Rare Earths (La, Y, and Nd) Adsorption Behaviour towards Mineral Clays and Organoclays: Monoionic and Trionic Solutions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
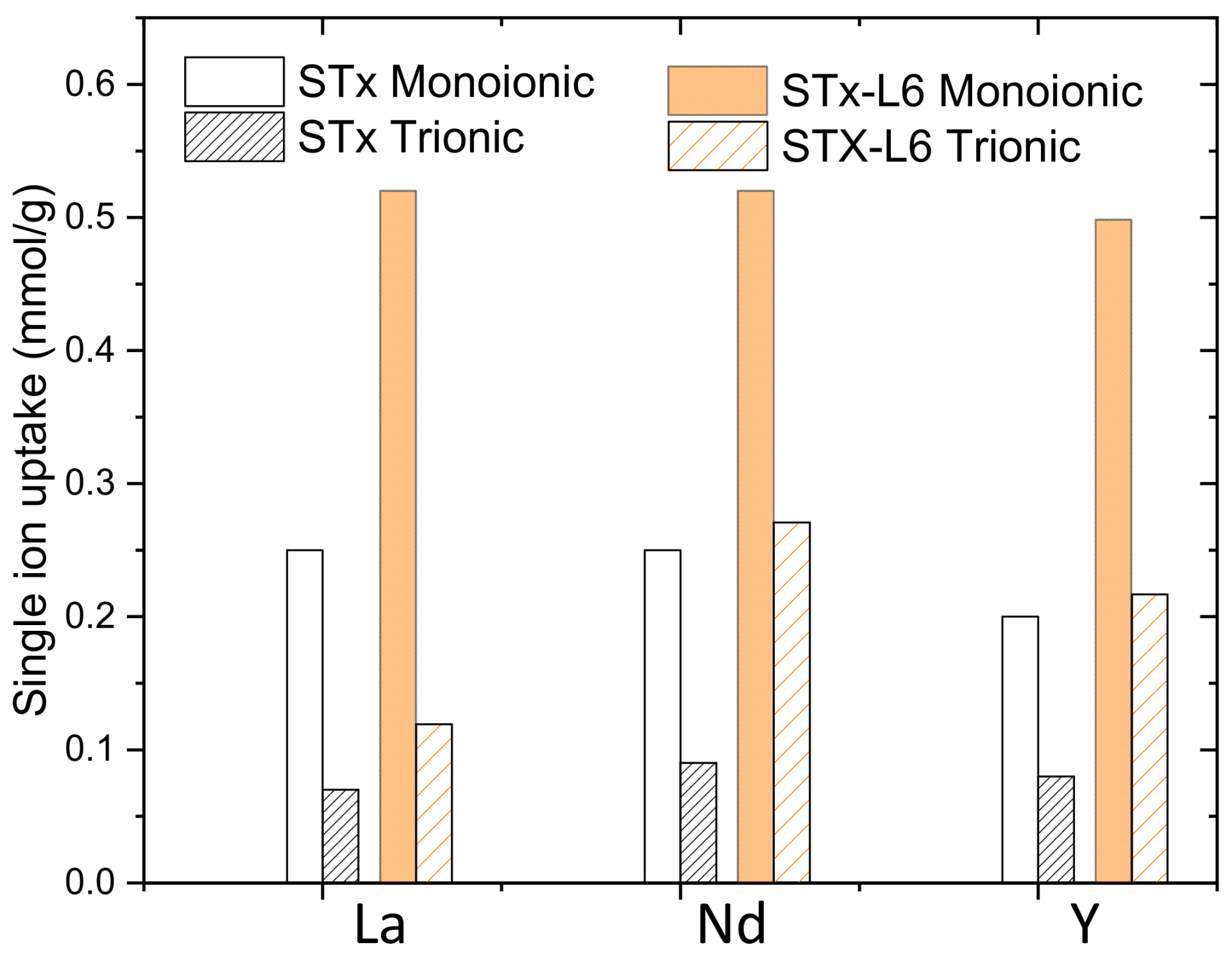
3.1. Monoionic Solutions
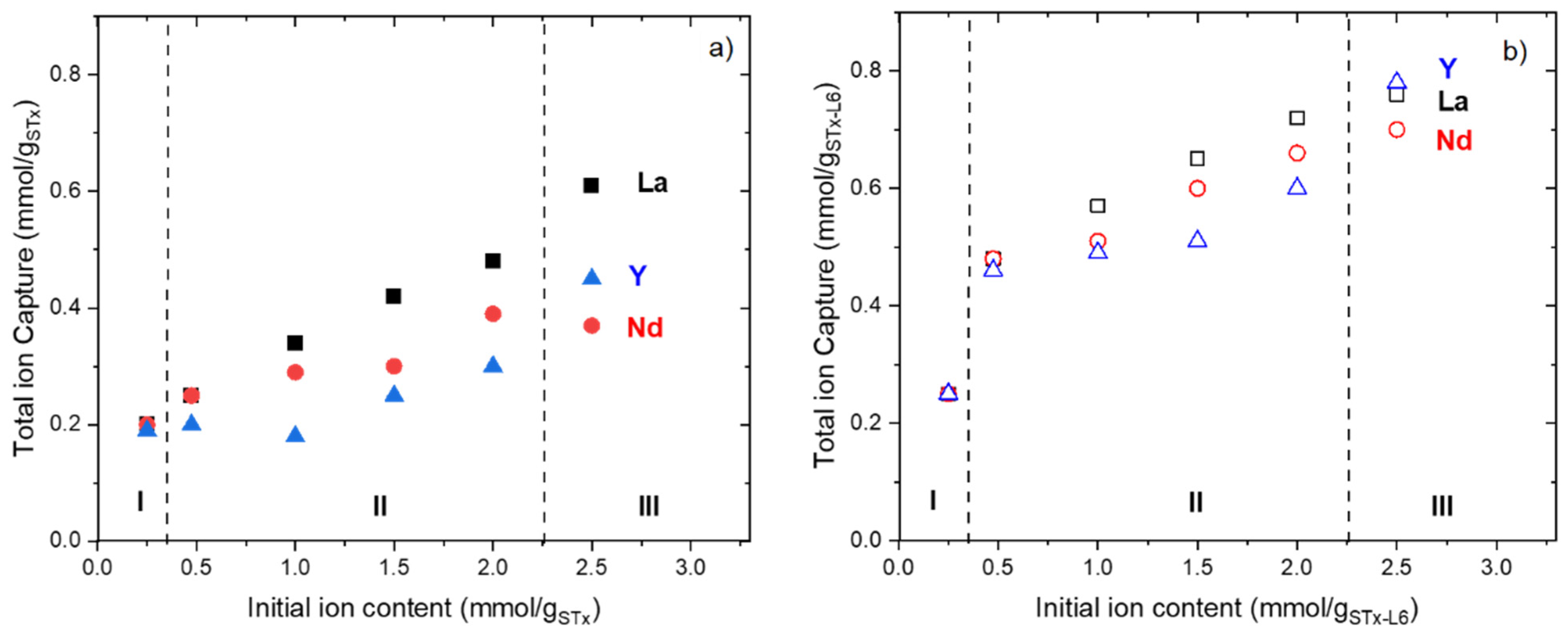
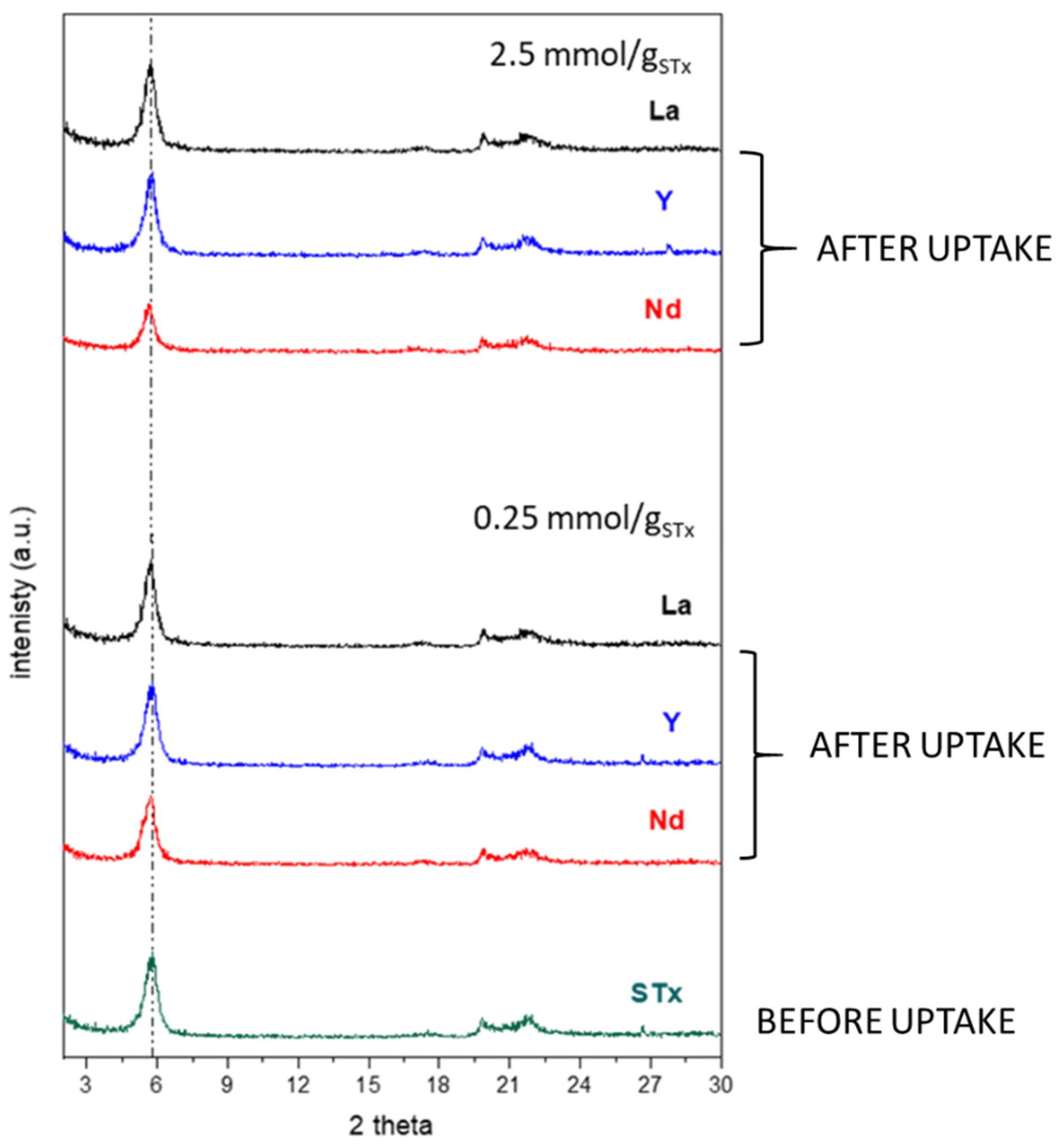
3.1.1. Uptake
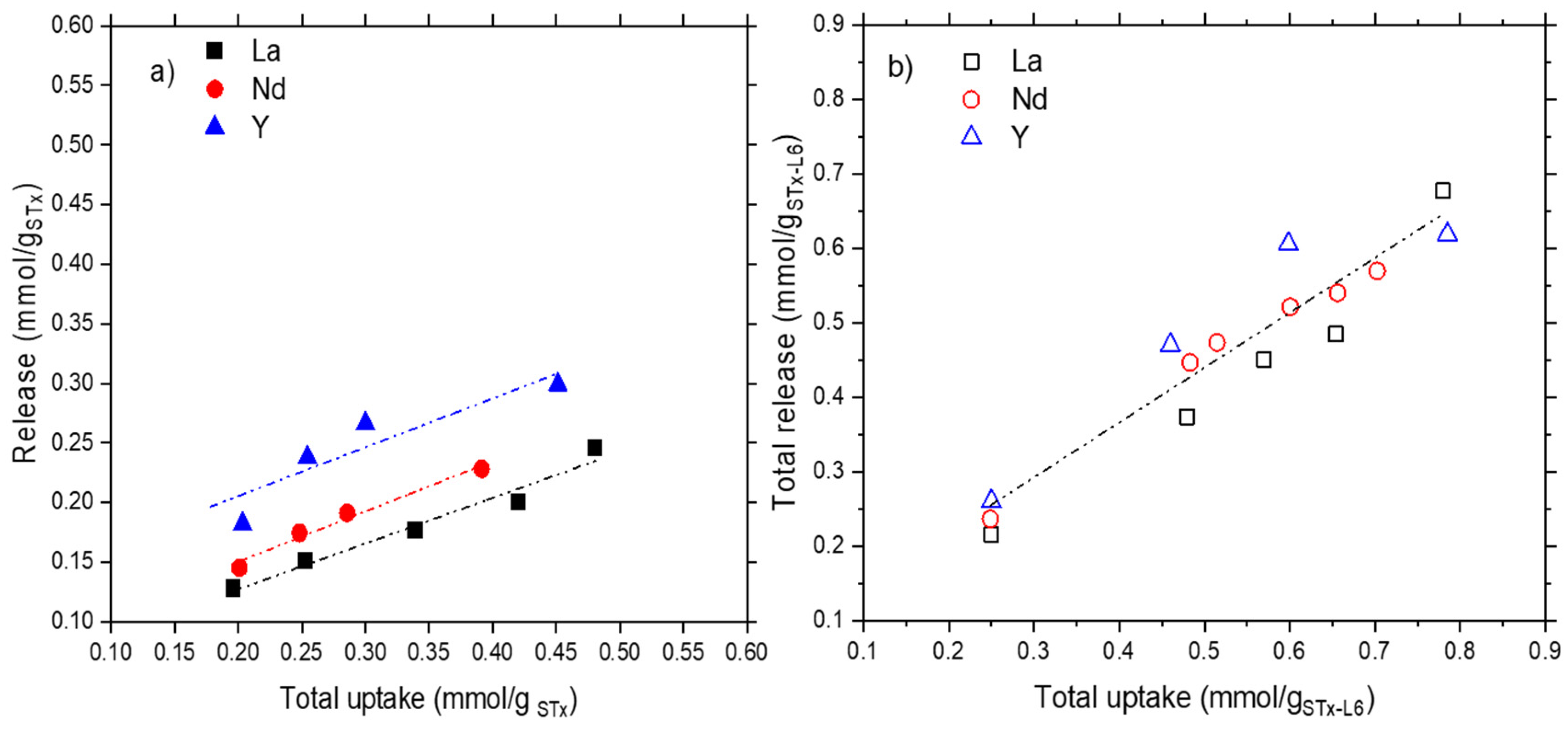
3.1.2. Release
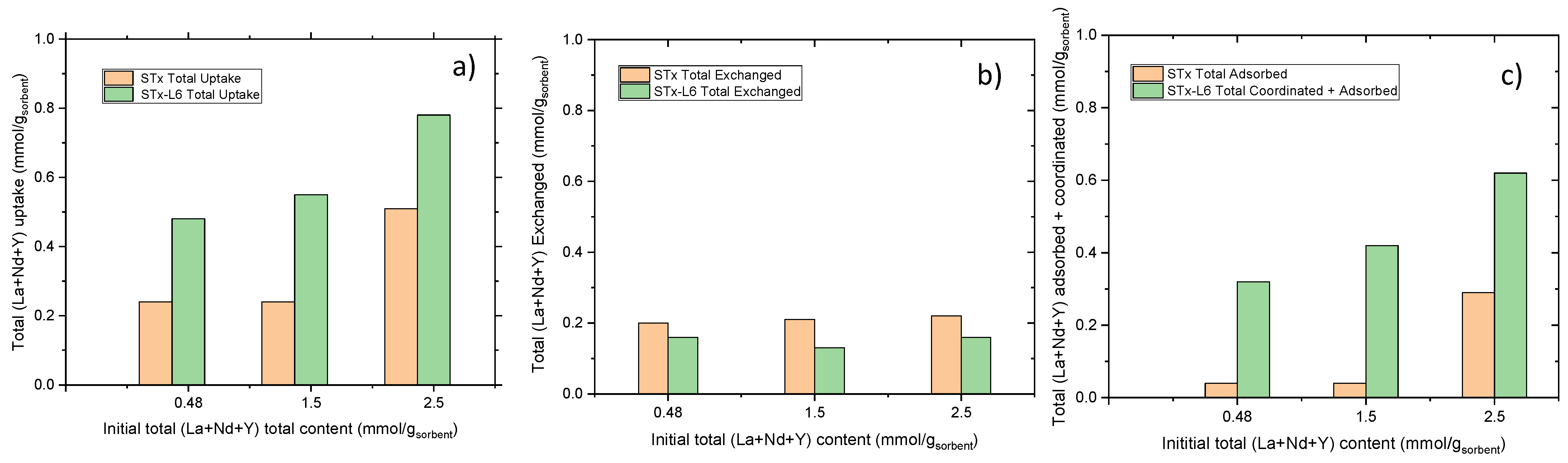
3.2. Trioninc Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Chen, Z. Global rare earth resources and scenarios of future rare earth industry. J. Rare Earths 2011, 29, 1–6. [Google Scholar] [CrossRef]
- European Commission. Report on Critical Raw Materials for the EU—Ad Hoc Working Group on Defining Critical Raw Materials; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Kegl, T.; Košak, A.; Lobnik, A.; Novak, Z.; Kralj, A.K.; Ban, I. Adsorption of rare earth metals from wastewater by nanomaterials: A review. J. Hazard. Mater. 2020, 386, 121632. [Google Scholar] [CrossRef] [PubMed]
- Kulaksiz, S.; Bau, M. Anthropogenic dissolved and colloid/nanoparticle-bound samarium, lanthanum and gadolinium in the Rhine River and the impending destruction of the natural rare earth element distribution in rivers. Earth Planet. Sci. Lett. 2013, 362, 43–50. [Google Scholar] [CrossRef]
- Funari, V.; Bokhari, S.N.H.; Vigliotti, L.; Meisel, T.; Braga, R. The rare earth elements in municipal solid waste incinerators ash and promising tools for their prospecting. J. Hazard. Mater. 2016, 301, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Funari, V.; Braga, R.; Bokhari, S.N.H.; Dinelli, E.; Meisel, T. Solid residues from Italian municipal solid waste incinerators: A source for “‘critical’” raw materials. Waste Manag. 2015, 45, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Tsamis, A.; Coyne, M. Recovery of Rare Earths from Electronic Wastes: An Opportunity for High-Tech SMEs; Directorate-General for Internal Policies of the Union (European Parliament): Brussels, Belgium, 2015. [Google Scholar]
- Making Raw Materials Available for Europe’s Future Wellbeing Proposal for a European Innovation Partnership on Raw Materials European Innovation Partnership on Raw Materials-COM(2012) 82 Final; European Commission: Brussels, Belgium, 2012.
- Ilyas, S.; Lee, J.C.; Kim, B.S. Bioremoval of heavy metals from recycling industry electronic waste by a consortium of moderate thermophiles: Process development and optimization. J. Clean. Prod. 2014, 70, 197–202. [Google Scholar] [CrossRef]
- Bigum, M.; Brogaard, L.; Christensen, T.H. Metal recovery from high-grade WEEE: A life cycle assessment. J. Hazard. Mater. 2012. [Google Scholar] [CrossRef]
- Menikpura, S.N.M.; Santo, A.; Hotta, Y. Assessing the climate co-benefits from Waste Electrical and Electronic Equipment (WEEE) recycling in Japan. J. Clean. Prod. 2014, 74, 183–190. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Xavier, L.H.; Giese, E.C.; Ribeiro-Duthie, A.C.; Lins, F.A.F. Sustainability and the circular economy: A theoretical approach focused on e-waste urban mining. Resour. Policy 2019, 101467. [Google Scholar] [CrossRef]
- Sabot, J.-L.; Maestro, P. Lanthanides. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Iannicelli-Zubiani, E.M.; Giani, M.I.; Recanati, F.; Dotelli, G.; Puricelli, S.; Cristiani, C. Environmental impacts of a hydrometallurgical process for electronic waste treatment: A life cycle assessment case study. J. Clean. Prod. 2017, 140, 1204–1216. [Google Scholar] [CrossRef]
- Lourenço, M.A.O.; Figueira, P.; Pereira, E.; Gomes, J.R.B.; Lopes, C.B.; Ferreira, P. Simple, mono and bifunctional periodic mesoporous organosilicas for removal of priority hazardous substances from water: The case of mercury(II). Chem. Eng. J. 2017, 322, 263–274. [Google Scholar] [CrossRef]
- Eivazihollagh, A.; Svanedal, I.; Edlund, H.; Norgren, M. On chelating surfactants: Molecular perspectives and application prospects. J. Mol. Liq. 2019, 278, 688–705. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Cristiani, C.; Dotelli, G.; Stampino, P.G.; Pelosato, R.; Mesto, E.; Schingaro, E.; Lacalamita, M. Use of natural clays as sorbent materials for rare earth ions: Materials characterization and set up of the operative parameters. Waste Manag. 2015, 46, 546–556. [Google Scholar] [CrossRef]
- Iannicelli-Zubiani, E.M.; Cristiani, C.; Dotelli, G.; Stampino, P.G. Recovery of valuable metals from electronic scraps by clays and organo-clays: Study on bi-ionic model solutions. Waste Manag. 2017, 60, 582–590. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Chen, G.; Zhang, J.; Slaný, M. Synthesis, property and mechanism analysis of a novel polyhydroxy organic amine shale hydration inhibitor. Minerals 2020, 10, 128. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Cristiani, C.; Iannicelli-Zubiani, E.M.; Bellotto, M.; Dotelli, G.; Finocchio, E.; Latorrata, S.; Ramis, G.; Gallo Stampino, P. Capture and release mechanism of La ions by new polyamine-based organoclays: A model system for rare-earths recovery in urban mining process. J. Environ. Chem. Eng. 2020, 104730. [Google Scholar] [CrossRef]
- Galamboš, M.; Suchánek, P.; Rosskopfová, O. Sorption of anthropogenic radionuclides on natural and synthetic inorganic sorbents. J. Radioanal. Nucl. Chem. 2012, 293, 613–633. [Google Scholar] [CrossRef]
- Viglašová, E.; Daňo, M.; Galamboš, M.; Krajňák, A.; Rosskopfová, O.; Rajec, P. Investigation of Cu(II) adsorption on Slovak bentonites and illite/smectite for agricultural applications. J. Radioanal. Nucl. Chem. 2017, 314, 2425–2435. [Google Scholar] [CrossRef]
- Krajňák, A.; Viglašová, E.; Galamboš, M.; Krivosudský, L. Kinetics, thermodynamics and isotherm parameters of uranium(Vi) adsorption on natural and hdtma-intercalated bentonite and zeolite. Desalin. Water Treat. 2018, 127, 272–281. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Q.; Zhou, Q.; Xi, Y.; Zhu, J.; He, H. Adsorbents based on montmorillonite for contaminant removal from water: A review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, F.; Chi, R.; Feng, J.; Ding, Y.; Liu, Q. Preparation of modified montmorillonite and its application to rare earth adsorption. Minerals 2019, 9, 747. [Google Scholar] [CrossRef]
- Alshameri, A.; He, H.; Xin, C.; Zhu, J.; Xinghu, W.; Zhu, R.; Wang, H. Understanding the role of natural clay minerals as effective adsorbents and alternative source of rare earth elements: Adsorption operative parameters. Hydrometallurgy 2019, 185, 149–161. [Google Scholar] [CrossRef]
- Yu, B.; Hu, Z.; Zhou, F.; Feng, J.; Chi, R. Lanthanum (III) and Yttrium (III) Adsorption on Montmorillonite: The Role of Aluminum Ion in Solution and Minerals. Miner. Process. Extr. Metall. Rev. 2020, 41, 107–116. [Google Scholar] [CrossRef]
- Cristiani, C.; Iannicelli-Zubiani, E.M.; Dotelli, G.; Finocchio, E.; Stampino, P.G.; Licchelli, M. Polyamine-based organo-clays for polluted water treatment: Effect of polyamine structure and content. Polymers 2019, 11, 897. [Google Scholar] [CrossRef]
- Kühnel, R.A. Handbook of determinative methods in clay mineralogy. Appl. Clay Sci. 1990, 5, 190–191. [Google Scholar] [CrossRef]
- Pokrić, B.; Branica, M.; Furedi, H.; Orhanović, Z. Precipitation and Hydrolysis of Metallic Ions. III. Studies on the Solubility of Yttrium and Some Rare Earth Hydroxides. Croat. Chem. Acta 1966, 38, 269. [Google Scholar]
- Han, K.N. Characteristics of precipitation of rare earth elements with various precipitants. Minerals 2020, 10, 178. [Google Scholar] [CrossRef]
- Finocchio, E.; Baccini, I.; Cristiani, C.; Dotelli, G.; Gallo Stampino, P.; Zampori, L. Hybrid organo-inorganic clay with nonionic interlayers. Mid- and near-IR spectroscopic studies. J. Phys. Chem. A 2011, 115, 7484–7493. [Google Scholar] [CrossRef] [PubMed]
- Malamis, S.; Katsou, E. A review on zinc and nickel adsorption on natural and modified zeolite, bentonite and vermiculite: Examination of process parameters, kinetics and isotherms. J. Hazard. Mater. 2013, 252–253, 428–461. [Google Scholar] [CrossRef] [PubMed]
- Kinraide, T.B.; Yermiyahu, U. A scale of metal ion binding strengths correlating with ionic charge, Pauling electronegativity, toxicity, and other physiological effects. J. Inorg. Biochem. 2007, 101, 1201–1213. [Google Scholar] [CrossRef]
- Smith, D.W. Ionic hydration enthalpies. J. Chem. Educ. 1977, 54, 540–542. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, Y.; Zheng, B.; Cai, X. Competitive removal of Cd(II) and Pb(II) by biochars produced from water hyacinths: Performance and mechanism. RSC Adv. 2016, 6, 5223–5232. [Google Scholar] [CrossRef]
- Padilla-Ortega, E.; Leyva-Ramos, R.; Flores-Cano, J.V. Binary adsorption of heavy metals from aqueous solution onto natural clays. Chem. Eng. J. 2013, 225, 535–546. [Google Scholar] [CrossRef]
- Ferreira, L.S.; Rodrigues, M.S.; de Carvalho, J.C.M.; Lodi, A.; Finocchio, E.; Perego, P.; Converti, A. Adsorption of Ni2+, Zn2+ and Pb2+ onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris. I. Single metal systems. Chem. Eng. J. 2011, 173, 326–333. [Google Scholar] [CrossRef]
- Cristiani, C.; Bellotto, M.; Dotelli, G.; Finocchio, E.; Latorrata, S.; Ramis, G.; Gallo Stampino, P.; Iannicelli-Zubiani, E.M. Rivalorizzazione di metalli da RAEE (Rivalorization of metals from WEEE). La Chim. e l’Industria Online 2020, IV, 30–34. (In Italian) [Google Scholar] [CrossRef]
- Vázquez, G.; González-Álvarez, J.; Freire, S.; López-Lorenzo, M.; Antorrena, G. Removal of cadmium and mercury ions from aqueous solution by sorption on treated Pinus pinaster bark: Kinetics and isotherms. Bioresour. Technol. 2002, 82, 247–251. [Google Scholar] [CrossRef]
- McKay, G.; Porter, J.F. Equilibrium Parameters for the Sorption of Copper, Cadmium and Zinc Ions onto Peat. J. Chem. Technol. Biotechnol. 1997, 69, 309–320. [Google Scholar] [CrossRef]
- Ma, J.; Luo, J.; Liu, Y.; Wei, Y.; Cai, T.; Yu, X.; Liu, H.; Liu, C.; Crittenden, J.C. Pb(ii), Cu(ii) and Cd(ii) removal using a humic substance-based double network hydrogel in individual and multicomponent systems. J. Mater. Chem. A 2018, 6, 20110–20120. [Google Scholar] [CrossRef]
- Zhou, G.; Luo, J.; Liu, C.; Chu, L.; Crittenden, J. Efficient heavy metal removal from industrial melting effluent using fixed-bed process based on porous hydrogel adsorbents. Water Res. 2018, 131, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Hadi, P.; Barford, J.; McKay, G. Toxic heavy metal capture using a novel electronic waste-based material—Mechanism, modeling and comparison. Environ. Sci. Technol. 2013, 47, 8248–8255. [Google Scholar] [CrossRef]
- Ni, B.J.; Huang, Q.S.; Wang, C.; Ni, T.Y.; Sun, J.; Wei, W. Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere 2019, 219, 351–357. [Google Scholar] [CrossRef]
- Ricordel, S.; Taha, S.; Cisse, I.; Dorange, G. Heavy metals removal by adsorption onto peanut husks carbon: Characterization, kinetic study and modeling. Sep. Purif. Technol. 2001, 24, 389–401. [Google Scholar] [CrossRef]
- Qu, J.; Tian, X.; Jiang, Z.; Cao, B.; Akindolie, M.S.; Hu, Q.; Feng, C.; Feng, Y.; Meng, X.; Zhang, Y. Multi-component adsorption of Pb(II), Cd(II) and Ni(II) onto microwave-functionalized cellulose: Kinetics, isotherms, thermodynamics, mechanisms and application for electroplating wastewater purification. J. Hazard. Mater. 2020, 387, 121718. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristiani, C.; Bellotto, M.; Dotelli, G.; Latorrata, S.; Ramis, G.; Gallo Stampino, P.; Zubiani, E.M.I.; Finocchio, E. Rare Earths (La, Y, and Nd) Adsorption Behaviour towards Mineral Clays and Organoclays: Monoionic and Trionic Solutions. Minerals 2021, 11, 30. https://doi.org/10.3390/min11010030
Cristiani C, Bellotto M, Dotelli G, Latorrata S, Ramis G, Gallo Stampino P, Zubiani EMI, Finocchio E. Rare Earths (La, Y, and Nd) Adsorption Behaviour towards Mineral Clays and Organoclays: Monoionic and Trionic Solutions. Minerals. 2021; 11(1):30. https://doi.org/10.3390/min11010030
Chicago/Turabian StyleCristiani, Cinzia, Maurizio Bellotto, Giovanni Dotelli, Saverio Latorrata, Gianguido Ramis, Paola Gallo Stampino, Elena Maria Iannicelli Zubiani, and Elisabetta Finocchio. 2021. "Rare Earths (La, Y, and Nd) Adsorption Behaviour towards Mineral Clays and Organoclays: Monoionic and Trionic Solutions" Minerals 11, no. 1: 30. https://doi.org/10.3390/min11010030
APA StyleCristiani, C., Bellotto, M., Dotelli, G., Latorrata, S., Ramis, G., Gallo Stampino, P., Zubiani, E. M. I., & Finocchio, E. (2021). Rare Earths (La, Y, and Nd) Adsorption Behaviour towards Mineral Clays and Organoclays: Monoionic and Trionic Solutions. Minerals, 11(1), 30. https://doi.org/10.3390/min11010030













