Experimental Modeling of Ankerite–Pyrite Interaction under Lithospheric Mantle P–T Parameters: Implications for Graphite Formation as a Result of Ankerite Sulfidation
Abstract
:1. Introduction
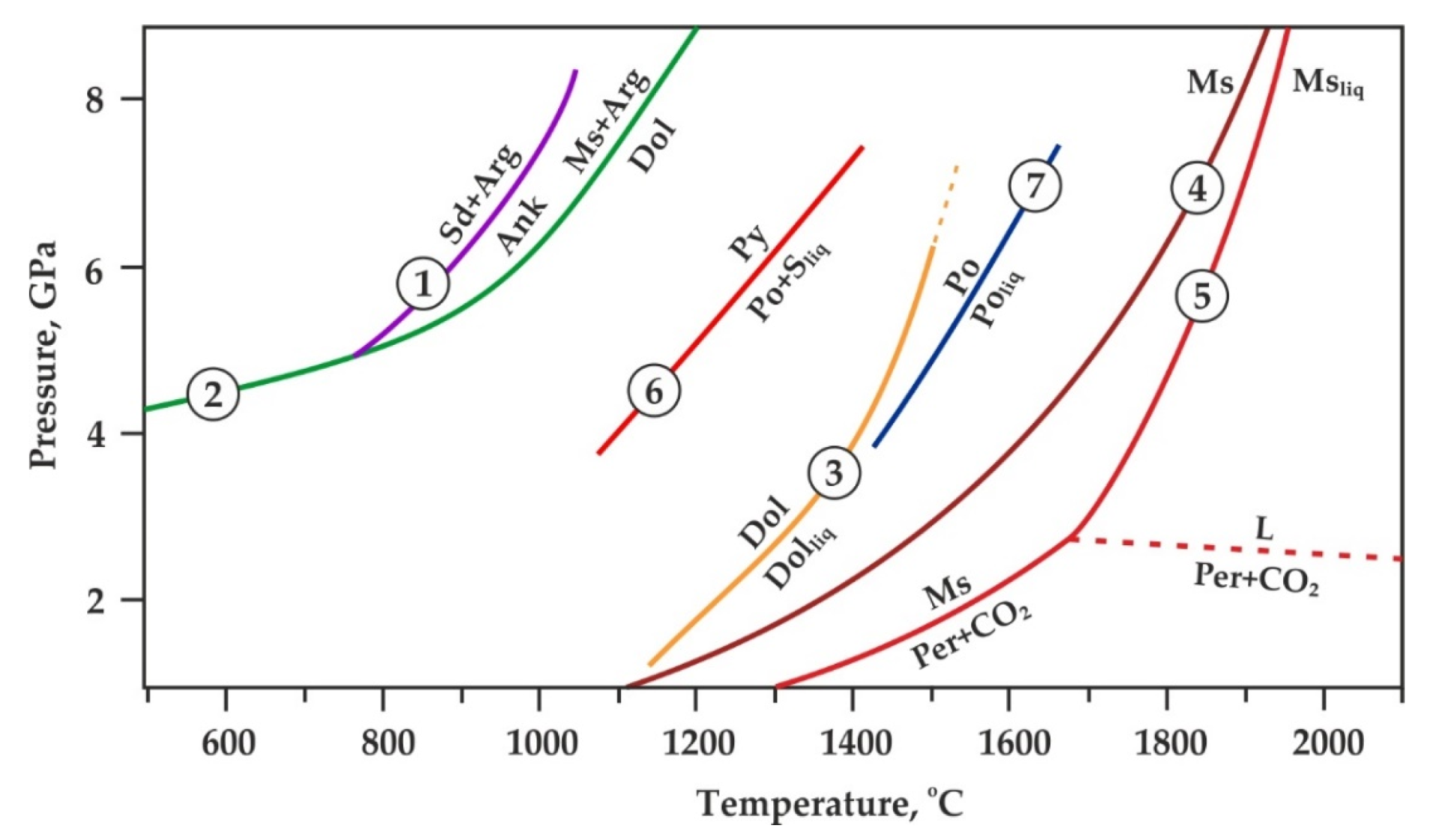
2. Materials and Methods
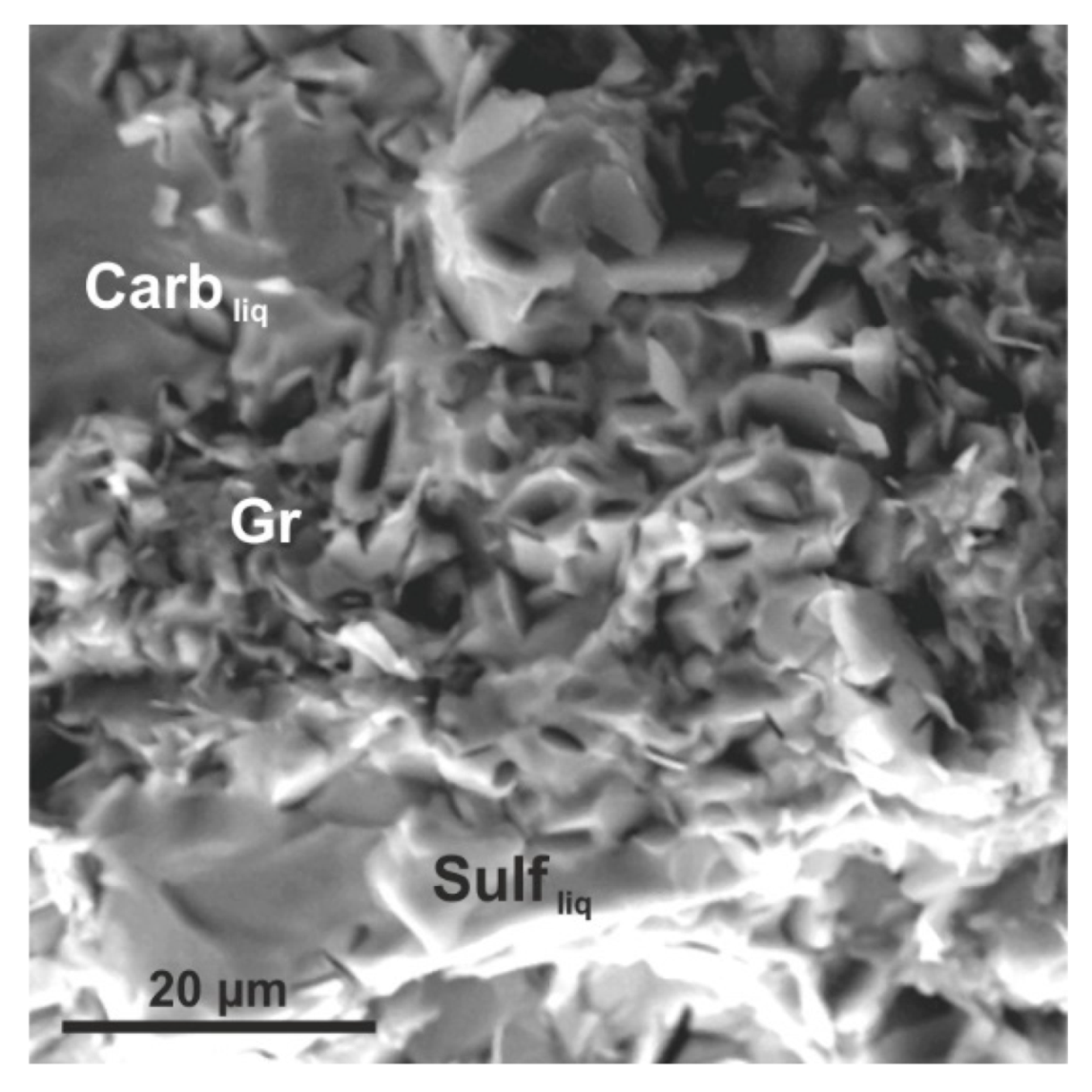
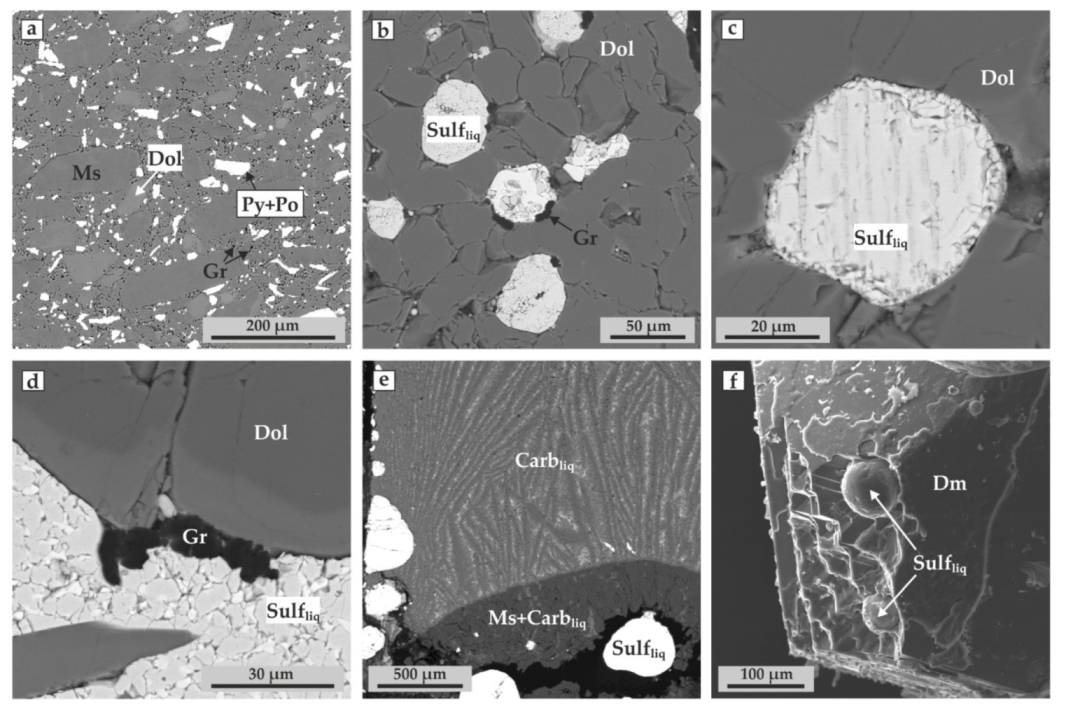
3. Results
4. Discussion
4.1. Reconstruction of Ankerite–Pyrite Interaction Processes at Mantle P–T Parameters
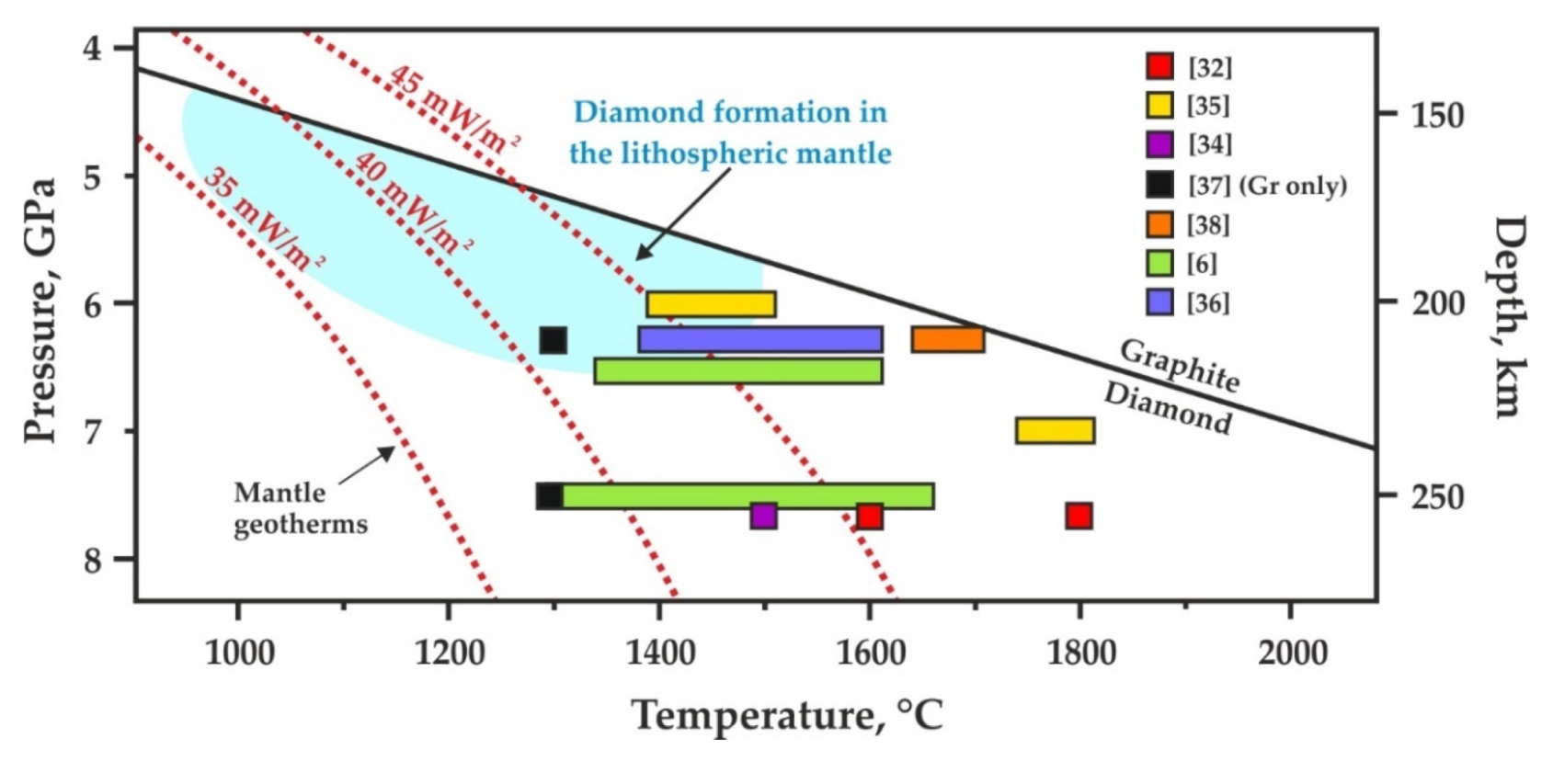
4.2. Implications of the Results of Ankerite–Pyrite Interaction to Graphite Formation via Ankerite Sulfidation in the Lithospheric Mantle and under Subduction Settings
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stachel, T.; Harris, J.W. The origin of cratonic diamonds—Constraints from mineral inclusions. Ore Geol. Rev. 2008, 34, 5–32. [Google Scholar] [CrossRef]
- Shirey, S.; Smit, K.; Pearson, D.; Walter, M.; Aulbach, S.; Brenker, F.; Bureau, H.; Burnham, A.; Cartigny, P.; Chacko, T.; et al. Diamonds and the mantle geodynamics of carbon: Deep mantle carbon evolution from the diamond record. In Deep Carbon: Past to Present; Orcutt, B., Daniel, I., Dasgupta, R., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 89–128. [Google Scholar]
- Shirey, S.B.; Cartigny, P.; Frost, D.J.; Keshav, S.; Nestola, F.; Nimis, P.; Pearson, D.G.; Sobolev, N.V.; Walter, M.J. Diamonds and the Geology of Mantle Carbon. Rev. Mineral. Geochem. 2013, 75, 355–421. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.M.; Shirey, S.B.; Nestola, F.; Bullock, E.S.; Wang, J.; Richardson, S.H.; Wang, W. Large gem diamonds from metallic liquid in Earth’s deep mantle. Science 2016, 354, 1403–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal’yanov, Y.N.; Sokol, A.G.; Khokhryakov, A.F.; Pal’yanova, G.A.; Borzdov, Y.M.; Sobolev, N.V. Diamond and graphite crystallization in COH fluid at PT parameters of the natural diamond formation. Dokl. Earth Sci. 2000, 375, 1395–1398. [Google Scholar]
- Palyanov, Y.N.; Bataleva, Y.V.; Sokol, A.G.; Borzdov, Y.M.; Kupriyanov, I.N.; Reutsky, V.N.; Sobolev, N.V. Mantle-slab interaction and redox mechanism of diamond formation. Proc. Natl. Acad. Sci. USA 2013, 110, 20408–20413. [Google Scholar] [CrossRef] [Green Version]
- Palyanov, Y.N.; Borzdov, Y.M.; Khokhryakov, A.F.; Bataleva, Y.V.; Kupriyanov, I.N. Effect of sulfur on diamond growth and morphology in metal-carbon systems. CrystEngComm. 2020, 22, 5497–5508. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Kupriyanov, I.N.; Bataleva, Y.V.; Nechaev, D.V. Effect of oxygen on diamond crystallization in metal-carbon systems. ACS Omega 2020, 5, 18376–18383. [Google Scholar] [CrossRef] [PubMed]
- Stachel, T.; Brey, G.P.; Harris, J.W. Inclusions in sublithospheric diamonds: Glimpses of deep Earth. Elements 2005, 1, 73–78. [Google Scholar] [CrossRef]
- Ryabchikov, I.D. Mechanisms of diamond formation: Reduction of carbonates or partial oxidation of hydrocarbons. Dokl. Earth Sci. 2009, 429, 1346–1349. [Google Scholar] [CrossRef]
- Luth, R. Diamond formation during partial melting in the Earth’s mantle. Abstr. Progr. Geol. Soc. Am. 2017, 49, 20–26. [Google Scholar]
- Palyanov, Y.N.; Borzdov, Y.M.; Sokol, A.G.; Bataleva, Y.V.; Kupriyanov, I.N.; Reutsky, V.N.; Wiedenbeck, M.; Sobolev, N.V. Diamond formation in an electric field under deep Earth conditions. Sci. Adv. 2021, 7, eabb4644. [Google Scholar] [CrossRef]
- Sverjensky, D.A.; Huang, F. Diamond formation due to a pH drop during fluid-rock interactions. Nat. Commun. 2015, 6, 8702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Pasteris, J.D.; Meyer, H.O.A.; DeleDuboi, M.L. Magnesite-bearing inclusion assemblage in natural diamond. Earth Planet. Sci. Lett. 1996, 141, 293–306. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P. Rare and unusual mineral inclusions in diamonds from Mwadui, Tanzania. Contrib. Mineral. Petrol. 1998, 132, 34–47. [Google Scholar] [CrossRef]
- Brenker, F.E.; Vollmer, C.; Vincze, L.; Vekemans, B.; Szymanski, A.; Janssens, K.; Szaloki, I.; Nasdala, L.; Joswig, W.; Kaminsky, F. Carbonates from the lower part of transition zone or even the lower mantle. Earth Planet. Sci. Lett. 2007, 260, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Wirth, R.; Kaminsky, F.; Matsyuk, S.; Schreiber, A. Unusual micro- and nanoinclusions in diamonds from the Juina Area, Brazil. Earth Planet. Sci. Lett. 2009, 286, 292–303. [Google Scholar] [CrossRef]
- Logvinova, A.M.; Taylor, L.A.; Fedorova, E.N.; Yelisseyev, A.P.; Wirth, R.; Howarth, G.; Reutsky, V.N.; Sobolev, N.V. A unique diamondiferous peridotite xenolith from the Udachnaya kimberlite pipe, Yakutia: Role of subduction in diamond formation. Russ. Geol. Geophys. 2015, 56, 306–320. [Google Scholar] [CrossRef]
- Izraeli, E.S.; Harris, J.W.; Navon, O. Fluid and mineral inclusions in cloudy diamonds from Koffiefontein, South Africa. Geochim. Cosmochim. Acta 2004, 68, 2561–2575. [Google Scholar] [CrossRef]
- Kopylova, M.; Navon, O.; Dubrovinsky, L.; Khachatryan, G. Carbonatitic mineralogy of natural diamond-forming fluids. Earth Planet. Sci. Lett. 2010, 291, 126–137. [Google Scholar] [CrossRef]
- Navon, O.; Hutcheon, I.D.; Rossman, G.R.; Wasserburg, G.J. Mantle-derived fluids in diamond microinclusions. Nature 1988, 335, 784–789. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatsky, V.S.; Araujo, D.; Griffin, W.L.; Kagi, H. Mg and Fe-rich carbonate-silicate high-density fluids in cuboid diamonds from the Internationalnaya kimberlite pipe (Yakutia). Lithos 2009, 112S, 638–647. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Logvinova, A.; Schrauder, M.; Spetius, Z.V.; Weiss, Y.; Hauri, E.H.; Kaminsky, F.V.; Sobolev, N.V.; Navon, O. High-Mg carbonatitic microinclusions in some Yakutian diamonds—A new type of diamond-forming fluid. Lithos 2009, 112, 648–659. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatsky, V.S.; Araujo, D.; Griffin, W.L. Fibrous diamonds from the placers of the northeastern Siberian Platform: Carbonate and silicate crystallization media. Russ. Geol. Geophys. 2011, 52, 1298–1309. [Google Scholar] [CrossRef]
- Weiss, Y.; Kiflawi, I.; Davies, N.; Navon, O. High-density fluids and the growth of monocrystalline diamonds. Geochim. Cosmochim. Acta 2014, 141, 145–159. [Google Scholar] [CrossRef]
- Weiss, Y.; Griffin, W.L.; Bell, D.R.; Navon, O. High-Mg carbonatitic melts in diamonds, kimberlites and the sub-continental lithosphere. Earth Planet. Sci. Lett. 2011, 309, 337–347. [Google Scholar] [CrossRef]
- Cartigny, P. Stable isotopes and the origin of diamond. Elements 2005, 1, 79–84. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Green, D.H. Experimental demonstration of refractory carbonate-bearing eclogite and siliceous melts in the subduction regime. Earth Planet. Sci. Lett. 1994, 128, 313–325. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M. The deep carbon cycle and melting in Earth’s interior. Earth Planet. Sci. Lett. 2010, 298, 1–13. [Google Scholar] [CrossRef]
- Walter, M.J.; Kohn, S.C.; Araujo, D.; Bulanova, G.P.; Smith, C.B.; Gaillou, E.; Wang, J.; Steele, A.; Shirey, S.B. Deep mantle cycling of oceanic crust: Evidence from diamonds and their mineral inclusions. Science 2011, 334, 54–57. [Google Scholar] [CrossRef] [Green Version]
- Morlidge, M.; Pawley, A.; Droop, G. Double carbonate breakdown reactions at high pressures: An experimental study in the system CaO-MgO-FeO-MnO-CO2. Contrib. Mineral. Petrol. 2006, 152, 365–373. [Google Scholar] [CrossRef]
- Logvinov, V.M.; Doroshev, A.M. Phase change in Mg, Ca, Sr and Ba carbonates at pressures to 160 kbar. In High-Pressure Silicate Systems; IGiG SO AN SSSR: Novosibirsk, Russia, 1983; pp. 47–56. (In Russian) [Google Scholar]
- Shatskiy, A.F.; Litasov, K.D.; Palyanov, Y.N. Phase relations in carbonate systems at pressures and temperatures of lithospheric mantle: Review of experimental data. Russ. Geol. Geophys. 2015, 56, 113–142. [Google Scholar] [CrossRef]
- Sharp, W.E. Melting curves of sphalerite, galena, and pyrrhotite and the decomposition curve of pyrite between 30 and 65 kilobars. J. Geophys. Res. 1969, 74, 1645–1652. [Google Scholar] [CrossRef]
- Arima, M.; Kozai, Y.; Akaishi, M. Diamond nucleation and growth by reduction of carbonate melts under high-pressure and high-temperature conditions. Geology 2002, 30, 691–694. [Google Scholar] [CrossRef]
- Siebert, J.; Guyot, F.; Malavergne, V. Diamond formation in metal-carbonate interactions. Earth Planet. Sci. Lett. 2005, 229, 940–950. [Google Scholar] [CrossRef]
- Yamaoka, S.; Shaji Kumar, M.D.; Kanda, H.; Akaishi, M. Formation of diamond from CaCO3 in a reduced C-O-H fluid at HP-HT. Diam. Relat. Mater. 2002, 11, 1496–1504. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Khokhryakov, A.F.; Sobolev, N.V. Diamond formation through carbonate-silicate interaction. Am. Miner. 2002, 87, 1009–1013. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Novoselov, I.D.; Bayukov, O.A. An effect of reduced S-rich fluids on diamond formation under mantle-slab interaction. Lithos 2019, 336–337, 27–39. [Google Scholar] [CrossRef]
- Gunn, S.C.; Luth, R.W. Carbonate reduction by Fe-S-O melts at high pressure and high temperature. Am. Miner. 2006, 91, 1110–1116. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Bataleva, Y.V.; Sokol, A.G.; Palyanova, G.A.; Kupriyanov, I.N. Reducing role of sulfides and diamond formation in the Earth’s mantle. Earth Planet. Sci. Lett. 2007, 260, 242–256. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Kupriyanov, I.N.; Sokol, A.G. Synthesis of diamonds with mineral, fluid and melt inclusions. Lithos 2016, 265, 292–303. [Google Scholar] [CrossRef]
- Zdrokov, E.; Novoselov, I.; Bataleva, Y.; Borzdov, Y.; Palyanov, Y. Experimental modeling of silicate and carbonate sulfidation under lithospheric mantle P, T-parameters. Minerals 2019, 9, 425. [Google Scholar] [CrossRef] [Green Version]
- Palyanov, Y.N.; Borzdov, Y.M.; Khokhryakov, A.F.; Kupriyanov, I.N.; Sokol, A.G. Effect of Nitrogen Impurity on Diamond Crystal Growth Processes. Cryst. Growth Des. 2010, 10, 3169–3175. [Google Scholar] [CrossRef]
- Sokol, A.G.; Borzdov, Y.M.; Palyanov, Y.N.; Khokhryakov, A.F. High-temperature calibration of a multi-anvil high pressure apparatus. High Press. Res. 2015, 35, 139–147. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Sokol, A.G. The effect of composition of mantle fluids/melts on diamond formation processes. Lithos 2009, 112, 690–700. [Google Scholar] [CrossRef]
- Dasgupta, R.; Buono, A.; Whelan, G.; Walker, D. High-pressure melting relations in Fe-C-S systems: Implications for formation, evolution, and structure of metallic cores in planetary bodies. Geochim. Cosmochim. Acta 2009, 73, 6678–6691. [Google Scholar] [CrossRef]
- Tomkins, A.; Evans, K.A. Separate zones of sulfate and sulfide release from subducted mafic oceanic crust. Earth Planet. Sci. Lett. 2015, 428, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Wallace, M.; Green, D.H. An experimental determination of primary carbonatite composition. Nature 1988, 335, 343–345. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Ryabchikov, I.D. Volatile components, magmas and critical fluids in upwelling mantle. J. Petrol. 2000, 41, 1195–1206. [Google Scholar] [CrossRef] [Green Version]
- Kamenetsky, M.B.; Sobolev, A.V.; Kamenetsky, V.S.; Maas, R.; Danyushevsky, L.V.; Thomas, R.; Pokhilenko, N.P.; Sobolev, N.V. Kimberlite melts rich in alkali chlorides and carbonates: A potential metasomatic agent in the mantle. Geology 2004, 32, 845–848. [Google Scholar] [CrossRef]
- Kelley, K.A.; Cottrell, E. Water and the oxidation state of subduction zone magmas. Science 2009, 325, 605–607. [Google Scholar] [CrossRef] [Green Version]
- Foley, S.F. A reappraisal of redox melting in the Earth’s mantle as a function of tectonic setting and time. J. Petrol. 2011, 2, 1363–1391. [Google Scholar] [CrossRef]
- O’Reilly, S.Y.; Griffin, W.L. Mantle metasomatism. In Metasomatism and the Chemical Transformation of Rock. The Role of Fluids in Terrestrial and Extraterrestrial Processes; Harlov, D.E., Austrheim, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 471–533. [Google Scholar]
- Alt, J.C.; Shanks, W.C.; Jackson, M.C. Cycling of sulfur in subduction zones: The geochemistry of sulfur in the Mariana-Island Arc. Earth Planet. Sci. Lett. 1993, 119, 477–494. [Google Scholar] [CrossRef] [Green Version]
- Jégo, S.; Dasgupta, R. The fate of sulfur during fluid-present melting of subducting basaltic crust at variable oxygen fugacity. J. Petrol. 2014, 55, 1019–1050. [Google Scholar] [CrossRef]
- Labidi, J.; Cartigny, P.; Jackson, M.G. Multiple sulfur isotope composition of oxidized Samoan melts and the implications of a sulfur isotope ‘mantle array’ in chemical geodynamics. Earth Planet. Sci. Lett. 2015, 417, 28–39. [Google Scholar] [CrossRef]
- Lorand, J.-P.; Grégoire, M. Petrogenesis of base metal sulphide assemblages of some peridotites from the Kaapvaal craton (South Africa). Contrib. Mineral. Petrol. 2006, 151, 521–538. [Google Scholar] [CrossRef]
- Andersen, T.; Neumann, E. Fluid inclusions in mantle xenoliths. Lithos 2001, 55, 301–320. [Google Scholar] [CrossRef]
- Newton, R.C.; Manning, C.E. Solubility of anhydrite, CaSO4, in NaCl-H2O solutions at high pressures and temperatures: Applications to fluid-rock interaction. J. Petrol. 2005, 46, 701–716. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, A.; Phillips, D.; Fiorentini, M.L.; Kendrick, M.A.; Maas, R.; Wing, B.A.; Woodhead, J.D.; Bui, T.H.; Kamenetsky, V.S. Mantle oddities: A sulphate fluid preserved in a MARID xenolith from Bultfontein kimberlite (South Africa). Earth Planet. Sci. Lett. 2013, 376, 74–86. [Google Scholar] [CrossRef]
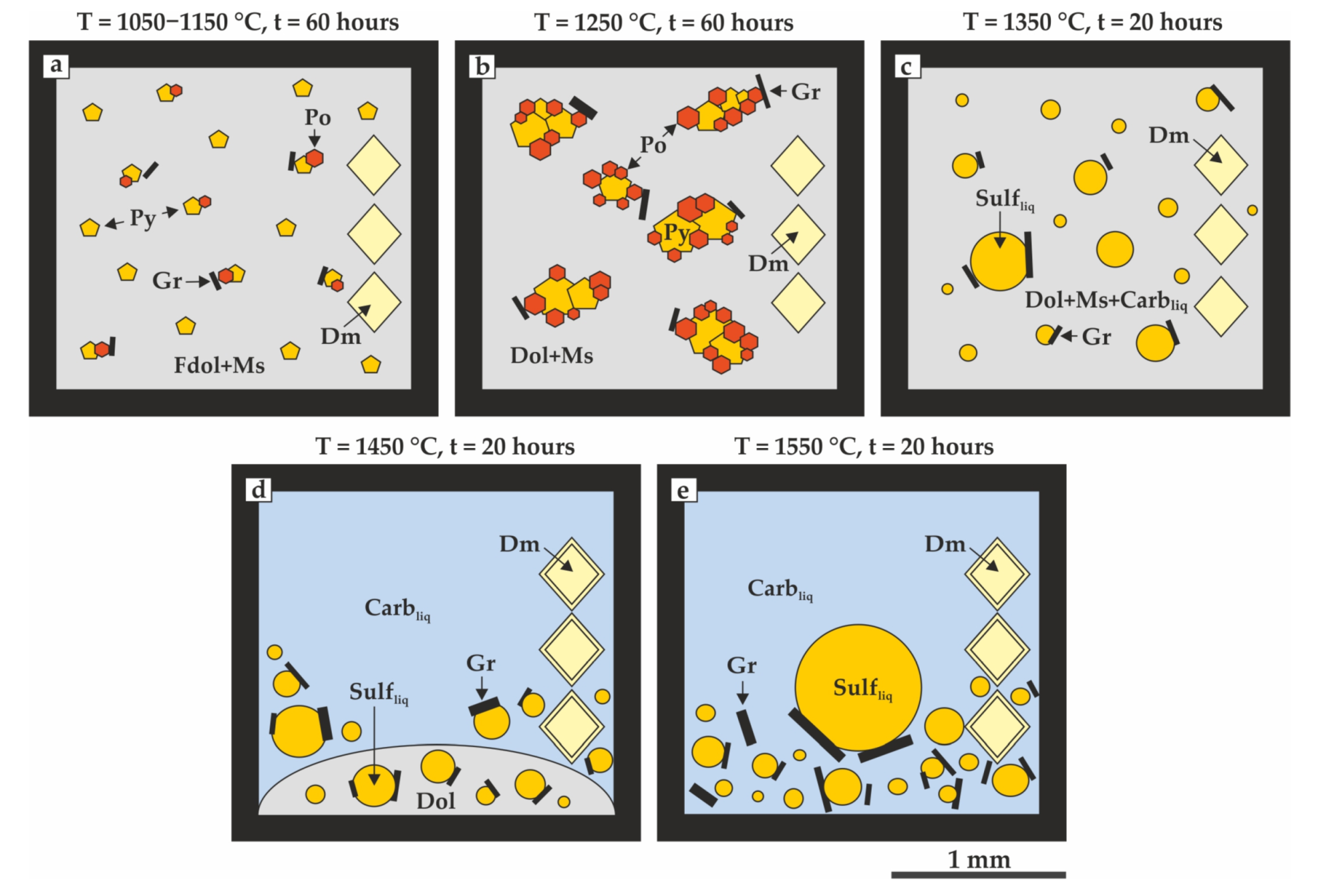
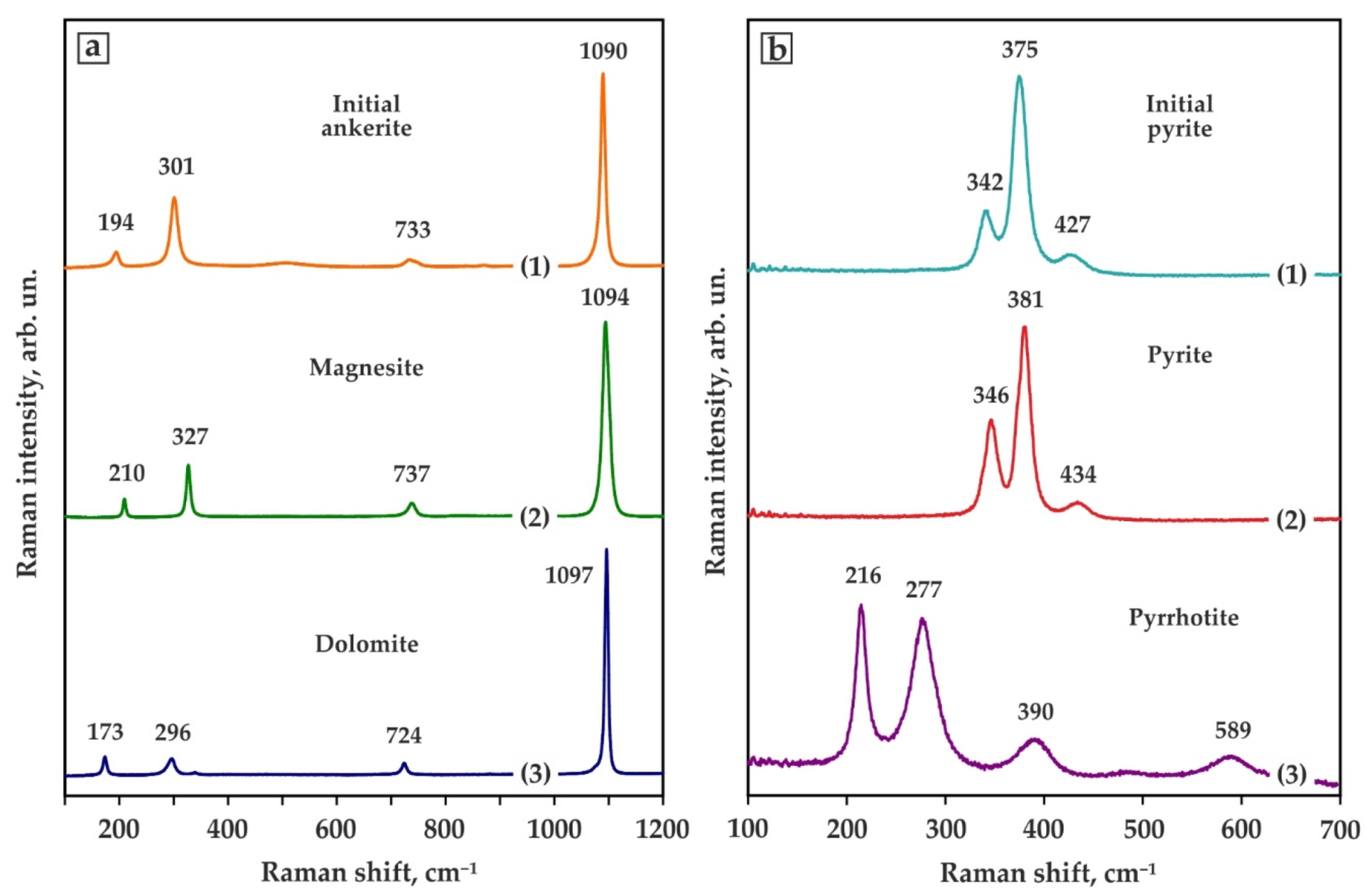
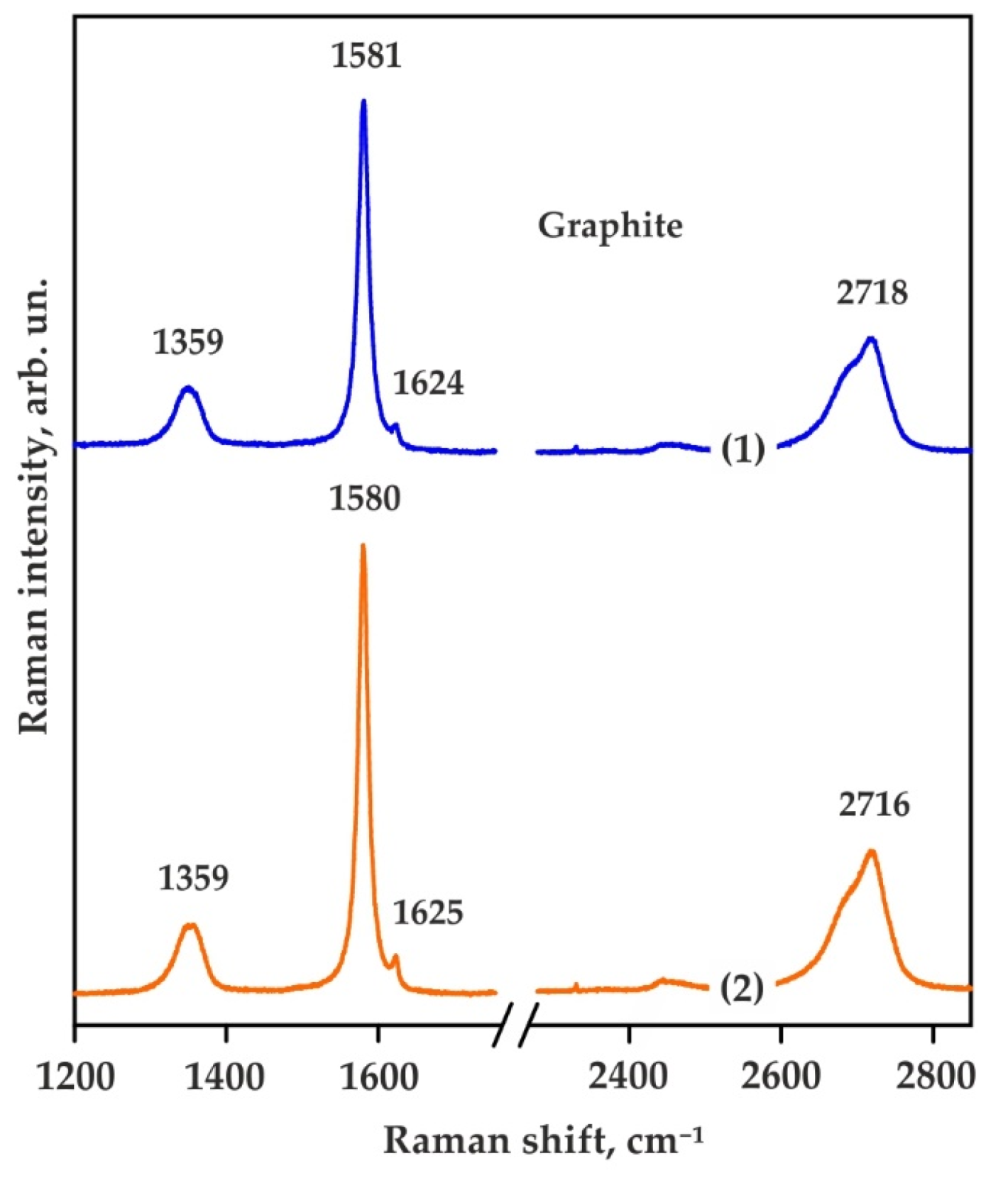
| Run No. | T, °C | t, h | Capsule Material | Phase Assemblage | Diamond Growth on Seeds |
|---|---|---|---|---|---|
| 932-4 | 1050 | 60 | Gr | Ms, Dol, Py, Po, Gr | no |
| 1476-4 | 1150 | 60 | Gr | Ms, Dol, Py, Po, Gr | no |
| 1997-4 | 1250 | 60 | Gr | Ms, Dol, Po, Py, Gr | no |
| 2000-4 | 1350 | 20 | Gr | Ms, Dol, Gr, Carb liq, Sulf liq | yes |
| 2001-4 | 1450 | 20 | Gr | Ms, Gr, Carb liq, Sulf liq | yes |
| 1811-4 | 1550 | 20 | Gr | Gr, Carb liq, Sulf liq | yes |
| 2002-M | 1350 | 20 | MgO | Carb liq, Sulf liq, Gr | No seeds |
| 2003-M | 1450 | 20 | MgO | Carb liq, Sulf liq, Gr | No seeds |
| 2004-T | 1550 | 20 | Ta ceramics | Ol, Carb liq, Sulf liq, Gr | No seeds |
| Run No. | T, °C | Phase | NA | Composition, wt % | n(O) | Cations per Formula Unit (p.f.u.) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FeO | MgO | CaO | CO2 * | Total | Fe | Mg | Ca | C ** | ∑cat | |||||
| n/a | n/a | Initial ankerite | 12 | 18.6 | 9.8 | 27.0 | 43.9 | 99.3 | 6 | 0.49 | 0.49 | 1 | 2 | 3.98 |
| 932 | 1050 | Ms | 10 | 0.8 | 41.6 | 3.8 | 53.8 | 100.0 | 3 | 0.01 | 0.89 | 0.06 | 1 | 1.98 |
| Dol | 10 | 2.6 | 20.3 | 27.9 | 49.2 | 100.0 | 6 | 0.07 | 0.92 | 0.91 | 2.05 | 3.95 | ||
| Dol | 10 | 11.5 | 14.0 | 27.6 | 46.4 | 100.0 | 6 | 0.31 | 0.67 | 0.95 | 2.03 | 3.96 | ||
| 1476 | 1150 | Ms | 7 | 0.6(3) | 40.1(5) | 6(1) | 52.9(5) | 100.0 | 3 | 0.010(5) | 0.85(1) | 0.09(2) | 1.02(1) | 1.97 |
| Dol | 10 | 0.7(4) | 20.9(9) | 29(1) | 48(1) | 100.0 | 6 | 0.02(1) | 0.96(4) | 0.97(5) | 2.05(1) | 3.98 | ||
| 1997 | 1250 | Dol | 25 | 3.0(5) | 19.8(5) | 28.4(4) | 48.2(7) | 100.0 | 6 | 0.08(1) | 0.91(2) | 0.94(2) | 2.03(2) | 3.96 |
| 2000 | 1350 | Dol | 16 | 2.7(4) | 22.0(4) | 26.1(6) | 49.0(6) | 100.0 | 6 | 0.07(1) | 1.00(2) | 0.85(2) | 2.04(2) | 3.96 |
| Carb liq | 18 | 1.8(6) | 13(1) | 37.5(9) | 47.6(4) | 100.0 | - | - | - | - | - | - | ||
| 2001 | 1450 | Ms | 12 | 1.7(2) | 40.9(1) | 3.6(3) | 53.8(8) | 100.0 | 3 | 0.02(0) | 0.86(0) | 0.05(0) | 1.03(1) | 1.97 |
| Carb liq | 20 | 3.0(3) | 15.6(7) | 24.0(8) | 57.4(9) | 100.0 | - | - | - | - | - | - | ||
| 1811 | 1550 | Carb liq | 24 | 6(2) | 21(6) | 17(3) | 52(7) | 100.0 | - | - | - | - | - | - |
| Run No | T, °C | Phase | NA | Composition, wt % | Formula Units | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | S | O | Total | Fe | n (S) | ||||
| n/a | n/a | Initial pyrite | 10 | 46.8 | 53.0 | bdl | 99.8 | 1.00 | 2 |
| 932-4 | 1050 | Po | 15 | 60.2(5) | 39.6(5) | bdl | 100.3 | 0.87(1) | 1 |
| Py | 18 | 46.3(3) | 53.4(3) | bdl | 100.1 | 1.00(1) | 2 | ||
| 1476-4 | 1150 | Po | 7 | 59(2) | 39(2) | bdl | 100.0 | 0.87(3) | 1 |
| Py | 5 | 46.7(2) | 53.3(2) | bdl | 100.0 | 1.01(1) | 2 | ||
| 1997-4 | 1250 | Po | 13 | 60.3(5) | 39.1(7) | bdl | 100.1 | 0.88(2) | 1 |
| Py | 15 | 45.6(3) | 53.5(3) | bdl | 100.0 | 0.98(1) | 2 | ||
| 2000-4 | 1350 | Sulf liq | 37 | 60.9(5) | 39.7(6) | 0.6(4) | 100.9 | - | - |
| 2001-4 | 1450 | Sulf liq | 18 | 60.8(6) | 39.3(5) | 1.3(6) | 100.1 | - | - |
| 1811-4 | 1550 | Sulf liq | 22 | 63.1(5) | 35(1) | 2.0(5) | 100.0 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bataleva, Y.V.; Novoselov, I.D.; Borzdov, Y.M.; Furman, O.V.; Palyanov, Y.N. Experimental Modeling of Ankerite–Pyrite Interaction under Lithospheric Mantle P–T Parameters: Implications for Graphite Formation as a Result of Ankerite Sulfidation. Minerals 2021, 11, 1267. https://doi.org/10.3390/min11111267
Bataleva YV, Novoselov ID, Borzdov YM, Furman OV, Palyanov YN. Experimental Modeling of Ankerite–Pyrite Interaction under Lithospheric Mantle P–T Parameters: Implications for Graphite Formation as a Result of Ankerite Sulfidation. Minerals. 2021; 11(11):1267. https://doi.org/10.3390/min11111267
Chicago/Turabian StyleBataleva, Yuliya V., Ivan D. Novoselov, Yuri M. Borzdov, Olga V. Furman, and Yuri N. Palyanov. 2021. "Experimental Modeling of Ankerite–Pyrite Interaction under Lithospheric Mantle P–T Parameters: Implications for Graphite Formation as a Result of Ankerite Sulfidation" Minerals 11, no. 11: 1267. https://doi.org/10.3390/min11111267
APA StyleBataleva, Y. V., Novoselov, I. D., Borzdov, Y. M., Furman, O. V., & Palyanov, Y. N. (2021). Experimental Modeling of Ankerite–Pyrite Interaction under Lithospheric Mantle P–T Parameters: Implications for Graphite Formation as a Result of Ankerite Sulfidation. Minerals, 11(11), 1267. https://doi.org/10.3390/min11111267







