Atypical Mineralization Involving Pd-Pt, Au-Ag, REE, Y, Zr, Th, U, and Cl-F in the Oktyabrsky Deposit, Norilsk Complex, Russia
Abstract
:1. Introduction and Geological Context
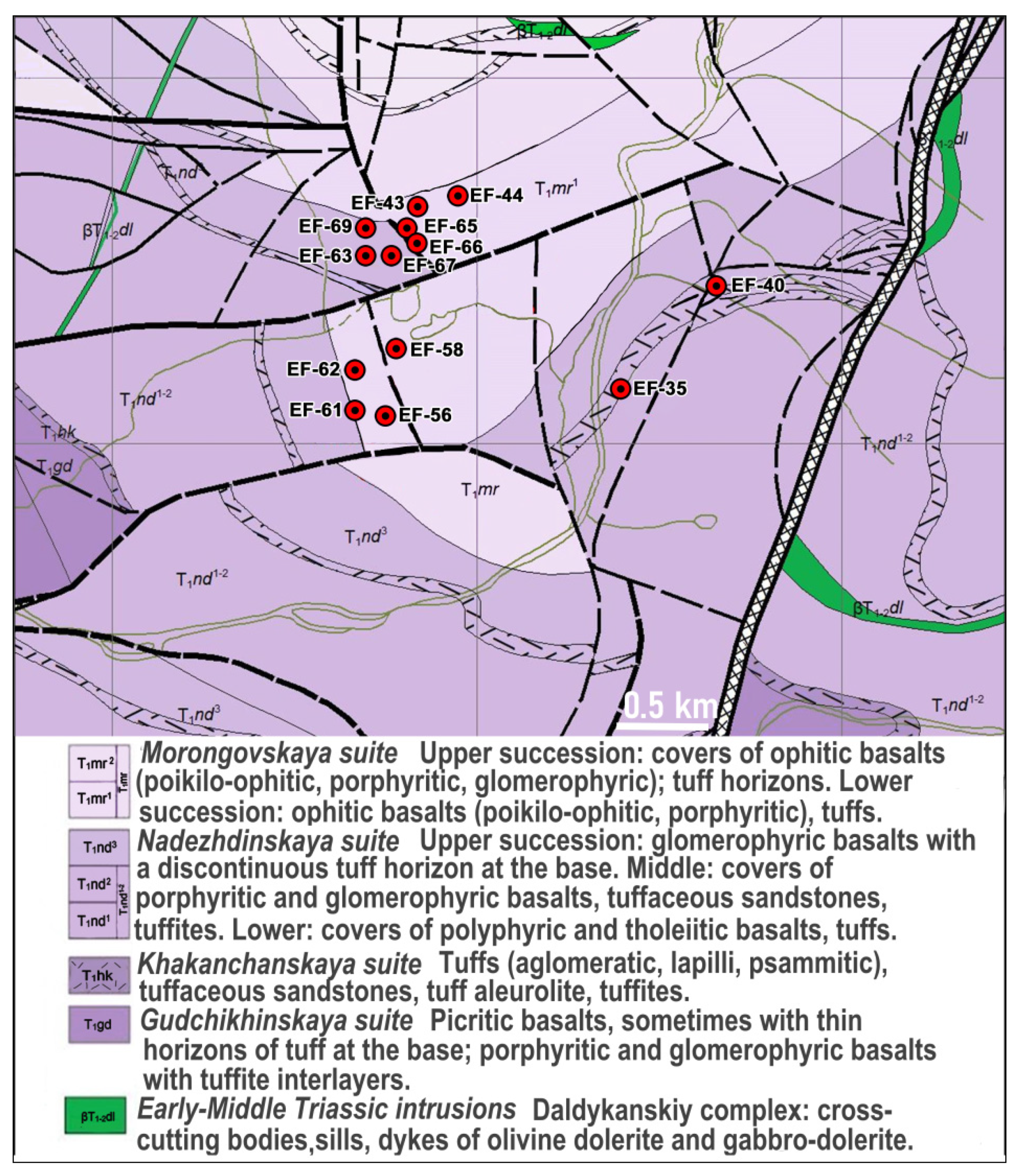
2. Materials and Methods
3. Results and Observations
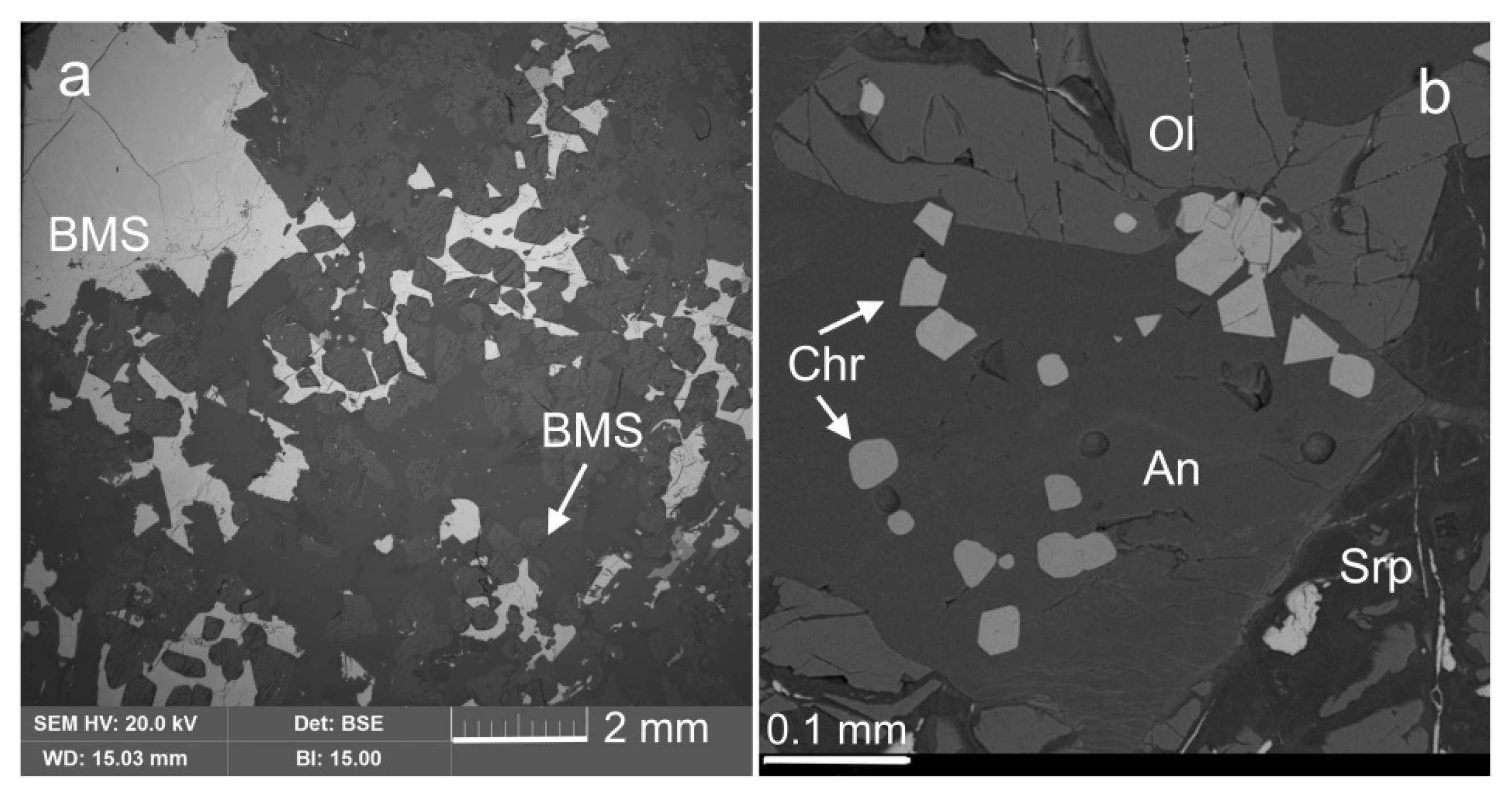
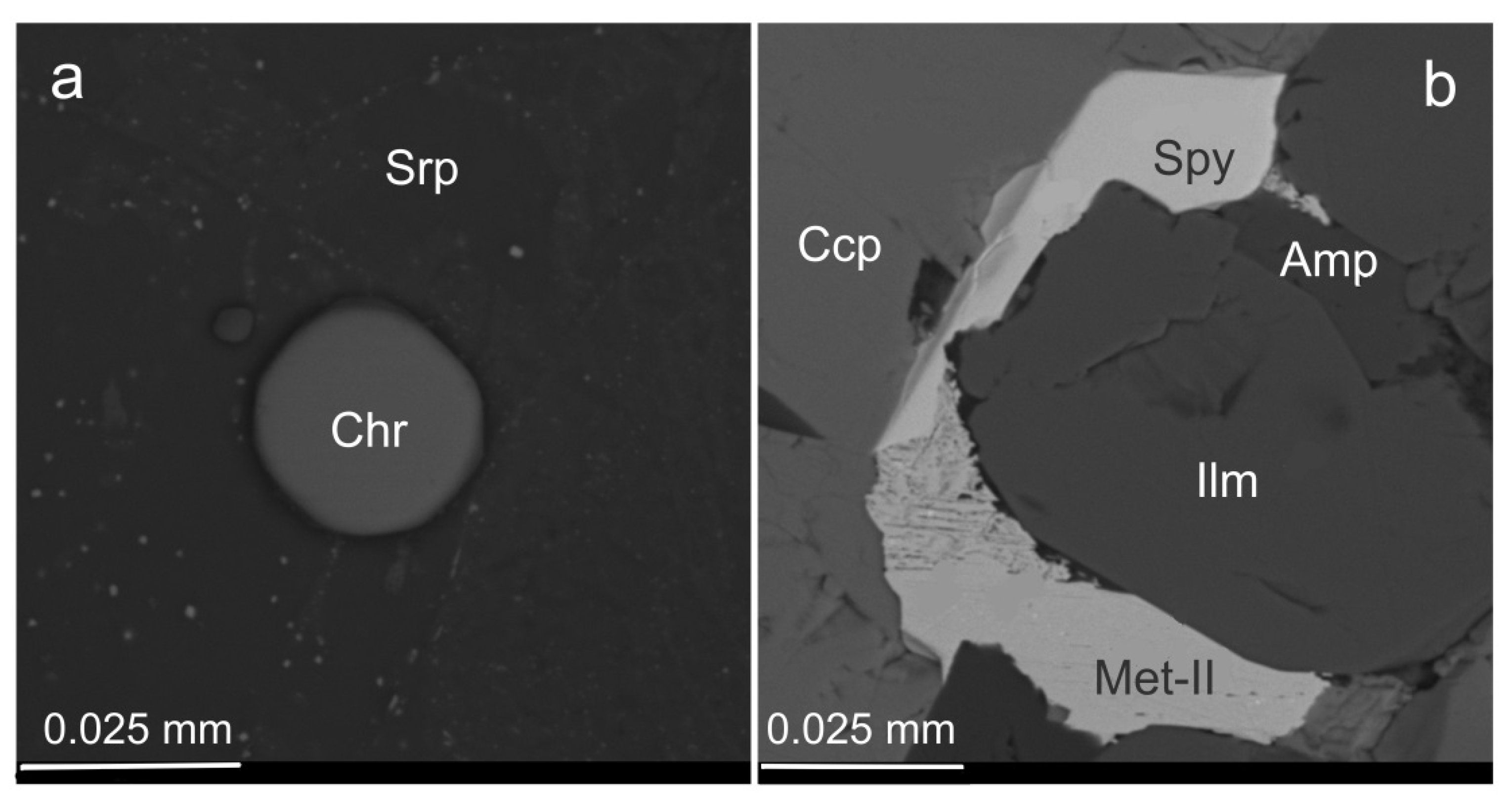
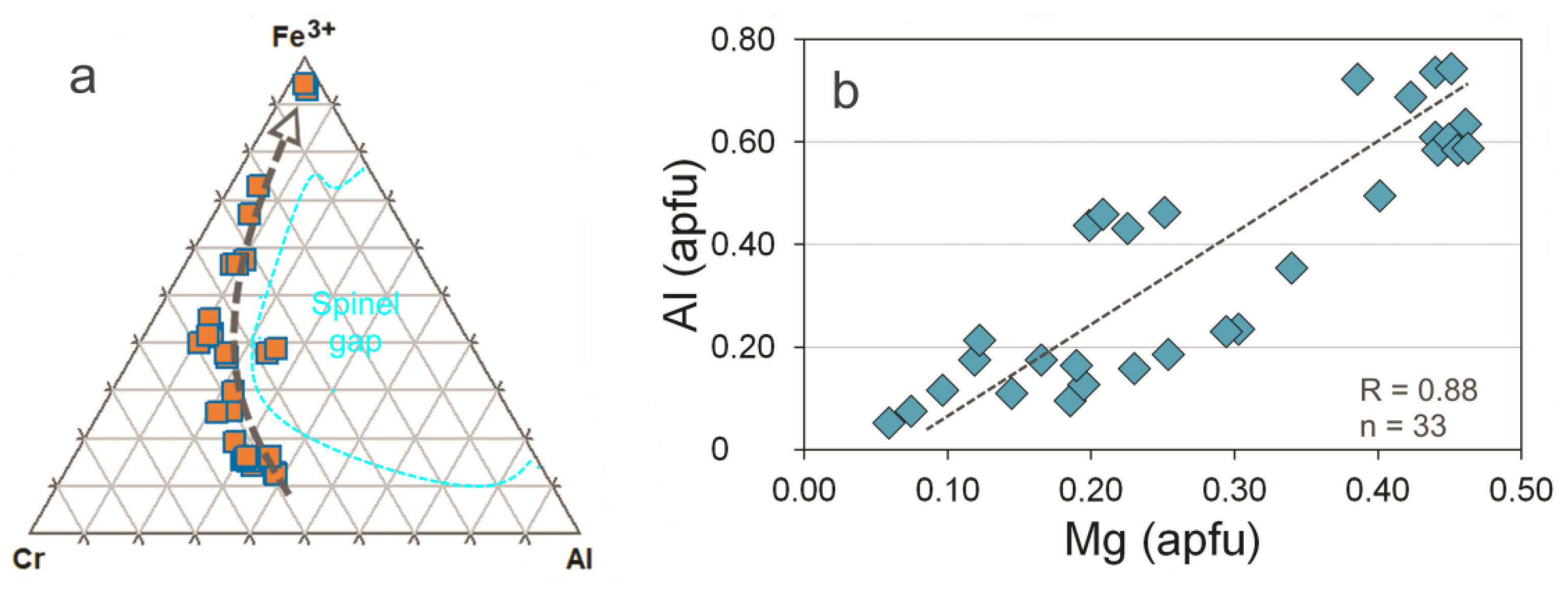
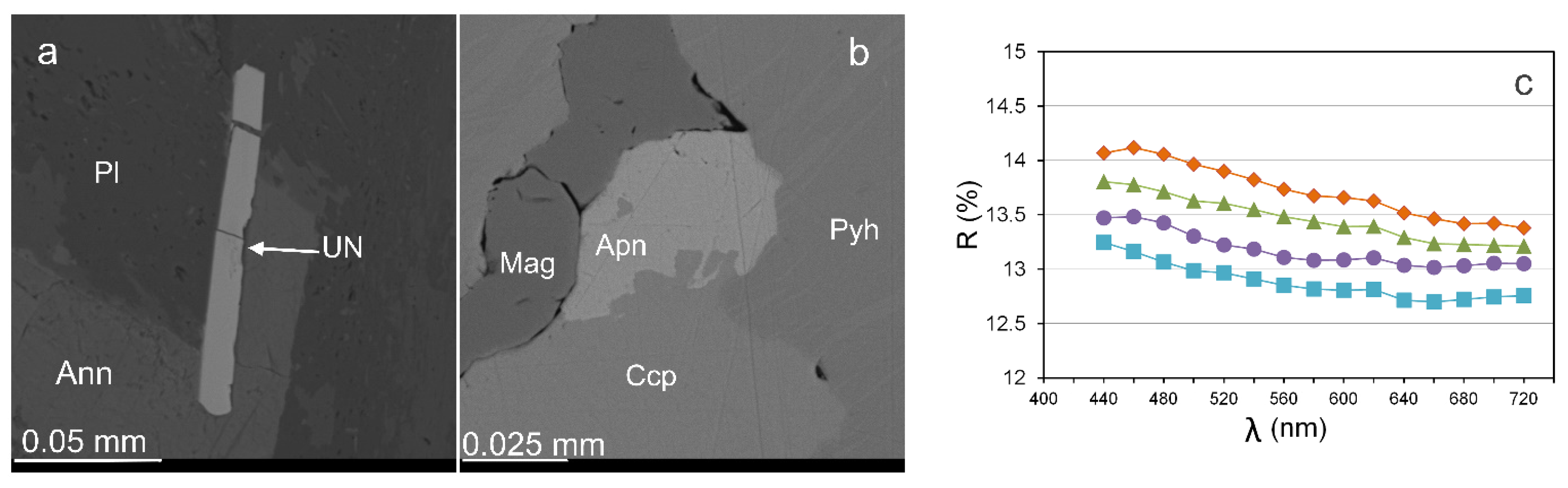
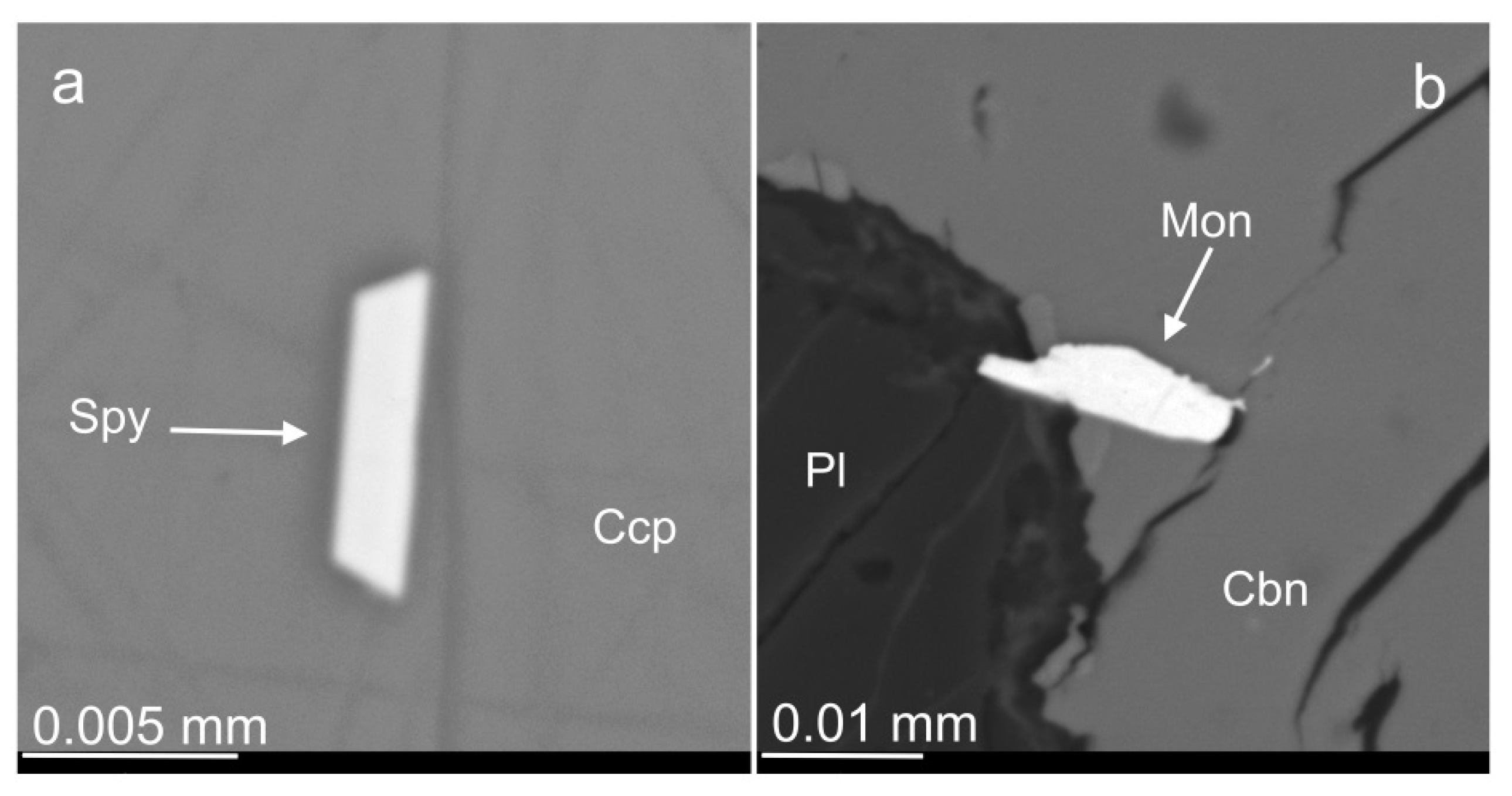
3.1. Rock-Forming Minerals and the Accessory Fe-Cr-(Ti) Oxides
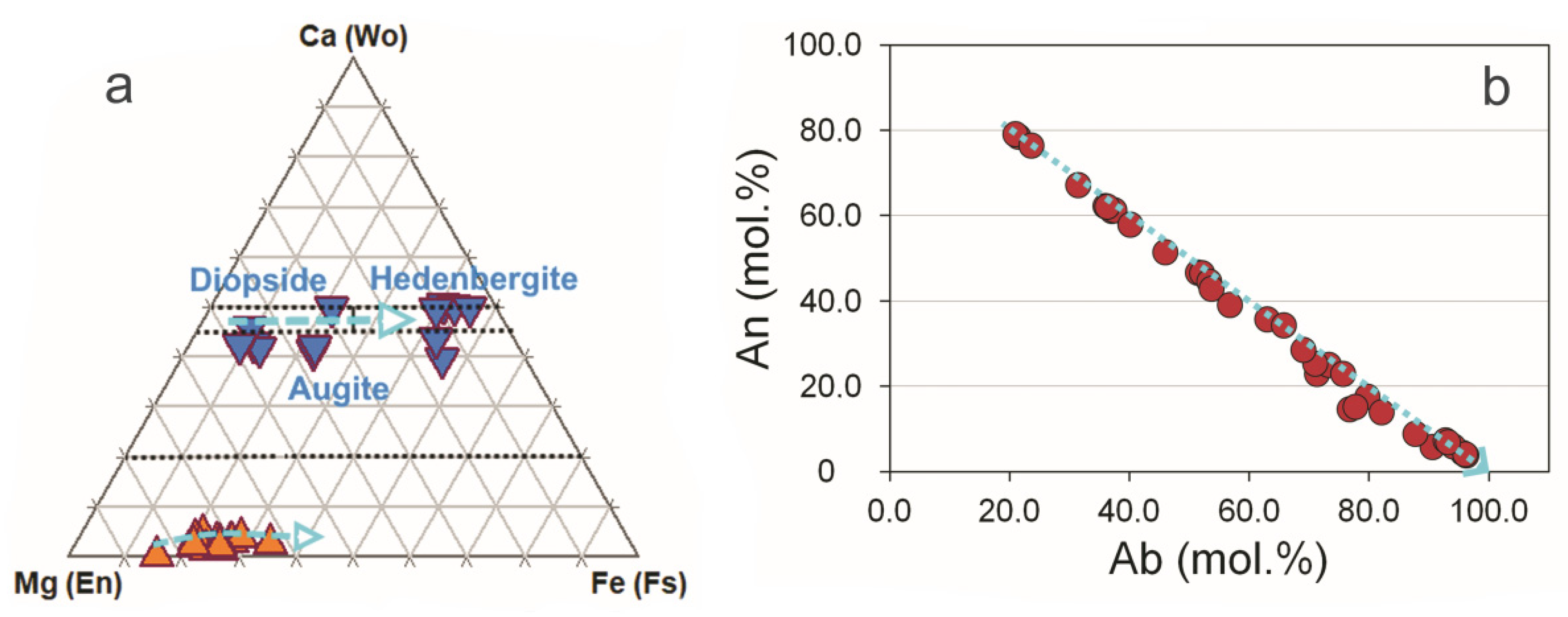
3.2. Amphiboles and Micas
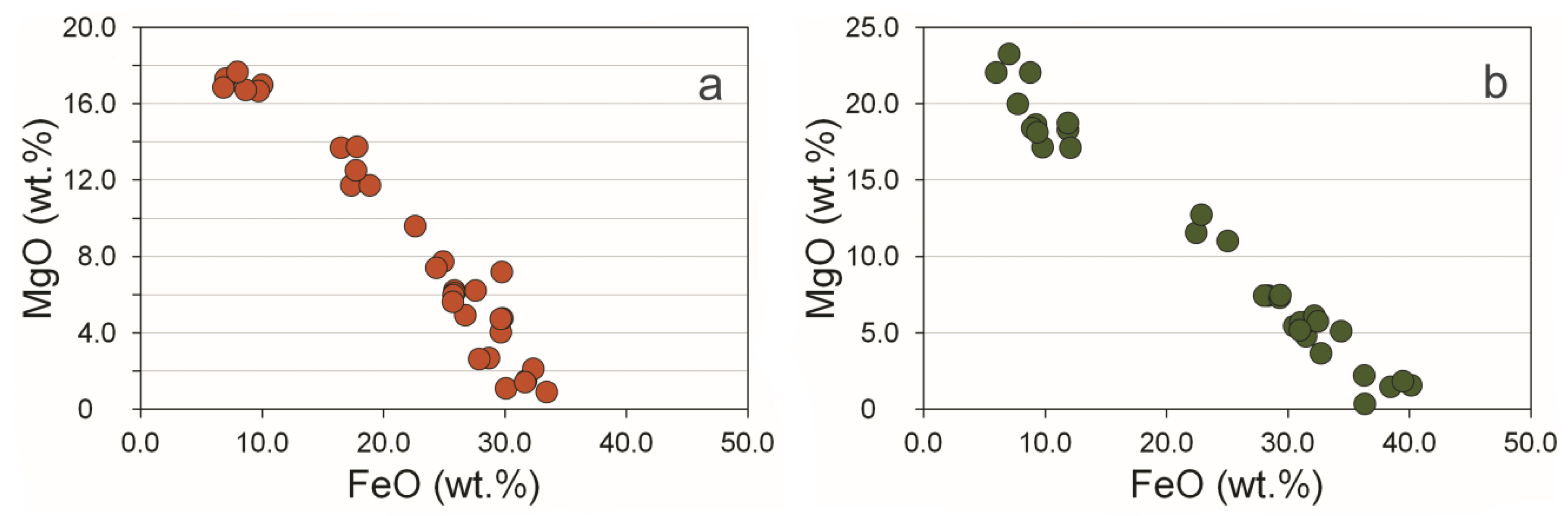
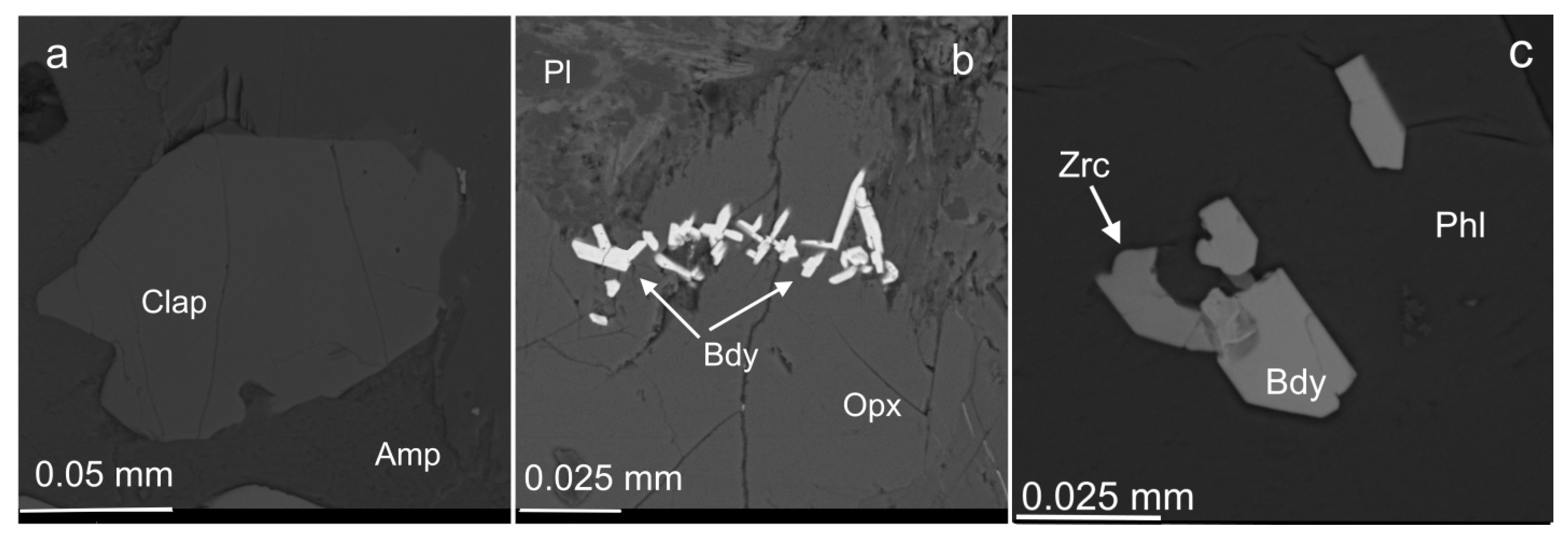
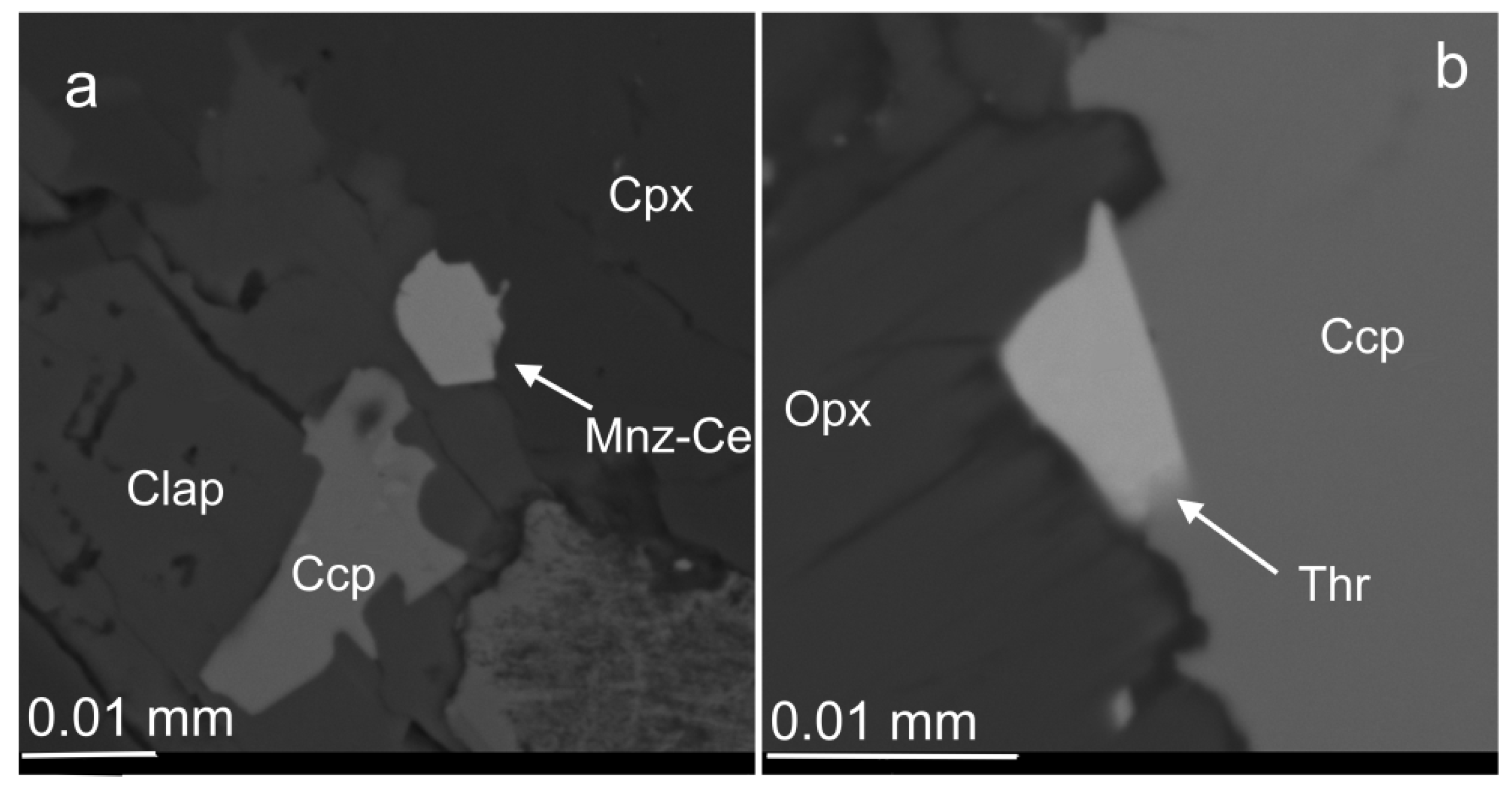
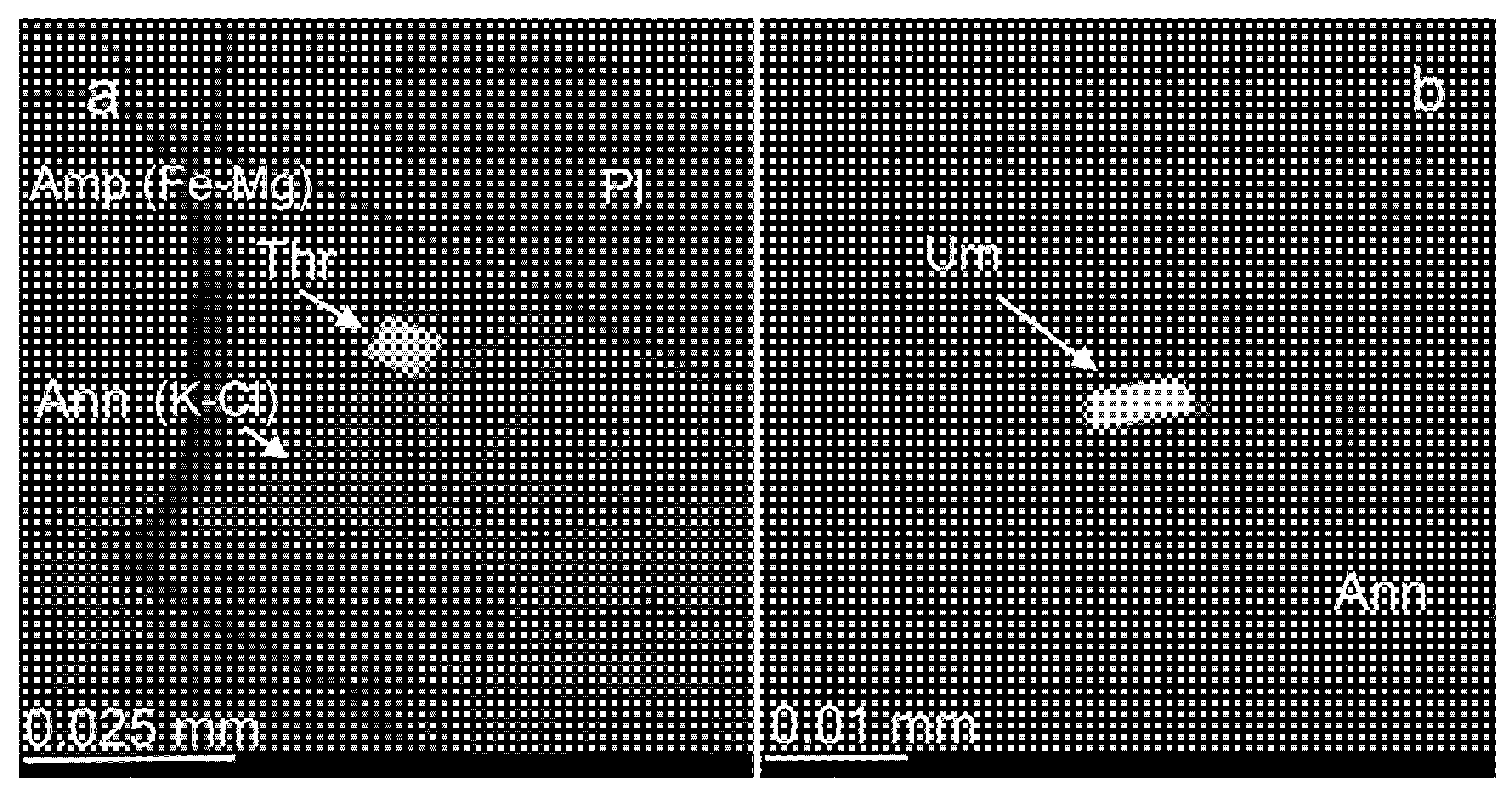
3.3. Compositional Variations of Apatite and Minerals of Zr, REE, Y, Th and U
3.4. Unnamed (Y,Ca,REE)2Zr2 (Ti,Nb)2TiFe2+O14
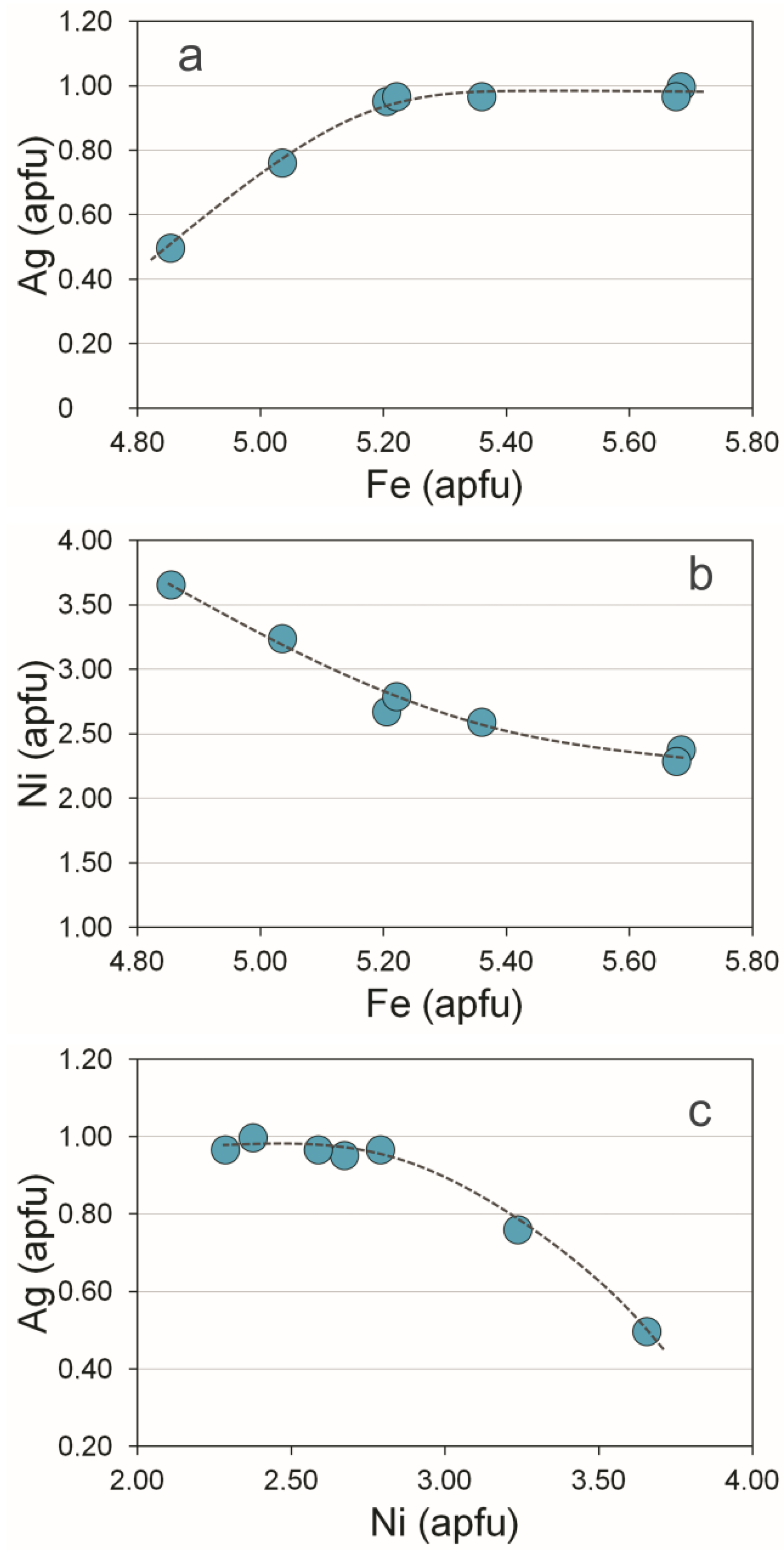
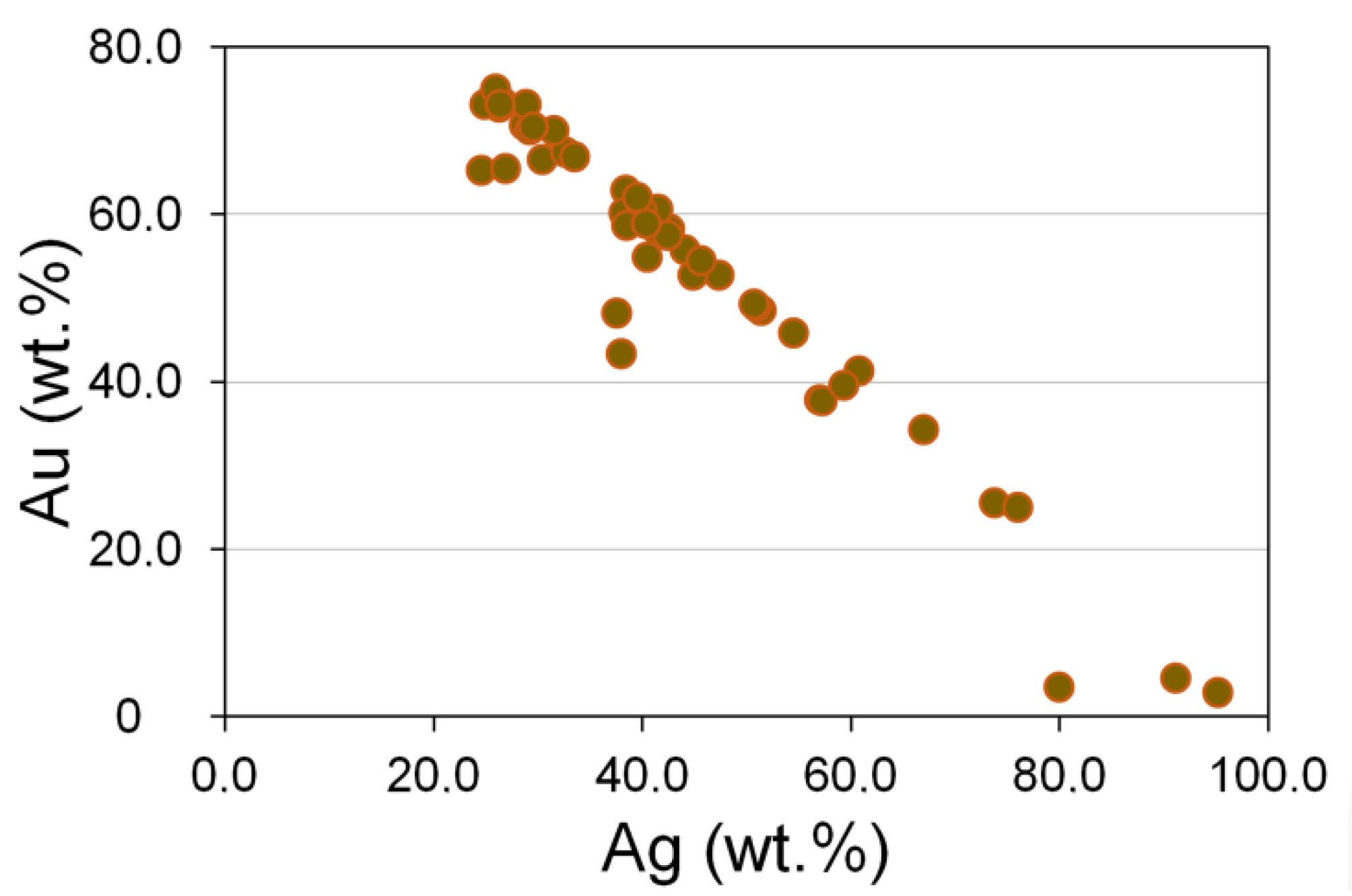
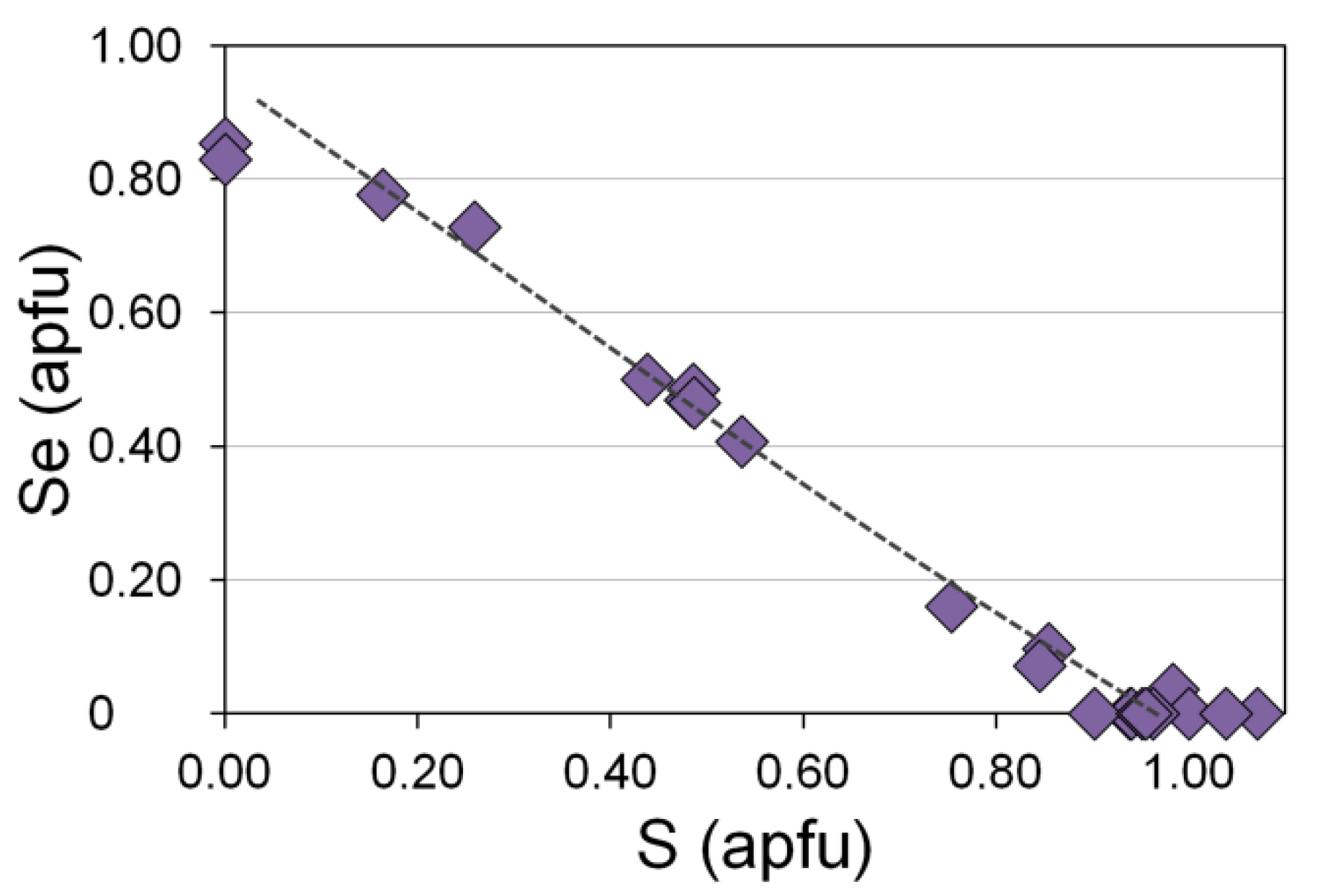
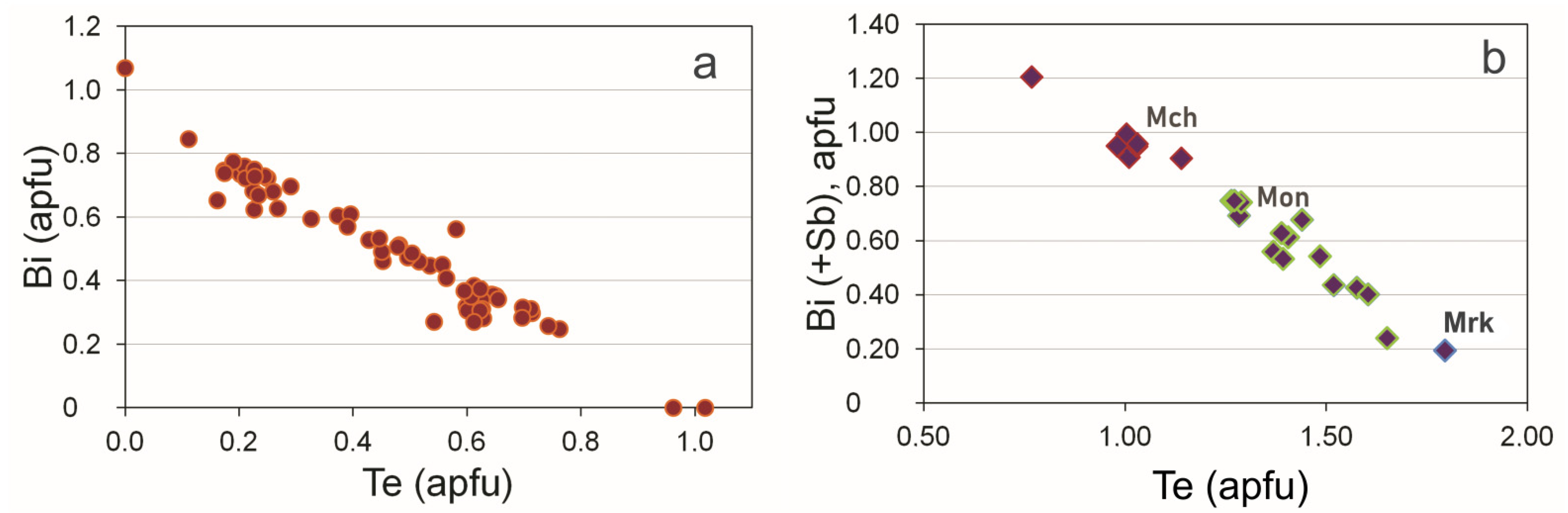
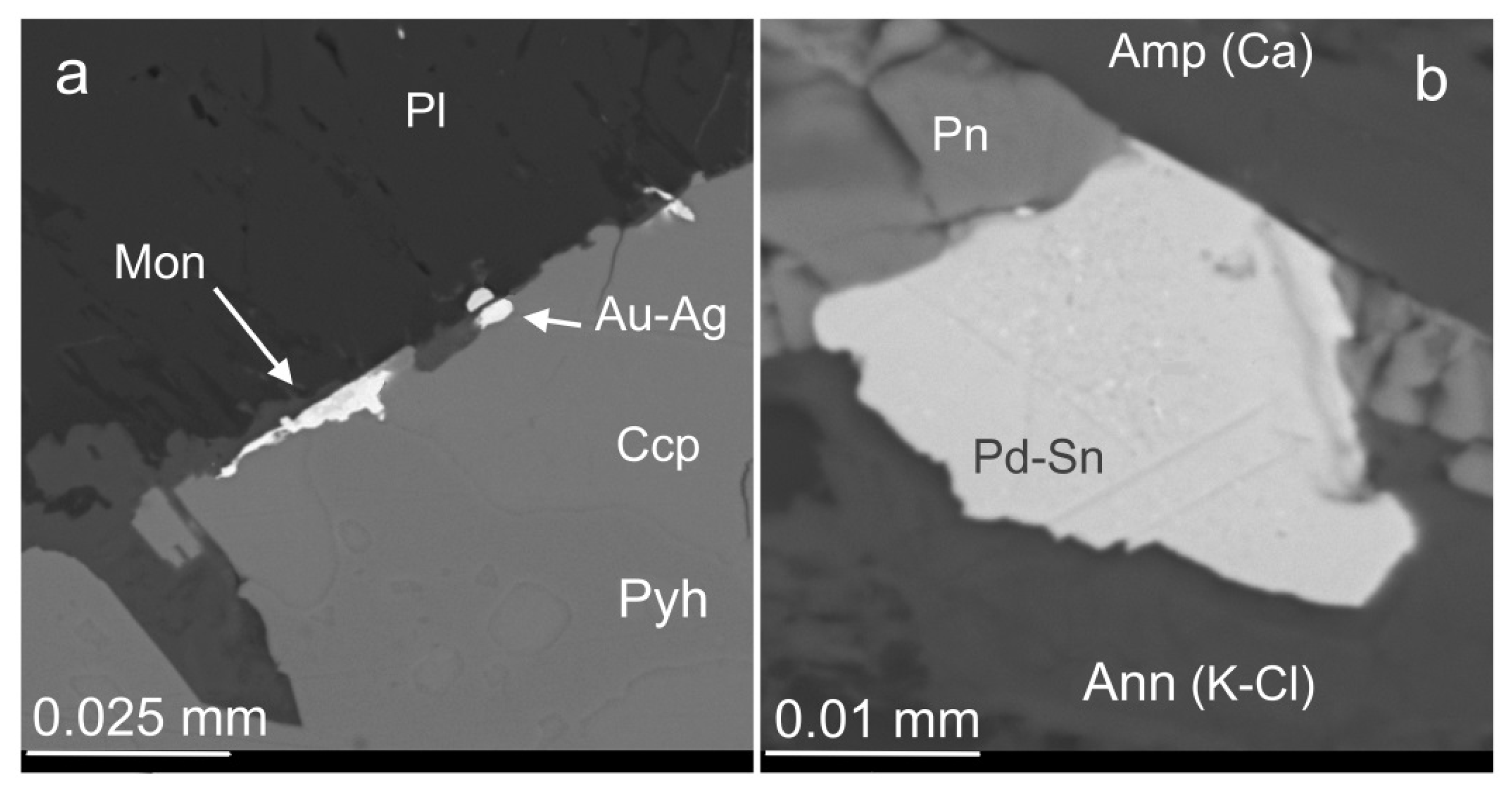
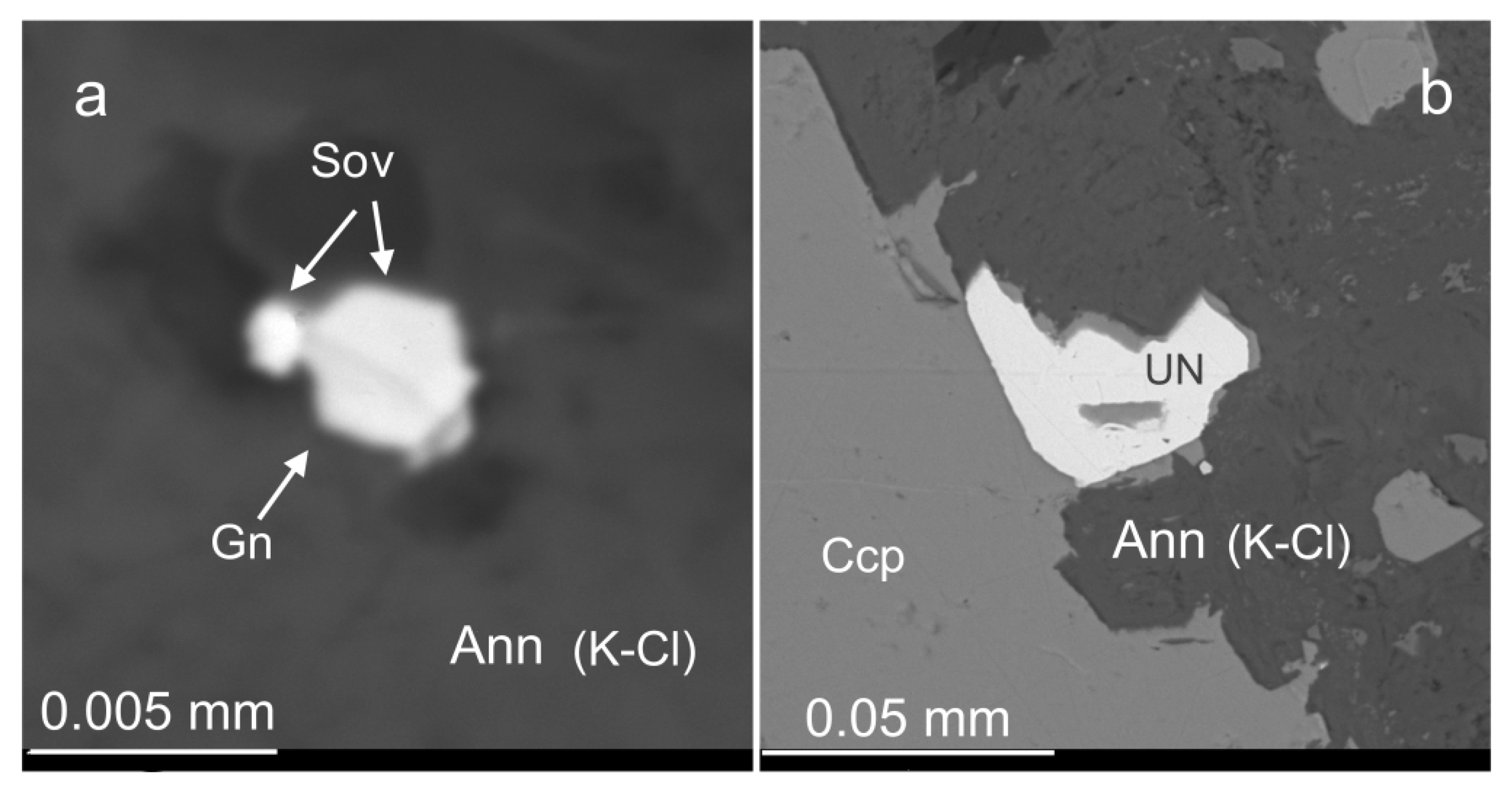
3.5. Au-Ag Minerals and Variations in the System PbS-PbSe-PbTe
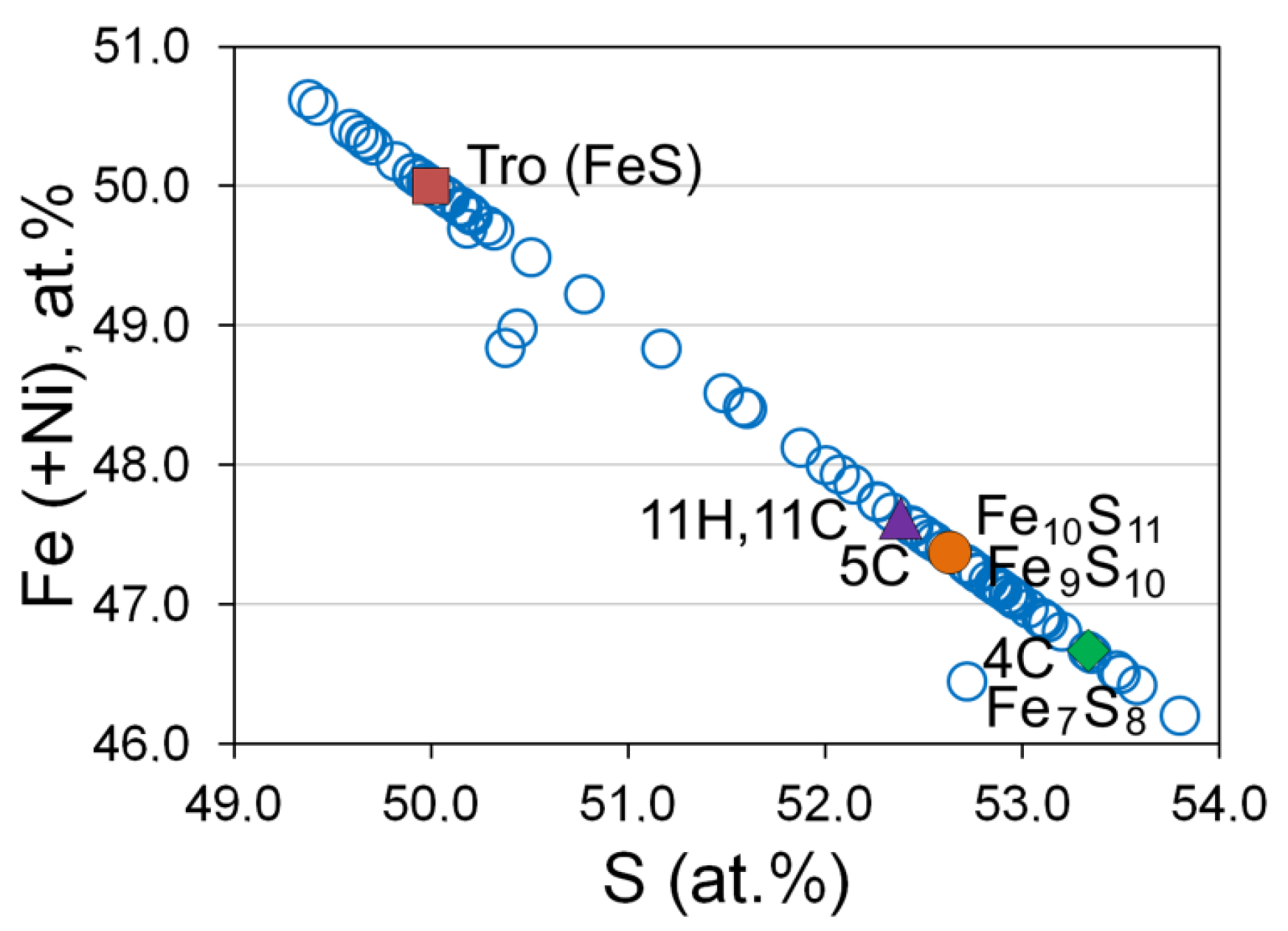
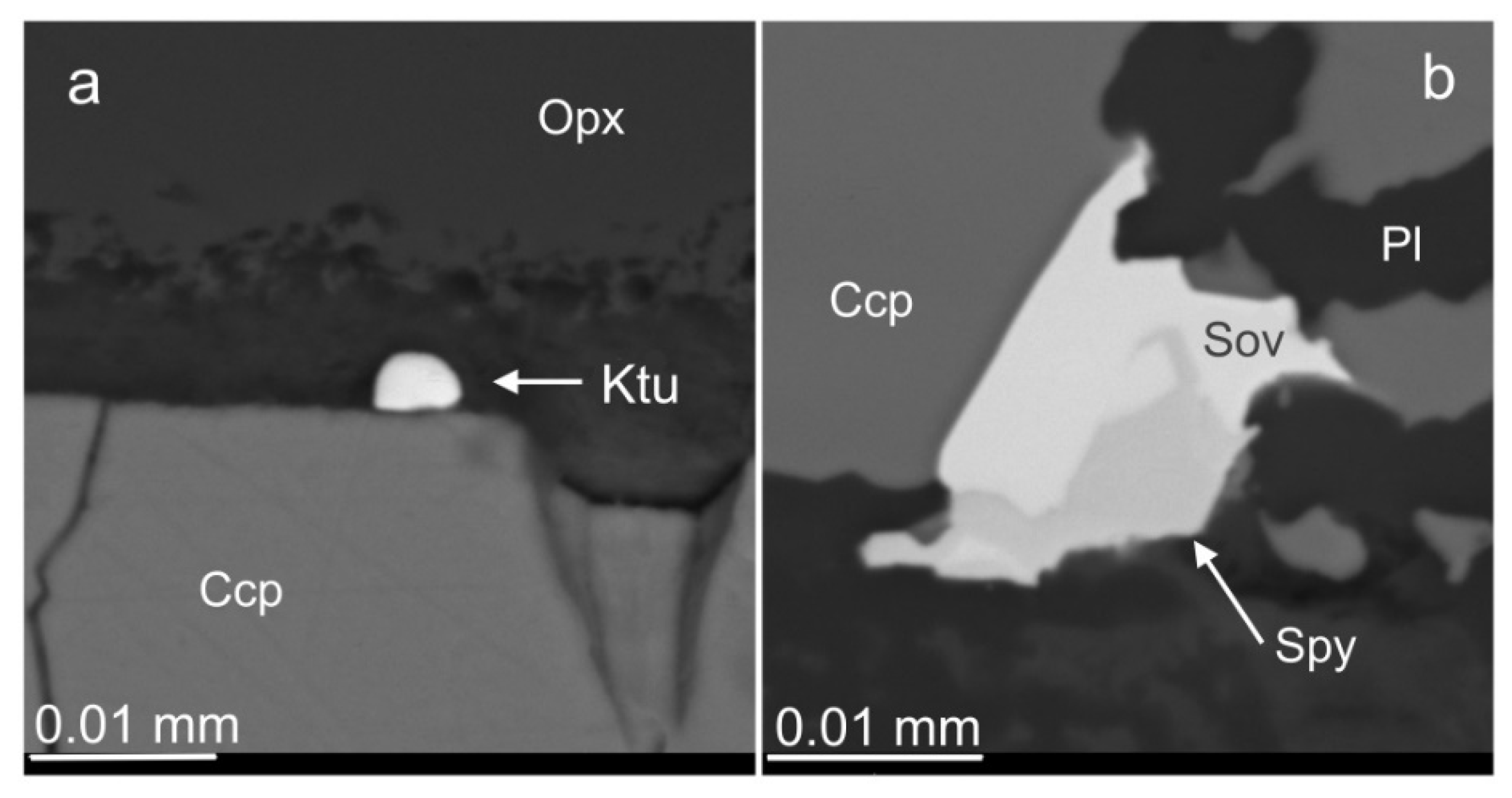
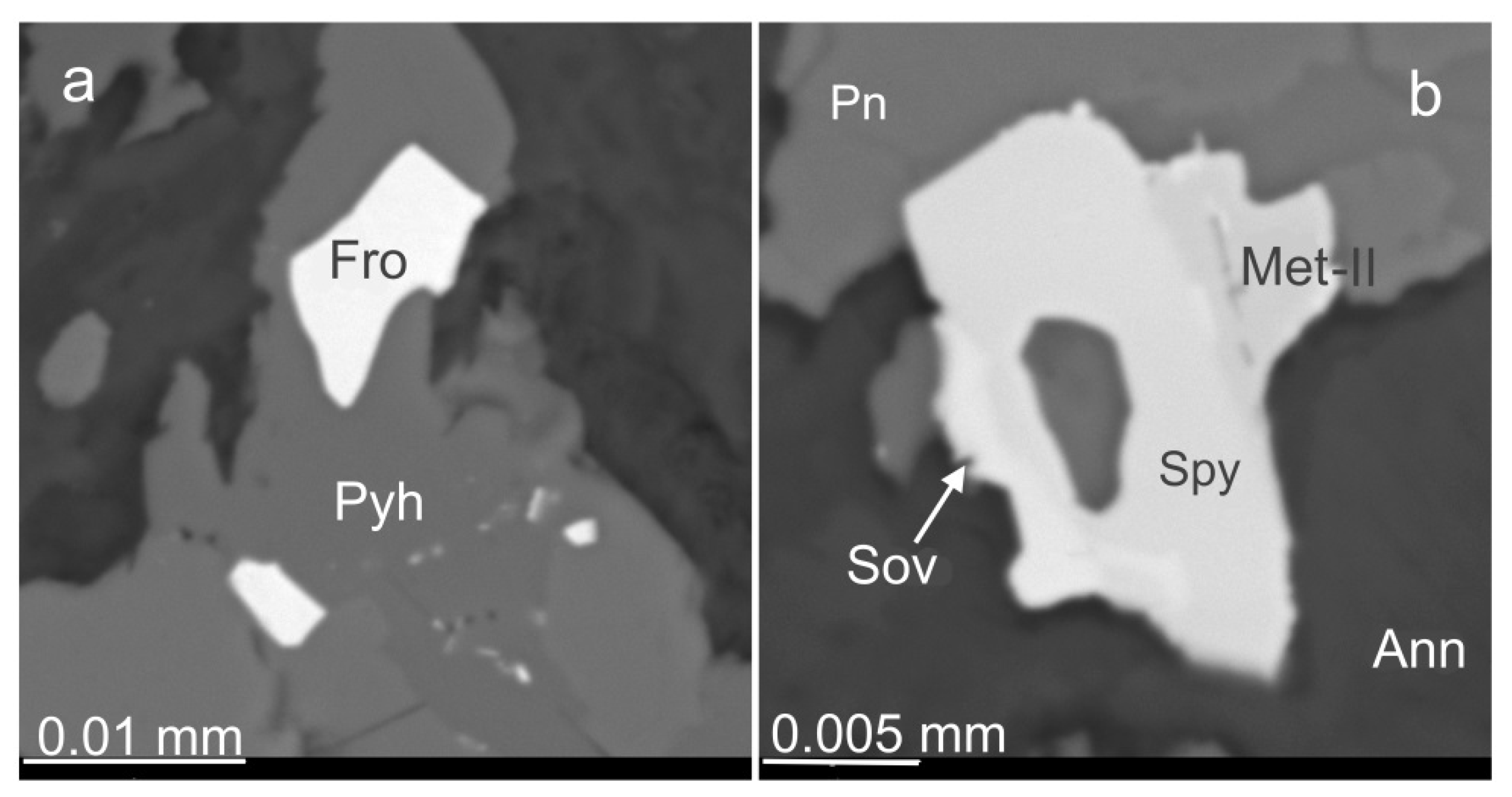
3.6. Base Metal Sulfides and Nickeline
3.7. Platinum Group Minerals
4. Discussion
4.1. Evidence for Extensive Differentiation in the Ore-Bearing Sequences
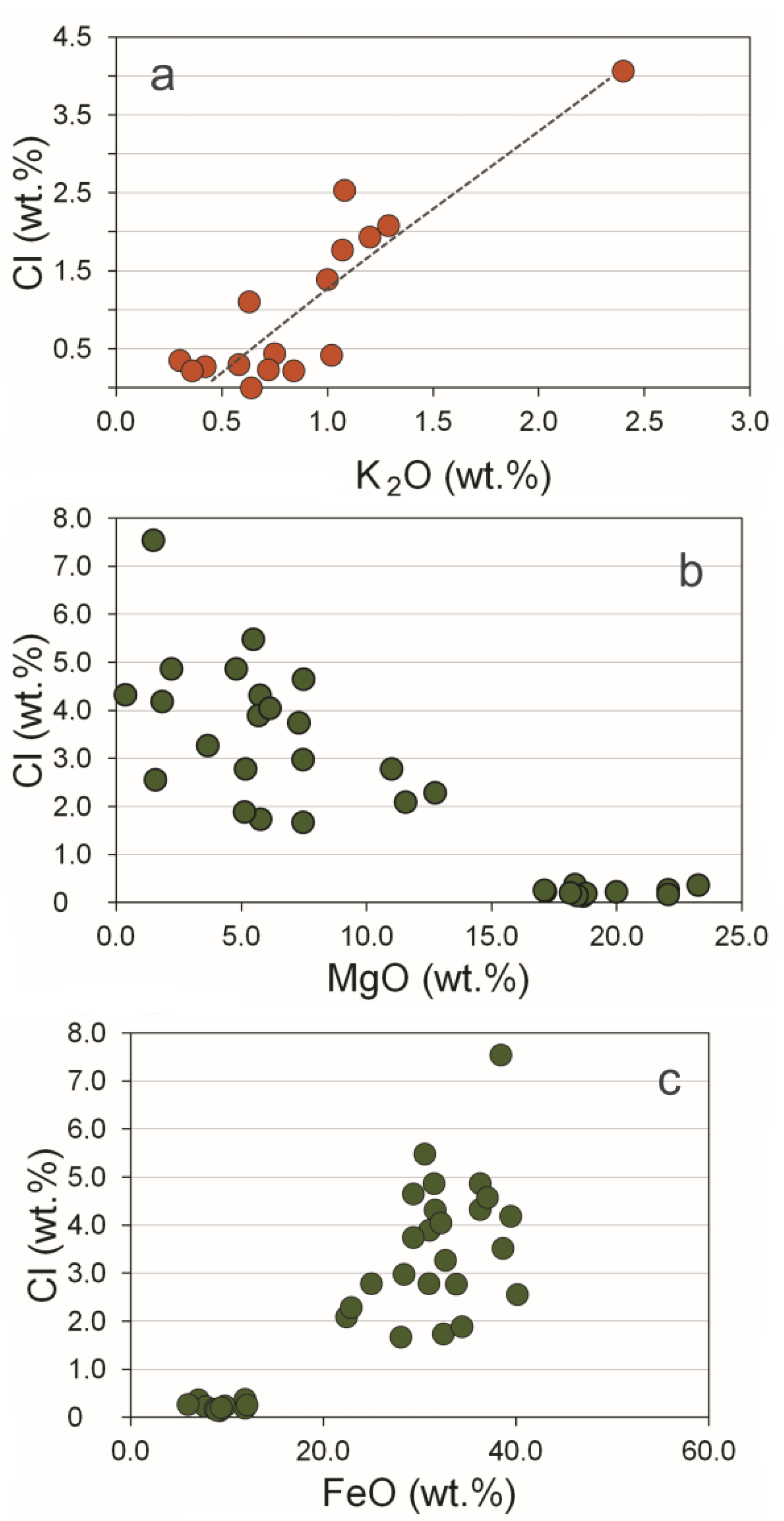
4.2. Evidence for Cl-Enrichment in Ore-Forming Fluids
4.3. Characteristic Features of Pd-Pt Mineralization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikulin, I.I. Geology of the Norilsk Metallogenic Province; The Public Joint Stock Company “Mining and Metallurgical Company “NORILSK NICKEL”; OOO Maks Press: Moscow, Russia, 2020; p. 524. (In Russian) [Google Scholar]
- Genkin, A.D.; Distler, V.V.; Gladyshev, D.G.; Filimonova, A.A.; Evstigneeva, T.L.; Kovalenker, V.A.; Laputina, I.P.; Smirnov, A.V.; Grokhovskaya, T.L. Suphide Copper-Nickel Ores of the Noril’sk Deposits; Publishing House Nauka: Moscow, Russia, 1981; p. 238. (In Russian) [Google Scholar]
- Campbell, I.H.; Czamanske, G.K.; Fedorenko, V.A.; Hill, R.I.; Stepanov, V. Synchronism of the Siberian traps and the Permian–Triassic boundary. Science 1992, 258, 1760–1763. [Google Scholar] [CrossRef]
- Naldrett, A.J. A model for the Ni-Cu-PGE ores of the Noril’sk region and its application to other areas of flood basalt. Econ. Geol. 1992, 87, 1945–1962. [Google Scholar] [CrossRef]
- Naldrett, A.J. World-class Ni-Cu-PGE deposits: Key factors in their genesis. Mineral. Depos. 1999, 34, 227–240. [Google Scholar] [CrossRef]
- Sobolev, A.V.; Krivolutskaya, N.A.; Kuzmin, D.V. Petrology of the parental melts and mantle sources of Siberian trap magmatism. Petrology 2009, 17, 253–286. [Google Scholar] [CrossRef] [Green Version]
- Cabri, L.J.; Wilson, J.M.D.; Distler, V.V.; Kingston, D.; Nejedlý, Z.; Sluzhenikin, S.F. Mineralogical distribution of trace platinum group elements in the disseminated sulphide ores of Noril’sk 1 layered intrusion. Appl. Earth Sci. 2002, 111, 15–22. [Google Scholar] [CrossRef]
- Arndt, N.T.; Czamanske, G.K.; Walker, R.J.; Chauvel, C.; Fedorenko, V.A. Geochemistry and origin of the intrusive hosts of the Noril’sk-Talnakh Cu-Ni-PGE sulfide deposits. Econ. Geol. 2003, 98, 495–515. [Google Scholar] [CrossRef]
- Sluzhenikin, S.F.; Mokhov, A.V. Gold and silver in PGE-Cu-Ni and PGE ores of the Noril’sk deposits, Russia. Mineral. Depos. 2015, 50, 465–492. [Google Scholar] [CrossRef]
- Tolstykh, N.D.; Zhitova, L.M.; Shapovalova, M.O.; Chayka, I.F. The evolution of the ore-forming system in the low sulfide horizon of the Noril’sk 1 intrsion, Russia. Mineral. Mag. 2019, 83, 673–694. [Google Scholar] [CrossRef]
- Morimoto, N. Nomenclature of pyroxenes. Can. Mineral. 1989, 27, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Barnes, S.J.; Kunilov, V.Y. Spinels and Mg ilmenites from the Noril’sk 1 and Talnakh intrusions and other mafic rocks of the Siberian Flood Basalt Province. Econ. Geol. 2000, 95, 1701–1717. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Zubkova, N.V.; Pekov, I.V.; Vigasina, M.F.; Polekhovsky, Y.S.; Ternes, B.; Schüller, W.; Britvin, S.N.; Pushcharovsky, D.Y. Stefanweissite, (Ca,REE)2Zr2(Nb,Ti)(Ti,Nb)2Fe2+O14, a new zirconolite-related mineral from the Eifel paleovolcanic region, Germany. Mineral. Mag. 2019, 83, 607–614. [Google Scholar] [CrossRef]
- Chukanov, N.; Zubkova, N.; Britvin, S.; Pekov, I.; Vigasina, M.; Schäfer, C.; Ternes, B.; Schüller, W.; Polekhovsky, Y.; Ermolaeva, V.; et al. Nöggerathite-(Ce), (Ce,Ca)2Zr2(Nb,Ti)(Ti,Nb)2Fe2+O14, a new zirconolite-related mineral from the Eifel volcanic region, Germany. Minerals 2018, 8, 449. [Google Scholar] [CrossRef] [Green Version]
- Chukanov, N.V.; Krivovichev, S.V.; Pakhomova, A.S.; Pekov, I.V.; Schäfer, C.; Vigasina, M.F.; Van, K.V. Laachite, (Ca,Mn)2Zr2Nb2TiFeO14, a new zirconolite-related mineral from the Eifel volcanic region, Germany. Eur. J. Mineral. 2014, 26, 103–111. [Google Scholar] [CrossRef]
- Hall, S.R.; Stewart, J.M. The crystal structure of argentian pentlandite (Fe,Ni)8AgS8, compared with the refined structure of pentlandite (Fe,Ni)9S8. Can. Mineral. 1973, 12, 169–177. [Google Scholar]
- Groves, D.I.; Hall, S.R. Argentian pentlandite with parkerite, joseite A and the probable Bi-analogue of ullmannite from Mount Windarra, Western Australia. Can. Mineral. 1978, 16, 1–7. [Google Scholar]
- Morales-Ruano, S.; Hach-Alí, P.F. Hydrothermal argentopentlandite at El Charcón, southeastern Spain: Mineral chemistry and conditions of formation. Can. Mineral. 1996, 34, 939–947. [Google Scholar]
- Bud’ko, I.A.; Kulagov, E.A. The new mineral talnakhite, a cubic variety of chalcopyrite. Zap. Vses. Mineral. Obshch 1968, 97, 63. (In Russian) [Google Scholar]
- Cabri, L.J.; Hall, S. Mooihoekite and haycockite, two new copper-iron sulfides, and their relationship to chalcopyrite and talnakhite. Am. Mineral. 1972, 57, 5–6. [Google Scholar]
- Rowland, J.F.; Hall, S.R. Haycockite, Cu4Fe5S8: A superstructure in the chalcopyrite series. Acta Crystallogr. B 1975, 31, 2105–2112. [Google Scholar] [CrossRef]
- Cabri, L.J.; Harris, D.C. New compositional data for talnakhite, Cu18(Fe,Ni)16S32. Econ. Geol. 1971, 66, 673–675. [Google Scholar] [CrossRef]
- Kravchenko, T.A.; Nenasheva, S.N. An experimental investigation of phase co^#mposition in areas of crystallization of the chalcopyrite solid solution. Vestn. Otd. Nauk Zemle RAN 2012, 4, NZ9001. [Google Scholar] [CrossRef]
- Makovicky, E. Crystal structures of sulfides and other chalcogenides. Rev. Mineral. Geochem. 2006, 61, 7–125. [Google Scholar] [CrossRef]
- Brese, N.E.; von Schnering, H.G. Bonding trends in pyrites and a reinvestigation of the structures of PdAs2, PdSb2, PtSb2 and PtBi2. Z. Anorg. Allg. Chem. 1994, 620, 393–404. [Google Scholar] [CrossRef]
- Genkin, A.D.; Evstigneeva, T.L. Associations of platinum group minerals of the Noril’sk copper-nickel sulfide ores. Econ. Geol. 1986, 81, 1203–1212. [Google Scholar] [CrossRef]
- McDonald, A.M.; Ames, D.E.; Kjarsgaard, I.M.; Cabri, L.J.; Zhe, W.; Ross, K.C.; Good, D.J. Marathonite, Pd25Ge9, and palladogermanide, Pd2Ge, two new platinum group minerals from the Marathon deposit, Coldwell Complex, Ontario, Canada: Descriptions, crystal chemical considerations and genetic implications. Can. Mineral. 2021, 59. in press. [Google Scholar]
- Ames, D.E.; Kjarsgaard, I.M.; McDonald, A.M.; Good, D.J. Insights into the extreme PGE enrichment of the W horizon, Marathon Cu-Pd deposit, Coldwell alkaline complex, Canada: Platinum group mineralogy, compositions and genetic implications. Ore Geol. Rev. 2017, 90, 723–747. [Google Scholar] [CrossRef]
- Cawthorn, R.G.; Ashwal, L.D. Origin of anorthosite and magnetitite layers in the Bushveld Complex, constrained by major element compositions of plagioclase. J. Petrol. 2009, 50, 1607–1637. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Sharkov, E.V.; Nikiforov, A.A.; Korolyuk, V.N.; Silyanov, S.A.; Lobastov, B.M. Compositional variations of apatite and REE-bearing minerals in relation to crystallization trends in the Monchepluton layered complex (Kola Peninsula). Russ. Geol. Geophys. 2021, 62, 427–444. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Nikiforov, A.A. Compositional variations of apatite, fractionation trends, and a PGE-bearing zone in the Kivakka layered intrusion, northern Karelia, Russia. Can. Mineral. 2016, 54, 475–490. [Google Scholar] [CrossRef]
- Jamieson, H.E.; Roeder, P.L. The distribution of Mg and Fe2+ between olivine and spinel at 1300 °C. Am. Mineral. 1984, 69, 283–291. [Google Scholar]
- Barkov, A.Y.; Nikiforov, A.A.; Barkova, L.P.; Korolyuk, V.N.; Martin, R.F. Zones of PGE-chromite mineralization in relation to crystallization of the Pados-Tundra ultramafic complex, Serpentinite Belt, Kola Peninsula, Russia. Minerals 2021, 11, 68. [Google Scholar] [CrossRef]
- Harris, D.C.; Nickel, E.H. Pentlandite compositions and associations in some mineral deposits. Can. Mineral. 1972, 11, 861–878. [Google Scholar]
- Harney, D.M.W.; Merkle, R.K.W. Sulfide mineralogy at the main magnetite layer, Upper Zone, eastern Bushveld Complex, and the effect of hydrothermal processes on pentlandite composition. Eur. J. Mineral. 1992, 4, 557–570. [Google Scholar] [CrossRef]
- Kolonin, G.R.; Orsoev, D.A.; Sinyakova, E.F.; Kislov, E.V. The use of Ni: Fe ratio in pentlandite for estimation of sulfur fugacity during the formation of PGE-bearing sulfide mineralization of Yoko-Dovyren Massif. Dokl. Earth Sci. 2000, 370, 75–79. (In Russian) [Google Scholar]
- Tolstykh, N.; Shvedov, G.; Polonyankin, A.; Korolyuk, V. Geochemical features and mineral associations of differentiated rocks of the Norilsk 1 intrusion. Minerals 2020, 10, 688. [Google Scholar] [CrossRef]
- Kosyakov, V.I.; Sinyakova, E.F. Study of crystallization of nonstoichiometric isocubanite Cu1.1Fe2.0S3.0 from melt in the system Cu–Fe–S. J. Therm. Anal. Calorim. 2017, 129, 623–628. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Shvedov, G.; Silyanov, S.; Martin, R.F. Mineralogy of platinum group elements and gold in the ophiolite-related placer of the River Bolshoy Khailyk, western Sayans, Russia. Minerals 2018, 8, 247. [Google Scholar] [CrossRef] [Green Version]
- Oberti, R.; Ungaretti, L.; Cannillo, E.; Hawthorne, F.C. The mechanism of Cl incorporation in amphibole. Am. Mineral. 1993, 78, 746–752. [Google Scholar]
- Barkov, A.Y.; Martin, R.F.; Tarkian, M.; Poirier, G.; Thibault, Y. Pd–Ag tellurides from a Cl-rich environment in the Lukkulaisvaara layered intrusion, northern Russian Karelia. Can. Mineral. 2001, 39, 639–653. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Martin, R.F.; Laajoki, K.V.O.; Alapieti, T.T.; Iljina, M.J. Paragenesis and origin of staurolite from a palladium-rich gabbronorite: An unusual occurrence from the Lukkulaisvaara layered intrusion, Russian Karelia. Neues Jahrb. Mineral. Abh. 1999, 175, 191–222. [Google Scholar] [CrossRef]
- Vymazalová, A.; Grokhovskaya, T.L.; Laufek, F.; Rassulov, V.A. Lukkulaisvaaraite, Pd14Ag2Te9, a new mineral from Lukkulaisvaara intrusion, northern Russian Karelia, Russia. Min. Mag. 2014, 78, 1743–1754. [Google Scholar] [CrossRef]
- Duesterhoeft, E.; Raase, P.; Gremler, P. A new occurrence of extremely rare ferro-chloro-pargasite in Tudor, Ontario. Int. J. Earth Sci. 2017, 106, 2815–2816. [Google Scholar] [CrossRef]
- Belkin, H.E.; Grosz, A.E. Platinum and gold placer from Tugidak Island, Alaska: Platinum group minerals and their inclusions, gold, and chromite mineralogy. Can. Mineral. 2021, 59, 667–712. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Nikiforov, A.A. A new criterion of searching for zones of PGE mineralization of the Kivakka Reef type. Vestnik VGU Geol 2015, 4, 75–83. (In Russian) [Google Scholar]
- Meurer, W.P.; Hellström, F.A.; Claeson, D.T. The relationship between chlorapatite and PGE-rich cumulates in layered intrusions: The Kläppsjö gabbro, north-central Sweden, as a case study. Can. Mineral. 2004, 42, 279–289. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Martin, R.F.; Kaukonen, R.J.; Alapieti, T.T. The occurrence of Pb-Cl-(OH) and Pt-Sn-S compounds in the Merensky Reef, Bushveld layered complex, South Africa. Can. Mineral. 2001, 39, 1397–1403. [Google Scholar] [CrossRef] [Green Version]
- Vymazalová, A.; Zaccarini, F.; Garuti, G.; Laufek, F.; Mauro, D.; Stanley, C.J.; Biagioni, C. Bowlesite, PtSnS, a new platinum group mineral (PGM) from the Merensky Reef of the Bushveld Complex, South Africa. Mineral. Mag. 2020, 84, 468–476. [Google Scholar] [CrossRef]
- Boudreau, A.E.; McCallum, I.S. Investigations of the Stillwater Complex. V. Apatites as indicators of evolving fluid composition. Contrib. Mineral. Petrol. 1989, 102, 138–153. [Google Scholar] [CrossRef]
- Boudreau, A.E.; Mathez, E.A.; McCallum, I.S. Halogen geochemistry of the Stillwater and Bushveld complexes: Evidence for transport of the platinum group elements by Cl-rich fluids. J. Petrol. 1986, 27, 967–986. [Google Scholar] [CrossRef]
- Cawthorn, R.G. Formation of chlor- and fluorapatite in layered intrusions. Mineral. Mag. 1994, 58, 299–306. [Google Scholar] [CrossRef]
- Karpenkov, A.M.; Rudashevsky, N.S.; Shumskaya, N.I. Natural palladium bismuth chloride phase of Pd4Bi5Cl3 composition. Zap. Vses. Mineral. Obshch. 1981, 110, 86–91. (In Russian) [Google Scholar] [CrossRef]
- Estigneeva, T.L.; Genkin, A.D. Cabriite, Pd2SnCu, a new mineral species in the mineral group of palladium, tin and copper compounds. Can. Mineral. 1983, 21, 481–487. [Google Scholar]
- Barkov, A.Y.; Martin, R.F.; Poirier, G.; Yakovlev, Y.N. The taimyrite-tatyanaite series and zoning in intermetallic compounds of Pt, Pd, Cu, and Sn from Noril’sk, Siberia, Russia. Can. Mineral. 2000, 38, 599–609. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Martin, R.F.; Poirier, G.; Tarkian, M.; Pakhomovskii, Y.A.; Men’shikov, Y.P. Tatyanaite, a new platinum group mineral, the Pt analogue of taimyrite, from the Noril’sk complex (northern Siberia, Russia). Eur. J. Mineral. 2000, 12, 391–396. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Martin, R.F. Compositional variations in natural intermetallic compounds of Pd, Pt, Cu, and Sn: New data and implications. Can. Mineral. 2016, 54, 453–460. [Google Scholar] [CrossRef]
- Spiridonov, E.M.; Orsoev, D.A.; Ariskin, A.A.; Kislov, E.V.; Korotaeva, N.N.; Nikolaev, G.S.; Yapaskurt, V.O. Germanium-rich palladium minerals of palladogermanide Pd2Ge, paolovite Pd2(Sn, Ge), and zvyagintsevite in sulfide-bearing anorthosites of the Yoko-Dovyren pluton, Baikal area. Geochem. Int. 2019, 57, 600–603. [Google Scholar] [CrossRef]
- Augé, T.; Legendre, O. Platinum group element oxides from the Pirogues ophiolitic mineralization, New Caledonia; origin and significance. Econ. Geol. 1994, 89, 1454–1468. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Halkoaho, T.A.A.; Roberts, A.C.; Criddle, A.J.; Martin, R.F.; Papunen, H. New Pd-Pb and Pb-V oxides from a bonanza-type PGE-rich, nearly BMS-free deposit in the Penikat layered complex, Finland. Can. Mineral. 1999, 37, 1507–1524. [Google Scholar]
- Barkov, A.Y.; Fleet, M.E.; Martin, R.F.; Halkoaho, T.A.A. New data on “bonanza”-type PGE mineralization in the Kirakkajuppura PGE deposit, Penikat layered complex, Finland. Can. Mineral. 2005, 43, 1663–1686. [Google Scholar] [CrossRef]
- Tolstykh, N.D.; Krivenko, A.P.; Lavrent’ev, Y.G.; Tolstykh, O.N.; Korolyuk, V.N. Oxides of the Pd–Sb–Bi system from the Chiney massif (Aldan Shield, Russia). Eur. J. Mineral. 2000, 12, 431–440. [Google Scholar] [CrossRef]
- Shcheka, G.G.; Lehmann, B.; Solianik, A.N. Pd-bearing oxides and hydrated oxides in mertieite-II crystals from alluvial sediments of the Darya river, Aldan Shield, Russia. Mineral. Mag. 2005, 69, 981–994. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Shvedov, G.I.; Polonyankin, A.A.; Martin, R.F. New and unusual Pd–Tl-bearing mineralization in the Anomal’nyi deposit, Kondyor concentrically zoned complex, northern Khabarovskiy kray, Russia. Mineral. Mag. 2017, 81, 679–688. [Google Scholar] [CrossRef]
- Hattori, K.H.; Takahashi, Y.; Augé, T. Mineralogy and origin of oxygen-bearing platinum–iron grains based on an X-ray absorption spectroscopy study. Am. Mineral. 2010, 95, 622–630. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.S.; Chao, G.Y.; Cabri, L.J. Phase relations in the Pd–Te system. J. Less Common Met. 1990, 162, 61–74. [Google Scholar] [CrossRef]
- Hoffman, E.L.; MacLean, W.H. Phase relations of michenerite and merenskyite in the Pd–Bi–Te system. Econ. Geol. 1976, 71, 1461–1468. [Google Scholar] [CrossRef]
- Kristavchuk, A.V.; Zabolotskaya, A.V.; Voronin, M.V.; Chareev, D.A.; Osadchii, E.G. Temperature dependence of tellurium fugacity for the kotulskite (PdTe)—merenskyite (PdTe2) equilibrium determined by the method of a solid-state galvanic cell. Phys. Chem. Miner. 2021, 48, 16. [Google Scholar] [CrossRef]
- Vymazalová, A.; Laufek, F.; Kristavchuk, A.V.; Chareev, D.A.; Drábek, M. The system Ag–Pd–Te: Phase relations and mineral assemblages. Mineral. Mag. 2015, 79, 1813–1832. [Google Scholar] [CrossRef]
- Vymazalová, A.; Drábek, M. The system Pd–Sn–Te at 400 °C and mineralogical implications. I. The binary phases. Can. Mineral. 2010, 48, 1041–1050. [Google Scholar] [CrossRef]
- Bai, L.; Barnes, S.-J.; Baker, D.R. Sperrylite saturation in magmatic sulfide melts: Implications for formation of PGE-bearing arsenides and sulfarsenides. Am. Mineral. 2017, 102, 966–974. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Fleet, M.E. An unusual association of hydrothermal platinum group minerals from the Imandra layered complex, Kola Peninsula, northwestern Russia. Can. Mineral. 2004, 42, 455–467. [Google Scholar] [CrossRef]
- Magyarosi, Z.; Watkinson, D.H.; Jones, P.C. Mineralogy of Ni-Cu-platinum group element sulfide ore in the 800 and 810 orebodies, Copper Cliff South mine, and P-T-X conditions during the formation of platinum group minerals. Econ. Geol. 2002, 97, 147–1486. [Google Scholar] [CrossRef]






| # | Sample | SiO2 | TiO2 | Al2O3 | FeO | MnO | MgO | CaO | Total (wt.%) | Mg# | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0040-1706.4 | Ol | 39.00 | 0 | 0 | 21.91 | 0.48 | 38.80 | 0.08 | 100.27 | 75.5 |
| 2 | EF0040-1706.4 | 38.68 | 0 | 0 | 22.33 | 0.35 | 39.10 | 0.20 | 100.66 | 75.4 | |
| 3 | EF0043-1748.4 | 39.62 | 0 | 0 | 23.43 | 0.39 | 38.14 | 0.15 | 101.73 | 74.1 | |
| 4 | EF0065-1714.8 | 39.21 | 0 | 0 | 21.15 | 0.49 | 39.62 | 0 | 100.47 | 76.5 | |
| 5 | EF0066-1684.4 | 39.92 | 0 | 0 | 20.88 | 0.45 | 39.90 | 0 | 101.15 | 76.9 | |
| 6 | EF0066-1684.4 | 40.20 | 0 | 0 | 18.73 | 0.36 | 42.32 | 0 | 101.61 | 79.8 | |
| 7 | EF0069-1743.6 | 39.09 | 0 | 0 | 21.68 | 0 | 38.89 | 0 | 99.66 | 76.2 | |
| 8 | EF8057-1572.3 | 39.22 | 0 | 0 | 19.96 | 0.35 | 40.49 | 0 | 100.01 | 78.0 | |
| 9 | EF0040-1706.4 | Opx | 54.60 | 0 | 0 | 18.51 | 0 | 26.86 | 1.51 | 101.48 | 75.6 |
| 10 | EF0040-1706.4 | 55.45 | 0 | 0.36 | 15.80 | 0.37 | 27.08 | 0.76 | 99.82 | 73.9 | |
| 11 | EF0042-1722.3 | 52.84 | 0 | 0 | 17.32 | 0.56 | 25.77 | 1.43 | 97.92 | 74.9 | |
| 12 | EF0042-1722.3 | 56.05 | 0.68 | 0.96 | 14.04 | 0 | 27.03 | 2.18 | 100.94 | 74.3 | |
| 13 | EF0043-1764.3 | 52.52 | 0.68 | 0.77 | 17.69 | 0.48 | 24.13 | 1.82 | 98.09 | 70.4 | |
| 14 | EF0062-1597.4 | 54.64 | 0.37 | 0 | 15.48 | 0 | 27.73 | 0.57 | 98.79 | 76.1 | |
| 15 | EF0063-1744.3 | 53.36 | 0 | 0 | 21.09 | 0.87 | 22.77 | 1.25 | 99.34 | 65.3 | |
| 16 | EF0065-1714.8 | 56.97 | 0 | 0 | 13.69 | 0 | 28.34 | 1.75 | 100.75 | 76.7 | |
| 17 | EF0065-1714.8 | 54.75 | 0 | 0 | 16.22 | 0 | 26.73 | 1.44 | 99.14 | 74.9 | |
| 18 | EF0065-1714.8 | 57.01 | 0 | 0 | 14.87 | 0 | 28.80 | 1.22 | 101.90 | 77.1 | |
| 19 | EF67-1707.8 | 54.83 | 0 | 1.10 | 16.79 | 0 | 26.12 | 0.64 | 99.48 | 71.9 | |
| 20 | EF67-1707.8 | 56.29 | 0 | 0 | 16.31 | 0.39 | 26.81 | 1.36 | 101.16 | 72.9 | |
| 21 | EF0069-1743.6 | 57.33 | 0.45 | 0 | 13.83 | 0.52 | 29.43 | 1.11 | 102.67 | 77.7 | |
| 22 | EF0035-1553.75 | Cpx | 51.19 | 0 | 0 | 23.32 | 1.32 | 2.98 | 23.93 | 102.74 | 17.4 |
| 23 | EF0040-1706.4 | 55.49 | 0 | 0 | 7.19 | 0 | 16.90 | 21.91 | 101.49 | 78.7 | |
| 24 | EF0043-1764.3 | 51.84 | 0 | 0.77 | 13.88 | 0 | 12.60 | 19.97 | 99.06 | 61.7 | |
| 25 | EF0061-1603.3 | 48.41 | 0 | 0 | 26.45 | 0.71 | 4.89 | 18.54 | 99.00 | 25.5 | |
| 26 | EF0065-1713.3 | 55.54 | 0 | 0.81 | 5.87 | 0 | 16.37 | 23.28 | 101.87 | 79.8 | |
| 27 | EF0066-1684.4 | 52.38 | 0 | 0 | 12.42 | 0.85 | 10.05 | 24.34 | 100.03 | 56.9 | |
| 28 | EF67-1707.8 | 48.99 | 0 | 0 | 24.25 | 0.87 | 2.35 | 23.14 | 99.60 | 14.5 | |
| 29 | EF0069-1757.8 | 50.47 | 0 | 0 | 22.54 | 0.85 | 3.53 | 22.85 | 100.24 | 20.2 | |
| 30 | EF8057-1572.3 | 56.52 | 0 | 0.94 | 5.76 | 0 | 17.81 | 21.74 | 102.77 | 79.9 | |
| 31 | EF00566-1568.7 | 52.82 | 0 | 0 | 13.30 | 0.65 | 12.57 | 20.67 | 100.01 | 61.1 | |
| 32 | EF00566-1568.7 | 48.24 | 0 | 0.77 | 23.86 | 2.40 | 1.48 | 22.60 | 99.35 | 9.2 |
| # | Sample | SiO2 | Al2O3 | FeO | CaO | Na2O | K2O | Total (wt.%) | An (mol.%) | Ab | Or |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0035-1546.2 | 52.95 | 28.89 | 1.51 | 12.22 | 3.91 | 0.29 | 99.77 | 62.2 | 36.0 | 1.8 |
| 2 | EF0035-1553.75 | 69.25 | 20.09 | 0.57 | 1.12 | 9.81 | 0.61 | 101.45 | 5.7 | 90.6 | 3.7 |
| 3 | EF0035-1553.75 | 68.67 | 18.71 | 0 | 0.71 | 10.14 | 0 | 98.23 | 3.7 | 96.3 | 0.0 |
| 4 | EF0040-1706.4 | 62.70 | 22.56 | 0.49 | 4.39 | 7.59 | 0.95 | 98.68 | 22.8 | 71.3 | 5.9 |
| 5 | EF0040-1706.4 | 66.08 | 21.58 | 0.39 | 2.94 | 8.59 | 1.49 | 101.07 | 14.5 | 76.7 | 8.8 |
| 6 | EF0040-1706.4 | 53.23 | 29.59 | 0.81 | 12.61 | 4.23 | 0.31 | 100.78 | 61.1 | 37.1 | 1.8 |
| 7 | EF0043-1748.4 | 64.27 | 23.85 | 0.59 | 5.12 | 8.33 | 0.30 | 102.46 | 24.9 | 73.3 | 1.7 |
| 8 | EF0043-1748.4 | 60.93 | 25.32 | 0.67 | 7.18 | 7.00 | 0.22 | 101.32 | 35.7 | 63.0 | 1.3 |
| 9 | EF0043-1764.3 | 54.60 | 28.81 | 0.62 | 12.79 | 4.33 | 0.24 | 101.39 | 61.2 | 37.5 | 1.4 |
| 10 | EF0061-1603.3 | 66.98 | 22.28 | 0.32 | 3.11 | 8.83 | 1.23 | 102.75 | 15.1 | 77.7 | 7.1 |
| 11 | EF0061-1603.3 | 70.26 | 20.52 | 0 | 0.91 | 11.05 | 0 | 102.74 | 4.4 | 95.6 | 0.0 |
| 12 | EF0063-1744.3 | 57.04 | 27.13 | 0.85 | 9.47 | 5.88 | 0.25 | 100.62 | 46.4 | 52.1 | 1.5 |
| 13 | EF0065-1713.3 | 64.52 | 22.64 | 0.53 | 4.44 | 8.13 | 0.24 | 100.50 | 22.8 | 75.7 | 1.5 |
| 14 | EF0065-1714.8 | 52.31 | 30.91 | 0.60 | 13.73 | 3.56 | 0.25 | 101.36 | 67.1 | 31.5 | 1.5 |
| 15 | EF0065-1714.8 | 58.21 | 26.68 | 0.58 | 9.46 | 6.28 | 0.39 | 101.60 | 44.4 | 53.4 | 2.2 |
| 16 | EF0066-1684.4 | 48.95 | 32.14 | 0.42 | 16.19 | 2.45 | 0 | 100.15 | 78.5 | 21.5 | 0.0 |
| 17 | EF0066-1726.05 | 58.23 | 25.96 | 0.75 | 8.73 | 6.05 | 0.60 | 100.32 | 42.8 | 53.7 | 3.5 |
| 18 | EF67-1707.8 | 67.15 | 21.45 | 0.94 | 2.83 | 9.26 | 0.69 | 102.32 | 13.9 | 82.1 | 4.0 |
| 19 | EF67-1712.0 | 53.08 | 28.53 | 1.26 | 11.53 | 4.42 | 0.33 | 99.15 | 57.9 | 40.2 | 2.0 |
| 20 | EF67-1724.65 | 53.01 | 29.46 | 0.72 | 12.55 | 4.06 | 0.27 | 100.07 | 62.1 | 36.3 | 1.6 |
| 21 | EF67-1724.65 | 59.58 | 25.26 | 0.64 | 7.89 | 6.34 | 0.71 | 100.42 | 39.0 | 56.8 | 4.2 |
| 22 | EF0069-1743.6 | 61.83 | 23.81 | 0.51 | 5.74 | 7.70 | 0.42 | 100.01 | 28.5 | 69.1 | 2.5 |
| 23 | EF0069-1743.6 | 49.48 | 32.93 | 0 | 15.71 | 2.31 | 0 | 100.43 | 79.0 | 21.0 | 0.0 |
| 24 | EF0069-1743.6 | 51.26 | 32.46 | 0.54 | 15.81 | 2.71 | 0 | 102.78 | 76.3 | 23.7 | 0.0 |
| 25 | EF0062-1615.4 | 65.81 | 20.35 | 0.78 | 1.37 | 9.75 | 0 | 98.06 | 7.2 | 92.8 | 0.0 |
| 26 | EF0066-1717 | 67.75 | 19.54 | 0.54 | 0.78 | 10.43 | 0 | 99.04 | 4.0 | 96.0 | 0.0 |
| 27 | EF0066-1717 | 65.79 | 20.20 | 2.47 | 1.75 | 9.61 | 0.57 | 100.39 | 8.8 | 87.7 | 3.4 |
| # | Speciation | Mineral | Compositional Variety | Main or Common | Subordinate | Minor or Rare |
|---|---|---|---|---|---|---|
| 1 | Base metal sulfides | Pyrrhotite; Fe1 − xS | Ni-Cu-bearing Troilite; FeS | × | ||
| 2 | Chalcopyrite; CuFeS2 | × | ||||
| 3 | Pentlandite (Fe,Ni)9S8 | Co-bearing | × | |||
| 4 | Cubanite (and/or isocubanite); CuFe2S3 | × | ||||
| 5 | Bornite; Cu5FeS4 | × | ||||
| 6 | Pyrite; FeS2 | Co-bearing | × | |||
| 7 | Pb sulfide | Galena; PbS | Se-bearing | × | ||
| 8 | Ag-Fe-Ni sulfide | Argentopentlandite; Ag(Fe,Ni)8S8 | × | |||
| 9 | Zn sulfide | Sphalerite (and/or wurtzite); ZnS | Fe-Cd-bearing | × | ||
| 10 | Ag sulfide | Acanthite; Ag2S | × | |||
| 11 | Ag telluride | Hessite; Ag2Te | × | |||
| 12 | Pb telluride | Altaite; PbTe | Se-bearing | × | ||
| 13 | Pb selenide | Clausthalite; PbSe | S-bearing | × | ||
| 14 | Ni arsenide | Nickeline NiAs | × | |||
| 15 | Au-Ag alloys | Native silver | Au-bearing | × | ||
| 16 | Native gold | Ag-bearing | × | |||
| 17 | Fe-Cr-Ti oxides | Chromite; FeCr2O4 | Mg-(Ti)-bearing | × | ||
| 18 | Magnetite; Fe3O4 | Cr-(Ti)-bearing | × | |||
| 19 | Ilmenite; FeTiO3 | Mg-Mn-bearing | × | |||
| 20 | Zr silicate | Zircon; ZrSiO4 | Hf-bearing | × | ||
| 21 | Th silicate | Thorite; ThSiO4 | Solid solution with coffinite; U(SiO4)1 − x(OH)4x | × | ||
| 22 | Zr oxide | Baddeleyite; ZrO2 | Hf-bearing | × | ||
| 23 | Zr-Ti oxide | Zirconolite; (Ca,Y)ZrTi2O7 | Th-(Fe)-bearing | × | ||
| 24 | Th-U oxydes | Thorianite; ThO2 | × | |||
| 25 | Uraninite; UO2 | U(SiO4)1 − x(OH)4x | × | |||
| 26 | Ce-REE phosphate | Monazite-(Ce); (Ce,La,Nd,Th)PO4 | × | |||
| 27 | A zirconolite-type oxide (Ca-dominant) | Unnamed (Ca,Y,REE)2Zr2 Ti(Ti,Nb)2Fe2+O14 | × | |||
| 28 | A zirconolite-type oxide (Y-dominant) | Unnamed (Y,Ca,REE)2Zr2 Ti(Ti,Nb)2Fe2+O14 | × | |||
| 29 | La-dominant carbonate-fluoride | Bastnäsite(?) (La,Ce,Y)CO3F | × | |||
| 30 | Pd-(Ag)-Pt tellurides, bismuthotellurides and bismuthides | Kotulskite; PdTe | Bi-Sb-bearing | × | ||
| 31 | Sobolevskite; PdBi | Te-Sb-bearing | × | |||
| 32 | Merenskyite; PdTe2 | Pt-(Bi)-bearing | × | |||
| 33 | Moncheite; PtTe2 | Pd-(Bi)-bearing | × | |||
| 34 | Michenerite; PdBiTe | × | ||||
| 35 | Froodite; PdBi2 | × | ||||
| 36 | Sopcheite; Ag4Pd3Te4 | × | ||||
| 37 | Pd-Pt stannides | Paolovite; Pd2Sn | Pt-bearing | × | ||
| 38 | Atokite; Pd3Sn | × | ||||
| 39 | Rustenburgite; Pt3Sn | Pd-bearing | × | |||
| 40 | Niggliite; PtSn | |||||
| 41 | Pd antimonides | Mertieite-II; Pd8Sb3 | × | |||
| 42 | Naldrettite; Pd2Sb | × | ||||
| 43 | Pd plumbides | Zvyagintsevite; Pd3Pb | × | |||
| 44 | Plumbopalladinite; Pd3Pb2 | × | ||||
| 45 | Pt arsenide | Sperrylite; PtAs2 | Sb-bearing | × | ||
| 46 | Pd-Ni arsenide | Majakite; PdNiAs | × | |||
| 47 | Pd germanide-arsenide | Unnamed Pd11Ge3As2 | × | |||
| 48 | Pd stannoarsenide | Unnamed Pd6Sn2As | × | |||
| 49 | Pt-Pd stannide | Unnamed (Pt, Pd)2Sn | Pd-bearing | × | ||
| 50 | Pt-Cu arseno-oxysulfide | Unnamed PtCu2AsSO3 | × |
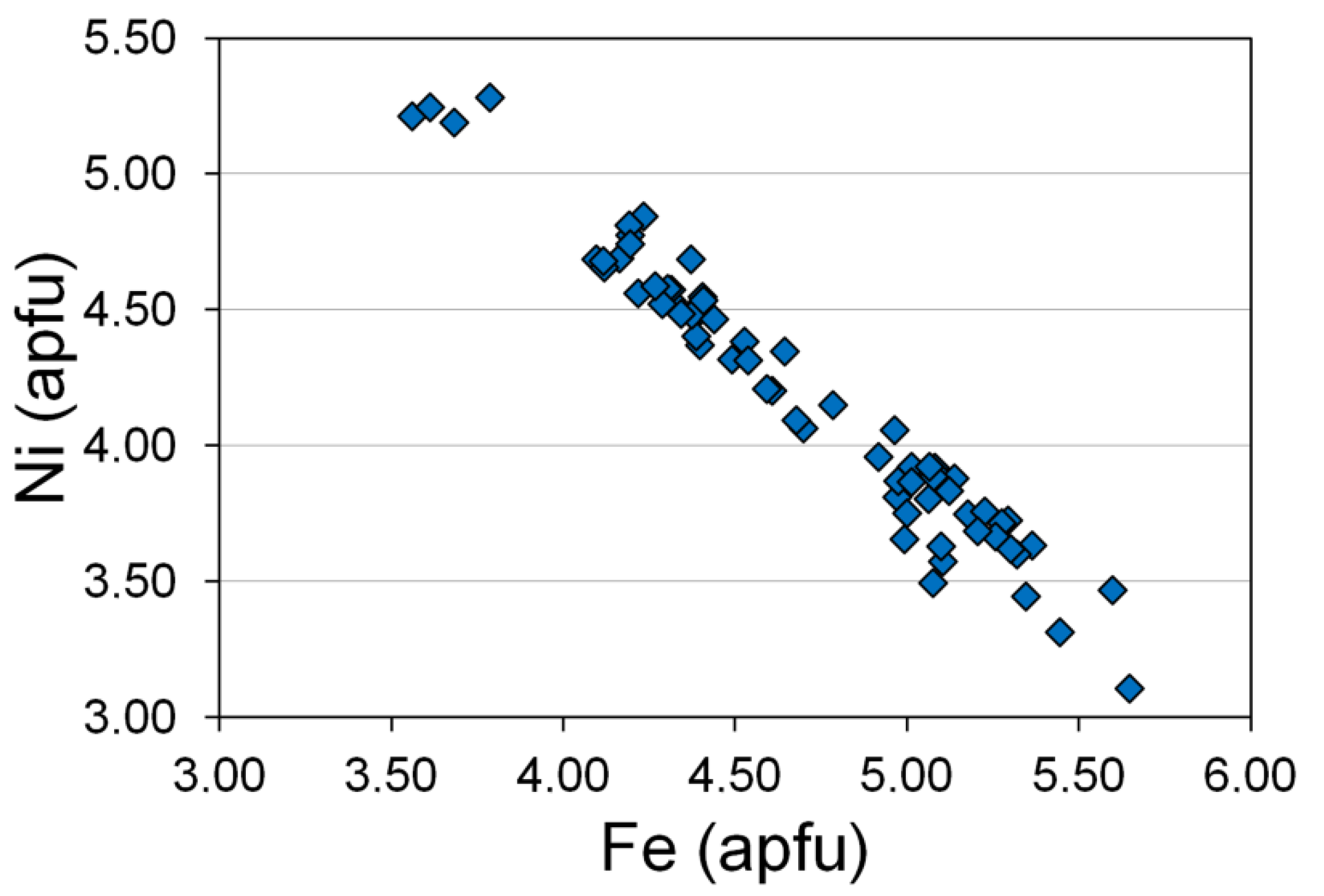
| # | Sample | TiO2 | Al2O3 | Cr2O3 | V2O3 | FeO Total | FeO Calc. | Fe2O3 Calc. | MnO | MgO | Total (wt.%) | Mg# | Cr# | Fe3+# | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0035-1534.1 | Chr | 3.24 | 2.15 | 13.43 | 0 | 71.19 | 28.91 | 46.98 | 0 | 3.32 | 98.04 | 17.0 | 80.7 | 59.4 |
| 2 | EF0040-1706.4 | 3.37 | 2.68 | 16.28 | 0.97 | 71.19 | 32.38 | 43.13 | 0 | 1.76 | 100.57 | 8.8 | 80.3 | 54.5 | |
| 3 | EF0062-1597.4 | 2.94 | 1.70 | 1.77 | 0 | 87.13 | 32.33 | 60.90 | 0 | 1.34 | 100.98 | 6.9 | 41.1 | 62.9 | |
| 4 | EF0065-1713.3 | 4.50 | 4.84 | 26.44 | 1.00 | 55.20 | 32.12 | 25.65 | 0 | 2.19 | 96.74 | 10.8 | 78.6 | 41.8 | |
| 5 | EF0065-1714.8 | 1.87 | 16.02 | 39.07 | 0.53 | 33.22 | 22.45 | 11.97 | 0 | 9.15 | 101.06 | 42.1 | 62.1 | 32.4 | |
| 6 | EF0065-1714.8 | 2.02 | 15.46 | 39.96 | 0.46 | 33.55 | 22.63 | 12.14 | 0 | 9.24 | 101.91 | 42.1 | 63.4 | 32.6 | |
| 7 | EF0065-1714.8 | 3.90 | 12.77 | 36.47 | 0.71 | 37.50 | 24.87 | 14.03 | 0.43 | 8.19 | 101.38 | 36.6 | 65.7 | 33.7 | |
| 8 | EF0066-1684.4 | 2.05 | 16.68 | 38.98 | 0 | 31.79 | 21.80 | 11.10 | 0 | 9.57 | 100.18 | 43.9 | 61.1 | 31.4 | |
| 9 | EF0066-1684.4 | 2.32 | 15.72 | 38.07 | 0.38 | 32.74 | 22.24 | 11.67 | 0 | 9.22 | 99.62 | 42.5 | 61.9 | 32.1 | |
| 10 | EF0066-1684.4 | 1.02 | 10.62 | 34.07 | 0.47 | 47.33 | 27.29 | 22.27 | 0.54 | 4.39 | 100.67 | 21.9 | 68.3 | 42.3 | |
| 11 | EF0069-1743.6 | 1.37 | 19.42 | 37.12 | 0.35 | 31.27 | 22.08 | 10.22 | 0 | 9.19 | 99.74 | 42.6 | 56.2 | 29.4 | |
| 12 | EF0069-1743.6 | 1.40 | 20.03 | 37.87 | 0.28 | 31.30 | 22.11 | 10.21 | 0 | 9.62 | 101.52 | 43.7 | 55.9 | 29.4 | |
| 13 | EF0069-1743.6 | 2.49 | 15.19 | 38.34 | 0.53 | 32.91 | 22.21 | 11.89 | 0 | 9.37 | 100.02 | 42.9 | 62.9 | 32.5 | |
| 14 | EF0062-1615.4 | 3.74 | 10.58 | 24.44 | 0.26 | 53.98 | 30.71 | 25.86 | 0 | 3.81 | 99.40 | 18.1 | 60.8 | 43.1 | |
| 15 | EF-0040-1706.4 | Ilm | 54.01 | 0 | 0 | 0 | 42.34 | 0 | 0 | 0.89 | 4.26 | 101.50 | 14.9 | - | - |
| 16 | EF0040-1706.4 | 47.64 | 0 | 0 | 0 | 49.25 | 0 | 0 | 0 | 2.11 | 99.00 | 7.1 | - | - | |
| 17 | EF0040-1706.4 | 48.07 | 0 | 0 | 0 | 47.00 | 0 | 0 | 0 | 3.66 | 98.73 | 12.2 | - | - | |
| 18 | EF0043-1764.3 | 51.23 | 0 | 0 | 1.24 | 43.52 | 0 | 0 | 0.65 | 2.5 | 99.14 | 9.2 | - | - | |
| 19 | EF0066-1726.05 | 52.44 | 0 | 0 | 0 | 46.70 | 0 | 0 | 1.10 | 0 | 100.24 | 0.0 | - | - | |
| 20 | EF67-1724.65 | 54.66 | 0 | 0 | 0 | 40.82 | 0 | 0 | 5.05 | 0 | 100.53 | 0.0 | - | - | |
| 21 | EF67-1724.65 | 54.20 | 0 | 0 | 0 | 42.21 | 0 | 0 | 4.52 | 0 | 100.93 | 0.0 | - | - | |
| 22 | EF8057-1572.3 | 54.83 | 0 | 1.15 | 0 | 40.58 | 0 | 0 | 0.71 | 5.42 | 102.69 | 19.0 | - | - | |
| 23 | EF0066-1717 | 53.08 | 0 | 0 | 0 | 46.73 | 0 | 0 | 0.62 | 0 | 100.43 | 0.0 | - | - |
| # | Group | Name | Sample | SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | Cl | O ≡ Cl | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Calcic | Ferro-actinolite | EF0035-1553.75 | 51.39 | 0 | 0.83 | 0 | 27.57 | 0.40 | 6.22 | 11.98 | 0 | 0 | 0 | 0.00 | 98.39 |
| 2 | Calcic | Ferro-actinolite | EF0035-1553.75 | 51.90 | 0 | 3.12 | 0 | 24.89 | 0 | 7.73 | 12.09 | 0 | 0.30 | 0.35 | 0.08 | 100.38 |
| 3 | Calcic | Magnesio-hornblende | EF0040-1706.4 | 47.04 | 0.77 | 8.77 | 0 | 10.00 | 0 | 17.00 | 11.82 | 2.20 | 0.42 | 0.27 | 0.06 | 98.29 |
| 4 | Calcic | Magnesio-hornblende | EF0040-1706.4 | 47.30 | 0.45 | 7.43 | 0 | 9.71 | 0 | 16.68 | 11.80 | 1.97 | 0.36 | 0.22 | 0.05 | 95.92 |
| 5 | Calcic chloro potassic | Hastingsite | EF0043-1764.3 | 38.72 | 0.73 | 9.03 | 0 | 28.69 | 0.43 | 2.69 | 10.72 | 0.90 | 2.40 | 4.06 | 0.92 | 98.37 |
| 6 | Calcic | Ferro-hornblende | EF0043-1764.3 | 44.93 | 0 | 7.10 | 0 | 27.85 | 1.42 | 2.64 | 10.34 | 0 | 0.75 | 0.44 | 0.10 | 95.47 |
| 7 | Calcic chlorian | Ferro-hornblende | EF0043-1764.3 | 44.35 | 0.57 | 6.97 | 0 | 25.83 | 0 | 6.22 | 10.03 | 0 | 1.08 | 2.53 | 0.57 | 97.58 |
| 8 | Calcic | Actinolite | EF0061-1603.3 | 54.17 | 0 | 0 | 16.48 | 0 | 13.71 | 13.17 | 0 | 0 | 0 | 0.00 | 97.53 | |
| 9 | Calcic | Ferro-hornblende | EF0061-1603.3 | 47.15 | 0 | 4.72 | 0 | 31.73 | 0.85 | 1.51 | 11.94 | 0 | 1.02 | 0.42 | 0.10 | 99.34 |
| 10 | Calcic | Magnesio-hastingsite | EF0062-1597.41 | 44.80 | 3.20 | 8.71 | 0 | 8.65 | 0 | 16.72 | 11.46 | 2.76 | 0.64 | 0 | 0.00 | 96.94 |
| 11 | Calcic | Edenite | EF0066-1684.4 | 47.73 | 2.27 | 9.33 | 1.36 | 6.97 | 0 | 17.31 | 11.36 | 3.68 | 0 | 0 | 0.00 | 100.01 |
| 12 | Sodic-calcic | Barroisite | EF0066-1684.4 | 45.95 | 1.80 | 9.50 | 1.84 | 6.79 | 0 | 16.85 | 8.70 | 4.00 | 0 | 0 | 0.00 | 95.43 |
| 13 | Calcic chlorian potassian | Ferro-hornblende | EF0066-1726.05 | 44.37 | 0 | 7.22 | 0 | 26.72 | 0.26 | 4.94 | 11.49 | 0.90 | 1.29 | 2.08 | 0.47 | 99.27 |
| 14 | Calcic chlorian | Ferro-hornblende | EF0066-1726.05 | 45.65 | 0.30 | 6.08 | 0 | 25.81 | 0 | 6.09 | 11.43 | 0.74 | 1.07 | 1.77 | 0.40 | 98.94 |
| 15 | Calcic chlorian | Ferro-hornblende | EF0066-1726.05 | 46.94 | 0 | 5.86 | 0 | 25.74 | 0.34 | 5.99 | 11.74 | 0.74 | 1.20 | 1.93 | 0.44 | 100.48 |
| 16 | Calcic | Magnesio-hornblende | EF0069-1743.6 | 47.90 | 2.39 | 8.86 | 0 | 7.94 | 0 | 17.68 | 11.57 | 2.62 | 0 | 0.23 | 0.05 | 99.19 |
| 17 | Calcic | Ferro-actinolite | EF0069-1757.8 | 50.06 | 0 | 2.63 | 0 | 29.64 | 0 | 4.05 | 12.33 | 0 | 0.45 | 0 | 0.00 | 99.16 |
| 18 | Calcic | Ferro-actinolite | EF0069-1757.8 | 52.54 | 0 | 1.13 | 0 | 29.76 | 0.41 | 4.76 | 12.27 | 0 | 0 | 0 | 0.00 | 100.87 |
| 19 | Na-Ca-Mg-Fe | Ferro-actinolite | EF0069-1757.8 | 47.07 | 0 | 3.44 | 0 | 29.71 | 0 | 7.20 | 8.48 | 0 | 0 | 0 | 0.00 | 95.90 |
| 20 | Calcic | Ferro-actinolite | EF0057-1590.5 | 47.52 | 0 | 0 | 0 | 33.41 | 0.87 | 0.91 | 11.43 | 0 | 0 | 0 | 0.00 | 94.14 |
| 21 | Calcic | Actinolite | EF0062-1615.4 | 53.85 | 0 | 0.72 | 0 | 17.35 | 0.67 | 11.72 | 13.85 | 0 | 0 | 0 | 0.00 | 98.16 |
| 22 | Calcic | Actinolite | EF0062-1615.4 | 54.62 | 0 | 0.81 | 0 | 18.86 | 0.44 | 11.72 | 12.65 | 0 | 0 | 0 | 0.00 | 99.10 |
| 23 | Calcic | Actinolite | EF0062-1615.4 | 51.92 | 0 | 2.85 | 0 | 17.79 | 0.49 | 13.75 | 11.57 | 0 | 0 | 0 | 0.00 | 98.37 |
| 24 | Calcic | Ferro-actinolite | EF0066-1717 | 48.24 | 0 | 2.95 | 0 | 30.07 | 1.45 | 1.09 | 14.57 | 0 | 0.58 | 0.30 | 0.07 | 99.25 |
| 25 | Calcic | Ferro-actinolite | EF0066-1717 | 50.23 | 0 | 1.62 | 0 | 32.32 | 0.61 | 2.12 | 11.64 | 0 | 0.36 | 0 | 0.00 | 98.90 |
| 26 | Calcic | Magnesio-hornblende | EF0066-1717 | 47.60 | 1.18 | 5.31 | 0 | 17.73 | 0.52 | 12.50 | 10.48 | 1.62 | 0.84 | 0.22 | 0.05 | 98.00 |
| 27 | Calcic chlorian | Ferro-hornblende | EF0066-1717 | 43.49 | 0.62 | 7.27 | 0 | 25.70 | 0.28 | 5.64 | 11.43 | 1.12 | 1.00 | 1.39 | 0.31 | 97.94 |
| 28 | Calcic | Ferro-actinolite | EF0066-1717 | 46.89 | 0 | 4.29 | 0 | 31.62 | 0.30 | 1.41 | 11.49 | 0 | 0.72 | 0.23 | 0.05 | 96.95 |
| 29 | Mg-Fe calcian | Ferro-gedrite | EF0066-1717 | 29.25 | 0 | 0.38 | 0 | 48.99 | 0.61 | 0 | 14.31 | 0 | 0 | 0 | 0.00 | 93.54 |
| 30 | Mg-Fe calcian | Ferro-gedrite | EF0066-1717 | 29.97 | 1.35 | 0 | 0 | 49.63 | 0.70 | 0 | 14.01 | 0 | 0 | 0 | 0.00 | 95.66 |
| 31 | Mg-Fe calcian | Ferro-gedrite | EF0066-1717 | 28.86 | 0 | 0.85 | 0 | 47.88 | 0.96 | 0 | 14.69 | 0 | 0 | 0 | 0.00 | 93.24 |
| 32 | Mg-Fe calcian | Ferro-gedrite | EF0066-1717 | 29.29 | 0 | 0 | 0 | 49.71 | 0.74 | 0 | 14.19 | 0 | 0 | 0 | 0.00 | 93.93 |
| 33 | Calcic | Ferro-actinolite | EF0056-1568.7 | 51.05 | 0 | 0.83 | 0 | 24.34 | 0.53 | 7.41 | 13.22 | 0 | 0 | 0 | 0.00 | 97.38 |
| 34 | Calcic | Ferro-hornblende | EF0056-1568.7 | 49.93 | 0 | 5.27 | 0 | 22.59 | 0.40 | 9.60 | 11.64 | 0 | 0.63 | 1.10 | 0.25 | 101.16 |
| 35 | Calcic | Ferro-actinolite | EF0056-1568.7 | 52.86 | 0 | 2.36 | 0 | 29.64 | 0.71 | 4.73 | 11.88 | 0 | 0 | 0 | 0.00 | 102.18 |
| # | Sample | SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO | MnO | MgO | CaO | NiO | Na2O | K2O | Cl | O≡Cl | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0035-1534.1 | 39.66 | 2.30 | 13.64 | 0 | 7.01 | 0 | 23.25 | 0 | 0 | 0.78 | 9.48 | 0.36 | 0.08 | 96.48 |
| 2 | EF0035-1546.2 | 37.22 | 1.83 | 12.11 | 0 | 22.42 | 0 | 11.56 | 0 | 0 | 0 | 11.03 | 2.09 | 0.47 | 98.26 |
| 3 | EF0040-1706.4 | 38.49 | 4.15 | 13.40 | 0 | 7.76 | 0 | 19.98 | 0 | 0 | 0 | 11.14 | 0.22 | 0.05 | 95.14 |
| 4 | EF0043-1748.4 | 38.96 | 6.52 | 12.22 | 0.50 | 9.24 | 0 | 18.66 | 0 | 0 | 0.98 | 9.77 | 0.14 | 0.03 | 96.99 |
| 5 | EF044-1801.05 | 33.07 | 0.73 | 11.45 | 0 | 31.49 | 0 | 4.79 | 0 | 0 | 0 | 10.67 | 4.86 | 1.10 | 97.06 |
| 6 | EF0061-1603.3 | 37.16 | 0.55 | 10.64 | 0 | 30.49 | 0 | 5.47 | 0 | 0 | 0 | 10.89 | 5.48 | 1.24 | 100.68 |
| 7 | EF0061-1603.3 | 36.78 | 0 | 10.64 | 0 | 31.57 | 0.27 | 5.74 | 0.60 | 0 | 0 | 9.07 | 4.31 | 0.97 | 98.98 |
| 8 | EF0062-1597.4 | 40.69 | 3.40 | 11.58 | 0 | 5.94 | 0 | 22.06 | 0 | 0 | 0.59 | 10.76 | 0.27 | 0.06 | 95.29 |
| 9 | EF0062-1597.4 | 40.41 | 3.25 | 12.43 | 0 | 8.75 | 0 | 22.06 | 0 | 0 | 0 | 10.94 | 0.17 | 0.04 | 98.01 |
| 10 | EF0063-1744.3 | 40.11 | 3.94 | 14.89 | 0 | 11.87 | 0 | 18.32 | 0 | 0 | 0 | 10.30 | 0.37 | 0.08 | 99.8 |
| 11 | EF0065-1714.8 | 35.56 | 3.09 | 11.92 | 0.51 | 9.76 | 0 | 17.15 | 0 | 0 | 1.15 | 9.48 | 0.23 | 0.05 | 88.85 |
| 12 | EF0065-1714.8 | 38.25 | 4.82 | 12.7 | 0.50 | 11.86 | 0 | 18.76 | 0 | 0 | 0 | 9.56 | 0.19 | 0.04 | 96.64 |
| 13 | EF0066-1726.05 | 34.53 | 0.58 | 12.11 | 0 | 31.02 | 0 | 5.69 | 0 | 0 | 0 | 10.96 | 3.89 | 0.88 | 98.78 |
| 14 | EF0066-1726.05 | 35.75 | 0 | 12.28 | 0 | 32.16 | 0 | 6.14 | 0 | 0 | 0 | 7.73 | 4.04 | 0.91 | 98.1 |
| 15 | EF67-1707.8 | 37.87 | 4.65 | 12.34 | 0 | 12.07 | 0 | 17.11 | 0 | 0.46 | 0 | 11.84 | 0.25 | 0.06 | 96.59 |
| 16 | EF67-1724.65 | 30.40 | 1.67 | 11.30 | 0 | 32.46 | 0.56 | 5.77 | 0 | 0 | 0 | 6.53 | 1.73 | 0.39 | 90.42 |
| 17 | EF67-1724.65 | 33.31 | 2.09 | 11.79 | 0 | 36.29 | 0 | 0.36 | 0 | 0 | 0 | 10.49 | 4.32 | 0.98 | 98.65 |
| 18 | EF67-1724.65 | 37.59 | 0 | 8.71 | 0 | 38.43 | 0.50 | 1.48 | 0 | 0 | 0 | 7.42 | 7.54 | 1.70 | 101.67 |
| 19 | EF67-1724.65 | 34.64 | 0 | 11.20 | 0 | 36.25 | 0.35 | 2.21 | 0 | 0 | 0 | 10.23 | 4.86 | 1.10 | 99.74 |
| 20 | EF67-1724.65 | 32.48 | 0 | 13.74 | 0 | 37.00 | 0.39 | 0 | 0 | 0.41 | 0 | 10.9 | 4.57 | 1.03 | 99.49 |
| 21 | EF67-1724.65 | 31.28 | 1.42 | 12.64 | 0 | 38.65 | 0.37 | 0 | 0 | 0 | 0 | 8.14 | 3.52 | 0.79 | 96.02 |
| 22 | EF67-1724.65 | 30.51 | 0.53 | 11.71 | 0 | 40.14 | 0.50 | 1.56 | 0 | 0.39 | 0 | 5.70 | 2.55 | 0.58 | 93.59 |
| 23 | EF67-1724.65 | 35.51 | 0.25 | 12.58 | 0 | 28.35 | 0.25 | 7.46 | 0 | 0.51 | 0 | 11.18 | 2.98 | 0.67 | 99.07 |
| 24 | EF67-1724.65 | 35.06 | 0 | 13.25 | 0 | 39.44 | 0.40 | 1.84 | 0.67 | 0 | 0 | 6.43 | 4.18 | 0.94 | 101.27 |
| 25 | EF0069-1743.6 | 38.66 | 7.06 | 13.15 | 0.56 | 8.95 | 0 | 18.41 | 0 | 0 | 1.00 | 11.00 | 0.15 | 0.03 | 98.94 |
| 26 | EF8057-1572.3 | 38.66 | 7.26 | 13.47 | 1.07 | 9.37 | 0 | 18.13 | 0 | 0 | 1.13 | 10.38 | 0.20 | 0.05 | 99.67 |
| 27 | EF0066-1717 | 38.81 | 0 | 11.79 | 0 | 30.95 | 0 | 5.17 | 0 | 0 | 0 | 9.72 | 2.78 | 0.63 | 99.22 |
| 28 | EF0066-1717 | 34.32 | 0.35 | 12.57 | 0 | 34.36 | 0.43 | 5.12 | 0 | 0 | 0 | 6.70 | 1.89 | 0.43 | 95.74 |
| 29 | EF0066-1717 | 38.44 | 0.37 | 12.57 | 0 | 28.02 | 0 | 7.46 | 0 | 0 | 0 | 10.02 | 1.67 | 0.38 | 98.55 |
| 30 | EF0066-1717 | 33.42 | 0 | 11.17 | 0 | 32.68 | 0 | 3.66 | 0 | 0 | 0 | 9.60 | 3.27 | 0.74 | 93.8 |
| 31 | EF0056-1568.7 | 33.42 | 0 | 16.48 | 0 | 33.81 | 0.53 | 0 | 0 | 0 | 0 | 10.83 | 2.77 | 0.63 | 97.84 |
| 32 | EF0056-1568.7 | 38.57 | 0.98 | 11.13 | 0 | 29.32 | 0 | 7.30 | 0 | 0 | 0 | 10.61 | 3.74 | 0.84 | 101.65 |
| 33 | EF0056-1568.7 | 39.60 | 0 | 12.32 | 0 | 25.00 | 0 | 11.01 | 0 | 0 | 0 | 9.87 | 2.78 | 0.63 | 100.58 |
| 34 | EF0056-1568.7 | 36.73 | 0 | 10.15 | 0 | 29.33 | 0 | 7.48 | 0 | 0 | 0 | 10.64 | 4.65 | 1.05 | 98.98 |
| 35 | EF0056-1568.7 | 43.19 | 0 | 9.54 | 0 | 22.86 | 0 | 12.74 | 0 | 0 | 0 | 8.34 | 2.28 | 0.51 | 98.95 |
| # | Sample | P2O5 | SiO2 | Ce2O3 | La2O3 | FeO | CaO | Na2O | F | Cl | O≡F | O≡Cl | Total (wt.%) | F (apfu) | Cl (apfu) | OH (calc.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0035-1534.1 | 42.94 | 0 | 0 | 0 | 0 | 54.89 | 0 | 0 | 0.69 | 0.00 | 0.16 | 98.62 | 0.00 | 0.19 | 1.81 |
| 2 | EF0035-1534.1 | 41.96 | 0.36 | 0.20 | 0 | 0 | 54.51 | 0 | 0.98 | 1.26 | 0.41 | 0.28 | 98.57 | 0.51 | 0.35 | 1.13 |
| 3 | EF0035-1534.1 | 41.66 | 0 | 0 | 0.26 | 0 | 53.48 | 0 | 0 | 6.97 | 0.00 | 1.58 | 100.53 | 0.00 | 1.95 | 0.05 |
| 4 | EF0035-1534.1 | 42.46 | 0 | 0 | 0 | 0 | 54.79 | 0 | 1.62 | 3.00 | 0.68 | 0.68 | 100.51 | 0.83 | 0.83 | 0.34 |
| 5 | EF0035-1534.1 | 42.37 | 0 | 0 | 0 | 0 | 54.81 | 0 | 1.31 | 3.76 | 0.55 | 0.85 | 100.85 | 0.67 | 1.04 | 0.29 |
| 6 | EF0035-1534.1 | 40.95 | 0 | 0 | 0 | 0 | 53.23 | 0 | 0 | 6.87 | 0.00 | 1.55 | 99.50 | 0.00 | 1.95 | 0.05 |
| 7 | EF0035-1534.1 | 41.68 | 0.34 | 0 | 0 | 0.73 | 54.53 | 0 | 0 | 1.99 | 0.00 | 0.45 | 98.82 | 0.00 | 0.56 | 1.44 |
| 8 | EF0035-1534.1 | 40.76 | 0 | 0.50 | 0 | 0.77 | 52.89 | 0 | 0 | 6.83 | 0.00 | 1.54 | 100.21 | 0.00 | 1.93 | 0.07 |
| 9 | EF0035-1534.1 | 42.14 | 0.79 | 0 | 0 | 0.44 | 55.06 | 0 | 1.37 | 1.13 | 0.58 | 0.26 | 100.10 | 0.71 | 0.31 | 0.98 |
| 10 | EF0035-1534.1 | 41.38 | 0 | 0.83 | 0 | 0 | 53.38 | 0 | 0 | 6.49 | 0.00 | 1.47 | 100.61 | 0.00 | 1.82 | 0.18 |
| 11 | EF0035-1534.1 | 40.83 | 0.60 | 0 | 0 | 0 | 53.13 | 0 | 0 | 5.84 | 0.00 | 1.32 | 99.08 | 0.00 | 1.66 | 0.34 |
| 12 | EF0035-1534.1 | 41.73 | 0.81 | 0 | 0 | 0.89 | 54.90 | 0 | 1.69 | 0.87 | 0.71 | 0.20 | 99.98 | 0.87 | 0.24 | 0.89 |
| 13 | EF0035-1534.1 | 42.28 | 0 | 0 | 0 | 54.48 | 0 | 0 | 6.55 | 0.00 | 1.48 | 101.83 | 0.00 | 1.81 | 0.19 | |
| 14 | EF0035-1553.75 | 42.00 | 0.51 | 0 | 0 | 0 | 54.46 | 0 | 2.92 | 1.45 | 1.23 | 0.33 | 99.78 | 1.50 | 0.40 | 0.10 |
| 15 | EF0035-1553.75 | 41.91 | 0.43 | 0 | 0 | 0.57 | 55.49 | 0 | 0.29 | 3.53 | 0.12 | 0.80 | 101.30 | 0.15 | 0.98 | 0.87 |
| 16 | EF0035-1553.75 | 42.55 | 0.39 | 0 | 0 | 0 | 55.00 | 0 | 0.98 | 5.34 | 0.41 | 1.21 | 102.64 | 0.50 | 1.45 | 0.05 |
| 17 | EF0035-1553.75 | 42.28 | 0 | 0 | 0 | 0 | 55.24 | 0 | 0 | 6.74 | 0.00 | 1.52 | 102.74 | 0.00 | 1.85 | 0.15 |
| 18 | EF0035-1553.75 | 43.56 | 0 | 0 | 0 | 0 | 56.79 | 0 | 3.15 | 0.54 | 1.33 | 0.12 | 102.59 | 1.57 | 0.14 | 0.28 |
| 19 | EF0040-1706.4 | 41.04 | 0 | 0 | 0 | 0.42 | 53.55 | 0 | 0 | 6.11 | 0.00 | 1.38 | 99.74 | 0.00 | 1.73 | 0.27 |
| 20 | EF0040-1706.4 | 42.18 | 0.41 | 0 | 0 | 0.35 | 54.71 | 0 | 2.71 | 0.44 | 1.14 | 0.10 | 99.56 | 1.39 | 0.12 | 0.48 |
| 21 | EF0040-1706.4 | 42.99 | 0 | 0 | 0 | 0 | 54.93 | 0 | 2.53 | 0.80 | 1.07 | 0.18 | 100.00 | 1.29 | 0.22 | 0.49 |
| 22 | EF0040-1706.4 | 40.99 | 0.32 | 0 | 0 | 0 | 53.13 | 0 | 0 | 6.44 | 0.00 | 1.46 | 99.42 | 0.00 | 1.82 | 0.18 |
| 23 | EF0040-1706.4 | 40.56 | 0 | 0 | 0 | 0 | 52.82 | 0 | 0 | 6.56 | 0.00 | 1.48 | 98.46 | 0.00 | 1.88 | 0.12 |
| 24 | EF0043-1748.4 | 41.96 | 0.36 | 0.19 | 0 | 0.78 | 53.07 | 0.34 | 0.76 | 3.36 | 0.32 | 0.76 | 99.74 | 0.40 | 0.94 | 0.66 |
| 25 | EF0043-1748.4 | 41.86 | 0.39 | 0.47 | 0 | 0.75 | 53.38 | 0.38 | 0 | 4.09 | 0.00 | 0.92 | 100.40 | 0.00 | 1.15 | 0.85 |
| 26 | EF0043-1748.4 | 41.52 | 0.62 | 0.53 | 0 | 0.45 | 52.62 | 0.34 | 0.25 | 3.87 | 0.11 | 0.87 | 99.22 | 0.13 | 1.09 | 0.78 |
| 27 | EF0043-1748.4 | 40.74 | 0.62 | 0.35 | 0 | 0.98 | 52.50 | 0.13 | 0 | 5.93 | 0.00 | 1.34 | 99.91 | 0.00 | 1.68 | 0.32 |
| 28 | EF0061-1603.3 | 43.01 | 0 | 0 | 0 | 0.49 | 55.09 | 0 | 2.43 | 0.45 | 1.02 | 0.10 | 100.35 | 1.24 | 0.12 | 0.63 |
| 29 | EF0061-1603.3 | 42.83 | 0 | 0 | 0 | 0.6 | 54.41 | 0 | 2.68 | 0.54 | 1.13 | 0.12 | 99.81 | 1.37 | 0.15 | 0.48 |
| 30 | EF0061-1603.3 | 42.48 | 0 | 0 | 0 | 0.5 | 54.09 | 0 | 3.23 | 0.58 | 1.36 | 0.13 | 99.39 | 1.66 | 0.16 | 0.18 |
| 31 | EF0061-1603.3 | 42.14 | 0 | 0 | 0 | 0.51 | 54.81 | 0 | 3.14 | 0.64 | 1.32 | 0.14 | 99.77 | 1.61 | 0.18 | 0.21 |
| 32 | EF0062-1591.3 | 41.66 | 0 | 0 | 0 | 0.58 | 53.94 | 0 | 0 | 4.17 | 0.00 | 0.94 | 99.41 | 0.00 | 1.18 | 0.82 |
| 33 | EF0065-1714.8 | 41.91 | 0.36 | 0 | 0 | 0 | 54.39 | 0 | 0.74 | 3.45 | 0.31 | 0.78 | 99.76 | 0.39 | 0.96 | 0.65 |
| 34 | EF0065-1714.8 | 41.63 | 0.41 | 0.61 | 0 | 0 | 53.38 | 0 | 1.86 | 1.22 | 0.78 | 0.28 | 98.05 | 0.98 | 0.34 | 0.68 |
| 35 | EF0065-1714.8 | 41.47 | 0.60 | 0 | 0 | 0.68 | 53.74 | 0 | 1.90 | 2.40 | 0.80 | 0.54 | 99.45 | 0.99 | 0.67 | 0.34 |
| 36 | EF0065-1714.8 | 41.54 | 0.39 | 0 | 0 | 0.49 | 53.20 | 0 | 0.75 | 3.92 | 0.32 | 0.89 | 99.09 | 0.39 | 1.10 | 0.50 |
| 37 | EF0066-1726.05 | 42.53 | 0 | 0 | 0 | 0 | 54.85 | 0 | 2.85 | 1.71 | 1.20 | 0.39 | 100.35 | 1.46 | 0.47 | 0.08 |
| 38 | EF67-1707.8 | 41.38 | 0.60 | 1.07 | 0 | 0 | 52.32 | 0.44 | 0.80 | 2.99 | 0.34 | 0.68 | 99.09 | 0.42 | 0.84 | 0.73 |
| 39 | EF67-1707.8 | 40.05 | 0.68 | 1.02 | 0 | 0.84 | 51.32 | 0.63 | 0.65 | 2.97 | 0.27 | 0.67 | 97.60 | 0.35 | 0.86 | 0.79 |
| 40 | EF67-1724.65 | 42.07 | 0 | 0 | 0.5 | 0.98 | 53.24 | 0 | 2.55 | 1.75 | 1.07 | 0.40 | 99.12 | 1.32 | 0.49 | 0.19 |
| 41 | EF0069-1743.6 | 41.41 | 0.73 | 0.84 | 0.38 | 0.36 | 52.54 | 0.32 | 1.45 | 3.09 | 0.61 | 0.70 | 99.43 | 0.76 | 0.86 | 0.38 |
| 42 | EF0069-1743.6 | 41.36 | 0.73 | 0.80 | 0 | 0.57 | 52.50 | 0.34 | 0.97 | 3.12 | 0.41 | 0.71 | 99.28 | 0.51 | 0.88 | 0.61 |
| 43 | EF0069-1743.6 | 41.80 | 0.58 | 1.00 | 0 | 1.00 | 53.00 | 0 | 0.44 | 3.71 | 0.19 | 0.84 | 100.51 | 0.23 | 1.04 | 0.73 |
| 44 | EF0069-1743.6 | 41.93 | 0 | 0 | 0 | 0.91 | 53.21 | 0.39 | 0.38 | 4.80 | 0.16 | 1.08 | 100.38 | 0.20 | 1.34 | 0.46 |
| 45 | EF0069-1757.8 | 42.67 | 0 | 0 | 0 | 0.64 | 54.58 | 0 | 3.51 | 0.31 | 1.48 | 0.07 | 100.16 | 1.79 | 0.08 | 0.13 |
| 46 | EF0069-1757.8 | 42.48 | 0 | 0 | 0 | 0.58 | 54.79 | 0 | 3.40 | 0.37 | 1.43 | 0.08 | 100.10 | 1.74 | 0.10 | 0.16 |
| 47 | EF8057-1572.3 | 43.17 | 0 | 0 | 0 | 0 | 54.30 | 0 | 2.05 | 1.74 | 0.86 | 0.39 | 100.00 | 1.05 | 0.48 | 0.47 |
| 48 | EF8057-1572.3 | 43.15 | 0.34 | 0 | 0 | 0.45 | 54.72 | 0 | 1.32 | 2.48 | 0.56 | 0.56 | 101.34 | 0.67 | 0.68 | 0.65 |
| 49 | EF8057-1572.3 | 42.41 | 0.32 | 0 | 0 | 0 | 53.93 | 0.27 | 0.98 | 3.94 | 0.41 | 0.89 | 100.55 | 0.51 | 1.09 | 0.41 |
| 50 | EF8057-1572.3 | 41.45 | 0.58 | 0 | 0 | 0.4 | 53.14 | 0.36 | 0.66 | 4.47 | 0.28 | 1.01 | 99.77 | 0.34 | 1.25 | 0.40 |
| 51 | EF0057-1590.5 | 41.82 | 0.17 | 0 | 0 | 0.48 | 53.24 | 0 | 3.43 | 0.21 | 1.44 | 0.05 | 97.86 | 1.79 | 0.06 | 0.16 |
| 52 | EF0062-1615.4 | 42.85 | 0 | 0.64 | 0 | 0 | 53.73 | 0.53 | 2.97 | 0.70 | 1.25 | 0.16 | 100.01 | 1.52 | 0.19 | 0.29 |
| 53 | EF0066-1717 | 42.67 | 0 | 0 | 0 | 0.50 | 54.67 | 0 | 3.47 | 0.76 | 1.46 | 0.17 | 100.44 | 1.77 | 0.21 | 0.03 |
| # | Sample | P2O5 | SiO2 | ThO2 | UO2 | Ce2O3 | La2O3 | Pr2O3 | Nd2O3 | Sm2O3 | Gd2O3 | Dy2O3 | Al2O3 | PbO | FeO | CaO | Total (wt.%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0040-1706.4 | Mnz-Ce | 29.54 | 0 | 13.22 | 0 | 29.43 | 20.99 | 0 | 7.49 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100.67 |
| 2 | EF0040-1706.4 | 31.07 | 0.47 | 0 | 0 | 32.96 | 15.11 | 4.15 | 13.83 | 1.53 | 1.26 | 0.55 | 0 | 0 | 0 | 0.71 | 101.64 | |
| 3 | EF0062-1597.4 | 32.13 | 0.77 | 0 | 0 | 34.47 | 18.25 | 2.88 | 9.39 | 0 | 0 | 0 | 0 | 0 | 0.94 | 0.85 | 99.68 | |
| 4 | EF67-1707.8 | 26.26 | 0 | 19.57 | 0 | 27.95 | 13.56 | 2.67 | 9.30 | 0 | 0 | 0 | 0 | 0 | 0 | 0.60 | 99.91 | |
| 5 | EF0065-1714.8 | Thr | 5.25 | 14.80 | 68.37 | 0 | 5.55 | 2.33 | 0 | 1.89 | 0 | 0 | 0 | 0 | 0 | 1.96 | 1.44 | 101.59 |
| 6 | EF0065-1714.8 | 5.45 | 14.38 | 67.47 | 0 | 6.21 | 3.01 | 0 | 1.47 | 0 | 0 | 0 | 0 | 0 | 2.07 | 1.23 | 101.29 | |
| 7 | EF67-1707.8 | 2.29 | 24.56 | 54.26 | 0 | 1.66 | 0 | 0 | 0 | 0 | 0 | 0 | 3.65 | 0 | 3.45 | 0 | 89.87 | |
| 8 | EF67-1724.65 | 0.76 | 17.59 | 53.72 | 23.64 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.41 | 1.93 | 0 | 98.05 | |
| 9 | EF67-1724.65 | 0 | 17.37 | 51.98 | 25.30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.01 | 2.68 | 0 | 98.34 | |
| 10 | EF67-1707.8 | Tho | 0 | 0 | 91.29 | 3.90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 95.19 |
| 11 | EF67-1724.65 | Urn | 0 | 0 | 0 | 93.74 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.10 | 0 | 0 | 96.84 |
| # | P (apfu) | Si | Th | U | Ce | La | Pr | Nd | Sm | Gd | Dy | Al | Pb | Fe | Ca | Σ | ||
| 1 | Mnz-Ce | 1.00 | 0.00 | 0.12 | 0.00 | 0.43 | 0.31 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.96 | |
| 2 | 1.01 | 0.00 | 0.00 | 0.00 | 0.46 | 0.21 | 0.06 | 0.19 | 0.02 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | 0.03 | 1.00 | ||
| 3 | 1.03 | 0.00 | 0.00 | 0.00 | 0.48 | 0.26 | 0.04 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.03 | 0.97 | ||
| 4 | 0.94 | 0.00 | 0.19 | 0.00 | 0.43 | 0.21 | 0.04 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 1.04 | ||
| 5 | Thr | 0.22 | 0.74 | 0.77 | 0 | 0.10 | 0.04 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 0.08 | - | |
| 6 | 0.23 | 0.72 | 0.77 | 0 | 0.11 | 0.06 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.09 | 0.07 | - | ||
| 7 | 0.09 | 1.10 | 0.56 | 0 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.19 | 0.00 | 0.13 | 0.00 | - | ||
| 8 | 0.04 | 0.96 | 0.67 | 0.29 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.09 | 0.00 | - | ||
| 9 | 0 | 0.96 | 0.66 | 0.31 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.12 | 0.00 | - | ||
| 10 | Tho | 0.00 | 0.00 | 0.96 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | - | |
| 11 | Urn | 0.00 | 0.00 | 0 | 0.98 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | - |
| # | Sample | Nb2O5 | TiO2 | ZrO2 | ThO2 | UO2 | Y2O3 | Ce2O3 | Nd2O3 | Sm2O3 | Gd2O3 | Dy2O3 | Er2O3 | Yb2O3 | FeO | CaO | Total (wt.%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF00401706.4 | 0 | 30.68 | 33.50 | 2.22 | 2.16 | 9.65 | 1.17 | 1.85 | 1.01 | 0.67 | 1.61 | 1.25 | 0 | 9.58 | 4.84 | 100.19 |
| 2 | EF0040-1706.4 | 0 | 29.67 | 33.27 | 1.31 | 4.57 | 6.20 | 0.40 | 0.62 | 0 | 0.35 | 0 | 0.67 | 0.73 | 8.12 | 6.37 | 92.28 |
| 3 | EF0061-1603.3 | 3.99 | 28.74 | 33.35 | 0.89 | 0 | 10.50 | 1.93 | 3.57 | 1.06 | 1.18 | 0 | 0 | 0 | 8.74 | 4.07 | 98.02 |
| 4 | EF0069-1743.6 | 1.99 | 27.77 | 35.58 | 8.55 | 2.09 | 3.77 | 1.15 | 0.79 | 0 | 0 | 0 | 0 | 0 | 5.72 | 5.93 | 93.34 |
| 5 | EF0066-1717 | 0 | 28.71 | 34.81 | 1.15 | 0 | 8.90 | 2.79 | 3.52 | 1.11 | 0.68 | 1.62 | 0 | 1.17 | 9.51 | 3.71 | 97.68 |
| 6 | EF0066-1717 | 0 | 29.57 | 34.54 | 1.31 | 0 | 8.83 | 2.64 | 3.58 | 0.80 | 0.56 | 1.39 | 0 | 1.22 | 9.49 | 3.74 | 97.67 |
| 7 | EF0066-1717 | 3.49 | 27.24 | 32.16 | 1.70 | 1.36 | 11.86 | 1.71 | 2.44 | 1.16 | 1.37 | 2.10 | 0 | 1.43 | 9.39 | 2.78 | 100.19 |
| 8 | EF0066-1717 | 1.70 | 28.57 | 32.76 | 0.90 | 0 | 12.47 | 1.52 | 2.96 | 1.11 | 1.29 | 1.84 | 0 | 0.84 | 9.10 | 3.06 | 98.12 |
| 9 | EF0066-1717 | 1.97 | 31.48 | 34.74 | 1.18 | 0 | 7.20 | 3.91 | 3.16 | 0.79 | 0.21 | 0 | 0.51 | 0.54 | 8.49 | 5.41 | 99.59 |
| 10 | EF0066-1717 | 1.93 | 30.01 | 35.12 | 1.08 | 0.36 | 5.51 | 4.71 | 2.67 | 0.77 | 0.39 | 0.50 | 0.61 | 0.71 | 9.17 | 5.26 | 98.80 |
| # | Nb (apfu) | Ti | Zr | Th | U | Y | Ce | Nd | Sm | Gd | Dy | Er | Yb | Fe2+ | Ca | ||
| 1 | 0.00 | 3.05 | 2.16 | 0.07 | 0.06 | 0.68 | 0.06 | 0.09 | 0.05 | 0.06 | 0.07 | 0.05 | 0.00 | 1.06 | 0.69 | - | |
| 2 | 0.00 | 3.13 | 2.28 | 0.04 | 0.14 | 0.46 | 0.02 | 0.03 | 0.00 | 0.03 | 0.00 | 0.03 | 0.03 | 0.95 | 0.96 | - | |
| 3 | 0.24 | 2.87 | 2.16 | 0.03 | 0.00 | 0.74 | 0.09 | 0.17 | 0.05 | 0.10 | 0.00 | 0.00 | 0.00 | 0.97 | 0.58 | - | |
| 4 | 0.13 | 2.96 | 2.46 | 0.28 | 0.07 | 0.28 | 0.06 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.68 | 0.90 | - | |
| 5 | 0.00 | 2.95 | 2.31 | 0.04 | 0.00 | 0.65 | 0.14 | 0.17 | 0.05 | 0.06 | 0.07 | 0.00 | 0.05 | 1.08 | 0.54 | - | |
| 6 | 0.00 | 3.01 | 2.28 | 0.04 | 0.00 | 0.64 | 0.13 | 0.17 | 0.04 | 0.05 | 0.06 | 0.00 | 0.05 | 1.08 | 0.54 | - | |
| 7 | 0.21 | 2.76 | 2.12 | 0.05 | 0.04 | 0.85 | 0.08 | 0.12 | 0.05 | 0.12 | 0.09 | 0.00 | 0.06 | 1.06 | 0.40 | - | |
| 8 | 0.10 | 2.90 | 2.16 | 0.03 | 0.00 | 0.90 | 0.08 | 0.14 | 0.05 | 0.11 | 0.08 | 0.00 | 0.03 | 1.03 | 0.44 | - | |
| 9 | 0.12 | 3.08 | 2.20 | 0.03 | 0.00 | 0.50 | 0.19 | 0.15 | 0.04 | 0.02 | 0.00 | 0.02 | 0.02 | 0.92 | 0.75 | - | |
| 10 | 0.12 | 2.99 | 2.27 | 0.03 | 0.01 | 0.39 | 0.23 | 0.13 | 0.04 | 0.03 | 0.02 | 0.03 | 0.03 | 1.02 | 0.75 | - |
| Λ, nm | R, % | R, % | R, % | R, % |
|---|---|---|---|---|
| 440 | 13.8 | 14.1 | 13.5 | 13.2 |
| 460 | 13.8 | 14.1 | 13.5 | 13.2 |
| 480 | 13.7 | 14.1 | 13.4 | 13.1 |
| 500 | 13.6 | 14.0 | 13.3 | 13.0 |
| 520 | 13.6 | 13.9 | 13.2 | 13.0 |
| 540 | 13.5 | 13.8 | 13.2 | 12.9 |
| 560 | 13.5 | 13.7 | 13.1 | 12.9 |
| 580 | 13.4 | 13.7 | 13.1 | 12.8 |
| 600 | 13.4 | 13.7 | 13.1 | 12.8 |
| 620 | 13.4 | 13.6 | 13.1 | 12.8 |
| 640 | 13.3 | 13.5 | 13.0 | 12.7 |
| 660 | 13.2 | 13.5 | 13.0 | 12.7 |
| 680 | 13.2 | 13.4 | 13.0 | 12.7 |
| 700 | 13.2 | 13.4 | 13.1 | 12.7 |
| 720 | 13.2 | 13.4 | 13.1 | 12.8 |
| # | Sample | Pd | Fe | Ni | Ag | Cu | S | Te | Total (wt.%) | Pd (apfu) | Fe | Ni | Cu | Ag | ΣMe | S | Te | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0057-1590.5 | Sop | 25.32 | 0 | 0 | 33.87 | 0 | 0 | 40.80 | 99.99 | 3.00 | 0.00 | 0.00 | 0.00 | 3.96 | - | 0.00 | 4.04 |
| 2 | EF0061-1603.3 | 26.32 | 0 | 0 | 33.93 | 0 | 0 | 41.90 | 102.15 | 3.06 | 0.00 | 0.00 | 0.00 | 3.89 | - | 0.00 | 4.06 | |
| 3 | EF0035-1546.2 | Apn | 0 | 34.96 | 23.62 | 10.19 | 0 | 31.75 | 0 | 100.52 | 0.00 | 5.04 | 3.24 | 0.00 | 0.76 | 9.03 | 7.97 | 0.00 |
| 4 | EF0042-1722.3 | 0 | 39.24 | 17.24 | 13.30 | 0 | 31.47 | 0 | 101.25 | 0.00 | 5.68 | 2.38 | 0.00 | 1.00 | 9.06 | 7.94 | 0.00 | |
| 5 | EF0061-1603.3 | 0 | 36.12 | 19.49 | 12.75 | 1.35 | 31.87 | 0 | 101.58 | 0.00 | 5.21 | 2.67 | 0.17 | 0.95 | 9.00 | 8.00 | 0.00 | |
| 6 | EF0063-1744.3 | 0 | 38.60 | 16.34 | 12.69 | 0 | 31.51 | 0 | 99.14 | 0.00 | 5.68 | 2.29 | 0.00 | 0.97 | 8.93 | 8.07 | 0.00 | |
| 7 | EF0069-1757.8 | 0 | 36.89 | 18.72 | 12.84 | 0 | 31.95 | 0 | 100.40 | 0.00 | 5.36 | 2.59 | 0.00 | 0.97 | 8.91 | 8.09 | 0.00 | |
| 8 | EF0069-1757.8 | 0 | 36.40 | 20.44 | 13.00 | 0 | 32.11 | 0 | 101.95 | 0.00 | 5.22 | 2.79 | 0.00 | 0.97 | 8.98 | 8.02 | 0.00 | |
| 9 | EF0069-1757.8 | 0 | 34.93 | 27.65 | 6.89 | 0 | 33.02 | 0 | 102.49 | 0.00 | 4.85 | 3.66 | 0.00 | 0.50 | 9.01 | 7.99 | 0.00 | |
| 10 | EF0040-1706.4 | Hes | 0 | 0 | 0 | 60.50 | 0 | 0 | 36.04 | 96.54 | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 | 2.00 | 0.00 | 1.00 |
| 11 | EF0043-1764.3 | 0 | 0 | 0 | 61.59 | 0 | 0 | 38.41 | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.96 | 1.96 | 0.00 | 1.04 | |
| 12 | EF044-1801.05 | 0 | 0 | 0 | 59.78 | 0 | 0 | 37.80 | 97.58 | 0.00 | 0.00 | 0.00 | 0.00 | 1.95 | 1.95 | 0.00 | 1.05 | |
| 13 | EF0063-1744.3 | 0 | 0 | 0 | 62.73 | 0 | 0 | 37.27 | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 | 2.00 | 0.00 | 1.00 | |
| 14 | EF0063-1744.3 | 0 | 0 | 0 | 62.70 | 0 | 0 | 37.78 | 100.48 | 0.00 | 0.00 | 0.00 | 0.00 | 1.99 | 1.99 | 0.00 | 1.01 | |
| 15 | EF0066-1726.05 | 0 | 0 | 0 | 63.74 | 0 | 0 | 37.91 | 101.65 | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 | 2.00 | 0.00 | 1.00 | |
| 16 | EF0062-1597.4 | Aca | 0 | 0 | 0 | 85.83 | 0 | 13.70 | 0 | 99.53 | 0.00 | 0.00 | 0.00 | 0.00 | 1.95 | 1.95 | 1.05 | 0.00 |
| # | Sample | Au | Ag | Cu | Total (wt.%) | Au (at.%) | Ag | Cu |
|---|---|---|---|---|---|---|---|---|
| 1 | EF0035-1534.1 | 58.26 | 42.56 | 0 | 100.82 | 42.8 | 57.2 | 0.0 |
| 2 | EF0035-1534.1 | 57.99 | 41.49 | 0 | 99.48 | 43.4 | 56.6 | 0.0 |
| 3 | EF0035-1534.1 | 58.09 | 41.53 | 0 | 99.62 | 43.4 | 56.6 | 0.0 |
| 4 | EF0035-1534.1 | 62.95 | 38.36 | 0 | 101.31 | 47.3 | 52.7 | 0.0 |
| 5 | EF0042-1722.3 | 66.84 | 33.51 | 0 | 100.35 | 52.2 | 47.8 | 0.0 |
| 6 | EF0042-1722.3 | 55.78 | 44.04 | 0 | 99.82 | 41.0 | 59.0 | 0.0 |
| 7 | EF0043-1748.4 | 41.33 | 60.72 | 0 | 102.05 | 27.2 | 72.8 | 0.0 |
| 8 | EF0043-1748.4 | 45.81 | 54.49 | 0 | 100.30 | 31.5 | 68.5 | 0.0 |
| 9 | EF0043-1764.3 | 70.68 | 28.69 | 0 | 99.37 | 57.4 | 42.6 | 0.0 |
| 10 | EF0043-1764.3 | 70.02 | 31.55 | 0 | 101.57 | 54.9 | 45.1 | 0.0 |
| 11 | EF044-1801.05 | 73.14 | 25.94 | 0 | 99.08 | 60.7 | 39.3 | 0.0 |
| 12 | EF044-1801.05 | 60.22 | 38.23 | 0 | 98.45 | 46.3 | 53.7 | 0.0 |
| 13 | EF044-1801.05 | 73.66 | 25.50 | 0 | 99.16 | 61.3 | 38.7 | 0.0 |
| 14 | EF0061-1603.3 | 72.83 | 26.25 | 0 | 99.08 | 60.3 | 39.7 | 0.0 |
| 15 | EF0061-1603.3 | 73.14 | 28.85 | 0 | 101.99 | 58.1 | 41.9 | 0.0 |
| 16 | EF0061-1603.3 | 70.10 | 29.23 | 0 | 99.33 | 56.8 | 43.2 | 0.0 |
| 17 | EF0061-1603.3 | 70.42 | 29.49 | 0 | 99.91 | 56.7 | 43.3 | 0.0 |
| 18 | EF0062-1597.4 | 0 | 98.98 | 0 | 98.98 | 0.0 | 100.0 | 0.0 |
| 19 | EF0062-1597.4 | 60.58 | 41.46 | 0 | 102.04 | 44.5 | 55.5 | 0.0 |
| 20 | EF0062-1597.4 | 0 | 99.88 | 0 | 99.88 | 0.0 | 100.0 | 0.0 |
| 21 | EF0062-1597.4 | 0 | 100.55 | 0 | 100.55 | 0.0 | 100.0 | 0.0 |
| 22 | EF0062-1597.4 | 0 | 101.71 | 0 | 101.71 | 0.0 | 100.0 | 0.0 |
| 23 | EF0062-1597.4 | 0 | 100.86 | 0 | 100.86 | 0.0 | 100.0 | 0.0 |
| 24 | EF0062-1597.4 | 0 | 98.46 | 0 | 98.46 | 0.0 | 100.0 | 0.0 |
| 25 | EF0062-1597.4 | 0 | 99.81 | 0 | 99.81 | 0.0 | 100.0 | 0.0 |
| 26 | EF0063-1744.3 | 60.48 | 39.92 | 0 | 100.40 | 45.3 | 54.7 | 0.0 |
| 27 | EF0065-1713.3 | 39.61 | 59.26 | 1.74 | 100.61 | 25.9 | 70.6 | 3.5 |
| 28 | EF0065-1713.3 | 0 | 98.74 | 0 | 98.74 | 0.0 | 100.0 | 0.0 |
| 29 | EF0065-1713.3 | 52.74 | 44.85 | 0 | 97.59 | 39.2 | 60.8 | 0.0 |
| 30 | EF0065-1713.3 | 34.34 | 66.98 | 0 | 101.32 | 21.9 | 78.1 | 0.0 |
| 31 | EF0065-1714.8 | 58.90 | 40.38 | 0 | 99.28 | 44.4 | 55.6 | 0.0 |
| 32 | EF0065-1714.8 | 62.06 | 39.51 | 0 | 101.57 | 46.2 | 53.8 | 0.0 |
| 33 | EF0066-1726.05 | 2.88 | 95.11 | 0 | 97.99 | 1.6 | 98.4 | 0.0 |
| 34 | EF67-1724.65 | 0 | 99.22 | 0 | 99.22 | 0.0 | 100.0 | 0.0 |
| 35 | EF67-1724.65 | 25.55 | 73.71 | 0 | 99.26 | 16.0 | 84.0 | 0.0 |
| 36 | EF67-1724.65 | 24.98 | 75.96 | 0 | 100.94 | 15.3 | 84.7 | 0.0 |
| 37 | EF67-1724.65 | 0 | 100.62 | 0.28 | 100.90 | 0.0 | 99.5 | 0.5 |
| 38 | EF0069-1757.8 | 73.18 | 24.92 | 1.29 | 99.39 | 59.7 | 37.1 | 3.3 |
| 39 | EF0069-1757.8 | 74.98 | 25.94 | 0 | 100.92 | 61.3 | 38.7 | 0.0 |
| 40 | EF0069-1757.8 | 73.12 | 26.37 | 0 | 99.49 | 60.3 | 39.7 | 0.0 |
| # | Sample | Pb | Fe | Te | Se | S | Total (wt.%) | Pb (apfu) | Fe | Te | Se | S | S + Se + Te | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0040-1706.4 | Alt | 62.45 | 0 | 36.84 | 2.04 | 0 | 101.33 | 0.98 | 0.00 | 0.94 | 0.08 | 0.00 | 1.02 | |
| 2 | EF0040-1706.4 | 63.22 | 0 | 38.01 | 0 | 0 | 101.23 | 1.01 | 0.00 | 0.99 | 0.00 | 0.00 | 0.99 | ||
| 3 | EF0040-1706.4 | 61.94 | 0 | 37.98 | 0 | 0 | 99.92 | 1.00 | 0.00 | 1.00 | 0.00 | 0.00 | 1.00 | ||
| 4 | EF0040-1706.4 | 61.71 | 0 | 38.16 | 0 | 0 | 99.87 | 1.00 | 0.00 | 1.00 | 0.00 | 0.00 | 1.00 | ||
| 5 | EF0043-1748.4 | 58.14 | 1.82 | 36.83 | 0 | 0 | 96.79 | 0.93 | 0.11 | 0.96 | 0.00 | 0.00 | 0.96 | ||
| 6 | EF0043-1748.4 | Gn | 85.55 | 0 | 0 | 1.18 | 13.27 | 100.00 | 0.98 | 0.00 | 0.00 | 0.04 | 0.98 | 1.02 | |
| 7 | EF0043-1764.3 | Cth-Gn | 74.03 | 0 | 1.21 | 21.25 | 1.82 | 98.31 | 1.03 | 0.00 | 0.03 | 0.78 | 0.16 | 0.97 | |
| 8 | EF0061-1603.3 | 87.00 | 0 | 0 | 3.07 | 10.97 | 101.04 | 1.05 | 0.00 | 0.00 | 0.10 | 0.85 | 0.95 | ||
| 9 | EF0061-1603.3 | 82.52 | 0 | 0 | 14.06 | 5.88 | 102.46 | 1.05 | 0.00 | 0.00 | 0.47 | 0.48 | 0.95 | ||
| 10 | EF0063-1744.3 | Gn | 86.14 | 0 | 0 | 0 | 11.79 | 97.93 | 1.06 | 0.00 | 0.00 | 0.00 | 0.94 | 0.94 | |
| 11 | EF0065-1714.8 | 87.62 | 0 | 0 | 0 | 12.03 | 99.65 | 1.06 | 0.00 | 0.00 | 0.00 | 0.94 | 0.94 | ||
| 12 | EF0066-1726.05 | 82.41 | 1.71 | 0 | 5.09 | 9.75 | 98.96 | 1.00 | 0.08 | 0.00 | 0.16 | 0.76 | 0.93 | ||
| 13 | EF67-1707.8 | 83.85 | 1.99 | 0 | 2.28 | 11.01 | 99.13 | 1.00 | 0.09 | 0.00 | 0.07 | 0.85 | 0.92 | ||
| 14 | EF0069-1743.6 | 85.68 | 0 | 0 | 0 | 14.32 | 100.00 | 0.96 | 0.00 | 0.00 | 0.00 | 1.04 | 1.04 | ||
| 15 | EF0069-1757.8 | Gn-Cth | 78.89 | 0 | 0.69 | 14.37 | 5.84 | 99.79 | 1.01 | 0.00 | 0.01 | 0.49 | 0.49 | 0.99 | |
| 16 | EF0069-1757.8 | Gn | 88.93 | 0 | 0 | 12.78 | 101.71 | 1.04 | 0.00 | 0.00 | 0.00 | 0.96 | 0.96 | ||
| 17 | EF0069-1757.8 | Gn-Cth | 80.84 | 0 | 0.81 | 13.87 | 5.90 | 101.42 | 1.03 | 0.00 | 0.02 | 0.46 | 0.49 | 0.97 | |
| 18 | EF0069-1757.8 | 79.73 | 0 | 0.95 | 14.62 | 5.20 | 100.50 | 1.04 | 0.00 | 0.02 | 0.50 | 0.44 | 0.96 | ||
| 19 | EF0069-1757.8 | Gn | 87.73 | 0 | 0 | 0 | 12.43 | 100.16 | 1.04 | 0.00 | 0.00 | 0.00 | 0.96 | 0.96 | |
| 20 | EF0069-1757.8 | Cth-Gn | 81.50 | 0 | 0.34 | 12.03 | 6.45 | 100.32 | 1.05 | 0.00 | 0.01 | 0.41 | 0.54 | 0.95 |
| # | Sample | Fe | Ni | S | Total (wt.%) | Fe (at.%) | Ni | Fe + Ni | S | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0035-1534.1 | 60.01 | 0 | 38.77 | 98.78 | 47.0 | 0.00 | 47.0 | 53.0 | |
| 2 | EF0035-1534.1 | 60.33 | 0 | 40.33 | 100.66 | 46.2 | 0.00 | 46.2 | 53.8 | |
| 3 | EF0035-1546.2 | 60.63 | 0 | 39.14 | 99.77 | 47.1 | 0.00 | 47.1 | 52.9 | |
| 4 | EF0035-1546.2 | 60.75 | 0.78 | 39.98 | 101.51 | 46.3 | 0.57 | 46.9 | 53.1 | |
| 5 | EF0035-1553.75 | 60.71 | 0.42 | 39.35 | 100.48 | 46.8 | 0.31 | 47.1 | 52.9 | |
| 6 | EF0035-1553.75 | 60.54 | 0 | 39.39 | 99.93 | 46.9 | 0.00 | 46.9 | 53.1 | |
| 7 | EF0035-1553.75 | 60.93 | 0 | 39.33 | 100.26 | 47.1 | 0.00 | 47.1 | 52.9 | |
| 8 | EF0040-1706.4 | 63.44 | 0 | 36.38 | 99.82 | 50.0 | 0.00 | 50.0 | 50.0 | |
| 9 | EF0040-1706.4 | 63.41 | 0 | 35.93 | 99.34 | 50.3 | 0.00 | 50.3 | 49.7 | |
| 10 | EF0040-1706.4 | 63.38 | 0 | 36.68 | 100.06 | 49.8 | 0.00 | 49.8 | 50.2 | |
| 11 | EF0042-1722.3 | 61.85 | 0 | 39.14 | 100.99 | 47.6 | 0.00 | 47.6 | 52.4 | |
| 12 | EF0042-1722.3 | 61.54 | 0 | 38.38 | 99.92 | 47.9 | 0.00 | 47.9 | 52.1 | |
| 13 | EF0043-1748.4 | 62.79 | 0 | 38.25 | 101.04 | 48.5 | 0.00 | 48.5 | 51.5 | |
| 14 | EF0043-1764.3 | 60.23 | 0 | 39.30 | 99.53 | 46.8 | 0.00 | 46.8 | 53.2 | |
| 15 | EF0043-1764.3 | 60.72 | 0 | 39.46 | 100.18 | 46.9 | 0.00 | 46.9 | 53.1 | |
| 16 | EF0043-1764.3 | 60.41 | 0 | 38.08 | 98.49 | 47.7 | 0.00 | 47.7 | 52.3 | |
| 17 | EF044-1801.05 | 60.69 | 0.83 | 38.64 | 100.16 | 47.1 | 0.61 | 47.7 | 52.3 | |
| 18 | EF0061-1603.3 | 60.90 | 0.62 | 39.35 | 100.87 | 46.8 | 0.45 | 47.3 | 52.7 | |
| 19 | EF0061-1603.3 | 60.28 | 0 | 39.94 | 100.22 | 46.4 | 0.00 | 46.4 | 53.6 | |
| 20 | EF0062-1591.3 | 63.45 | 0 | 36.84 | 100.29 | 49.7 | 0.00 | 49.7 | 50.3 | |
| 21 | EF0062-1591.3 | 60.32 | 0 | 38.32 | 98.64 | 47.5 | 0.00 | 47.5 | 52.5 | |
| 22 | EF0062-1591.3 | 64.37 | 0 | 36.11 | 100.48 | 50.6 | 0.00 | 50.6 | 49.4 | |
| 23 | EF0062-1597.4 | 61.75 | 1.78 | 35.52 | 99.05 | 49.3 | 1.35 | 50.6 | 49.4 | |
| 24 | EF0062-1597.4 | 64.37 | 0 | 36.52 | 100.89 | 50.3 | 0.00 | 50.3 | 49.7 | |
| 25 | EF0062-1597.4 | 63.54 | 0 | 36.76 | 100.30 | 49.8 | 0.00 | 49.8 | 50.2 | |
| 26 | EF0063-1744.3 | 62.05 | 0 | 38.59 | 100.64 | 48.0 | 0.00 | 48.0 | 52.0 | |
| 27 | EF0063-1744.3 | 63.78 | 0 | 36.91 | 100.69 | 49.8 | 0.00 | 49.8 | 50.2 | |
| 28 | EF0065-1713.3 | 64.20 | 0 | 36.84 | 101.04 | 50.0 | 0.00 | 50.0 | 50.0 | |
| 29 | EF0065-1714.8 | 63.41 | 0 | 36.63 | 100.04 | 49.8 | 0.00 | 49.8 | 50.2 | |
| 30 | EF0065-1714.8 | 64.34 | 0 | 36.67 | 101.01 | 50.2 | 0.00 | 50.2 | 49.8 | |
| 31 | EF0066-1684.4 | 64.07 | 0 | 36.90 | 100.97 | 49.9 | 0.00 | 49.9 | 50.1 | |
| 32 | EF67-1712.0 | 60.50 | 0.75 | 39.67 | 100.92 | 46.4 | 0.55 | 47.0 | 53.0 | |
| 33 | EF0069-1743.6 | 63.14 | 0 | 36.71 | 99.85 | 49.7 | 0.00 | 49.7 | 50.3 | |
| 34 | EF0069-1743.6 | 63.93 | 0 | 36.80 | 100.73 | 49.9 | 0.00 | 49.9 | 50.1 | |
| 35 | EF0069-1743.6 | 63.35 | 0 | 36.65 | 100.00 | 49.8 | 0.00 | 49.8 | 50.2 | |
| 36 | EF0069-1757.8 | 61.02 | 0.71 | 39.51 | 101.24 | 46.8 | 0.52 | 47.3 | 52.7 | |
| 37 | EF0069-1757.8 | 60.68 | 0.79 | 40.30 | 101.77 | 46.1 | 0.57 | 46.7 | 53.3 | |
| 38 | EF8057-1572.3 | 63.66 | 0 | 36.68 | 100.34 | 49.9 | 0.00 | 49.9 | 50.1 |
| # | Sample | Fe | Ni | Co | Cu | S | Total (wt.%) | Fe (apfu) | Ni | Co | Cu | ΣM | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0035-1534.1 | 32.00 | 34.40 | 0 | 0 | 33.83 | 100.23 | 4.40 | 4.50 | 0.00 | 0.00 | 8.90 | 8.10 |
| 2 | EF0035-1546.2 | 30.55 | 36.18 | 1.76 | 0 | 33.38 | 101.87 | 4.16 | 4.69 | 0.23 | 0.00 | 9.08 | 7.92 |
| 3 | EF0035-1546.2 | 29.85 | 35.49 | 2.17 | 0 | 33.03 | 100.54 | 4.12 | 4.66 | 0.28 | 0.00 | 9.06 | 7.94 |
| 4 | EF0035-1553.75 | 31.87 | 35.91 | 0 | 0 | 33.24 | 101.02 | 4.37 | 4.69 | 0.00 | 0.00 | 9.06 | 7.94 |
| 5 | EF0035-1553.75 | 30.38 | 36.36 | 0 | 0 | 33.39 | 100.13 | 4.19 | 4.78 | 0.00 | 0.00 | 8.97 | 8.03 |
| 6 | EF0035-1553.75 | 31.01 | 37.32 | 0 | 0 | 33.31 | 101.64 | 4.23 | 4.85 | 0.00 | 0.00 | 9.08 | 7.92 |
| 7 | EF0040-1706.4 | 37.09 | 30.07 | 0 | 0 | 33.53 | 100.69 | 5.08 | 3.92 | 0.00 | 0.00 | 9.00 | 8.00 |
| 8 | EF0040-1706.4 | 35.89 | 28.94 | 1.15 | 0 | 33.44 | 99.42 | 4.97 | 3.81 | 0.15 | 0.00 | 8.93 | 8.07 |
| 9 | EF0042-1722.3 | 33.81 | 33.28 | 0 | 0 | 33.47 | 100.56 | 4.64 | 4.35 | 0.00 | 0.00 | 8.99 | 8.01 |
| 10 | EF0042-1722.3 | 33.00 | 33.58 | 1.04 | 0 | 33.29 | 100.91 | 4.53 | 4.38 | 0.14 | 0.00 | 9.04 | 7.96 |
| 11 | EF0042-1722.3 | 33.36 | 31.99 | 1.46 | 0 | 33.24 | 100.05 | 4.61 | 4.20 | 0.19 | 0.00 | 9.00 | 8.00 |
| 12 | EF0043-1748.4 | 36.93 | 30.23 | 0 | 0 | 34.11 | 101.27 | 5.02 | 3.91 | 0.00 | 0.00 | 8.93 | 8.07 |
| 13 | EF0043-1748.4 | 37.75 | 28.74 | 0 | 0 | 33.80 | 100.29 | 5.18 | 3.75 | 0.00 | 0.00 | 8.93 | 8.07 |
| 14 | EF0043-1764.3 | 32.01 | 33.45 | 1.45 | 0 | 33.62 | 100.53 | 4.40 | 4.37 | 0.19 | 0.00 | 8.96 | 8.04 |
| 15 | EF0043-1764.3 | 32.27 | 34.91 | 0 | 0 | 33.87 | 101.05 | 4.41 | 4.54 | 0.00 | 0.00 | 8.94 | 8.06 |
| 16 | EF044-1801.05 | 30.85 | 33.49 | 2.18 | 0 | 32.14 | 98.66 | 4.34 | 4.49 | 0.29 | 0.00 | 9.12 | 7.88 |
| 17 | EF044-1801.05 | 31.18 | 34.57 | 1.82 | 0 | 33.20 | 100.77 | 4.29 | 4.52 | 0.24 | 0.00 | 9.05 | 7.95 |
| 18 | EF0061-1603.3 | 30.48 | 36.23 | 0 | 0 | 33.66 | 100.37 | 4.19 | 4.74 | 0.00 | 0.00 | 8.93 | 8.07 |
| 19 | EF0061-1603.3 | 30.62 | 34.8 | 1.55 | 0 | 33.42 | 100.39 | 4.22 | 4.56 | 0.20 | 0.00 | 8.98 | 8.02 |
| 20 | EF0062-1591.3 | 37.29 | 27.44 | 0 | 2.09 | 33.86 | 100.68 | 5.10 | 3.57 | 0.00 | 0.25 | 8.93 | 8.07 |
| 21 | EF0062-1597.4 | 36.25 | 28.64 | 0 | 0.87 | 33.00 | 98.76 | 5.06 | 3.80 | 0.00 | 0.11 | 8.97 | 8.03 |
| 22 | EF0062-1597.4 | 36.88 | 29.27 | 0 | 0 | 32.90 | 99.05 | 5.14 | 3.88 | 0.00 | 0.00 | 9.02 | 7.98 |
| 23 | EF0062-1597.4 | 35.97 | 27.69 | 0 | 2.39 | 33.34 | 99.39 | 4.99 | 3.66 | 0.00 | 0.29 | 8.94 | 8.06 |
| 24 | EF0063-1744.3 | 33.35 | 32.14 | 1.41 | 0 | 33.42 | 100.32 | 4.59 | 4.21 | 0.18 | 0.00 | 8.99 | 8.01 |
| 25 | EF0063-1744.3 | 34.79 | 31.72 | 1.17 | 0 | 33.05 | 100.73 | 4.78 | 4.15 | 0.15 | 0.00 | 9.08 | 7.92 |
| 26 | EF0065-1713.3 | 36.92 | 26.73 | 0 | 3.46 | 33.45 | 100.56 | 5.08 | 3.50 | 0.00 | 0.42 | 8.99 | 8.01 |
| 27 | EF0065-1714.8 | 36.54 | 29.64 | 0 | 1.14 | 33.42 | 100.74 | 5.01 | 3.87 | 0.00 | 0.14 | 9.02 | 7.98 |
| 28 | EF0065-1714.8 | 36.08 | 31.02 | 0 | 0 | 33.31 | 100.41 | 4.96 | 4.06 | 0.00 | 0.00 | 9.02 | 7.98 |
| 29 | EF0065-1714.8 | 36.76 | 29.93 | 0 | 0 | 33.41 | 100.10 | 5.06 | 3.92 | 0.00 | 0.00 | 8.98 | 8.02 |
| 30 | EF0066-1684.4 | 38.75 | 28.41 | 0.95 | 0 | 33.68 | 101.79 | 5.26 | 3.67 | 0.12 | 0.00 | 9.04 | 7.96 |
| 31 | EF0066-1684.4 | 38.47 | 28.65 | 0.80 | 0 | 33.97 | 101.89 | 5.20 | 3.69 | 0.10 | 0.00 | 8.99 | 8.01 |
| 32 | EF0066-1684.4 | 38.30 | 27.26 | 0 | 0 | 33.42 | 98.98 | 5.32 | 3.60 | 0.00 | 0.00 | 8.92 | 8.08 |
| 33 | EF0066-1726.05 | 32.01 | 33.86 | 0 | 0 | 33.53 | 99.40 | 4.44 | 4.47 | 0.00 | 0.00 | 8.90 | 8.10 |
| 34 | EF67-1724.65 | 26.24 | 40.08 | 1.38 | 0 | 33.22 | 100.92 | 3.61 | 5.25 | 0.18 | 0.00 | 9.04 | 7.96 |
| 35 | EF67-1724.65 | 26.81 | 39.33 | 0 | 0 | 32.24 | 98.38 | 3.79 | 5.28 | 0.00 | 0.00 | 9.07 | 7.93 |
| 36 | EF67-1724.65 | 27.04 | 40.07 | 1.34 | 0 | 33.54 | 101.99 | 3.68 | 5.19 | 0.17 | 0.00 | 9.05 | 7.95 |
| 37 | EF0069-1743.6 | 39.71 | 25.88 | 0 | 0 | 32.31 | 97.90 | 5.60 | 3.47 | 0.00 | 0.00 | 9.07 | 7.93 |
| 38 | EF0069-1743.6 | 38.93 | 27.95 | 0 | 0 | 34.08 | 100.96 | 5.30 | 3.62 | 0.00 | 0.00 | 8.92 | 8.08 |
| 39 | EF0069-1757.8 | 31.53 | 35.63 | 1.51 | 0 | 33.73 | 102.40 | 4.27 | 4.59 | 0.19 | 0.00 | 9.05 | 7.95 |
| 40 | EF8057-1572.3 | 41.61 | 24.08 | 1.26 | 0 | 34.21 | 101.16 | 5.64 | 3.11 | 0.16 | 0.00 | 8.92 | 8.08 |
| # | Sample | Pd | Te | Bi | Sb | Total (wt.%) | Pd (apfu) | Te | Bi | Sb | Te + Bi + Sb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0035-1534.1 | 38.54 | 29.12 | 29.84 | 1.50 | 99.00 | 0.97 | 0.61 | 0.38 | 0.03 | 1.03 | |
| 2 | EF0035-1546.2 | 37.37 | 8.82 | 52.37 | 1.44 | 100.00 | 1.03 | 0.20 | 0.73 | 0.03 | 0.97 | |
| 3 | EF0040-1706.4 | 44.09 | 29.22 | 25.45 | 0 | 98.76 | 1.08 | 0.60 | 0.32 | 0.00 | 0.92 | |
| 4 | EF0040-1706.4 | 40.79 | 31.38 | 28.40 | 0 | 100.57 | 1.00 | 0.64 | 0.36 | 0.00 | 1.00 | |
| 5 | EF0040-1706.4 | 41.20 | 32.20 | 27.49 | 0 | 100.89 | 1.00 | 0.65 | 0.34 | 0.00 | 1.00 | |
| 6 | EF0042-1722.3 | 39.15 | 14.96 | 44.57 | 2.51 | 101.19 | 1.02 | 0.33 | 0.59 | 0.06 | 0.98 | |
| 7 | EF0043-1748.4 | 40.21 | 24.29 | 35.50 | 0 | 100.00 | 1.02 | 0.52 | 0.46 | 0.00 | 0.98 | |
| 8 | EF0043-1748.4 | 37.55 | 10.95 | 51.79 | 0 | 100.29 | 1.03 | 0.25 | 0.72 | 0.00 | 0.97 | |
| 9 | EF0043-1748.4 | 38.53 | 16.84 | 44.58 | 0 | 99.95 | 1.02 | 0.37 | 0.60 | 0.00 | 0.98 | |
| 10 | EF044-1801.05 | 37.39 | 12.86 | 50.40 | 0 | 100.65 | 1.01 | 0.29 | 0.70 | 0.00 | 0.99 | |
| 11 | EF044-1801.05 | 38.02 | 9.27 | 54.96 | 0 | 102.25 | 1.03 | 0.21 | 0.76 | 0.00 | 0.97 | |
| 12 | EF044-1801.05 | 39.22 | 12.37 | 47.34 | 3.73 | 102.66 | 1.02 | 0.27 | 0.63 | 0.08 | 0.98 | |
| 13 | EF0061-1603.3 | 41.38 | 38.24 | 20.39 | 0 | 100.01 | 0.99 | 0.76 | 0.25 | 0.00 | 1.01 | |
| 14 | EF0061-1603.3 | 40.36 | 34.24 | 25.40 | 0 | 100.00 | 0.99 | 0.70 | 0.32 | 0.00 | 1.01 | |
| 15 | EF0061-1603.3 | 44.94 | 55.85 | 0 | 0 | 100.79 | 0.98 | 1.02 | 0.00 | 0.00 | 1.02 | |
| 16 | EF0061-1603.3 | 40.92 | 23.59 | 36.82 | 0 | 101.33 | 1.03 | 0.50 | 0.47 | 0.00 | 0.97 | |
| 17 | EF0062-1591.3 | 42.87 | 21.40 | 35.73 | 0 | 100.00 | 1.09 | 0.45 | 0.46 | 0.00 | 0.91 | |
| 18 | EF0062-1591.3 | 38.12 | 25.61 | 33.85 | 0 | 97.58 | 0.99 | 0.56 | 0.45 | 0.00 | 1.01 | |
| 19 | EF0063-1744.3 | 39.21 | 11.51 | 49.28 | 0 | 100.00 | 1.06 | 0.26 | 0.68 | 0.00 | 0.94 | |
| 20 | EF0063-1744.3 | 37.48 | 7.57 | 53.20 | 2.02 | 100.27 | 1.03 | 0.17 | 0.75 | 0.05 | 0.97 | |
| 21 | EF0066-1684.4 | 39.96 | 29.81 | 29.32 | 0 | 99.09 | 1.00 | 0.62 | 0.37 | 0.00 | 1.00 | |
| 22 | EF0066-1684.4 | 41.86 | 34.31 | 22.88 | 0 | 99.05 | 1.02 | 0.70 | 0.28 | 0.00 | 0.98 | |
| 23 | EF0066-1726.05 | 37.41 | 8.22 | 54.84 | 0 | 100.47 | 1.04 | 0.19 | 0.77 | 0.00 | 0.96 | |
| 24 | EF0066-1726.05 | 37.75 | 10.02 | 54.20 | 0 | 101.97 | 1.02 | 0.23 | 0.75 | 0.00 | 0.98 | |
| 25 | EF67-1712.0 | 37.94 | 18.06 | 45.56 | 0 | 101.56 | 1.00 | 0.40 | 0.61 | 0.00 | 1.00 | |
| 26 | EF67-1712.0 | 37.75 | 7.69 | 53.47 | 2.70 | 101.61 | 1.02 | 0.17 | 0.74 | 0.06 | 0.98 | |
| 27 | EF67-1724.65 | 40.03 | 26.32 | 31.22 | 0 | 97.57 | 1.03 | 0.56 | 0.41 | 0.00 | 0.97 | |
| 28 | EF0069-1743.6 | 39.86 | 20.85 | 40.83 | 0 | 101.54 | 1.02 | 0.45 | 0.53 | 0.00 | 0.98 | |
| 29 | EF0069-1743.6 | 39.18 | 22.12 | 38.41 | 0 | 99.71 | 1.02 | 0.48 | 0.51 | 0.00 | 0.98 | |
| 30 | EF8057-1572.3 | 39.51 | 23.64 | 37.35 | 0 | 100.50 | 1.01 | 0.50 | 0.49 | 0.00 | 0.99 | |
| 31 | EF8057-1572.3 | 39.56 | 17.82 | 42.63 | 0 | 100.01 | 1.04 | 0.39 | 0.57 | 0.00 | 0.96 |
| # | Sample | Pt | Pd | Te | Bi | Sb | Total (wt.%) | Pt (apfu) | Pd | Pt + Pd | Te | Bi | Sb | Te + Bi + Sb | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0042-1722.3 | Mch | 0 | 23.75 | 34.00 | 38.74 | 3.16 | 99.65 | 0.00 | 0.96 | 0.96 | 1.14 | 0.79 | 0.11 | 2.04 | |
| 2 | EF0042-1722.3 | 19.19 | 12.08 | 20.21 | 46.48 | 3.15 | 101.11 | 0.48 | 0.55 | 1.03 | 0.77 | 1.08 | 0.13 | 1.97 | ||
| 3 | EF0043-1764.3 | Mon | 39.33 | 0 | 32.20 | 28.47 | 0 | 100.00 | 1.02 | 0.00 | 1.02 | 1.28 | 0.69 | 0.00 | 1.98 | |
| 4 | EF044-1801.05 | Mch | 0 | 24.12 | 28.92 | 46.95 | 0 | 99.99 | 0.00 | 1.00 | 1.00 | 1.00 | 0.99 | 0.00 | 2.00 | |
| 5 | EF044-1801.05 | 0 | 24.99 | 30.19 | 45.58 | 0 | 100.76 | 0.00 | 1.02 | 1.02 | 1.03 | 0.95 | 0.00 | 1.98 | ||
| 6 | EF0061-1603.3 | 0 | 24.81 | 30.25 | 46.05 | 0 | 101.11 | 0.00 | 1.01 | 1.01 | 1.03 | 0.96 | 0.00 | 1.99 | ||
| 7 | EF0061-1603.3 | Mrk | 0 | 28.83 | 61.31 | 10.85 | 0 | 100.99 | 0.00 | 1.01 | 1.01 | 1.79 | 0.19 | 0.00 | 1.99 | |
| 8 | EF0057-1590.5 | 0 | 30.01 | 71.65 | 0 | 0 | 101.66 | 0.00 | 1.00 | 1.00 | 2.00 | 0.00 | 0.00 | 2.00 | ||
| 9 | EF0061-1603.3 | Mch | 0 | 25.47 | 29.87 | 47.15 | 0 | 102.49 | 0.00 | 1.03 | 1.03 | 1.00 | 0.97 | 0.00 | 1.97 | |
| 10 | EF0061-1603.3 | 0 | 24.44 | 29.35 | 45.76 | 0 | 99.55 | 0.00 | 1.02 | 1.02 | 1.02 | 0.97 | 0.00 | 1.98 | ||
| 11 | EF0062-1597.4 | Mon | 30.66 | 7.76 | 42.67 | 20.10 | 0 | 101.19 | 0.71 | 0.33 | 1.04 | 1.52 | 0.44 | 0.00 | 1.96 | |
| 12 | EF0065-1713.3 | 38.06 | 0 | 35.60 | 25.46 | 0 | 99.12 | 0.98 | 0.00 | 0.98 | 1.40 | 0.61 | 0.00 | 2.02 | ||
| 13 | EF0065-1714.8 | 39.03 | 0 | 38.91 | 23.36 | 0 | 101.30 | 0.97 | 0.00 | 0.97 | 1.48 | 0.54 | 0.00 | 2.03 | ||
| 14 | EF0065-1714.8 | 38.37 | 0 | 35.41 | 26.27 | 0 | 100.05 | 0.98 | 0.00 | 0.98 | 1.39 | 0.63 | 0.00 | 2.02 | ||
| 15 | EF0065-1714.8 | 37.98 | 0 | 32.95 | 31.13 | 0 | 102.06 | 0.97 | 0.00 | 0.97 | 1.29 | 0.74 | 0.00 | 2.03 | ||
| 16 | EF0069-1743.6 | 37.13 | 2.43 | 42.99 | 19.11 | 0 | 101.66 | 0.89 | 0.11 | 1.00 | 1.58 | 0.43 | 0.00 | 2.00 | ||
| 17 | EF0069-1743.6 | 37.87 | 1.72 | 43.33 | 17.83 | 0 | 100.75 | 0.92 | 0.08 | 0.99 | 1.60 | 0.40 | 0.00 | 2.01 | ||
| 18 | EF0069-1743.6 | 37.31 | 0 | 31.20 | 30.27 | 0 | 98.78 | 0.99 | 0.00 | 0.99 | 1.26 | 0.75 | 0.00 | 2.01 | ||
| 19 | EF0069-1743.6 | 38.12 | 0 | 32.32 | 31.20 | 0 | 101.64 | 0.98 | 0.00 | 0.98 | 1.27 | 0.75 | 0.00 | 2.02 | ||
| 20 | EF044-1801.05 | Fro | 0 | 21.82 | 0 | 79.10 | 0 | 100.92 | 0.00 | 1.05 | 1.05 | 0.00 | 1.95 | 0.00 | 1.95 | |
| 21 | EF044-1801.05 | 0 | 21.53 | 0 | 80.92 | 0 | 102.45 | 0.00 | 1.03 | 1.03 | 0.00 | 1.97 | 0.00 | 1.97 | ||
| 22 | EF0063-1744.3 | 7.87 | 17.16 | 0 | 72.86 | 0 | 97.89 | 0.22 | 0.88 | 1.10 | 0.00 | 1.90 | 0.00 | 1.90 |
| # | Sample | Pt | Pd | Au | Sn | Sb | As | Total (wt.%) | Pt (apfu) | Pd | Pd + Pt | Au | Sn | Sb | As | Sn + Sb + As | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0035-1546.2 | Plv-Unamed (Pt, Pd)2Sn | 5.43 | 59.36 | 0 | 32.11 | 3.19 | 0 | 100.09 | 0.09 | 1.90 | 1.99 | 0.00 | 0.92 | 0.09 | 0.00 | 1.01 |
| 2 | EF0035-1546.2 | 7.21 | 57.80 | 0 | 32.61 | 1.72 | 0 | 99.34 | 0.13 | 1.88 | 2.00 | 0.00 | 0.95 | 0.05 | 0.00 | 1.00 | |
| 3 | EF0040-1706.4 | 0 | 64.27 | 0 | 36.18 | 0 | 0 | 100.45 | 0.00 | 1.99 | 1.99 | 0.00 | 1.01 | 0.00 | 0.00 | 1.01 | |
| 4 | EF0043-1764.3 | 0 | 64.05 | 0 | 36.59 | 0 | 0 | 100.64 | 0.00 | 1.98 | 1.98 | 0.00 | 1.02 | 0.00 | 0.00 | 1.02 | |
| 5 | EF0043-1764.3 | 1.98 | 62.59 | 0 | 35.28 | 0 | 0 | 99.85 | 0.03 | 1.97 | 2.00 | 0.00 | 1.00 | 0.00 | 0.00 | 1.00 | |
| 6 | EF044-1801.05 | 4.48 | 60.53 | 0 | 34.98 | 0 | 0 | 99.99 | 0.08 | 1.93 | 2.00 | 0.00 | 1.00 | 0.00 | 0.00 | 1.00 | |
| 7 | EF044-1801.05 | 4.59 | 59.87 | 0 | 35.54 | 0 | 0 | 100.00 | 0.08 | 1.91 | 1.99 | 0.00 | 1.01 | 0.00 | 0.00 | 1.01 | |
| 8 | EF0061-1603.3 | 0 | 63.75 | 0 | 36.23 | 0 | 0 | 99.98 | 0.00 | 1.99 | 1.99 | 0.00 | 1.01 | 0.00 | 0.00 | 1.01 | |
| 9 | EF0061-1603.3 | 0 | 64.90 | 0 | 36.22 | 0 | 0 | 101.12 | 0.00 | 2.00 | 2.00 | 0.00 | 1.00 | 0.00 | 0.00 | 1.00 | |
| 10 | EF0063-1744.3 | 11.94 | 51.86 | 0 | 33.27 | 0.49 | 0.41 | 97.97 | 0.22 | 1.74 | 1.96 | 0.00 | 1.00 | 0.01 | 0.03 | 1.04 | |
| 11 | EF0063-1744.3 | 5.52 | 57.99 | 0 | 33.47 | 0 | 0 | 96.98 | 0.10 | 1.91 | 2.01 | 0.00 | 0.99 | 0.00 | 0.00 | 0.99 | |
| 12 | EF0065-1713.3 | 19.92 | 48.38 | 0 | 31.89 | 0 | 0 | 100.19 | 0.37 | 1.65 | 2.02 | 0.00 | 0.98 | 0.00 | 0.00 | 0.98 | |
| 13 | EF0065-1713.3 | 0 | 65.31 | 0 | 36.52 | 0 | 0 | 101.83 | 0.00 | 2.00 | 2.00 | 0.00 | 1.00 | 0.00 | 0.00 | 1.00 | |
| 14 | EF0065-1714.8 | 15.28 | 53.35 | 0 | 31.37 | 0 | 0 | 100.00 | 0.28 | 1.78 | 2.06 | 0.00 | 0.94 | 0.00 | 0.00 | 0.94 | |
| 15 | EF0065-1714.8 | 46.18 | 26.11 | 0 | 27.70 | 0 | 0 | 99.99 | 0.99 | 1.03 | 2.02 | 0.00 | 0.98 | 0.00 | 0.00 | 0.98 | |
| 16 | EF0065-1714.8 | 25.21 | 41.59 | 0 | 33.20 | 0 | 0 | 100.00 | 0.48 | 1.47 | 1.95 | 0.00 | 1.05 | 0.00 | 0.00 | 1.05 | |
| 17 | EF0066-1726.05 | 0 | 64.94 | 0 | 36.29 | 0 | 0 | 101.23 | 0.00 | 2.00 | 2.00 | 0.00 | 1.00 | 0.00 | 0.00 | 1.00 | |
| 18 | EF67-1707.8 | 39.24 | 32.06 | 0 | 26.44 | 0 | 0 | 97.74 | 0.83 | 1.25 | 2.08 | 0.00 | 0.92 | 0.00 | 0.92 | ||
| 19 | EF67-1707.8 | 0 | 64.49 | 0 | 35.51 | 0 | 0 | 100.00 | 0.00 | 2.01 | 2.01 | 0.00 | 0.99 | 0.00 | 0.99 | ||
| 20 | EF0035-1546.2 | UN Pd6Sn2As | 0 | 65.39 | 0 | 27.99 | 0 | 6.21 | 99.59 | 0.00 | 5.93 | 0.00 | 2.27 | 0.00 | 0.80 | 0.00 | |
| 21 | EF0043-1748.4 | 68.11 | 0 | 21.64 | 0 | 10.25 | 100.00 | 0.00 | 6.01 | 0.00 | 1.71 | 0.00 | 1.28 | 0.00 | |||
| 22 | EF0061-1603.3 | 68.96 | 0 | 22.71 | 0 | 8.46 | 100.13 | 0.00 | 6.12 | 0.00 | 1.81 | 0.00 | 1.07 | 0.00 | |||
| 23 | EF67-1724.65 | 67.74 | 0.71 | 24.16 | 0 | 7.51 | 100.12 | 0.00 | 6.07 | 0.03 | 1.94 | 0.00 | 0.96 | 0.00 | |||
| 24 | EF67-1724.65 | 66.58 | 1.00 | 24.56 | 0 | 7.12 | 99.26 | 0.00 | 6.04 | 0.05 | 2.00 | 0.00 | 0.92 | 0.00 | |||
| 25 | WDS | 0.22 | 65.76 | 1.20 | 24.30 | 0 | 7.58 | 99.06 | 0.01 | 5.97 | 0.06 | 1.98 | 0.00 | 0.98 | 0.00 |
| # | Sample | Pt | Pd | Au | Sn | Pb | Sb | Bi | Te | As | Total (wt.%) | Pt (apfu) | Pd | Au | Pd + Pt (+Au) | Sn | Pb | Sb | Bi | Te | As | Sn + Sb (+Bi + As) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0065-1714.8 | Ato- Rsb | 48.44 | 28.07 | 2.41 | 21.08 | 0 | 0 | 0 | 0 | 0 | 100.00 | 1.42 | 1.50 | 0.07 | 2.99 | 1.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.01 |
| 2 | EF0065-1714.8 | 48.42 | 30.26 | 0 | 21.32 | 0 | 0 | 0 | 0 | 0 | 100.00 | 1.39 | 1.60 | 0.00 | 2.99 | 1.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.01 | |
| 3 | EF0065-1714.8 | 41.57 | 35.75 | 0 | 22.68 | 0 | 0 | 0 | 0 | 0 | 100.00 | 1.15 | 1.82 | 0.00 | 2.97 | 1.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.03 | |
| 4 | EF0069-1743.6 | Nig | 63.66 | 0 | 0 | 33.25 | 0 | 3.09 | 0 | 0 | 0 | 100.00 | 1.03 | 0.00 | 0.00 | 1.03 | 0.89 | 0.00 | 0.08 | 0.00 | 0.00 | 0.00 | 0.97 |
| 5 | EF0063-1744.3 | 63.55 | 0 | 0 | 36.45 | 0 | 0 | 0 | 0 | 0 | 100.00 | 1.03 | 0.00 | 0.00 | 1.03 | 0.97 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.97 | |
| 6 | EF0065-1714.8 | 58.92 | 0 | 0 | 28.78 | 0 | 2.58 | 9.73 | 0 | 0 | 100.01 | 0.99 | 0.00 | 0.00 | 0.99 | 0.79 | 0.00 | 0.07 | 0.15 | 0.00 | 0.00 | 1.01 | |
| 7 | EF0042-1722.3 | Nld | 0 | 64.80 | 0 | 0 | 0 | 22.99 | 8.93 | 3.87 | 2.35 | 102.94 | 0.00 | 2.02 | 0.00 | 2.02 | 0.00 | 0.00 | 0.63 | 0.14 | 0.10 | 0.10 | 0.97 |
| 8 | EF0069-1743.6 | Zv | 0 | 60.43 | 0 | 0 | 39.57 | 0 | 0 | 0 | 0 | 100.00 | 0.00 | 2.99 | 0.00 | 0.00 | 0.00 | 1.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 9 | EF8057-1572.3 | 0 | 61.94 | 0 | 0 | 38.88 | 0 | 0 | 0 | 0 | 100.82 | 0.00 | 3.02 | 0.00 | 0.00 | 0.00 | 0.98 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 10 | EF8057-1572.3 | 0 | 60.65 | 0 | 0 | 36.81 | 0 | 0 | 0 | 0 | 97.46 | 0.00 | 3.05 | 0.00 | 0.00 | 0.00 | 0.95 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 11 | EF8057-1572.3 | Ppdn | 0 | 42.21 | 0 | 0 | 58.05 | 0 | 0 | 0 | 0 | 100.26 | 0.00 | 2.93 | 0.00 | 0.00 | 0.00 | 2.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 12 | EF8057-1572.3 | 0 | 40.32 | 0 | 0 | 58.30 | 0 | 0 | 0 | 0 | 98.62 | 0.00 | 2.87 | 0.00 | 0.00 | 0.00 | 2.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 13 | EF044-1801.05 | Met-II | 0 | 71.07 | 0 | 4.69 | 0 | 22.36 | 0 | 0 | 2.35 | 101.78 | 0.00 | 7.77 | 0.00 | 0.00 | 0.46 | 0.00 | 2.14 | 0.00 | 0.00 | 0.36 | 2.96 |
| 14 | EF0061-1603.3 | 0 | 70.93 | 0 | 0 | 0 | 25.11 | 0 | 0 | 3.58 | 99.62 | 0.00 | 7.96 | 0.00 | 0.00 | 0.00 | 0.00 | 2.46 | 0.00 | 0.00 | 0.57 | 3.04 | |
| 15 | EF0061-1603.3 | 0 | 71.90 | 0 | 0 | 0 | 24.73 | 0 | 0 | 2.98 | 101.84 | 0.00 | 7.75 | 0.00 | 0.00 | 0.00 | 0.00 | 2.33 | 0.00 | 0.00 | 0.46 | 2.79 | |
| 16 | EF0062-1591.3 | 0 | 69.84 | 0 | 0 | 0 | 29.68 | 0 | 0 | 0 | 99.52 | 0.00 | 8.02 | 0.00 | 0.00 | 0.00 | 0.00 | 2.98 | 0.00 | 0.00 | 0.00 | 2.98 | |
| 17 | EF0062-1591.3 | 0 | 69.73 | 0 | 0 | 0 | 30.08 | 0 | 0 | 0.76 | 100.57 | 0.00 | 7.90 | 0.00 | 0.00 | 0.00 | 0.00 | 2.98 | 0.00 | 0.00 | 0.12 | 3.10 | |
| 18 | EF0062-1591.3 | 0 | 68.85 | 0 | 0 | 0 | 29.39 | 0 | 0 | 0.67 | 98.91 | 0.00 | 7.93 | 0.00 | 0.00 | 0.00 | 0.00 | 2.96 | 0.00 | 0.00 | 0.11 | 3.07 | |
| 19 | EF0062-1591.3 | 0 | 68.11 | 0 | 0 | 0 | 29.43 | 0 | 0 | 0 | 98.43 | 0.00 | 7.84 | 0.00 | 0.00 | 0.00 | 0.00 | 2.96 | 0.00 | 0.00 | 0.00 | 2.96 | |
| 20 | EF0066-1726.05 | 0 | 68.72 | 0 | 0 | 0 | 30.28 | 0 | 0 | 0.86 | 99.86 | 0.00 | 7.84 | 0.00 | 0.00 | 0.00 | 3.02 | 0.00 | 0.00 | 0.14 | 3.16 | ||
| 21 | EF67-1724.65 | 0 | 70.28 | 0 | 9.12 | 0 | 16.48 | 0 | 0 | 3.30 | 99.18 | 0.00 | 7.93 | 0.00 | 0.00 | 0.92 | 0.00 | 1.62 | 0.00 | 0.00 | 0.53 | 3.07 | |
| 22 | EF67-1724.65 | 0 | 67.15 | 0 | 14.4 | 0 | 11.41 | 0 | 0 | 3.12 | 96.08 | 0.00 | 7.82 | 0.00 | 0.00 | 1.50 | 0.00 | 1.16 | 0.00 | 0.00 | 0.52 | 3.18 |
| # | Sample | Pt | Pd | Ni | As | Sb | Ge | Total (wt.%) | Pt (apfu) | Pd | Ni | As | Sb | As + Sb | Ge | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | EF0035-1546.2 | Spy | 56.36 | 0 | 0 | 41.47 | 1.41 | 0 | 99.24 | 1.01 | 0.00 | 0.00 | 1.94 | 0.04 | 1.98 | 0.00 |
| 2 | EF0035-1546.2 | 57.86 | 0 | 0 | 42.12 | 0.99 | 0 | 100.97 | 1.03 | 0.00 | 0.00 | 1.95 | 0.03 | 1.98 | 0.00 | |
| 3 | EF044-1801.05 | 53.68 | 1.20 | 0 | 40.88 | 1.30 | 0 | 97.06 | 0.98 | 0.04 | 0.00 | 1.94 | 0.04 | 1.98 | 0.00 | |
| 4 | EF044-1801.05 | 56.17 | 0 | 0 | 40.19 | 2.62 | 0 | 98.98 | 1.02 | 0.00 | 0.00 | 1.90 | 0.08 | 1.98 | 0.00 | |
| 5 | EF0062-1591.3 | 57.74 | 0 | 0 | 39.76 | 3.19 | 0 | 100.69 | 1.04 | 0.00 | 0.00 | 1.87 | 0.09 | 1.96 | 0.00 | |
| 6 | EF0063-1744.3 | 55.74 | 0 | 0 | 35.66 | 7.79 | 0 | 99.19 | 1.04 | 0.00 | 0.00 | 1.73 | 0.23 | 1.96 | 0.00 | |
| 7 | EF0063-1744.3 | 57.28 | 0 | 0 | 38.64 | 4.08 | 0 | 100.00 | 1.05 | 0.00 | 0.00 | 1.84 | 0.12 | 1.96 | 0.00 | |
| 8 | EF0066-1684.4 | 57.34 | 0 | 0 | 41.92 | 0.74 | 0 | 100.00 | 1.03 | 0.00 | 0.00 | 1.95 | 0.02 | 1.97 | 0.00 | |
| 9 | EF67-1712.0 | 57.97 | 0 | 0 | 43.48 | 1.04 | 0 | 102.49 | 1.01 | 0.00 | 0.00 | 1.96 | 0.03 | 1.99 | 0.00 | |
| 10 | EF67-1724.65 | Mjk | 0 | 43.50 | 20.57 | 31.18 | 0 | 0 | 95.25 | 0.00 | 1.04 | 0.89 | 1.06 | 0.00 | - | 0.00 |
| 11 | EF67-1724.65 | UN Pd11Ge3As2 | 0 | 75.44 | 0 | 10.12 | 0 | 14.06 | 99.62 | 0.00 | 10.93 | 0.00 | 2.08 | 0.00 | 2.08 | 2.99 |
| 12 | EF67-1724.65 | 0 | 72.60 | 0 | 10.46 | 0 | 14.23 | 97.29 | 0.00 | 10.72 | 0.00 | 2.19 | 0.00 | 2.19 | 3.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkov, A.Y.; Nikulin, I.I.; Nikiforov, A.A.; Lobastov, B.M.; Silyanov, S.A.; Martin, R.F. Atypical Mineralization Involving Pd-Pt, Au-Ag, REE, Y, Zr, Th, U, and Cl-F in the Oktyabrsky Deposit, Norilsk Complex, Russia. Minerals 2021, 11, 1193. https://doi.org/10.3390/min11111193
Barkov AY, Nikulin II, Nikiforov AA, Lobastov BM, Silyanov SA, Martin RF. Atypical Mineralization Involving Pd-Pt, Au-Ag, REE, Y, Zr, Th, U, and Cl-F in the Oktyabrsky Deposit, Norilsk Complex, Russia. Minerals. 2021; 11(11):1193. https://doi.org/10.3390/min11111193
Chicago/Turabian StyleBarkov, Andrei Y., Ivan I. Nikulin, Andrey A. Nikiforov, Boris M. Lobastov, Sergey A. Silyanov, and Robert F. Martin. 2021. "Atypical Mineralization Involving Pd-Pt, Au-Ag, REE, Y, Zr, Th, U, and Cl-F in the Oktyabrsky Deposit, Norilsk Complex, Russia" Minerals 11, no. 11: 1193. https://doi.org/10.3390/min11111193






