Linking Automated Scanning Electron Microscope Based Investigations to Chemical Analysis for an Improved Understanding of Ash Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Description
2.2. X-ray Fluorescence (XRF)
2.3. X-ray Diffraction (XRD)
2.4. Scanning Electron Microscopy with Energy Dispersive X-ray Analysis (SEM-EDX)
2.5. Inductively Coupled Plasma—Optical Emission Spectrometry (ICP-OES)
2.6. Laser Diffractometry
3. Results and Discussion
3.1. Elemental Compositions
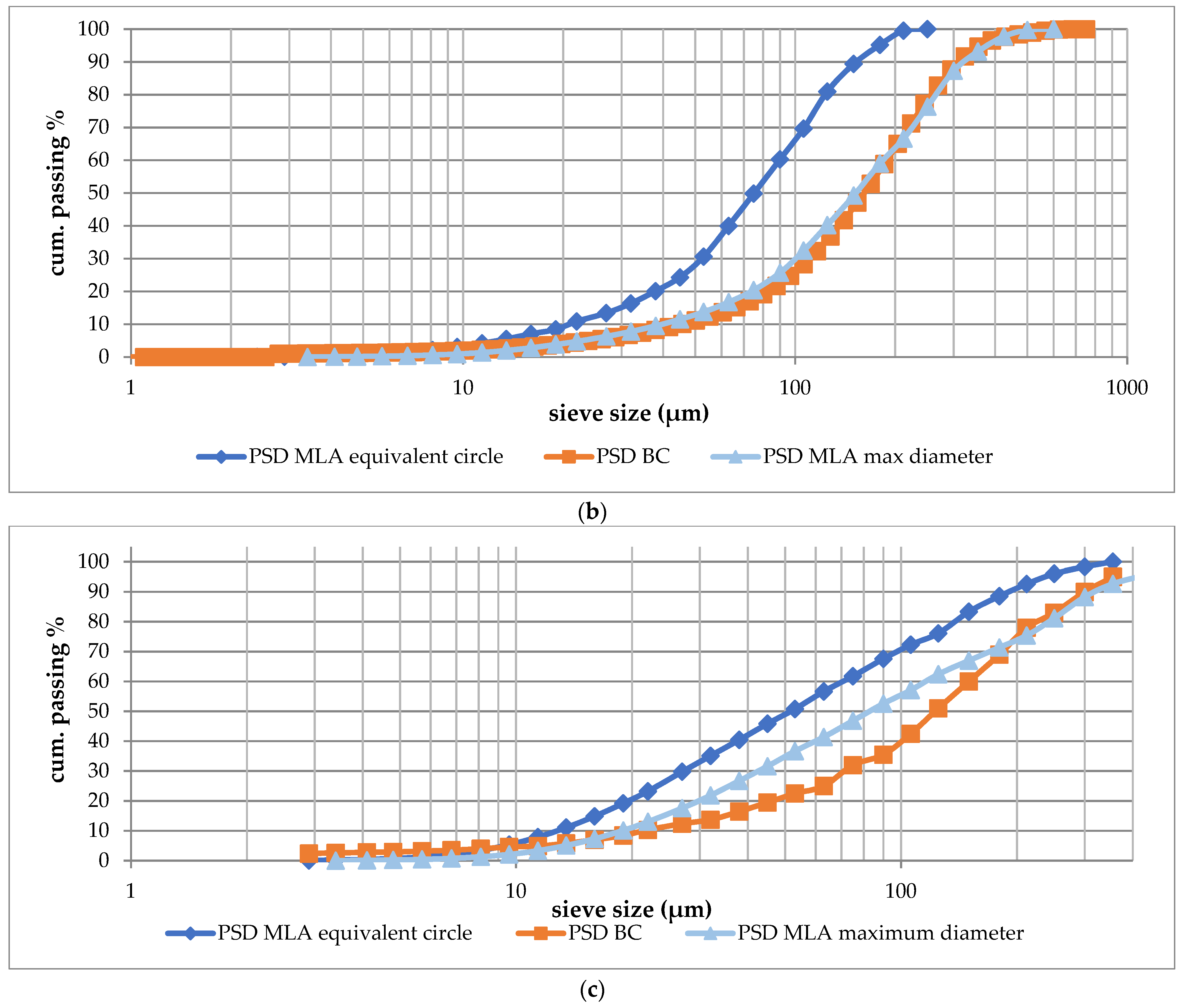
3.2. Particle Size Distribution Curves (PSD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berna, F.; Goldberg, P.; Horwitz, L.K.; Brink, J.; Holt, S.; Bamford, M.; Chazan, M. Microstrati-graphic evidence of in situ fire in the Acheulean strata of Wonderwerk Cave, Northern Cape province, South Africa. Proc. Natl. Acad. Sci. USA 2012, 10, E1215–E1220. [Google Scholar] [CrossRef] [Green Version]
- Hakbijl, T. The Traditional, Historical and Prehistoric Use of Ashes as an Insecticide, with an Experimental Study on the Insecticidal Efficacy of Washed Ash. Environ. Archaeol. 2002, 7, 13–22. [Google Scholar] [CrossRef]
- Palmer, T. On the ASH of tillandsia usneoides. J. Frankl. Inst. 1889, 127, 131–133. [Google Scholar] [CrossRef]
- Berzelius, J. Analyse der Asche. Arch. Pharm. Pharm. Med. Chem. 1847, 100, 177–181. [Google Scholar]
- Nadkarni, R.A. Multitechnique multielemental analysis of coal and fly ash. Anal. Chem. 1980, 52, 929–935. [Google Scholar] [CrossRef]
- Chand, P.; Kumar, A.; Gaur, A.; Mahna, S.K. Elemental analysis of ash using X-ray fluo-rescence technique. Asian J. Chem. 2009, 21, 220–224. [Google Scholar]
- An, S.; Norlin, B.; Hummelgård, M.; Thungström, G. Comparison of Elemental Analysis Tech-niques for Fly Ash from Municipal Solid Waste Incineration using X-rays and Electron Beams. IOP Conf. Ser. Earth Environ. Sci. 2019, 337, 12007. [Google Scholar] [CrossRef]
- Schulz, B.; Sandmann, D.; Gilbricht, S. SEM-based Automated Mineralogy and its Application in Geo- and Material Sciences. Minerals 2020, 10, 1004. [Google Scholar] [CrossRef]
- Guhl, A.C.; Greb, V.-G.; Schulz, B.; Bertau, M. An Improved Evaluation Strategy for Ash Analysis Using Scanning Electron Microscope Automated Mineralogy. Minerals 2020, 10, 484. [Google Scholar] [CrossRef]
- Kamon, M.; Hartlen, J.; Katsumi, T. Reuse of waste and its environmental impact. In Proceedings of the International Conference on Geotechnical and Geological Engineering (GeoEng2000), Melbourne, Australia, 19–24 November 2000; Volume 1, pp. 1095–1123. [Google Scholar]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Keller, V.; Stopic, S.; Xakalashe, B.; Ma, Y.; Ndlovu, S.; Mwewa, B.; Simate, G.; Friedrich, B. Effectiveness of Fly Ash and Red Mud as Strategies for Sustainable Acid Mine Drainage Management. Minerals 2020, 10, 707. [Google Scholar] [CrossRef]
- US Geological Survey. Mineral Commodity Summaries 2020; US Geological Survey: Reston, VA, USA, 2020. Available online: https://pubs.usgs.gov/periodicals/mcs2020/mcs2020.pdf (accessed on 18 October 2021).
- Guo, C.; Zou, J.; Ma, S.; Yang, J.; Wang, K. Alumina Extraction from Coal Fly Ash via Low-Temperature Potassium Bisulfate Calcination. Minerals 2019, 9, 585. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Joshi, P.; Preda, D.V.; Gamliel, D.P.; Mohler, D.T.; Wiseman, J.D.; Hopps, S.D.; Morgan, T.D.; Beers, T.; et al. Distribution of Lanthanides, Yttrium, and Scandium in the Pilot-Scale Beneficiation of Fly Ashes Derived from Eastern Kentucky Coals. Minerals 2020, 10, 105. [Google Scholar] [CrossRef] [Green Version]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Bartzsch, A.; Gilbricht, S.; Bachmann, K.; Heinig, T. A Method of Preparing a Sample Preparation, Sample Preparation, and Method of Assaying a Sample Material; Helmholtz-Zentrum Dresden Rossendorf: Dresden, Germany, 2017; eV on 17.01.2017. [Google Scholar]
- Kleemann, R.; Chenoweth, J.; Clift, R.; Morse, S.; Pearce, P.; Sajorj, D. Comparison of phosphorus recovery from incinerated sewage sludge ash (ISSA) and pyrolysed sewage sludge char (PSSC). Waste Manag. 2016, 60, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Benassi, L.; Zanoletti, A.; Depero, L.; Bontempi, E. Sewage sludge ash recovery as valuable raw material for chemical stabilization of leachable heavy metals. J. Environ. Manag. 2019, 245, 464–470. [Google Scholar] [CrossRef]
- Anderson, M. Encouraging prospects for recycling incinerated sewage sludge ash (ISSA) into clay-based building products. J. Chem. Technol. Biotechnol. 2002, 77, 352–360. [Google Scholar] [CrossRef]
- Smol, M.; Adam, C.; Kugler, S.A. Inventory of Polish municipal sewage sludge ash (SSA)—Mass flows, chemical composition, and phosphorus recovery potential. Waste Manag. 2020, 116, 31–39. [Google Scholar] [CrossRef]
- Baldi, M.; Martinotti, A.; Sorlini, S.; Katsoyiannis, I.; Abbà, A.; Miino, M.C.; Collivignarelli, M. Extraction and Purification of Phosphorus from the Ashes of Incinerated Biological Sewage Sludge. Water 2021, 13, 1102. [Google Scholar] [CrossRef]
- Prabhakar, A.K.; Mohan, B.C.; Tay, T.S.; Lee, S.S.C.; Teo, S.L.M.; Wang, C.H. Incinerated Sewage Sludge Bottom Ash- Chemical pro-cessing, Leaching patterns and Toxicity testing. J. Hazard. Mater. 2020, 402, 123350. [Google Scholar] [CrossRef]
- Guhl, A.C.; Gilbricht, S.; Pätzold, C.; Schulz, B.; Bertau, M. Wastewater treatment plant processes affect P-phases in sewage sludge ashes. Miner. Eng. 2021, 173, 107138. [Google Scholar] [CrossRef]





| Sample | Particles, Horizontal | Particles, Vertical | Grains, Horizontal | Grains, Vertical |
|---|---|---|---|---|
| Leipzig | 17,536 | 23,803 | 142,299 | 188,810 |
| Dresden | 14,139 | 15,742 | 170,342 | 187,086 |
| Chemnitz | 30,217 | 32,452 | 169,603 | 173,353 |
| Phase | XRD | MLA | ||
|---|---|---|---|---|
| Leipzig | Dresden | Chemnitz | ||
| Quartz | detected | 7.58% | 10.54% | 9.09% |
| Cristobalite high | detected | |||
| Iron Oxide | detected | 0.46% | 0.25% | 0.63% |
| Ca9Al(PO4)7 | detected | Not found | Not found | Not found |
| Ca9Fe(PO4)7 | detected | 0.59% | 0.51% | 0.23% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guhl, A.C.; Pavón, S.; Schulz, B.; Bertau, M. Linking Automated Scanning Electron Microscope Based Investigations to Chemical Analysis for an Improved Understanding of Ash Characteristics. Minerals 2021, 11, 1182. https://doi.org/10.3390/min11111182
Guhl AC, Pavón S, Schulz B, Bertau M. Linking Automated Scanning Electron Microscope Based Investigations to Chemical Analysis for an Improved Understanding of Ash Characteristics. Minerals. 2021; 11(11):1182. https://doi.org/10.3390/min11111182
Chicago/Turabian StyleGuhl, Andrea C., Sandra Pavón, Bernhard Schulz, and Martin Bertau. 2021. "Linking Automated Scanning Electron Microscope Based Investigations to Chemical Analysis for an Improved Understanding of Ash Characteristics" Minerals 11, no. 11: 1182. https://doi.org/10.3390/min11111182
APA StyleGuhl, A. C., Pavón, S., Schulz, B., & Bertau, M. (2021). Linking Automated Scanning Electron Microscope Based Investigations to Chemical Analysis for an Improved Understanding of Ash Characteristics. Minerals, 11(11), 1182. https://doi.org/10.3390/min11111182







