recovery of copper and molybdenum in the bulk flotation circuit, but silicate recovery can be high at the beginning of Cu-Mo separation flotation circuit (see
Table 7). There is a probability distribution of particle-bubble collision and adhesion depending on many affecting factors, of which surface hydrophobicity definitely contributes. The activated and hydrophobic silicate particles are concentrated in bulk circuit and these particles can behave highly probable of collision and adhesion at the beginning of molybdenum circuit. In most cases of copper-molybdenum beneficiation plants, the recovery of silicate minerals is low, less than 20% by using fresh water [
23]. The inadvertent activation of silicate minerals in Wushan’s case should be a due to the presence of cations in processed water and the recent use of novel copper collectors.
Moreover, flocculants are also used at Wushan’s flotation circuit due to the increasing number of fine particles released by grinding. It is seen from
Figure 5 that Wushan’s flotation feed contains quite amount of fine chalcopyrite particles less than 10 μm. Mineralogy shows the mass fraction of ≤10 μm accounts for 27.58% of copper minerals and as high as 36.45% of molybdenum minerals. Low flotation efficiency has been suffered in the beneficiation of low-grade molybdenum ores due to finer size liberation and crystal anisotropy [
24,
25]. Finer sizes for a high degree of liberation were required for finely disseminated low-grade ores, which reduced the collision and adhesion probabilities of particles to bubbles. As reported, polyethylene oxide (PEO) has been proved to improve flotation efficiency of micro-fine molybdenite particles visibly [
23,
26,
27]. Such flocculants adsorbed onto the mineral surface while bridging particles by the hydrophobic long hydrocarbon chain. Thus, flocculants not only induce the flocculation of fine particles but also improve the surface hydrophobicity, both of which improve the flotation performances of molybdenite and chalcopyrite fine particles. However, the selectivity of flocculants is normally poor, anchoring on silicate and other minerals as well [
27,
28]. These minerals can then be activated in the floc-flotation. Moreover, molybdenite and quartz fine particles may induce heterogeneous flocculation, contaminating the concentrate.
4.3. Surface Analysis
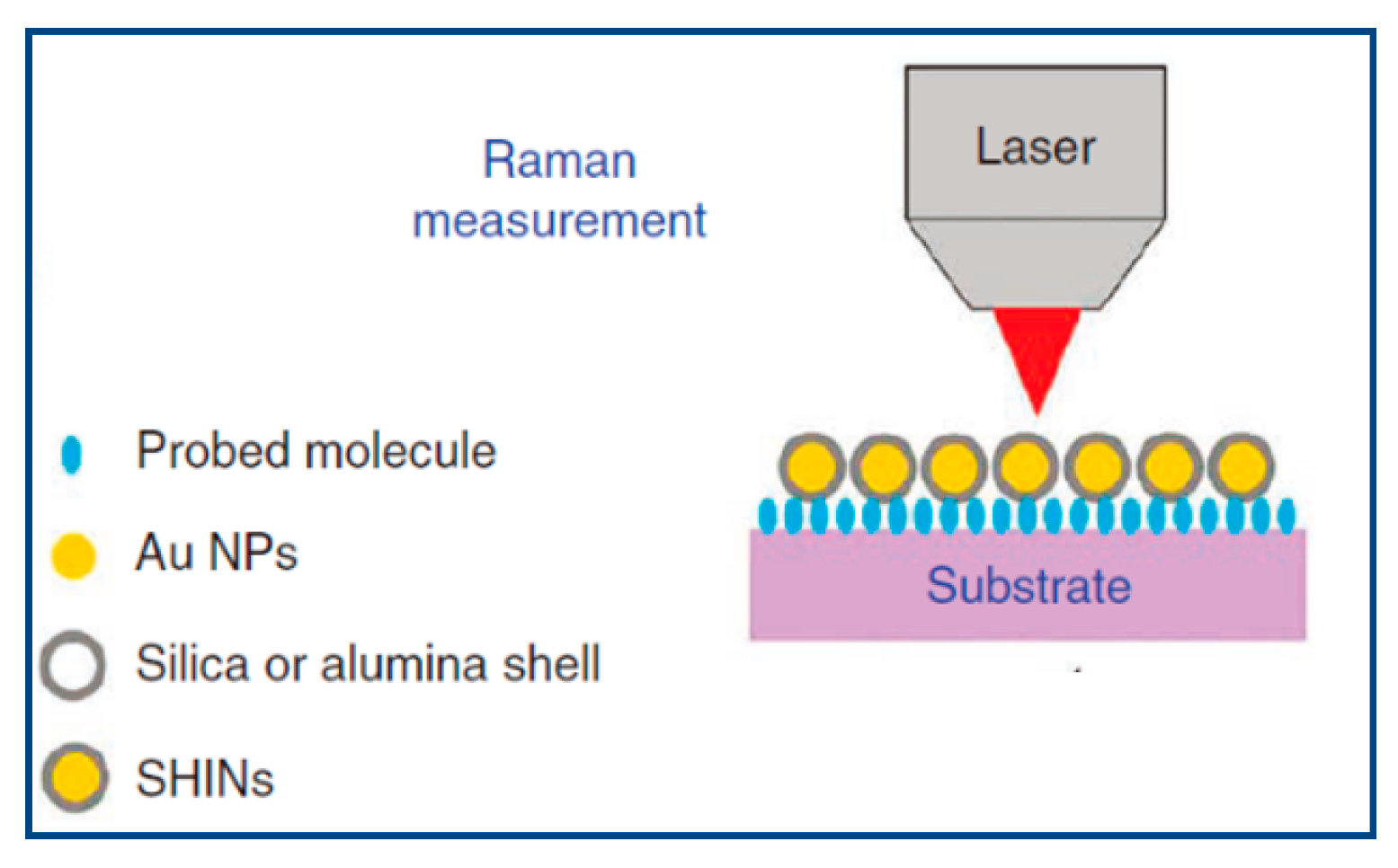
In order to prove the proposed hypothesis regarding the inadvertent activation of silicate minerals in flotation, surface properties of typical quartz mineral were analyzed in response to CaCl2 and collectors by using in-situ SHINERS technique.
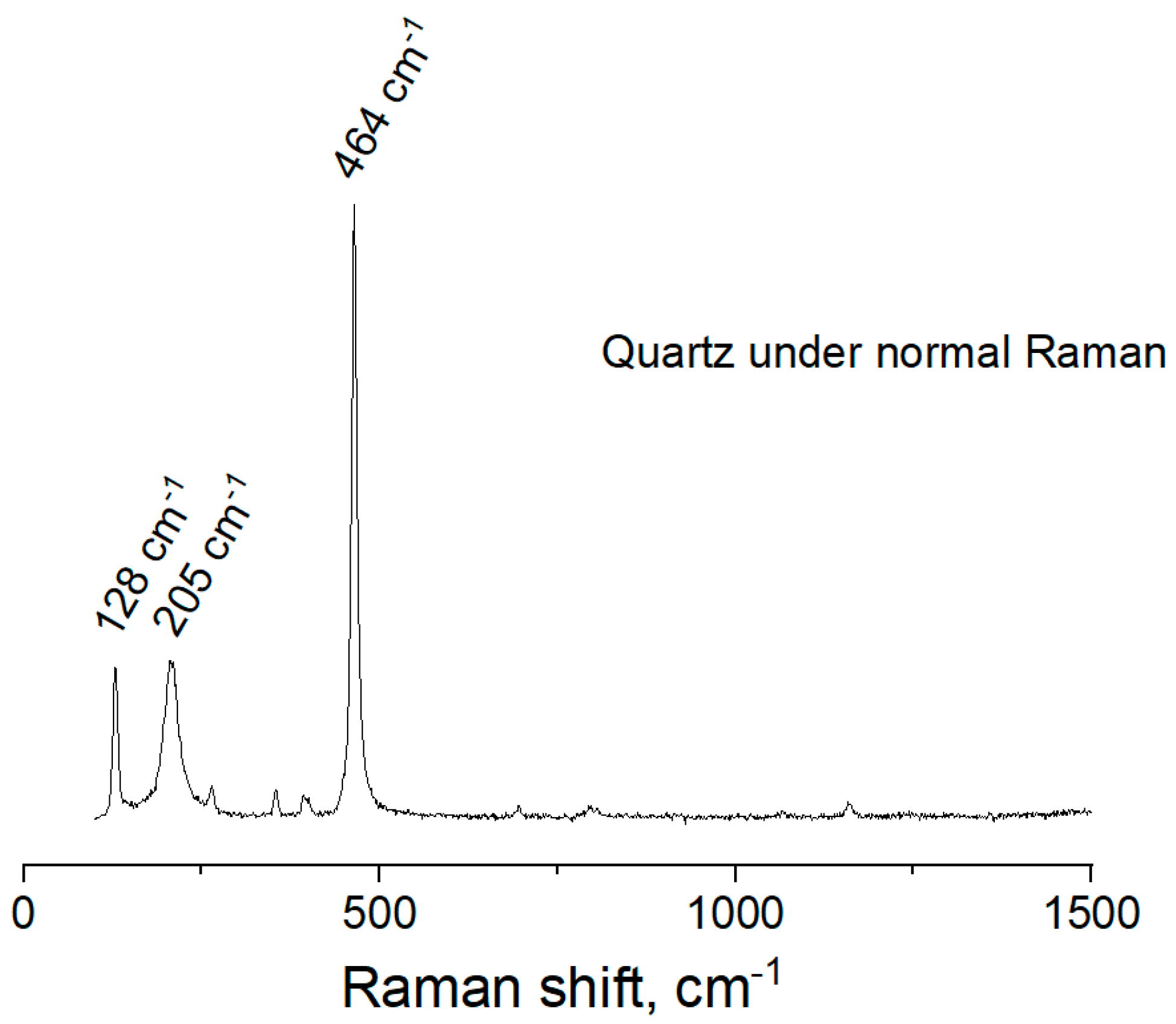
The normal Raman spectra of bulk quartz sample after dry abrading is shown in
Figure 6. The intense Raman bands at 128 cm
−1, 205 cm
−1 and 464 cm
−1 are indicative of quartz. For example, the strong peaks at 205 cm
−1 and 464 cm
−1 can be assigned to Raman active modes of type A1 in quartz crystal. The band at 128 cm
−1, corresponds to the Si-O-Si bending vibration, as previously described by Koshchug et al. [
29] and Dračínský et al. [
30].
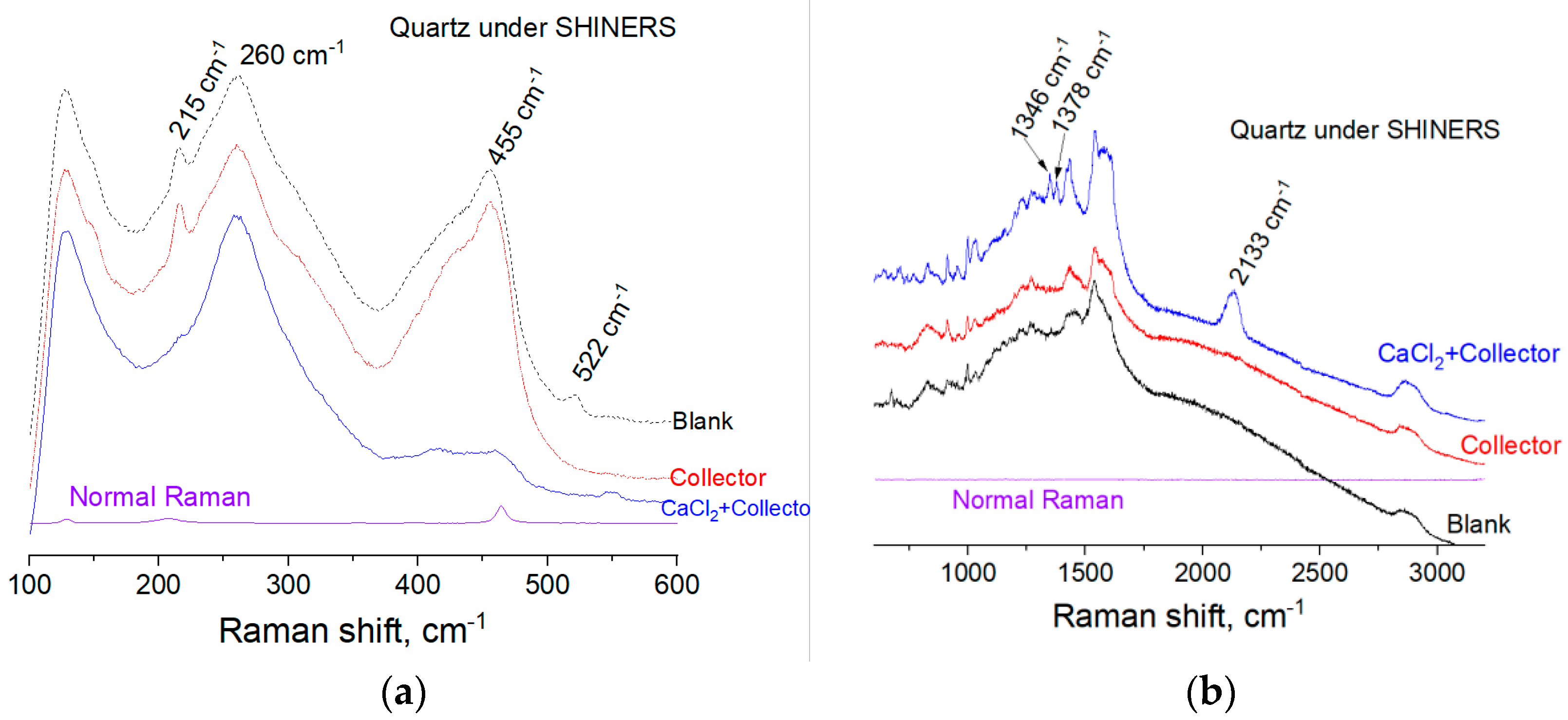
The SHINERS spectra of quartz after dry abrading and SHINs deposition is shown in
Figure 7. In
Figure 7, normal Raman vibrational modes become less obvious in the SHINERS spectra since the SERS signal is a great deal more intense than the bulk mineral signal. The blank sample’s spectra in
Figure 7 were obtained with Raman laser focusing on SHINs deposited area exposing to air, without any presence of aqueous solution. The peaks at 260 cm
−1, 455 cm
−1 and between 2800~3000 cm
−1, a typical region for hydrocarbon vibrations, are probably corresponding to the adsorbed polluting species. These polluting species exist when sample was exposed to air during pre-treatment and physical adsorption may occur as a result of electrostatic attraction, since the quartz surface is largely negatively charged.
Copper collectors implemented at Wushan Operation include SFJ CIBA and RJ-053. A pre-determined concentration of these collector mixture (10
−4 M SFJ CIBA and 10
−4 M RJ-053) was reacted with the SHINs deposited quartz surface for 20 min before SHINERS spectra can be taken. Upon reaction with the collector mixture, the SHINERS spectra is similar to the blank spectra, indicating the absence of collector adsorption on inactivated quartz surface. The SHINs deposited quartz sample was also treated with 0.005 M CaCl
2 solution for 20 min and subsequently treated with 0.005M CaCl
2 + 10
−4 M SFJ CIBA + 10
−4 M RJ-053 mixture solution for another 20 min. The SHINERS spectra were carried out in-situ with a thin film of corresponding solution covering the sample surface. It is seen from
Figure 8 that new peaks appear on the spectra compared to the sample treated with collector only. A full list of Raman bands assignment can be difficult as the molecule structure of the marketed products are unknown. However, the vibrational bands at 1346 cm
−1, 1378 cm
−1 and 2133 cm
−1 should be a result of collectors’ adsorption onto quartz surface. Moreover, characteristic bands at 215 cm
−1 and 455 cm
−1 for polluting species are greatly diminished, which is associated with the adsorption of collectors.
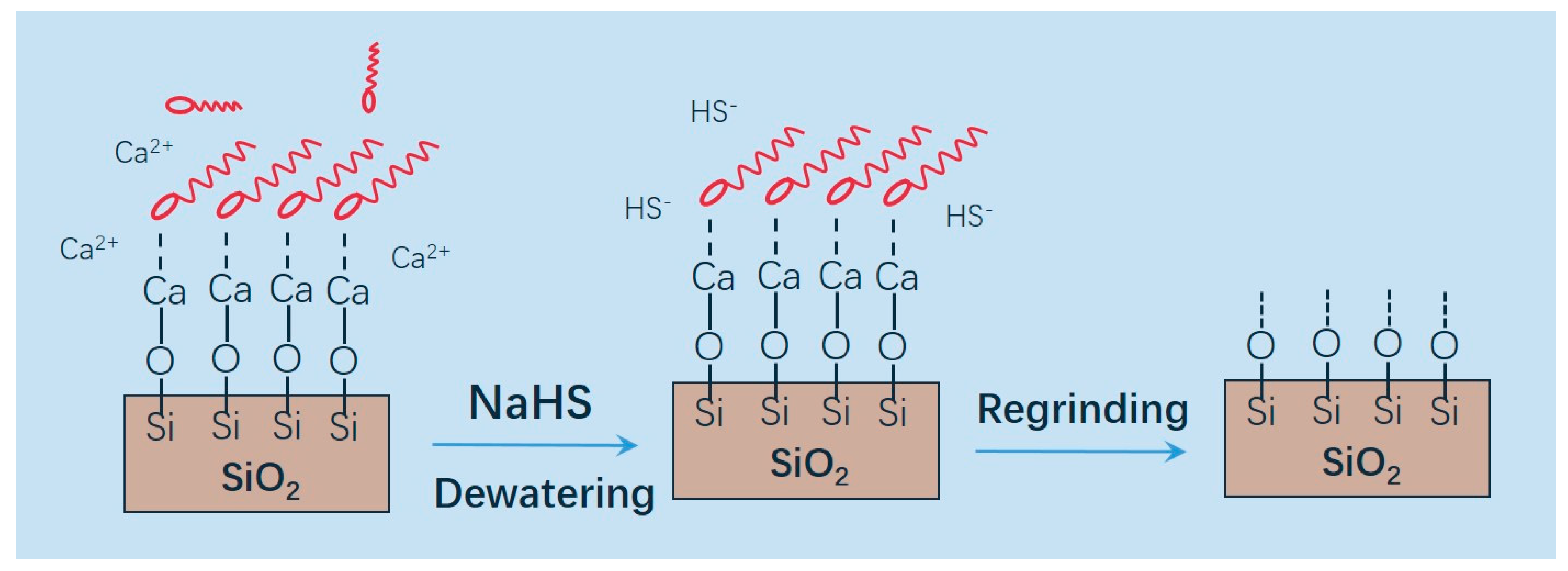
It is implicated that the novel copper collectors at Wushan Operation can also adsorb onto Ca
2+-activated quartz, rendering its surface hydrophobic. As HS
− does not complex with Ca
2+ or quartz, the activated surface can retain its surface hydrophobicity during the process of NaHS depression. According to the water analysis in
Table 6, calcium ions concentration was largely higher than iron and copper ions, reasonaly accounting for the activation of silicate minerals. Mechanical damage during regrinding may remove the surface layer, in addition to particle size reduction. The proposed mechanism of quartz activation and depression is schematically illustrated in
Figure 8.
4.4. Depression by Water Glass
Sodium silicate solution (commonly known as water glass) is a typical depressant for silicate minerals’ flotation [
31,
32,
33]. Molecular dynamics simulations and density functional theory calculations demonstrated that water glass formed a dense adsorption layer on quartz, with both strong hydrogen bond and covalent bond being the main driving forces for adsorption [
34]. The stable adsorption layer prevents Ca
2+, Mg
2+, and collectors from contacting quartz. Meanwhile, adsorption increases the electronegativity of quartz particles and enhances their dispersion. The aggregation between valuable minerals and fine gangue minerals can then be greatly reduced, avoiding the deterioration of concentrate grade.
The rougher flotation tests carried out are summarized in
Table 7. Adding appropriate amount of water glass is beneficial to reduce silica in the concentrate without compromising molybdenum recovery too much. The silica grade of molybdenum concentrate is reduced from 52.39% to 35.12% by merely using rougher flotation and the quality of molybdenum concentrate could be further improved if cleaner stage is included.
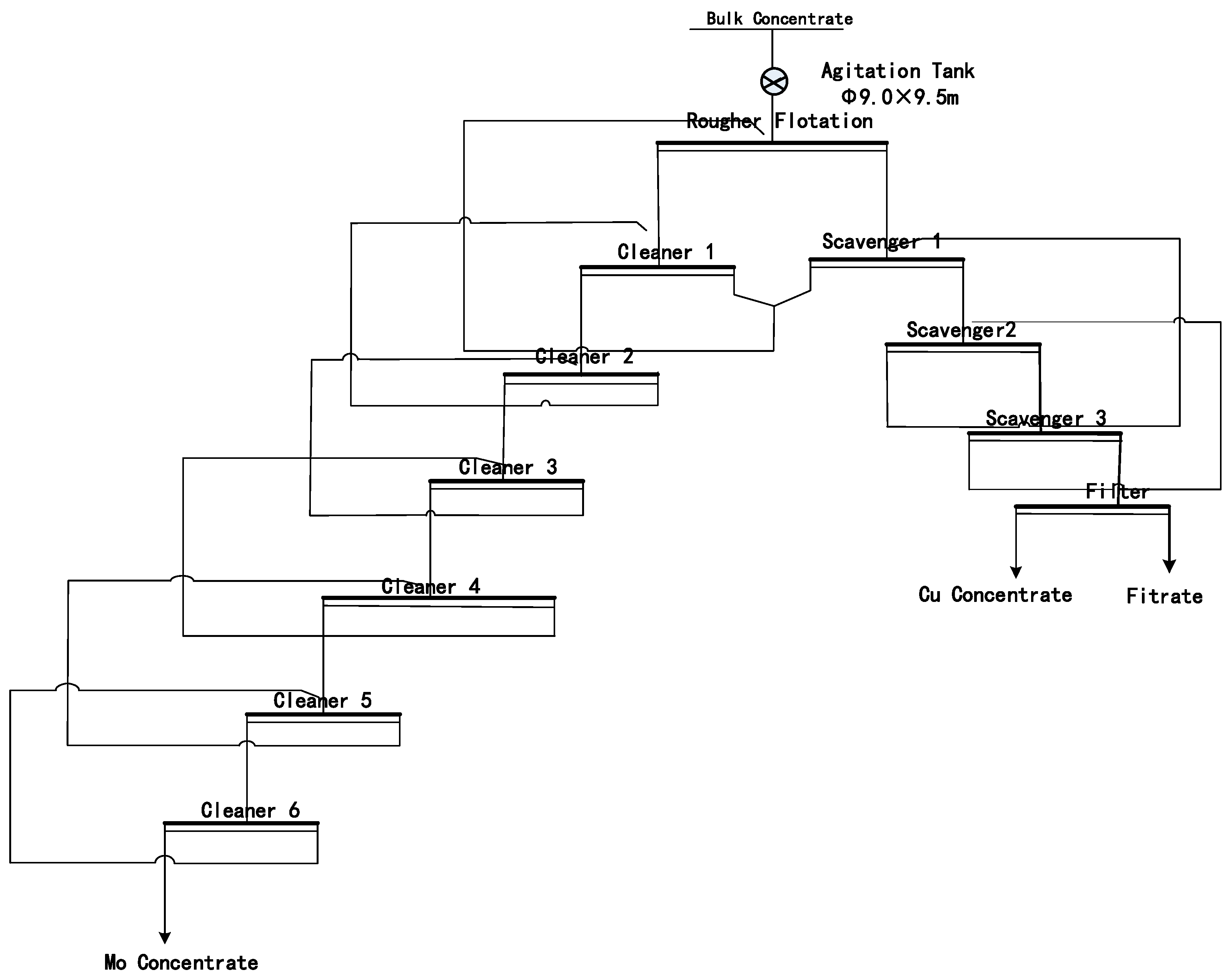
4.5. Depression by Regrinding
As presented in the process flowsheet, the regrind mill is operating in open circuit. Open circuit grinding is very well-known for producing a flat size distribution with a lot of unnecessary ultra-fines. It is seen from
Table 8 that regrinding for 2 min only makes a mass fraction of −325 mesh increasing from 34% to 50%, while the silica content in molybdenum concentrate has been effectively reduced. Molybdenum grade in the concentrate after one stage of rougher and two stages of cleaner operation has been increased to 41.96%, which is 3.79% higher than that without regrinding. It is also seen that the recovery of molybdenite and the mass recovery slightly decreased to some extent, compromising the separation efficiency. Therefore, great caution needs to be taken for the use of regrinding in molybdenum circuit.
In the grinding process, both abrasion/attrition and breakage could be the mechanisms of comminution actions taking place. Attrition may be helpful to obtain freshly fractured surface without reducing particle size too much [
35]. The contaminated silicate mineral particles surface can then be cleaned, exhibiting their naturally owned hydrophilic characteristics. Size-by-size analysis of the feed in
Table 9 indicates molybdenite particle size is slightly reduced as +325 mesh mass fraction decreasing from 43.00% to 36.96%, while its mass fraction of −600 mesh remains unchanged. However, silicate particle size significantly reduced as +325 mesh mass fraction decreasing from 84.10% to 62.98%. In particular, the mass fraction of −600 mesh increased from 4.64% to 14.18%. The size-by-size analysis implicates that silicate minerals are easier to grind compared to molybdenite. The blade-shaped grains of molybdenite are largely restored in grinding.
Most minerals exhibit the typical size dependent recovery as illustrated by Trahar [
36] with the maximum recovery being achieved in the intermediate size fractions and the coarse and fine particles being recovered at a lower rate. For both molybdenum and copper, their recoveries are highest in the range of 20~150 μm [
36]. In the case of molybdenum, the finer than 20 μm fraction recovers 10% lower than the optimum size range. The more fine silicate mineral particles that grinding produces, the more difficult their flotation. In contrast, the effects of grinding on molybdenite particle size and the subsequent flotation performance are insignificant.
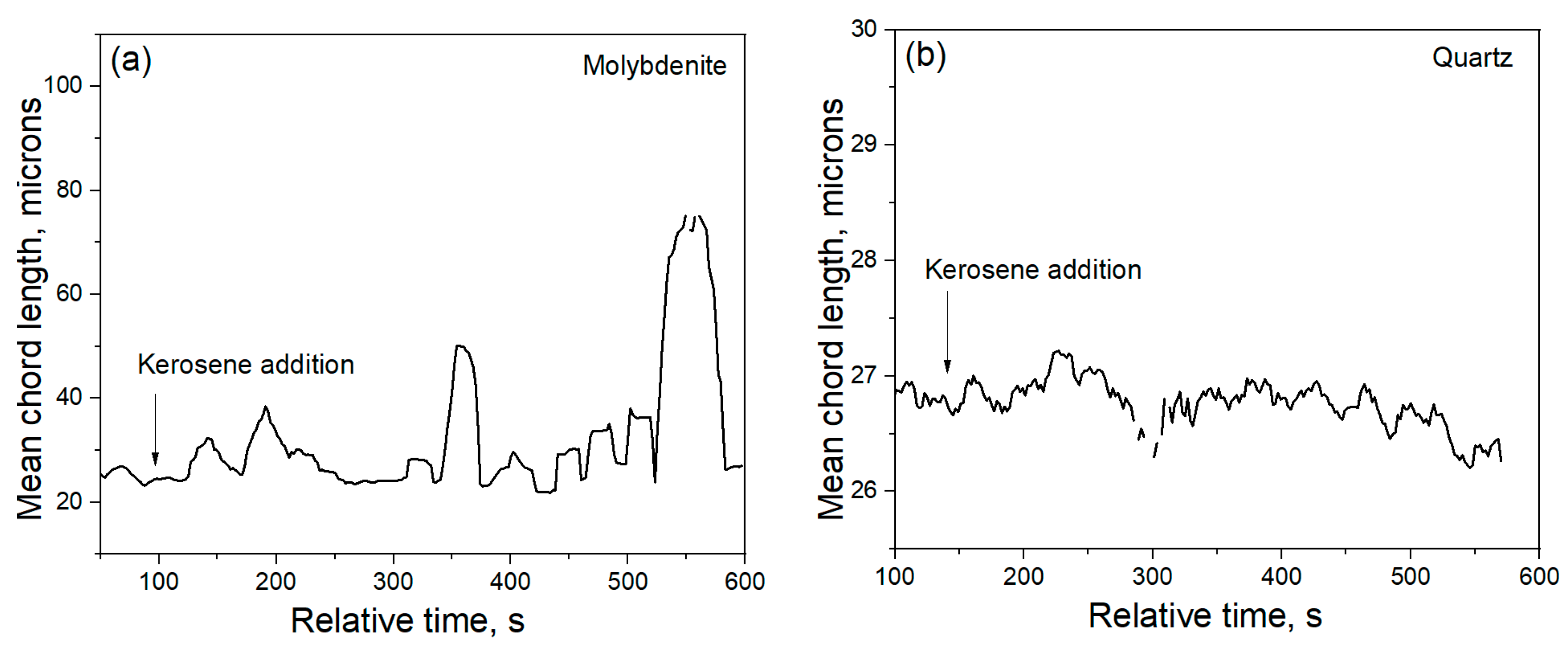
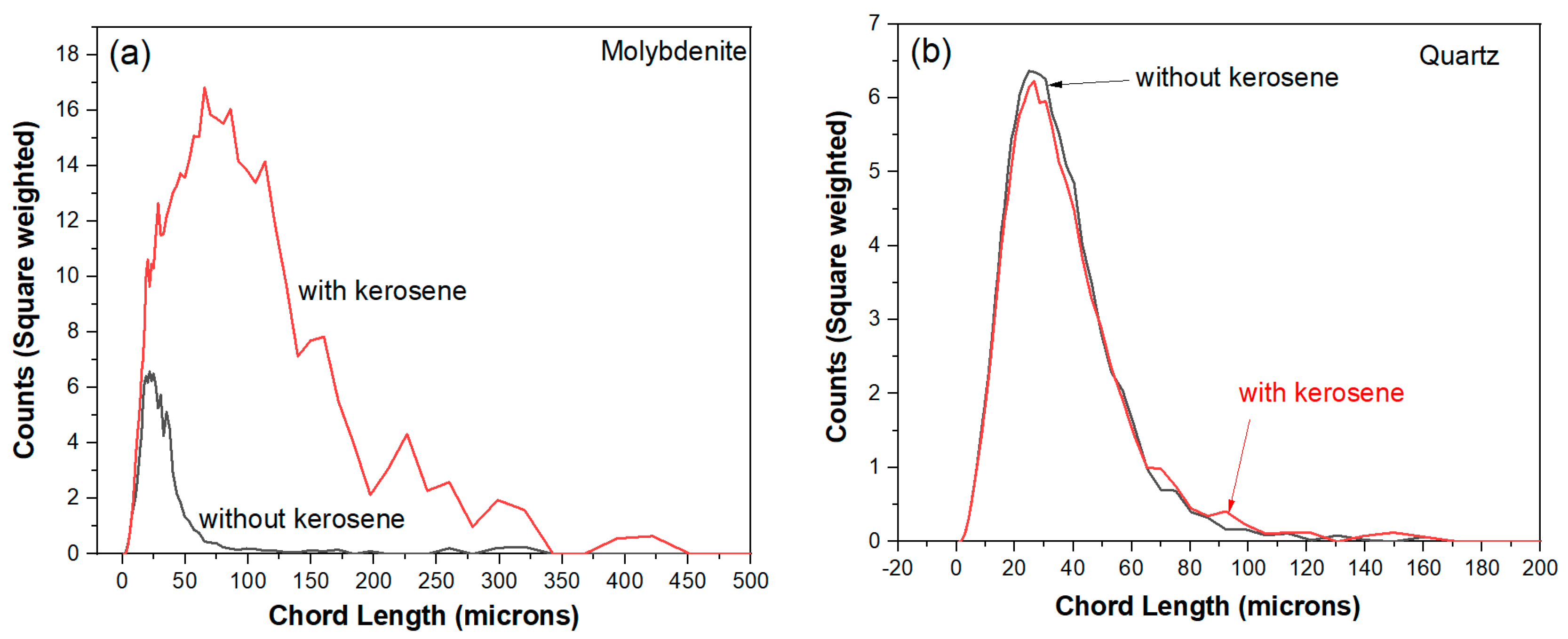
Flocculants has a prominent effect in the microfine molybdenite flotation. In addition, kerosene not only acts at collector, but also induce the formation of large flocs due to the hydrophobic force attraction between molybdenite fine particles. The advanced FBRM instrument can give an online measurement of particle size, rendering flocculation study easier. As seen in
Figure 9, molybdenite fine particles exhibited a mean chord length of 26 μm before the addition of any reagent, but it increased to 75 μm shortly after the addition of 0.35 g/L kerosene.
Figure 10 shows that, in square weighted mode, large flocs above average chord length are commonly seen. These molybdenite flocs are easily floated onto the surface of slurry even without the presence of dedicated bubbling. The high points of chord length in
Figure 9 were obtained when FBRM probe was lifted adjacent to the surface of slurry, where large flocs are concentrated. Flocs of quartz particles were hardly seen with the addition of kerosene, in accordance with the unchanged mean chord length and its distribution as shown in
Figure 9 and
Figure 10, respectively.
Hydrophilic silicate mineral particles are not prone to be attractive to each other, therefore flocculation can hardly occur between these particles. The flocs size is conducive to the flotation of microfine molybdenite, leading to enhanced selectivity against silicate minerals. In conclusion, slightly regrinding is beneficial for improving the quality of molybdenum concentrate for two main reasons: firstly, the exposure of nature hydrophilic silicate particle surface upon attrition. Secondly, relatively finer silicate particles lead to poorer recovery. The different particle size was due to differences of grindability between molybdenite and quartz, in addition to flocculation between molybdenite and silicate minerals. The different performances of quartz and molybdenite particles subjecting to regrinding and kerosene are schematically illustrated in
Figure 11 for better understanding.