Rare Earths’ Recovery from Phosphogypsum: An Overview on Direct and Indirect Leaching Techniques
Abstract
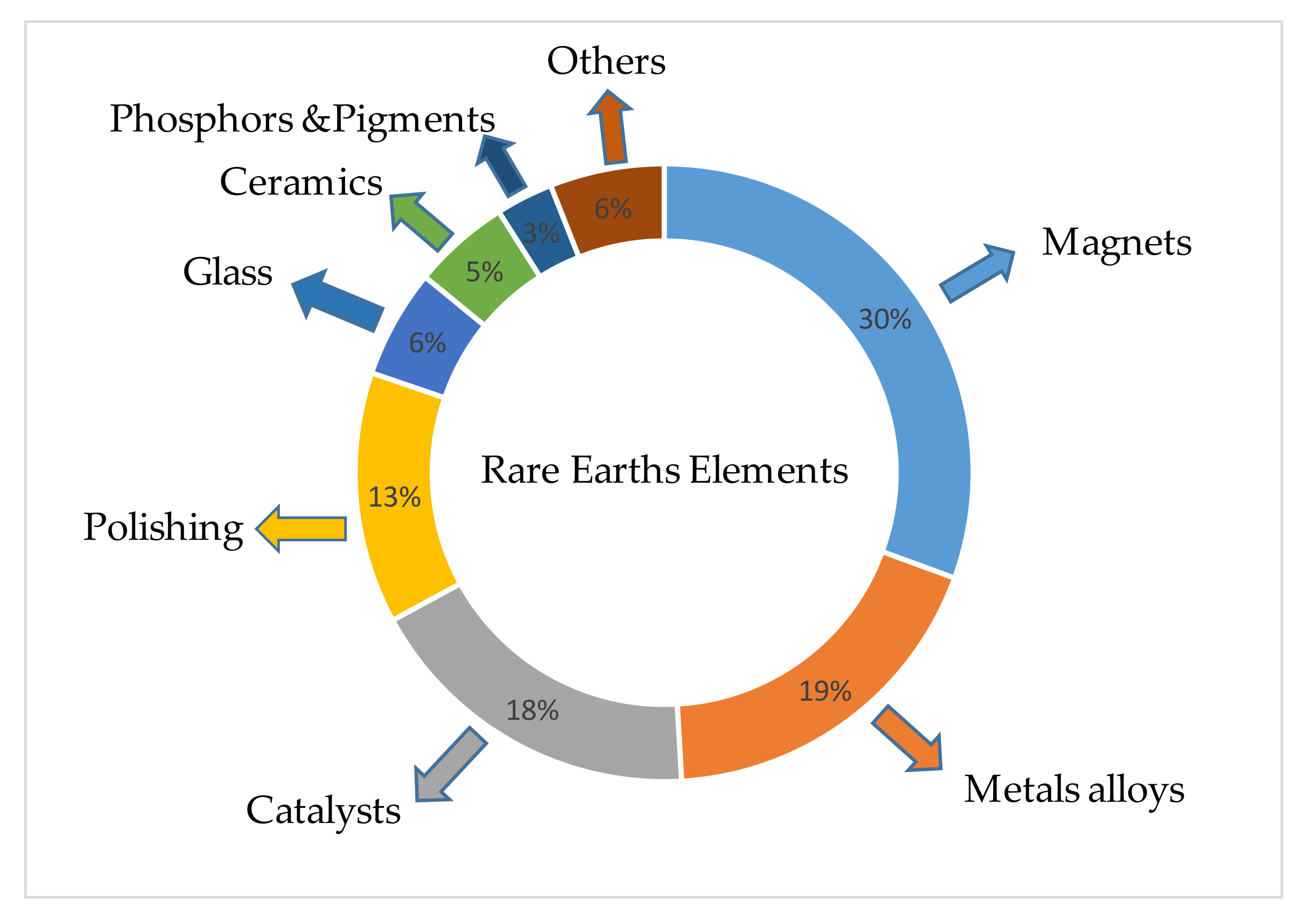
:1. Introduction
2. Phosphogypsum
3. Phosphogypsum Disposal and Use Worldwide
4. Beneficiation Methods
4.1. Direct Leaching
4.2. Indirect Leaching
4.2.1. Carbonation
| Approach | REE Content (wt. %) | Lixiviant | S:L Ratio | Time (h) | Temperature (°C) | Efficiency (%) | Ref |
|---|---|---|---|---|---|---|---|
| Inorganic acid leaching | 0.041 | 15% H2SO4 | 1:3 | 2 | 100 | 60 | [67] |
| 0.035 | 3 M HNO3 | 1:30 | 8 | 25 | 85 | [11] | |
| 0.17 * | 2.5% HNO3 | 1:7.5 | 0.25 | 25 | 59 | [68] | |
| 0.032 | 1.5 M HCl | 1:15 | 1 | 85 | Nd: 80 Dy: 99 Y: 99 | [50] | |
| 2.6 | H2SO4 | 1:6 | 4.3 | 275 | 95 | [18] | |
| 0.19 | 0.01 M H2SO4 | 1:20 | 20–22 | 24 | 15 | [68] | |
| 0.44 | 10% H2SO4 | 1:3 | 20 | 2 | 52 | [69] | |
| 1.7 | 1 g/L H2SO4 | 1:8 | 24 | - | 45–75 | [70] | |
| 0.44 | 10–30% H2SO4 | 1:7.5 | 2 | 50 | 72 | [17] | |
| 0.022 | 10% H2SO4 | 1:1.3 | 1–2 | 60 | 50 | [37] | |
| 0.020 | 1.5 M HCl 1.5 M H2SO4 1.5 M HNO3 | 1:8 | 0.33 | 80 | 51 23 57 | [32] | |
| 0.042 0.034 | 36% HNO3 90% H2SO4 + 10% H3PO4 | 1:4 1:6.7 | 1 1 | 72 72 | 58 49 | [30] | |
| 0.048 | 3 M HNO3 2 M HCl 4 M H2SO4 | 1:2 | 3 | 25 | 43 12 13 | [33] | |
| 0.048 | 1 M C6H8O7 | 1:5 | 0.25 | 85 | 83.4 | [71] | |
| Organic leaching | 0.040 | 0.7 M TBP- 0.9M TOPO 0.5 M Na2CO3 + TPB-TOPO | 1:1 1:1 | 2 2 | 55 55 | 70 80 | [72] |
| Bioleaching | 1.3 | Spent medium (Gluconobacter oxydans, 220 mM gluconic acid) Gluconobacter oxydants NRRL, B85 | 1:50 | 24 | 25 | Y: 91.2 Ce:36.7 Nd: 42.8 Sm: 73.2 Eu: 50 Yb: 83.7 | [53] |
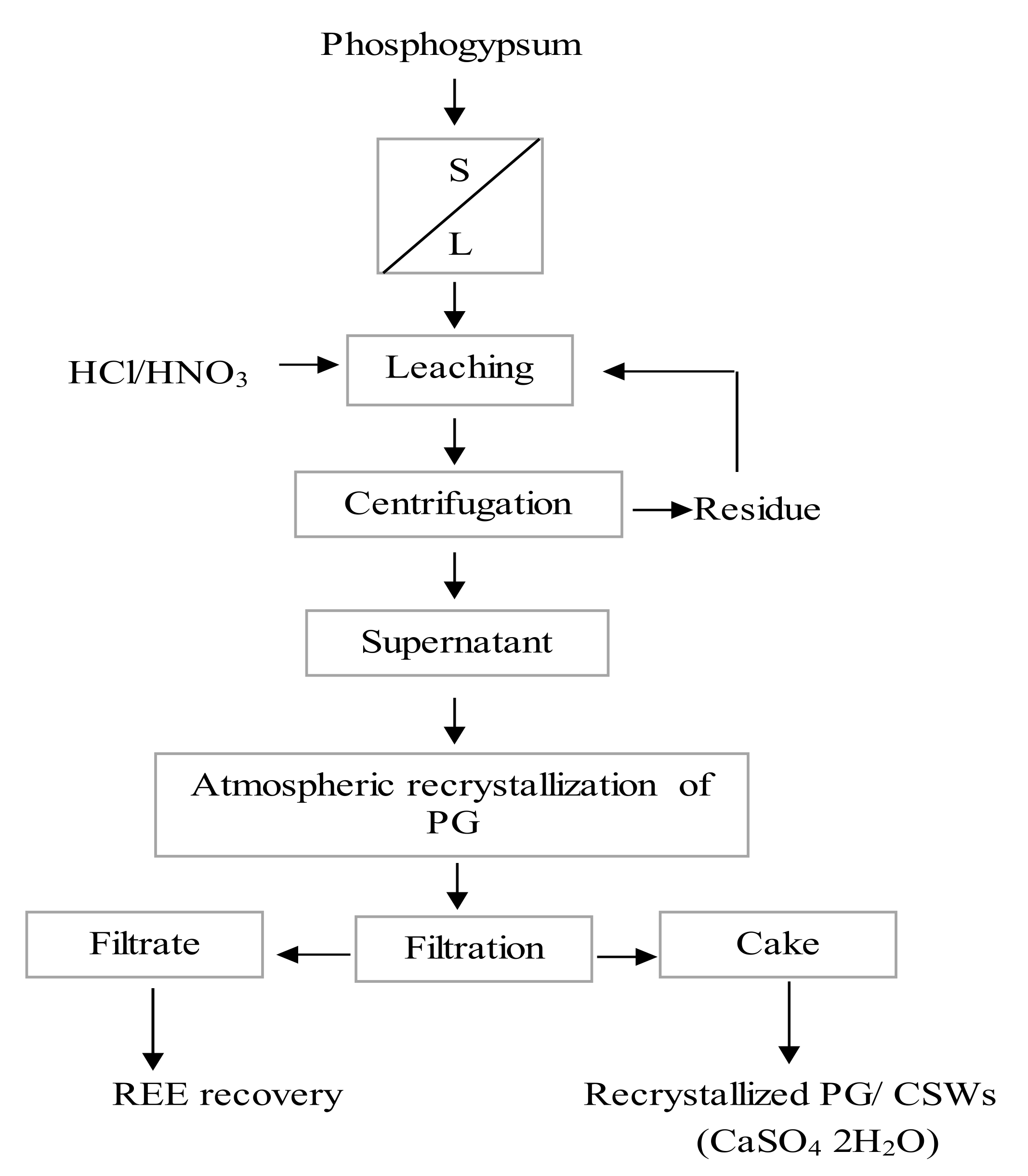
4.2.2. Recrystallization
4.3. Organic Liquid
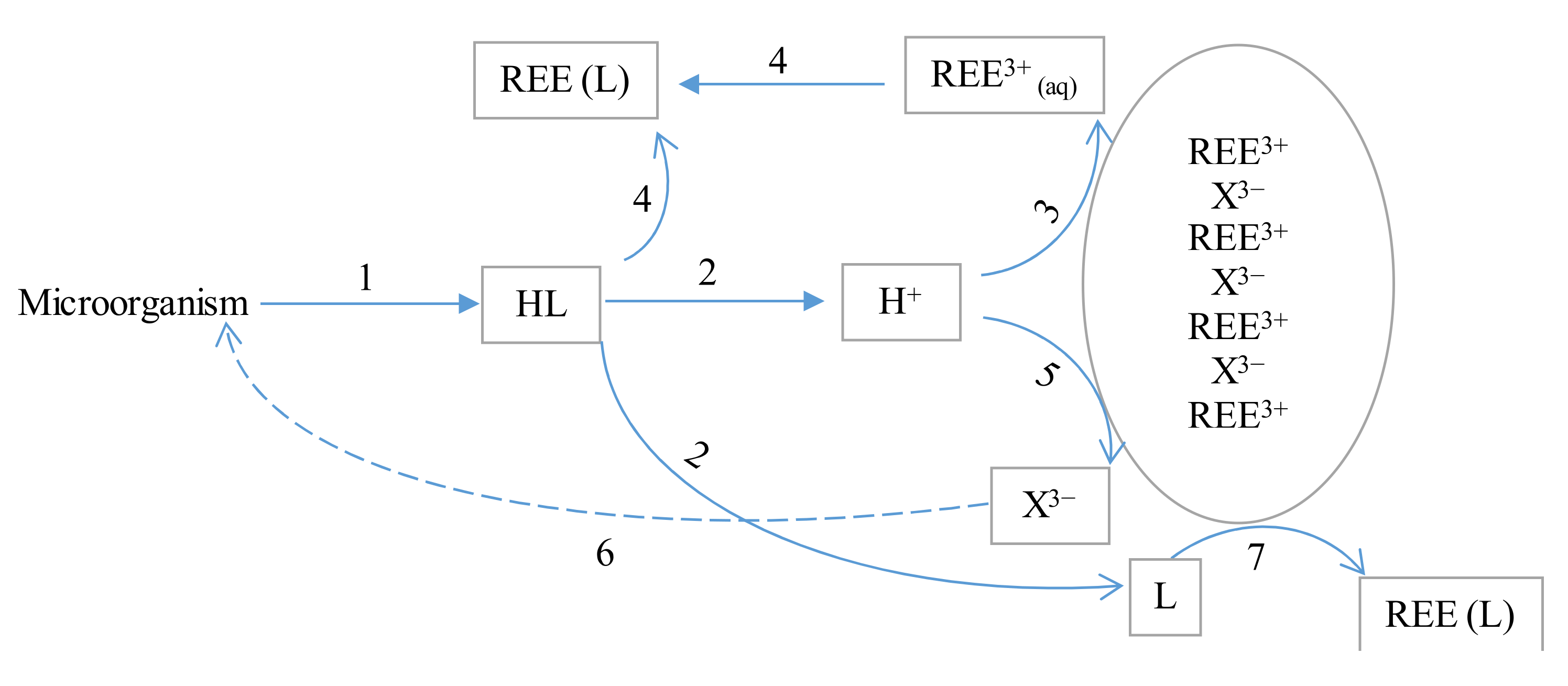
4.4. Bioleaching
5. Methods of REE Recovery
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dushyantha, N.; Batapola, N.; Ilankoon, I.M.S.K.; Rohitha, S.; Premasiri, R.; Abeysinghe, B.; Ratnayake, N.; Dissanayake, K. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol. Rev. 2020, 122, 103521. [Google Scholar] [CrossRef]
- Golev, A.; Scott, M.; Erskine, P.D.; Ali, S.H.; Ballantyne, G.R. Rare earths supply chains: Current status, constraints and opportunities. Res. Policy 2014, 41, 52–59. [Google Scholar] [CrossRef]
- Peiravi, M.; Dehghani, F.; Ackah, L.; Baharlouei, A.; Godbold, J.; Liu, J.; Mohanty, M.; Ghosh, T. A Review of Rare-Earth Elements Extraction with Emphasis on Non-Conventional Sources: Coal and Coal by products, Iron Ore Tailings, Apatite, and Phosphate by products. Min. Metal. Explor. 2021, 38, 1–26. [Google Scholar]
- Goodenough, K.M.; Wall, F.; Merriman, D. The rare earth elements: Demand, global resources, and challenges for resourcing future generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Mancheri, N.A.; Sprecher, B.; Bailey, G.; Ge, J.; Tukker, A. Effect of Chinese policies on rare earth supply chain resilience. Res. Conserv. Recycl. 2019, 142, 101–112. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165, 2–26. [Google Scholar] [CrossRef]
- Tunsu, C.; Petranikova, M.; Gergorić, M.; Ekberg, C.; Retegan, T. Reclaiming rare earth elements from end-of-life products: A review of the perspectives for urban mining using hydrometallurgical unit operations. Hydrometallurgy 2015, 156, 239–258. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Blissett, R.S.; Smalley, N.; Rowson, N.A. An investigation into six coal fly ashes from the United Kingdom and Poland to evaluate rare earth element content. Fuel 2014, 119, 236–239. [Google Scholar] [CrossRef] [Green Version]
- Seredin, V.V.; Dai, S.; Sun, Y.; Chekryzhov, I.Y. Coal deposits as promising sources of rare metals for alternative power and energy-efficient technologies. Appl. Geochem. 2013, 31, 1–11. [Google Scholar] [CrossRef]
- Cánovas, C.R.; Chapron, S.; Arrachart, G.; Pellet-Rostaing, S. Leaching of rare earth elements (REEs) and impurities from phosphogypsum: A preliminary insight for further recovery of critical raw materials. J. Clean. Prod. 2019, 219, 225–235. [Google Scholar] [CrossRef]
- McLellan, B.C.; Corder, G.D.; Ali, S.H. Sustainability of rare earths—An overview of the state of knowledge. Minerals 2013, 3, 304–317. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Innocenzi, V.; De Michelis, I.; Kopacek, B.; Veglio, F. Yttrium recovery from primary and secondary sources: A review of main hydrometallurgical processes. Waste Manag. 2014, 34, 1237–1250. [Google Scholar] [CrossRef]
- Canovas, C.R.; Perez-Lopez, R.; Macías, F.; Chapron, S.; Nieto, J.M.; Pellet-Rostaing, S. Exploration of fertilizer industry wastes as potential source of critical raw materials. J. Clean. Prod. 2017, 143, 497–505. [Google Scholar] [CrossRef]
- Kulczycka, J.; Kowalski, Z.; Smol, M.; Wirth, H. Evaluation of the recovery of rare earth elements (REE) from phosphogypsum waste case study of the WIZOW chemical plant (Poland). J. Clean. Prod. 2016, 113, 345–354. [Google Scholar] [CrossRef]
- Rychkov, V.N.; Kirillov, E.V.; Kirillov, S.V.; Semenishchev, V.S.; Bunkov, G.M.; Botalov, M.S.; Smyshlyaev, D.V.; Malyshev, A.S. Recovery of rare earth elements from phosphogypsum. J. Clean. Prod. 2018, 196, 674–681. [Google Scholar] [CrossRef]
- Brückner, L.; Elwert, T.; Schirmer, T. Extraction of rare earth elements from phosphogypsum: Concentrate digestion, leaching, and purification. Metals 2020, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wu, P. Geochemical characteristics of dissolved rare earth elements in acid mine drainage from abandoned high-As coal mining area, southwestern China. Environ. Sci. Pollut. Res. 2017, 24, 20540–20555. [Google Scholar] [CrossRef]
- Pan, J.; Zhou, C.; Liu, C.; Tang, M.; Cao, S.; Hu, T.; Ji, W.; Luo, Y.; Wen, M.; Zhang, N. Modes of occurrence of rare earth elements in coal fly ash: A case study. Energy Fuels 2018, 32, 9738–9743. [Google Scholar] [CrossRef]
- Peelman, S.; Kooijman, D.; Sietsma, J.; Yang, Y. Hydrometallurgical recovery of rare earth elements from mine tailings and WEEE. J. Sustain. Met. 2018, 4, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Vander Hoogerstraete, T.; Blanpain, B.; Van Gerven, T.; Binnemans, K. From NdFeB magnets towards the rare-earth oxides: A recycling process consuming only oxalic acid. RSC Adv. 2014, 4, 64099–64111. [Google Scholar] [CrossRef] [Green Version]
- Maroufi, S.; Nekouei, R.K.; Hossain, R.; Assefi, M.; Sahajwalla, V. Recovery of rare earth (i.e., La, Ce, Nd, and Pr) oxides from end-of-life Ni-MH battery via thermal isolation. ACS Sustain. Chem. Eng. 2018, 6, 11811–11818. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, B.; Blanpain, T.; Van Gerven, Y.; Pontikes, Y. Towards zerowaste valorisation of rare-earth-containing industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef] [Green Version]
- Innocenzi, V.; Ippolito, N.M.; Pietrelli, L.; Centofanti, M.; Piga, L.; Veglio, F. Application of solvent extraction operation to recover rare earths from fluorescent lamps. J. Clean. Prod. 2018, 172, 2840–2852. [Google Scholar] [CrossRef]
- Roszczynialski, W.; Gawlicki, M.; Nocuń-Wczelik, W. Production and use of by-product gypsum in the construction industry. In Waste Materials Used in Concrete Manufacturing; William Andrew Publishing: Norwich, NY, USA, 1996; pp. 53–141. [Google Scholar]
- Rutherford, P.M.; Dudas, M.J.; Samek, R.A. Environmental impacts of phosphogypsum. Sci. Total Environ. 1994, 149, 1–38. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, L.; Wang, L.; Huang, X.; Zhang, Y.; Feng, Z.; Cui, D. Simultaneous recovery of rare earth elements and phosphorus from phosphate rock by phosphoric acid leaching and selective precipitation: Towards green process. J. Rare Earths 2019, 37, 652–658. [Google Scholar] [CrossRef]
- Koopman, C.; Witkamp, G.J. Extraction of lanthanides from the phosphoric acid production process to gain a purified gypsum and a valuable lanthanide by-product. Hydrometallurgy 2000, 58, 51–60. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Zhang, P. REE extraction from phosphoric acid, phosphoric acid sludge, and phosphogypsum. Min. Proc. Ext. Met. 2015, 124, 143–150. [Google Scholar] [CrossRef]
- Santos, A.; Mazzilli, B.; Fávaro, D.; Silva, P. Partitioning of radionuclides and trace elements in phosphogypsum and its source materials based on sequential extraction methods. J. Environ. Radioactiv. 2006, 87, 52–61. [Google Scholar] [CrossRef]
- Walawalkar, M.; Nichol, C.K.; Gisele Azimi, G. Process Investigation of the Acid Leaching of Rare Earth Elements from Phosphogypsum Using HCl, HNO3 and H2SO4. Hydrometallurgy 2016, 166, 195–204. [Google Scholar] [CrossRef]
- Ismail, Z.; Abu Elgoud, E.; Gasser, M.; Aly, H.; Abdel Hai, F.; Ali, I. Leaching of some lanthanides from phosphogypsum fertilizers by mineral acids. Arab J. Nucl. Sci. Appl. 2015, 48, 37–50. [Google Scholar]
- Lokshin, E.; Tareeva, O.; Elizarova, I. On integrated processing of phosphogypsum, Russ. J. Appl. Chem. 2013, 86, 463–468. [Google Scholar]
- Kybartiene, N.; Valancius, Z.; Leskeviciene, V.; Urbonas, L. Influence of the composition of phosphate rock on the amount of water-insoluble phosphate impurities in semi-hydrate phosphogypsum. Ceram.-Silikáty 2015, 59, 29–36. [Google Scholar]
- Samonov, A. New data on mineral forms of rare metals in phosphogypsum wastes. In Doklady Earth Sciences; Springer: Berlin/Heidelberg, Germany, 2011; Volume 440, pp. 1312–1315. [Google Scholar]
- Hammas-Nasri, I.; Horchani-Naifer, K.; Férid, M.; Barca, D. Rare earths concentration from phosphogypsum waste by two-step leaching method. Int. J. Miner. Process. 2016, 149, 78–83. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Z.; Zhao, G.; Liu, Z. Utilization of phosphogypsum for backfilling, way to relieve its environmental impact. Gospod. Surowcami Miner. 2008, 24, 226–232. [Google Scholar]
- Oliveira, S.M.B.; Da Silva, P.S.C.; Mazzilli, B.P.; Favaro, D.I.T.; Saueia, C.H. Rare earth elements as tracers of sediment contamination by phosphogypsum in the Santos estuary, southern Brazil. Appl. Geochem. 2007, 22, 837–850. [Google Scholar] [CrossRef]
- Fuleihan, N.F. Phosphogypsum disposal-the pros & cons of wet versus dry stacking. Procedia Eng. 2012, 46, 195–205. [Google Scholar]
- Oliveira, K.; Menezes, M.; Von Sperling, E.; Jacomino, V. Transfer factor of rare earth elements from phosphogypsum amended Brazilian tropical soils to lettuce, corn and soybean. J. Solid Waste Technol. Manag. 2012, 38, 202–210. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [Green Version]
- Masmoudi-Soussi, A.; Hammas-Nasri, I.; Horchani-Naifer, K.; Ferid, M. Study of rare earths leaching after hydrothermal conversion of phosphogypsum. Chem. Afr. 2019, 2, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Dutrizac, J.E. The Behaviour of the Rare Earth Elements during Gypsum (CaSO4·2H2O) Precipitation. Hydrometallurgy 2017, 174, 38–46. [Google Scholar] [CrossRef]
- Borges, R.C.; Favaro, D.I.T.; Caldas, V.G.; Lauria, D.C.; Bernedo, A.V.B. Instrumental Neutron Activation Analysis, Gamma Spectrometry and Geographic Information System Techniques in the Determination and Mapping of Rare Earth Element in Phosphogypsum Stacks. Environ. Earth Sci. 2016, 75, 705. [Google Scholar] [CrossRef]
- Yang, X.; Salvador, D.; Makkonen, H.T.; Pakkanen, L. Phosphogypsum processing for rare earths recovery—A review. Nat. Resour. 2019, 10, 325. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Makkonen, H.T.; Pakkanen, L. Rare Earth Occurrences in Streams of Processing a Phosphate Ore. Minerals 2019, 9, 262. [Google Scholar] [CrossRef] [Green Version]
- Yang, J. Reprocessing of Phosphogypsum for Recovery of REE. In Proceedings of the Prometia Scientific Seminar, Barcelona, Spain, 28–29 November 2017; pp. 28–29. [Google Scholar]
- Reid, S.; Walawalkari, M.; Azimi, G. Valorization of Rare Earth-Containing Landfilled Stocks of Industrial Process Residues: Phosphogypsum and Red Mud. In Proceedings of the The European Real Estate Society (ERES), Santorini, Greece, 28–31 May 2017; pp. 164–165. [Google Scholar]
- Lambert, A.; Anawati, J.; Walawalkar, M.; Tam, J.; Azimi, G. Innovative application of microwave treatment for recovering of rare earth elements from phosphogypsum. ACS Sustain. Chem. Eng. 2018, 6, 16471–16481. [Google Scholar] [CrossRef]
- Hammas-Nasri, I.; Horchani-Naifer, K.; Férid, M.; Barca, D. Production of a rare earths concentrate after phosphogypsum treatment with dietary NaCl and Na2CO3 solutions. Miner. Eng. 2019, 132, 169–174. [Google Scholar] [CrossRef]
- Jyothi, R.K.; Thenepalli, T.; Ahn, J.W.; Parhi, P.K.; Chung, K.W.; Lee, J.Y. Review of rare earth elements recovery from secondary resources for clean energy technologies: Grand opportunities to create wealth from waste. J. Clean. Prod. 2020, 267, 122048. [Google Scholar] [CrossRef]
- Antonick, P.J.; Hu, Z.; Fujita, Y.; Reed, D.W.; Das, G.; Wu, L.; Shivaramaiah, R.; Kim, P.; Eslamimanesh, A.; Lencka, M.M.; et al. Bio-and mineral acid leaching of rare earth elements from synthetic phosphogypsum. J. Chem. Thermodyn. 2019, 132, 491–496. [Google Scholar] [CrossRef]
- Kouraim, M.N.; Fawzy, M.M.; Helaly, O.S. Leaching of lanthanides from phosphogypsum waste using nonyl phenol ethoxylate associated with HNO3 and HCl. Int. J. Sci. Basic Appl. Res. 2014, 16, 31–44. [Google Scholar]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare earths recovery and gypsum upgrade from Florida phosphogypsum. Miner. Metall. Process. 2017, 34, 201–206. [Google Scholar] [CrossRef]
- Lokshin, E.; Tareeva, O.; Elizarova, I. A study of the sulfuric acid leaching of rare-earth elements, phosphorus, and alkali metals from phosphodihydrate. Russ. J. Appl. Chem. 2010, 83, 958–964. [Google Scholar] [CrossRef]
- Valkov, A.V.; Andreev, V.A.; Anufrieva, A.V.; Makaseev, Y.N.; Bezrukova, S.A.; Demyanenko, N.V. Phosphogypsum Technology with the Extraction of Valuable Components. Procedia Chem. 2014, 11, 176–181. [Google Scholar] [CrossRef] [Green Version]
- El-Reefy, S.; Nayl, A.; Aly, H. Leaching and group separation of lanthanides from phosphogypsum. In Proceedings of the 9th. International Conference for Nuclear Sciences and Applications, Sharm Al Sheikh, Egypt, 11–14 February 2008; pp. 11–14. [Google Scholar]
- Lokshin, E.; Vershkova, Y.A.; Vershkov, A.; Tareeva, O. Leaching of lanthanides from phosphohemihydrate with nitric acid. Russ. J. Appl. Chem. 2002, 75, 1753–1759. [Google Scholar] [CrossRef]
- Salo, M.; Knauf, O.; Mäkinen, J.; Yang, X.; Koukkari, P. Integrated acid leaching and biological sulfate reduction of phosphogypsum for REE recovery. Miner. Eng. 2020, 155, 106408. [Google Scholar] [CrossRef]
- Yahorava, V.; Bazhko, V.; Freeman, M. Viability of phosphogypsum as a secondary resource of rare earth elements. In Proceedings of the XXVIII International Mineral Processing Congress Proceedings, Quebec City, QC, Canada, 11–15 September 2016; pp. 1–16, ISBN 9781926872292. [Google Scholar]
- Kolokolnikov, V.A.; Kovalev, M.I. Technology for processing technical calcium carbonate obtained from phosphogypsum into pure calcium carbonate and rare-earth element concentrate. Chem. Sustain. Dev. 2009, 17, 387–393. [Google Scholar]
- De Beer, M.; Maree, J.P.; Liebenberg, L.; Doucet, F.J. Conversion of calcium sulphide to calcium carbonate during the process of recovery of elemental sulphur from gypsum waste. Waste Manag. 2014, 34, 2373–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandil, A.H.T.; Cheira, M.F.; Gado, H.S.; Soliman, M.H.; Akl, H.M. Ammonium sulfate prepar tion from phosphogypsum waste. J. Radiat. Res. Appl. Sci. 2017, 10, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Safiulina, A.M.; Matveeva, A.G.; Evtushenko, A.V.; Lizunov, A.V.; Goryunov, E.I.; Goryunova, I.B.; Brel, V.K. Recovery of lanthanides from digested phosphogypsum solutions using a new organophosphorus extractant, 5-(diphenylphosphoryl) hexan-3-one. Russ. J. Gen. Chem. 2015, 85, 2128–2134. [Google Scholar] [CrossRef]
- Mattila, H.P.; Zevenhoven, R. Mineral carbonation of phosphogypsum waste for production of useful carbonate and sulfate salts. Front. Energy Res. 2015, 3, 48. [Google Scholar] [CrossRef] [Green Version]
- Masmoudi-Soussi, A.; Hammas-Nasri, I.; Horchani-Naifer, K.; Ferid, M. Rare earths recovery by fractional precipitation from a sulfuric leach liquor obtained after phosphogypsum processing. Hydrometallurgy 2020, 191, 105253. [Google Scholar] [CrossRef]
- Laurino, J.P.; Mustacato, J.; Huba, Z.J. Rare earth element recovery from acidic extracts of Florida phosphate mining materials using chelating polymer 1-octadecene, polymer with 2,5-furandione sodium salt. Minerals 2019, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Grabas, K.; Pawelczyk, A.; Strek, W.; Szeleg, E.; Strek, S. Study on the properties of waste apatite phosphogypsum as a raw material of prospective application. Waste Biomass Valor. 2019, 10, 3143–3155. [Google Scholar] [CrossRef] [Green Version]
- Virolainen, S.; Repo, E.; Sainio, T. Recovering rare earth elements from phosphogypsum using a resin-in-leach process: Selection of resin, leaching agent, and eluent. Hydrometallurgy 2019, 189, 105125. [Google Scholar] [CrossRef]
- Gasser, M.S.; Ismail, Z.H.; Abu Elgoud, A.; Abdel Hai, F.; Ali, O.I.; Aly, H.F. Process for Lanthanides-Y leaching from phosphogypsum fertilizers using weak acids. J. Hazard. Mater. 2019, 378, 12762. [Google Scholar] [CrossRef] [PubMed]
- El-Didamony, H.; Ali, M.M.; Awwad, N.S.; Fawzy, M.M.; Attallah, M.F. Treatment of phosphogypsum waste using suitable organic extractants. J. Radioanal. Nucl. Chem. 2012, 291, 907–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genkin, M.V.; Evtushenko, A.V.; Komkov, A.A.; Safiulina, A.M.; Spiridonov, V.S.; Shvetsov, S.V.; Uralchem, J.S.C. Methods for Extracting Rare-Earth Metals and Preparing Gypsum Plaster from Phosphogypsum Hemihydrate. U.S. Patent 9,657,371, 25 April 2017. [Google Scholar]
- Kanzel, A.V.; Mazurkevich, P.A.; Bortkov, I.A.; Hares, N.K. Method for Complex Processing of Phosphogypsum. Patent RU 2639394, 30 December 2017. [Google Scholar]
- Bouchhima, L.; Rouis, M.J.; Choura, M. A study of phosphogypsum-crushing sand based bricks grade negligible weathering. Rom. J. Mater. 2017, 47, 316–323. [Google Scholar]
- Campos, M.P.; Costa, L.J.P.; Nisti, M.B.; Mazzilli, B.P. Phosphogypsum recycling in the building materials industry: Assessment of the radon exhalation rate. J. Environ. Radioact. 2017, 1, 232–236. [Google Scholar] [CrossRef]
- Sheng, Z.; Zhou, J.; Shu, Z.; Yakubu, Y.; Chen, Y.; Wang, W.; Wang, Y. Calcium sulfate whisker reinforced non-fired ceramic tiles prepared from phosphogypsum. Boletín Soc. Española Cerámica Vidr. 2018, 57, 73–78. [Google Scholar] [CrossRef]
- Sun, H.; Tan, D.; Peng, T.; Liang, Y. Preparation of calcium sulfate whisker by atmospheric acidification method from flue gas desulfurization gypsum. Procedia Environ. Sci. 2016, 31, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Miao, M.; Feng, X.; Wang, G.; Cao, S.; Shi, W.; Shi, L. Direct transformation of FGD gypsum to calcium sulfate hemihydrate whiskers: Preparation, simulations, and process analysis. Particuology 2015, 19, 53–59. [Google Scholar] [CrossRef]
- Qi, Y.; Zeng, C.; Wang, C.; Ke, X.; Zhang, L. Continuous fabrication of calcium sulfate whiskers with adjustable aspect ratio in microdroplets. Mater. Lett. 2017, 194, 231–233. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wang, Y.; Tan, R.; Ke, X.; Zhou, X.; Geng, J.; Hou, H.; Zhou, M. Calcium sulfate hemihydrate whiskers obtained from flue gas desulfurization gypsum and used for the adsorption removal of lead. Crystals 2017, 7, 270. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Wei, R.; Zhu, Y.; Long, H.; Huang, B.; Wang, Y.; Wu, S. Calcium sulfate whisker one-step preparation using semi-dry flue gas desulfurization ash and directional growth control. J. Clean. Prod. 2021, 290, 125754. [Google Scholar] [CrossRef]
- Canovas, C.R.; Macias, F.; Perez-Lopez, R.; Basallote, M.D.; Millan-Becerro, R. Valorization of wastes from the fertilizer industry: Current status and future trends. J. Clean. Prod. 2018, 174, 678–690. [Google Scholar] [CrossRef]
- El-Didamony, H.; Gado, H.S.; Awwad, N.S.; Fawzy, M.M.; Attallah, M.F. Treatment of phosphogypsum waste produced from phosphate ore processing. J. Hazard. Mater. 2013, 244, 596–602. [Google Scholar] [CrossRef]
- Pollmann, K.; Kutschke, S.; Matys, S.; Kostudis, S.; Hopfe, S.; Raff, J. Novel biotechnological approaches for the recovery of metals from primary and secondary resources. Minerals 2016, 6, 54. [Google Scholar] [CrossRef]
- Corbett, M.K.; Eksteen, J.J.; Niu, X.Z.; Croue, J.P.; Watkin, E.L.J. Interactions of phosphate solubilising microorganisms with natural rare-earth phosphate minerals: A study utilizing Western Australian monazite. Bioprocess Biosyst. Eng. 2017, 40, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Hopfe, S.; Konsulke, S.; Barthen, R.; Lehmann, F.; Kutschke, S.; Pollmann, K. Screening and selection of technologically applicable microorganisms for recovery of rare earth elements from fluorescent powder. Waste Manag. 2018, 79, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Dev, S.; Sachan, A.; Dehghani, F.; Ghosh, T.; Briggs, B.R.; Aggarwal, S. Mechanisms of biological recovery of rare-earth elements from industrial and electronic wastes: A review. Chem. Eng. J. 2020, 397, 124596. [Google Scholar] [CrossRef]
- Fathollahzadeh, H.; Eksteen, J.J.; Kaksonen, A.H.; Watkin, E.L.J. Role of microorganisms in bioleaching of rare earth elements from primary and secondary resources. Appl. Microbiol. Biotechnol. 2019, 103, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Glombitza, F.; Reichel, S. Metal-containing residues from industry and in the environment: Geobiotechnological urban mining. In Geobiotechnology I; Springer: Berlin/Heidelberg, Germany, 2013; pp. 49–107. [Google Scholar]
- Rasoulnia, P.; Barthen, R.; Lakaniemi, A.M. A critical review of bioleaching of rare earth elements: The mechanisms and effect of process parameters. Crit. Rev. Environ. Sci. Technol. 2021, 51, 378–427. [Google Scholar] [CrossRef]
- Barmettler, F.; Castelberg, C.; Fabbri, C.; Brandl, H. Microbial mobilization of rare earth elements (REE) from mineral solids—A mini review. AIMS Microbiol. 2016, 2, 190–204. [Google Scholar] [CrossRef]
- Costis, S.; Mueller, K.K.; Coudert, L.; Neculita, C.M.; Reynier, N.; Blais, J.F. Recovery potential of rare earth elements from mining and industrial residues: A review and cases studies. J. Geochem. Explor. 2020, 221, 106699. [Google Scholar]
- Pereao, O.; Bode-Aluko, C.; Fatoba, O.; Laatikainen, K.; Petrik, L. Rare earth elements removal techniques from water/wastewater: A review. Desalin. Water Treat 2018, 130, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.M.; Florek, J.; Lariviere, D.; Fontaine, F.G.; Kleitz, F. Recent Advances in the Separation of Rare Earth Elements Using Mesoporous Hybrid Materials. Chem. Rec. 2018, 18, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Hidayah, N.N.; Abidin, S.Z. The evolution of mineral processing in extraction of rare earth elements using solid-liquid extraction over liquid-liquid extraction: A review. Miner. Eng. 2017, 112, 103–113. [Google Scholar] [CrossRef] [Green Version]

| Bearing Wastes | REEs | Ref | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y | Sc | ||
| Acid mine drainage | 0.9 | 0.09 | 0.03 | 0.1 | 0.06 | 0.02 | 0.1 | 0.02 | 0.07 | 0.01 | 0.03 | 0.002 | 0.01 | 0.002 | NR | NR | [19] |
| Fly ash | 91 | 196 | 24 | 89 | 18 | 3 | 16 | 3 | 13 | 3 | 7 | 1 | 7 | 1 | 62 | NR | [20] |
| Mine tailings | 903 | 2047 | 239 | 906 | 148 | 19 | 138 | 16 | 101 | 17 | 54 | 5 | 38 | 4 | 664 | NR | [21] |
| NdFeB magnet | NR | NR | 3 | 260 | NR | NR | NR | NR | 42 | NR | NR | NR | NR | NR | NR | NR | [22] |
| NiMH batteries | 237 | 67 | NR | 36 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | [23] |
| Phosphogypsum | 1450 | 2310 | 235 | 899 | 163 | 35 | 99 | 7 | 46 | 7 | 16 | 1 | 6 | 0.6 | 180 | 1 | [24] |
| Phosphor | 4 | 5 | NR | NR | NR | 3 | 3 | NR | NR | NR | NR | NR | NR | NR | 112 | NR | [25] |
| Country of Origin | Type of PG | REO Content (% wt) | Ref |
|---|---|---|---|
| America | DH | 0.034 | [30] |
| Belgium | DH + HH | 0.55 | [24] |
| Brazil | DH | 0.52–0.54 | [31] |
| Canada | DH | 0.02 | [32] |
| Egypt | DH | 0.048 | [33] |
| Poland | DH + HH | 0.11–0.65 | [16] |
| Russia | DH | 0.47 | [34] |
| Russia | HH | 0.40–0.43 | [34] |
| Russia | HH | 0.59 | [34] |
| Russia | HH | 0.11 | [35] |
| Russia | HH | 0.46 | [35] |
| Russia | DH + HH | 0.3–0.9 | [36] |
| Tunisia | DH | 0.022 | [37] |
| Approach | Advantages | Disadvantages |
|---|---|---|
| Direct acid leaching |
|
|
| Organic liquid |
|
|
| Bioleaching |
|
|
| Carbonation |
|
|
| Atmospheric recrystallization |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukaba, J.-L.; Eze, C.P.; Pereao, O.; Petrik, L.F. Rare Earths’ Recovery from Phosphogypsum: An Overview on Direct and Indirect Leaching Techniques. Minerals 2021, 11, 1051. https://doi.org/10.3390/min11101051
Mukaba J-L, Eze CP, Pereao O, Petrik LF. Rare Earths’ Recovery from Phosphogypsum: An Overview on Direct and Indirect Leaching Techniques. Minerals. 2021; 11(10):1051. https://doi.org/10.3390/min11101051
Chicago/Turabian StyleMukaba, Jean-Luc, Chuks Paul Eze, Omoniyi Pereao, and Leslie Felicia Petrik. 2021. "Rare Earths’ Recovery from Phosphogypsum: An Overview on Direct and Indirect Leaching Techniques" Minerals 11, no. 10: 1051. https://doi.org/10.3390/min11101051
APA StyleMukaba, J.-L., Eze, C. P., Pereao, O., & Petrik, L. F. (2021). Rare Earths’ Recovery from Phosphogypsum: An Overview on Direct and Indirect Leaching Techniques. Minerals, 11(10), 1051. https://doi.org/10.3390/min11101051






