The Application of Thermal Decomposition for Determination of Carbonate Acid-Neutralising Capacity for Improved Acid Mine Drainage Prediction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineralogy and Bulk Assay
2.2. Acid Neutralisation Capacity by Titration
2.2.1. ANC Titration Methodology (1)—ANCtitrate-all
2.2.2. ANC Titration Methodology (2)—ANCtitrate-carb
2.3. Thermal Decomposition
3. Results
3.1. Single Minerals
3.1.1. Mineralogy and Bulk Assay
3.1.2. ANC by Titration—Methodology 1
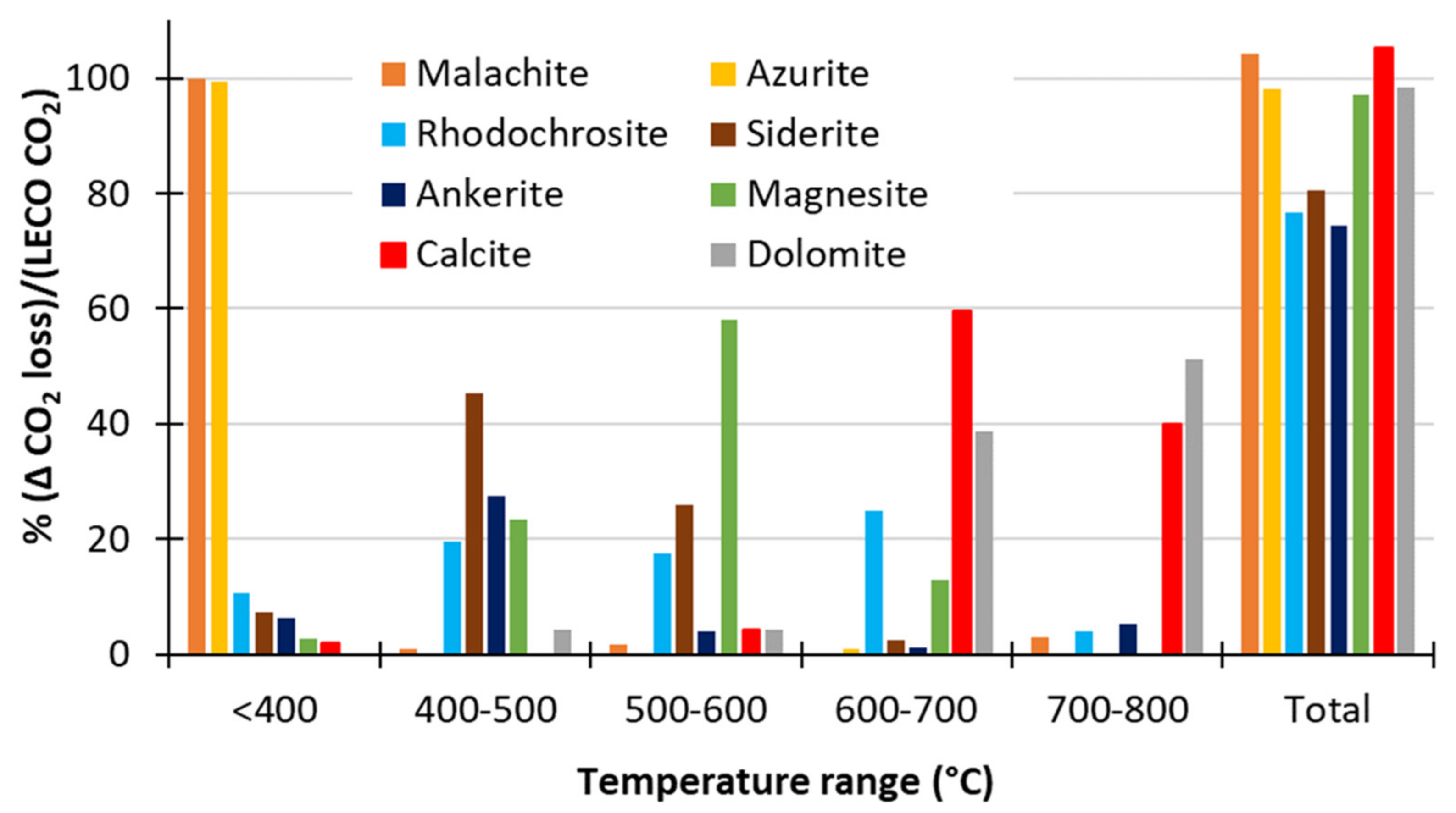
3.1.3. Thermal Decomposition
Using O2 as the Carrier Gas
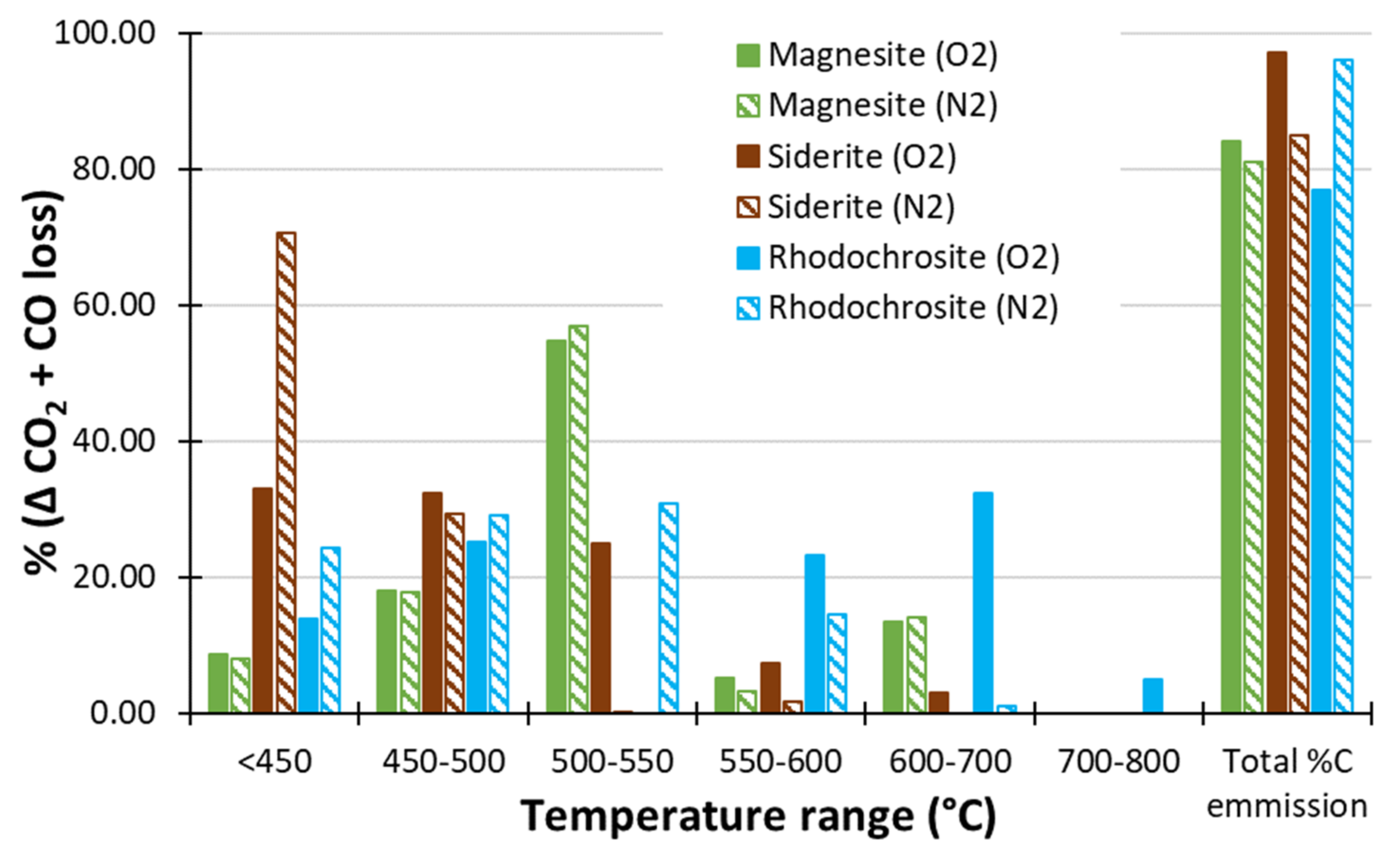
Using N2 as the Carrier Gas
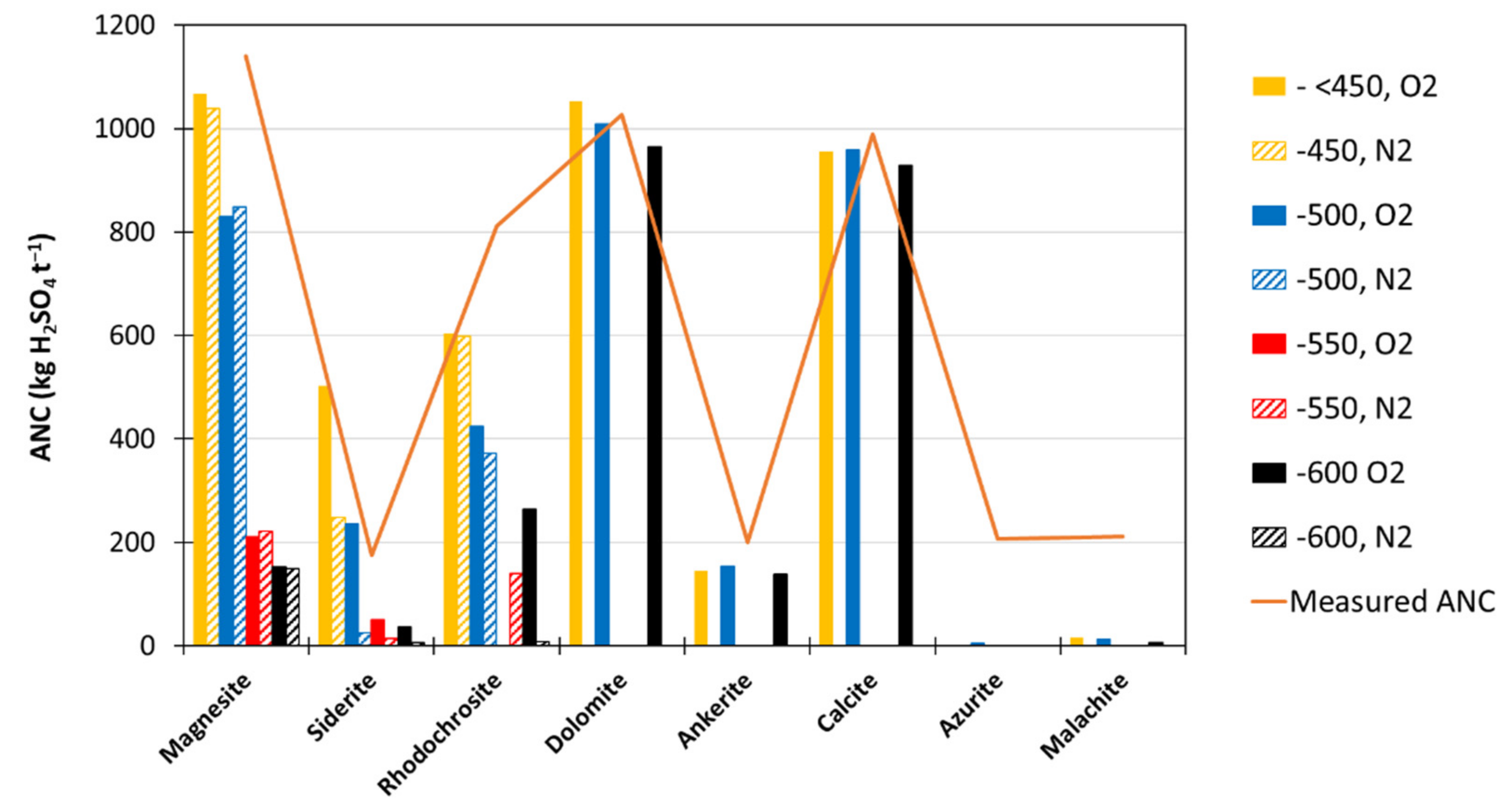
3.1.4. Thermal Decomposition ANC of Single-Carbonate Minerals
3.2. Mine Site Project Waste Rock Samples
- (1)
- Samples with the greatest difference between ANCtitrate-carb and ANCIC were chosen as these were likely to provide the most information on what is contributing to the ANC values. When ANCtitrate-carb minus ANCIC is positive it may be due to non-carbonate neutralisation and when this value is negative it may be due to the presence of siderite.
- (2)
- Samples that were categorised as uncertain where chosen, i.e., either NAG pH <4.5 and NAPP <0, or NAG pH >4.5 and NAPP >0 (for a description of the net acid generation, NAG, test refer to [4]).
- (3)
- Samples with either low or high NAG pH with little difference between ANCtitrate-carb and ANCIC were not chosen.
4. Conclusions
- Thermal decomposition of single-mineral carbonates in O2 carrier gas was found to give good separation of the decomposition of malachite and azurite (<400 °C, considered to be non-neutralising for the purposes of these analyses) and siderite (<600 °C) from calcite and dolomite (>600 °C).
- It was not possible to separate the decomposition events of siderite from rhodochrosite and magnesite in O2 carrier gas (400−600 °C).
- Thermal decomposition of single-mineral carbonates in N2 carrier gas depressed the thermal decomposition temperature of siderite (70% decomposition at <450 °C) enabling improved differentiation of siderite from rhodochrosite and magnesite.
- C emission due to ‘neutralising’ carbonates was calculated by subtracting carbon emission at 450 °C where available, otherwise 400 °C, from the carbon emission at the greatest temperature applied (800 or 1000 °C). Data collected using N2 as the carrier gas were used if available. Thermal ANC (ANCtherm-carb) was thereafter calculated as 30.6 × C wt% × 32.065/12.
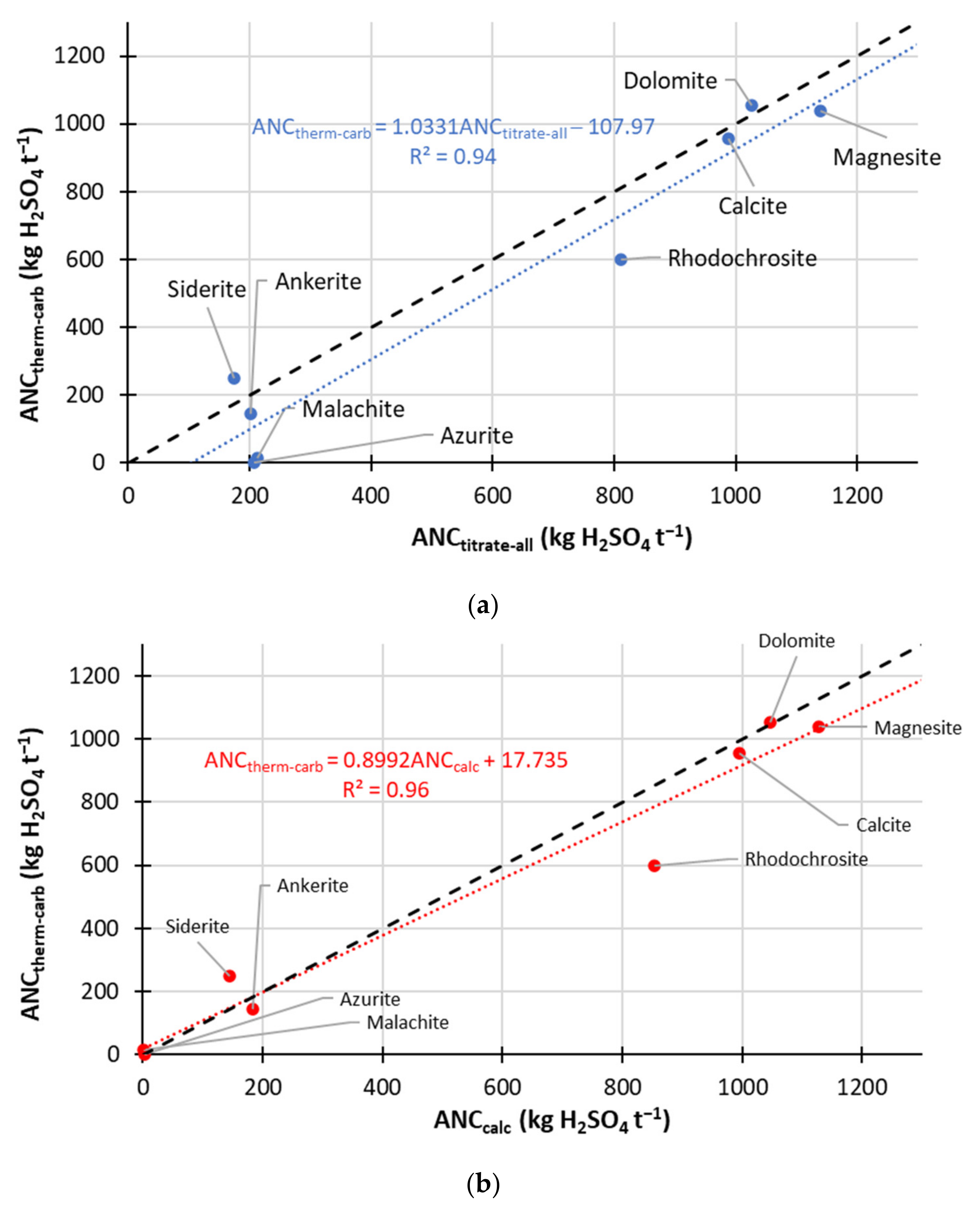
- Correlation of ANCcarb to calculated ANC (using Mg, K, Na, Ca and Mn) gave rise to the line of best fit of:with R2 of 0.96.ANCtherm-carb = (0.8992 × ANCcalc) + 17.735
- Based on this outcome the conditions of thermal decompositions for each sample at 450 and 1000 °C for 30 min in N2 carrier gas at flow rate of 100 mL min−1 were defined for examination of mine project waste rock samples.
- ANCtherm-carb determination using decomposition measurements at 450 °C (and 500 °C for one high siderite sample) and 1000 °C in N2 carrier gas (flow rate 100 mL min−1) gave excellent correlation to the titration ANCtitrate-carb (available for 18 samples, methodology (2)) values (that focused on carbonate ANC):ANCtherm-carb = (0.85 × ANCtitrate-carb) − 3.4691 (R2 = 0.96)
- This correlation is valid for samples containing both non-neutralising carbonates and sources of neutralisation arising from silicates and ion exchange.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- INAP. Global Acid Rock Drainage Guide. 2010. Available online: http://www.gardguide.com/index.php/Main_Page (accessed on 13 October 2021).
- Parbhakar-Fox, A.; Lottermoser, B.G. A critical review of acid rock drainage prediction methods and practices. Miner. Eng. 2015, 82, 107–124. [Google Scholar] [CrossRef]
- Smart, R.S.C.; Skinner, W.M.; Levay, G.; Gerson, A.R.; Thomas, J.E.; Sobieraj, H.; Schumann, R.; Weisener, C.G.; Weber, P.A.; Miller, S.D.; et al. ARD Test Handbook: Project P387, A Prediction and Kinetic Control of Acid Mine Drainage; AMIRA International Ltd.: Melbourne, Australia, 2002. [Google Scholar]
- INAP. 2009. Available online: http://www.gardguide.com/index.php?title=Chapter_6#6.6.7_Water_Cover_Methods (accessed on 13 October 2021).
- Jambor, J.L.; Dutrizac, J.E.; Raudsepp, M.; Groat, L.A. Effect of peroxide on neutralization-potential values of siderite and other carbonate minerals. J. Environ. Qual. 2003, 32, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.A.; Thomas, J.E.; Skinner, W.M.; Smart, R.S.C. Improved acid neutralisation capacity assessment of iron carbonates by titration and theoretical calculation. Appl. Geochem. 2004, 19, 687–694. [Google Scholar] [CrossRef]
- Dyantyi, N. Application of Mineralogy in the Interpretation of Laboratory Scale Acid Rock Drainage (ARD) Prediction Tests: A Gold Case Study. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2014. [Google Scholar]
- Gerson, A.R.; Rolley, P.J.; Davis, C.; Feig, S.T.; Doyle, S.; Smart, R.S.C. Unexpected non-acid drainage from sulfidic rock waste. Sci. Rep. 2019, 9, 4357. [Google Scholar] [CrossRef] [PubMed]
- Masindi, V.; Gitari, M.W.; Tutu, H.; DeBeer, M. Efficiency of ball milled South African bentonite clay for remediation of acid mine drainage. J. Water Process Eng. 2015, 8, 227–240. [Google Scholar] [CrossRef]
- Beck, C.W. Differential thermal analysis curves of carbonate minerals. Am. Mineral. 1950, 35, 985–1013. [Google Scholar]
- García, A.C.; Latifi, M.; Chaouki, J. Kinetics of calcination of natural carbonate minerals. Miner. Eng. 2020, 150, 106279. [Google Scholar] [CrossRef]
- Cuthbert, F.L.; Rowland, R.A. Differential thermal analysis of some carbonate minerals. Am. Mineral. 1947, 32, 111–116. [Google Scholar]
- Rowland, R.A. Differential thermal analysis of clays and carbonates. Clays Clay Technol. 1952, 1, 151–163. [Google Scholar] [CrossRef]
- Tian, L.; Tahmasebi, A.; Yu, J. An experimental study of the thermal decomposition behaviour of magnesite. J. Therm. Anal. Calorim. 2014, 118, 1577–1584. [Google Scholar] [CrossRef]
- Hammack, R.W. Evolved-gas analysis: A method for determining pyrite, marcasite, and alkaline-earth carbonates. In Environmental Geochemistry of Sulfide Oxidation; Alpers, C.N., Blowes, D.W., Eds.; American Chemical Society: Washington, DC, USA, 1994; Volume 550, pp. 431–444. [Google Scholar]
- LaCount, R.B.; Anderson, R.R.; Friedman, S.; Blaustein, B.D. Sulphur in coal by programmed-temperature oxidation. Fuel 1987, 66, 909–913. [Google Scholar] [CrossRef]
- LaCount, R.B.; Kern, D.G.; King, W.P.; LaCount, R.B.J.; Miltz, D.J.J.; Stewart, A.L.; Trulli, T.K.; Walker, D.K.; Wicker, R.K. Advances in coal characterization by programmed-temperature oxidation. Fuel 1993, 72, 1203–1208. [Google Scholar] [CrossRef]
- Dubrawski, J.V.; Warnes, S.S.J. Use of differential scanning calorimetry in measuring the thermal decomposition of mineral carbonates occurring in coal. Fuel 1987, 66, 1733–1736. [Google Scholar] [CrossRef]
- Kern, D.; LaCount, R.B.; Hammack, R.W. Improving the acid-base account by use programmed temperature oxidation and evolved gas analysis. In Proceedings of the 21st West Virginia Surface Mine Drainage Task Force Symposium, Morgantown, WV, USA, 4–5 April 2000. [Google Scholar]
- Kemp, S.J.; Wagner, D.; Mounteney, I. Low Level Detection and Quantification of Carbonate Species Using Thermogravimetric and Differential Thermal Analysis; British Geological Survey: Nottingham, UK, 2010; p. 35. [Google Scholar]
- Geelhoed, J.S.; Meeussen, J.C.L.; Hillier, S.; Lumsdon, D.G.; Thomas, R.P.; Farmer, J.G.; Paterson, E. Identification and geochemical modeling of processes controlling leaching of Cr(VI) and other major elements from chromite ore processing residue. Geochim. Cosmochim. Acta 2002, 66, 3927–3942. [Google Scholar] [CrossRef]
- Takeno, N. Atlas of Eh-pH Diagrams Intercomparison of Thermodynamic Databases; Geological Survey of Japan Open File Report No.419; National Institute of Advanced Industrial Science and Technology: Tokyo, Japan, 2005.

| Mineral Phase | Standard Stoichiometry | Magnesite | Siderite | Rhodochrosite | Dolomite | Calcite | Azurite | Malachite | Ankerite |
|---|---|---|---|---|---|---|---|---|---|
| Quartz | SiO2 | 0.2 | 2.5 | 4.3 | 0.5 | 0.2 | 0.8 | 0.3 | 44 |
| Calcite | CaCO3 | 1.1 | 99.8 | ||||||
| Dolomite | CaMg(CO3)2 | 17.9 | 98.5 | 7.6 | |||||
| Rhodochrosite | MnCO3 | 95.7 | |||||||
| Azurite | Cu3(CO3)2(OH)2 | 99.2 | |||||||
| Magnesite | MgCO3 | 81.9 | |||||||
| Malachite | Cu2CO3(OH)2 | 99.7 | |||||||
| Siderite | FeCO3 | 0.1 | 91.5 | 27.3 | |||||
| Ankerite | Ca(Fe2+,Mg)(CO3)2 | 7.2 | |||||||
| Hematite | Fe2O3 | 5.9 | |||||||
| Sphalerite | ZnS | 13.8 |
| ANCIC (LECO Total Carbon) | ANC (Ca, Mg Assay) | ANC (Ca, Mg, Na, K Assay) | ANCcalc (Ca, Mg, Na, K, Mn Assay) | ANCtitrate-all | |
|---|---|---|---|---|---|
| Dolomite | 1070 | 1027 | 1045 | 1047 | 1027 |
| Magnesite | 1168 | 1125 | 1127 | 1127 | 1140 |
| Calcite | 923 | 993 | 994 | 994 | 989 |
| Siderite | 907 | 144 | 144 | 144 | 175 |
| Malachite | 346 | 1 | 2 | 2 | 212 |
| Azurite | 433 | 3 | 3 | 3 | 207 |
| Rhodochrosite | 915 | 81 | 81 | 853 | 811 |
| Ankerite | 382 | 168 | 169 | 184 | 201 |
| Temperature (°C) | Magnesite | Dolomite | Calcite | Siderite | ||||
| Meas | Calc | Meas | Calc | Meas | Calc | Meas | Calc | |
| 400 | 1 | 1 | 0 | 0 | 1 | 1 | 3 | 3 |
| 500 | 14 | 14 | 2 | 2 | 1 | 1 | 21 | 21 |
| 600 | 44 | 44 | 4 | 4 | 2 | 2 | 32 | 32 |
| 700 | 51 | 51 | 23 | 23 | 27 | 27 | 33 | 33 |
| 800 | 51 | 51 | 47 | 47 | 44 | 44 | 33 | 33 |
| Temperature (°C) | Ankerite | Rhodochrosite | Azurite | Malachite | ||||
| Meas | Calc | Meas | Calc | Meas | Calc | Meas | Calc | |
| 400 | 1 | 1 | 4 | 4 | 31 | 23 | 28 | 22 |
| 500 | 6 | 6 | 12 | 12 | 30 | 23 | 28 | 22 |
| 600 | 7 | 7 | 20 | 20 | 31 | 23 | 28 | 22 |
| 700 | 7 | 7 | 30 | 30 | 30 | 23 | 28 | 22 |
| 800 | 8 | 8 | 31 | 32 | 30 | 23 | 29 | 23 |
| Temperature (°C) | Rhodochrosite | Magnesite | Siderite | |||
|---|---|---|---|---|---|---|
| Meas | Calc | Meas | Calc | Meas | Calc | |
| 450 | 8 | 7 | 3.3 | 3 | 24 | 23 |
| 500 | 17 | 18 | 12 | 12 | 34 | 32 |
| 550 | 27 | 28 | 43 | 40 | 35 | 32 |
| 600 | 34 | 34 | 44 | 43 | 35 | 32 |
| 700 | 35 | 34 | 50 | 50 | 36 | 34 |
| Sample # | Ankerite (wt%) | Calcite (wt%) | Dolomite (wt%) | Siderite (wt%) | Total C (wt%) | ANCIC | ANCtitrate-carb | ANCtitrate-carb/ANCIC |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.1 | 0.2 | 0.1 | 0.08 | 7 | 8 | 1.2 | |
| 2 | 1.3 | 0.24 | 20 | 16 | 0.8 | |||
| 3 | 5.8 | 1.4 | 1.04 | 85 | 118 | 1.4 | ||
| 4 | 4.5 | 0.9 | 4.6 | 1.26 | 103 | 100 | 1.0 | |
| 5 | 2.2 | 1.7 | 0.64 | 52 | 60 | 1.1 | ||
| 6 | 2.7 | 8.5 | 1.62 | 132 | 138 | 1.0 | ||
| 7 | 1.3 | 1.2 | 2.6 | 0.1 | 0.70 | 57 | NA | NA |
| 8 | 1.6 | 2.9 | 0.1 | 0.78 | 64 | NA | NA | |
| 9 | 3.1 | 12.7 | 2.36 | 193 | 170 | 0.9 | ||
| 10 | 5.9 | 3.4 | 1.28 | 105 | 99 | 0.9 | ||
| 11 | 6.3 | 1.5 | 7.2 | 2.14 | 175 | 148 | 0.8 | |
| 12 | 1.5 | 0.24 | 20 | 12 | 0.6 | |||
| 13 | 2.1 | 0.28 | 23 | 12 | 0.5 | |||
| 14 | 4.0 | 0.44 | 36 | 11 | 0.3 | |||
| 15 | 8.6 | 1.06 | 87 | 16 | 0.2 | |||
| 16 | 4.6 | 0.60 | 49 | 8 | 0.2 | |||
| 17 | 2.8 | 0.48 | 39 | 9 | 0.2 | |||
| 18 | 2.1 | 0.34 | 28 | 8 | 0.3 | |||
| 19 | 1.6 | 0.26 | 21 | 3 | 0.1 | |||
| 20 | 2.3 | 0.34 | 28 | 11 | 0.4 |
| Sample # | 450 °C | 1000 °C | ANCtherm-carb (kg H2SO4 t−1) | ||||
|---|---|---|---|---|---|---|---|
| Generated CO (% of Possible Total) | Generated CO2 (% of Possible Total) | CO + CO2 (% as CO2 Combined, of Possible Total) | Generated CO (% of Possible Total) | Generated CO2 (% of Possible Total) | CO + CO2 (% as CO2 Combined, of Possible Total) | ||
| 1 | 5.6 | 61 | 70 | 0.0 | 93 | 93 | 2 |
| 2 | 2.2 | 69 | 72 | 0.0 | 96 | 96 | 5 |
| 3 | 0.3 | 17 | 17 | 0.1 | 97 | 97 | 68 |
| 4 | 0.4 | 8.5 | 9.2 | 0.0 | 91 | 91 | 84 |
| 5 | 0.7 | 11 | 12 | 0.1 | 102 | 102 | 47 |
| 6 | 0.5 | 14 | 15 | 0.6 | 90 | 91 | 101 |
| 7 | 0.8 | 13 | 14 | 0.0 | 95 | 95 | 46 |
| 8 | 0.5 | 14 | 15 | 0.0 | 96 | 96 | 52 |
| 9 | 0.4 | 9.2 | 10 | 1.5 | 83 | 86 | 147 |
| 10 | 0.2 | 10 | 10 | 0.5 | 97 | 98 | 92 |
| 11 | 0.1 | 6.5 | 6.7 | 0.00 | 89 | 89 | 144 |
| 12 | 1.2 | 75 | 77 | 0.5 | 99 | 100 | 5 |
| 13 | 0.6 | 60 | 61 | 0.5 | 98 | 98 | 8 |
| 14 | 1.4 | 89 | 92 | 0.0 | 97 | 97 | 2 |
| 15 | 0.7 | 45 | 46 | 0.5 | 97 | 98 | 45 |
| 15 * | 0.4 | 82 | 83 | 0.5 | 97 | 98 | 13 |
| 16 | 2.7 | 82 | 87 | 0.0 | 101 | 101 | 7 |
| 17 | 1.9 | 88 | 91 | 0.0 | 97 | 97 | 2 |
| 18 | 2.0 | 90 | 94 | 0.2 | 108 | 108 | 4 |
| 19 | 3.5 | 75 | 81 | 1.5 | 98 | 100 | 4 |
| 20 | 1.9 | 63 | 66 | 1.4 | 98 | 100 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerson, A.R.; Weber, P.; Smart, R.S.C.; Levay, G.; Hutton-Ashkenny, M.; Green, R. The Application of Thermal Decomposition for Determination of Carbonate Acid-Neutralising Capacity for Improved Acid Mine Drainage Prediction. Minerals 2021, 11, 1181. https://doi.org/10.3390/min11111181
Gerson AR, Weber P, Smart RSC, Levay G, Hutton-Ashkenny M, Green R. The Application of Thermal Decomposition for Determination of Carbonate Acid-Neutralising Capacity for Improved Acid Mine Drainage Prediction. Minerals. 2021; 11(11):1181. https://doi.org/10.3390/min11111181
Chicago/Turabian StyleGerson, Andrea R., Paul Weber, Roger St. C. Smart, George Levay, Mike Hutton-Ashkenny, and Rosalind Green. 2021. "The Application of Thermal Decomposition for Determination of Carbonate Acid-Neutralising Capacity for Improved Acid Mine Drainage Prediction" Minerals 11, no. 11: 1181. https://doi.org/10.3390/min11111181
APA StyleGerson, A. R., Weber, P., Smart, R. S. C., Levay, G., Hutton-Ashkenny, M., & Green, R. (2021). The Application of Thermal Decomposition for Determination of Carbonate Acid-Neutralising Capacity for Improved Acid Mine Drainage Prediction. Minerals, 11(11), 1181. https://doi.org/10.3390/min11111181







