Chitosan with Bentonite and Biochar in Ni-Affected Soil Reduces Grain Ni Concentrations, Improves Soil Enzymes and Grain Quality in Lentil
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Analysis of Ni-Affected Soil
2.2. Acquirement of Immobilizing Amendments
2.2.1. Preparation and Characteristics of BR
2.2.2. Purchase of CN and BE
2.3. Pot Trial
2.4. Reaping of Lentil Plants and Analysis
2.5. Determination of Ni in Soil and Plant Parts and Micro-Nutrients in Grain
2.6. Biochemical Spectrum of Grain
2.7. Activities of Antioxidants and Oxidative Injury in the Leaves
2.8. Soil Enzymatic Activities
2.9. Statistical Analysis of Experimental Data
3. Results
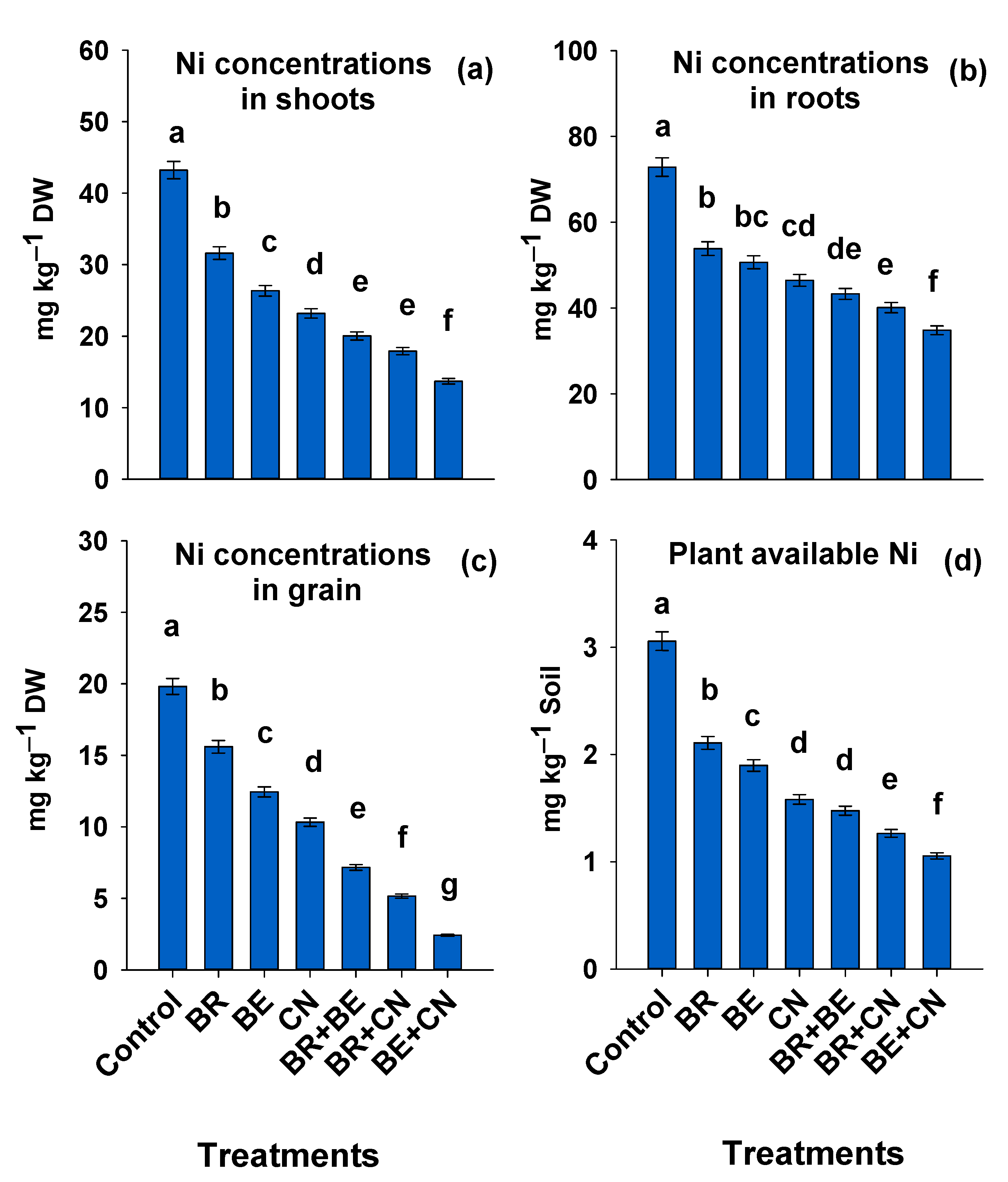
3.1. Nickel Distribution in Lentil Plant and Soil DTPA-Extractable Ni
3.2. Biomass, Growth, Chlorophyll Contents, and RWC of Lentil
3.3. Biochemical Compounds and Micronutrients in the Grain and Soil pH
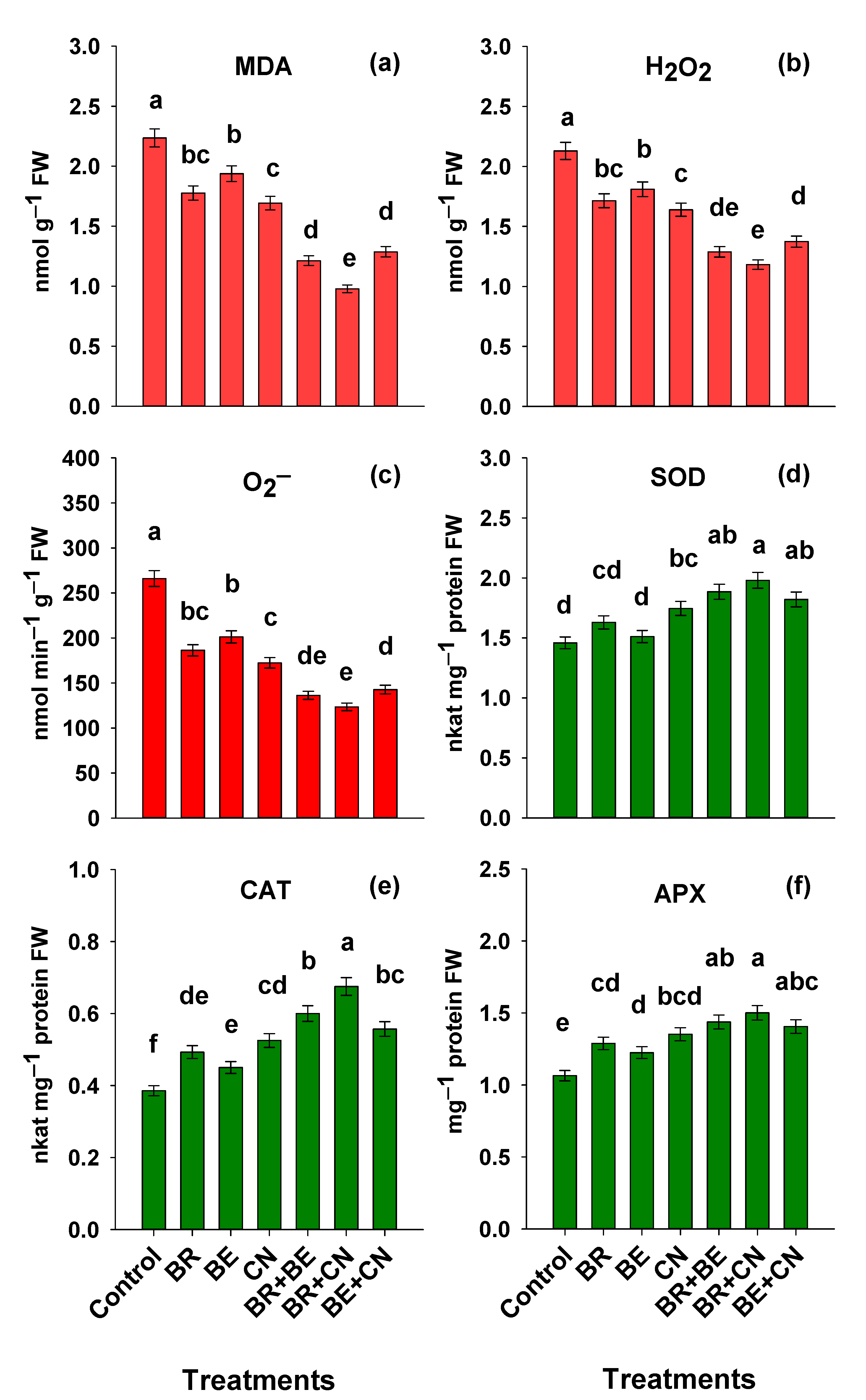
3.4. Oxidative Injury and the Status of Antioxidant Defense Machinery in Lentil
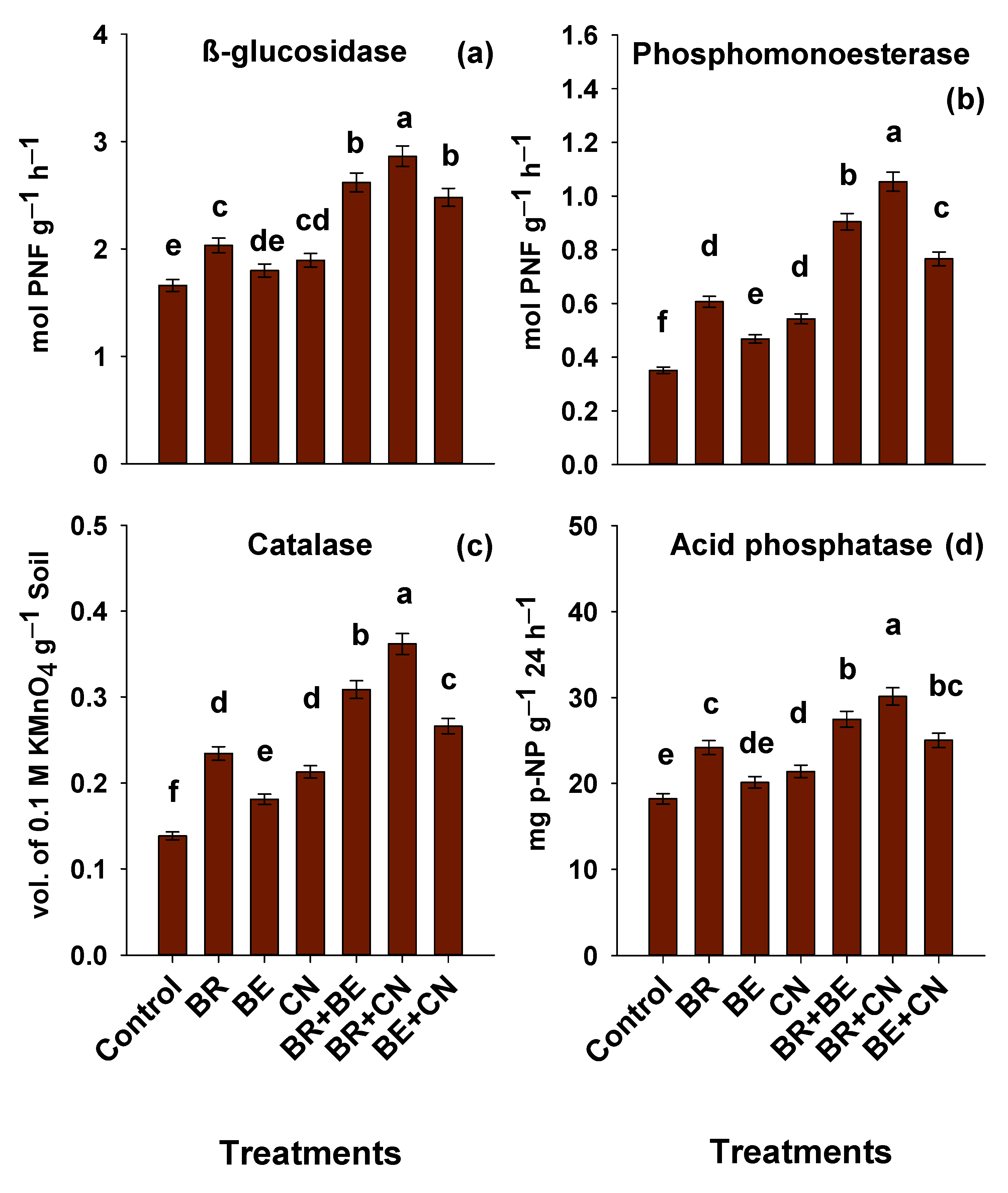
3.5. Enzymatic Activities in Soil
4. Discussion
4.1. Nickel Distribution in Lentil Plant, Bioavailable Ni in Soil, and Soil pH
4.2. Biomass, Growth, Chlorophyll Contents, and RWC of Lentil
4.3. Biochemical Compounds and Micronutrients in the Grain
4.4. Oxidative Injury and the Status of Antioxidant Defense Machinery in Lentil
4.5. Enzymatic Activities in the Soil
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alloway, B.J. Heavy Metals in Soils; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. [Google Scholar]
- Cempel, M.; Nikel, G. Nickel: A review of its sources and environmental toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- Iyaka, Y.A. Nickel in soils: A review of its distribution and impacts. Sci. Res. Essays 2011, 6, 6774–6777. [Google Scholar]
- Shahbaz, A.K.; Lewińska, K.; Iqbal, J.; Ali, Q.; Iqbal, M.; Abbas, F.; Tauqeer, H.M.; Ramzani, P.M.A. Improvement in productivity, nutritional quality, and antioxidative defense mechanisms of sunflower (Helianthus annuus L.) and maize (Zea mays L.) in nickel contaminated soil amended with different biochar and zeolite ratios. J. Environ. Manag. 2018, 218, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, A.K.; Iqbal, M.; Jabbar, A.; Hussain, S.; Ibrahim, M. Assessment of nickel bioavailability through chemical extractants and red clover (Trifolium pratense L.) in an amended soil: Related changes in various parameters of red clover. Ecotoxicol. Environ. Saf. 2018, 149, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Holm, O.; Hansen, E.; Lassen, C.; Stuer-Lauridsen, F.; Kjølholt, J. Heavy Metals in Wastes; Project ENV.E.3/ETU/2000/, COWI A/S; European Commission on Environment: Copenhagen, Denmark, 2002; Available online: https://ec.europa.eu/environment/waste/studies/pdf/heavy_metalsreport.pdf (accessed on 8 December 2020).
- Mahmood, A.; Malik, R.N. Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arabian J. Chem. 2014, 7, 91–99. [Google Scholar] [CrossRef]
- WHO/FAO Codex Alimentarius Commission. Food Additives and Contaminants. Joint FAO/WHO Food Standards Programme, ALINORM 10/12A. 2001. Available online: www.transpaktrading.com/static/pdf/research/achemistry/introTofertilizers.pdf (accessed on 8 December 2020).
- Abbasi, M.N.; Tufail, M.; Mashiatullah, A.; Chaudhary, M.Z. A Review of Heavy Metal Pollution in the Soil of Pakistan. Sci. Int. 2014, 26, 2201–2205. [Google Scholar]
- Mashiatullah, A.; Chaudhary, M.Z.; Ahmad, N.; Javed, T.; Ghaffar, A. Metal pollution and ecological risk assessment in marine sediments of Karachi Coast, Pakistan. Environ. Monit. Assess. 2012, 185, 1555–1565. [Google Scholar] [CrossRef]
- Khan, W.U.D.; Ramzani, P.M.A.; Anjum, S.; Abbas, F.; Iqbal, M.; Yasar, A.; Ihsan, M.Z.; Anwar, M.N.; Baqar, M.; Taqueer, H.M.; et al. Potential of miscanthus biochar to improve sandy soil health, in situ nickel immobilization in soil and nutritional quality of spinach. Chemosphere 2017, 185, 1144–1156. [Google Scholar] [CrossRef]
- Pivarčiová, L.; Rosskopfová, O.; Galamboš, M.; Rajec, P. Sorption of nickel on chitosan. J. Radioanal. Nucl. Chem. 2014, 300, 361–366. [Google Scholar] [CrossRef]
- Mousavi, H.Z.; Seyedi, S.R. Nettle ash as a low cost adsorbent for the removal of nickel and cadmium from wastewater. Int. J. Environ. Sci. Technol. 2011, 8, 195–202. [Google Scholar] [CrossRef][Green Version]
- Ahmad, I.Z.; Ahmad, A.; Mabood, A.; Tabassum, H. Effects of different metal stresses on the antioxidant defense systems of medicinal plants. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Khan, M.I.R., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 215–256. [Google Scholar]
- Kotapati, K.V.; Palaka, B.K.; Ampasala, D.R. Alleviation of nickel toxicity in finger millet (Eleusine coracana L.) germinating seedlings by exogenous application of salicylic acid and nitric oxide. Crop J. 2017, 5, 240–250. [Google Scholar] [CrossRef]
- Soares, C.; de Sousa, A.; Pinto, A.; Azenha, M.; Teixeira, J.; Azevedo, R.A.; Fidalgo, F. Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ. Exp. Botany 2016, 122, 115–125. [Google Scholar] [CrossRef]
- Sachan, P.; Lal, N. An overview of nickel (Ni2+) essentiality, toxicity and tolerance strategies in plants. Asian J. Biol. 2017, 2, 1–15. [Google Scholar] [CrossRef]
- Pandey, N.; Pathak, G.C. Nickel alters antioxidative defense and water status in green gram. Indian J. Plant Physiol. 2006, 11, 113. [Google Scholar]
- Pandey, N.; Sharma, C.P. Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci. 2002, 163, 753–758. [Google Scholar] [CrossRef]
- Pandey, N.; Pathak, G.C.; Pandey, D.K.; Pandey, R. Heavy metals, Co, Ni, Cu, Zn and Cd, produce oxidative damage and evoke differential antioxidant responses in spinach. Braz. J. Plant Physiol. 2009, 21, 103–111. [Google Scholar] [CrossRef]
- Demirezen Yilmaz, D.; Uruç Parlak, K. Antioxidative parameters in the opposite-leaved pondweed (Gronlendia densa) in response to nickel stress. Chem. Spec. Bioavail. 2011, 23, 71–79. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, H.; Deng, Z.; Tsao, R. Phytochemicals of lentil (Lens culinaris) and their antioxidant and anti-inflammatory effects. J. Food Bioact. 2018, 1, 93–103. [Google Scholar] [CrossRef]
- Shahbaz, A.K.; Ramzani, P.M.A.; Saeed, R.; Turan, V.; Iqbal, M.; Lewińska, K.; Abbas, F.; Saqib, M.; Tauqeer, H.M.; Iqbal, M.; et al. Effects of biochar and zeolite soil amendments with foliar proline spray on nickel immobilization, nutritional quality and nickel concentrations in wheat. Ecotoxicol. Environ. Saf. 2019, 173, 182–191. [Google Scholar] [CrossRef]
- Beri, A.; Sharma, R. Nickel toxicity to photosynthetic attributes in the leaves of lentil (Lens culnaris Medic. Masar). IJAR 2016, 2, 239–242. [Google Scholar]
- Saad, R.; Kobaissi, A.; Robin, C.; Echevarria, G.; Benizri, E. Nitrogen fixation and growth of Lens culinaris as affected by nickel availability: A pre-requisite for optimization of agromining. Environ. Exp. Bot. 2016, 131, 1–9. [Google Scholar] [CrossRef]
- Puschenreiter, M. Sustainable Management of Trace Element Contaminated Soils-Development of a Decision Tool System and Its Evaluation for Practical Application. 2; Snowman: Vienna, Austria, 2008; pp. 1–318. [Google Scholar]
- Turan, V.; Ramzani, P.M.A.; Ali, Q.; Abbas, F.; Iqbal, M.; Irum, A.; Khan, W.U.D. Alleviation of nickel toxicity and an improvement in zinc bioavailability in sunflower seed with chitosan and biochar application in pH adjusted nickel contaminated soil. Arch. Agron. Soil Sci. 2018, 64, 1053–1067. [Google Scholar] [CrossRef]
- Turan, V.; Khan, S.A.; Iqbal, M.; Ramzani, P.M.A.; Fatima, M. Promoting the productivity and quality of brinjal aligned with heavy metals immobilization in a wastewater irrigated heavy metal polluted soil with biochar and chitosan. Ecotoxicol. Environ. Saf. 2018, 161, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Ramzani, P.M.A.; Shan, L.; Anjum, S.; Ronggui, H.; Iqbal, M.; Virk, Z.A.; Kausar, S. Improved quinoa growth, physiological response, and seed nutritional quality in three soils having different stresses by the application of acidified biochar and compost. Plant Physiol. Biochem. 2017, 116, 127–138. [Google Scholar] [CrossRef]
- Ramzani, P.M.A.; Khalid, M.; Anjum, S.; Khan, W.U.D.; Iqbal, M.; Kausar, S. Improving iron bioavailability and nutritional value of maize (Zea mays L.) in sulfur-treated calcareous soil. Arch. Agron. Soil Sci. 2017, 63, 1255–1266. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Rinklebe, J.; Selim, M.H. Impact of various amendments on immobilization and phytoavailability of nickel and zinc in a contaminated floodplain soil. Inter. J. Environ. Sci. Tech. 2015, 12, 2765–2776. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, L.; Yan, H.; Kennedy, J.F.; Meng, X. Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohyd. Polym. 2013, 94, 272–277. [Google Scholar] [CrossRef]
- Pandey, P.; De, N. Effect of Chitosan Based Superabsorbent on Water Retention Behaviour of Soil and Seedling Growth of Alfalfa (Medicago sativa L.). Ind. J. Ecol. 2017, 44, 456–460. [Google Scholar]
- Khati, P.; Chaudhary, P.; Gangola, S.; Bhatt, P.; Sharma, A. Nanochitosan supports growth of Zea mays and also maintains soil health following growth. Biotech 2017, 7, 81. [Google Scholar] [CrossRef]
- Tahervand, S.; Jalali, M. Sorption and desorption of potentially toxic metals (Cd, Cu, Ni and Zn) by soil amended with bentonite, calcite and zeolite as a function of pH. J. Geochem. Exp. 2017, 181, 148–159. [Google Scholar] [CrossRef]
- Mi, J.; Gregorich, E.G.; Xu, S.; McLaughlin, N.B.; Liu, J. Effects of a one-time application of bentonite on soil enzymes in a semi-arid region. Canad. J. Soil Sci. 2018, 98, 1–14. [Google Scholar] [CrossRef]
- Kumararaja, P.; Shabeer, T.A.; Manjaiah, K.M. Effect of bentonite on heavy metal uptake by amaranth (Amaranthus blitum cv. Pusa Kirti) grown on metal contaminated soil. Horti. Soc. Ind. 2016, 73, 224–228. [Google Scholar]
- Śnioszek, M.; Telesiński, A.; Smolik, B.; Zakrzewska, H. Effect of Fluoride and Bentonite on Biochemical Aspects of Oxidative Stress in Pisum sativum L. J. Ecol. Eng. 2018, 19, 164–171. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle-Size Analysis. In Methods of Soil Analsysis, Part 1: Physical and Mineralogical Methods; Klute, A., Ed.; SSSA, ASA: Madison, WI, USA, 1986; pp. 383–409. [Google Scholar]
- Rhoades, J.D. Cation exchange capacity. In Methods of Soil Analysis. Part 2, Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy Inc.: Madison, WI, USA, 1982; pp. 149–157. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Constable and Company: London, UK, 1962; p. 498. [Google Scholar]
- Watanabe, F.S.; Olsen, S.R. Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts. Soil Sci. Soc. Am. Proc. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; Handbook No. 60; USDA: Washington, DC, USA, 1954; pp. 83–126. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis. Part 2, 2nd ed.; Page, A.L., Miller, R.H., Eds.; Agron. Monogr. 9; ASA and SSSA: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Allison, L.E.; Moodie, C.D. Carbonate. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Black, C.A., Ed.; ASA, SSSA: Madison, WI, USA, 1965; pp. 1379–1396. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese and copper. Soil. Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Chen, M.; Ma, L.Q. Comparison of three aqua regia digestion methods for twenty Florida soils. Soil Sci. Soc. Am. J. 2001, 65, 491–499. [Google Scholar] [CrossRef]
- Mullan, D.; Pietragalla, J. Leaf relative water content. In Physiological Breeding II: A Field Guide to Wheat Phenotyping; Pask, A.J.D., Pietragalla, J., Mullan, D.M., Reynolds, M.P., Eds.; Cimmyt: Mexico City, Mexico, 2012; pp. 25–27. [Google Scholar]
- Aydinalp, C.; Katkat, A.V. The comparison of extraction methods for evaluating some heavy metals in polluted soils. Plant Soil Environ. 2004, 50, 212–217. [Google Scholar] [CrossRef]
- Jalali, M.; Hourseresht, Z. Metal Extractability in Binary and Multi-metals Spiked Calcareous Soils. Commun. Soil Sci.Plant Anal. 2017, 48, 1089–1104. [Google Scholar] [CrossRef]
- Quevauviller, P.; Lachica, M.; Barahona, E.; Rauret, G.; Ure, A.; Gomez, A.; Muntau, H. Interlaboratory comparison of EDTA and DTPA procedures prior to certification of extractable trace elements in calcareous soil. Sci. Total Environ. 1996, 178, 127–132. [Google Scholar] [CrossRef]
- Jones, J.R.J.; Case, V.W. Sampling, handling, and analyzing plant tissue samples. In Soil Testing and Plant Analysis; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; pp. 389–428. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official’s Analytical Chemists, 17th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2003. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Roth, E.F., Jr.; Gilbert, H.S. The pyrogallol assay for superoxide dismutase: Absence of a glutathione artifact. Anal. Biochem. 1984, 137, 50–53. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of endogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Method Mol. Biol. 2010, 639, 292–298. [Google Scholar]
- Auclair, C.; Voisin, E. Nitroblue tetrazolium reduction. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 123–132. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Xu, G.H.; Zheng, H.Y. Handbook of Soil Microbiology Analysis Method; Chinese Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Paz-Ferreiro, J.; Fu, S.; Méndez, A.; Gascó, G. Interactive effects of biochar and the earthworm Pontoscolex corethrurus on plant productivity and soil enzyme activities. J. Soils Sed. 2014, 14, 483–494. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Turan, V. Confident performance of chitosan and pistachio shell biochar on reducing Ni bioavailability in soil and plant plus improved the soil enzymatic activities, antioxidant defense system and nutritional quality of lettuce. Ecotoxicol. Environ. Saf. 2019, 183, 109594. [Google Scholar] [CrossRef]
- Ali, U.; Shaaban, M.; Bashir, S.; Fu, Q.; Zhu, J.; Islam, M.S.; Hu, H. Effect of rice straw, biochar and calcite on maize plant and Ni bio-availability in acidic Ni contaminated soil. J. Environ. Manag. 2020, 259, 109674. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Malik, Z.; Parveen, A.; Huang, L.; Riaz, M.; Bashir, S.; Mustafa, A.; Abbasi, G.H.; Xue, B.; Ali, U. Ameliorative Effects of Biochar on Rapeseed (Brassica napus L.) Growth and Heavy Metal Immobilization in Soil Irrigated with Untreated Wastewater. J. Plant Growth Regul. 2020, 39, 266–281. [Google Scholar] [CrossRef]
- Khan, M.A.; Ramzani, P.M.A.; Zubair, M.; Rasool, B.; Khan, M.K.; Ahmed, A.; Khan, S.A.; Turan, V.; Iqbal, M. Associative effects of lignin-derived biochar and arbuscular mycorrhizal fungi applied to soil polluted from Pb-acid batteries effluents on barley grain safety. Sci. Total Environ. 2020, 710, 136294. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Bashir, S.; Khan, I.; Hussain, Q.; Gao, R.; Hu, H. Biochar induced Pb and Cu immobilization, phytoavailability attenuation in Chinese cabbage, and improved biochemical properties in naturally co-contaminated soil. J. Soils Sediments 2019, 19, 2381–2392. [Google Scholar] [CrossRef]
- Lahori, A.H.; Zhang, Z.; Shaheen, S.M.; Rinklebe, J.; Guo, Z.; Li, R.; Mahar, A.; Wang, Z.; Ren, C.; Mi, S.; et al. Mono-and co-applications of Ca-bentonite with zeolite, Ca-hydroxide, and tobacco biochar affect phytoavailability and uptake of copper and lead in a gold mine-polluted soil. J. Hazard. Mater. 2019, 374, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Vrinceanu, N.O.; Motelică, D.M.; Calciu, I.; Tănase, V.; Preda, M.; Plopeanu, G.; Ivana, I. Influence of bentonite, dolomite, natural zeolite and manure on heavy metal immobilization in a contaminated soil. AgroLife Sci. J. 2017, 6, 227–234. [Google Scholar]
- Zulqurnain Haider, M.; Hussain, S.; Muhammad Adnan Ramzani, P.; Iqbal, M.; Iqbal, M.; Shahzad, T.; Fatima, M.; Khan, S.A.; Khan, I.; Shahid, M.; et al. Bentonite and Biochar Mitigate Pb Toxicity in Pisum sativum by Reducing Plant Oxidative Stress and Pb Translocation. Plants 2019, 8, 571. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and the biomass production of rapeseed (Brassica napus L.). Biomass Bioenergy 2013, 57, 196–204. [Google Scholar] [CrossRef]
- Kim, J.M.; Roh, A.-S.; Choi, S.-C.; Kim, E.-J.; Choi, M.-T.; Ahn, B.-K.; Kim, S.-K.; Lee, Y.-H.; Joa, J.-H.; Kang, S.-S.; et al. Soil pH and electrical conductivity are key edaphic factors shaping bacterial communities of greenhouse soils in Korea. J. Microbiol. 2016, 54, 838–845. [Google Scholar] [CrossRef]
- Bandara, T.; Herath, I.; Kumarathilaka, P.; Seneviratne, M.; Seneviratne, G.; Rajakaruna, N.; Vithanage, M.; Ok, Y.S. Role of woody biochar and fungal-bacterial co-inoculation on enzyme activity and metal immobilization in serpentine soil. J. Soils Sediments 2017, 17, 665–673. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng.J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Tauqeer, H.M.; ur-Rahman, M.; Hussain, S.; Abbas, F.; Iqbal, M. The potential of an energy crop “Conocarpus erectus” for lead phytoextraction and phytostabilization of chromium, nickel, and cadmium: An excellent option for the management of multi-metal contaminated soils. Ecotoxicol. Environ. Saf. 2019, 173, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Song, W.; Tian, J. Biochar-facilitated soil remediation: Mechanisms and efficacy variations. Front. Environ. Sci. 2020, 8, 183. [Google Scholar] [CrossRef]
- Deshpande, P.; Dapkekar, A.; Oak, M.D.; Paknikar, K.M.; Rajwade, J.M. Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohyd. Poly. 2017, 165, 394–401. [Google Scholar] [CrossRef]
- Xu, G.; Wei, L.L.; Sun, J.N.; Shao, H.B.; Chang, S.X. What is more important for enhancing nutrient bioavailability with biochar application into a sandy soil: Direct or indirect mechanism? Ecol. Eng. 2013, 52, 119–124. [Google Scholar] [CrossRef]
- Hafeez, Y.; Iqbal, S.; Jabeen, K.; Shahzad, S.; Jahan, S.; Rasul, F. Effect of biochar application on seed germination and seedling growth of Glycine max (L.) Merr. Under drought stress. Pak. J. Bot. 2017, 49, 7–13. [Google Scholar]
- Kizito, S.; Luo, H.; Lu, J.; Bah, H.; Dong, R.; Wu, S. Role of nutrient-enriched biochar as a soil amendment during maize growth: Exploring practical alternatives to recycle agricultural residuals and to reduce chemical fertilizer demand. Sustainability 2019, 11, 3211. [Google Scholar] [CrossRef]
- Naeem, I.; Masood, N.; Turan, V.; Iqbal, M. Prospective usage of magnesium potassium phosphate cement combined with Bougainvillea alba derived biochar to reduce Pb bioavailability in soil and its uptake by Spinacia oleracea L. Ecotoxicol. Environ. Saf. 2021, 208, 111723. [Google Scholar] [CrossRef]
- Georgiadou, E.C.; Kowalska, E.; Patla, K.; Kulbat, K.; Smolinska, B.; Leszczynska, J.; Fotopoulos, V. Influence of heavy metals (Ni, Cu, and Zn) on nitro-oxidative stress responses, proteome regulation and allergen production in basil (Ocimum basilicum L.). Front. Plant Sci. 2018, 9, 862. [Google Scholar] [CrossRef] [PubMed]
- Abderrahim, F.; Huanatico, E.; Segura, R.; Arribas, S.; Gonzalez, M.C.; Condezo-Hoyos, L. Physical features, phenolic compounds, betalains and total antioxidant capacity of coloured quinoa seeds (Chenopodium quinoa Willd.) from Peruvian Altiplano. Food Chem. 2015, 183, 83–90. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; González-Morales, S.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effects of chitosan–PVA and Cu nanoparticles on the growth and antioxidant capacity of tomato under saline stress. Molecules 2018, 23, 178. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, H.; Al-Tabbaa, A. Leachability and heavy metal speciation of 17-year old stabilised/solidified contaminated site soils. J. Hazard. Mater. 2014, 278, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.J.; Hu, J.; Wang, X.J.; Shao, C.X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Hu, B.; Wang, Y.; Zeng, X. Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. J. Agric. Food Chem. 2014, 62, 9128–9136. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Liu, L.; Huang, C.; Zhou, D.; Fu, L. Characterization and toxicology evaluation of chitosan nanoparticles on the embryonic development of zebrafish, Danio rerio. Carbohyd. Polym. 2016, 141, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Kuziemska, B. Enzymatic activity of nickel-contaminated soil. Teka Kom. Ochr. Kszt. Środ. Przyr. OL PAN 2014, 11, 77–89. [Google Scholar]
- Strachel, R.; Wyszkowska, J.; Baćmaga, M. An Evaluation of the Effectiveness of Sorbents in the Remediation of Soil Contaminated with Zinc. Water Air Soil Pollut. 2018, 229, 235. [Google Scholar] [CrossRef]
| Treatments | Abbreviations | % of the Amendment Incorporated in the Soil | Amounts of Amendments Incorporated in the Soil (g pot−1) |
|---|---|---|---|
| Control | Control | − | − |
| Biochar | BR | 2% | 100 |
| Bentonite | BE | 2% | 100 |
| Chitosan | CN | 2% | 100 |
| Biochar+Bentonite | BR+BE | 1% + 1% | 50 + 50 |
| Biochar+Chitosan | BR+CN | 1% + 1% | 50 + 50 |
| Bentonite+Chitosan | BE+CN | 1% + 1% | 50 + 50 |
| Treatments | Growth | Chlorophyll | Relative Water Content (RWC) | |||
|---|---|---|---|---|---|---|
| Plant Height | Shoot Dry Biomass | Root Dry Biomass | Chl-a | Chl-b | ||
| (cm) | (g pot−1) | (mg g−1 FW) | (%) | |||
| Control | 45.4 ± 1.16 e | 3.1 ± 0.08 f | 1.9 ± 0.03 e | 0.65 ± 0.02 e | 0.53 ± 0.02 f | 73.8 ± 2.08 e |
| BR | 59.8 ± 1.52 c | 4.3 ± 0.11 d | 1.6 ± 0.04 c | 0.83 ± 0.03 bc | 0.67 ± 0.02 cd | 83.3 ± 2.35 b-d |
| BE | 52.0 ± 1.33 d | 3.6 ± 0.09 e | 1.3 ± 0.04 d | 0.72 ± 0.02 de | 0.58 ± 0.02 ef | 78.0 ± 2.20 de |
| CN | 56.4 ± 1.44 cd | 3.9 ± 0.10 de | 1.4 ± 0.04 d | 0.76 ± 0.02 cd | 0.63 ± 0.02 df | 81.2 ± 2.29 cd |
| BR+BE | 69.7 ± 1.78 b | 5.3 ± 0.13 b | 1.7 ± 0.05 b | 0.89 ± 0.03 b | 0.77 ± 0.02 ab | 88.5 ± 2.50 ab |
| BR+CN | 76.4 ± 1.95 a | 5.7 ± 0.15 a | 1.9 ± 0.05 a | 0.98 ± 0.03 a | 0.83 ± 0.03 a | 91.7 ± 2.58 a |
| BE+CN | 66.4 ± 1.70 b | 4.9 ± 0.12 c | 1.6 ± 0.05 bc | 0.86 ± 0.03 b | 0.73 ± 0.02 bc | 85.4 ± 2.41 a-c |
| Treatments | Biochemical Compounds | Micronutrients | Soil pH after Plant Harvest | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein | Fiber | Fat | Total Sugar | Polyphenols | Fe | Zn | Mn | Mg | Ca | Na | ||
| (mg g−1 FW) | (mg g−1 DW) | Values | ||||||||||
| Control | 17.6 ± 0.59 e | 3.9 ± 0.13 f | 0.9 ± 0.03 d | 1.9 ± 0.06 e | 5.9 ± 0.20 a | 47.9 ± 1.35 de | 29.7 ± 0.84 e | 12.2 ± 0.34 f | 390.0 ± 11.0 d | 321.5 ± 9.06 e | 39.9 ± 1.13 d | 7.03 ± 0.04c |
| BR | 20.6 ± 0.69 cd | 5.4 ± 0.18 cd | 1.3 ± 0.04 bc | 2.8 ± 0.09 b | 4.9 ± 0.16 bc | 57.6 ± 1.62 b | 41.6 ± 1.17 bc | 17.8 ± 0.50 b | 495.4 ± 13.9 ab | 423.8 ± 11.9 ab | 49.4 ± 1.39 ab | 7.27 ± 0.05b |
| BE | 18.8 ± 0.63 de | 4.7 ± 0.16 e | 1.2 ± 0.04 c | 2.2 ± 0.08 d | 5.2 ± 0.18 b | 45.1 ± 1.27 e | 35.9 ± 1.01 d | 12.8 ± 0.36 ef | 436.4 ± 12.3 c | 357.3 ± 10.0 d | 43.5 ± 1.23 cd | 7.78 ± 0.05a |
| CN | 19.4 ± 0.65 de | 5.1 ± 0.17 de | 1.3 ± 0.04 c | 2.5 ± 0.09 bc | 4.6 ± 0.15 c | 52.5 ± 1.48 cd | 39.6 ± 1.12 c | 14.6 ± 0.41 cd | 458.5 ± 12.9 bc | 377.4 ± 10.6 cd | 47.0 ± 1.33 bc | 7.12 ± 0.05c |
| BR+BE | 22.9 ± 0.77 ab | 6.3 ± 0.21 b | 1.5 ± 0.05 a | 2.4 ± 0.08 cd | 3.7 ± 0.13 de | 53.9 ± 1.52 bc | 43.6 ± 1.23 b | 15.2 ± 0.43 c | 479.6 ± 13.5 ab | 394.2 ± 11.1 bc | 47.9 ± 1.35 b | 7.64 ± 0.05ab |
| BR+CN | 24.9 ± 0.84 a | 7.0 ± 0.24 a | 1.5 ± 0.05 a | 3.1 ± 0.10 a | 3.3 ± 0.11 e | 68.2 ± 1.92 a | 48.4 ± 1.36 a | 20.9 ± 0.59 a | 515.5 ± 14.5 a | 443.8 ± 12.5 a | 52.9 ± 1.49 a | 7.19 ± 0.05bc |
| BE+CN | 21.6 ± 0.73 bc | 5.7 ± 0.19 bc | 1.4 ± 0.05 ab | 2.3 ± 0.08 cd | 4.0 ± 0.14 d | 55.8 ± 1.57 bc | 40.5 ± 1.14b c | 13.6 ± 0.38 de | 466.9 ± 13.1 bc | 384.8 ± 10.8 cd | 45.6 ± 1.28 bc | 7.54 ± 0.05ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanzeem-ul-Haq, H.S.; Rasool, B.; Ehtisham-ul-Haque, S.; Saif, S.; Zafar, S.; Younis, T.; Akhtar, I.; Jafri, L.; Iqbal, N.; Masood, N.; et al. Chitosan with Bentonite and Biochar in Ni-Affected Soil Reduces Grain Ni Concentrations, Improves Soil Enzymes and Grain Quality in Lentil. Minerals 2021, 11, 11. https://doi.org/10.3390/min11010011
Tanzeem-ul-Haq HS, Rasool B, Ehtisham-ul-Haque S, Saif S, Zafar S, Younis T, Akhtar I, Jafri L, Iqbal N, Masood N, et al. Chitosan with Bentonite and Biochar in Ni-Affected Soil Reduces Grain Ni Concentrations, Improves Soil Enzymes and Grain Quality in Lentil. Minerals. 2021; 11(1):11. https://doi.org/10.3390/min11010011
Chicago/Turabian StyleTanzeem-ul-Haq, Hafiz Syed, Bilal Rasool, Syed Ehtisham-ul-Haque, Sadia Saif, Sadia Zafar, Tahira Younis, Imran Akhtar, Laila Jafri, Naeem Iqbal, Nasir Masood, and et al. 2021. "Chitosan with Bentonite and Biochar in Ni-Affected Soil Reduces Grain Ni Concentrations, Improves Soil Enzymes and Grain Quality in Lentil" Minerals 11, no. 1: 11. https://doi.org/10.3390/min11010011
APA StyleTanzeem-ul-Haq, H. S., Rasool, B., Ehtisham-ul-Haque, S., Saif, S., Zafar, S., Younis, T., Akhtar, I., Jafri, L., Iqbal, N., Masood, N., Lewińska, K., & Iqbal, M. (2021). Chitosan with Bentonite and Biochar in Ni-Affected Soil Reduces Grain Ni Concentrations, Improves Soil Enzymes and Grain Quality in Lentil. Minerals, 11(1), 11. https://doi.org/10.3390/min11010011









