High-Gradient Magnetic Separation of Compact Fluorescent Lamp Phosphors: Elucidation of the Removal Dynamics in a Rotary Permanent Magnet Separator
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of the Phosphors’ Chemical Compositions
2.2. Characterization of the Phosphors’ Physical Properties
2.3. Development of a Time-Resolved Laser-Induced Fluorescence Spectroscopic Quantification Method
2.4. Removal Experiment with a High-Gradient Magnetic Separator
2.5. Magnetic Field Configuration within the Applied Separation Cell
3. Results
3.1. Microwave-Assisted Acid Digestion with Subsequent ICP-MS Measurement
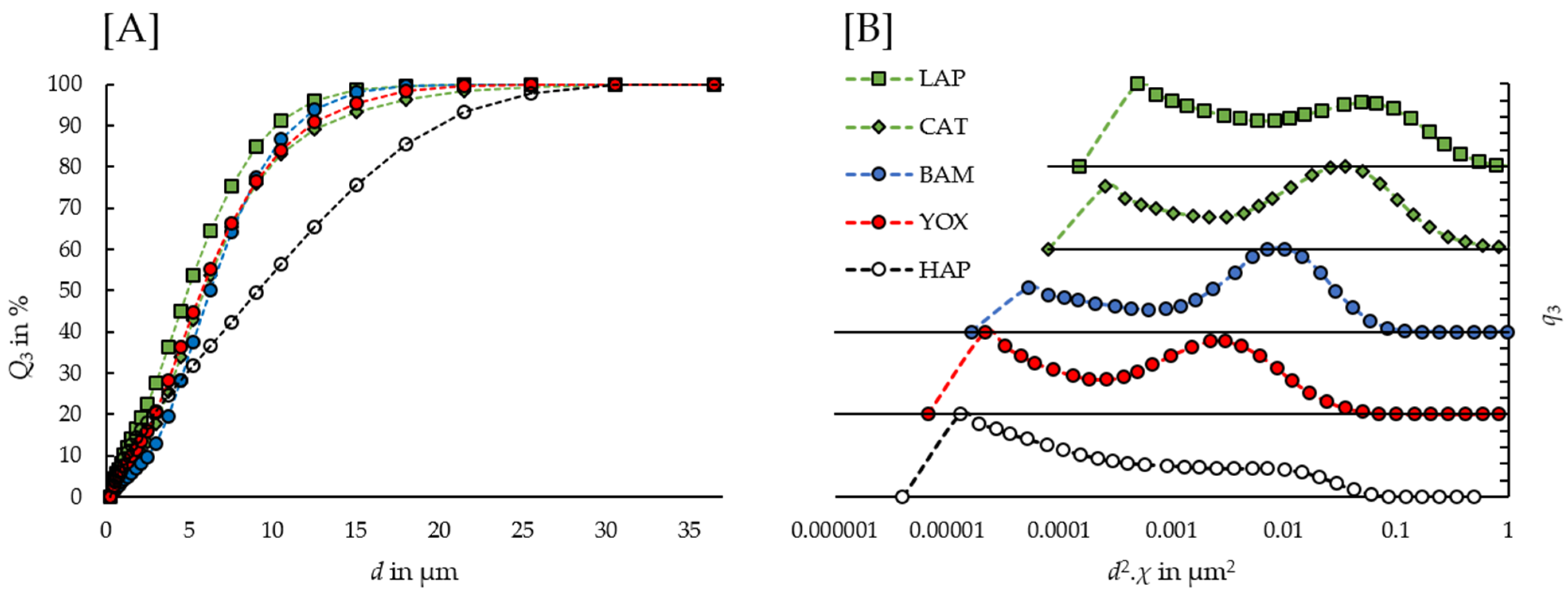
3.2. Physical Particle Characterization
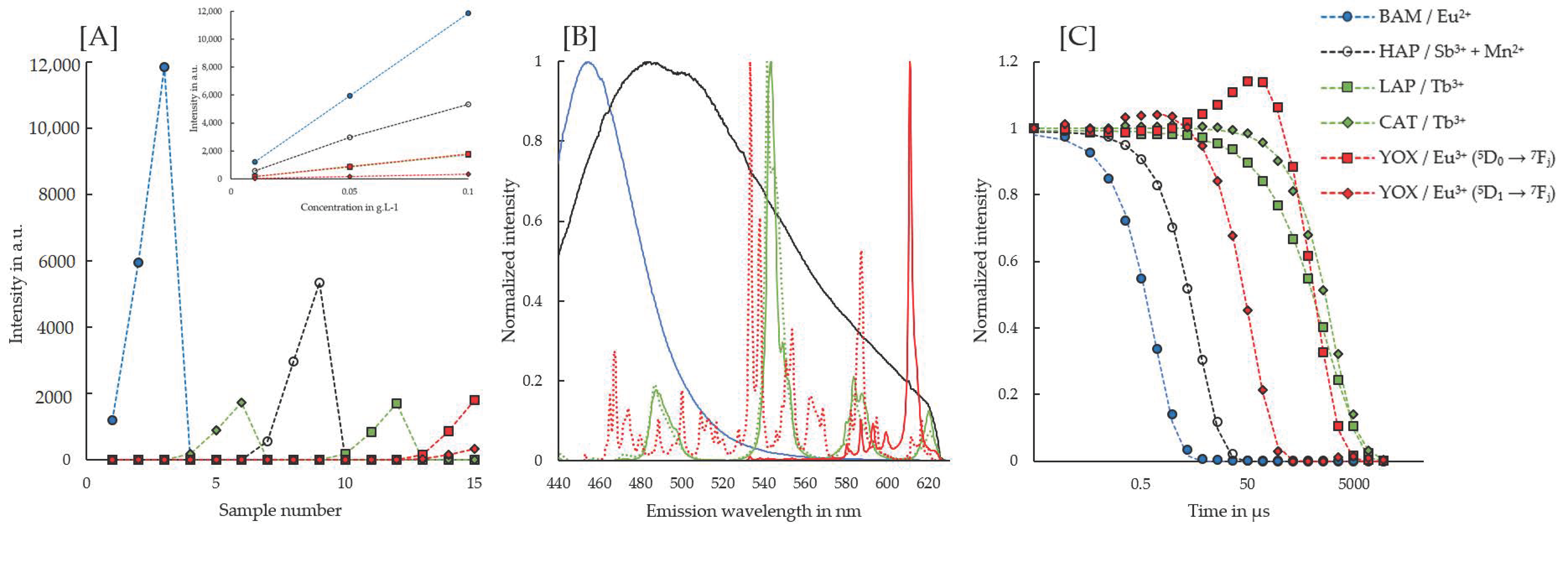
3.3. Time-Resolved Laser-Induced Fluorescence Spectroscopy
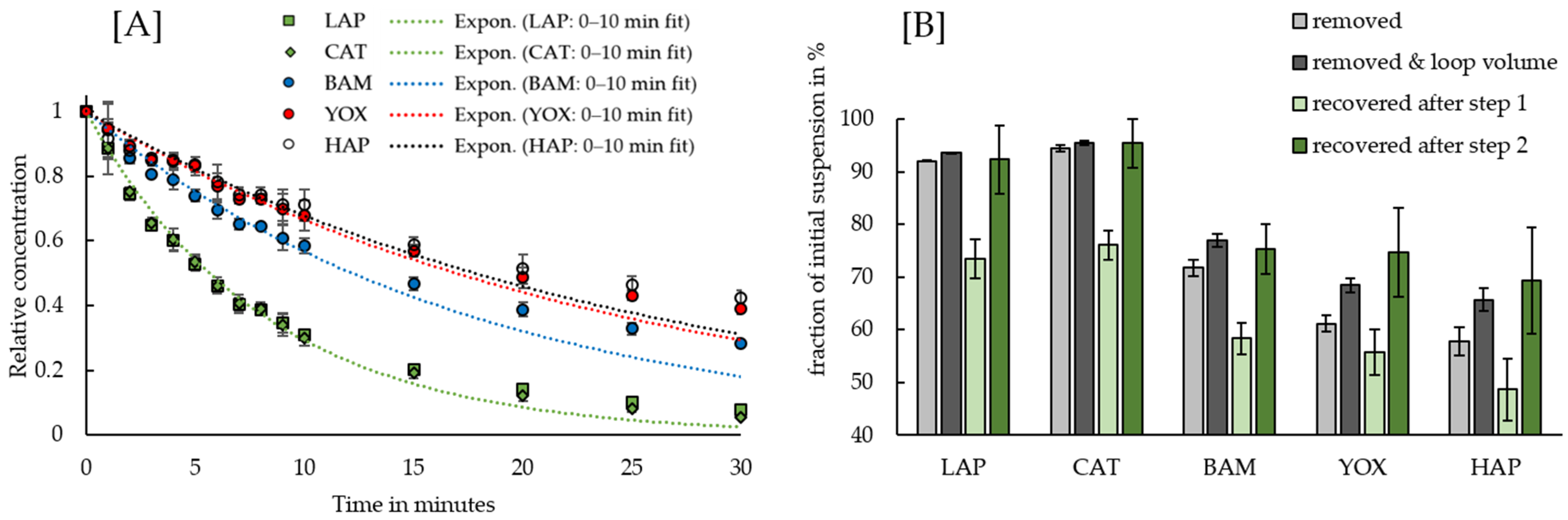
3.4. High-Gradient Magnetic Separation
4. Discussion
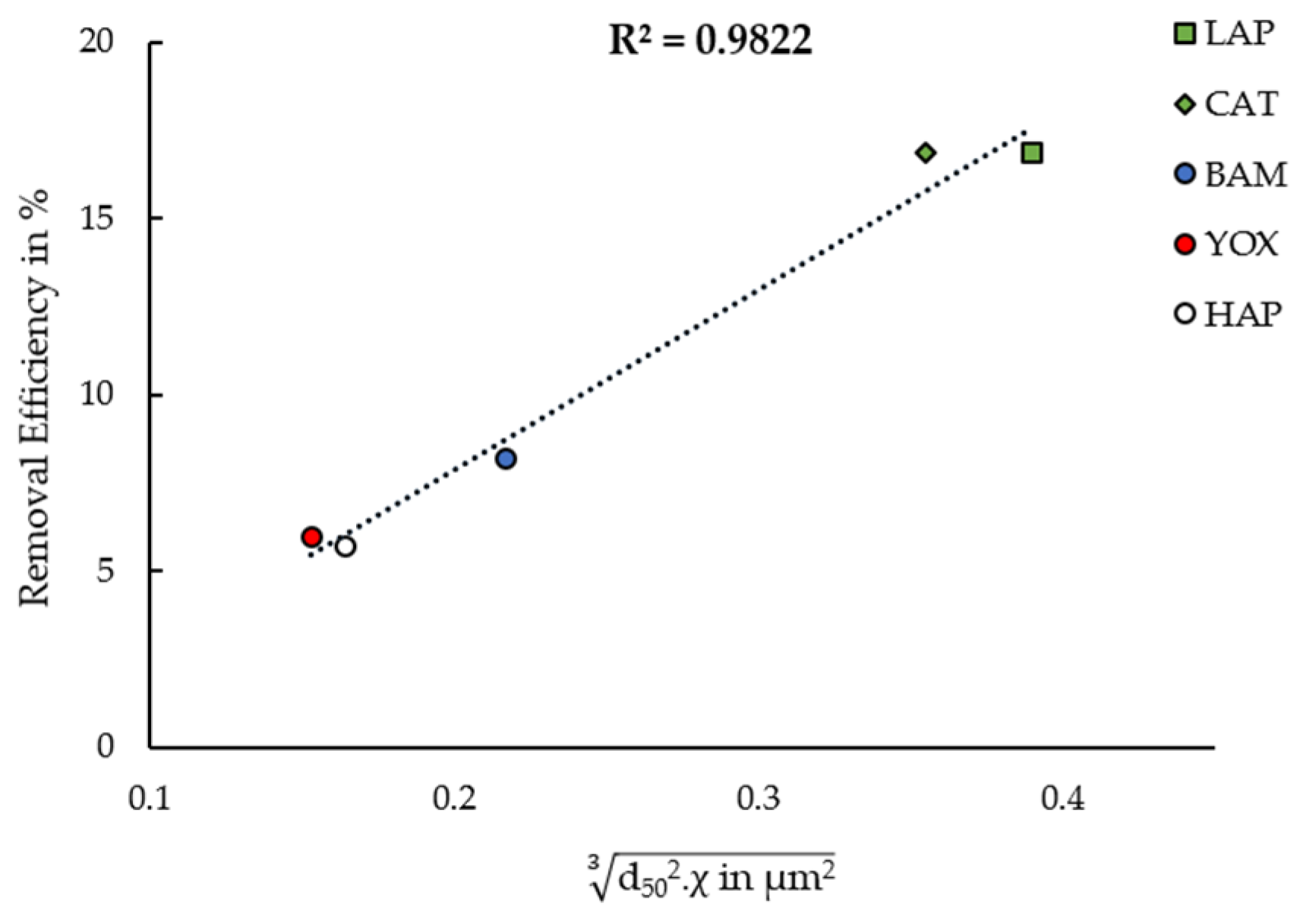
4.1. Magnetophoretic Velocity and High-Gradient Magnetic Separation
4.2. Elucidation of the Empirically Observed Removal Dynamics
4.3. Compensation Demanding Role of Broad Particle Size Distributions
4.4. Potential of the HGF-10 for a Peptide-Based Magnetic Phosphor Separation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binnemans, K.; Jones, P.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of Rare Earths a Critical Review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Lee, J. Rare Earths from Mines to Metals: Comparing Environmental Impacts from China’s Main Production Pathways: Environmental Impacts of Rare Earths in China. J. Ind. Ecol. 2016, 21, 1277–1290. [Google Scholar] [CrossRef]
- European Commission. Study on the EU’s List of Critical Raw Materials—Final Report (2020); European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Binnemans, K.; Jones, P. Perspectives for the recovery of rare earths from end-of-life fluorescent lamps. J. Rare Earths 2014, 32, 195–200. [Google Scholar] [CrossRef]
- Dupont, D.; Binnemans, K. Rare-earth recycling using a functionalized ionic liquid for the selective dissolution and revalorization of Y2O3:Eu3+ from lamp phosphor waste. Green Chem. 2015, 17, 856–868. [Google Scholar] [CrossRef]
- Hopfe, S.; Flemming, K.; Lehmann, F.; Möckel, R.; Kutschke, S.; Pollmann, K. Leaching of rare earth elements from fluorescent powder using the tea fungus Kombucha. Waste Manag. 2017, 62, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Takano, A.; Saitoh, T.; Nagano, N.; Hirai, S.; Shimakage, K. Separation and Recovery of Rare Earth Elements from Phosphor Sludge in Processing Plant of Waste Fluorescent Lamp by Pneumatic Classification and Sulfuric Acidic Leaching. Shigen-to-Sozai 2001, 117, 579–585. [Google Scholar] [CrossRef]
- Hirajima, T.; Sasaki, K.; Abel, B.; Hirai, H.; Hamada, M.; Tsunekawa, M. Feasibility of an efficient recovery of rare earth-activated phosphors from waste fluorescent lamps through dense-medium centrifugation. Sep. Purif. Technol. 2005, 44, 197–204. [Google Scholar] [CrossRef]
- Hirajima, T.; Abel, B.; Sasaki, K.; Nakayama, K.; Hirai, H.; Tsunekawa, M. Floatability of rare earth phosphors from waste fluorescent lamps. Int. J. Miner. Process. 2005, 77, 187–198. [Google Scholar] [CrossRef]
- Otsuki, A.; Dodbiba, G.; Shibayama, A.; Sadaki, J.; Mei, G.; Fujita, T. Separation of Rare Earth Fluorescent Powders by Two-Liquid Flotation using Organic Solvents. Jpn. J. Appl. Phys. 2008, 47, 5093–5099. [Google Scholar] [CrossRef]
- Mei, G.; Rao, P.; Mitsuaki, M.; Fujita, T. Separation of red (Y2O3: Eu3+), blue (Sr, Ca, Ba)10(PO4)6Cl2: Eu2+ and green (LaPO4: Tb3+, Ce3+) rare earth phosphors by liquid/liquid extraction. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 418–423. [Google Scholar] [CrossRef]
- Wada, K.; Mishima, F.; Akiyama, Y.; Nishijima, S. The Development of the Separation Apparatus of Phosphor by Controlling the Magnetic Force. Phys. Procedia 2014, 58, 252–255. [Google Scholar] [CrossRef]
- Yamashita, M.; Akai, T.; Imamura, T.; Murakami, M.; Oki, T. Recycling of waste phosphors using high gradient magnetic separation method. Trans. Mater. Res. Soc. Jpn. 2014, 39, 35–37. [Google Scholar] [CrossRef][Green Version]
- Akai, T.; Ataka, M.; Yamashita, M. Recycling Method of Waste Phosphor. Japan Patent JP 5120963, 16 January 2013. [Google Scholar]
- Yamashita, M.; Akai, T.; Murakami, M.; Oki, T. Recovery of LaPO4:Ce,Tb from waste phosphors using high-gradient magnetic separation. Waste Manag. 2018, 79, 164–168. [Google Scholar] [CrossRef]
- Lederer, F.; Curtis, S.; Bachmann, S.; Dunbar, S.; MacGillivray, R. Identification of lanthanum-specific peptides for future recycling of rare earth elements from compact fluorescent lamps: Peptides for Rare Earth Recycling. Biotechnol. Bioeng. 2016, 114, 1016–1024. [Google Scholar] [CrossRef]
- Pollmann, K.; Kutschke, S.; Matys, S.; Raff, J.; Hlawacek, G.; Lederer, F. Bio-recycling of metals: Recycling of technical products using biological applications. Biotechnol. Adv. 2018, 36, 1048–1062. [Google Scholar] [CrossRef] [PubMed]
- Vreuls, C.; Genin, A.; Zocchi, G.; Boschini, F.; Cloots, R.; Gilbert, B.; Martial, J.; Weerdt, C. Genetically engineered polypeptides as a new tool for inorganic nano-particles separation in water based media. J. Mater. Chem. 2011, 21, 13841–13846. [Google Scholar] [CrossRef]
- Essinger-Hileman, E.; Popczun, E.; Schaak, R. Magnetic separation of colloidal nanoparticle mixtures using a material specific peptide. Chem. Commun. 2013, 49, 5471. [Google Scholar] [CrossRef] [PubMed]
- Cetinel, S.; Shen, W.-Z.; Aminpour, M.; Bhomkar, P.; Wang, F.; Borujeny, E.; Sharma, K.; Nayebi, N.; Montemagno, C. Biomining of MoS2 with Peptide-based Smart Biomaterials. Sci. Rep. 2018, 8, 3374. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Franzreb, M.; Holl, W.H. A novel high-gradient magnetic separator (HGMS) design for biotech applications. IEEE Trans. Appl. Supercond. 2002, 12, 963–966. [Google Scholar] [CrossRef]
- Andersson, C.A.; Bro, R. The N-way Toolbox for MATLAB. Chemom. Intell. Lab. Syst. 2000, 52, 1–4. [Google Scholar] [CrossRef]
- Drobot, B.; Bauer, A.; Steudtner, R.; Tsushima, S.; Bok, F.; Patzschke, M.; Raff, J.; Brendler, V. Speciation Studies of Metals in Trace Concentrations: The Mononuclear Uranyl(VI) Hydroxo Complexes. Anal. Chem. 2016, 88, 3548–3555. [Google Scholar] [CrossRef] [PubMed]
- Drobot, B.; Schmidt, M.; Mochizuki, Y.; Abe, T.; Okuwaki, K.; Brulfert, F.; Falke, S.; Samsonov, S.A.; Komeiji, Y.; Betzel, C.; et al. Cm3+/Eu3+ induced structural, mechanistic and functional implications for calmodulin. Phys. Chem. Chem. Phys. 2019, 21, 21213–21222. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Encinas, A.; Araujo, E.; Song, S. Magnetic matrices used in high gradient magnetic separation (HGMS): A review. Results Phys. 2017, 7, 4278–4286. [Google Scholar] [CrossRef]
- Svoboda, J. Magnetic Techniques for the Treatment of Materials; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Franzreb, M. Magnettechnologie in der Verfahrenstechnik Wässriger Medien; University of Karlsruhe: Karlsruhe, Germany, 2003. [Google Scholar]
- Wadell, H. Volume, Shape, and Roundness of Quartz Particles. J. Geol. 1935, 43, 250–280. [Google Scholar] [CrossRef]
- Tanaka, M.; Oki, T.; Koyama, K.; Narita, H.; Oishi, T. Recycling of rare earths from scrap. In Handbook on the Physics; and Chemistry of Rare Earths; Bunzli, J.C.G., Pecharsky, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 43, pp. 159–211. [Google Scholar]
- Srivastava, A.M.; Ronda, C.R. Phosphors. In The Electrochemical Society Interface; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2003; Volume 12, p. 48. [Google Scholar]
- Greben, M.; Khoroshyy, P.; Sychugov, I.; Valenta, J. Non-exponential decay kinetics: Correct assessment and description illustrated by slow luminescence of Si nanostructures. Appl. Spectrosc. Rev. 2019, 54, 758–801. [Google Scholar] [CrossRef]
- Zatsepin, A.; Kuznetsova, Y. Kinetic selection of nonradiative excitation in photonic nanoparticles Gd2O3:Er. Phys. Chem. Chem. Phys. 2020, 22, 6818–6825. [Google Scholar] [CrossRef] [PubMed]
- Kampeis, P.; Bewer, M.; Rogin, S. Einsatz von Magnetfiltern in der Bioverfahrenstechnik Teil 1: Vergleich Verschiedener Verfahren zum Rückspülen der Magnetfilter. Chem. Ing. Tech. 2009, 81, 275–281. [Google Scholar] [CrossRef]
- Wills, B.; Napier-Munn, T. Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Leong, S.; Yeap, S.P.; Lim, J.K. Working principle and application of magnetic separation for biomedical diagnostic at high- and low-field gradients. Interface Focus 2016, 6, 20160048. [Google Scholar] [CrossRef]
- Birss, R.; Dennis, B.; Gerber, R. Particle capture on the upstream and downstream side of wires in HGMS. IEEE Trans. Magn. 1979, 15, 1362–1363. [Google Scholar] [CrossRef]
- Uchiyama, S.; Hayashi, K. Analytical Theory of Magnetic Particle Capture Process and Capture Radius in High Gradient Magnetic Separation. In Proceedings of the International Conference on Industrial Applications of Magnetic Separation, Rindge, NH, USA, 30 July–4 August 1978; p. 169. [Google Scholar]
- Shaikh, Y.; Kampeis, P. Development of a novel disposable filter bag for separation of biomolecules with functionalized magnetic particles. Eng. Life Sci. 2017, 17, 817–828. [Google Scholar] [CrossRef]
- Schultz Jensen, N.; Syldatk, C.; Franzreb, M.; Hobley, T. Integrated processing and multiple re-use of immobilised lipase by magnetic separation technology. J. Biotechnol. 2007, 132, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-F.; Chang, C.-Y.; Hsu, T.-L. Removal of fluoride from aqueous solution with the superparamagnetic zirconia material. Desalination 2011, 279, 375–382. [Google Scholar] [CrossRef]

| ICP-MS Analysis | LAP | CAT | BAM | YOX | HAP | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| measured | La | 44.8 ± 0.3 | Ce | 6.2 ± 1.1 | Ba | 16.9 ± 0.3 | Y | 74.1 | Ca | 38.6 ± 0.2 |
| Ce | 7.6 ± 0.1 | Mg | 3.3 ± 0.2 | Mg | 3.6 ± 0.4 | Eu | 4.1 | P | 18.9 ± 0.2 | |
| Tb | 11.4 ± 0.1 | Al | 39.1 ± 0.2 | Al | 36.0 ± 3.0 | Mn | 0.3 ± 0.1 | |||
| Tb | 5.2 ± 0.2 | Eu | 2.3 ± 0.1 | Sb | 0.6 ± 0.1 | |||||
| assumed | LaPO4 | 75.4 ± 0.5 | Ce2O3 | 7.3 ± 1.2 | BaO | 18.9 ± 0.3 | Y2O3 | 94.1 | Ca | 38.6 ± 0.2 |
| CePO4 | 12.7 ± 0.1 | MgO | 5.5 ± 0.3 | MgO | 6.0 ± 0.7 | Eu2O3 | 4.7 | PO4 | 57.5 ± 0.5 | |
| TbPO4 | 18.1 ± 0.1 | Al2O3 | 73.9 ± 0.3 | Al2O3 | 68.0 ± 5.7 | Mn | 0.3 ± 0.1 | |||
| Tb2O3 | 6.0 ± 0.3 | EuO | 2.5 ± 0.1 | Sb | 0.6 ± 0.1 | |||||
| total | 106.2 ± 0.5 | 92.6 ± 1.4 | 95.4 ± 6.1 | 98.8 | 97.4 ± 0.7 | |||||
| Variable | Unit | LAP | CAT | BAM | YOX | HAP |
|---|---|---|---|---|---|---|
| d10 | μm | 1.1 | 2.0 | 2.6 | 1.6 | 1.2 |
| d50 | μm | 4.9 | 5.9 | 6.2 | 5.8 | 9.1 |
| d90 | μm | 10.2 | 13.0 | 11.4 | 12.3 | 20.1 |
| Sv, volume equivalent spheres | m2·m−3 | 2.41 | 1.75 | 1.56 | 2.00 | 1.90 |
| ρ | kg·m−3 | 5276 | 4216 | 3764 | 5115 | 3079 |
| Sm | m2·g−1 | 0.76 | 0.45 | 0.62 | 0.53 | 0.77 |
| χ·10−4 | / | 24.4 | 12.8 | 2.61 | 1.08 | 0.64 |
| ψ | / | 0.60 | 0.92 | 0.67 | 0.74 | 0.40 |
| Variable | LAP | CAT | BAM | YOX | HAP 1 |
|---|---|---|---|---|---|
| k/min−1 | 0.123 | 0.123 | 0.057 | 0.041 | 0.039 |
| R2 | 0.9886 | 0.9926 | 0.9766 | 0.9718 | 0.9093 |
| Removal Efficiency | 17% | 17% | 8% | 6% | 6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boelens, P.; Lei, Z.; Drobot, B.; Rudolph, M.; Li, Z.; Franzreb, M.; Eckert, K.; Lederer, F. High-Gradient Magnetic Separation of Compact Fluorescent Lamp Phosphors: Elucidation of the Removal Dynamics in a Rotary Permanent Magnet Separator. Minerals 2021, 11, 1116. https://doi.org/10.3390/min11101116
Boelens P, Lei Z, Drobot B, Rudolph M, Li Z, Franzreb M, Eckert K, Lederer F. High-Gradient Magnetic Separation of Compact Fluorescent Lamp Phosphors: Elucidation of the Removal Dynamics in a Rotary Permanent Magnet Separator. Minerals. 2021; 11(10):1116. https://doi.org/10.3390/min11101116
Chicago/Turabian StyleBoelens, Peter, Zhe Lei, Björn Drobot, Martin Rudolph, Zichao Li, Matthias Franzreb, Kerstin Eckert, and Franziska Lederer. 2021. "High-Gradient Magnetic Separation of Compact Fluorescent Lamp Phosphors: Elucidation of the Removal Dynamics in a Rotary Permanent Magnet Separator" Minerals 11, no. 10: 1116. https://doi.org/10.3390/min11101116
APA StyleBoelens, P., Lei, Z., Drobot, B., Rudolph, M., Li, Z., Franzreb, M., Eckert, K., & Lederer, F. (2021). High-Gradient Magnetic Separation of Compact Fluorescent Lamp Phosphors: Elucidation of the Removal Dynamics in a Rotary Permanent Magnet Separator. Minerals, 11(10), 1116. https://doi.org/10.3390/min11101116







