Abstract
Flotation is the most often employed process to achieve the selective removal of contaminants from the raw materials used in the manufacturing of phosphate fertilizer. However, sodium oleate (NaOL), as a typical collector, is ineffective because of its low collecting ability under low temperature. As a result, developing and implementing feasible alternatives is critical for the long-term output of mines. In this study, sodium dodecyl benzene sulfonate (SDBS), a low-cost and freely soluble reagent under low temperature was used to examine its collecting ability and selectivity in a fluorapatite-dolomite system by means of single and artificially mixed minerals flotation. The adsorption mechanism was evaluated with the help of XPS analyses. The flotation results demonstrate that SDBS could float both fluorapatite and dolomite, but show a higher affinity towards fluorapatite instead of dolomite. Moreover, SDBS could preferred adsorb onto fluorapatite surface when fluorapatite and dolomite coexist. SDBS is more suitable than NaOL for satisfactory recovery of fluorapatite under low temperature in terms of the higher recovery obtained. The XPS analyses results demonstrate that the adsorption of SDBS on fluorapatite surface was more intensively as opposed to that on dolomite surface and Ca active sites on fluorapatite surface are supposed to be the main location for SDBS attachment.
1. Introduction
Since China is an agricultural country with a vast population, agriculture is the backbone of the country’s economy. Chemical fertilizer has long been a vital component in food production. It is also crucial for China’s food supply, which feeds over one billion people. Phosphate rock deposits are an essential raw material for the manufacturing of phosphate fertilizers [] (Li et al., 2021), therefore they have become a vital mineral resource for food security, and even a role in defining the country’s stability []. The wet and pyrogenic processes will be utilized to extract phosphoric acid and phosphorus from high-grade phosphate ore, which can then be used to make different phosphate fertilizers and phosphates []. However, as the world’s population grows and phosphate demand rises, high-grade phosphate ores with low impurity are becoming scarce. Since they have a low P2O5 grade and typically contain a variety of gangue minerals, such as quartz, mica, feldspar, dolomite, calcite, clays, and so on, most phosphate ores are not appropriate for direct use in the acidulation process. As a result, the phosphate beneficiation sector has a significant challenge: how to economically and efficiently use these low-grade phosphate ores [].
Since low-grade phosphate ores must be processed to produce a high-quality phosphate concentrate, beneficiation procedures and reagents are crucial for achieving phosphate mineral separation. apatite is commonly linked with gangue minerals such as quartz, iron oxides, clays, and calcareous minerals in phosphate ores because it is one of the most important phosphorus-bearing minerals (e.g., dolomite and calcite) [,]. In the mineral processing, dolomite as a low-value mineral is usually removed from valuable minerals, such as apatite [], magnesite [], and rare earth minerals (e.g., monazite and bastnasite) []. However, due to their comparable physicochemical properties, successful separation of apatite and dolomite remains a major difficulty. As a result, it is critical to find effective ways for recovering apatite from dolomite [].
Flotation is a separation and concentrate method that uses the physicochemical features of minerals to separate valuable minerals from gangue minerals [,,], is regarded as the most effective method for removing dolomite from apatite []. Traditional fatty acid collectors, particularly oleic acid or its salts, are still the most widely used to separate apatite from dolomite because they are simple to make and inexpensive [,]. However, because of the non-selective adsorption behavior of these collectors towards both minerals by interacting with adsorption sites, the separation efficiency is still not sufficient [,]. Furthermore, due to their limited solubility and dispersion in solution at low temperatures, these collectors are completely ineffective, resulting in an unsatisfactory effect under the same flotation circumstances [,]. Research shows that mixed surfactant systems using fatty acids and nonionic surfactant with higher surface activities can improve the flotation performance of minerals at low temperature, and have been successfully applied in actual production []. However, the use of additional reagents would result in an extra cost, which is not highly effective. As a result, developing cost-effective and selective collectors that can be employed at low temperatures is critical for the flotation of phosphate ore [].
As a traditional anionic surfactant, SDBS was used to reduce the interfacial tension of oil/water because of its freely solubility and dispersibility, as well as to collect metal ions. To our best of knowledge, the flotation performance of SDBS as a single collector has not been proposed yet in an apatite-dolomite mixed system under low temperature. As such, the objective of this work is to evaluate the collecting ability and selectivity of SDBS on apatite and dolomite under low temperature and then describe the adsorption mechanism with the help of XPS analyses to make this study more comprehensive. In this work, fluorapatite and dolomite were selected as the typical valuable and gangue minerals for flotation experiments.
2. Materials and Methods
2.1. Mineral Samples
Fluorapatite and dolomite with high purity were purchased from Guangdong and Hubei, China. Firstly, both minerals were broken and powdered carefully, and subsequently wet sieved and dried to generate desired size fraction for further experiments. The size fraction of 38–75 μm was applied for micro-flotation experiments, whereas the −5 μm size fraction samples were used for XPS analyses. The X-ray diffraction patterns and chemical compositions of pure fluorapatite and dolomite are presented in Figure 1 and Table 1 and Table 2. X-ray diffraction (XRD) analysis was performed by a Bruker D8 ADVANCE X-ray diffractometer at a voltage of 40 kV and a current of 40 mA with Cu K radiation []. The chemical compositions were detected by using X-ray fluorescence (Zetium analyzer manufactured by Panalytical B. V company, Netherlands). From the results, it can be inferred that both fluorapatite and dolomite are of high purity as the characteristic peaks belonged to fluorapatite and dolomite are sharp and intensive [], some of the peaks cannot be detected are ascribed to the impurities.
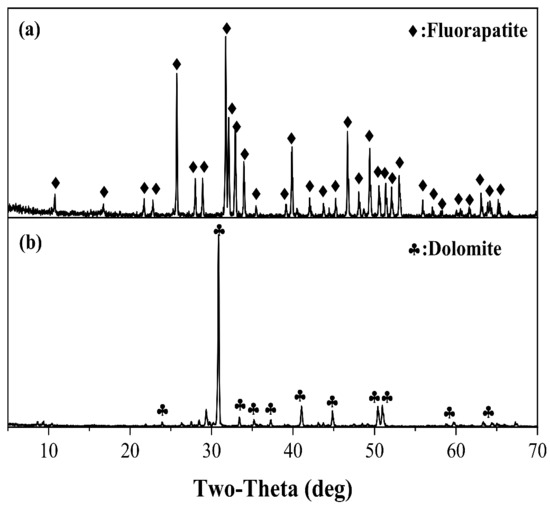
Figure 1.
XRD patterns of (a) Fluorapatite and (b) Dolomite.

Table 1.
Chemical composition of fluorapatite (wt.%).

Table 2.
Chemical composition of dolomite.
2.2. Chemical Reagents
In this work, sodium dodecyl benzene sulfonate (SDBS) and sodium oleate (NaOL) with analytical purity were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China and used as collector. Diluted sodium carbonate (Na2CO3) and hydrochloric acid (HCl) were used as pH regulators. Moreover, deionized water (18.25 MΩ) obtained from a Milli-Q Direct 16 (Millipore Q, Burlington, MA, USA) was used throughout the experiments.
2.3. Flotation Tests
The micro flotation experiments were conducted using an XFG-type flotation machine (Wuhan Exploration Machinery Co., Ltd., Wuhan, China) with a 50 mL flotation cell and an impeller speed of 1800 rpm, Figure 2 presents the schematic of XFG-II flotation machine [,]. As for the single mineral flotation, the procedure was described as follows: firstly, 2 g of fluorapatite/dolomite was added in the flotation cell with 50 mL pure water, and then stirred 2 min for dispersion. After that the regulator (HCl/NaOH) was added to adjust the pH to desired value with an interval of 2 min. The pH device PB-10 used here was made by Sartorius, pH value and temperature were tested using such device. Then, NaOL/SDBS with certain concentration was added and agitated for another 2 min. Finally, the froth above the solution was collected using a scraper blade as concentrate and the sink product was filtrated as tailing. The floatability was calculated based on the dry weights of the concentrates (m1) and tails (m2) and given as Equation (1):
Floatability = m1/(m1 + m2)

Figure 2.
Schematic of XFG-II flotation machine.
As for the artificially mixed minerals flotation, the procedure was the same as mentioned above, the only difference was: 2 g minerals were made up of fluorapatite and dolomite with a mass ratio of 2:1. Through this mass ratio, a mixed mineral with P2O5 contents 25% could be generated (25% is the P2O5 contents of most of the low and medium phosphate ore). In mixed minerals flotation system, the concentrate collected contains both fluorapatite and dolomite, so here, P2O5 contents and recovery were used to evaluate the performance. The P2O5 contents was measured by acid-base titration and the recovery was calculated based on the Equation (2):
where, c is the P2O5 contents in the concentrate; t is the P2O5 contents in the tailing [,].
Recovery = [cm1/(cm1 + tm2)] × 100
2.4. XPS Analysis
XPS detection is commonly employed to characterize the changes in surface elements of mineral specimens after interaction with flotation chemicals. The mineral sample was analyzed by using a Thermo Scientific ESCALAB 250Xi instrument under 10−9 bar of air pressure [,]. Both dolomite and fluorapatite minerals with size fraction below 5 μm were added into the flotation cell and stirred for 2 min. Then 30 mg·L−1 SDBS collector was added into the pulp, the pH was adjusted to 9.6 with the help of diluted Na2CO3. The solution was then filtered and washed twice using pure water to remove the weak adsorption of collector. The filtered solids were dried in a vacuum drying oven and then analyzed by XPS instrument. The peaks fitting and data analysis were carried out with XPS software Thermo Advantage, and the standard Carbon binding energy was calibrated to 284.80 eV.
3. Results and Discussion
3.1. Effect of pH
pH is an important factor that affects the forms of chemicals existing in the solution, then changing the performance of the reagents in response [,,]. Dolomite is prone to being dissolved in acidic environment because of the presence of CO32−. Therefore, for dolomite, only alkaline environment has been tested here. As shown in Figure 3 it can be seen that the floatability of fluorapatite grew progressively from 65.33% to 93.86% across the pH range tested. Dolomite, on the other hand, had a lower floatability, with the highest floatability being about 63.57% at pH 7.28. The optimal pH value for flotation was around 9.6, at which point fluorapatite had a floatability of around 93% and dolomite had a floatability of 53.14%. The results demonstrate that when SDBS was applied as a collector, both dolomite and fluorapatite could float. The floatability of fluorapatite was always higher than that of dolomite over the pH range tested, indicating that SDBS showed a higher affinity towards fluorapatite rather than dolomite. Moreover, it should be noted that SDBS still exhibited high collecting ability even at low temperature, demonstrating that it could be employed as the potential alternative for effective fluorapatite recovery in hard area.
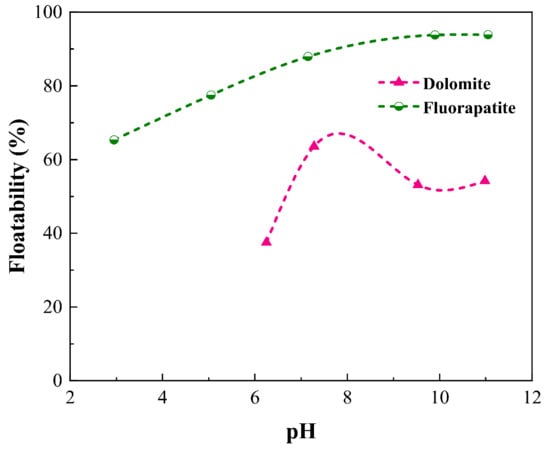
Figure 3.
Effect of pH on the floatability of fluorapatite and dolomite (CSDBS = 50 mg·L−1, temperature = 15 °C ± b 0.2).
3.2. Effect of Collector Concentration
The flotation performance of NaOL and SDBS was evaluated and compared with respect to collector concentration under low temperature and the results are presented in Figure 4. When NaOL was used as collector, the floatability of fluorapatite and dolomite both experienced an upward trend. The floatability of fluorapatite reached its biggest value when the NaOL concentration was 30 mg·L−1, assaying by 62%. A further increase of concentration caused negligible effect on the floatability improvement. The floatability of dolomite under this condition was 27.9%, which was 34.1% lower than that of fluorapatite. The results indicate that NaOL cannot successfully collect fluorapatite under low temperature in terms of the low floatability, it is because the dispersion and activity of NaOL in solution were affected by temperature []. In comparison with NaOL, the floatability of fluorapatite was always above 85% across the concentration tested when SDBS was applied. It should be noted that dolomite could also be collected with a relatively high floatability. It reached its biggest value when the SDBS concentration was 30 mg·L−1, coming to 61.56%, which was 27.54% lower than that of fluorapatite under the same condition. Based on the flotation results, it can be concluded that both SDBS and NaOL showed the collecting ability toward fluorapatite and dolomite, the floatability of fluorapatite was always higher than that of dolomite under the same collector concentration. The difference between SDBS and NaOL was that the former exhibited a stronger performance under low temperature in terms of the higher floatability of fluorapatite and dolomite, indicating that SDBS was more suitable to be used under hard condition.
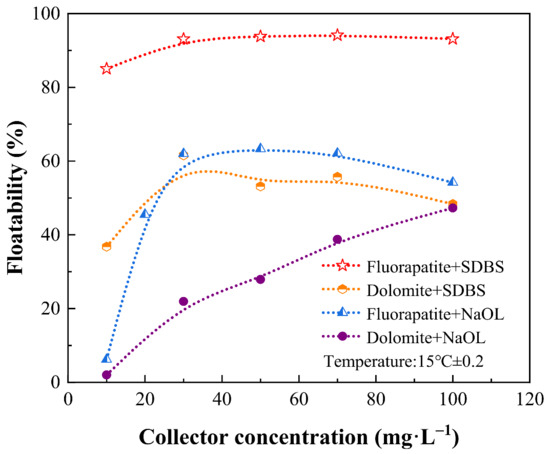
Figure 4.
Effect of SDBS concentration on the floatability of fluorapatite and dolomite (pH = 9.6).
3.3. Artificially Mixed Minerals Flotation
The selectivity of NaOL and SDBS under low temperature was evaluated with the help of artificially mixed minerals flotation, the results are presented in Figure 5a,b. In the presence of NaOL, the P2O5 contents kept stable around 36% without any big change over the concentration tested, indicating that NaOL exhibited an excellent selectivity in fluorapatite-dolomite system. However, the recovery was relatively low when the NaOL concentration was 30 mg·L−1, reaching 45.79%, and further gradually increased to 72.46% when the NaOL concentration was 100 mg·L−1 (Figure 5b). The results indicate that NaOL showed a high selectivity in mixed minerals system, but cannot collect valuable minerals as much as possible because of the limited temperature condition. Moreover, a further improvement of recovery would increase the addition of collector dosage, thereby causing an extra cost. As for SDBS collector, it can be seen that with the increase of collector concentration from 0 to 100 mg·L−1, the P2O5 contents gradually increased from 25% to 34.05% (Figure 5a), the recovery was almost stable around 90% without any big variation (Figure 5b). When the SDBS concentration was 30 mg·L−1, a concentrate with P2O5 contents of 32.8% and recovery of 96.63% could be obtained. The results indicate that SDBS exhibited a good selectivity in fluorapatite-dolomite system and can collect most of the fluorapatite. Moreover, the results obtained from mixed minerals flotation further prove that SDBS showed a high affinity towards fluorapatite rather than dolomite and it can preferred adsorb onto fluorapatite surface when fluorapatite and dolomite coexist. Regarding on the flotation results of SDBS and NaOL, it can be concluded that SDBS is more suitable than NaOL for satisfactory recovery of fluorapatite under low temperature.
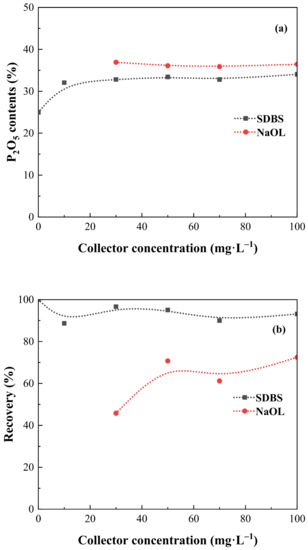
Figure 5.
Effect of SDBS concentration on the (a) P2O5 contents and (b) recovery (pH = 9.6, temperature = 15 °C ± 0.2).
3.4. XPS Analyses
In order to find the detailed adsorption mechanism of SDBS on minerals surfaces, XPS analyses were conducted. The Ca 2p, S 2p and O 1s XPS spectra and the fitting curves of fluorapatite before and after the treatment of SDBS were evaluated. The Ca 2p spectrum for pure fluorapatite contains two peaks of Ca 2p3/2 and Ca 2p1/2 at binding energy locations of 347.35 eV and 350.84 eV, respectively, as shown in Figure 6a []. Since the presence of SDBS affected the density of the electron atmosphere in the Ca atomic perimeter of the fluorapatite surface, the binding energy of Ca 2p shifted to 347.45 eV and 351.00 eV (shifted by 0.2 eV) when it was treated with SDBS. In comparison with other researches [,,], because the absolute value of repulsive potential on fluorapatite surface atoms varied under different chemical conditions, the variations in binding energy of Ca 2p were more noticeable than matching values obtained in this work. As a result, the hydrophobic flotation of fluorapatite may be attributed mostly to the active components RSO3− in the collector interacting with the Ca sites, resulting in the formation of a hydrophobic layer coverage. The peak at 169.27 eV after the addition of SDBS has shifted to 169.58 eV (shifted by 0.31 eV), indicating that SDBS has successfully adsorbed onto fluorapatite surface and the chemical surrounding of S atomic has experienced a large change, which may be attributed to chemical bonding between sulfo group and Ca sites []. As for O 1s, from Figure 6c it can be seen that the O 1s spectrum of fluorapatite after the treatment of SDBS was fitted into two peaks: PO43− (531.27 eV), -O-S=O (532.06 eV). The presence of -O-S=O indicates that chemical adsorption occurred at Ca sites on the fluorapatite surface and the reaction involved the sulfonate species of SDBS. The XPS analyses strongly demonstrate that SDBS showed a high affinity on fluorapatite and the interaction is mainly due the chemical bonding between sulfo groups and Ca sites.
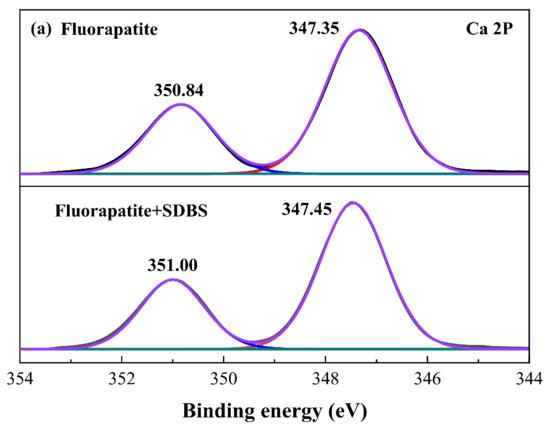
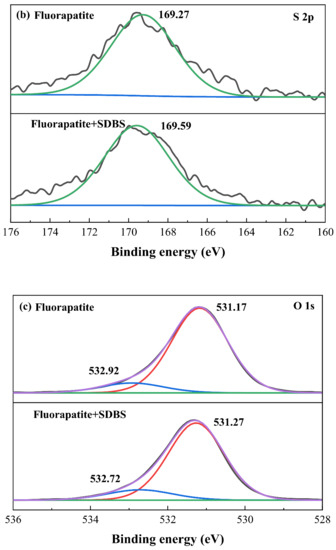
Figure 6.
(a) Ca 2p, (b) S 2p, and (c) O 1s of fluorapatite sample with and without the addition of SDBS.
As for dolomite, the S 2p, C 1s, Ca 2p, Mg 1s and O 1s XPS spectra were presented in Figure 7, respectively. From Figure 7a it can be seen that with and without the pretreatment of SDBS, there are no S peaks have been detected on dolomite surface, suggesting that the adsorption of SDBS on dolomite was weak. As depicted in Figure 7b, for untreated dolomite, three well-fitting bands at 284.86 eV, 286.17 eV, and 289.92 eV separated from the C 1s spectrum. The fitted bands at 284.86 eV and 286.17 eV on the dolomite surface were assigned to the adventitious contaminants of hydrocarbon and carbon-oxygen [], while the separated peak at 289.92 eV was derived from the carbon of dolomite itself []. After the dolomite conditioned with SDBS, no significant shift and new peaks occurred, indicating that the chemical environment of carbon element kept stable. The Ca 2p and Mg 1s spectra of dolomite are displayed in Figure 7c,d. The two strong double peaks at 347.37 eV and 350.94 eV were derived from Ca 2p3/2 and Ca 2p1/2 in dolomite, the peak located at 352.24 eV was attributed to Mg KLL Auger due to magnesium element existed in dolomite. The peak of Mg 1s spectrum was located at 1304.25 eV. After the addition of SDBS, the binding energy of both Ca 2p and Mg 1s shifted slightly, indicating that the chemical environment of Ca and Mg on dolomite surface were not affected and there is no chemical bonding between SDBS and Ca, Mg active sites. Moreover, the O 1s peak of bare dolomite was divided into two bands recorded at 531.87 eV and 533.41 eV, which separately belonged to the oxygen element of CO32− in dolomite itself and adventitious carbon oxides (contamination). After the addition of SDBS, the binding energy of these two characteristic peaks shifted slightly, indicating that the weak adsorption of SDBS on dolomite surface. The XPS results of dolomite are in good agreement with the experimental phenomenon that SDBS has a certain collecting ability towards dolomite, but the adsorption is relatively weak compared to that on fluorapatite surface.
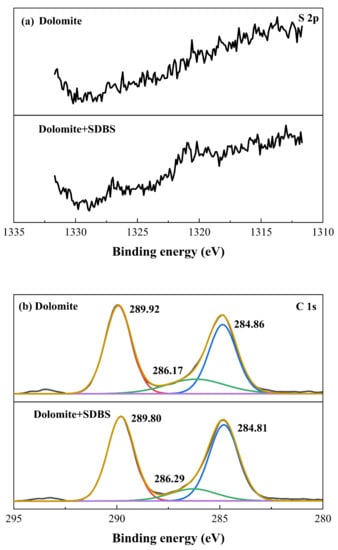
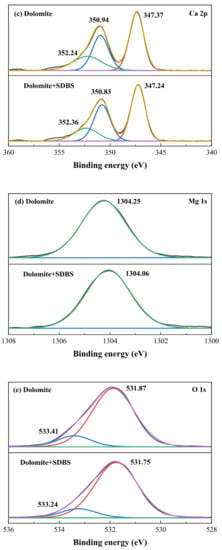
Figure 7.
(a) S 2p, (b) C 1s, (c) Ca 2p, (d) Mg 1s and (e) O 1s of dolomite sample with and without the addition of SDBS.
4. Conclusions
In this study, SDBS was selected as the collector to replace the traditional collector sodium oleate for effective recovery of fluorapatite under low temperature owing to its low-cost freely soluble capacity. Single minerals and artificially mixed minerals flotation were conducted to evaluate the collecting ability and selectivity of SDBS toward minerals under low temperature. The adsorption mechanism was described by means of XPS analyses. The flotation results demonstrate that SDBS collector exhibited a stronger harvesting effect on fluorapatite as opposed to dolomite, an effective separation of dolomite from fluorapatite could be achieved under low temperature, a concentrate with P2O5 grade of 32.8% and recovery of 96.63% was obtained. Moreover, SDBS collector could preferred adsorb on fluorapatite surface in the mixed flotation system. Compared to NaOL, SDBS is more suitable for satisfactory recovery of fluorapatite under low temperature. The results obtained from XPS analyses demonstrate that SDBS collector showed a higher affinity towards fluorapatite as RSO3− components could interact with Ca active sites on fluorapatite surface through chemical bonding.
Author Contributions
Investigation, X.W.; methodology, X.W.; resources, H.Z.; supervision, H.Z.; writing—original draft, H.Z.; writing—review & editing, H.Z., Y.C., X.W., Y.J., R.M.K. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Natural Science Foundation of China (projects Nos.51974205), The Open Foundation of State Key Laboratory of Mineral Processing (BGRIMM-KJSKL-2021-16) and Utilization of Ministry of Education (project No.201904). The APC was funded by the Natural Science Foundation of China (project Nos.51904208).
Data Availability Statement
Not applicable.
Acknowledgments
Thanks for the two reviewers and academic editor who provided so much meaningful and helpful suggestions. We also appreciate for the financial supports from Open Project of Engineering Research Center of Phosphorus Resources Development.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, H.; Chen, Y.; Zheng, H.; Huang, P.; Yang, P.; Chen, Q.; Weng, X.; He, D.; Song, S. Effect of geological origin of apatite on reverse flotation separation of phosphate ores using phosphoric acid as depressant. Miner. Eng. 2021, 172, 107182. [Google Scholar] [CrossRef]
- Ruan, Y.; He, D.; Chi, R. Review on Beneficiation Techniques and Reagents Used for Phosphate Ores. J. Miner. 2019, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Zafar, Z.I.; Anwar, M.M.; Pritchard, D.W. Innovations in beneficiation technology for low grade phosphate rocks. Nutr. Cycl. Agroecosyst. 1996, 46, 135–151. [Google Scholar] [CrossRef]
- Amirech, A.; Bouhenguel, M.; Kouachi, S. Two-stage reverse flotation process for removal of carbonates and silicates from phosphate ore using anionic and cationic collectors. Arab. J. Geosci. 2018, 11, 593. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, Z.; Sun, H.; Yin, W.; Hong, J.; Cao, S.; Tang, Y.; Zhao, C.; Yao, J. Improving flotation separation of apatite from dolomite using PAMS as a novel eco-friendly depressant. Miner. Eng. 2020, 156, 106492. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, Z.; Yin, W.; Sun, Q.; Sun, H.; Han, H.; Sheng, Q.; Yao, J. Selective adsorption of an eco-friendly and efficient depressant PBTCA onto dolomite for effective flotation of fluorapatite from dolomite. Chem. Eng. J. 2020, 400, 125780. [Google Scholar] [CrossRef]
- Yang, B.; Sun, H.; Wang, D.; Yin, W.; Cao, S.; Wang, Y.; Zhu, Z.; Jiang, K.; Yao, J. Selective adsorption of a new depressant Na2ATP on dolomite: Implications for effective separation of magnesite from dolomite via froth flotation. Sep. Purif. Technol. 2020, 250, 117278. [Google Scholar] [CrossRef]
- Espiritu, E.; da Silva, G.; Azizi, D.; Larachi, F.; Waters, K. The effect of dissolved mineral species on bastnäsite, monazite and dolomite flotation using benzohydroxamate collector. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 319–334. [Google Scholar] [CrossRef]
- Yang, B.; Yin, W.; Zhu, Z.; Sun, H.; Sheng, Q.; Fu, Y.; Yao, J.; Zhao, K. Differential adsorption of hydrolytic polymaleic anhydride as an eco-friendly depressant for the selective flotation of apatite from dolomite. Sep. Purif. Technol. 2021, 256, 117803. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, M.; Kasomo, R.M.; Li, H.; Zheng, H.; Song, S.; Luo, H.; He, D. Depression effect of Al(Ⅲ) and Fe(Ⅲ) on rutile flotation using dodecylamine polyxyethylene ether as collector. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125269. [Google Scholar] [CrossRef]
- Chen, Q.; Tian, M.; Zheng, H.; Luo, H.; Li, H.; Song, S.; He, D.; Jiang, X. Flotation of rutile from almandine using sodium fluorosilicate as the depressant. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124918. [Google Scholar] [CrossRef]
- Kasomo, R.M.; Li, H.; Zheng, H.; Chen, Q.; Weng, X.; Mwangi, A.D.; Kiamba, E.; Song, S. Depression of the selective separation of rutile from almandine by Sodium Hexametaphosphate. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124631. [Google Scholar] [CrossRef]
- Mohammadkhani, M.; Noaparast, M.; Shafaei, S.; Amini, A.; Amini, E.; Abdollahi, H. Double reverse flotation of a very low grade sedimentary phosphate rock, rich in carbonate and silicate. Int. J. Miner. Process. 2011, 100, 157–165. [Google Scholar] [CrossRef]
- Filippova, I.V.; Filippov, L.O.; Lafhaj, Z.; Barres, O.; Fornasiero, D. Effect of calcium minerals reactivity on fatty acids adsorption and flotation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 157–166. [Google Scholar] [CrossRef]
- Ge, Y.-Y.; Gan, S.-P.; Zeng, X.-B.; Yu, Y.-F. Double reverse flotation process of collophanite and regulating froth action. Trans. Nonferrous Met. Soc. China 2008, 18, 449–453. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, W.; Chen, C.; Liu, R. Interactions Between Sodium Oleate and Polyoxyethylene Ether and the Application in the Low-Temperature Flotation of Scheelite at 283 K. J. Surfactants Deterg. 2016, 19, 1289–1295. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Luo, H.; Cheng, R.; Liu, F. Selective reverse flotation of apatite from dolomite in collophanite ore using saponified gutter oil fatty acid as a collector. Int. J. Miner. Process. 2017, 165, 20–27. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, T.; Li, H.; Yi, H.; Song, S. Can carboxymethyl cellulose molecules bind swelling montmorillonite layers in Water? Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 515–519. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, B.; Zhang, L.; Zhang, W.; Wang, H.; Gao, Z. Flotation separation of apatite and calcite using gum arabic as a depressant. Colloids Surf. A Physicochem. Eng. Asp. 2021, 632, 127723. [Google Scholar] [CrossRef]
- Gorain, B.; Franzidis, J.; Manlapig, E. FLOTATION|Flotation Cell Design: Application of Fundamental Principles. In Encyclopedia of Separation Science; Elsevier: Amsterdam, The Netherlands, 2000; pp. 1502–1512. [Google Scholar]
- Sheng, Q.; Yin, W.; Ma, Y.; Liu, Y.; Wang, L.; Yang, B.; Sun, H.; Yao, J. Selective depression of talc in azurite sulfidization flotation by tamarind polysaccharide gum: Flotation response and adsorption mechanism. Miner. Eng. 2022, 178, 107393. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, Y.; Chen, T.; Zhang, T.; Song, S. Synthesis of montmorillonite-chitosan hollow and hierarchical mesoporous spheres with single-template layer-by-layer assembly. J. Mater. Sci. Technol. 2019, 35, 2325–2330. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, Y.; Yang, S.; Fu, W.; Chen, X. Exploration of a novel depressant polyepoxysuccinic acid for the flotation separation of pentlandite from lizardite slimes. Appl. Clay Sci. 2021, 202, 105939. [Google Scholar] [CrossRef]
- Li, H.; Mao, Y.; Zheng, H.; Kasomo, R.M.; Huang, P.; Chen, Y.; Chen, Q.; Weng, X.; He, D.; Song, S. Impact of geological origin on flotation separation of apatite from dolomite using β-naphthyl sulfonate formaldehyde condensate as depressant. Miner. Eng. 2021, 176, 107323. [Google Scholar] [CrossRef]
- Liu, S.; Mao, Y.; Luo, Y.; Weng, X.; Chen, Q.; Li, H.; Yi, H.; Song, S.; He, D. Selective flotation separation of bastnaesite from dolomite using β-naphthyl sulfonate formaldehyde condensate as depressant: Experimental and calculational studies. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128380. [Google Scholar] [CrossRef]
- Cao, S.; Yin, W.; Yang, B.; Zhu, Z.; Sun, H.; Sheng, Q.; Chen, K. Insights into the influence of temperature on the adsorption behavior of sodium oleate and its response to flotation of quartz. Int. J. Min. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, B.; Zhang, W.; Zhang, L.; Guo, Y.; Wang, T.; Wang, H. The role of sodium alginate in the flotation separation of apatite and dolomite. Powder Technol. 2020, 373, 620–626. [Google Scholar] [CrossRef]
- Cao, Q.; Cheng, J.; Wen, S.; Li, C.; Bai, S.; Liu, D. A mixed collector system for phosphate flotation. Miner. Eng. 2015, 78, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Li, J.; Bi, Y.; Yu, P.; Dai, H.; Wen, S.; Bai, S. The adsorption mechanism of synergic reagents and its effect on apatite flotation in oleamide-sodium dodecyl benzene sulfonate (SDBS) system. Miner. Eng. 2021, 170, 107070. [Google Scholar] [CrossRef]
- Jong, K.; Han, Y.; Ryom, S. Flotation mechanism of oleic acid amide on apatite. Colloids Surf. A Physicochem. Eng. Asp. 2017, 523, 127–131. [Google Scholar] [CrossRef]
- Deng, R.; Yang, X.; Hu, Y.; Ku, J.; Zuo, W.; Ma, Y. Effect of Fe(II) as assistant depressant on flotation separation of scheelite from calcite. Miner. Eng. 2018, 118, 133–140. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).