Mineralogy, Fluid Inclusions, and Isotopic Study of the Kargah Cu-Pb Polymetallic Vein-Type Deposit, Kohistan Island Arc, Northern Pakistan: Implication for Ore Genesis
Abstract
:1. Introduction
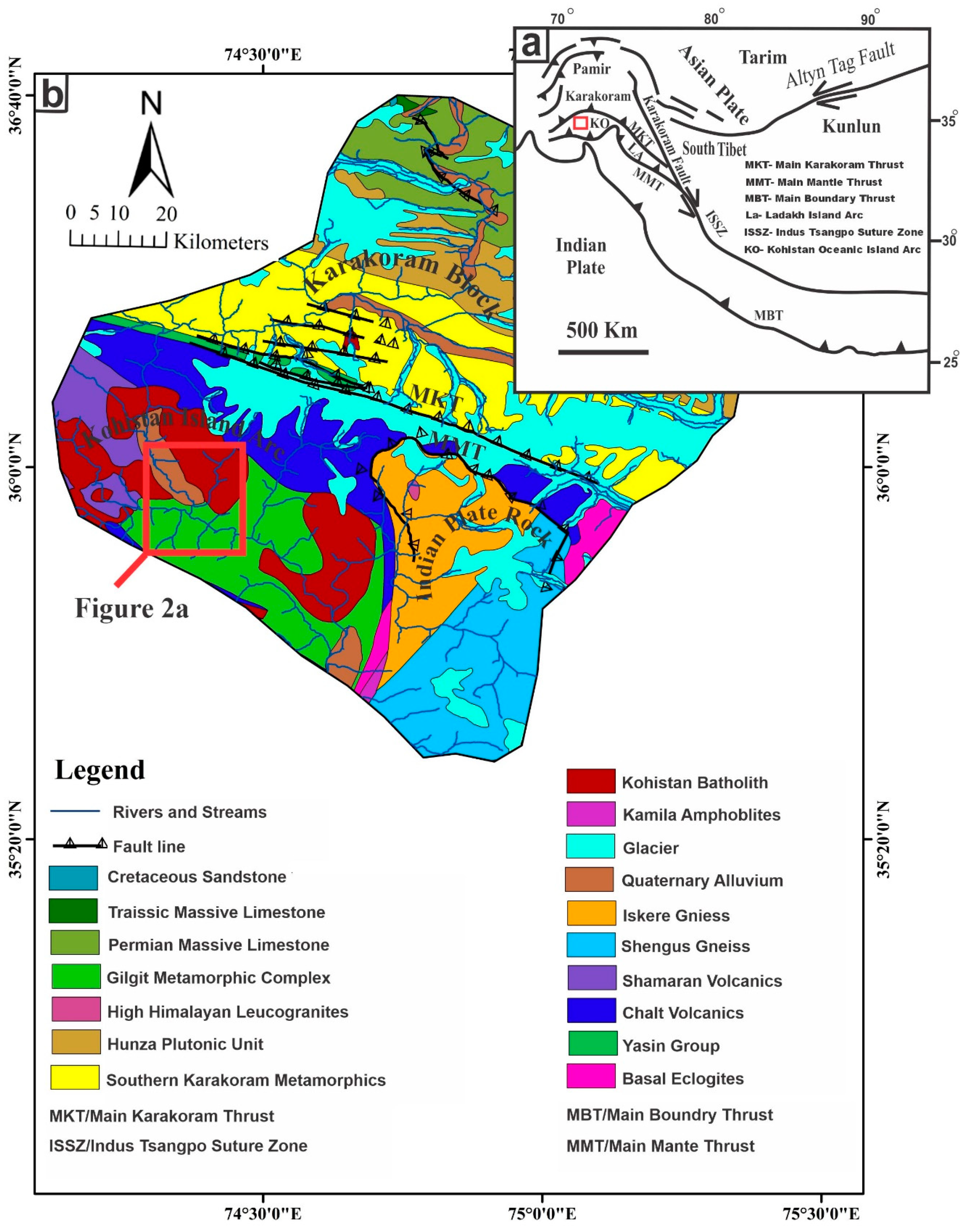
2. Geological Setting
2.1. Regional Geology
2.2. Geology of the Kargah Valley
3. Sampling and Analytical Methods
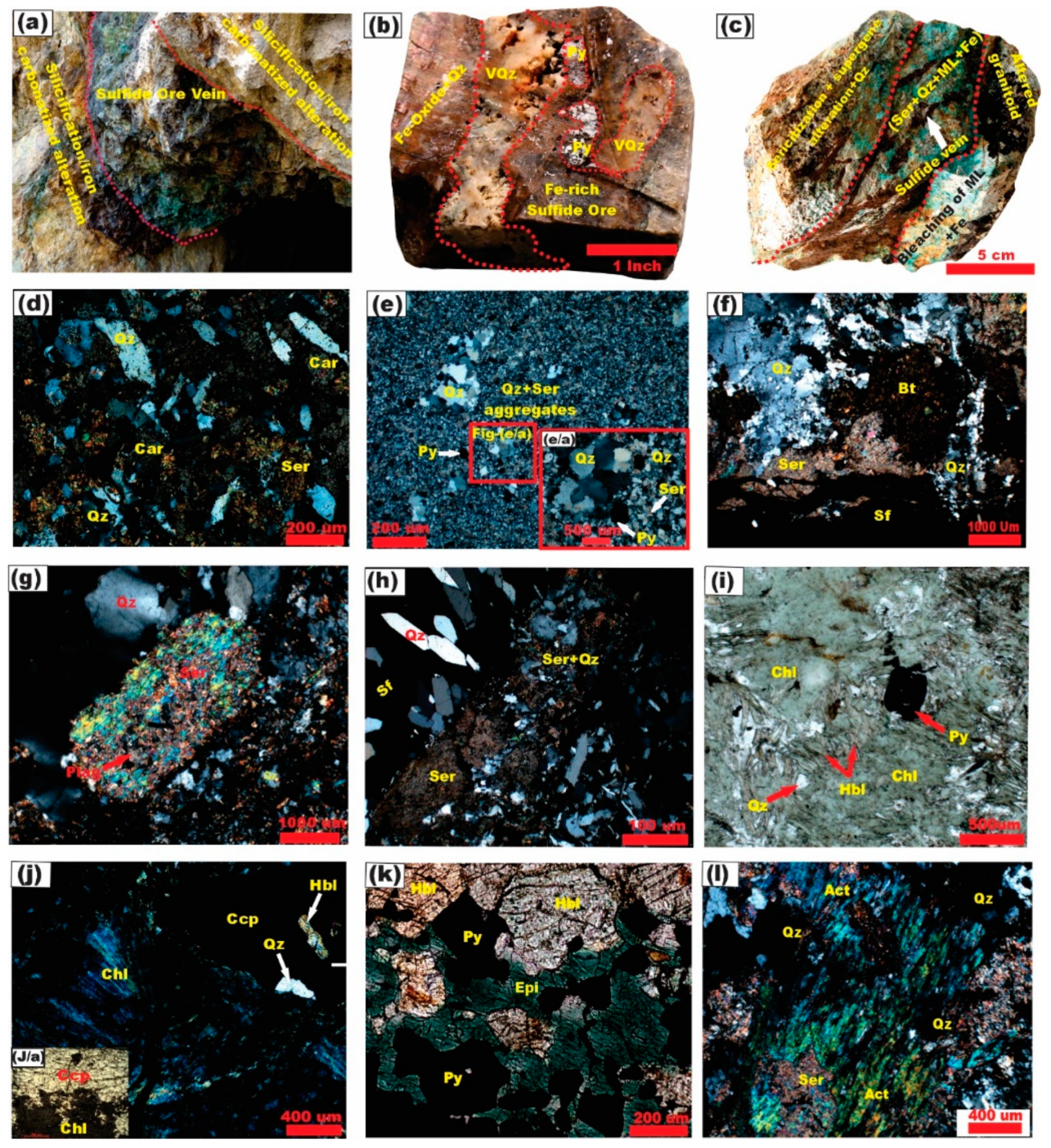
4. Ore Geology
5. Results
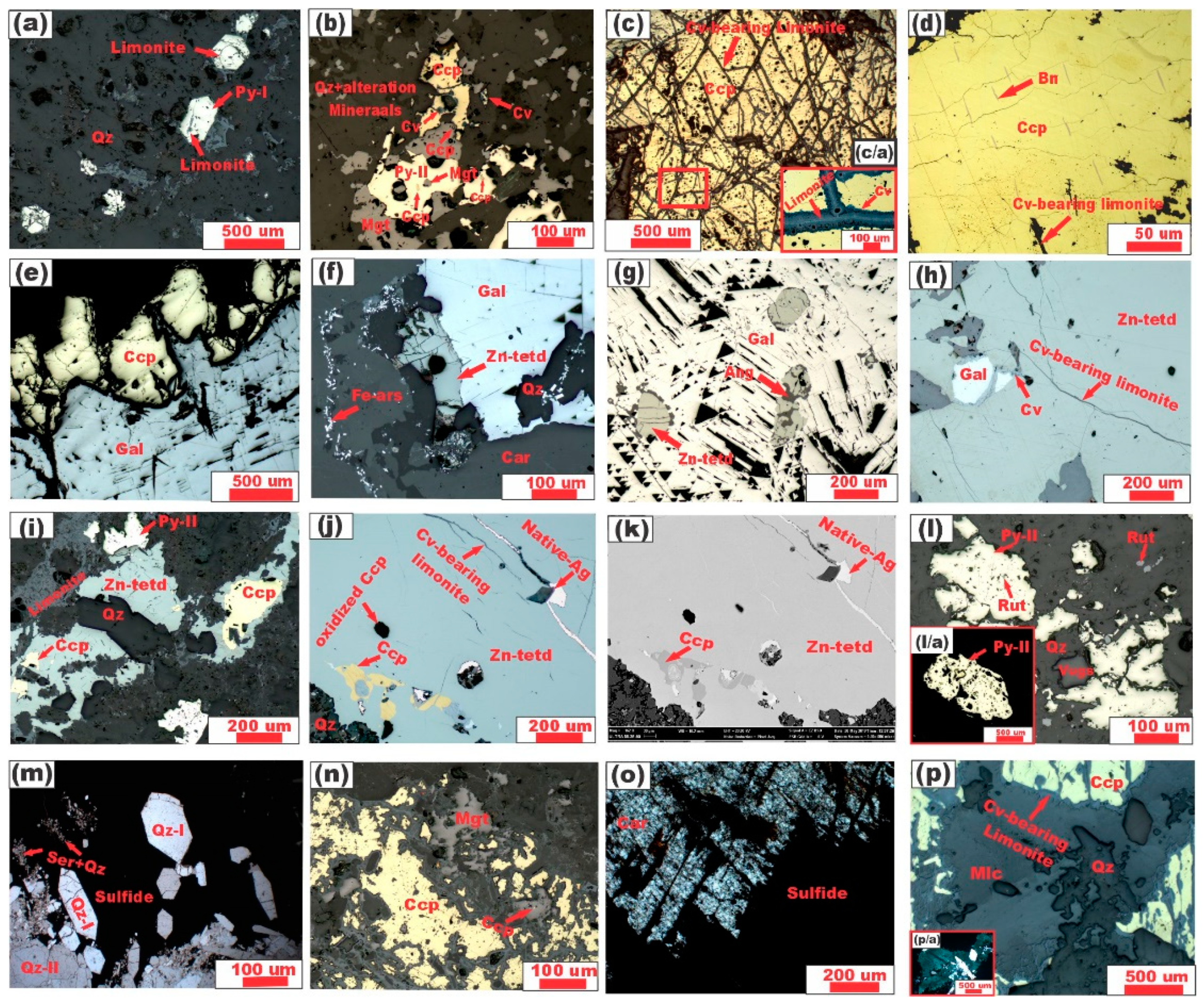
5.1. Mineralogy and Mineral Chemistry
5.1.1. Ore Minerals
Pyrite
Chalcopyrite
Magnetite
Bornite
Galena
Zincian Tetrahedrite
Native Silver
Iron-Arsenide (Lollingite)
5.2. Fluid Inclusion Study
5.2.1. Petrography and Classification
Type I Inclusions
Type II Inclusions
Type III Inclusions
Type IV Inclusions
Type V Inclusions
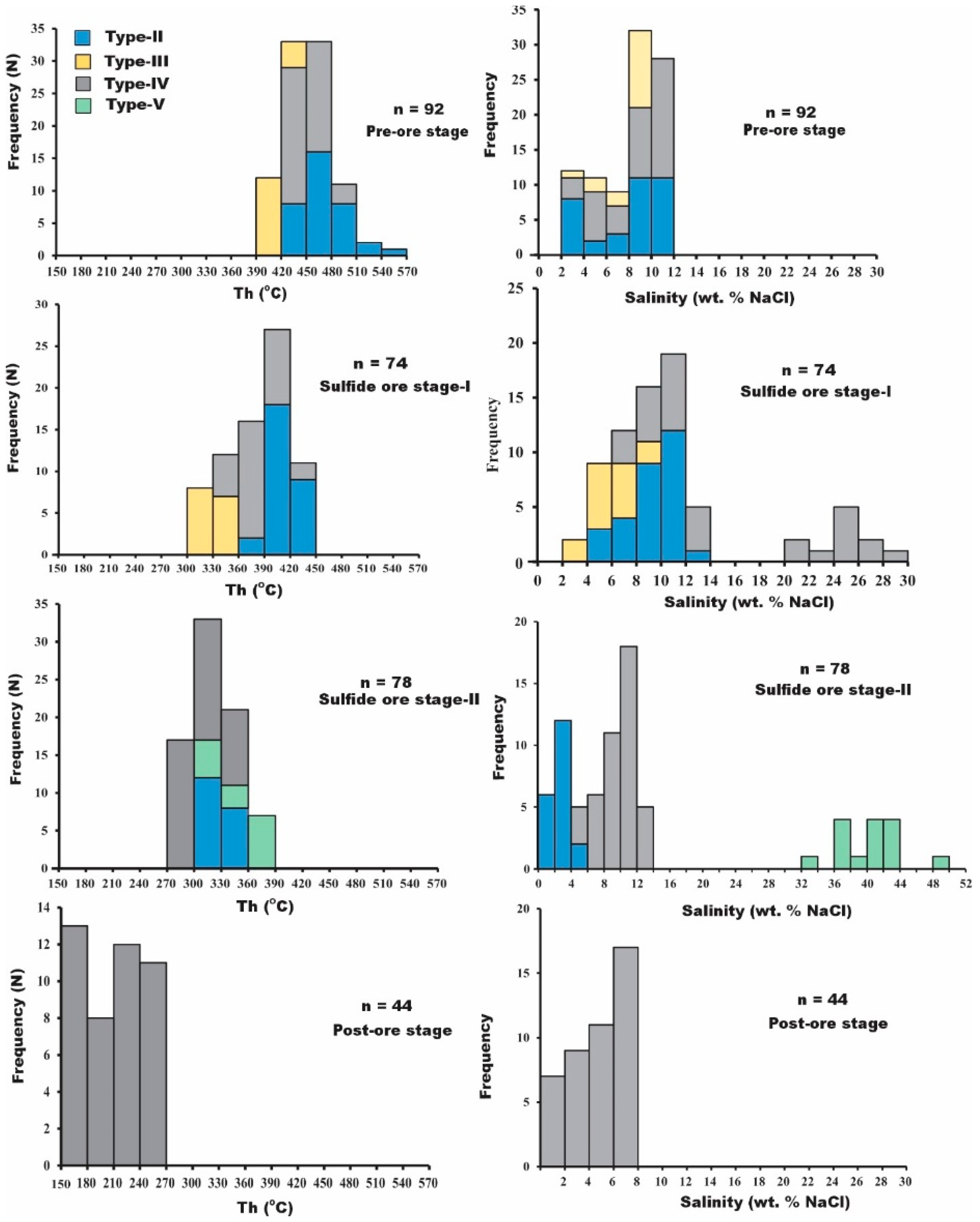
5.2.2. Microthermometry
Pre-Ore Stage
Sulfide Ore Stage I
Sulfide Ore Stage II
Post-Ore Stage
5.2.3. Laser Raman Spectroscopy
5.3. Sulfur Isotopes
5.4. Pb Isotopes
6. Discussion
6.1. Trace Elements Incorporation within Pyrite
6.2. Evolution of Ore-Forming Fluids
6.3. Composition and Implication for Ore-Forming Fluids
6.4. Source of the Ore-Forming Materials
6.5. Precipitation Mechanism for Ore Minerals
6.6. Deposit Genesis
7. Conclusions
- Three main stages of mineralization were identified, including the pre-ore (quartz-pyrite), main sulfide ore, and post-ore (quartz-carbonate) stages. Pyrite, chalcopyrite, galena, and zincian tetrahedrite are major sulfides, with accessory mineral native silver and gold mainly distributed in pyrite.
- High Co/Ni ratios, low Mo/Ni ratios, and low Ni and Mo contents in pyrites suggest a mafic source for mineralization.
- Five types of fluid inclusions were identified. At the pre-ore stage, the fluid was observed to show critical behaviors at temperatures between 434 and 555 °C. Chalcopyrite/magnetite ± bornite were primarily precipitated at temperatures ranging from 300 to 438 °C (>45 Mpa and depth > 2 km); galena/zincian tetrahedrite + native silver were deposited at temperatures ranging from 280 to 390 °C (<20 Mpa and depth < 2 km).
- The sulfur and lead isotope data of sulfide minerals (pyrite, chalcopyrite, and galena) suggest that the ore-forming fluid of the Kargah Cu-Pb polymetallic deposit was mainly derived from a deep magma source, accompanied by crustal-derived materials, and the Kohistan Batholith provided metals for mineralization.
- The ore-forming fluids contained both magmatic and meteoric waters. High to moderate temperatures with contrasting salinities, solid-bearing inclusions, less abundant CO2 components, and a range of δ34S values, in this study, (from −2.80 to 6.41‰, average 3.38‰) probably suggest an intrusion-related magmatic-hydrothermal deposit for the studied area.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tahirkheli, R.K. Geology of Kohistan and adjoining Eurasian and Indo-Pakistan continents, Pakistan. Geol. Bull. Univ. Peshawar 1979, 11, 1–30. [Google Scholar]
- Ziabrev, S.V.; Aitchison, J.C.; Abrajevitch, A.V.; Davis, A.M.; Luo, H. Bainang Terrane, Yarlung–Tsangpo suture, southern Tibet (Xizang, China): A record of intra-Neotethyan subduction–accretion processes preserved on the roof of the world. J. Geol. Soc. 2004, 161, 523–539. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Goswami, A.; Scotese, C.R. The longest voyage: Tectonic, magmatic, and paleoclimatic evolution of the Indian plate during its northward flight from Gondwana to Asia. Gondwana Res. 2013, 23, 238–267. [Google Scholar] [CrossRef]
- Jan, M.Q.; Howie, R. The mineralogy and geochemistry of the metamorphosed basic and ultrabasic rocks of the Jijal complex, Kohistan, NW Pakistan. J. Petrol. 1981, 22, 85–126. [Google Scholar] [CrossRef]
- Calkins, J.A.; Jamiluddin, S.; Bhuyan, K.; Hussain, A. Geology and Mineral Resources of the Chitral-Partsan Area, Hindu Kush Range, Northern Pakistan; US Govt. Print. Off: Washington, DC, USA, 1980.
- Hussain, A.; Inayat, U.; Mughal, M.S.; Hussain, S.A.; Arif, M. Comment on: “Platinum-group element geochemistry and geodynamic evolution of Chilas complex gabbros, Kohistan Island Arc NE Pakistan”. Lithos 2021, 106031. [Google Scholar] [CrossRef]
- Rehman, H.U.; Seno, T.; Yamamoto, H.; Khan, T. Timing of collision of the Kohistan–Ladakh Arc with India and Asia: Debate. Isl. Arc 2011, 20, 308–328. [Google Scholar] [CrossRef]
- Halfpenny, R.; Mazzucchelli, R.H. Regional multi-element drainage geochemistry in the Himalayan Mountains, northern Pakistan. J. Geochem. Explor. 1999, 67, 223–233. [Google Scholar] [CrossRef]
- Alam, M.; Li, S.-R.; Santosh, M.; Shah, A.; Yuan, M.-W.; Khan, H.; Qureshi, J.A.; Zeng, Y.-J. Morphological, thermoelectrical, geochemical and isotopic anatomy of auriferous pyrite from the Bagrote valley placer deposits, North Pakistan: Implications for ore genesis and gold exploration. Ore Geol. Rev. 2019, 112, 103008. [Google Scholar] [CrossRef]
- Austromineral. Final Report (feasibility Study), Indus Gold Project Submitted to Pakistan Mineral Development Corporation by Austromineral Vienna; Austromineral: Vienna, Austria, 1976. [Google Scholar]
- PMDC. Final Report on Geochemical Exploration and Evaluation of Gold and Base Metals, Northern Area, Pakistan; Pakistan Mineral Development Corporation: Islamabad, Pakistan, 2001; (Unpublished Results). [Google Scholar]
- Kausar, A.B. Petrology of the Kohistan Arc and hosted hydrothermal sulfides, Gilgit Area, Pakistan. Master’s Thesis, The Oregon State University, Corvallis, OR, USA, 28 May 1991. [Google Scholar]
- Shah, M.T.; Khan, S.D.; Ahmad, L. Investigation of gold and base metals mineralization and the petrochemical studies of the associated host rocks, Shagari Bala area, Skardu Gilgit-Baltistan, Pakistan. J. Himal. Earth Sci. 2015, 48, 26–40. [Google Scholar]
- Ahmad, L.; Khan, S.D.; Shah, M.T.; Jehan, N. Gold mineralization in Bubin area, Gilgit-Baltistan, Northern Areas, Pakistan. Arab. J. Geosci. 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Hussain, A.; Zhao, K.-D.; Arif, M.; Palmer, M.R.; Chen, W.; Zhang, Q.; Li, Q.; Jiang, S.-Y.; Girei, M.B. Geochronology, mineral chemistry and genesis of REE mineralization in alkaline rocks from the Kohistan Island Arc, Pakistan. Ore Geol. Rev. 2020, 126, 103749. [Google Scholar] [CrossRef]
- Mahéo, G.; Pêcher, A.; Guillot, S.; Rolland, Y.; Delacourt, C. Exhumation of Neogene Gneiss Domes between Oblique Crustal Boundaries in South Karakorum (Northwest Himalaya, Pakistan); Geological Society of America: Boulder, CO, USA, 2004; pp. 141–154. [Google Scholar]
- Pecher, A.; Seeber, L.; Guillot, S.; Jouanne, F.; Kausar, A.; Latif, M.; Majid, A.; Mahéo, G.; Mugnier, J.-L.; Rolland, Y. Stress field evolution in the northwest Himalayan syntaxis, northern Pakistan. Tectonics 2008, 27. [Google Scholar] [CrossRef]
- Searle, M.P.; Khan, M.A. Geological Map of North Pakistan. Scale 1:650,000; Shell International Exploration and Production: The Hague, The Netherlands, 1996. [Google Scholar]
- Khan, T.; Latif, M.; Khan, N.; Shah, S. Geology and petrography of the rock outcrops in Shinghai Gah and Kiner Gah, Gilgit Chilas districts, Northern Areas. Pak. Geol. Surv. Pak. I. R 1989, 446, 16. [Google Scholar]
- Khan, T.; Asif Khan, M.; Qasim Jan, M.; Naseem, M. Back-arc basin assemblages in Kohistan, Northern Pakistan. Geodin. Acta 1996, 9, 30–40. [Google Scholar] [CrossRef]
- Khan, T.; Jan, M.Q.; Khan, M.A.; Kausar, A.B. High-grade metasedimentary rocks (Gilgit Formation) in the vicinity of Gilgit, Kohistan, northern Pakistan. J. Mineral. Petrol. Geol. 1997, 92, 465–479. [Google Scholar] [CrossRef]
- Khan, T.; Latif, M.; Fayaz, A.; Shah, S.H. Geology of the Kar Gah Quadrangle, District Gilgit, Northern area Pakistan, by Geological Survey of Pakistan. Inf. Release Geol. Surv. Pak. 1999, 675, 1–9. [Google Scholar]
- Fraser, J.E.; Searle, M.P.; Parrish, R.R.; Noble, S.R. Chronology of deformation, metamorphism, and magmatism in the southern Karakoram Mountains. Geol. Soc. Am. Bull. 2001, 113, 1443–1455. [Google Scholar] [CrossRef]
- Zanchi, A.; Gaetani, M. The geology of the Karakoram range, Pakistan: The new 1: 100,000 geological map of Central-Western Karakoram. Ital. J. Geosci. 2011, 130, 161–262. [Google Scholar]
- Searle, M.P.; Robb, L.J.; Gardiner, N.J. Tectonic Processes and Metallogeny along the Tethyan Mountain Ranges of the Middle East and South Asia (Oman, Himalaya, Karakoram, Tibet, Myanmar, Thailand, Malaysia). Spec. Publ. 2016, 19, 301–32721. [Google Scholar]
- Hussain, A.; Shah, M.T.; Arif, M.; Agheem, M.H.; Mughal, M.S.; Ullah, S.; Hussain, S.A.; Sadiq, I. Chemical composition of gemstones and characterization of their host pegmatites and country rocks from Chumar Bakhoor, Gilgit-Baltistan, Pakistan: Implications for the source of gem-forming fluids. Arab. J. Geosci. 2021, 14, 1–15. [Google Scholar] [CrossRef]
- Pudsey, C.J. The Northern Suture, Pakistan: Margin of a Cretaceous island arc. Geol. Mag. 1986, 123, 405–423. [Google Scholar] [CrossRef]
- Petterson, M.G. A review of the geology and tectonics of the Kohistan island arc, north Pakistan. Geol. Soc. Lond. Spec. Publ. 2010, 338, 287–327. [Google Scholar] [CrossRef]
- Shah, S.Z.; Sayab, M.; Aerden, D.; Khan, M.A.J.T. Foliation intersection axes preserved in garnet porphyroblasts from the Swat area, NW Himalaya: A record of successive crustal shortening directions between the Indian plate and Kohistan–Ladakh Island Arc. Tectonophys 2011, 509, 14–32. [Google Scholar] [CrossRef]
- Jagoutz, O.; Schmidt, M.W. The formation and bulk composition of modern juvenile continental crust: The Kohistan arc. Chem. Geol. 2012, 298, 79–96. [Google Scholar] [CrossRef]
- Rowley, D.B. Age of initiation of collision between India and Asia: A review of stratigraphic data. Earth Planet. Sci. Lett. 1996, 145, 1–13. [Google Scholar] [CrossRef]
- Anczkiewicz, R.; Burg, J.-P.; Hussain, S.; Dawood, H.; Ghazanfar, M.; Chaudhry, M. Stratigraphy and structure of the Indus Suture in the lower Swat, Pakistan, NW Himalaya. J. Asian Earth Sci. 1998, 16, 225–238. [Google Scholar] [CrossRef]
- Treloar, P.; Rex, D. Cooling, uplift and exhumation rates in the crystalline thrust stack of the North Indian Plate, west of the Nanga Parbat syntaxis. Tectonophys 1990, 180, 323–349. [Google Scholar] [CrossRef]
- Kazmi, A.H.; Jan, M.Q. Geology and Tectonics of Pakistan; Graphic Publishers: Karachi, Pakistan, 1997; p. 554. [Google Scholar]
- Petterson, M.G.; Windley, B.F. RbSr dating of the Kohistan arc-batholith in the Trans-Himalaya of north Pakistan, and tectonic implications. Earth Planet. Sci. Lett. 1985, 74, 45–57. [Google Scholar] [CrossRef]
- Bodnar, R. Revised equation and table for determining the freezing point depression of H2O-NaCl solutions. Geochim. Et Cosmochim. Acta. 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Collins, P.L. Gas hydrates in CO2-bearing fluid inclusions and the use of freezing data for estimation of salinity. Econ. Geol. 1979, 74, 1435–1444. [Google Scholar] [CrossRef]
- Bodnar, R.J. Introduction to aqueous-electrolyte fluid inclusions. Fluid Incl. Anal. Interpret. 2003, 32, 81–100. [Google Scholar]
- Sterner, S.M.; Hall, D.L.; Bodnar, R.J. Synthetic fluid inclusions. V. Solubility relations in the system NaCl-KCl-H2O under vapor-saturated conditions. Geochim. Et Cosmochim. Acta 1988, 52, 989–1005. [Google Scholar] [CrossRef]
- Bodnar, R.J. A method of calculating fluid inclusion volumes based on vapor bubble diameters and PVTX properties of inclusion fluids. Econ. Geol. 1983, 78, 535–542. [Google Scholar] [CrossRef]
- Rajabzadeh, M.A.; Rasti, S. Investigation on mineralogy, geochemistry and fluid inclusions of the Goushti hydrothermal magnetite deposit, Fars Province, SW Iran: A comparison with IOCGs. Ore Geol. Rev. 2017, 82, 93–107. [Google Scholar] [CrossRef]
- Giesemann, A.; Jäger, H.-J.; Norman, A.; Krouse, H.; Brand, W. Online sulfur-isotope determination using an elemental analyzer coupled to a mass spectrometer. Anal. Chem. 1994, 66, 2816–2819. [Google Scholar] [CrossRef]
- Yuan, M.-W.; Li, L.; Li, S.-R.; Li, C.-L.; Santosh, M.; Alam, M.; Bao, X.-B. Mineralogy, fluid inclusions and S-Pb-HO isotopes of the Erdaokan Ag-Pb-Zn deposit, Duobaoshan metallogenic belt, NE China: Implications for ore genesis. Ore Geol. Rev. 2019, 113, 103074. [Google Scholar] [CrossRef]
- Yuan, M.-W.; Li, S.-R.; Li, C.-L.; Santosh, M.; Alam, M.; Zeng, Y.-J. Geochemical and isotopic composition of auriferous pyrite from the Yongxin gold deposit, Central Asian Orogenic Belt: Implication for ore genesis. Ore Geol. Rev. 2018, 93, 255–267. [Google Scholar] [CrossRef]
- Farhan, M.; Arif, M.; Ying, Y.; Chen, X.; Garbe-Schönberg, D.; Li, C.-F.; Hussain, Z.; Ullah, Z.; Zhang, P.; Khan, A. Fluid source, element mobility and physicochemical conditions of porphyry-style hydrothermal alteration-mineralization at Mirkhani, southern Chitral, Pakistan. Ore Geol. Rev. 2021, 104222. [Google Scholar] [CrossRef]
- Doyle, F.; Mirza, A.H. Electrochemical oxidation of pyrite samples with known composition and electrical properties. Electrochem. Proc. 1996, 96, 203–214. [Google Scholar]
- Roedder, E. Fluid Inclusions. In Reviews in Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1984; Volume 12, 646p. [Google Scholar]
- Roedder, E.; Bodnar, R.J. Fluid Inclusion Studies of Hydrothermal Ore Deposits; John Willey & Sons: New York, NY, USA, 1997. [Google Scholar]
- Cai, Y.-T.; Ni, P.; Wang, G.-G.; Pan, J.-Y.; Zhu, X.-T.; Chen, H.; Ding, J.-Y. Fluid inclusion and H–O–S–Pb isotopic evidence for the Dongxiang Manto-type copper deposit, South China. J. Geochem. Explor. 2016, 171, 71–82. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, S.-Y.; Chen, R.-S.; Li, X.-X.; Zhu, L.; Xiong, S.-F. Hydrothermal evolution and ore genesis of the Zhaiping Ag-Pb-Zn deposit in Fujian Province of Southeast China: Evidence from stable isotopes (H, O, C, S) and fluid inclusions. Ore Geol. Rev. 2019, 104, 246–265. [Google Scholar] [CrossRef]
- Zartman, R.; Doe, B. Plumbotectonics—The model. Tectonophys 1981, 75, 135–162. [Google Scholar] [CrossRef]
- Barker, S.L.; Hickey, K.A.; Cline, J.S.; Dipple, G.M.; Kilburn, M.R.; Vaughan, J.R.; Longo, A. Uncloaking invisible gold: Use of nanoSIMS to evaluate gold, trace elements, and sulfur isotopes in pyrite from Carlin-type gold deposits. Econ. Geol. 2009, 104, 897–904. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Ewing, R.C.; Chryssoulis, S.L.; Venter, D.; Kesler, S.E. Decoupled geochemical behavior of As and Cu in hydrothermal systems. Geology 2009, 37, 707–710. [Google Scholar] [CrossRef]
- Agangi, A.; Hofmann, A.; Wohlgemuth-Ueberwasser, C.C. Pyrite zoning as a record of mineralization in the Ventersdorp Contact Reef, Witwatersrand Basin, South Africa. Econ. Geol. 2013, 108, 1243–1272. [Google Scholar] [CrossRef]
- Reich, M.; Deditius, A.; Chryssoulis, S.; Li, J.-W.; Ma, C.-Q.; Parada, M.A.; Barra, F.; Mittermayr, F. Pyrite as a record of hydrothermal fluid evolution in a porphyry copper system: A SIMS/EMPA trace element study. Geochim. Cosmochim. Acta 2013, 104, 42–62. [Google Scholar] [CrossRef]
- Ingham, E.S.; Cook, N.J.; Cliff, J.; Ciobanu, C.L.; Huddleston, A. A combined chemical, isotopic and microstructural study of pyrite from roll-front uranium deposits, Lake Eyre Basin, South Australia. Geochim. Cosmochim. Acta 2014, 125, 440–465. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, J.; Chen, H.-Y.; Yang, L.-Q.; Cooke, D.; Danyushevsky, L.; Gong, Q.-J. LA-ICP-MS trace element analysis of pyrite from the Chang’an gold deposit, Sanjiang region, China: Implication for ore-forming process. Gondwana Res. 2014, 26, 557–575. [Google Scholar] [CrossRef]
- Thomas, H.V.; Large, R.R.; Bull, S.W.; Maslennikov, V.; Berry, R.F.; Fraser, R.; Froud, S.; Moye, R. Pyrite and pyrrhotite textures and composition in sediments, laminated quartz veins, and reefs at Bendigo gold mine, Australia: Insights for ore genesis. Econ. Geol. 2011, 106, 1–31. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Utsunomiya, S.; Kogagwa, M.; Green, L.; Gilbert, S.; Wade, B. Gold-telluride nanoparticles revealed in arsenic-free pyrite. Am. Mineral. 2012, 97, 1515–1518. [Google Scholar] [CrossRef]
- Chouinard, A.; Paquette, J.; Williams-Jones, A.E. Crystallographic controls on trace-element incorporation in auriferous pyrite from the Pascua epithermal high-sulfidation deposit, Chile–Argentina. Miner. Depos. 2005, 43, 951–963. [Google Scholar] [CrossRef] [Green Version]
- Deditius, A.P.; Utsunomiya, S.; Renock, D.; Ewing, R.C.; Ramana, C.V.; Becker, U.; Kesler, S.E. A proposed new type of arsenian pyrite: Composition, nanostructure and geological significance. Geochim. Et Cosmochim. Acta. 2008, 72, 2919–2933. [Google Scholar] [CrossRef]
- Tardani, D.; Reich, M.; Deditius, A.P.; Chryssoulis, S.; Sánchez-Alfaro, P.; Wrage, J.; Roberts, M.P. Copper–arsenic decoupling in an active geothermal system: A link between pyrite and fluid composition. Geochim. Et Cosmochim. Acta. 2017, 204, 179–204. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.; Utsunomiya, S. Solubility of Gold in Arsenian Pyrite. Geochim. Et Cosmochim. Acta. 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Sack, R.O.; Loucks, R.R. Thermodynamic properties of tetrahedrite-tennantite: Constraints on the interdependence of the Ag⇌ Cu, Fe⇌ Zn, Cu⇌ Fe, and As⇌ Sb exchange reactions. Am. Mineral. 1985, 70, 1270–1289. [Google Scholar]
- Franchini, M.; McFarlane, C.; Maydagán, L.; Reich, M.; Lentz, D.R.; Meinert, L.; Bouhier, V. Trace metals in pyrite and marcasite from the Agua Rica porphyry-high sulfidation epithermal deposit, Catamarca, Argentina: Textural features and metal zoning at the porphyry to epithermal transition. Ore Geol. Rev. 2015, 66, 366–387. [Google Scholar] [CrossRef]
- Grant, H.L.; Hannington, M.D.; Petersen, S.; Frische, M.; Fuchs, S.H. Constraints on the behavior of trace elements in the actively-forming TAG deposit, Mid-Atlantic Ridge, based on LA-ICP-MS analyses of pyrite. Chem. Geol. 2018, 498, 45–71. [Google Scholar] [CrossRef]
- Mehrabi, B.; Fazel, E.T.; Yardley, B. Ore geology, fluid inclusions and OS stable isotope characteristics of Shurab Sb-polymetallic vein deposit, eastern Iran. Geochemistry 2019, 79, 307–322. [Google Scholar] [CrossRef]
- Zhou, X.W.; Li, S.R.; Lu, L.; Li, J.J.; Wang, J. Study of Pyrite Typomorphic Characteristics of Wulong Quartz-Vein-Type Gold Deposit in Dandong, Liaoning Province, China. Geoscience 2005, 19, 231. [Google Scholar]
- Cline, J.S.; Bodnar, R.J. Direct evolution of brine from a crystallizing silicic melt at the Questa, New Mexico, molybdenum deposit. Econ. Geol. 1994, 89, 1780–1802. [Google Scholar] [CrossRef]
- Liao, S.; Chen, S.; Deng, X.; Li, P.; Zhao, J.; Liao, R. Fluid inclusion characteristics and geological significance of the Xi’ao copper–tin polymetallic deposit in Gejiu, Yunnan Province. J. Asian Earth Sci. 2014, 79, 455–467. [Google Scholar] [CrossRef]
- Roedder, E. Fluid inclusions as samples of ore fluids. Geochemistry of Hydrothermal Ore Deposit, 2nd ed.; Wiley: New York, NY, USA, 1979; pp. 684–737. [Google Scholar]
- Mei, W.; Lü, X.; Cao, X.; Liu, Z.; Zhao, Y.; Ai, Z.; Tang, R.; Abfaua, M.M. Ore genesis and hydrothermal evolution of the Huanggang skarn iron–tin polymetallic deposit, southern Great Xing’an Range: Evidence from fluid inclusions and isotope analyses. Ore Geol. Rev. 2015, 64, 239–252. [Google Scholar] [CrossRef]
- Ramboz, C.; Pichavant, M.; Weisbrod, A. Fluid immiscibility in natural processes: Use and misuse of fluid inclusion data: II. Interpretation of fluid inclusion data in terms of immiscibility. Chem. Geol. 1982, 37, 29–48. [Google Scholar] [CrossRef]
- Hayba, B. Geologic, mineralogic and geochemical characteristics of volcanic hosted epithermal precious metal deposits. In Geology and geochemistry of epithermal systems. Rev. Econ. Geol. 1985, 2, 129–167. [Google Scholar]
- Liu, L.; Gu, X.-X.; Zhang, Y.-M.; Ouyang, X.; Wang, L.-Z.; Gao, L.-Y. Genesis of the Jinchanggouliang gold deposit, Chifeng, China: Constraints from fluid inclusions and isotopic geochemistry. Ore Geol. Rev. 2019, 115, 103180. [Google Scholar] [CrossRef]
- Shepherd, T.; Rankin, A.; Alderton, D. A Practical Guide to Fluid Inclusion Studies; Blackie Academic & Professional: Glasgow, UK, 1985; p. 239. [Google Scholar]
- Driesner, T.; Heinrich, C.A. The system H2O–NaCl. Part I: Correlation formulae for phase relations in temperature–pressure–composition space from 0 to 1000 C, 0 to 5000 bar, and 0 to 1 XNaCl. Geochim. Et Cosmochim. Acta. 2007, 71, 4880–4901. [Google Scholar] [CrossRef]
- Henley, R.; McNabb, A. Magmatic vapor plumes and ground-water interaction in porphyry copper emplacement. Econ. Geol. 1978, 73, 1–20. [Google Scholar] [CrossRef]
- Wilkinson, J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Meinert, L.; Hedenquist, J.; Satoh, H.; Matsuhisa, Y. Formation of anhydrous and hydrous skarn in Cu-Au ore deposits by magmatic fluids. Econ. Geol. 2003, 98, 147–156. [Google Scholar] [CrossRef]
- Kontak, D.J.; Clark, A.H. Genesis of the giant, bonanza San Rafael lode tin deposit, Peru: Origin and significance of pervasive alteration. Econ. Geol. 2002, 97, 1741–1777. [Google Scholar] [CrossRef]
- Palme, H.; O’Neill, H.S.C. Cosmochemical estimates of mantle composition. Treatise Geochem. 2003, 2, 568. [Google Scholar]
- Rudnick, R.; Gao, S.; Holland, H.; Turekian, K.J. Composition of the continental crust. Crust 2003, 3, 1–64. [Google Scholar]
- Koglin, N.; Frimmel, H.E.; Minter, W.L.; Brätz, H. Trace-element characteristics of different pyrite types in Mesoarchaean to Palaeoproterozoic placer deposits. Mineral. Depos. 2010, 45, 259–280. [Google Scholar] [CrossRef]
- Niu, S.-D.; Li, S.-R.; Santosh, M.; Zhang, D.-H.; Li, Z.-D.; Shan, M.-J.; Lan, Y.-X.; Gao, D.-R.; Zhao, W.-B. Mineralogical and isotopic studies of base metal sulfides from the Jiawula Ag–Pb–Zn deposit, Inner Mongolia, NE China. J. Asian Earth Sci. 2016, 115, 480–491. [Google Scholar] [CrossRef]
- Li, S.-R.; Santosh, M.; Zhang, H.-F.; Luo, J.-Y.; Zhang, J.-Q.; Li, C.-L.; Song, J.-Y.; Zhang, X.-B. Metallogeny in response to lithospheric thinning and craton destruction: Geochemistry and U–Pb zircon chronology of the Yixingzhai gold deposit, central North China Craton. Ore Geol. Rev. 2014, 56, 457–471. [Google Scholar] [CrossRef]
- Hall, D.; Sterner, S. BO~ NAR RJ Freezingpoint depression of NaCI-KCEH20 solutions. Econ. Geol 1988, 83, 197–202. [Google Scholar] [CrossRef]
- Liu, J.; Mao, J.-W.; Wu, G.; Wang, F.; Luo, D.-F.; Hu, Y.-Q.; Li, T.-G. Fluid inclusions and H–O–S–Pb isotope systematics of the Chalukou giant porphyry Mo deposit, Heilongjiang Province, China. Ore Geol. Rev. 2014, 59, 83–96. [Google Scholar] [CrossRef]
- Mei, J. Chemical typomorphic characteristic of pyrites from Zhilingtou gold deposit, Suichang, Zhejiang. Geoscience 2000, 14, 51–55. [Google Scholar]
- Liu, Z.; Shao, Y.; Zhou, H.; Liu, N.; Huang, K.; Liu, Q.; Zhang, J.; Wang, C. Major and trace element geochemistry of pyrite and pyrrhotite from stratiform and lamellar orebodies: Implications for the ore genesis of the Dongguashan copper (gold) deposit, eastern China. Minerals 2018, 8, 380. [Google Scholar] [CrossRef] [Green Version]
- Román, N.; Reich, M.; Leisen, M.; Morata, D.; Barra, F.; Deditius, A.P. Geochemical and micro-textural fingerprints of boiling in pyrite. Geochim. Et Cosmochim. Acta 2019, 246, 60–85. [Google Scholar] [CrossRef]
- Liu, Z.; Mao, X.; Deng, H.; Li, B.; Zhang, S.; Lai, J.; Bayless, R.C.; Pan, M.; Li, L.; Shang, Q. Hydrothermal processes at the Axi epithermal Au deposit, western Tianshan: Insights from geochemical effects of alteration, mineralization and trace elements in pyrite. Ore Geol. Rev. 2018, 102, 368–385. [Google Scholar]
- Brill, B.A. Trace-element contents and partitioning of elements in ore minerals from the CSA Cu-Pb-Zn deposit, Australia, and implications for ore genesis. Can. Mineral. 1989, 27, 263–274. [Google Scholar]
- Yan, Y.; Li, S.; Jia, B.; Zhang, N.; Yan, L. Composition typomorphic characteristics and statistic analysis of pyrite in gold deposits of different genetic types. Earth Sci. Front. 2012, 19, 214–226. [Google Scholar]
- Large, R.R.; Danyushevsky, L.; Hollit, C.; Maslennikov, V.; Meffre, S.; Gilbert, S.; Bull, S.; Scott, R.; Emsbo, P.; Thomas, H. Gold and trace element zonation in pyrite using a laser imaging technique: Implications for the timing of gold in orogenic and Carlin-style sediment-hosted deposits. Econ. Geol. 2009, 104, 635–692. [Google Scholar]
- Bajwah, Z.; Seccombe, P.; Offler, R. Trace element distribution, Co: Ni ratios and genesis of the Big Cadia iron-copper deposit, New South Wales, Australia. Mineral. Depos. 1987, 22, 292–300. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Belin, German, 2009; Volume 285. [Google Scholar]
- Ohmoto, H.; Rye, R.O. Isotopes of sulfur and carbon. In Geochemistry of Hydrothermal Ore Deposits, 2nd ed.; Holt Rinehart and Winston: New York, NY, USA, 1979; pp. 509–567. [Google Scholar]
- Ohmoto, H.; Goldhaber, M.B. Sulfur and carbon isotopes. In Geochemistry of Hydrothermal Ore Deposit, 3rd ed.; Wilay: New York, NY, USA, 1997; pp. 517–611. [Google Scholar]
- Li, S.; Zhang, X.; Gao, L. Ore Genesis at the Jinchang Gold–Copper Deposit in Heilongjiang Province, Northeastern China: Evidence from Geology, Fluid Inclusions, and H–O–S Isotopes. Minerals 2019, 9, 99. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, S.-R.; Santosh, M.; Zhu, J.; Suo, X.-J. Early Jurassic decratonic gold metallogenesis in the eastern North China Craton: Constraints from S-Pb-CDO isotopic systematics and pyrite Rb-Sr geochronology of the Guilaizhuang Te-Au deposit. Ore Geol. Rev. 2018, 92, 558–568. [Google Scholar] [CrossRef]
- Duuring, P.; Rowins, S.M.; McKinley, B.S.; Dickinson, J.M.; Diakow, L.J.; Kim, Y.-S.; Creaser, R.A. Examining potential genetic links between Jurassic porphyry Cu–Au ± Mo and epithermal Au ± Ag mineralization in the Toodoggone district of north-central British Columbia, Canada. Mineral. Depos. 2009, 44, 463–496. [Google Scholar] [CrossRef]
- Seal, R.R. Sulfur isotope geochemistry of sulfide minerals. Rev. Mineral. Geochem. 2006, 61, 633–677. [Google Scholar] [CrossRef]
- Zhai, D.; Liu, J.; Cook, N.J.; Wang, X.; Yang, Y.; Zhang, A.; Jiao, Y. Mineralogical, textural, sulfur and lead isotope constraints on the origin of Ag-Pb-Zn mineralization at Bianjiadayuan, Inner Mongolia, NE China. Mineral. Depos. 2019, 54, 47–66. [Google Scholar] [CrossRef]
- Zhai, D.; Williams-Jones, A.E.; Liu, J.; Tombros, S.F.; Cook, N.J. Mineralogical, fluid inclusion, and multiple isotope (HOS-Pb) constraints on the genesis of the sandaowanzi epithermal Au-Ag-Te deposit, NE China. Econ. Geol. 2018, 113, 1359–1382. [Google Scholar] [CrossRef] [Green Version]
- Bozkaya, G. Sulphur-and lead-isotope geochemistry of the Arapuçandere lead–zinc–copper deposit, Biga Peninsula, northwest Turkey. Int. Geol. Rev. 2011, 53, 116–129. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Ayuso, R.; Miller, M.L.; Ebert, S.W.; Marsh, E.E.; Petsel, S.A.; Miller, L.D.; Bradley, D.; Johnson, C.; McClelland, W. The late cretaceous Donlin Creek gold deposit, Southwestern Alaska: Controls on epizonal ore formation. Econ. Geol. 2004, 99, 643–671. [Google Scholar] [CrossRef]
- Jagoutz, O.; Bouilhol, P.; Schaltegger, U.; Müntener, O. The isotopic evolution of the Kohistan Ladakh arc from subduction initiation to continent arc collision. Geol. Soc. Lond. Spec. Publ. 2019, 483, 165–182. [Google Scholar] [CrossRef]
- Zuo, C.H. Study of the Kangjiawan Lead–Zinc Deposit Genesis and Relationship with Surrounding Magmatism in Changning City; Nanjing University: Nanjing, China, 2015. [Google Scholar]
- Chi, G.; Lai, J. Roles of fluid inclusions in study of mineral deposits. Miner. Depos. 2009, 6. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-KCDZ200906012.htm (accessed on 7 November 2021).
- Fontboté, L.; Kouzmanov, K.; Chiaradia, M.; Pokrovski, G.S. Sulfide minerals in hydrothermal deposits. Elements 2017, 13, 97–103. [Google Scholar] [CrossRef]
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, C.A.; Candela, P.A. Fluids and ore formation in the Earth’s crust. In Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 13, pp. 1–28. [Google Scholar]
- Hedenquist, J.W.; Arribas, A.; Reynolds, T.J. Evolution of an intrusion-centered hydrothermal system; Far Southeast-Lepanto porphyry and epithermal Cu-Au deposits, Philippines. Econ. Geol. 1998, 93, 373–404. [Google Scholar] [CrossRef] [Green Version]
- Logan, M.A.V. Mineralogy and geochemistry of the Gualilán skarn deposit in the Precordillera of western Argentina. Ore Geol. Rev. 2000, 17, 113–138. [Google Scholar] [CrossRef]
- Heinrich, C.A. Fluid-fluid interactions in magmatic-hydrothermal ore formation. Rev. Mineral. Geochem. 2007, 65, 363–387. [Google Scholar] [CrossRef]
- Wagner, T.; Mlynarczyk, M.S.; Williams-Jones, A.E.; Boyce, A.J. Stable isotope constraints on ore formation at the San Rafael tin-copper deposit, Southeast Peru. Econ. Geol. 2009, 104, 223–248. [Google Scholar] [CrossRef]
- Audétat, A.; Günther, D.; Heinrich, C.A. Formation of a magmatic-hydrothermal ore deposit: Insights with LA-ICP-MS analysis of fluid inclusions. Science 1998, 279, 2091–2094. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.-Z.; Liu, Y.; Wang, C.; Xu, Y.; Li, H. Mineralization and fluid inclusion study of the Shizhuyuan W-Sn-Bi-Mo-F skarn deposit, Hunan Province, China. Econ. Geol. 2003, 98, 955–974. [Google Scholar] [CrossRef]
- Chen, Y.J. On epizonogenism and genetic classification of hydrothermal deposits. Earth Sci. Front. 2010, 17, 27. [Google Scholar]
- Han, J.-S.; Yao, J.-M.; Chen, H.-Y.; Deng, X.-H.; Ding, J.-Y. Fluid inclusion and stable isotope study of the Shagou Ag–Pb–Zn deposit, Luoning, Henan province, China: Implications for the genesis of an orogenic lode Ag–Pb–Zn system. Ore Geol. Rev. 2014, 62, 199–210. [Google Scholar] [CrossRef]
- Sabeva, R.; Mladenova, V.; Mogessie, A. Ore petrology, hydrothermal alteration, fluid inclusions, and sulfur stable isotopes of the Milin Kamak intermediate sulfidation epithermal Au-Ag deposit in Western Srednogorie, Bulgaria. Ore Geol. Rev. 2017, 88, 400–415. [Google Scholar] [CrossRef]
- Foster, R.P. Gold Metallogeny and Exploration; Springer Science & Business Media: Belin, Germany, 2012. [Google Scholar]
- Hedenquist, J.W.; Lowenstern, J.B. The role of magmas in the formation of hydrothermal ore deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Ramsay, W.; Bierlein, F.; Arne, D.; VandenBerg, A. Turbidite-hosted gold deposits of Central Victoria, Australia: Their regional setting, mineralising styles, and some genetic constraints. Ore Geol. Rev. 1998, 13, 131–151. [Google Scholar] [CrossRef]
- Melfos, V.; Voudouris, P. Fluid evolution in Tertiary magmatic-hydrothermal ore systems at the Rhodope metallogenic province, NE Greece. A review. Geol. Croat. 2016, 69, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Bralia, A.; Sabatini, G.; Troja, F. A revaluation of the Co/Ni ratio in pyrite as geochemical tool in ore genesis problems. Miner. Depos. 1979, 14, 353–374. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, J.; Yang, P.; Ma, D.; Xu, Z.; Zhang, R.; Cai, Y.; Ding, T. S, Pb, C and O isotopic characteristics and sources of metallogenic materials of Baoshan Pb–Zn deposit, southern Hunan Province. Miner. Depos. 2015, 34, 333–351. [Google Scholar]












| Minerals | Wt.% | S | Fe | As | Cu | Mn | Zn | Co | Ni | Ag | Sb | Pb | Au | Cd | Cr | Total | Representative Formulae |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyrite-I (n = 8) | Average | 53.04 | 45.41 | 0.01 | 0.02 | 0.00 | 0.01 | 0.12 | 0.02 | 0.00 | bdl | bdl | 0.00 | 0.02 | 0.00 | 98.66 | (Fe0.99 Co0.002)1.00S2.02 |
| St.dev. | 0.35 | 0.45 | 0.01 | 0.01 | 0.01 | 0.00 | 0.06 | 0.01 | 0.00 | bdl | bdl | 0.00 | 0.00 | 0.01 | |||
| Pyrite-II (n = 6) | Average | 51.65 | 46.39 | 0.40 | 0.62 | bdl | 0.03 | 0.04 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.000 | 99.20 | Fe0.98 S1.93 (Fe0.98As0.01Cu0.01)1.0S1.93 |
| St.dev. | 0.44 | 0.51 | 0.59 | 0.76 | bdl | 0.01 | 0.03 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Chalcopyrite (n = 13) | Average | 34.29 | 29.89 | 0.00 | 34.63 | 0.00 | 0.02 | 0.00 | 0.00 | 0.19 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 99.08 | Cu1.00 Fe1.00S2.00 (Cu1.01Ag0.003)1.01Fe 1.00S1.98 |
| St.dev. | 0.22 | 0.58 | 0.01 | 0.22 | 0.00 | 0.03 | 0.00 | 0.00 | 0.32 | 0.02 | 0.02 | 0.01 | 0.01 | 0.01 | |||
| Zincian Tetrahedrite (n = 11) | Average | 26.06 | 2.06 | 8.56 | 39.28 | 0.00 | 5.97 | 0.01 | 0.00 | 2.62 | 16.19 | 0.012 | 0.01 | 0.22 | 0.01 | 101.00 | Cu10Zn2Sb4S13 (Cu9.48Ag0.37)9.85 (Zn1.40 Fe0.57Cd0.03)2.00 (Sb2.04 As1.75)3.79S12.56 |
| St.dev. | 0.25 | 0.95 | 0.32 | 0.71 | 0.00 | 1.47 | 0.01 | 0.00 | 0.55 | 0.49 | 0.03 | 0.01 | 0.05 | 0.01 | |||
| Galena (n = 5) | Average | 12.85 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.43 | 0.55 | 85.95 | bdl | 0.04 | 0.00 | 99.83 | PbS (Pb1.04Ag0.01Sb0.01Cd0.001)1.06S1.00 |
| St.dev. | 0.20 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.17 | 0.20 | 0.51 | bdl | 0.02 | 0.00 | |||
| Native Silver (n = 4) | Average | 0.06 | 0.09 | 0.00 | 1.02 | 0.00 | 0.11 | 0.01 | 0.00 | 98.66 | bdl | 0.01 | 0.01 | 0.70 | 0.01 | 100.68 | (Ag0.92Cu0.02Cd0.01)0.9 |
| St.dev. | 0.03 | 0.05 | 0.00 | 0.27 | 0.00 | 0.01 | 0.01 | 0.00 | 0.52 | bdl | 0.00 | 0.01 | 0.04 | 0.02 | |||
| Bornite (n = 3) | Average | 25.42 | 11.02 | 0.01 | 62.83 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.01 | 0.03 | 99.31 | Cu5.0Fe1.0S4.0 |
| St.dev. | 0.42 | 0.41 | 0.01 | 0.62 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.01 | |||
| Iron-arsenide (Lollingite) (n = 2) | Average | 2.58 | 29.86 | 65.70 | 0.01 | 0.06 | 0.01 | bdl | 0.00 | 0.00 | bdl | 0.12 | 0.00 | 0.01 | 0.01 | 98.37 | FeAs2 (Fe1.08As1.75)2.83S0.16 |
| St.dev. | 0.28 | 0.19 | 0.22 | 0.00 | 0.01 | 0.01 | bdl | 0.00 | 0.00 | bdl | 0.02 | 0.00 | 0.00 | 0.01 | |||
| Covellite (n = 2) | Average | 33.32 | 1.31 | bdl | 66.13 | bdl | bdl | bdl | 0.00 | 0.10 | bdl | bdl | bdl | 0.02 | 0.00 | 100.88 | CuS (Cu1.00A0.01Fe0.02)1.03S1.00 |
| St.dev. | 0.01 | 0.50 | bdl | 0.49 | bdl | bdl | bdl | 0.00 | 0.14 | bdl | bdl | bdl | 0.01 | 0.00 |
| Pyrite Type | Py-I | Py-I | Py-I | Py-I | Py-I | Py-I | Py-I | Py-I | Py-II | Py-II | Py-II | Py-II | Py-II | Py-II |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spot | Core | Core | Core | Core | Core | Rim | Rim | Rim | Core | Core | Core | Rim | Rim | Rim |
| S | 52.69 | 53.23 | 53.82 | 52.83 | 53.04 | 52.85 | 52.91 | 52.93 | 51.50 | 51.80 | 52.45 | 51.32 | 51.79 | 51.06 |
| Fe | 45.185 | 45.39 | 46.43 | 45.45 | 45.15 | 45.53 | 45.17 | 45.01 | 46.58 | 46.38 | 47.14 | 46.41 | 45.43 | 46.40 |
| As | 60 | 214 | 45 | 151 | 200 | 64 | 143 | 40 | 16,940 | 2880 | 170 | 900 | 2650 | 240 |
| Cu | 100 | 290 | 80 | 210 | 280 | 110 | 200 | 62 | 2920 | 17640 | 290 | 90 | 15840 | 180 |
| Mn | 120 | bdl | bdl | 60 | 0.0 | 50 | 120 | 0.0 | bdl | bdl | bdl | bdl | bdl | bdl |
| Zn | 160 | 170 | 140 | 150 | 75 | 130 | 40 | 74 | 410 | 423 | 270 | 330 | 210 | 340 |
| Co | 1720 | 1900 | 500 | 810 | 1800 | 520 | 1640 | 720 | 310 | 320 | 130 | 380 | 210 | 230 |
| Ni | 110 | 310 | 100 | 120 | 320 | 130 | 100 | 140 | 240 | 200 | 100 | 120 | 110 | 120 |
| Ag | 40 | 80 | 55 | 37 | 23 | 76 | 20 | 0.0 | 410 | 160 | 200 | 170 | 310 | 210 |
| Sb | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 91 | 97 | 44 | 80 | 53 | 60 |
| Pb | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 100 | 140 | 51 | 83 | 42 | 94 |
| Au | 24 | 17 | 14 | 51 | 52 | 43 | bdl | bdl | 150 | 110 | 100 | 140 | 80 | 150 |
| Cd | 250 | 160 | 170 | 140 | 170 | 150 | 230 | 180 | 120 | 110 | 80 | 0.0 | 100 | 60 |
| Cr | 180 | bdl | bdl | bdl | bdl | bdl | 190 | bdl | 110 | bdl | bdl | bdl | bdl | bdl |
| Mo | 98 | 240 | 94 | 104 | 243 | 121 | 85 | 91 | 113 | 94 | 61 | 54 | 63 | 51 |
| Se | 187 | 253 | 163 | 124 | 217 | 106 | 98 | 124 | 73 | 102 | 67 | 93 | 64 | 86 |
| Pyrite Type | Spot | S (wt. %) | Fe (wt. %) | As (ppm) | Co (ppm) | Ni (ppm) | Mo (ppm) | S/Fe | Fe/(S + As) | Co/Ni | Mo/Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Py-I | Cores | 52.69 | 45.18 | 60 | 1720 | 110 | 98 | 1.17 | 0.857 | 15.64 | 0.89 |
| Py-I | Cores | 53.23 | 45.39 | 214 | 1900 | 310 | 240 | 1.17 | 0.853 | 6.13 | 0.77 |
| Py-I | Cores | 53.82 | 46.43 | 45 | 500 | 100 | 94 | 1.16 | 0.863 | 5.00 | 0.94 |
| Py-I | Cores | 52.83 | 45.45 | 151 | 810 | 120 | 104 | 1.16 | 0.860 | 6.75 | 0.87 |
| Py-I | Cores | 53.04 | 45.15 | 200 | 1800 | 320 | 243 | 1.17 | 0.851 | 5.63 | 0.76 |
| Py-I | Rim | 52.85 | 45.53 | 64 | 520 | 130 | 121 | 1.16 | 0.861 | 4.00 | 0.93 |
| Py-I | Rim | 52.91 | 45.17 | 143 | 1640 | 100 | 85 | 1.17 | 0.853 | 16.40 | 0.85 |
| Py-I | Rim | 52.93 | 45.01 | 40 | 720 | 140 | 91 | 1.18 | 0.850 | 5.14 | 0.65 |
| Py-II | Cores | 51.50 | 46.58 | 16,940 | 310 | 240 | 113 | 1.11 | 0.876 | 1.29 | 0.47 |
| Py-II | Cores | 51.79 | 46.38 | 2880 | 320 | 200 | 94 | 1.12 | 0.890 | 1.60 | 0.47 |
| Py-II | Cores | 52.45 | 47.14 | 170 | 130 | 100 | 61 | 1.11 | 0.899 | 1.30 | 0.61 |
| Py-II | Rim | 51.32 | 46.41 | 900 | 380 | 120 | 54 | 1.11 | 0.903 | 3.17 | 0.45 |
| Py-II | Rim | 51.79 | 45.43 | 2650 | 210 | 110 | 63 | 1.14 | 0.873 | 1.91 | 0.57 |
| Py-II | Rim | 51.06 | 46.40 | 240 | 230 | 120 | 51 | 1.10 | 0.908 | 1.92 | 0.43 |
| Stage | Host Mineral | Type | N | Size (um) | Tm,CO2 (oC) | Tm,cla (°C) | Th,CO2 (°C) | Tm.ice (°C) | Td (°C) | Th (°C) | W (wt.% NaCl) | ρ (g/cm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-ore | Quartz | II | 35 | 1–15 | From −8.3 to −1.8 (−5.42) | 434 –555 (470.49) | 3.06–12.05 (8.26) | 0.20–0.65 (0.43) | ||||
| III | 16 | 3–60 | From −57.0 to −54.0 (−55.71) | From 4.3 to −7.9 (5.25) | From 30 to 33 (32.0) | 399–425 (414.94) | 4.07–10.04 (8.53) | 0.55–0.70 (0.63) | ||||
| IV | 41 | 1–27 | From −8.3 to −2.0 (−5.62) | 442–495 (450.07) | 3.39–12.05 (9.10) | 0.40–0.70 (0.55) | ||||||
| Sulfide ore I | Quartz | II | 29 | 1–19 | From −8.7 to −3.0 (−6.55) | 380–438 (410) | 4.96–12.51 (9.87) | 0.55–0.75 (0.65) | ||||
| III | 15 | 2–45 | From −57.0 to −53.6 (−55.47) | From 4.8 to −8.2 (6.62) | From 30 to 36 (32.13) | 300–359 (327.47) | 3.52–9.28 (6.28) | 0.70–0.80 (0.39) | ||||
| IV | 30 | 1–37 | From −31.0 to −5.0 (−13.59) | 332–423 (380.8) | 7.86–29.26 (16.07) | 0.65–0.95 (0.80) | ||||||
| Sulfide ore II | Quartz | II | 20 | 1–22 | From −3.1 to −0.9 (−1.90) | 300–356 (331.95) | 1.57–6.45 (3.20) | 0.60–0.80 (0.70) | ||||
| IV | 43 | 1–39 | From −9.2 to −3.0 (−6.97) | 280–352 (308.67) | 4.96–13.07 (10.34) | 0.75–0.90 (0.83) | ||||||
| V | 15 | 5–31 | From 283 to 368 (325) | 313–390 (354.8) | 33.44–49.26 (40.94) | 1.0–1.30 (1.15) | ||||||
| Post-ore | Quartz, calcite | IV | 44 | 1–40 | From −5.3 to 4.5 (−3.13) | 155–256 (211.20) | 0.88–8.28 (5.37) | 0.85–1.0 (0.90) |
| Sample No | Minerals | δ34S (%) | 206Pb/204Pb | 2σ | 207Pb/204Pb | 2σ | 208Pb/204Pb | 2σ |
|---|---|---|---|---|---|---|---|---|
| KCZ-01 | Pyrite | 5.4 | 18.5 | 0.0 | 15.7 | 0.0 | 39.4 | 0.0 |
| KCZ-02 | Pyrite | −2.8 | 19.3 | 0.0 | 15.8 | 0.0 | 39.8 | 0.0 |
| KCZ-02-1 | Pyrite | −2.5 | 19.5 | 0.0 | 15.9 | 0.0 | 39.1 | 0.0 |
| KGH-04 | Pyrite | 4.9 | 20.2 | 0.0 | 15.9 | 0.0 | 40.7 | 0.0 |
| KSL-M-1 | Pyrite | 4.4 | 20.2 | 0.0 | 15.9 | 0.0 | 40.4 | 0.0 |
| KS-ad-01 | Pyrite | −0.1 | 20.1 | 0.0 | 15.9 | 0.0 | 40.1 | 0.0 |
| KCH-03 | Pyrite | 3.0 | 20.3 | 0.0 | 15.9 | 0.0 | 40.5 | 0.0 |
| KS-ad-01-1 | Pyrite | 5.8 | 20.2 | 0.0 | 16.0 | 0.0 | 40.9 | 0.0 |
| KS-ad-01-2 | Pyrite | 3.6 | 19.8 | 0.0 | 15.8 | 0.0 | 40.2 | 0.0 |
| KS-sv-30 | Pyrite | 2.9 | 20.4 | 0.0 | 16.0 | 0.0 | 40.2 | 0.0 |
| KJZ-07 | Galena | 0.7 | 20.3 | 0.0 | 15.9 | 0.0 | 40.3 | 0.0 |
| KS-sv-24 | Galena | 3.7 | 20.3 | 0.0 | 15.9 | 0.0 | 40.2 | 0.0 |
| KS-sv-T-1 | Galena | 3.4 | 20.2 | 0.0 | 16.0 | 0.0 | 40.8 | 0.0 |
| KSL-sv-01 | Galena | 3.4 | 20.2 | 0.0 | 15.9 | 0.0 | 40.2 | 0.0 |
| KGN-sv-02 | Galena | 3.4 | 20.3 | 0.0 | 15.9 | 0.0 | 40.6 | 0.0 |
| KCH-sv-01 | Galena | 3.6 | 20.3 | 0.0 | 15.9 | 0.0 | 40.3 | 0.0 |
| KS-sv-T-2 | Galena | 3.4 | 20.2 | 0.0 | 15.9 | 0.0 | 40.2 | 0.0 |
| KS-ad-01 | Chalcopyrite | 5.9 | 20.2 | 0.0 | 15.9 | 0.0 | 40.6 | 0.0 |
| KS-ad-01-2 | Chalcopyrite | 6.4 | 20.2 | 0.0 | 15.9 | 0.0 | 40.6 | 0.0 |
| KS-sv-30 | Chalcopyrite | 6.2 | 20.2 | 0.0 | 16.0 | 0.0 | 40.7 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, Z.; Tao, C.; Li, C.-F.; Liao, S.; Alam, M.; Farhan, M.; Zhang, H.; Hussain, A. Mineralogy, Fluid Inclusions, and Isotopic Study of the Kargah Cu-Pb Polymetallic Vein-Type Deposit, Kohistan Island Arc, Northern Pakistan: Implication for Ore Genesis. Minerals 2021, 11, 1266. https://doi.org/10.3390/min11111266
Hussain Z, Tao C, Li C-F, Liao S, Alam M, Farhan M, Zhang H, Hussain A. Mineralogy, Fluid Inclusions, and Isotopic Study of the Kargah Cu-Pb Polymetallic Vein-Type Deposit, Kohistan Island Arc, Northern Pakistan: Implication for Ore Genesis. Minerals. 2021; 11(11):1266. https://doi.org/10.3390/min11111266
Chicago/Turabian StyleHussain, Zahid, Chunhui Tao, Chun-Feng Li, Shili Liao, Masroor Alam, Muhammad Farhan, Huichao Zhang, and Amjad Hussain. 2021. "Mineralogy, Fluid Inclusions, and Isotopic Study of the Kargah Cu-Pb Polymetallic Vein-Type Deposit, Kohistan Island Arc, Northern Pakistan: Implication for Ore Genesis" Minerals 11, no. 11: 1266. https://doi.org/10.3390/min11111266
APA StyleHussain, Z., Tao, C., Li, C.-F., Liao, S., Alam, M., Farhan, M., Zhang, H., & Hussain, A. (2021). Mineralogy, Fluid Inclusions, and Isotopic Study of the Kargah Cu-Pb Polymetallic Vein-Type Deposit, Kohistan Island Arc, Northern Pakistan: Implication for Ore Genesis. Minerals, 11(11), 1266. https://doi.org/10.3390/min11111266








