Biogenic Platinum Nanoparticles’ Production by Extremely Acidophilic Fe(III)-Reducing Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Pt(IV) Tolerance Test
2.3. Pt(IV) Reduction for Bio-Pt(0)NPs’ Production
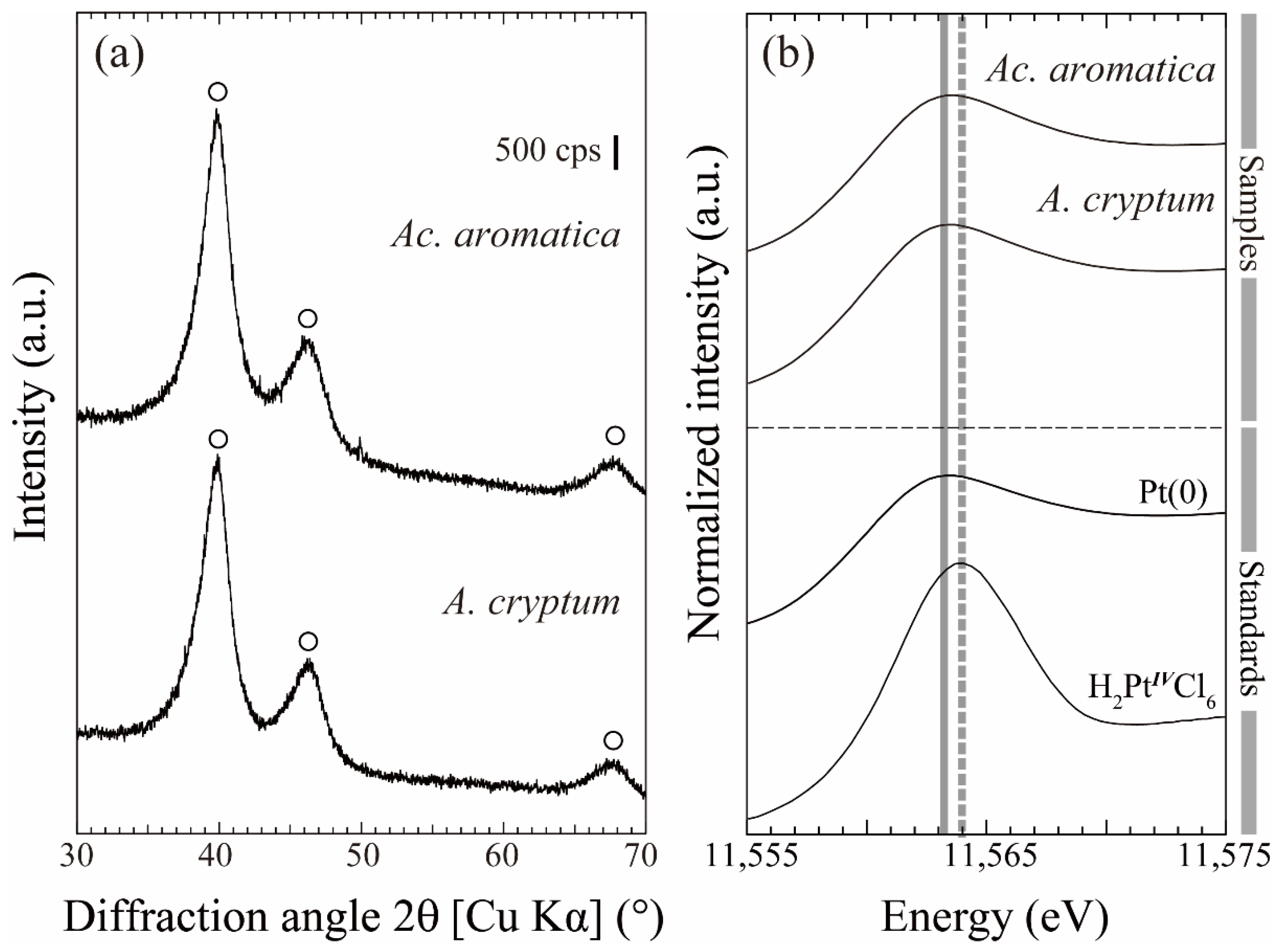
2.4. Characterization of Bio-Pt(0)NPs by X-ray Diffraction (XRD) and X-ray Absorption Fine Structure (XAFS)
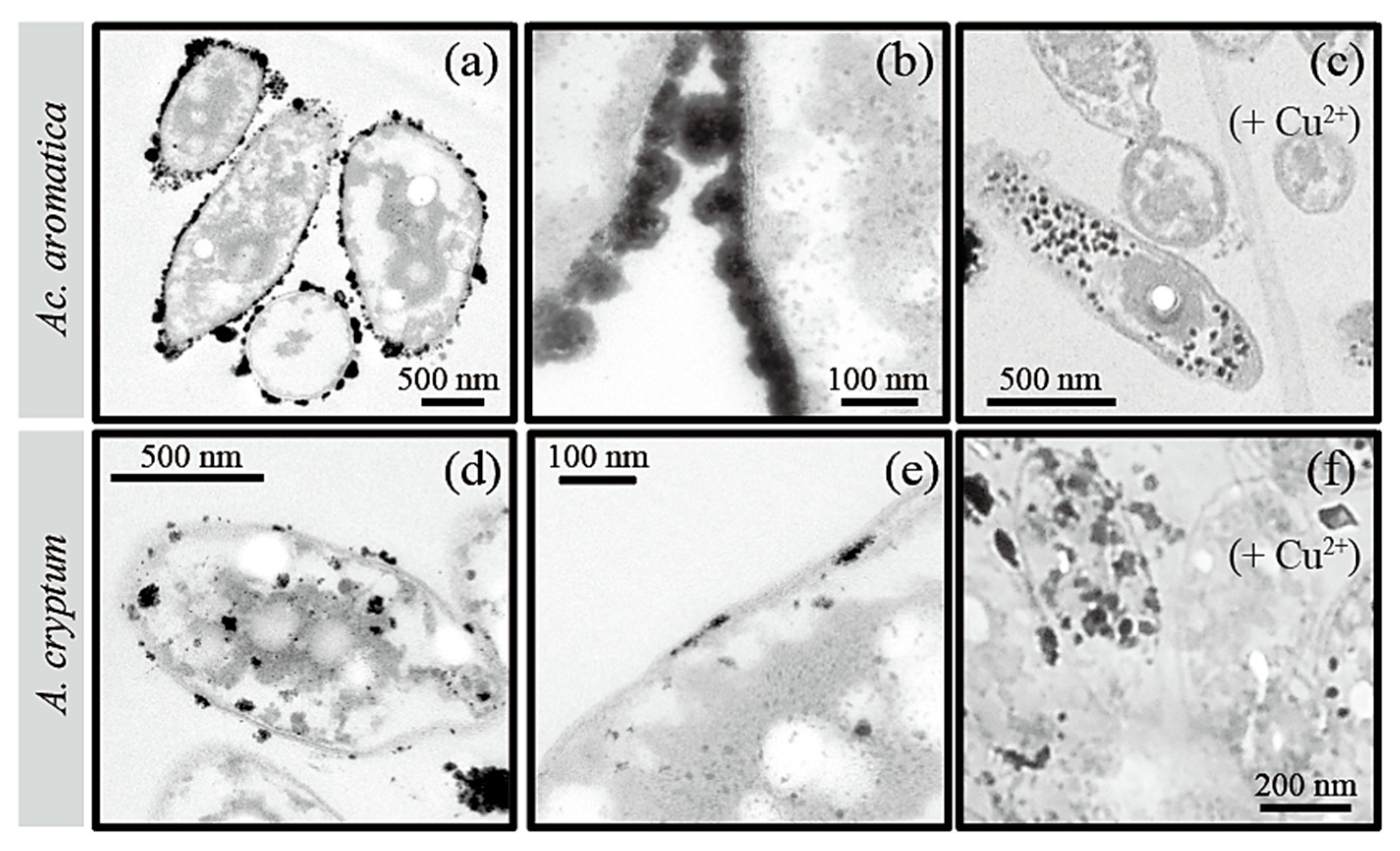
2.5. Ultra-Thin Section Transmission Electron Microscopy (TEM) Observation
2.6. Particle Size Analysis Using Image-J
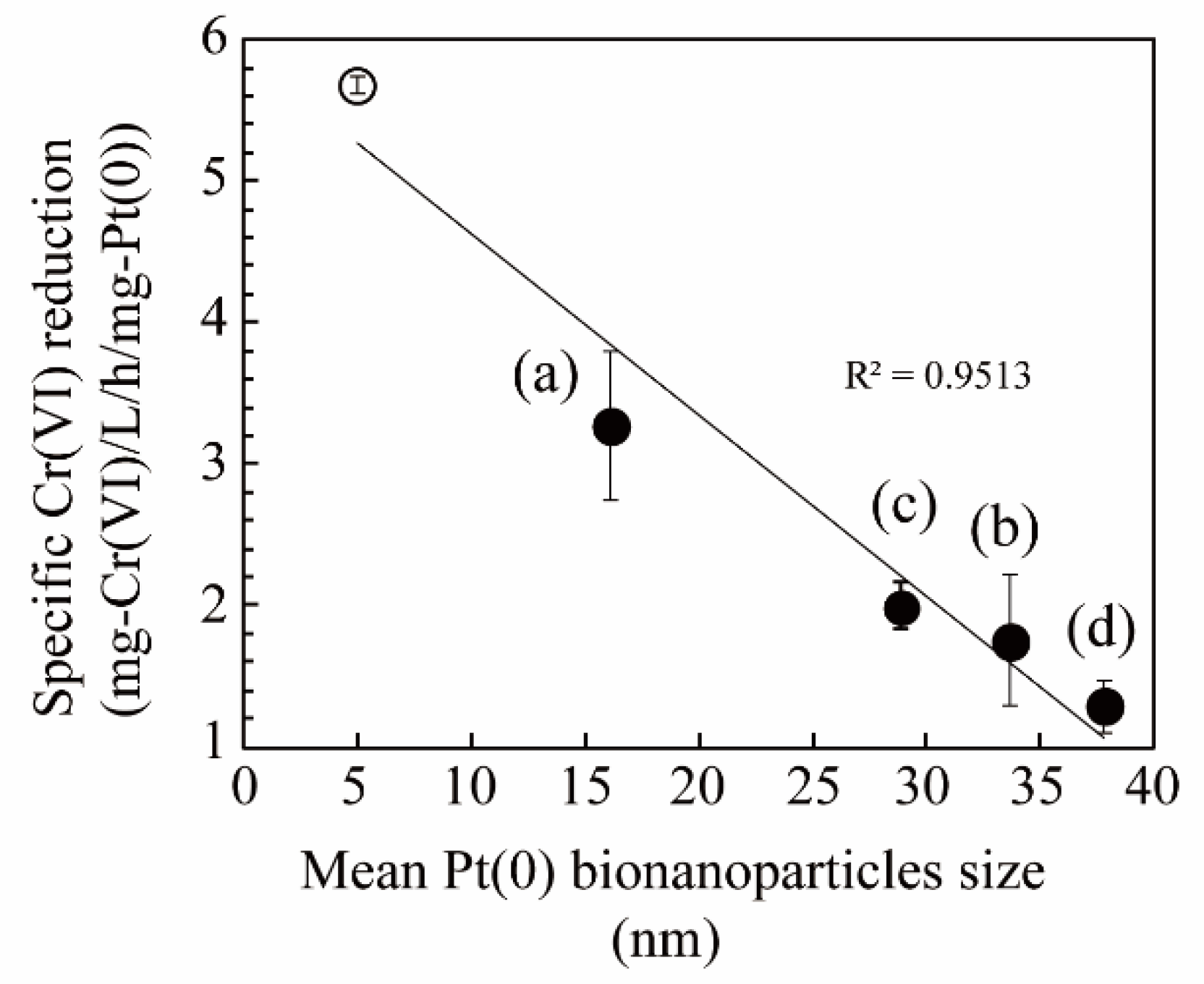
2.7. Catalytic Activity of Bio-Pt(0)NPs
3. Results and Discussion
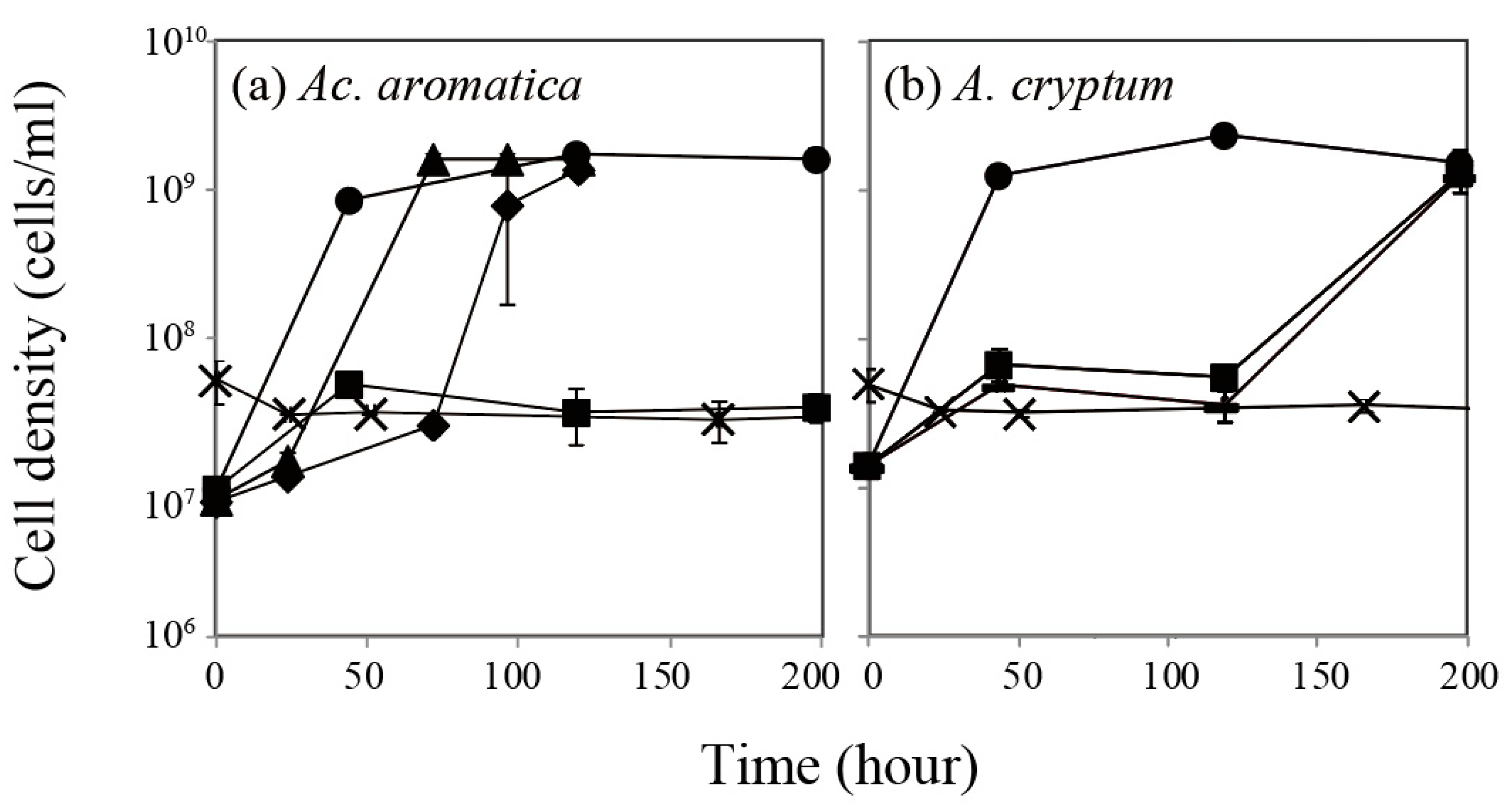
3.1. Effect of Pt(IV) on Bacterial Cell Growth
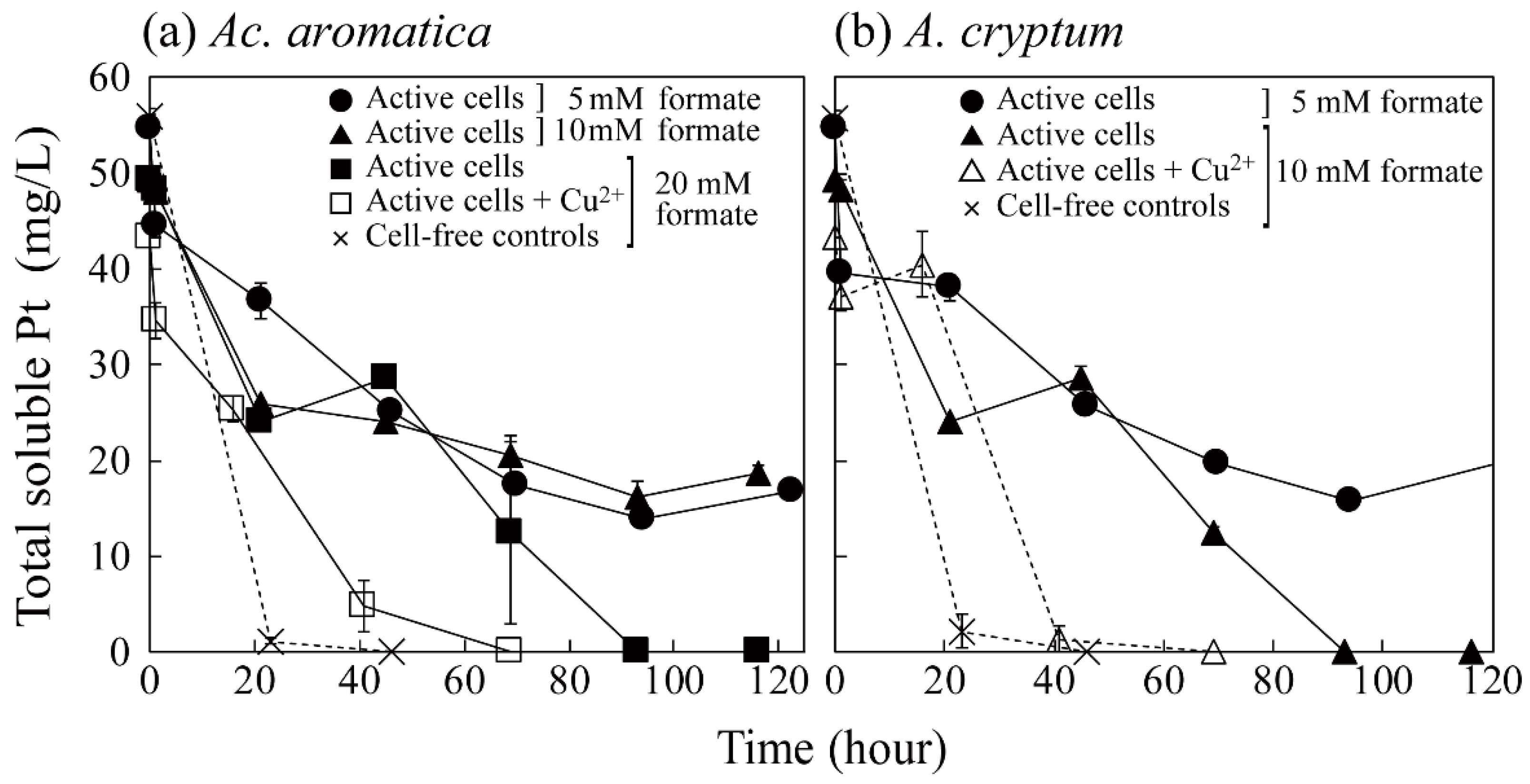
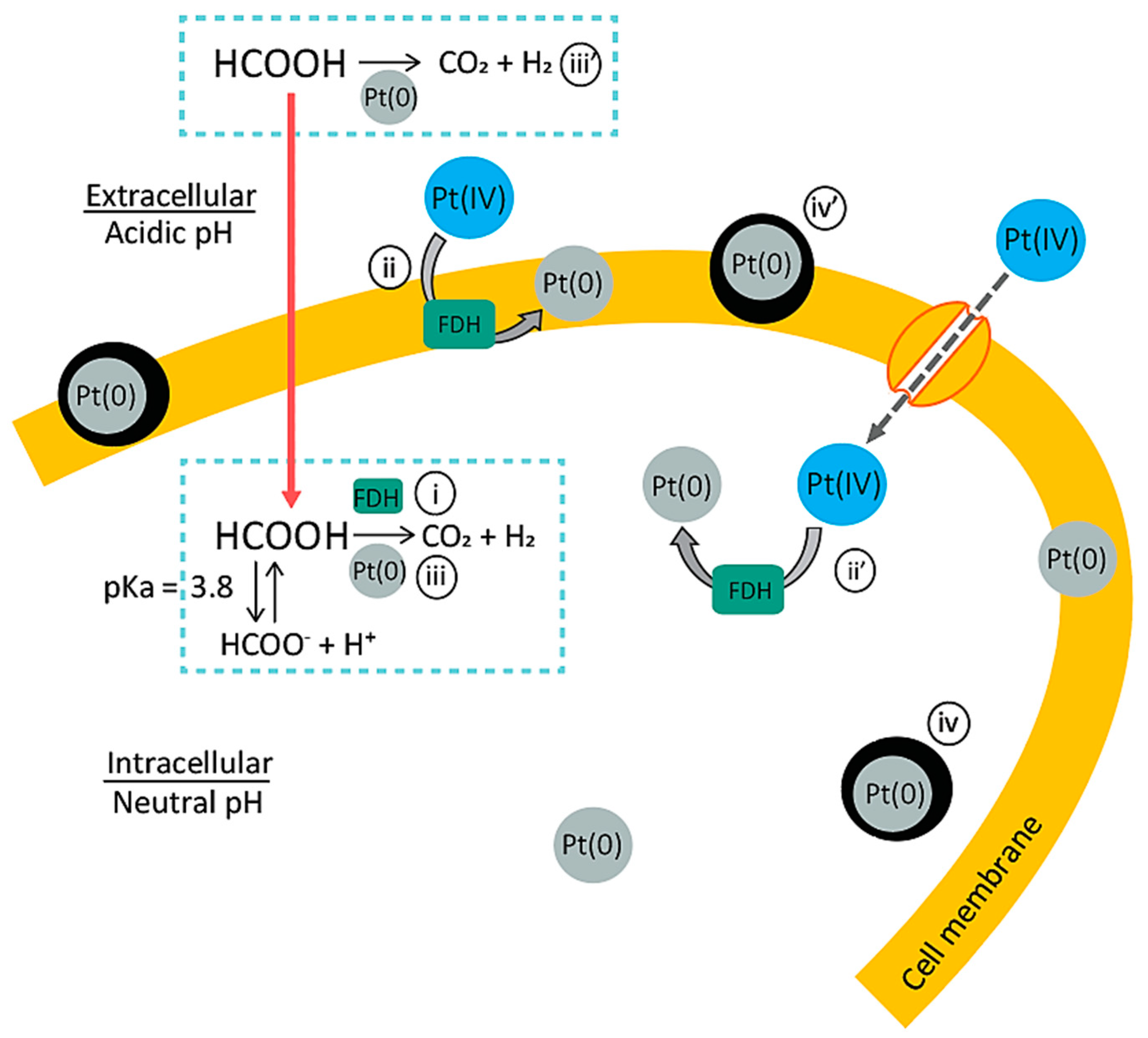
3.2. Pt(IV) Reduction for the Production of Bio-Pt(0)NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bönnemann, H.; Richards, R.M. Nanoscopic metal particles synthetic methods and potential applications. Eur. J. Inorg. Chem. 2001, 10, 2455–2480. [Google Scholar] [CrossRef]
- Bloch, K.; Pardesi, K.; Satriano, C.; Ghosh, S. Bacteriogenic platinum nanoparticles for application in nanomedicine. Front. Chem. 2021, 9, 624344. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Thakkar, M.S.; Snehit, S.; Mhatre, M.S.; Rasesh, Y.; Parikh, M.S. Biological synthesis of metallic nanoparticles. Nanomedicine 2010, 6, 257–262. [Google Scholar]
- Srivastava, S.K.; Constanti, M. Room temperature biogenic synthesis of multiple nanoparticles (Ag, Pd, Fe, Rh, Ni, Ru, Pt, Co, and Li) by Pseudomonas aeruginosa SM1. J. Nanoparticle Res. 2012, 14, 831. [Google Scholar] [CrossRef]
- Kitjanukit, S.; Sasaki, K.; Okibe, N. Production of highly catalytic, archaeal Pd(0) bionanoparticles using Sulfolobus tokodaii. Extremophiles 2019, 23, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Rizki, I.N.; Okibe, N. Size-controlled production of gold bionanoparticles using the extremely acidophilic Fe(III)-reducing bacterium, Acidocella aromatica. Minerals 2018, 8, 81. [Google Scholar] [CrossRef] [Green Version]
- Aritonang, H.F.; Onggo, D.; Ciptati, C.; Radiman, C.L. Synthesis of platinum nanoparticles from K2PtCl4 solution using bacterial cellulose matrix. J. Nanoparticles 2014, 2014, 285954. [Google Scholar] [CrossRef] [Green Version]
- Gaidhani, S.V.; Yeshvekar, R.K.; Shedbalkar, U.U.; Bellare, J.H.; Chopade, B.A. Bio-reduction of hexachloroplatinic acid to platinum nanoparticles employing Acinetobacter calcoaceticus. Process Biochem. 2014, 49, 2313–2319. [Google Scholar] [CrossRef]
- Martins, M.; Mourato, C.; Sanches, S.; Noronha, J.P.; Crespo, M.T.B.; Pereira, I.A.C. Biogenic platinum and palladium nanoparticles as new catalysts for the removal of pharmaceutical compounds. Water Res. 2017, 108, 160–168. [Google Scholar] [CrossRef]
- Capeness, M.J.; Edmundson, M.C.; Horsfall, L.E. Nickel and platinum group metal nanoparticle production by Desulfovibrio alaskensis G20. New Biotechnol. 2015, 32, 727–731. [Google Scholar] [CrossRef] [Green Version]
- Attard, G.; Casadesús, M.; Macaskie, L.E.; Deplanche, K. Biosynthesis of platinum nanoparticles by Escherichia coli MC4100: Can such nanoparticles exhibit intrinsic surface enantioselectivity? Langmuir 2012, 28, 5267–5274. [Google Scholar] [CrossRef]
- Konishi, Y.; Ohnoa, K.; Saitoh, N.; Nomura, T.; Nagamine, S.; Hishida, H.; Takahashi, Y.; Uruga, T. Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella Algae. J. Biotechnol. 2007, 128, 648–653. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, Y.; Xu, M.; Cui, H.; Tan, L.; Feng, N.; Liu, X.; Qiu, G.; Dong, H.; Xie, J. Microbial synthesis of Pd-Pt alloy nanoparticles using Shewanella oneidensis MR-1 with enhanced catalytic activity for nitrophenol and azo dyes reduction. Nanotechnology 2019, 30, 065607. [Google Scholar] [CrossRef] [PubMed]
- Eramabadi, P.; Masoudi, M.; Makhdoumi, A.; Mashreghi, M. Microbial cell lysate supernatant (CLS) alteration impact on platinum nanoparticles fabrication, characterization, antioxidant and antibacterial activity. Mater. Sci. Eng. C 2020, 117, 111292. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, B.; Muthukumarasamy, A.; Chidambaram, S.; Sugumaran, A.; Ramachandran, K.; Manimuthu, T.R. Cytotoxic potentials of biologically fabricated platinum nanoparticles from Streptomyces sp. on MCF-7 breast cancer cells. IET Nanobiotechnol. 2017, 11, 241–246. [Google Scholar] [CrossRef]
- Riddin, T.L.; Govender, Y.; Gericke, M.; Whiteley, C.G. Two different hydrogenase enzymes from sulphate-reducing bacteria are responsible for the bioreductive mechanism of platinum into nanoparticles. Enzyme Microb. Technol. 2009, 45, 267–273. [Google Scholar] [CrossRef]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Synthesis of platinum nanoparticles by reaction of filamentous cyanobacteria with platinum(IV)−chloride complex. Langmuir 2006, 22, 7318–7323. [Google Scholar] [CrossRef]
- Brayner, R.; Barberousse, H.; Hemadi, M.; Djedjat, C.; Yéprémian, C.; Coradin, T.; Livage, J.; Fiévet, F.; Couté, A. Cyanobacteria as bioreactors for the synthesis of Au, Ag, Pd, and Pt nanoparticles via an enzyme-mediated route. J. Nanosci. Nanotechnol. 2007, 7, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
- Maes, S.; Props, R.; Fitts, J.P.; De Smet, R.; Vilchez-Vargas, R.; Vital, M.; Pieper, D.H.; Vanhaecke, F.; Boon, N.; Hennebel, T. Platinum recovery from synthetic extreme environments by halophilic bacteria. Environ. Sci. Technol. 2016, 50, 2619–2626. [Google Scholar] [CrossRef]
- Okibe, N.; Nakayama, D.; Matsumoto, T. Palladium bionanoparticles production from acidic Pd(II) solutions and spent catalyst leachate using acidophilic Fe(III)-reducing bacteria. Extremophiles 2017, 21, 1091–1100. [Google Scholar] [CrossRef]
- Okibe, N.; Maki, M.; Nakayama, D.; Sasaki, K. Microbial recovery of vanadium by the acidophilic bacterium. Acidocella aromatica. Biotechnol. Lett. 2016, 38, 1475–1481. [Google Scholar] [CrossRef]
- Jones, R.M.; Hedrich, S.; Johnson, D.B. Acidocella aromatica sp. nov.: An acidophilic heterotrophic alphaproteobacterium with unusual phenotypic traits. Extremophiles 2013, 17, 841–850. [Google Scholar] [CrossRef]
- Johnson, D.B.; Roberto, F.F. Heterotrophic acidophiles and their roles in the bioleaching of sulfide minerals. In Biomining: Theory, Microbes and Industrial Processes; Rawlings, D.E., Ed.; Springer: New York, NY, USA, 1997; pp. 259–280. [Google Scholar]
- Noroozifar, M.; Khorasani-Motlagh, M. Specific extraction of chromium as tetrabutylammonium-chromate and spectrophotometric determination by diphenylcarbazide: Speciation of chromium in effluent streams. Anal. Sci. 2003, 19, 705–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UniProt. Available online: http://www.uniprot.org/ (accessed on 1 September 2021).
- Minami, Y.; Muroga, Y.; Yoshida, T.; Amao, Y. Selective hydrogen production from formate using nanoparticle with homogeneously polymer-dispersed platinum clusters. Chem. Lett. 2019, 48, 775–778. [Google Scholar] [CrossRef]
- Masaki, Y.; Hirajima, T.; Sasaki, K.; Okibe, N. Bioreduction and immobilization of hexavalent chromium by the extremely acidophilic Fe(III)-reducing bacterium Acidocella aromatica strain PFBC. Extremophiles 2015, 19, 495–503. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, T.; Phann, I.; Okibe, N. Biogenic Platinum Nanoparticles’ Production by Extremely Acidophilic Fe(III)-Reducing Bacteria. Minerals 2021, 11, 1175. https://doi.org/10.3390/min11111175
Matsumoto T, Phann I, Okibe N. Biogenic Platinum Nanoparticles’ Production by Extremely Acidophilic Fe(III)-Reducing Bacteria. Minerals. 2021; 11(11):1175. https://doi.org/10.3390/min11111175
Chicago/Turabian StyleMatsumoto, Takahiro, Idol Phann, and Naoko Okibe. 2021. "Biogenic Platinum Nanoparticles’ Production by Extremely Acidophilic Fe(III)-Reducing Bacteria" Minerals 11, no. 11: 1175. https://doi.org/10.3390/min11111175
APA StyleMatsumoto, T., Phann, I., & Okibe, N. (2021). Biogenic Platinum Nanoparticles’ Production by Extremely Acidophilic Fe(III)-Reducing Bacteria. Minerals, 11(11), 1175. https://doi.org/10.3390/min11111175






