Coupled Thermodynamics and Phase Diagram Analysis of Gas-Duct Concretion Formation in Pyro-Processing Ironmaking and Steelmaking Dust
Abstract
:1. Introduction
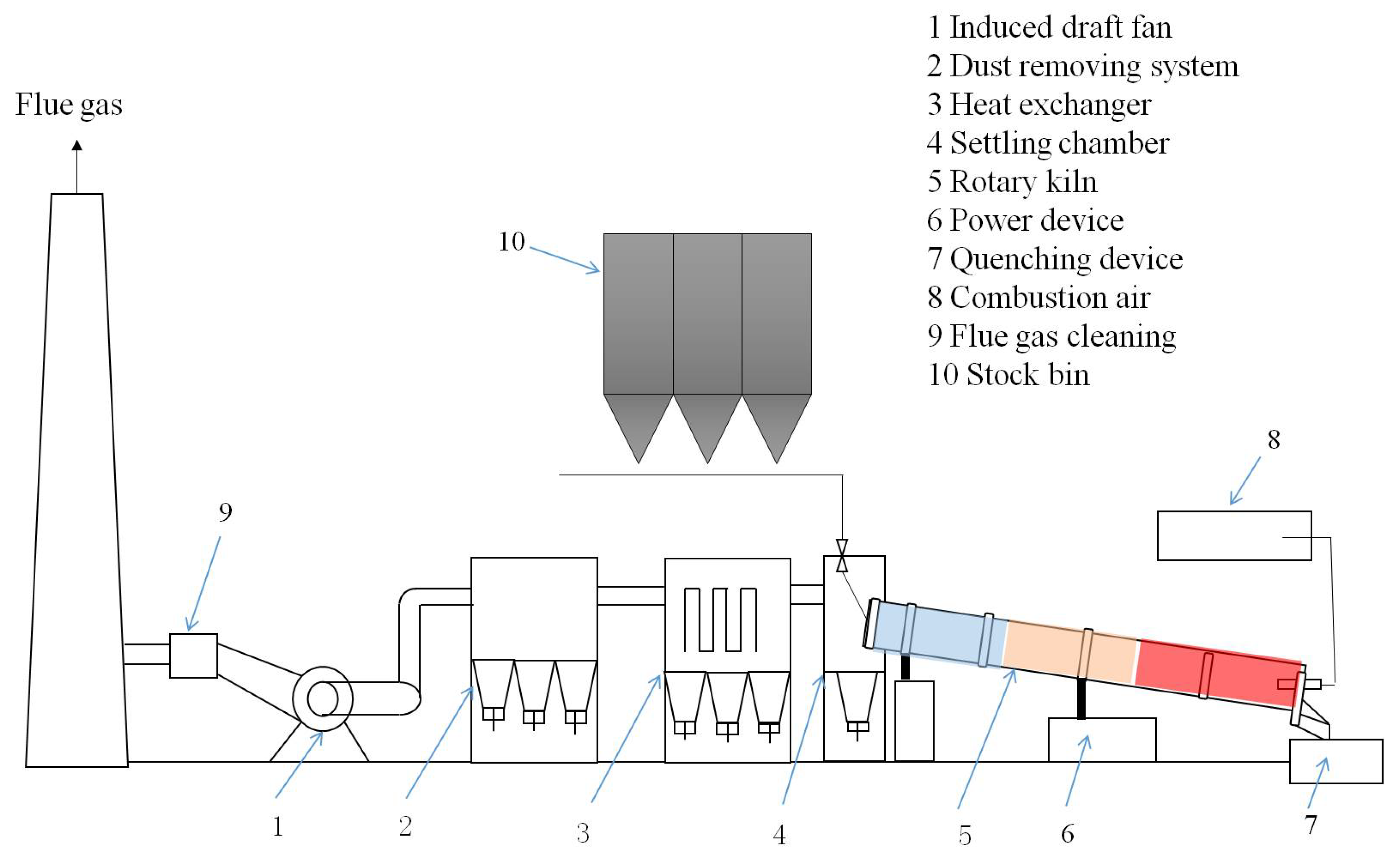
2. Materials and Methods
3. Results
4. Discussion
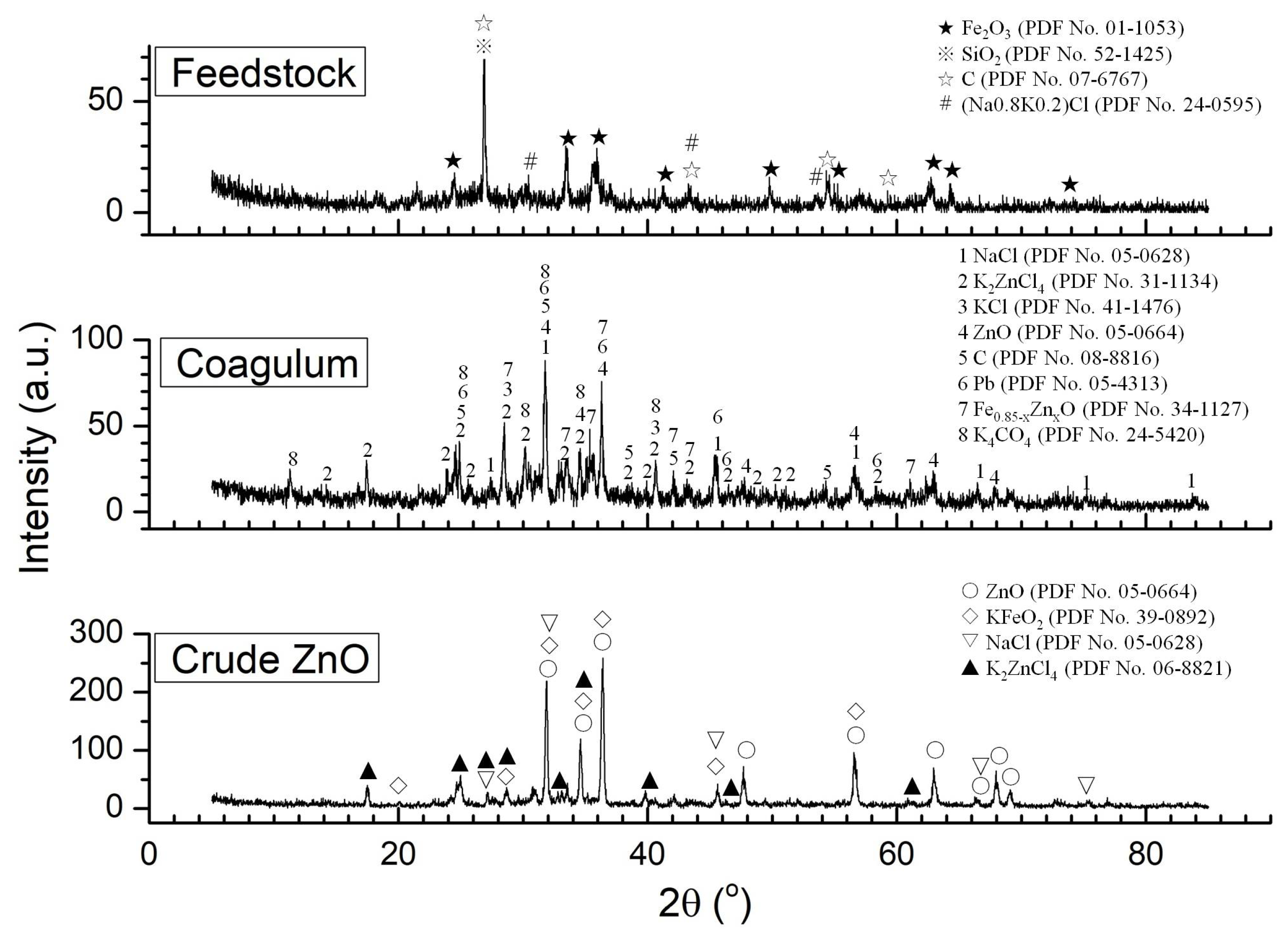
4.1. Substances in Feedstock Volatilization
4.2. Oxidation and Condensation of Substances in the Flue Gas
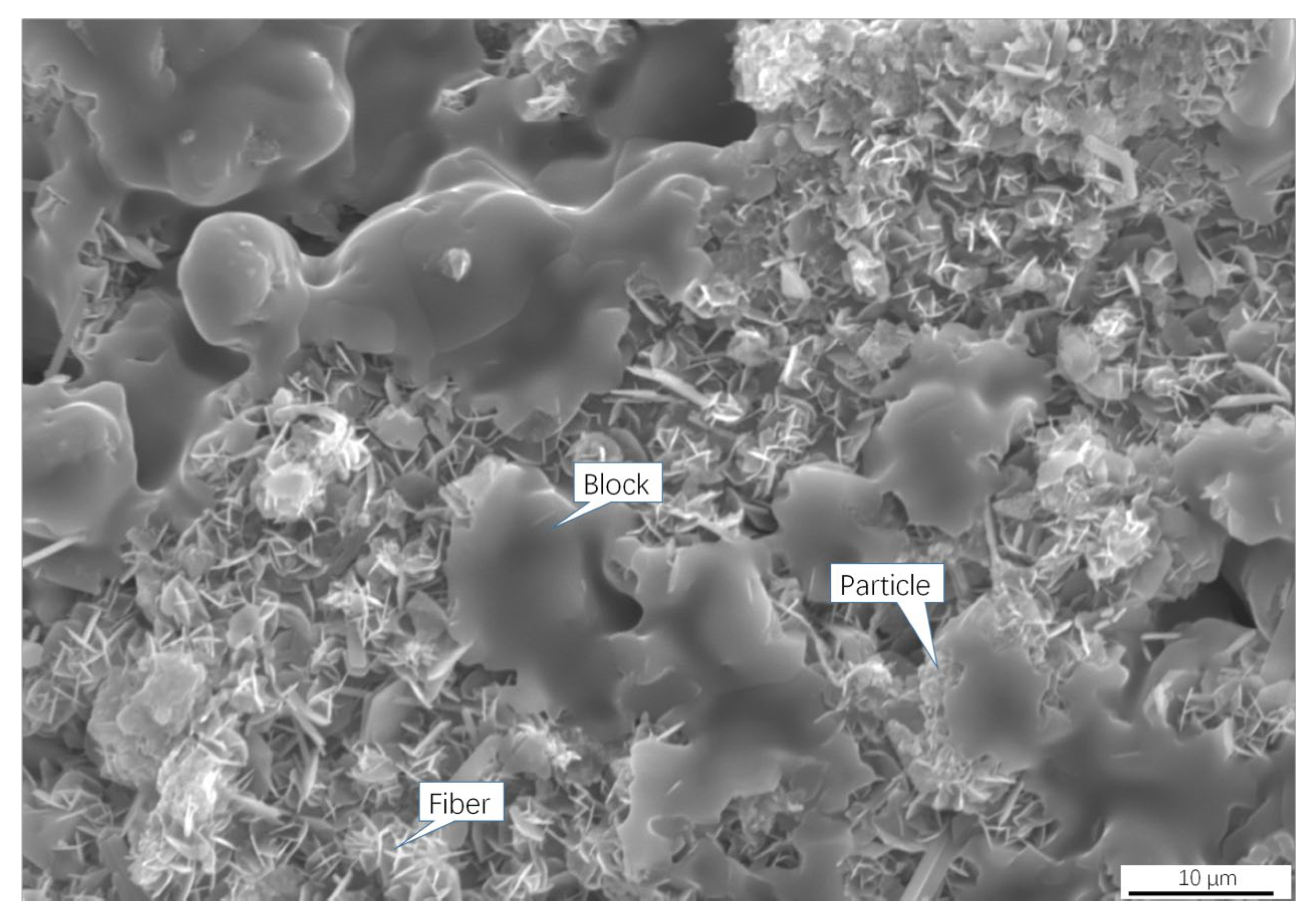
4.3. Formation Mechanism of Coagulum
5. Conclusions
- (1)
- Some of the oxides in the feedstock were reduced and chloridized to form metals and chlorides. These oxides volatilized into the flue gas at high temperatures. The produced metals were then re-oxidized, and the chlorides became condensed at lower temperatures, forming a coagulum on the cooling wall of the flue duct.
- (2)
- The coagulum consisted of chlorides (KCl, NaCl, and ZnCl2), oxides (ZnO, FeO), and carbon, exhibiting three structures: lumps, fibers, and particles. The liquid phase (eutectic system of KCl–NaCl–ZnCl2), dendrites (KCl, NaCl), and particles ((Zn, Fe)O) served as binders, stiffeners, and aggregates, respectively, constituting a composite structure.
- (3)
- Liquids, which were essential for the formation of coagulants, originated from the KCl–NaCl–ZnCl2 eutectic system. The eutectic system played a crucial role in coagulum formation, serving as a binder.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, X.L.; Peng, Z.W.; Yan, J.X.; Li, Z.Z.; Hwang, J.Y.; Zhang, Y.B.; Li, G.H.; Jiang, T. Pyrometallurgical recycling of electric arc furnace dust. J. Clean. Prod. 2017, 149, 1079–1100. [Google Scholar] [CrossRef]
- Stewart, D.J.C.; Barron, A.R. Pyrometallurgical removal of zinc from basic oxygen steelmaking dust—A review of best available technology. Resour. Conserv. Recycl. 2020, 157, e104746. [Google Scholar] [CrossRef]
- Mohammad, A.H.; Jomana, A.N.; Awni, A.O.; Isra, A.H.; Huda, A.J.; Mais, A.Z.; Shaima, A.A. Treatments of electric arc furnace dust and halogenated plastic wastes: A review. J. Environ. Chem. Eng. 2019, 7, e102856. [Google Scholar]
- Singh, P.K.; Katiyar, P.K.; Kumar, A.L.; Chaithnya, B.; Pramanik, S. Effect of sintering performance of the utilization of blast furnace solid wastes as pellets. Procedia Mater. Sci. 2014, 5, 2468–2477. [Google Scholar] [CrossRef] [Green Version]
- Bagatini, M.C.; Fernandes, T.; Silva, R.; Galvão, D.F.; Flores, I.V. Mill scale and flue dust briquettes as alternative burden to low height blast furnaces. J. Clean. Prod. 2020, 276, e124332. [Google Scholar] [CrossRef]
- Lanzerstorfer, C.; Kröppl, M. Air classification of blast furnace dust collected in a fabric filter for recycling to the sinter process. Resour. Conserv. Recycl. 2014, 86, 132–137. [Google Scholar] [CrossRef]
- Hu, W.T.; Xia, H.W.; Pan, D.L.; Wei, X.L.; Li, J.; Dai, X.J.; Yang, F.H.; Lu, X.; Wang, H.J. Difference of zinc volatility in diverse carrier minerals: The critical limit of blast furnace dust recycle. Miner. Eng. 2018, 116, 24–31. [Google Scholar] [CrossRef]
- Andrade, L.N.; Amorim, C.C.; Santos, S.V.; Teixeira, I.F.; Leão, M.M.D.; Lago, R.M. Efficient demulsification of wastewater by steel furnace dust with amphiphilic and surface charge properties. Chem. Eng. J. 2015, 271, 281–286. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Cortes, D.; McKay, G. Steel-Making dust as a potential adsorbent for the removal of lead (II) from an aqueous solution. Chem. Eng. J. 2018, 334, 837–844. [Google Scholar] [CrossRef]
- Amorim, C.C.; Leão, M.M.D.; Moreira, R.F.P.M.; Fabris, J.D.; Henriques, A.B. Performance of blast furnace waste for azo dye degradation through photo-Fenton-like processes. Chem. Eng. J. 2013, 224, 59–66. [Google Scholar] [CrossRef]
- Chang, Y.C.; Choi, D.B.; Takamizawa, K.; Kikuchi, S. Effect of blast furnace dust on the degradation of chlorinated organic and endocrine disrupting compounds. Process Biochem. 2013, 48, 694–702. [Google Scholar] [CrossRef]
- Yang, G.; Fang, H.Y.; Wang, J.; Jia, H.; Zhang, H.W. Enhanced anaerobic digestion of up-flow anaerobic sludge blanket (UASB) by blast furnace dust (BFD): Feasibility and mechanism. Int. J. Hydrogen Energy 2019, 44, 17709–17719. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J.; Zhang, H.W.; Jia, H.; Zhang, Y.; Gao, F. Applying bio-electric field of microbial fuel cell-upflow anaerobic sludge blanket reactor catalyzed blast furnace dusting ash for promoting anaerobic digestion. Water Res. 2019, 149, 215–224. [Google Scholar] [CrossRef]
- Pang, Y.J.; Wu, D.; Chen, Y.S.; Xu, J.; Wu, J.R.; Zhai, M.S. Pyrolysis of pine pellets catalyzed by blast furnace gas ash. Chem. Eng. Process. 2020, 156, e108094. [Google Scholar] [CrossRef]
- Silva, V.S.; Silva, J.S.; Costa, B.S.; Labes, C.; Oliveira, R.M.P.B. Preparation of glaze using electric-arc furnace dust as raw material. J. Mater. Res. Technol. 2019, 8, 5504–5514. [Google Scholar] [CrossRef]
- Magalhães, M.S.; Faleschini, F.; Pellegrino, C.; Brunelli, K. Cementing efficiency of electric arc furnace dust in mortars. Constr. Build. Mater. 2017, 157, 141–150. [Google Scholar] [CrossRef]
- Arnold, M.C.; Vargas, A.S.; Bianchini, L. Study of electric-arc furnace dust (EAFD) in fly ash and rice husk ash-based geopolymers. Adv. Powder Technol. 2017, 28, 2023–2034. [Google Scholar] [CrossRef]
- Lozano-Lunar, A.; Silva, P.R.; Brito, J.; Álvarez, J.I.; Fernández, J.M.; Jiménez, J.R. Performance and durability properties of self-compacting mortars with electric arc furnace dust as filler. J. Clean. Prod. 2019, 219, 818–832. [Google Scholar] [CrossRef]
- Zeydabadi, B.A.; Mowla, D.; Shariat, M.H.; Kalajahi, J.F. Zinc recovery from blast furnace flue dust. Hydrometallurgy 1997, 47, 113–125. [Google Scholar] [CrossRef]
- Oustadakis, P.; Tsakiridis, P.E.; Katsiapi, A.; Agatzini-Leonardou, S. Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD): Part I: Characterization and leaching by diluted sulphuric acid. J. Hazard. Mater. 2010, 179, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.C.; Zhang, X.W.; Yang, T.Z.; Rao, S.; Hu, W.; Liu, W.F.; Chen, L. Selective leaching of zinc from blast furnace dust with mono-ligand and mixed-ligand complex leaching systems. Hydrometallurgy 2017, 169, 219–228. [Google Scholar] [CrossRef]
- Steer, J.M.; Griffiths, A.J. Investigation of carboxylic acids and non-aqueous solvents for the selective leaching of zinc from blast furnace dust slurry. Hydrometallurgy 2013, 140, 34–41. [Google Scholar] [CrossRef]
- Luo, X.G.; Wei, C.; Li, X.B.; Deng, Z.G.; Li, M.T.; Fan, G. The use of carbon-dioxide to enhance the solvent extraction of zinc from ammonia leaching solutions of blast furnace dust. Hydrometallurgy 2010, 197, e105458. [Google Scholar] [CrossRef]
- Chairaksa-Fujimoto, R.; Maruyama, K.; Miki, T.; Nagasaka, T. The selective alkaline leaching of zinc oxide from Electric Arc Furnace dust pre-treated with calcium oxide. Hydrometallurgy 2016, 159, 120–125. [Google Scholar] [CrossRef]
- Christopher, H.; Marina, S.; Dirk, S.; Gerhard, A.; Eric, W.; Dominik, A.S.; Markus, O.; Christian, A. Recycling of blast-furnace sludge by thermochemical treatment with spent iron (II) chloride solution from steel pickling. J. Hazard. Mater. 2021, 402, e123511. [Google Scholar]
- Xu, J.; Wang, N.; Chen, M.; Zhou, Z.Y.; Yu, H.Y. Comparative investigation on the reduction behavior of blast furnace dust particles during in-flight process in hydrogen-rich and carbon monoxide atmospheres. Powder Technol. 2020, 366, 709–721. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T. Improved removal of zinc from blast furnace sludge by particle size separation and microwave heating. Miner. Eng. 2018, 127, 265–276. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T. Utilization of blast furnace sludge for the removal of zinc from steelmaking dusts using microwave heating. Sep. Purif. Technol. 2019, 210, 867–884. [Google Scholar] [CrossRef]
- Wu, Y.L.; Jiang, Z.Y.; Zhang, X.X.; Xue, Q.G.; Miao, Z.; Zhou, Z.Y.; Shen, Y.S. Process optimization of metallurgical dust recycling by direct reduction in rotary hearth furnace. Powder Technol. 2018, 326, 101–113. [Google Scholar] [CrossRef]
- AKamali, R.; Yang, J.G. Effect of molten salts on the structure, morphology and electrical conductivity of PET-derived carbon nanostructures. Polym. Degrad. Stab. 2020, 177, e109184. [Google Scholar] [CrossRef]
- Liu, H.C.; Song, H.; Hou, W.J.; Chang, Y.Z.; Zhang, Y.; Li, Y.P.; Zhao, Y.; Han, G.Y. Coal tar pitch-based hierarchical porous carbons prepared in molten salt for supercapacitors. Mater. Chem. Phys. 2021, 265, e124491. [Google Scholar] [CrossRef]
- Babushkina, O.B.; Volkov, S.V. Raman spectroscopy of the heteronuclear complexes in the ZnCl2-CdCl2-Li,K/Cl and AlCl3-MgCl2-Li,K/Cl melts. J. Mol. Liq. 1999, 83, 131–140. [Google Scholar] [CrossRef]
- Lutz, H.D.; Steiner, H.J. Phase diagrams of the systems Li2ZnCl4-Na2ZnCl4 Li2ZnCl4 Li2MnCl4. The spinel-olivine phase transition of Li2ZnCl4. Thermochim. Acta 1992, 211, 189–197. [Google Scholar] [CrossRef]
- Lima, L.; Caldas, L.S.; Alí, A.; Barreto, J.; Freitas, R.; Mazzarella, A.; Felix, G.; Carozo, V.; Stavale, F. Growth and Raman spectroscopy of ultrathin ZnO (0001) films on Ag (001). Surf. Sci. 2021, 704, e121748. [Google Scholar] [CrossRef]
- Hadzic, B.; Romcevic, N.; Romcevic, M.; Kuryliszyn-Kudelska, I.; Dobrowolski, W.; Narkiewicz, U.; Sibera, D. Influence of SOP modes on Raman spectra of ZnO(Fe) nanoparticles. Opt. Mater. 2015, 42, 118–123. [Google Scholar] [CrossRef]
- Airoldi, C.; Digiampietri, E.A. Thioamide adducts of zinc-group chlorides—A thermochemical study. J. Chem. Thermodyn. 1992, 24, 33–39. [Google Scholar] [CrossRef]
- Robelin, C.; Chartrand, P. Thermodynamic evaluation and optimization of the (NaCl + KCl + MgCl2 + CaCl2 + ZnCl2) system. J. Chem. Thermodyn. 2011, 43, 377–391. [Google Scholar] [CrossRef]
- Shaw, S.J.; Perry, G.S. NaCl-ZnCl2 phase diagram. Thermochim. Acta 1990, 157, 329–333. [Google Scholar] [CrossRef]
- Chartrand, C.; Chartrand, P.; Pelton, A.D. Thermodynamic evaluation and optimization of the (NaCl + KCl + MgCl2 + CaCl2 + MnCl2 + FeCl2 + CoCl2 + NiCl2) system. J. Chem. Thermodyn. 2004, 36, 809–828. [Google Scholar]
- Yin, C.; Liu, M.; Yang, J.; Ma, H.; Luo, Z. (Solid+liquid) phase equilibrium for the ternary system (K2CO3-Na2CO3-H2O) at T = (323.15, 343.15, and 363.15) K. J. Chem. Thermodyn. 2017, 108, 1–6. [Google Scholar] [CrossRef]
- Cao, J.; Ren, Y.; Yu, B.; Qiu, L.; Ma, F. Solid-liquid equilibrium for the ternary systems (Na2CO3 + NaCl + H2O) and (Na2CO3 + Na2SO4 + H2O) at 313.15K and atmospheric pressure. J. Chem. Thermodyn. 2019, 133, 181–193. [Google Scholar] [CrossRef]
- Lindberg, D.; Backman, R.; Chartrand, P. Thermodynamic evaluation and optimization of the (NaCl + Na2SO4 + Na2CO3 + KCl + K2SO4 + K2CO3) system. J. Chem. Thermodyn. 2007, 39, 1001–1021. [Google Scholar] [CrossRef]
- Lindberg, D.; Backman, R.; Chartrand, P. Thermodynamic evaluation and optimization of the (Na2CO3 + Na2SO4 + Na2S + K2CO3 + K2SO4 + K2S) system. J. Chem. Thermodyn. 2007, 39, 942–960. [Google Scholar] [CrossRef]

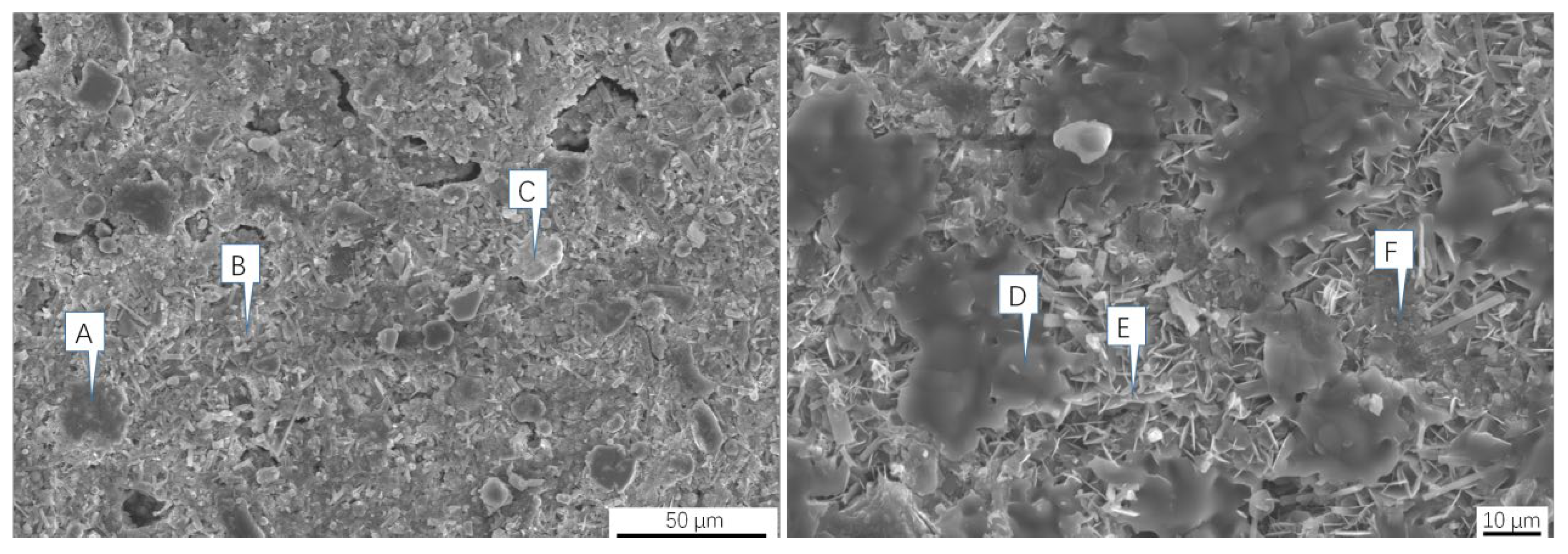

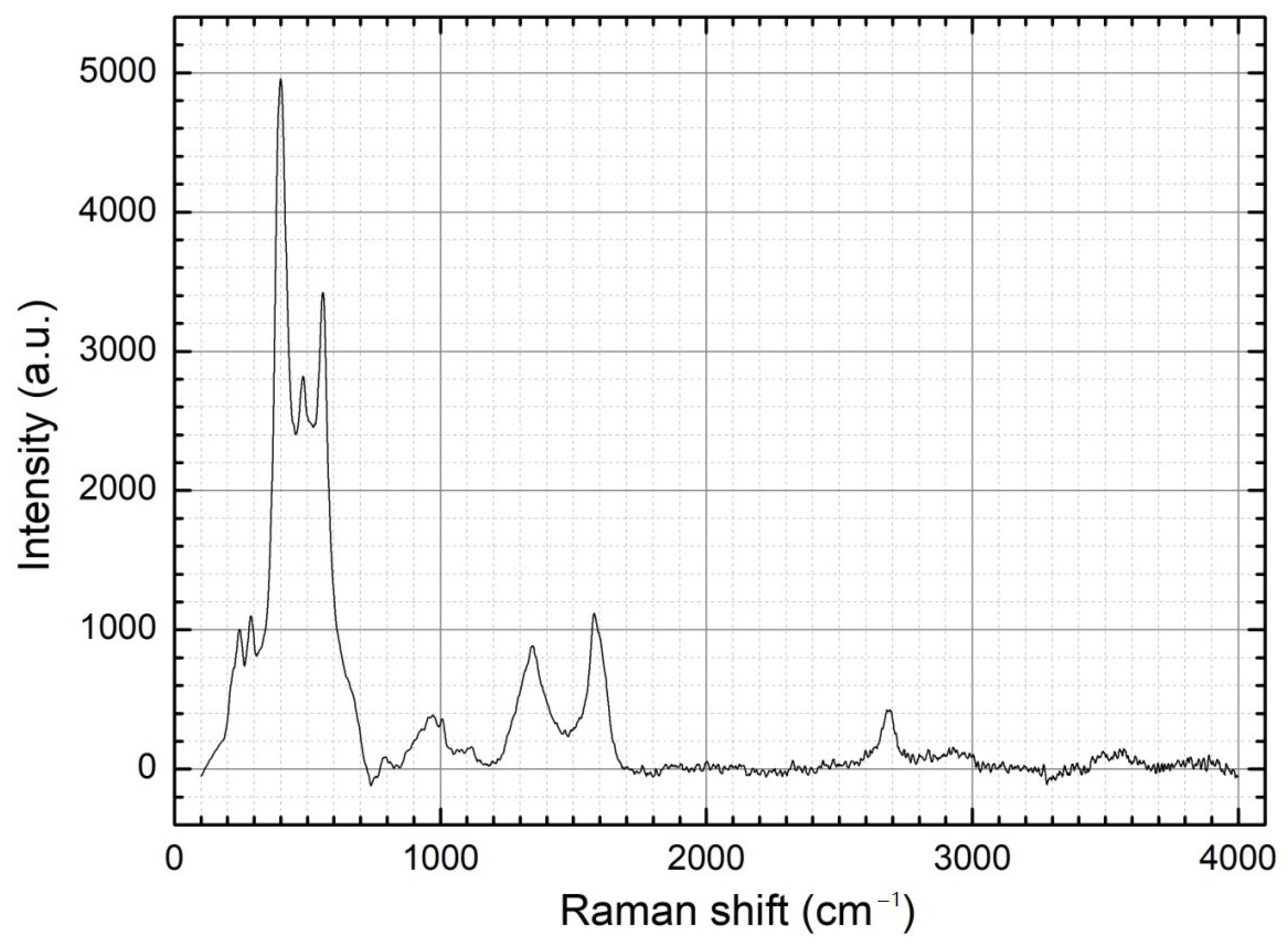
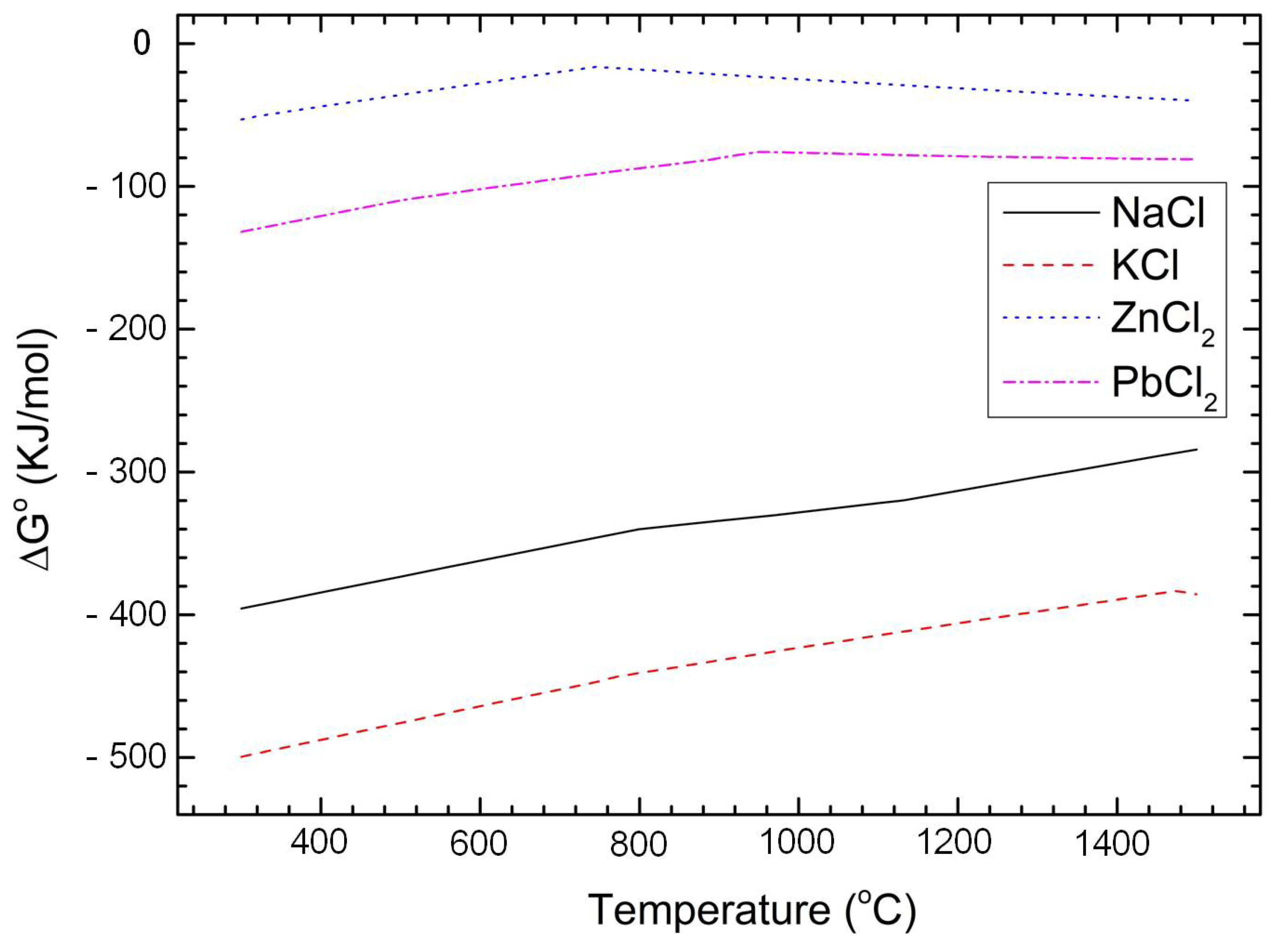
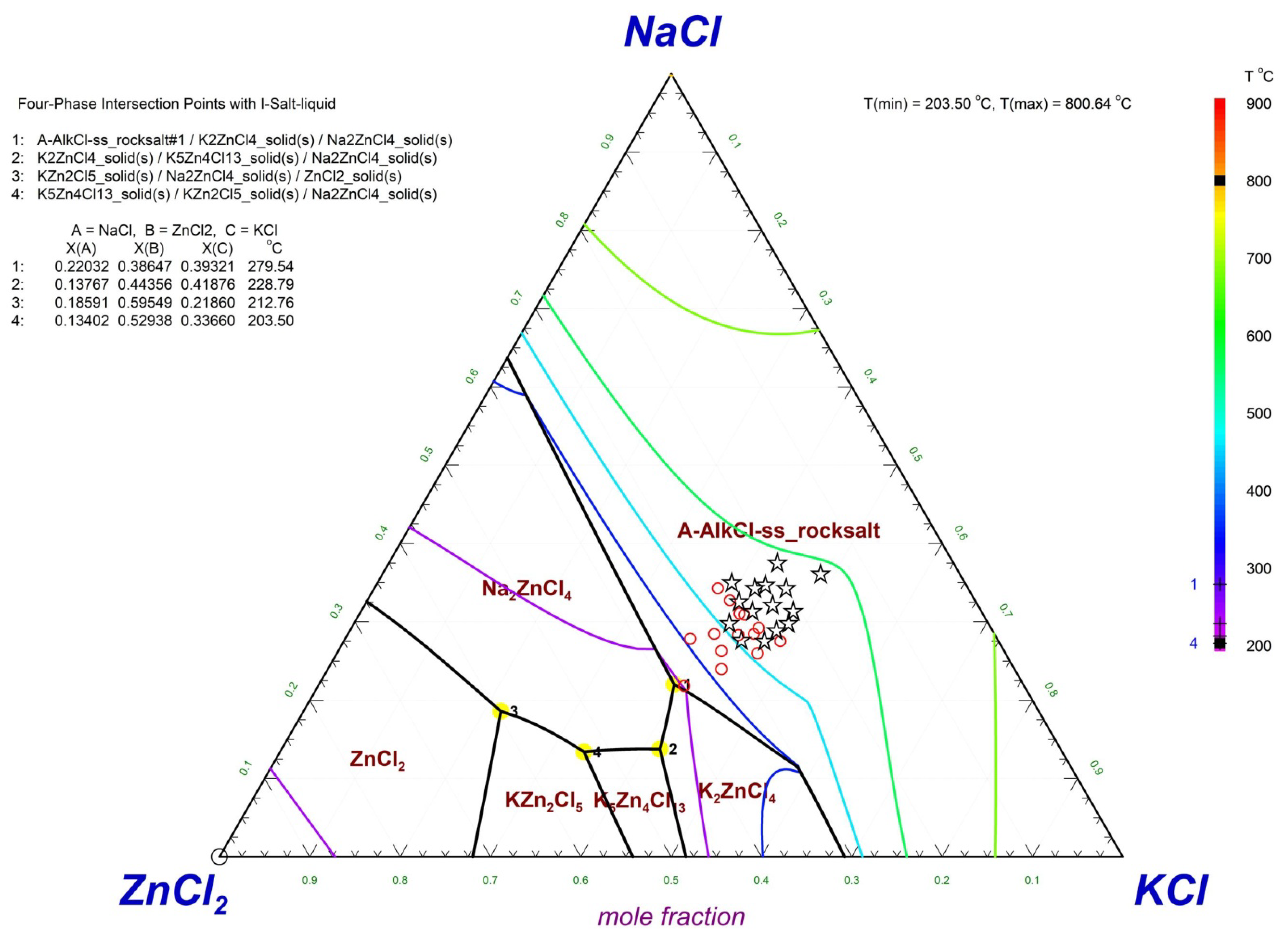
| Element | Fe | Cl | K | Zn | Ca | Na | Pb | Si |
|---|---|---|---|---|---|---|---|---|
| Feedstock | 30–35 | 6–10 | 5–7 | 5–8 | 4–6 | 1–6 | <1 | 2–5 |
| Coagulum | 9–12 | 25–28 | 9–12 | 20–23 | <1 | 2–7 | 4–5 | <1 |
| Crude zinc | 5–6 | 24–26 | 7–9 | 35–38 | <0.5 | 4–6 | 3–4 | <1 |
| Element | C | O | Al | Si | S | Cl | K | Ca | Fe | Zn | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 91.97 | 5.57 | – | – | 0.43 | 0.46 | 0.28 | 0.25 | 0.3 | 0.74 | – |
| B | 51.13 | 35.64 | – | – | 2.63 | 2.41 | 2.67 | 0.93 | 1.24 | 3.35 | – |
| C | 20.96 | 35.75 | 1.03 | 3.5 | – | 4.69 | 1.72 | – | 17.42 | 14.92 | – |
| D | 26.69 | 11.2 | – | – | – | 30.63 | 26.26 | – | 1.14 | 4.08 | – |
| E | 50.77 | 27.51 | – | – | 0.73 | 8.51 | 3.07 | – | 1.46 | 7.95 | – |
| F | 60.22 | 13.94 | 0.83 | 0.57 | 0.8 | 11.48 | 3.99 | – | 3.21 | 4.68 | 0.29 |
| Pressure | 0.1 | 0.01 | 0.001 | 4 × 10−4 | 3 × 10−4 | 1 × 10−5 | 5 × 10−6 | 1 × 10−6 | 1 × 10−7 | 1 × 10−8 |
|---|---|---|---|---|---|---|---|---|---|---|
| KCl | 1173 | 971 | 822 | 774 | 761 | 633 | 611 | 564 | 506 | 455 |
| NaCl | 1215 | 1008 | 856 | 806 | 792 | 662 | 639 | 591 | 531 | 478 |
| ZnCl2 | 600 | 494 | 412 | 385 | 376 | 296 | 284 | 257 | 222 | 192 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Hua, S.; Wu, L.; Liu, K.; Wang, H. Coupled Thermodynamics and Phase Diagram Analysis of Gas-Duct Concretion Formation in Pyro-Processing Ironmaking and Steelmaking Dust. Minerals 2021, 11, 1125. https://doi.org/10.3390/min11101125
Wang D, Hua S, Wu L, Liu K, Wang H. Coupled Thermodynamics and Phase Diagram Analysis of Gas-Duct Concretion Formation in Pyro-Processing Ironmaking and Steelmaking Dust. Minerals. 2021; 11(10):1125. https://doi.org/10.3390/min11101125
Chicago/Turabian StyleWang, Daya, Shaoguang Hua, Liushun Wu, Kunlong Liu, and Haichuan Wang. 2021. "Coupled Thermodynamics and Phase Diagram Analysis of Gas-Duct Concretion Formation in Pyro-Processing Ironmaking and Steelmaking Dust" Minerals 11, no. 10: 1125. https://doi.org/10.3390/min11101125
APA StyleWang, D., Hua, S., Wu, L., Liu, K., & Wang, H. (2021). Coupled Thermodynamics and Phase Diagram Analysis of Gas-Duct Concretion Formation in Pyro-Processing Ironmaking and Steelmaking Dust. Minerals, 11(10), 1125. https://doi.org/10.3390/min11101125





