Synergistic Effect of DBP with CTAB on Flotation Separation of Quartz from Collophane
Abstract
:1. Introduction
2. Experimental
2.1. Acquisition of Minerals and Reagents
2.2. Mineral Sample Characterization
2.3. Methods
2.3.1. Micro-Flotation Tests
2.3.2. Surface Tension Measurements
2.3.3. Aggregate Size Measurements
2.3.4. Froth Water Mass Fraction Measurements
3. Results and Discussion
3.1. Micro-Flotation Tests
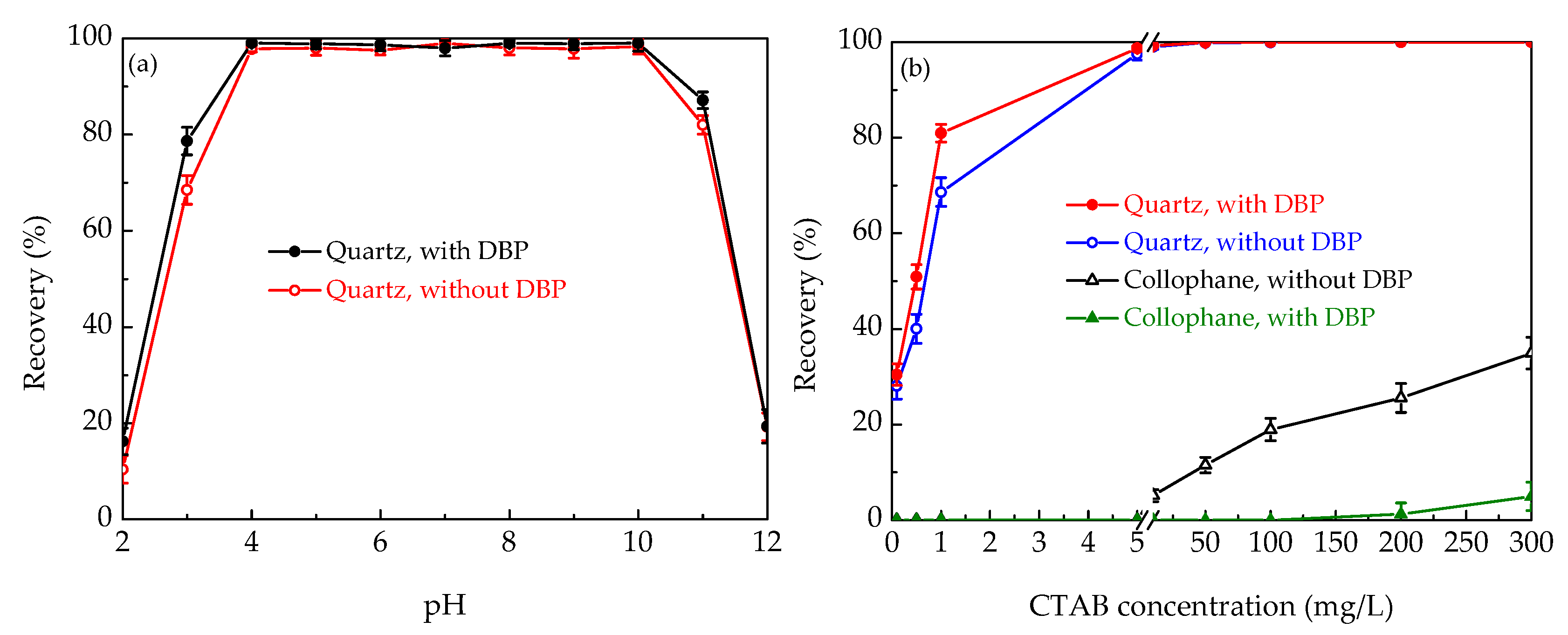
3.1.1. Flotation of Pure Minerals
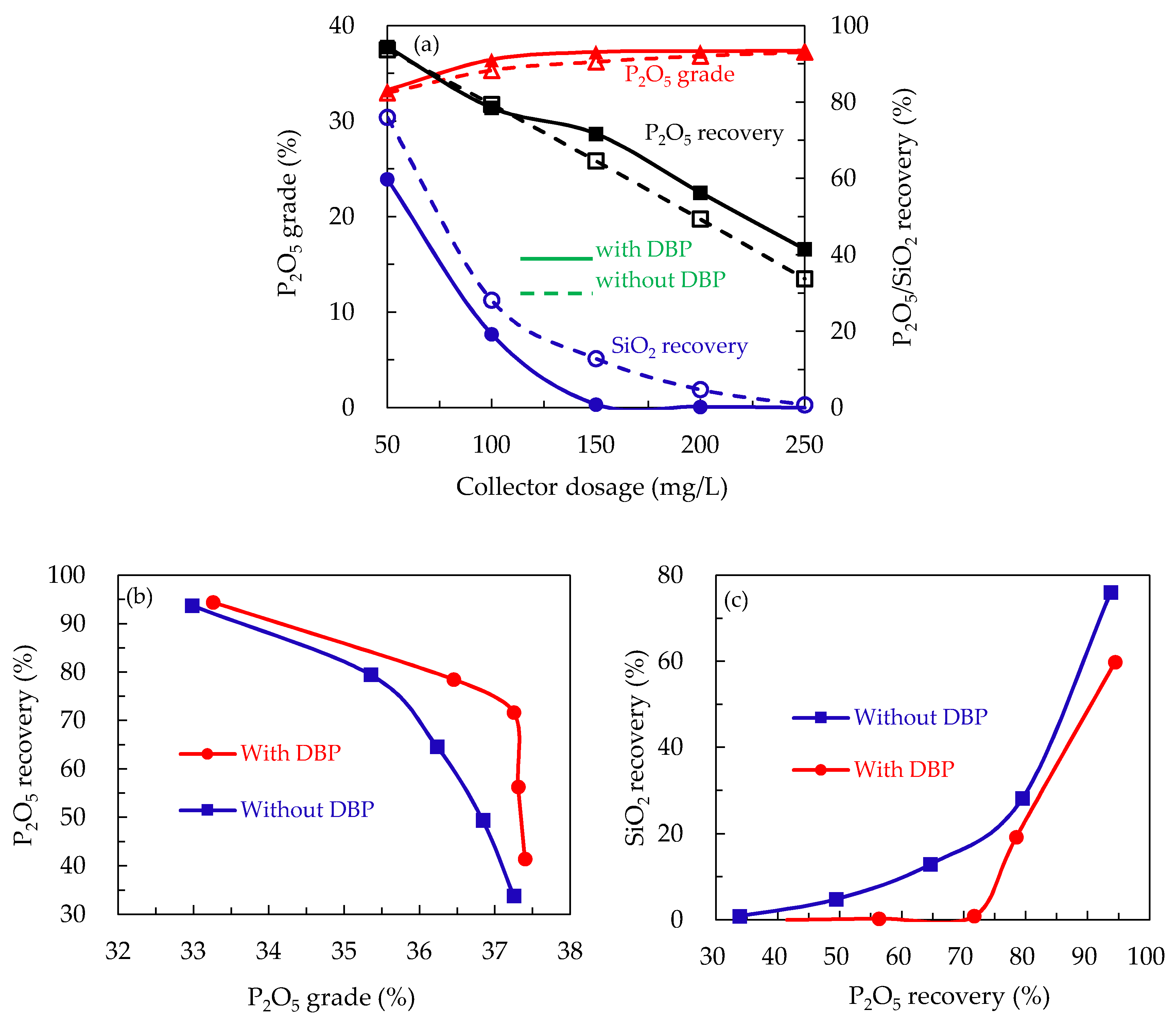
3.1.2. Flotation of Artificial Mineral Mixtures
3.2. Effect of DBP on Surface Tension
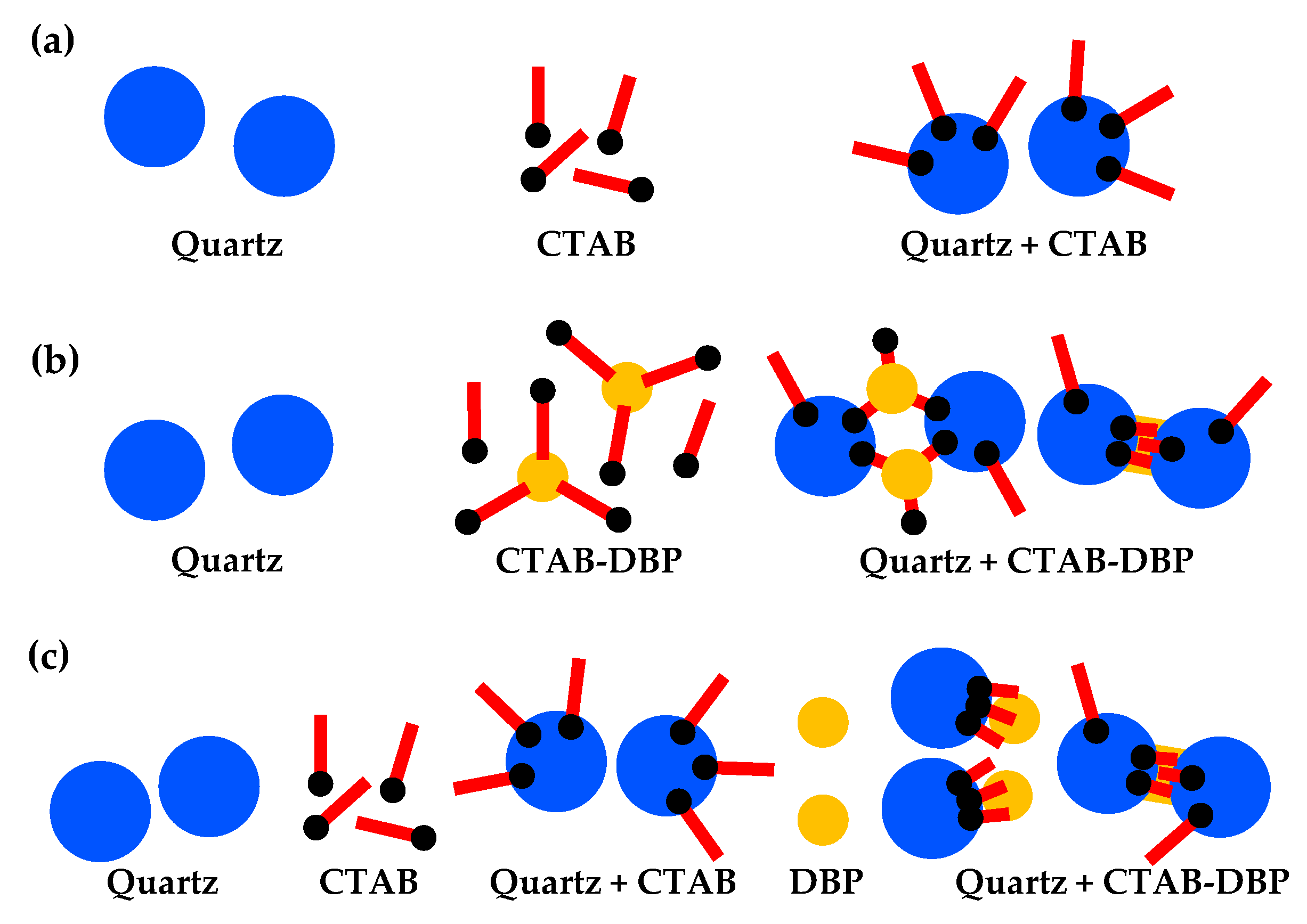
3.3. DBP-Assisted Aggregation of Quartz Particles
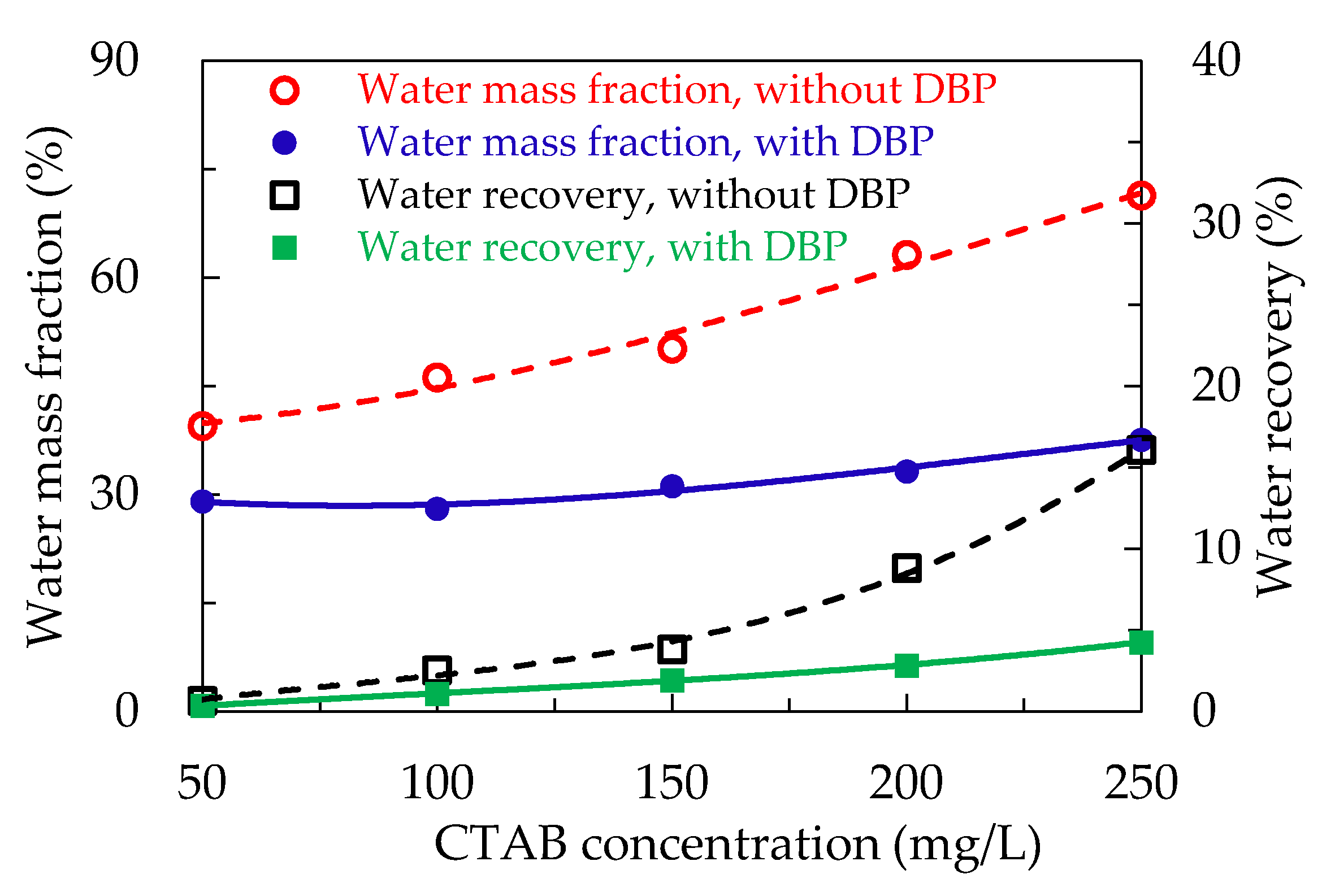
3.4. Effect of DBP on Entrainment in Froth
4. Conclusions
- The use of DBP in reverse flotation of collophane significantly improved the collecting potency and selectivity of CTAB for quartz at all pH’ and CTAB concentrations examined.
- Concentrate P2O5 grade and recovery obtained in the presence of DBP was substantially higher than that generated in the absence of DBP at the same CTAB concentration.
- The addition of DBP reduced the solution surface tension and the bubble size and improved the collision efficiency between the bubbles and the quartz particles.
- DBP improved the aggregation behavior of the coarse and fine quartz particles due to the strong hydrophobic interaction between the hydrophobic quartz particles and DBP droplets with stronger affinity for the quartz surface than for kerosene droplets.
- The introduction of DBP at a suitable dosage promoted defoaming and reduced the hydraulic entrainment of fine collophane particles.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abouzeid, A.Z.M. Physical and thermal treatment of phosphate ores—An overview. Int. J. Miner. Process. 2008, 85, 59–84. [Google Scholar] [CrossRef]
- Ministry of Natural Resources. China Mineral Resources; Geological Publishing House: Beijing, China, 2012; pp. 52–60. (In Chinese)
- Liu, X.; Li, C.X.; Luo, H.H.; Cheng, R.J.; Liu, F.Y. Selective reverse flotation of apatite from dolomite in collophanite ore using saponified gutter oil fatty acid as a collector. Int. J. Miner. Process. 2017, 165, 20–27. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Yang, H.Y.; Xiao, J.F.; Xia, Y.; Xie, Z.J. Origin of the Ediacaran Weng’an and Kaiyang phosphorite deposits in the Nanhua basin, SW China. J. Asian Earth Sci. 2019, 182, 103931. [Google Scholar] [CrossRef]
- Li, X.B.; Zhang, Q.; Hou, B.; Ye, J.J.; Mao, S.; Li, X.H. Flotation separation of quartz from collophane using an amine collector and its adsorption mechanisms. Powder Technol. 2017, 318, 224–229. [Google Scholar] [CrossRef]
- Wu, Z.X.; Tao, D.P.; Jiang, X.J. Experimental Study on a Novel Cationic Collector for Reverse Flotation of Collophane for Silica Removal. Multipurp. Util. Miner. Resour. 2020, 5, 92–100. (In Chinese) [Google Scholar]
- Fang, J.; Ge, Y.Y.; Yu, J. Adsorption behavior and mechanism of an ether amine collector on collophane and quartz. Physicochem. Probl. Miner. Process. 2019, 55, 301–310. [Google Scholar] [CrossRef]
- Han, Y.; Han, S.; Kim, B.; Yang, J. Flotation separation of quartz from apatite and surface forces in bubble-particle interactions: Role of pH and cationic amine collector contents. J. Ind. Eng. Chem. 2019, 70, 107–115. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Cheng, C.; Liu, Z.W.; Zeng, H.Q.; Feng, B.; Zhong, H.; Luo, W.H.; Hu, Y.J.; Guo, Z.Q.; He, G.C.; et al. Utilization of a new Gemini surfactant as the collector for the reverse froth flotation of phosphate ore in sustainable production of phosphate fertilizer. J. Clean. Prod. 2019, 221, 108–112. [Google Scholar] [CrossRef]
- Vieira, A.M.; Peres, A.E.C. The effect of amine type, pH, and size range in the flotation of quartz. Miner. Eng. 2007, 20, 1008–1013. [Google Scholar] [CrossRef]
- Sobhy, A.; Wu, Z.X.; Tao, D.P. Statistical analysis and optimization of reverse anionic hematite flotation integrated with nanobubbles. Miner. Eng. 2020, 163, 106799. [Google Scholar] [CrossRef]
- Tao, D.P.; Wu, Z.X.; Sobhy, A. Investigation of nanobubble enhanced reverse anionic flotation of hematite and associated mechanisms. Powder Technol. 2020, 379, 12–25. [Google Scholar] [CrossRef]
- Wu, Z.X.; Tao, D.P. Mineralogical analysis of collophane in Yunnan using AMICS and exploration of difficult flotation mechanisms. Chin. J. Eng. 2021, 43, 503–511. (In Chinese) [Google Scholar] [CrossRef]
- Xia, Y.C.; Zhang, R.; Xing, Y.W.; Gui, X.H. Improving the adsorption of oily collector on the surface of low-rank coal during flotation using a cationic surfactant: An experimental and molecular dynamics simulation study. Fuel 2019, 235, 687–695. [Google Scholar] [CrossRef]
- Li, H.; Liu, M.X.; Liu, Q. The effect of non-polar oil on fine hematite flocculation and flotation using sodium oleate or hydroxamic acids as a collector. Miner. Eng. 2018, 119, 105–115. [Google Scholar] [CrossRef]
- Ozkan, A.; Dudnik, V.; Esmeli, K. Hydrophobic flocculation of talc with kerosene and effects of anionic surfactants. Part. Sci. Technol. 2015, 34, 235–240. [Google Scholar] [CrossRef]
- Liu, A.; Fan, M.Q.; Li, Z.H.; Fan, J.C. Non-polar oil assisted DDA flotation of quartz I: Interfacial interaction between dodecane oil drop and mineral particle. Int. J. Miner. Process. 2017, 168, 1–8. [Google Scholar] [CrossRef]
- Qiao, X.X.; Liu, A.; Li, Z.H.; Fan, J.C.; Luo, C.J.; Fan, P.P.; Liu, Y.Z.; Fan, M.Q. A novel miscible collector DDA-2-octanol-kerosene: Properties and flotation performance. Miner. Eng. 2020, 156, 106475. [Google Scholar] [CrossRef]
- Nazari, S.; Shafaei, S.Z.; Gharabaghi, M.; Ahmadi, R.; Shahbazi, B.; Fan, M.M. Effects of nanobubble and hydrodynamic parameters on coarse quartz flotation. Int. J. Min. Sci. Technol. 2019, 29, 289–295. [Google Scholar] [CrossRef]
- Lin, D.; Zhong, J.M.; Ji, S.M.; Yuan, Z.X.; Xing, L.J.; He, Q.; Zhang, H.L.; Huo, Y.P. Highly efficient triplet-triplet annihilation upconversion in high viscosity phthalate ester media. Dye. Pigment. 2021, 185, 108912. [Google Scholar] [CrossRef]
- Liao, Y.F.; Yang, Z.; An, M.Y.; Cao, Y.J.; Hao, X.D.; Song, X.W.; Ren, H.R.; Yang, A.S.; Chen, L.J. Spreading behavior of dodecane-oleic acid collector mixture in low-rank coal flotation. Fuel 2022, 308, 122071. [Google Scholar] [CrossRef]
- Tan, J.L.; Cheng, H.Z.; Wei, L.B.; Gui, X.H.; Xing, Y.W. Investigation of CTAB and DBP esters on low-rank coal flotation selectivity. Energe Source Part A 2019, 42, 1–10. [Google Scholar] [CrossRef]
- Li, C.; Cao, Y.J.; Peng, W.J.; Shi, F.N. On the correlation between froth stability and viscosity in flotation. Miner. Eng. 2020, 149, 106269. [Google Scholar] [CrossRef]
- Li, C.; Runge, K.; Shi, F.N.; Farrokhpay, S. Effect of flotation froth properties on froth rheology. Powder Technol. 2016, 294, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.C.; Teng, Q.; Liu, J.; Yang, W.P.; Hu, D.H.; Liu, S.Y. Use of NaOL and CTAB mixture as collector in selective flotation separation of enstatite and magnetite. Colloids Surf. A Physicochem. Eng. Asp. 2019, 570, 481–486. [Google Scholar] [CrossRef]
- Carvalho, J.A.E.D.; Brando, P.; Henriques, A.B.; Oliveira, P.S.D.; Cançado, R.Z.L.; Silva, G.R.D. Selective flotation of apatite from micaceous minerals using patauá palm tree oil collector. Miner. Eng. 2020, 156, 106474. [Google Scholar] [CrossRef]
- Xu, T.H.; Long, B.W.; Zhang, Y.; Deng, F.L.; Dai, Y.F.; Zhang, L.L.; Ding, Y.G. Synthesis and Application of Silicon Removal Reagent for Reverse Flotation of Phosphate Rock. Multipurp. Util. Miner. Resour. 2021, 3, 57–63. (In Chinese) [Google Scholar]
- Chen, Y.M.; Li, H.; Feng, D.X.; Tong, X.; Hu, S.X.; Yang, F.; Wang, G.C. A recipe of surfactant for the flotation of fine cassiterite particles. Miner. Eng. 2021, 160, 106658. [Google Scholar] [CrossRef]
- Liao, Y.F.; Hao, X.D.; An, M.Y.; Yang, L.F.; Ren, H.R. Enhancing low-rank coal flotation using mixed collector of dodecane and oleic acid: Effect of droplet dispersion and its interaction with coal particle. Fuel 2020, 280, 118634. [Google Scholar] [CrossRef]
- Liu, A.; Fan, M.Q.; Fan, P.P. Interaction mechanism of miscible DDA-Kerosene and fine quartz and its effect on the reverse flotation of magnetic separation concentrate. Miner. Eng. 2014, 65, 41–50. [Google Scholar] [CrossRef]
- Cao, L.; Chen, X.M.; Peng, Y.J. The effect of polyglycol-type frothers on the interfacial characteristics of oily collector dispersion. Miner. Eng. 2020, 157, 106579. [Google Scholar] [CrossRef]
- Ma, F.Y.; Tao, D.P.; Tao, Y.J. Effects of nanobubbles in column flotation of Chinese sub-bituminous coal. Int. J. Coal Prep. Util. 2019, 1–17. [Google Scholar] [CrossRef]
- Yan, S.L.; Yang, X.Y.; Bai, Z.S.; Xu, X.; Wang, H.L. Drop attachment behavior of oil droplet-gas bubble interactions during flotation. Chem. Eng. Sci. 2020, 223, 115740. [Google Scholar] [CrossRef]
- Hoang, D.H.; Hassanzadeh, A.; Peuker, U.A.; Rudolph, M. Impact of flotation hydrodynamics on the optimization of fine-grained carbonaceous sedimentary apatite ore beneficiation. Powder Technol. 2019, 345, 223–233. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Wang, X.X.; Bu, X.N.; Shao, H.Z.; Hu, Y.; Alheshibri, M.; Li, B.; Chao, N.; Peng, Y.L.; Xie, G.Y. Effects of emulsified kerosene nanodroplets on the entrainment of gangue materials and selectivity index in aphanitic graphite flotation. Miner. Eng. 2020, 158, 106592. [Google Scholar] [CrossRef]
- Xu, M.D.; Xing, Y.W.; Jin, W.; Li, M.; Cao, Y.J.; Gui, X.H. Effect of diesel on the froth stability and its antifoam mechanism in fine coal flotation used MIBC as the frother. Powder Technol. 2019, 364, 183–188. [Google Scholar] [CrossRef]
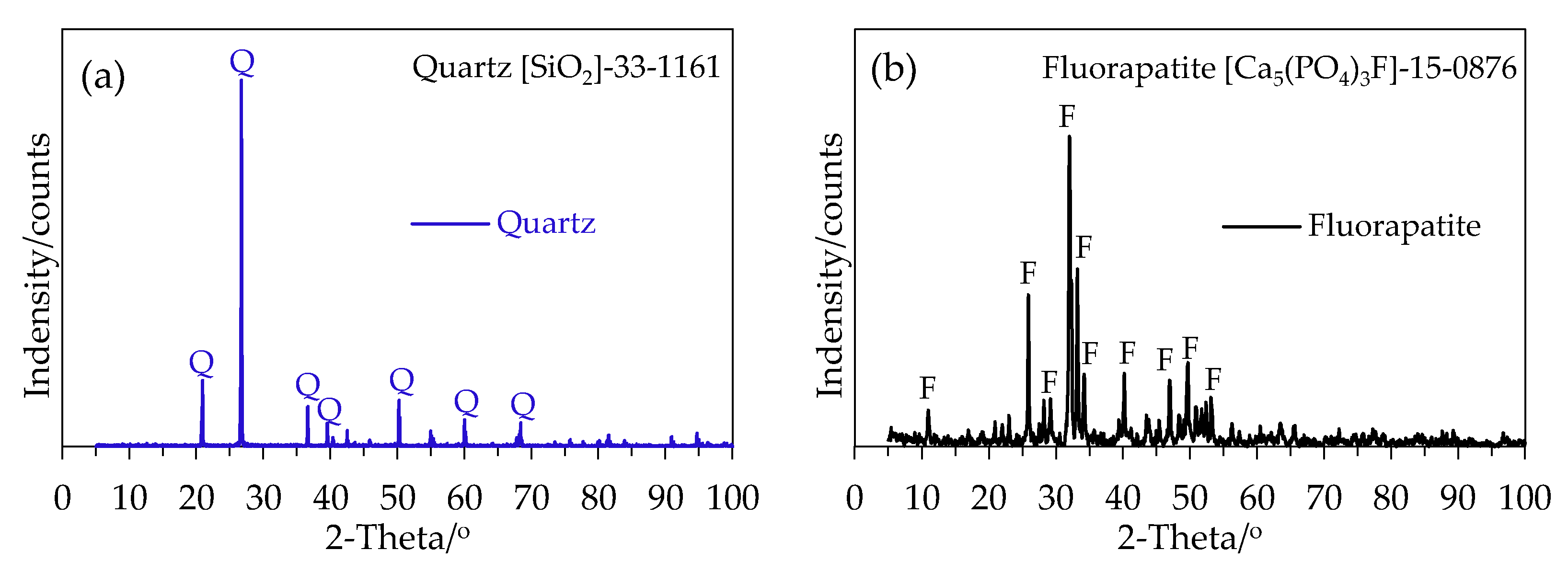


| Quartz | Name | SiO2 | Al2O3 | P2O5 | CaO | Fe2O3 | Cl |
| Composition (%) | 99.37 | 0.21 | 0.04 | 0.36 | 0.01 | 0.01 | |
| Fluorapatite | Name | MgO | Al2O3 | P2O5 | CaO | Fe2O3 | F |
| Composition (%) | 0.08 | 4.2 | 39.56 | 53.35 | 1.69 | 1.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Tao, D.; Zhang, P.; Jiang, X.; Jiang, M. Synergistic Effect of DBP with CTAB on Flotation Separation of Quartz from Collophane. Minerals 2021, 11, 1196. https://doi.org/10.3390/min11111196
Wu Z, Tao D, Zhang P, Jiang X, Jiang M. Synergistic Effect of DBP with CTAB on Flotation Separation of Quartz from Collophane. Minerals. 2021; 11(11):1196. https://doi.org/10.3390/min11111196
Chicago/Turabian StyleWu, Zhongxian, Dongping Tao, Patrick Zhang, Xiaojun Jiang, and Man Jiang. 2021. "Synergistic Effect of DBP with CTAB on Flotation Separation of Quartz from Collophane" Minerals 11, no. 11: 1196. https://doi.org/10.3390/min11111196
APA StyleWu, Z., Tao, D., Zhang, P., Jiang, X., & Jiang, M. (2021). Synergistic Effect of DBP with CTAB on Flotation Separation of Quartz from Collophane. Minerals, 11(11), 1196. https://doi.org/10.3390/min11111196







