Solidification Effect and Mechanism of Marine Muck Treated with Ionic Soil Stabilizer and Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
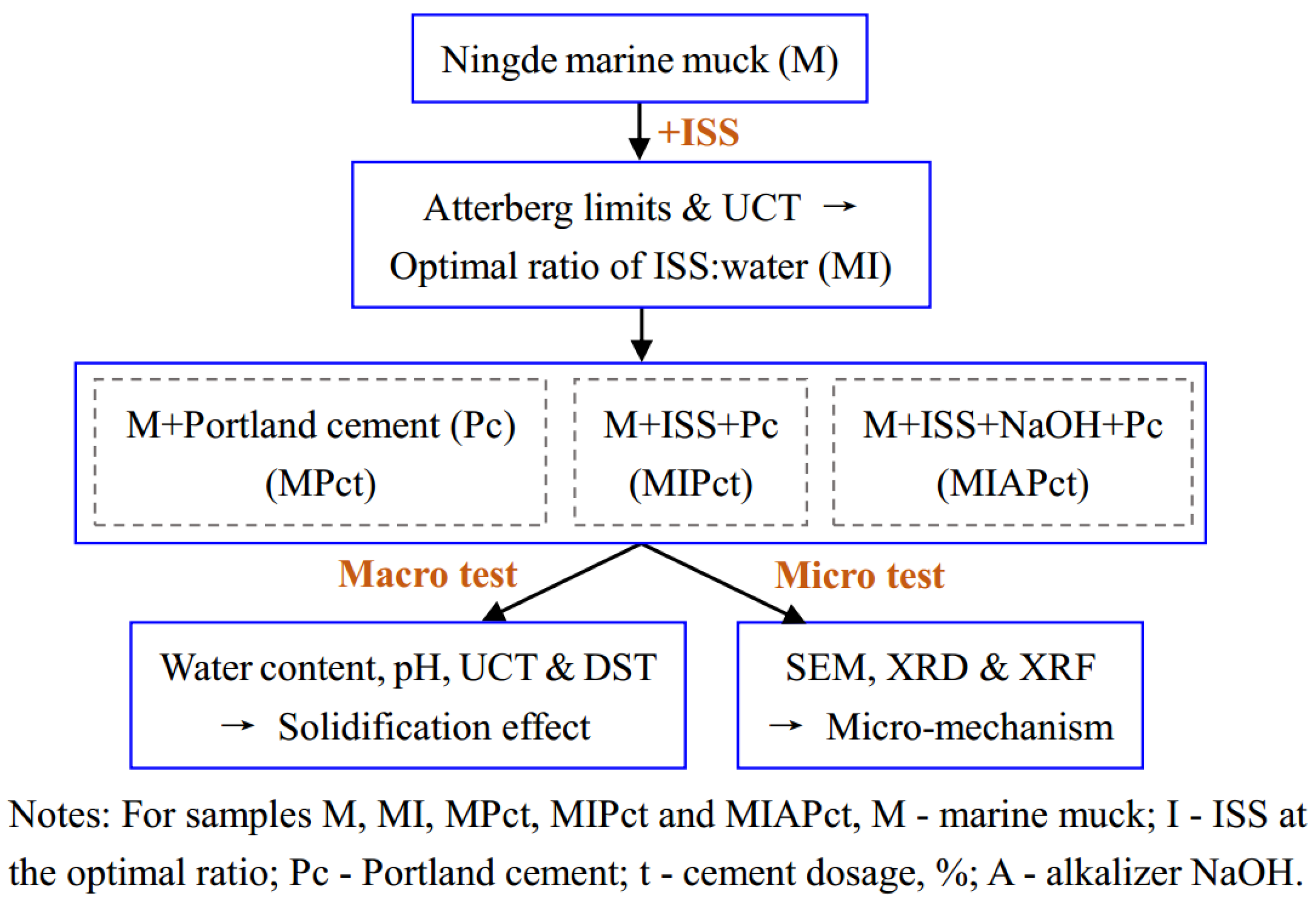
2.2. Sample Preparation
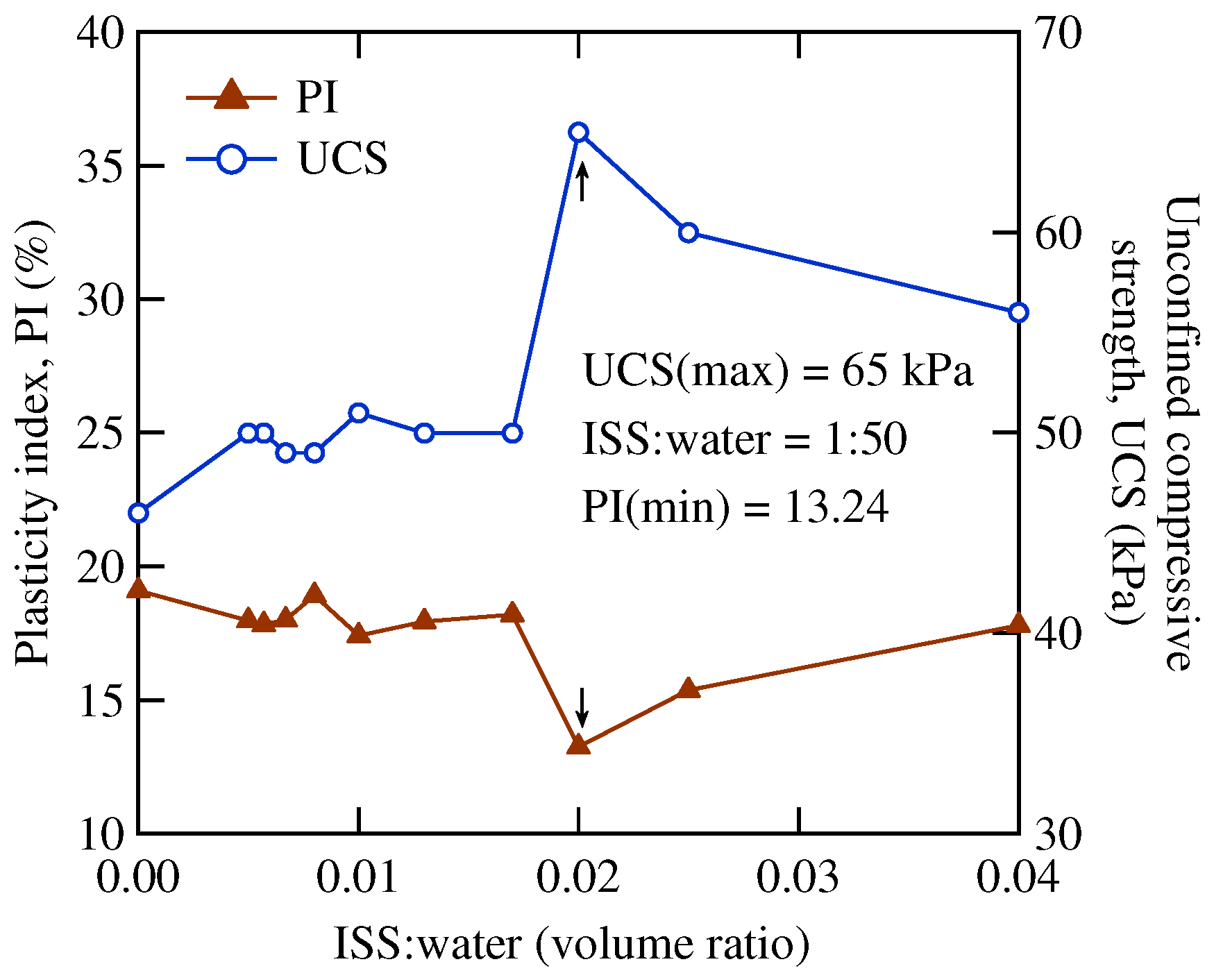
2.3. Experimental Methods
3. Results and Analysis
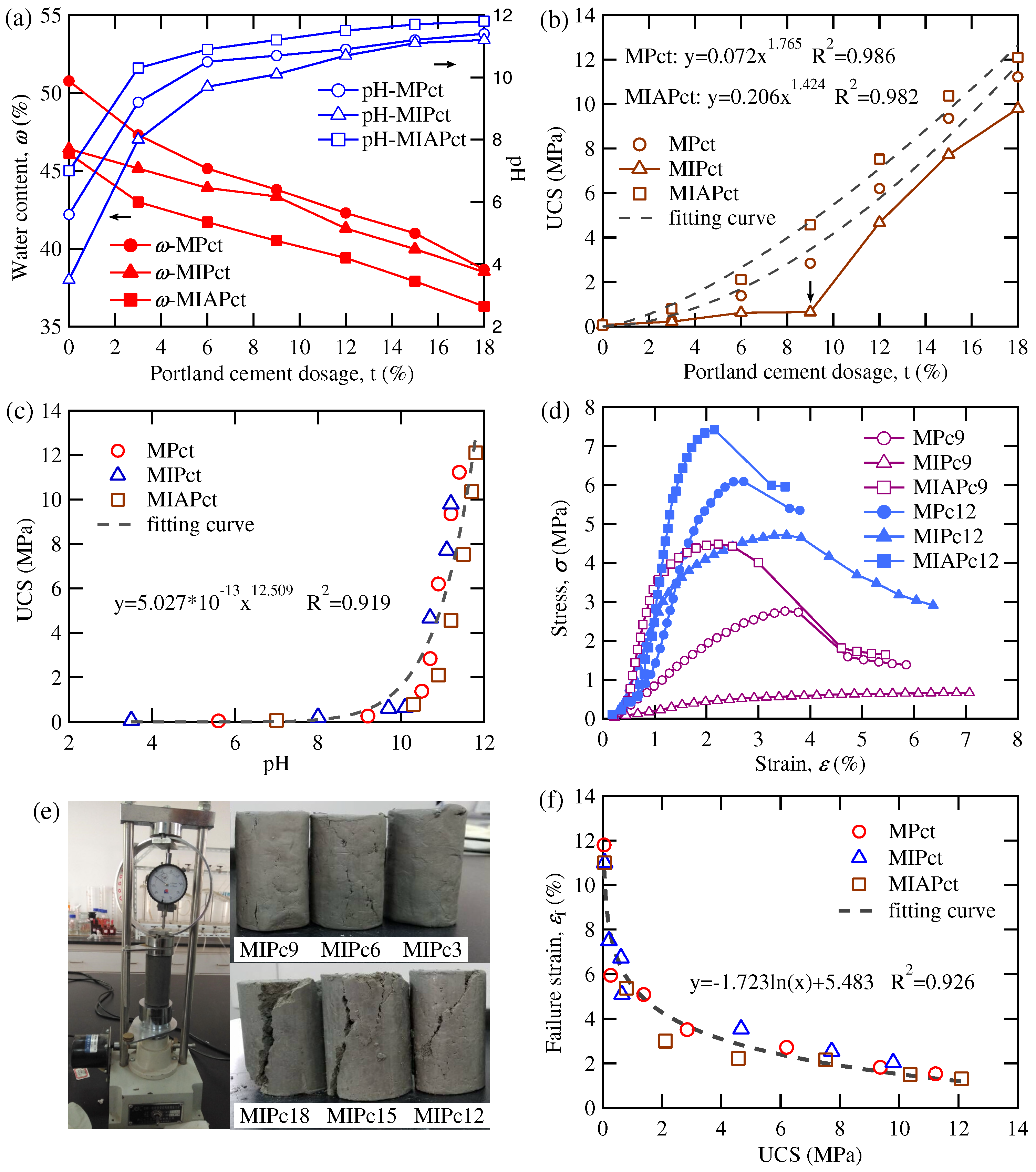
3.1. Macroscopic Analysis for Solidification Effect
3.2. Microscopic Analysis for Solidification Effect
3.3. Microscopic Analysis for Solidification Mechanism
3.3.1. SEM Results
3.3.2. XRD Results
3.3.3. XRF Results
4. Discussion
5. Conclusions
- ISS is an eco-friendly and effective stabilizer to enhance the strength of high-water-content soils (such as marine muck) when it was used combined with cement after adding the additives such as NaOH. Under the same Portland cement dosage, the strength of solidified samples ranged from more to less as: MIAPct > MPct > MIPct.
- There was an unified function relationship for all types of solidified samples (such as MPct, MIPct, and MIAPct) between UCS and pH-value by power function, UCS and failure strain by natural logarithmic function, and UCS and cohesion by linear function, respectively, which can be used to predict the strength of other solidified samples with different proportion of the additives of ISS, cement and NaOH. However, the relationship between UCS and Portland cement dosage cannot be described by an uniform functional equation, which was attributed to the different amount of cement involved in acid-base neutralization reaction or hydration reaction varying from sample to sample.
- With the exception of d-value decreasing for clay minerals, Ca and SO dissolved from ISS promoted the production of AFt, pozzolanic reaction and carbonation reaction of cement in the presence of NaOH. AFt efficiently stabilized soil by constructing a skeleton structure. In the meantime, cementitious hydration products aggregated soils, resulting in a sharp pore reduction and a high strength improvement for solidified soils.
- As a partial substitute, the best reinforcement effect of the ISS-NaOH-cement-solidified soil samples was determined. Furthermore, this combination of stabilizers can not only save the dosage of cement, but also accelerate the solidification speed, decrease the cement setting time within 7 days to meet the curing requirements, and improve the strength of solidified soils. Thus, this solidification method can be applied in engineering practice for the advantages of high strength, resource reuse and environmental protection.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, C.G.; Xie, X.Y.; Gen, J.; Wang, W.J. Effect of stabilization/solidification on mechanical and phase characteristics of organic river silt by a stabilizer. Constr. Build. Mater. 2020, 236, 117538. [Google Scholar] [CrossRef]
- Shirazi, M.G.; Rashid, A.S.B.A.; Nazir, R.B.; Rashid, A.H.B.A.; Moayedi, H.; Horpibulsuk, S.; Samingthong, W. Sustainable soil bearing capacity improvement using natural limited life geotextile reinforcement—A review. Minerals 2020, 10, 479. [Google Scholar] [CrossRef]
- Yang, Q.; Du, C.X. Experimental Study on the Effect of Plant Ash on Soft Clay Stabilized with Cement-Based Composites. Geotech. Geol. Eng. 2021, 39, 105–117. [Google Scholar] [CrossRef]
- Lorenzo, G.A.; Bergado, D.T. Fundamental characteristics of cement-admixed clay in deep mixing. J. Mater. Civ. Eng. 2006, 18, 161–174. [Google Scholar] [CrossRef]
- Fonseca, A.V.d.; Cruz, R.C.; Consoli, N.C. Strength properties of sandy soil-cement admixtures. Geotech. Geol. Eng. 2009, 27, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Qin, Z.; Zhuang, Y.; Chen, L.; Chen, B. Influence of sodium silicate and promoters on unconfined compressive strength of Portland cement-stabilized clay. Soils Found. 2015, 55, 1222–1232. [Google Scholar] [CrossRef] [Green Version]
- Ekinci, A.; Hanafi, M.; Aydin, E. Strength, Stiffness, and Microstructure of Wood-Ash Stabilized Marine Clay. Minerals 2020, 10, 796. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Bergado, D.T.; Lorenzo, G.A. Compressibility of cement-admixed clays at high water content. Geotechnique 2004, 54, 151–154. [Google Scholar] [CrossRef]
- Jauberthie, R.; Rendell, F.; Rangeard, D.; Molez, L. Stabilisation of estuarine silt with lime and/or cement. Appl. Clay Sci. 2010, 50, 395–400. [Google Scholar] [CrossRef]
- Ikhlef, N.S.; Ghembaza, M.S.; Dadouch, M. Effect of treatment with cement on the mechanical characteristics of silt from Telagh region of Sidi Belabes, Algeria. Geotech. Geol. Eng. 2015, 33, 1067–1079. [Google Scholar] [CrossRef]
- Higgins, D. Briefing: GGBS and sustainability. Constr. Mater. 2007, 160, 99–101. [Google Scholar] [CrossRef]
- Wang, D.X.; Gao, X.Y.; Wang, R.H.; Larsson, S.; Benzerzour, M. Elevated curing temperature-associated strength and mechanisms of reactive MgO-activated industrial by-products solidified soils. Mar. Georesources Geotechnol. 2020, 38, 659–671. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Rachan, R.; Raksachon, Y. Role of fly ash on strength and microstructure development in blended cement stabilized silty clay. Soils Found. 2009, 49, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Bian, X.; Ding, G.Q.; Wang, Z.F.; Cao, Y.P.; Ding, J.W. Compression and strength behavior of cement-lime-polymer-solidified dredged material at high water content. Mar. Georesources Geotechnol. 2017, 35, 840–846. [Google Scholar] [CrossRef]
- Jamsawang, P.; Poorahong, H.; Yoobanpot, N.; Songpiriyakij, S.; Jongpradist, P. Improvement of soft clay with cement and bagasse ash waste. Constr. Build. Mater. 2017, 154, 61–71. [Google Scholar] [CrossRef]
- Baldovino, J.A.; Izzo, R.L.; Silva, É.R.; Rose, J.L. Sustainable use of recycled-glass powder in soil stabilization. J. Mater. Civ. Eng. 2020, 32, 04020080. [Google Scholar] [CrossRef]
- Bahmani, S.H.; Huat, B.B.; Asadi, A.; Farzadnia, N. Stabilization of residual soil using SiO2 nanoparticles and cement. Constr. Build. Mater. 2014, 64, 350–359. [Google Scholar] [CrossRef]
- Dong, C.Q.; Zhang, R.J.; Zheng, J.J.; Jiang, W.H. Strength behavior of dredged mud slurry treated jointly by cement, metakaolin and flocculant. Appl. Clay Sci. 2020, 193, 105676. [Google Scholar] [CrossRef]
- Katz, L.E.; Rauch, A.F.; Liljestrand, H.M.; Harmon, J.S.; Shaw, K.S.; Albers, H. Mechanisms of soil stabilization with liquid ionic stabilizer. Transp. Res. Rec. 2001, 1757, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.T.; Xiang, W.; Zhang, M.X. Solidification of fluviatile-lacustrine facies silt with ionic soil stabilizer. Electron. J. Geotech. Eng. 2016, 21, 2071–2082. [Google Scholar]
- Arefin, S.; Al-Dakheeli, H.; Bulut, R. Stabilization of expansive soils using ionic stabilizer. Bull. Eng. Geol. Environ. 2021, 80, 4025–4033. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhang, H.; Zhang, J.M.; Chai, M.T. Effectiveness of ionic polymer soil stabilizers on warm frozen soil. KSCE J. Civ. Eng. 2019, 23, 2867–2876. [Google Scholar] [CrossRef]
- Cui, D.S.; Xiang, W. Pore diameter distribution test of red clay treated with ISS. Rock Soil Mech. 2010, 31, 3096–3100. [Google Scholar]
- Liu, Q.B.; Xiang, W.; Cui, D.S. Effect of ionic soil stabilizer on bound water of expansive soils. Chin. J. Geotech. Eng. 2012, 34, 1887–1895. [Google Scholar]
- He, S.; Yu, X.B.; Banerjee, A.; Puppala, A.J. Expansive soil treatment with liquid ionic soil stabilizer. Transp. Res. Rec. 2018, 2672, 185–194. [Google Scholar] [CrossRef]
- Wu, X.T.; Qi, Y.; Chen, B. Solidification effect and mechanism of ionic soil stabilizer applied on high-water-content clay. Bull. Eng. Geol. Environ. 2021, 80, 8583–8595. [Google Scholar] [CrossRef]
- Scholen, D.E. Stabilizer mechanisms in nonstandard stabilizers. In Proceedings of the Transportation Research Board Conference Proceedings, Minneapolis, MN, USA, 25–29 June 1995; pp. 252–260. [Google Scholar]
- Sarkar, S.L.; Herbert, B.E.; Scharlin, R.J. Injection stabilization of expansive clays using a hydrogen ion exchange chemical. In Advances in Unsaturated Geotechnics; ACSE: Reston, VA, USA, 2000; pp. 487–516. [Google Scholar]
- Xiang, W.; Cui, D.S.; Liu, Q.B.; Lu, X.S.; Cao, L.J. Theory and practice of ionic soil stabilizer reinforcing special clay. J. Earth Sci. 2010, 21, 882–887. [Google Scholar] [CrossRef]
- Miura, N.; Horpibulsuk, S.; Nagaraj, T.S. Engineering behavior of cement stabilized clay at high water content. Soils Found. 2001, 41, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Bian, X.; Wang, Z.F.; Ding, G.Q.; Cao, Y.P. Compressibility of cemented dredged clay at high water content with super-absorbent polymer. Eng. Geol. 2016, 208, 198–205. [Google Scholar] [CrossRef]
- Zhu, J.F.; Xu, R.Q.; Zhao, H.Y.; Luo, Z.Y.; Pan, B.J.; Rao, C.Y. Fundamental mechanical behavior of CMMOSC-SC composite stabilized marine soft clay. Appl. Clay Sci. 2020, 192, 105635. [Google Scholar] [CrossRef]
- Xu, G.Z.; Qiu, C.C.; Song, M.M.; Cao, Y.P.; Yin, J. Flocculant effects on fluidity and strength behavior of cemented dredged clay with high water content. Mar. Georesources Geotechnol. 2021, 39, 951–961. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, X.H.; Qiu, X.; Wu, J.H. Analysis of microstructure characteristics and stabilization mechanism of ionic soil stabilizer treated clay. J. Highw. Transp. Res. Dev. 2015, 32, 33–40. [Google Scholar]
- Wu, X.T.; Tang, S.; Cheng, M.F.; Wang, Z.H.; Xiang, W.; Chen, B. Mechanical properties of solidified silt with ISS and cement. China Harb. Eng. 2019, 39, 21–26. [Google Scholar]
- GB. Common Portland Cement; GB 175; Standards Press of China: Beijing, China, 2007. [Google Scholar]
- ASTM. Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System); D2487; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Zhang, T.; Cai, G.J.; Liu, S.Y. Reclaimed Lignin-Stabilized Silty Soil: Undrained Shear Strength, Atterberg Limits, and Microstructure Characteristics. J. Mater. Civ. Eng. 2018, 30, 1–9. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, Y.J.; Xu, J.X.; Yang, Q. Mechanical Characteristics of Stabilized Clay under the Influence of both Acidic and Alkalic Additives. J. Highw. Transp. Res. Dev. 2017, 11, 39–47. [Google Scholar] [CrossRef]
- Lu, X.S.; Xiang, W.; Fan, W.Y.; Yu, H.C. Experimental study on reinforcement of red clay with ionic soil stabilizer. Yellow River 2010, 32, 127–129. [Google Scholar]
- ASTM. Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils; D4318; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- GB/T 50123. Standard for Soil Test Method; China Planning Press: Beijing, China, 1999. [Google Scholar]
- Du, Y.J.; Jiang, N.J.; Liu, S.Y.; Jin, F.; Singh, D.N.; Puppala, A.J. Engineering properties and microstructural characteristics of cement-stabilized zinc-contaminated kaolin. Can. Geotech. J. 2014, 51, 289–302. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, G.; Liu, S.; Duan, W. Laboratory observation of engineering properties and deformation mechanisms of cemented rubber-sand mixtures. Constr. Build. Mater. 2016, 120, 514–523. [Google Scholar] [CrossRef]
- Lei, W.; Xiang, W. Mechanism of disturbed sliding zone soil improved by EN-1 ionic soil stabilizer. J. Yangtze River Sci. Res. Inst. 2014, 31, 47–51. [Google Scholar]
- Deng, W.J. Experimental study on expansive soil modified by EN-1 Ionic Soil Stabilizer. J. China Foreign Highw. 2015, 35, 248–250. [Google Scholar]
- Zhang, Z.L.; Zhang, J.M.; Zhang, H. Effects and mechanisms of ionic soil stabilizers on warm frozen soil. Arab. J. Sci. Eng. 2018, 43, 5657–5666. [Google Scholar] [CrossRef]
- Kannan, K.; Bindu, J.; Vinod, P. Engineering behaviour of MICP treated marine clays. Mar. Georesources Geotechnol. 2020, 38, 761–769. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.M.; Zhou, X.; Chen, C.; Zhang, X.W.; Gui, L. In-situ test of shear modulus decay characterisitics of lacustrine clay layer in Taihu Lake. Rock Soil Mech. 2021, 42, 2031–2040. [Google Scholar]
- Cheng, X.; Chen, Y.H.; Chen, G.; Li, B.Y. Characterization and prediction for the strength development of cement stabilized dredged sediment. Mar. Georesources Geotechnol. 2021, 39, 1015–1024. [Google Scholar] [CrossRef]
- Chen, M. Experimental Studies on Conditioning Solidification and Environmental Characteristics of Solidified Silt. Ph.D. Thesis, Wuhan University, Wuhan, China, 2014. [Google Scholar]
- Ning, J.G.; Huang, X.; Xu, s. Effect of pH value of soil on strength increasing of the stabilized soil. Chin. J. Geotech. Eng. 2007, 29, 98–102. [Google Scholar]
- Wang, D.X.; Wang, H.W.; Wang, X.Q. Compressibility and strength behavior of marine soils solidified with MgO—A green and low carbon binder. Mar. Georesources Geotechnol. 2017, 35, 878–886. [Google Scholar] [CrossRef]
- Liu, C.; Shi, B.; Zhou, J.; Tang, C.S. Quantification and characterization of microporosity by image processing, geometric measurement and statistical methods: Application on SEM images of clay materials. Appl. Clay Sci. 2011, 54, 97–106. [Google Scholar] [CrossRef]
- Liu, C.; Tang, C.S.; Shi, B.; Suo, W.B. Automatic quantification of crack patterns by image processing. Comput. Geosci. 2013, 57, 77–80. [Google Scholar] [CrossRef]
- Wu, X.T.; Sun, J.S.; Qi, Y.; Chen, B. Pore and compression characteristics of clay solidified by ionic soil stabilizer. Bull. Eng. Geol. Environ. 2021, 80, 5003–5019. [Google Scholar] [CrossRef]
- Du, C.X.; Zhang, J.L.; Zhang, T.T.; Yang, Q. Effect of salt on strength development of marine soft clay stabilized with cement-based composites. Mar. Georesources Geotechnol. 2020, 38, 672–685. [Google Scholar] [CrossRef]
- Gastaldi, D.; Paul, G.; Marchese, L.; Irico, S.; Boccaleri, E.; Mutke, S.; Buzzi, L.; Canonico, F. Hydration products in sulfoaluminate cements: Evaluation of amorphous phases by XRD/solid-state NMR. Cem. Concr. Res. 2016, 90, 162–173. [Google Scholar] [CrossRef]
- Chen, Y.J.; He, X.X.; Zhang, S.H.; Tan, X.; Wan, Y. Strength and microstructure properties of solidified sewage sludge with two types of cement-based binders. Sci. Rep. 2020, 10, 20769. [Google Scholar] [CrossRef]
- Pinto, C.A.; Sansalone, J.J.; Cartledge, F.K.; Dweck, J.; Diaz, F.R.; Büchler, P.M. Cement Stabilization of Runoff Residuals: A Study of Stabilization/Solidification of Urban Rainfall-Runoff Residuals in Type 1 Portland Cement by XRD and Si-29 NMR Analysis. Water Air Soil Pollut. 2008, 188, 261–270. [Google Scholar] [CrossRef]
- Yin, Z.H.; Zhang, H.; Zhang, J.M.; Chai, M.T. Mechanical behavior of frozen soil improved with sulphoaluminate cement and its microscopic mechanism. Sci. Rep. 2020, 10, 16297. [Google Scholar] [CrossRef] [PubMed]
- Du, C.X.; Yang, G.; Zhang, T.T.; Yang, Q. Multiscale study of the influence of promoters on low-plasticity clay stabilized with cement-based composites. Constr. Build. Mater. 2019, 213, 537–548. [Google Scholar] [CrossRef]
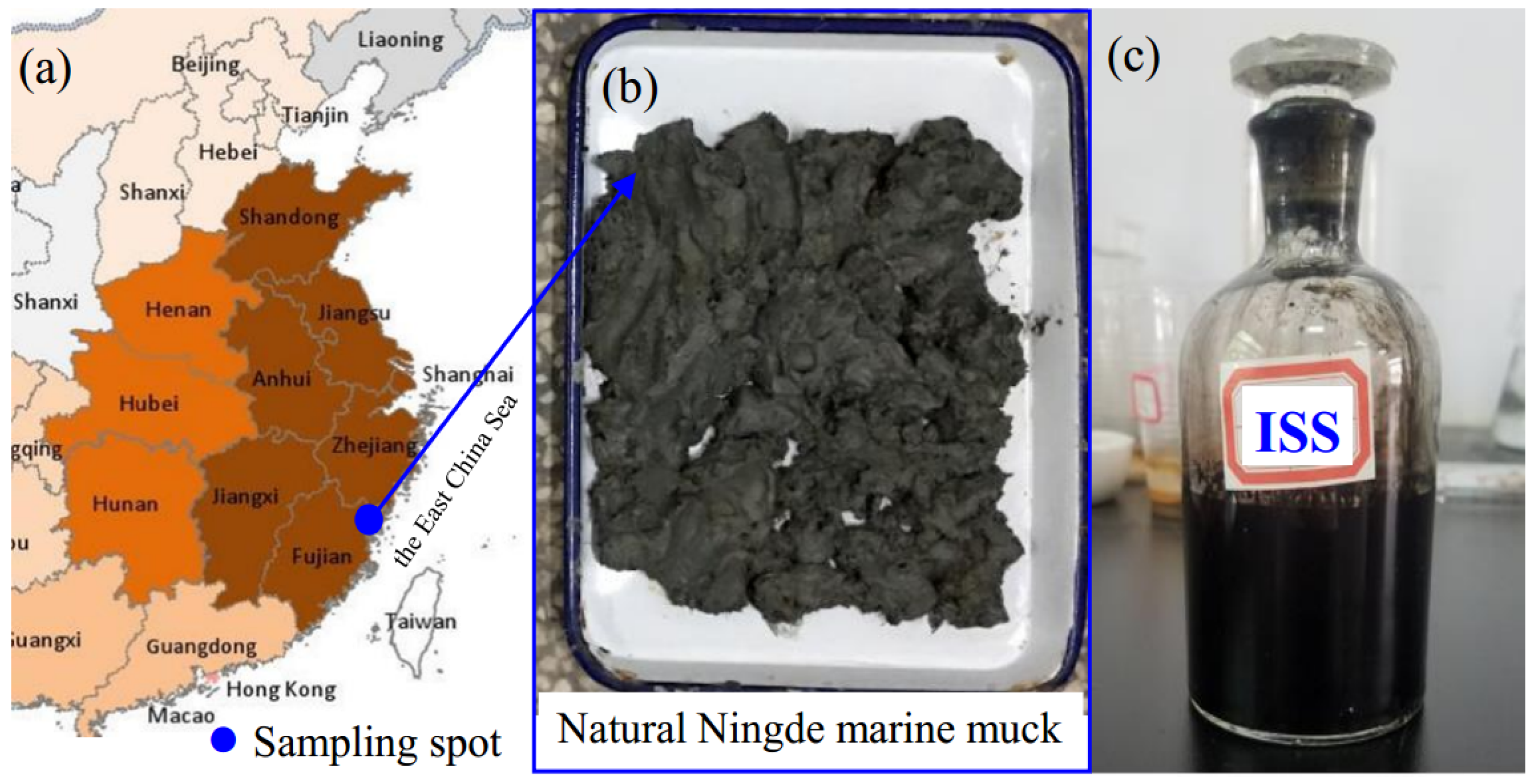

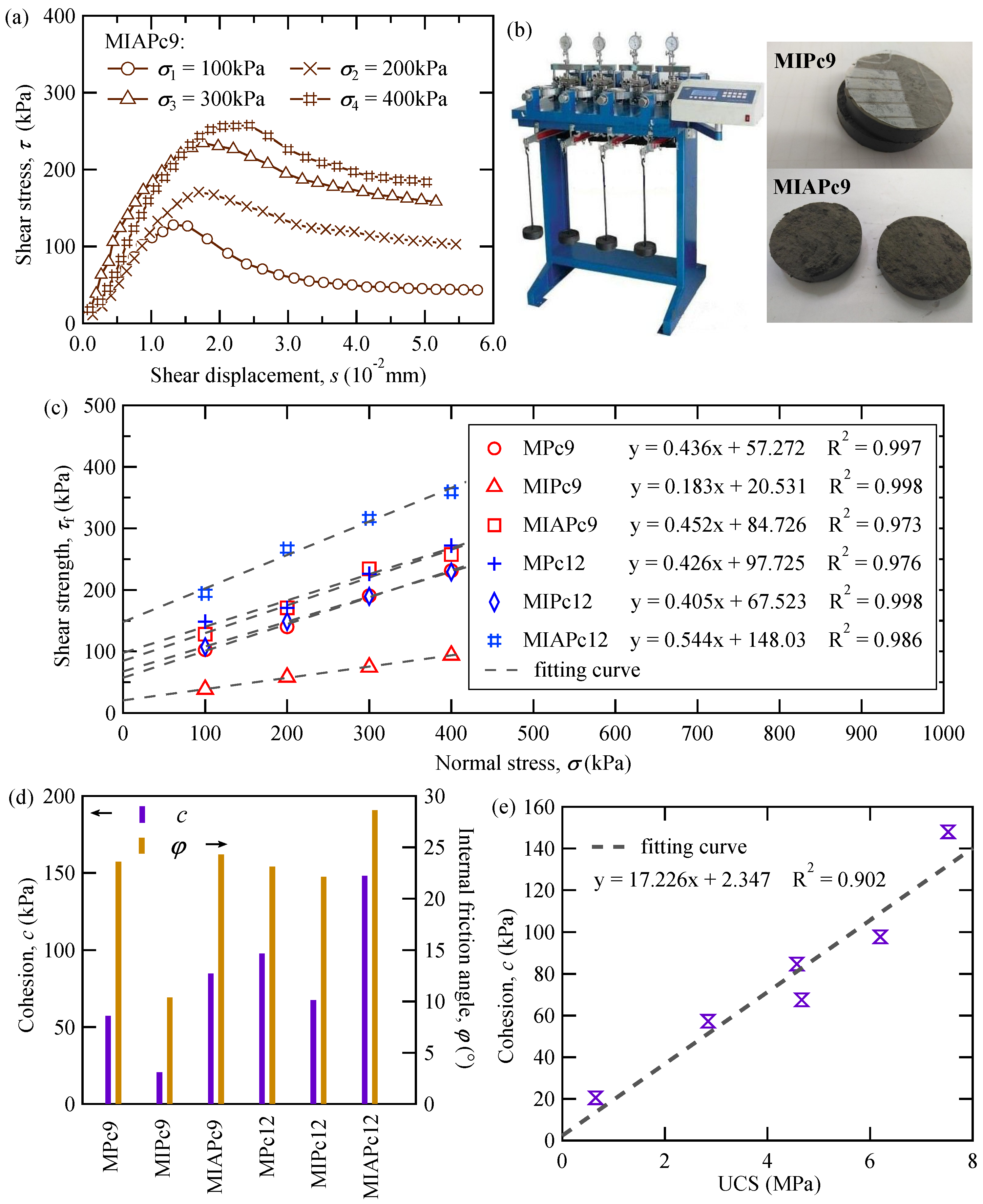

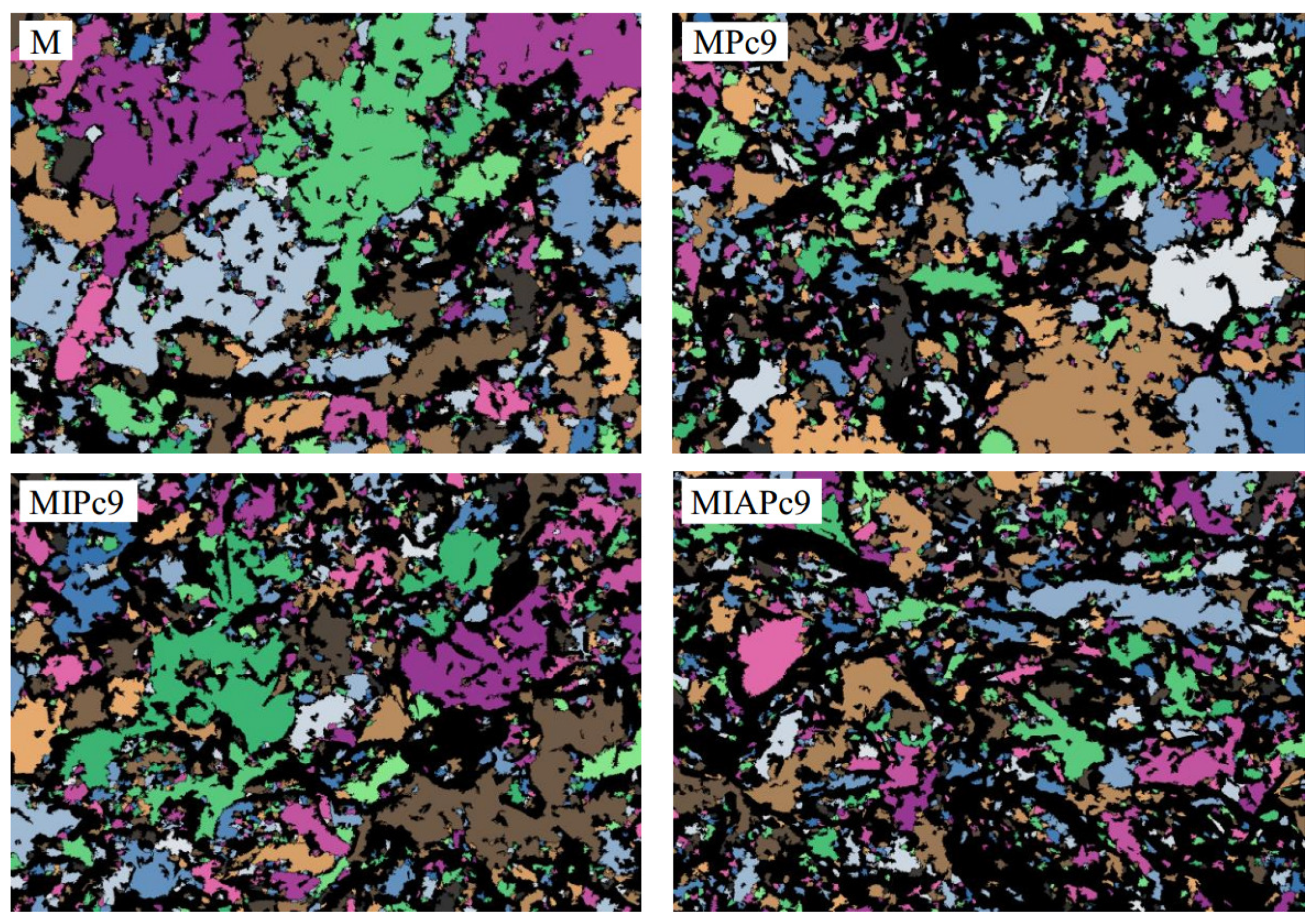

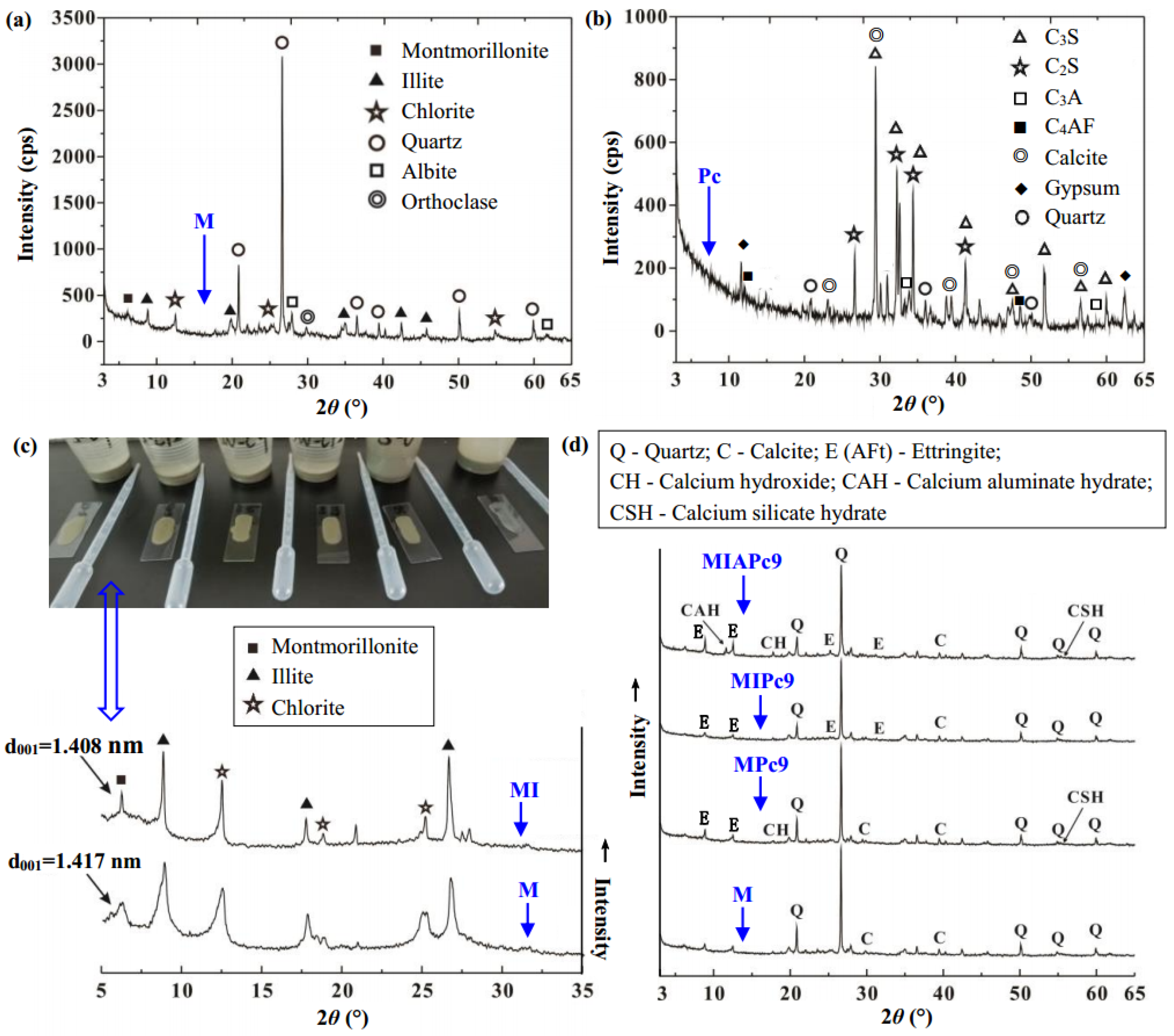
| Ion | Concentration (mg/L) |
|---|---|
| Fe | 2388 |
| Al | 807 |
| Ca | 10,662 |
| Mg | 1433 |
| K | 11,300 |
| Na | 16,128 |
| NO | 1920 |
| SO | 100,344 |
| Parameter | Value |
|---|---|
| Natural water content (%) | 65.02 |
| Natural density (g/cm) | 1.38 |
| Soil particle density (g/cm) | 2.73 |
| Initial void ratio | 2.26 |
| Liquid limit, LL (%) | 50.77 |
| Plastic limit, PL (%) | 31.67 |
| Plasticity index, PI (%) | 19.10 |
| pH | 5.6 |
| UCS (kPa) | 46 |
| Sample Symbol | Soil | ISS:HO Ratio | Cement Dosage (t%) | NaOH | Water Content (%) |
|---|---|---|---|---|---|
| M | Natural | 0 | 0 | 0 | Natural content |
| MIx | Powder | 1:x | 0 | 0 | LL |
| MI | Powder | Optimal ratio | 0 | 0 | LL |
| MPct | Powder | 0 | 0, 3, 6, 9, 12, 15, 18 | 0 | LL |
| MIPct | Powder | Optimal ratio | 0, 3, 6, 9, 12, 15, 18 | 0 | LL |
| MIAPct | Powder | Optimal ratio | 0, 3, 6, 9, 12, 15, 18 | A | LL |
| Sample Symbol | Actual Porosity (%) | Image Analytical (2000×) | ||
|---|---|---|---|---|
| Porosity (%) | Smax (µm) | S (µm) | ||
| M | 69.33 | 65.48 | 258.88 | 1860 |
| MPc9 | 50.50 | 50.01 | 160.87 | 1422 |
| MIPc9 | 52.38 | 51.06 | 165.75 | 1451 |
| MIAPc9 | 42.86 | 40.91 | 63.67 | 1164 |
| Sample Symbol | Oxide Composition and Content (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO | AlO | FeO | MgO | CaO | NaO | KO | MnO | TiO | PO | SO | LOI | |
| Pc | 22.72 | 6.70 | 3.40 | 2.72 | 53.76 | 0.14 | 0.65 | 0.13 | 0.29 | 0.12 | 2.63 | 6.64 |
| M | 61.15 | 17.58 | 5.46 | 1.82 | 0.56 | 1.45 | 3.49 | 0.05 | 0.79 | 0.09 | 0.64 | 6.84 |
| MI | 61.85 | 17.78 | 5.26 | 1.79 | 0.29 | 1.41 | 3.61 | 0.04 | 0.79 | 0.09 | 0.90 | 6.11 |
| MPc9 | 55.82 | 15.82 | 5.01 | 1.78 | 4.60 | 1.26 | 3.29 | 0.06 | 0.76 | 0.09 | 0.73 | 10.71 |
| MPc12 | 54.04 | 15.38 | 4.89 | 1.77 | 5.63 | 1.19 | 3.12 | 0.06 | 0.70 | 0.09 | 0.78 | 12.29 |
| MIPc9 | 56.12 | 16.20 | 4.98 | 1.83 | 4.88 | 1.30 | 3.17 | 0.06 | 0.73 | 0.09 | 0.97 | 9.59 |
| MIPc12 | 55.12 | 16.13 | 4.98 | 1.84 | 5.58 | 1.29 | 3.05 | 0.06 | 0.73 | 0.09 | 1.01 | 10.04 |
| MIAPc9 | 52.43 | 16.17 | 4.91 | 1.91 | 4.32 | 2.31 | 3.21 | 0.05 | 0.79 | 0.08 | 1.09 | 12.21 |
| MIAPc12 | 52.43 | 15.49 | 4.78 | 1.83 | 5.24 | 2.11 | 3.18 | 0.06 | 0.74 | 0.08 | 1.16 | 12.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.-T.; Qi, Y.; Liu, J.-N.; Chen, B. Solidification Effect and Mechanism of Marine Muck Treated with Ionic Soil Stabilizer and Cement. Minerals 2021, 11, 1268. https://doi.org/10.3390/min11111268
Wu X-T, Qi Y, Liu J-N, Chen B. Solidification Effect and Mechanism of Marine Muck Treated with Ionic Soil Stabilizer and Cement. Minerals. 2021; 11(11):1268. https://doi.org/10.3390/min11111268
Chicago/Turabian StyleWu, Xue-Ting, Yi Qi, Jun-Ning Liu, and Bin Chen. 2021. "Solidification Effect and Mechanism of Marine Muck Treated with Ionic Soil Stabilizer and Cement" Minerals 11, no. 11: 1268. https://doi.org/10.3390/min11111268
APA StyleWu, X.-T., Qi, Y., Liu, J.-N., & Chen, B. (2021). Solidification Effect and Mechanism of Marine Muck Treated with Ionic Soil Stabilizer and Cement. Minerals, 11(11), 1268. https://doi.org/10.3390/min11111268






