Recrystallization and Uptake of 226Ra into Ba-Rich (Ba,Sr)SO4 Solid Solutions
Abstract
1. Introduction
2. Materials and Methods
3. Results
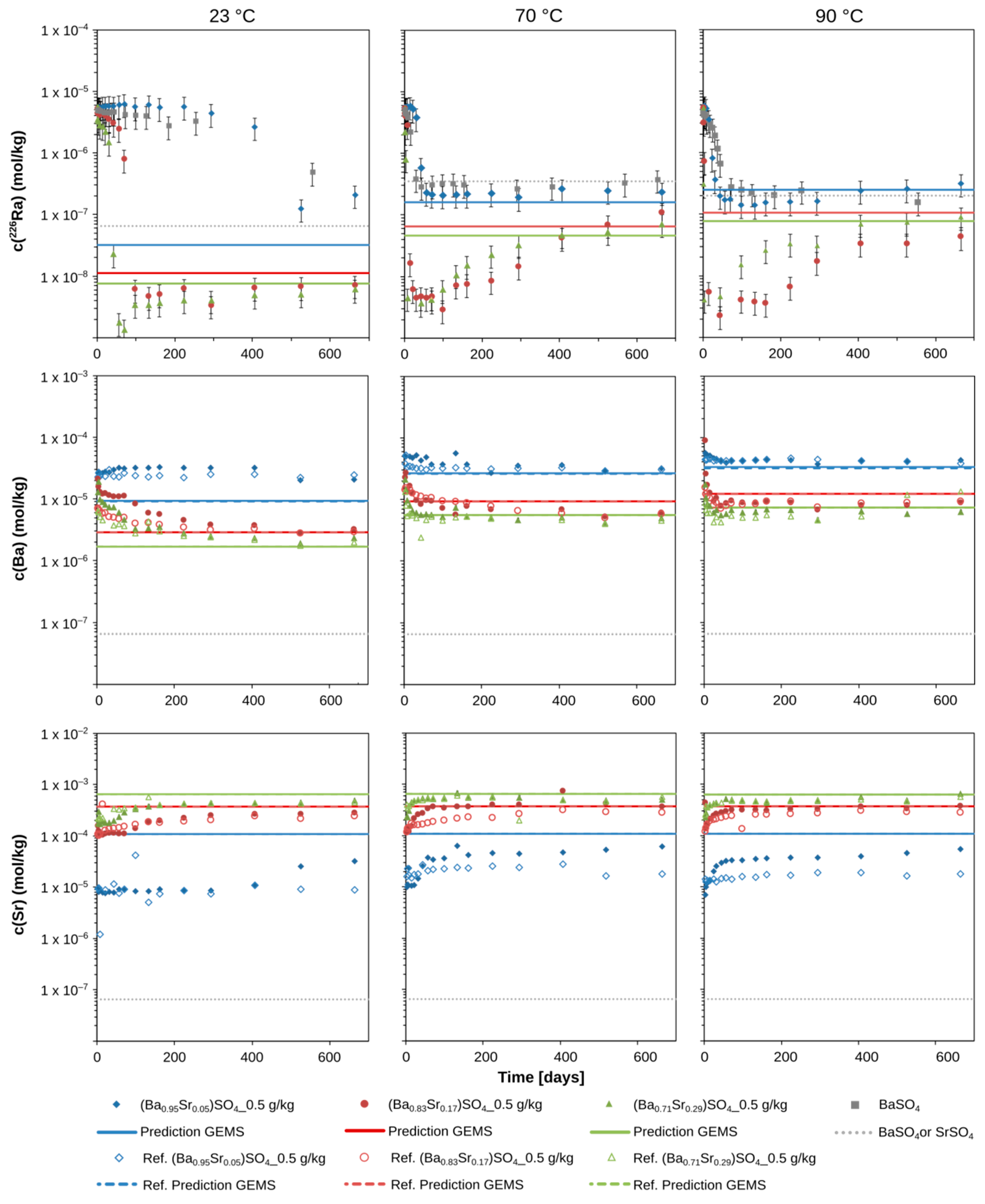
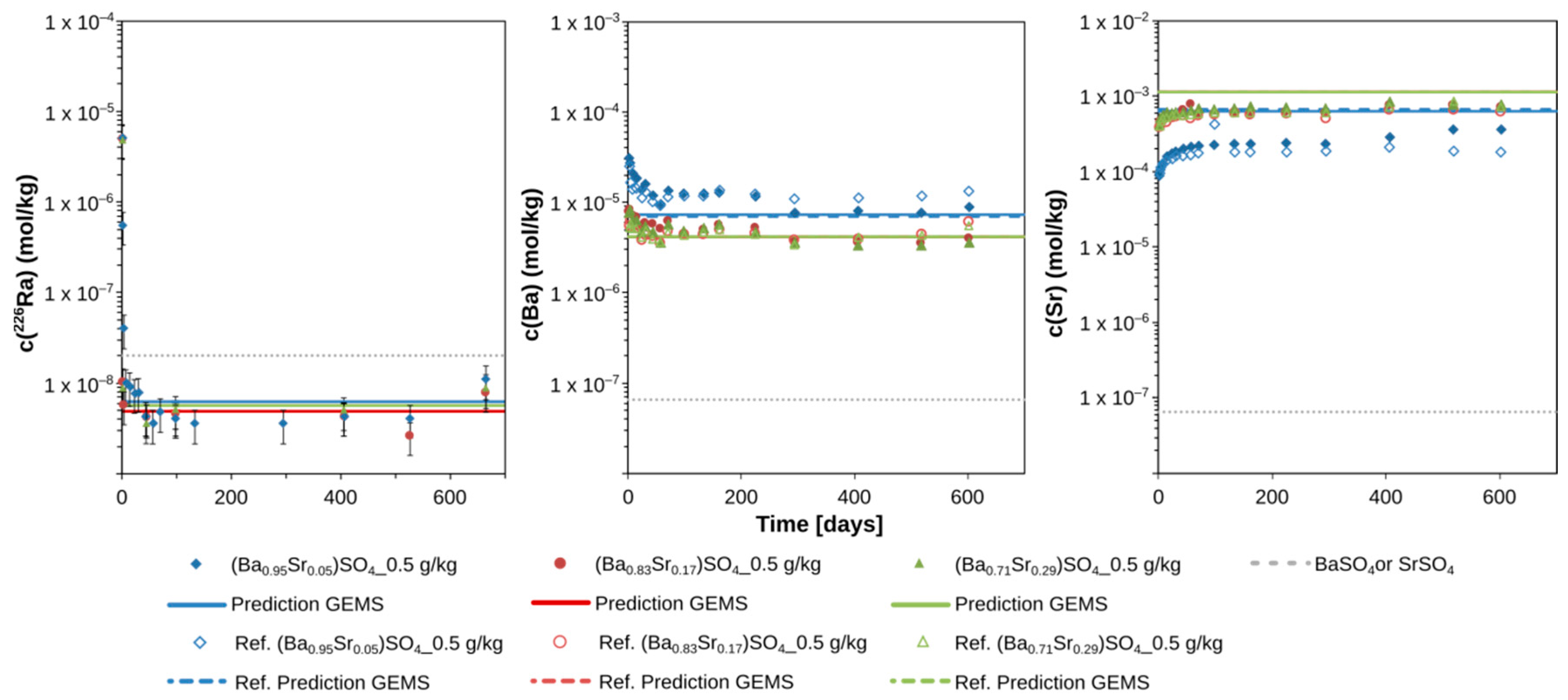
3.1. The Evolution of the 226Ra Concentration over Time
3.2. The Evolution of Ba and Sr Concentrations over Time
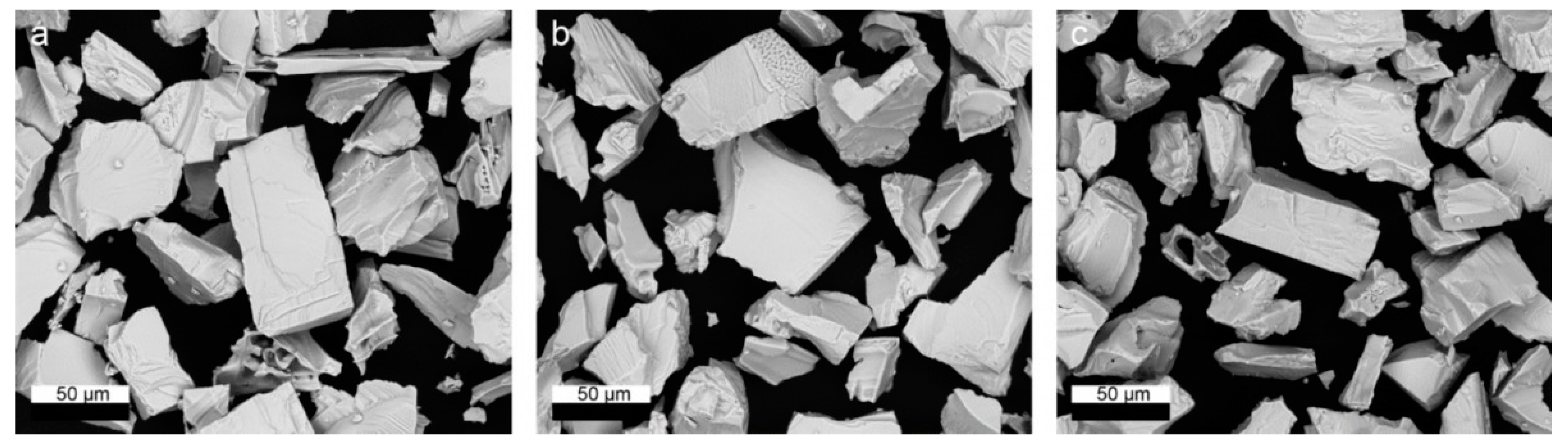
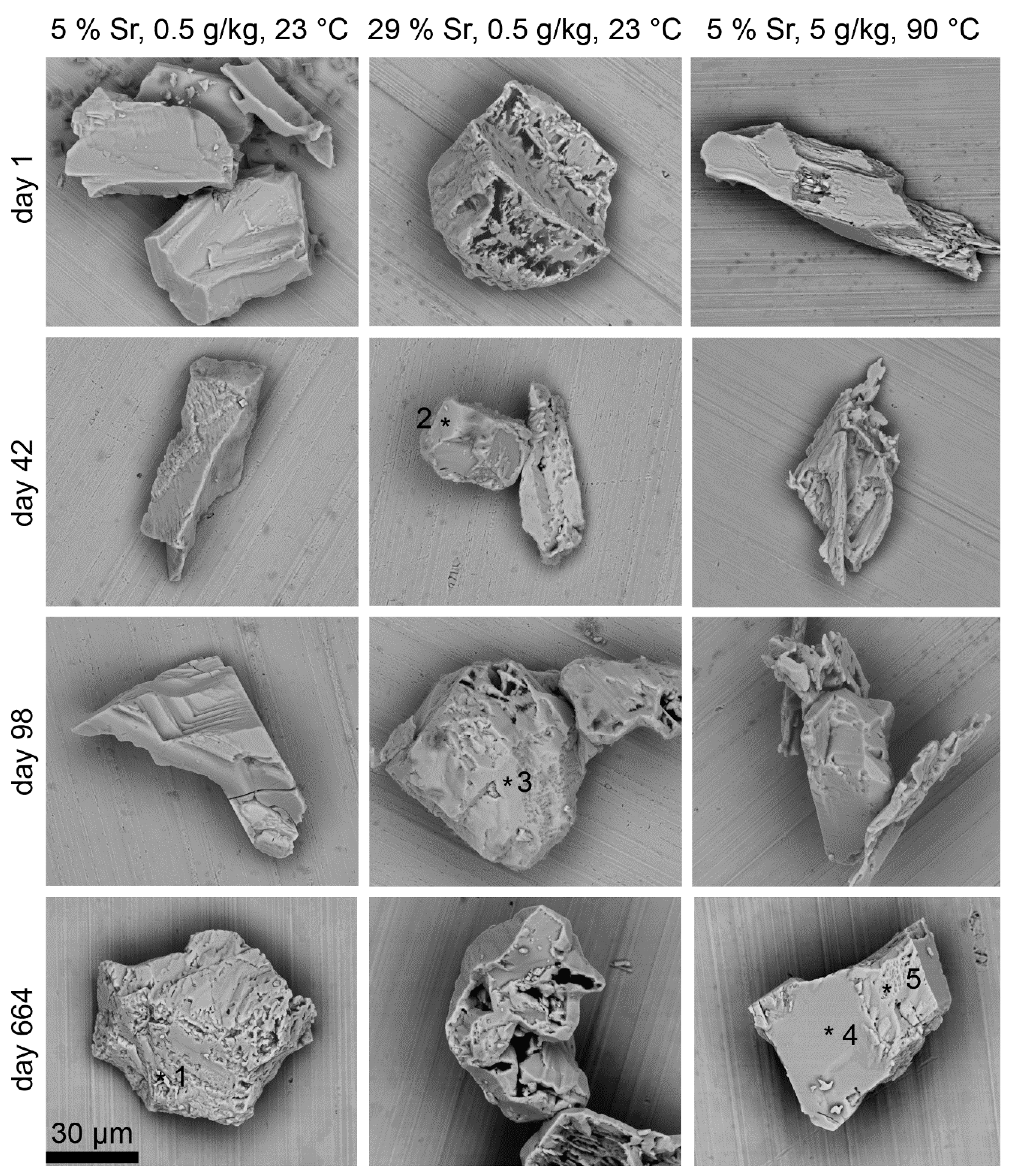
3.3. Chemical and Microstructural Evolution of the Solid
- (1)
- XSrSO4 = 5 mol%, 23 °C, S/L = 0.5 g/kg, slow macroscopic recrystallization kinetics;
- (2)
- XSrSO4 = 29 mol%, 23 °C, S/L = 0.5 g/kg, fast macroscopic recrystallization kinetics and 226Ra entrapment;
- (3)
- XSrSO4 = 5 mol%, 90 °C, 5 g/kg, fast macroscopic recrystallization kinetics, no entrapment of 226Ra.
4. Discussion
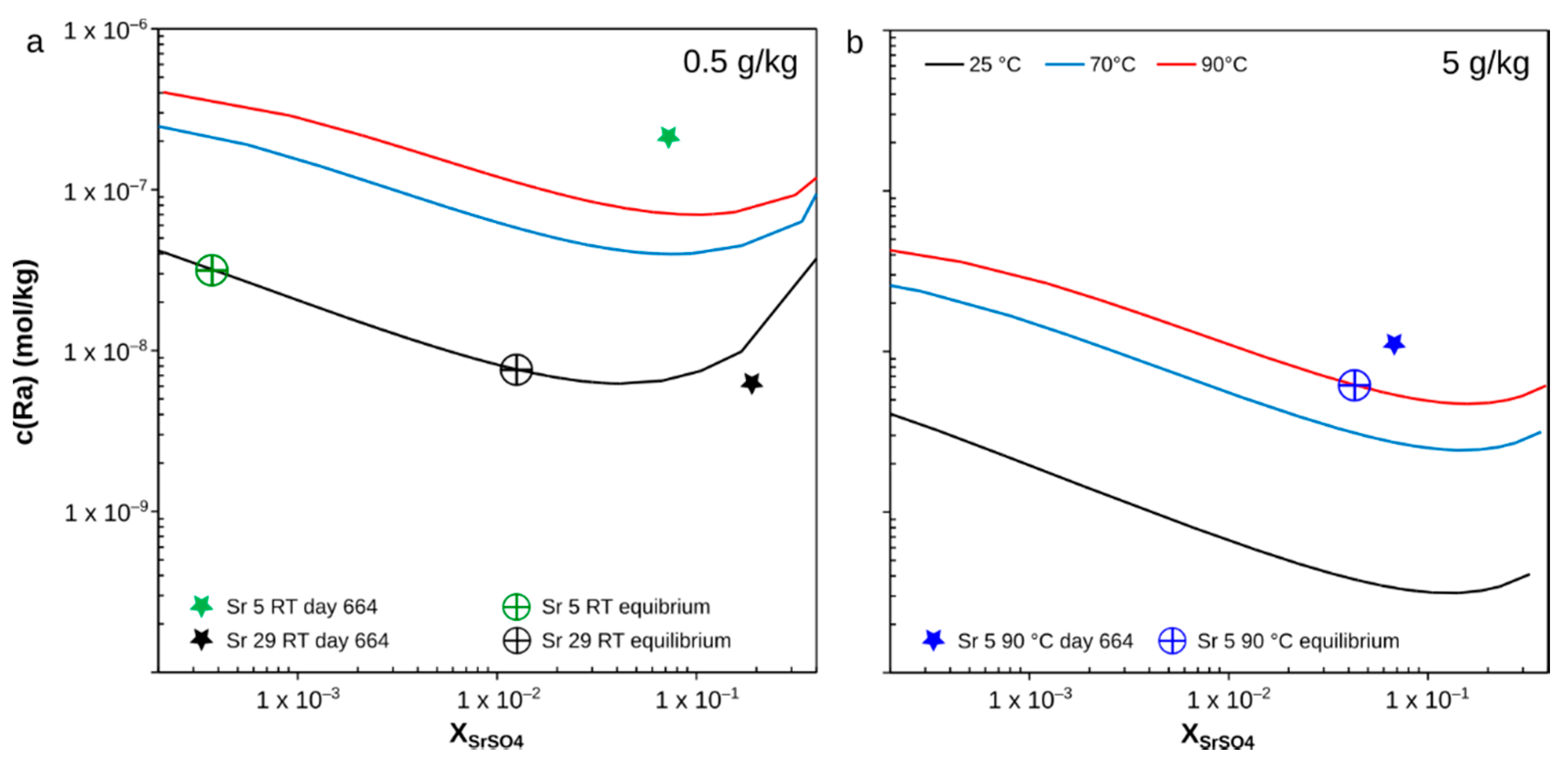
4.1. Effect of XSrSO4 upon the Solubility of 226Ra
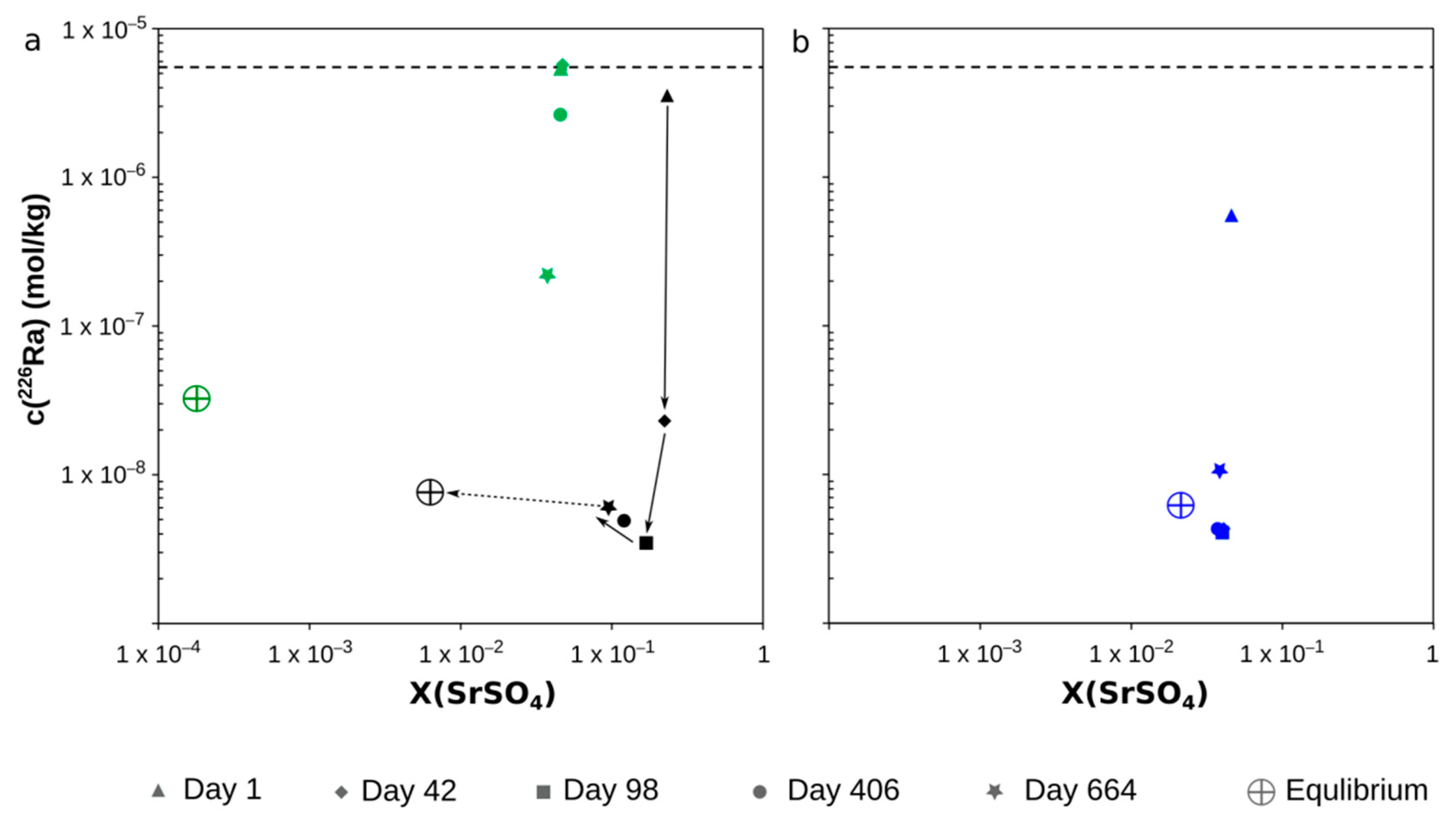
4.2. Kinetics of the Recrystallization from (Ba,Sr)SO4 to (Ba,Sr,Ra)SO4
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Day | Ra Concentration in Solution (10−8 mol/kg) | ||
|---|---|---|---|
| (Ba0.95Sr0.05)SO4_0.5 g/kg_RT | (Ba0.83Sr0.17)SO4_0.5 g/kg_RT | (Ba0.71Sr0.29)SO4_0.5 g/kg_RT | |
| 0.5 | 536 | 523 | 559 |
| 1 | 542 | 442 | 355 |
| 3 | 551 | 433 | 324 |
| 7 | 550 | 406 | 280 |
| 14 | 568 | 431 | 268 |
| 21 | 569 | 404 | 223 |
| 30 | 580 | 357 | 148 |
| 42 | 571 | 302 | 2.30 |
| 56 | 600 | 247 | 0.18 |
| 70 | 625 | 79.4 | 0.14 |
| 98 | 556 | 0.61 | 0.35 |
| 133 | 605 | 0.47 | 0.35 |
| 161 | 544 | 0.51 | 0.37 |
| 224 | 568 | 0.63 | 0.41 |
| 294 | 438 | 0.34 | 0.41 |
| 406 | 264 | 0.65 | 0.49 |
| 525 | 12.5 | 0.68 | 0.51 |
| 664 | 20.9 | 0.72 | 0.61 |
| Equilibrium (GEMS) | 3.24 | 1.13 | 0.76 |
| Day | Ra Concentration (10−8 mol/kg) | ||
|---|---|---|---|
| (Ba0.95Sr0.05)SO4_0.5 g/kg_70 | (Ba0.83Sr0.17)SO4_0.5 g/kg_70 | (Ba0.71Sr0.29)SO4_0.5 g/kg_70 | |
| 0.5 | 546 | 534 | 531 |
| 1 | 552 | 441 | 222 |
| 3 | 539 | 402 | 80.0 |
| 7 | 559 | 281 | 0.45 |
| 14 | 576 | 1.66 | |
| 21 | 519 | 0.61 | |
| 30 | 378 | 0.45 | |
| 42 | 58.3 | 0.47 | 0.37 |
| 56 | 23.1 | 0.45 | |
| 70 | 21.5 | 0.47 | |
| 98 | 20.9 | 0.29 | 0.61 |
| 133 | 21.3 | 0.72 | 1.06 |
| 161 | 21.7 | 0.76 | 1.51 |
| 224 | 22.5 | 0.85 | 2.25 |
| 294 | 19.2 | 1.46 | 3.27 |
| 406 | 26.4 | 4.30 | 4.70 |
| 525 | 24.7 | 6.95 | 5.32 |
| 664 | 23.5 | 0.11 | 7.16 |
| Equilibrium (GEMS) | 16.2 | 6.55 | 4.59 |
| Day | Ra Concentration (10−8 mol/kg) | ||
|---|---|---|---|
| (Ba0.95Sr0.05)SO4_0.5 g/kg_90 | (Ba0.83Sr0.17)SO4_0.5 g/kg_90 | (Ba0.71Sr0.29)SO4_0.5 g/kg_90 | |
| 0.5 | 546 | 541 | 532 |
| 1 | 547 | 308 | 30.5 |
| 3 | 576 | 72.2 | 0.41 |
| 7 | 522 | ||
| 14 | 344 | 0.55 | |
| 21 | 80.8 | ||
| 30 | 36.2 | ||
| 42 | 19.8 | 0.22 | 0.45 |
| 56 | 17.0 | ||
| 70 | 17.6 | ||
| 98 | 14.1 | 0.41 | 0.51 |
| 133 | 14.0 | 0.38 | |
| 161 | 15.7 | 0.36 | 2.64 |
| 224 | 15.8 | 0.67 | 3.35 |
| 294 | 16.1 | 1.70 | 3.11 |
| 406 | 24.2 | 3.35 | 6.95 |
| 525 | 25.6 | 3.35 | 7.43 |
| 664 | 31.6 | 4.31 | 9.10 |
| Equilibrium (GEMS) | 24.9 | 10.6 | 7.78 |
| Day | Ra Concentration (10−8 mol/kg) | ||
|---|---|---|---|
| (Ba0.95Sr0.05)SO4_5 g/kg_90 | (Ba0.83Sr0.17)SO4_5 g/kg_90 | (Ba0.71Sr0.29)SO4_5 g/kg_90 | |
| 0.5 | 507 | 503 | 490 |
| 1 | 55.4 | 1.02 | 0.86 |
| 42 | 0.43 | 0.41 | 0.37 |
| 98 | 0.41 | 0.45 | 0.51 |
| 406 | 0.43 | 0.43 | 0.51 |
| 664 | 1.10 | 0.79 | 0.89 |
| Equilibrium (GEMS) | 0.62 | 0.49 | 0.57 |
| Day | Ba Concentration (10−6 mol/kg) | ||
|---|---|---|---|
| (Ba0.95Sr0.05)SO4_0.5 g/kg_RT | (Ba0.83Sr0.17)SO4_0.5 g/kg_RT | (Ba0.71Sr0.29)SO4_0.5 g/kg_RT | |
| 1 | 20.9 | 12.9 | |
| 3 | 27.4 | 15.7 | 19.1 |
| 7 | 26.6 | 13.1 | 9.83 |
| 14 | 26.5 | 12.2 | 9.00 |
| 21 | 27.6 | 12.5 | 8.21 |
| 30 | 27.1 | 11.4 | 7.45 |
| 42 | 30.0 | 10.9 | 7.41 |
| 56 | 31.9 | 11.0 | 6.06 |
| 70 | 31.1 | 11.1 | 4.62 |
| 98 | 32.3 | 8.40 | 3.27 |
| 133 | 32.3 | 5.91 | 3.35 |
| 161 | 33.1 | 5.69 | 3.53 |
| 224 | 32.2 | 4.51 | 2.79 |
| 294 | 32.2 | 3.81 | 2.52 |
| 406 | 31.9 | 3.69 | 2.32 |
| 525 | 20.2 | 2.79 | 1.92 |
| 664 | 20.9 | 3.26 | 2.35 |
| Equilibrium (GEMS) | 9.40 | 2.90 | 1.70 |
| Day | Ba Concentration (10−6 mol/kg) | ||
|---|---|---|---|
| Reference (Ba0.95Sr0.05)SO4_0.5 g/kg_RT | Reference (Ba0.83Sr0.17)SO4_0.5 g/kg_RT | Reference (Ba0.71Sr0.29)SO4_0.5 g/kg_RT | |
| 1 | 22.7 | 7.06 | 6.39 |
| 3 | 26.3 | 6.70 | 6.11 |
| 7 | 23.3 | 6.41 | 5.10 |
| 14 | 1.82 | 5.83 | 4.59 |
| 21 | 23.1 | 5.90 | 0.08 |
| 30 | 29.9 | 5.17 | 0.17 |
| 42 | 23.6 | 4.95 | 3.75 |
| 56 | 22.7 | 4.81 | 3.88 |
| 70 | 26.2 | 4.95 | 3.60 |
| 98 | 24.2 | 4.00 | 2.77 |
| 133 | 22.6 | 4.08 | 4.35 |
| 161 | 24.0 | 3.86 | 3.02 |
| 224 | 22.4 | 3.50 | 2.53 |
| 294 | 25.0 | 3.13 | 2.41 |
| 406 | 25.0 | 3.27 | 2.18 |
| 525 | 22.4 | 2.77 | 1.74 |
| 664 | 24.4 | 2.92 | 1.94 |
| Equilibrium (GEMS) | 9.05 | 2.87 | 1.69 |
| Day | Ba Concentration (10−6 mol/kg) | ||
|---|---|---|---|
| (Ba0.95Sr0.05)SO4_0.5 g/kg_70 | (Ba0.83Sr0.17)SO4_0.5 g/kg_70 | (Ba0.71Sr0.29)SO4_0.5 g/kg_70 | |
| 1 | 50.0 | 22.7 | 21.3 |
| 3 | 49.3 | 26.7 | 14.5 |
| 7 | 13.9 | 9.69 | |
| 14 | 50.0 | 16.4 | 7.88 |
| 21 | 47.2 | 12.7 | 6.53 |
| 30 | 52.0 | 9.63 | 6.11 |
| 42 | 42.5 | 8.37 | 5.64 |
| 56 | 48.8 | 9.74 | 5.85 |
| 70 | 37.3 | 9.45 | 5.68 |
| 98 | 37.3 | 7.19 | 5.28 |
| 133 | 56.3 | 5.67 | 7.41 |
| 161 | 37.5 | 7.81 | 5.40 |
| 224 | 27.3 | 6.90 | 5.10 |
| 294 | 35.5 | 4.66 | |
| 406 | 36.1 | 6.83 | 5.19 |
| 525 | 29.3 | 5.26 | 4.26 |
| 664 | 31.7 | 6.08 | 4.98 |
| Equilibrium (GEMS) | 26.6 | 9.42 | 5.62 |
| Day | Ba Concentration (10−6 mol/kg) | ||
|---|---|---|---|
| Reference (Ba0.95Sr0.05)SO4_0.5 g/kg_70 | Reference (Ba0.83Sr0.17)SO4_0.5 g/kg_70 | Reference (Ba0.71Sr0.29)SO4_0.5 g/kg_70 | |
| 1 | 38.0 | 14.3 | 13.2 |
| 3 | 52.7 | 15.6 | 7.88 |
| 7 | 34.0 | 13.7 | 5.42 |
| 14 | 34.1 | 14.6 | 5.91 |
| 21 | 33.5 | 12.6 | 5.57 |
| 30 | 31.7 | 11.8 | 5.04 |
| 42 | 32.1 | 11.3 | 2.41 |
| 56 | 30.4 | 10.5 | 4.66 |
| 70 | 32.8 | 10.6 | 5.32 |
| 98 | 32.0 | 9.54 | 4.56 |
| 133 | 33.1 | 9.32 | 5.08 |
| 161 | 31.8 | 8.81 | 5.24 |
| 224 | 31.3 | 7.72 | 4.91 |
| 294 | 32.0 | 6.63 | 0.85 |
| 406 | 33.1 | 5.96 | 4.66 |
| 525 | 29.1 | 5.03 | 4.07 |
| 664 | 30.8 | 5.85 | 4.52 |
| Equilibrium (GEMS) | 2.58 | 9.29 | 5.58 |
| Day | Ba Concentration (10−6 mol/kg) | ||
|---|---|---|---|
| (Ba0.95Sr0.05)SO4_0.5 g/kg_90 | (Ba0.83Sr0.17)SO4_0.5 g/kg_90 | (Ba0.71Sr0.29)SO4_0.5 g/kg_90 | |
| 1 | 56.2 | 90.3 | 17.2 |
| 3 | 51.8 | 25.8 | 10.7 |
| 7 | 52.5 | 16.8 | 8.60 |
| 14 | 51.2 | 12.6 | 7.86 |
| 21 | 46.4 | 9.17 | 6.07 |
| 30 | 44.6 | 10.4 | 6.74 |
| 42 | 40.7 | 7.73 | 5.50 |
| 56 | 38.9 | 8.52 | 5.80 |
| 70 | 43.0 | 9.40 | 6.69 |
| 98 | 42.2 | 8.69 | 6.15 |
| 133 | 43.1 | 8.74 | 6.29 |
| 161 | 43.3 | 8.96 | 6.79 |
| 224 | 42.9 | 8.72 | 6.66 |
| 294 | 36.8 | 6.73 | 4.70 |
| 406 | 41.8 | 7.98 | 6.40 |
| 525 | 40.4 | 7.96 | 5.73 |
| 664 | 42.6 | 8.72 | 6.29 |
| Equilibrium (GEMS) | 32.9 | 12.2 | 7.39 |
| Day | Ba Concentration (10−6 mol/kg) | ||
|---|---|---|---|
| Reference (Ba0.95Sr0.05)SO4_0.5 g/kg_90 | Reference (Ba0.83Sr0.17)SO4_0.5 g/kg_90 | Reference (Ba0.71Sr0.29)SO4_0.5 g/kg_90 | |
| 1 | 43.5 | 15.6 | 8.69 |
| 3 | 43.3 | 12.1 | 6.74 |
| 7 | 44.1 | 11.0 | 5.94 |
| 14 | 45.2 | 10.4 | 5.87 |
| 21 | 42.0 | 7.99 | 4.23 |
| 30 | 43.1 | 8.69 | 5.07 |
| 42 | 41.8 | 7.09 | 4.20 |
| 56 | 42.2 | 7.72 | |
| 70 | 42.0 | 8.61 | 5.34 |
| 98 | 42.2 | 8.26 | 5.04 |
| 133 | 43.4 | 8.39 | 4.98 |
| 161 | 44.1 | 9.20 | 5.57 |
| 224 | 46.3 | 9.11 | 5.29 |
| 294 | 43.9 | 7.32 | 4.51 |
| 406 | 42.1 | 8.49 | 5.20 |
| 525 | 40.5 | 8.76 | 11.6 |
| 664 | 38.2 | 9.30 | 13.2 |
| Equilibrium (GEMS) | 32.0 | 12.1 | 0.32 |
| Day | Ba concentration (10−6 mol/kg) | ||
|---|---|---|---|
| (Ba0.95Sr0.05)SO4_5 g/kg_90 | (Ba0.83Sr0.17)SO4_5 g/kg_90 | (Ba0.71Sr0.29)SO4_5 g/kg_90 | |
| 1 | 30.9 | 7.81 | 7.59 |
| 3 | 27.2 | 8.33 | 8.32 |
| 7 | 20.7 | 7.14 | 6.45 |
| 14 | 18.6 | 6.81 | 6.28 |
| 21 | 13.8 | 5.04 | 4.70 |
| 30 | 15.8 | 5.93 | 5.46 |
| 42 | 12.1 | 5.72 | 4.67 |
| 56 | 9.66 | 5.12 | 3.60 |
| 70 | 13.6 | 6.11 | 5.77 |
| 98 | 12.5 | 0.00 | 4.82 |
| 133 | 12.6 | 4.95 | 5.15 |
| 161 | 12.6 | 5.59 | 5.72 |
| 224 | 11.6 | 5.19 | 4.75 |
| 294 | 7.72 | 3.60 | 3.63 |
| 406 | 8.15 | 3.56 | 3.33 |
| 525 | 7.64 | 3.51 | 3.35 |
| 664 | 8.97 | 3.99 | 3.60 |
| Equilibrium (GEMS) | 7.37 | 4.16 | 4.16 |
| Day | Ba concentration (10−6 mol/kg) | ||
|---|---|---|---|
| Reference (Ba0.95Sr0.05)SO4_5 g/kg_90 | Reference (Ba0.83Sr0.17)SO4_5 g/kg_90 | Reference (Ba0.71Sr0.29)SO4_5 g/kg_90 | |
| 1 | 25.2 | 5.46 | 5.25 |
| 3 | 16.7 | 6.30 | 5.80 |
| 7 | 14.0 | 5.26 | 5.09 |
| 14 | 14.2 | 5.18 | 5.10 |
| 21 | 11.1 | 3.86 | 4.09 |
| 30 | 12.9 | 4.34 | 4.85 |
| 42 | 10.2 | 4.17 | 3.97 |
| 56 | 9.22 | 3.60 | 3.63 |
| 70 | 11.6 | 4.81 | 4.70 |
| 98 | 11.9 | 4.46 | 4.38 |
| 133 | 11.7 | 4.46 | 4.63 |
| 161 | 13.8 | 4.96 | 5.00 |
| 224 | 12.3 | 4.52 | 4.43 |
| 294 | 10.9 | 3.80 | 3.41 |
| 406 | 11.2 | 3.91 | 4.09 |
| 525 | 11.6 | 4.46 | 4.31 |
| 664 | 13.2 | 6.05 | 5.57 |
| Equilibrium (GEMS) | 6.97 | 4.19 | 4.19 |
| Day | Sr Concentration (10−5 mol/kg) | ||
|---|---|---|---|
| (Ba0.95Sr0.05)SO4_0.5 g/kg_RT | (Ba0.83Sr0.17)SO4_0.5 g/kg_RT | (Ba0.71Sr0.29)SO4_0.5 g/kg_RT | |
| 1 | 0.92 | 15.7 | 17.5 |
| 3 | 0.81 | 12.2 | 29.7 |
| 7 | 0.85 | 10.6 | 17.3 |
| 14 | 0.79 | 10.3 | 18.9 |
| 21 | 0.77 | 10.9 | 17.9 |
| 30 | 0.82 | 11.2 | 18.3 |
| 42 | 0.78 | 11.2 | 19.6 |
| 56 | 0.90 | 11.1 | 23.7 |
| 70 | 0.87 | 11.1 | 28.9 |
| 98 | 0.84 | 13.9 | 33.2 |
| 133 | 0.84 | 18.1 | 37.7 |
| 161 | 0.90 | 19.8 | 40.2 |
| 224 | 0.85 | 22.3 | 42.3 |
| 294 | 0.86 | 25.3 | 44.2 |
| 406 | 1.13 | 26.6 | 44.0 |
| 525 | 2.51 | 26.6 | 45.1 |
| 664 | 3.16 | 28.2 | 49.3 |
| Equilibrium (GEMS) | 10.8 | 37.4 | 65.2 |
| Day | Sr Concentration (10−5 mol/kg) | ||
|---|---|---|---|
| Reference (Ba0.95Sr0.05)SO4_0.5 g/kg_RT | Reference (Ba0.83Sr0.17)SO4_0.5 g/kg_RT | Reference (Ba0.71Sr0.29)SO4_0.5 g/kg_RT | |
| 1 | 0.95 | 10.7 | 21.4 |
| 3 | 0.98 | 10.1 | 22.0 |
| 7 | 0.12 | 10.8 | 22.1 |
| 14 | 0.67 | 40.6 | 21.9 |
| 21 | 0.88 | 12.7 | 15.9 |
| 30 | 13.4 | 14.6 | |
| 42 | 1.17 | 13.9 | 33.6 |
| 56 | 0.77 | 14.4 | 32.4 |
| 70 | 0.90 | 15.3 | 34.2 |
| 98 | 4.20 | 16.6 | 35.0 |
| 133 | 0.51 | 18.9 | 56.1 |
| 161 | 0.74 | 17.9 | 39.9 |
| 224 | 0.85 | 19.3 | 42.3 |
| 294 | 0.75 | 20.2 | 43.5 |
| 406 | 1.08 | 24.4 | 43.7 |
| 525 | 0.90 | 21.9 | 42.7 |
| 664 | 0.88 | 24.2 | 44.6 |
| Equilibrium (GEMS) | 10.8 | 37.5 | 65.4 |
| Day | Sr Concentration (10−5 mol/kg) | ||
|---|---|---|---|
| (Ba0.95Sr0.05)SO4_0.5 g/kg_70 | (Ba0.83Sr0.17)SO4_0.5 g/kg_70 | (Ba0.71Sr0.29)SO4_0.5 g/kg_70 | |
| 1 | 1.00 | 11.6 | 23.7 |
| 3 | 1.10 | 14.45 | 23.1 |
| 7 | 2.32 | 12.1 | 30.7 |
| 14 | 1.05 | 15.0 | 44.3 |
| 21 | 1.09 | 21.6 | 47.1 |
| 30 | 1.45 | 25.8 | 50.5 |
| 42 | 2.52 | 27.3 | 52.7 |
| 56 | 3.73 | 33.2 | 54.3 |
| 70 | 3.42 | 36.6 | 51.9 |
| 98 | 3.65 | 34.4 | 55.1 |
| 133 | 6.22 | 36.3 | 68.0 |
| 161 | 4.13 | 36.8 | 55.2 |
| 224 | 4.61 | 39.5 | 57.6 |
| 294 | 4.49 | 39.7 | 55.8 |
| 406 | 4.75 | 74.0 | 50.4 |
| 525 | 5.32 | 42.8 | 48.5 |
| 664 | 6.21 | 36.2 | 50.9 |
| Equilibrium (GEMS) | 10.7 | 37.0 | 64.0 |
| Day | Sr Concentration (10−5 mol/kg) | ||
|---|---|---|---|
| Reference (Ba0.95Sr0.05)SO4_0.5 g/kg_70 | Reference (Ba0.83Sr0.17)SO4_0.5 g/kg_70 | Reference (Ba0.71Sr0.29)SO4_0.5 g/kg_70 | |
| 1 | 1.59 | 11.8 | 39.4 |
| 3 | 2.27 | 13.5 | 34.1 |
| 7 | 1.70 | 14.5 | 38.0 |
| 14 | 1.50 | 16.9 | 42.0 |
| 21 | 1.72 | 16.0 | 47.1 |
| 30 | 1.78 | 16.3 | 48.4 |
| 42 | 2.66 | 16.7 | 49.6 |
| 56 | 2.04 | 17.6 | 51.4 |
| 70 | 2.20 | 18.4 | 54.1 |
| 98 | 2.27 | 20.0 | 52.4 |
| 133 | 2.36 | 21.7 | 59.7 |
| 161 | 2.35 | 22.9 | 56.1 |
| 224 | 2.53 | 22.1 | 54.8 |
| 294 | 2.41 | 26.6 | 19.8 |
| 406 | 2.75 | 31.4 | 48.7 |
| 525 | 1.63 | 28.9 | 46.0 |
| 664 | 1.77 | 28.3 | 56.6 |
| Equilibrium (GEMS) | 10.7 | 37.1 | 64.4 |
| Day | Sr Concentration (10−5 mol/kg) | ||
|---|---|---|---|
| (Ba0.95Sr0.05)SO4_0.5 g/kg_90 | (Ba0.83Sr0.17)SO4_0.5 g/kg_90 | (Ba0.71Sr0.29)SO4_0.5 g/kg_90 | |
| 1 | 0.69 | 43.2 | 22.6 |
| 3 | 0.99 | 13.5 | 28.0 |
| 7 | 1.20 | 16.2 | 35.7 |
| 14 | 1.35 | 21.0 | 39.8 |
| 21 | 2.00 | 24.1 | 42.5 |
| 30 | 2.57 | 26.4 | 43.6 |
| 42 | 2.95 | 28.2 | |
| 56 | 3.19 | 29.6 | 52.5 |
| 70 | 3.35 | 31.1 | 49.9 |
| 98 | 3.32 | 31.2 | 47.9 |
| 133 | 3.53 | 31.4 | 47.4 |
| 161 | 3.57 | 31.1 | 43.3 |
| 224 | 3.72 | 32.9 | 47.5 |
| 294 | 3.74 | 32.9 | 47.2 |
| 406 | 3.98 | 37.0 | 56.1 |
| 525 | 4.60 | 33.7 | 48.7 |
| 664 | 5.40 | 37.9 | 65.3 |
| Equilibrium (GEMS) | 10.7 | 36.7 | 62.7 |
| Day | Sr Concentration (10−5 mol/kg) | ||
|---|---|---|---|
| Reference (Ba0.95Sr0.05)SO4_0.5 g/kg_90 | Reference (Ba0.83Sr0.17)SO4_0.5 g/kg_90 | Reference (Ba0.71Sr0.29)SO4_0.5 g/kg_90 | |
| 1 | 1.40 | 11.9 | 26.0 |
| 3 | 1.27 | 13.9 | 37.6 |
| 7 | 1.34 | 16.8 | 35.2 |
| 14 | 1.35 | 18.9 | 38.7 |
| 21 | 1.43 | 20.4 | 41.7 |
| 30 | 1.25 | 21.0 | 41.8 |
| 42 | 1.45 | 22.1 | 43.4 |
| 56 | 1.48 | 23.4 | |
| 70 | 1.40 | 24.5 | 47.4 |
| 98 | 1.60 | 13.3 | 48.6 |
| 133 | 1.54 | 26.0 | 46.1 |
| 161 | 1.75 | 25.6 | 45.6 |
| 224 | 1.66 | 26.8 | 48.4 |
| 294 | 1.91 | 27.0 | 48.7 |
| 406 | 1.90 | 31.1 | 50.6 |
| 525 | 1.63 | 28.9 | 46.0 |
| 664 | 1.77 | 28.3 | 56.6 |
| Equilibrium (GEMS) | 10.7 | 36.7 | 63.3 |
| Day | Sr Concentration (10−5 mol/kg) | ||
|---|---|---|---|
| (Ba0.95Sr0.05)SO4_5 g/kg_90 | (Ba0.83Sr0.17)SO4_5 g/kg_90 | (Ba0.71Sr0.29)SO4_5 g/kg_90 | |
| 1 | 8.81 | 37.3 | 42.1 |
| 3 | 10.6 | 45.0 | 51.7 |
| 7 | 12.7 | 48.2 | 55.2 |
| 14 | 16.0 | 51.0 | 60.7 |
| 21 | 17.5 | 52.6 | 59.9 |
| 30 | 18.5 | 54.9 | 61.5 |
| 42 | 20.3 | 64.6 | 61.5 |
| 56 | 21.3 | 77.7 | 64.1 |
| 70 | 21.9 | 56.9 | 68.3 |
| 98 | 22.2 | 66.6 | |
| 133 | 23.0 | 58.1 | 68.0 |
| 161 | 23.3 | 59.0 | 72.4 |
| 224 | 23.7 | 60.5 | 71.4 |
| 294 | 23.3 | 60.1 | 68.2 |
| 406 | 28.4 | 74.9 | 85.0 |
| 525 | 35.7 | 74.7 | 77.3 |
| 664 | 36.2 | 70.9 | 77.4 |
| Equilibrium (GEMS) | 63.1 | 113 | 113 |
| Day | Sr Concentration (10−5 mol/kg) | ||
|---|---|---|---|
| Reference (Ba0.95Sr0.05)SO4_5 g/kg_90 | Reference (Ba0.83Sr0.17)SO4_5 g/kg_90 | Reference (Ba0.71Sr0.29)SO4_5 g/kg_90 | |
| 1 | 9.60 | 38.9 | 40.1 |
| 3 | 11.3 | 40.6 | 46.3 |
| 7 | 12.8 | 47.1 | 50.8 |
| 14 | 14.0 | 44.9 | 52.0 |
| 21 | 14.7 | 51.2 | 55.3 |
| 30 | 16.1 | 53.5 | 58.2 |
| 42 | 16.1 | 55.0 | 55.7 |
| 56 | 16.6 | 50.9 | 57.5 |
| 70 | 17.5 | 55.1 | 59.2 |
| 98 | 42.7 | 57.0 | 59.3 |
| 133 | 18.0 | 60.4 | 60.1 |
| 161 | 17.9 | 56.8 | 60.0 |
| 224 | 18.3 | 58.7 | 60.0 |
| 294 | 18.4 | 50.7 | 60.8 |
| 406 | 20.8 | 66.1 | 71.7 |
| 525 | 18.7 | 65.9 | 85.9 |
| 664 | 18.2 | 61.3 | 71.1 |
| Equilibrium (GEMS) | 66.8 | 114 | 114 |
| Day | Particle | XSrSO4 | XRaSO4 | XBaSO4 |
|---|---|---|---|---|
| (%) | (%) | (%) | ||
| (Ba0.95Sr0.05)SO4_0.5 g/kg_RT | ||||
| 1 | P1 | 4.6 | <0.5 | 95.1 |
| P2 | 4.5 | 0.8 | 94.7 | |
| P3 | 4.8 | <0.5 | 95.0 | |
| P4 | 3.5 | 0.6 | 95.9 | |
| P5 | 9.8 | n.d. | 90.2 | |
| Average EDX | 5.4 | 94.2 | ||
| Average mass balance | 5.6 | 0.04 | 95.4 | |
| 42 | P1 | 7.3 | <0.5 | 92.5 |
| P1 | 3.9 | <0.5 | 95.8 | |
| P2 | 3.9 | <0.5 | 95.9 | |
| P3 | 3.5 | 0.5 | 96.0 | |
| P3 | 8.2 | 0.5 | 91.3 | |
| P4 | 6.0 | <0.5 | 93.8 | |
| P5 | 2.9 | <0.5 | 96.9 | |
| Average EDX | 5.1 | 94.6 | ||
| Average mass balance | 4.7 | 0.03 | 95.3 | |
| 98 | P1 | 3.7 | n.d. | 96.3 |
| P1 | 3.2 | n.d. | 96.8 | |
| P2 | 10.1 | <0.5 | 89.8 | |
| P2 | 10.3 | <0.5 | 89.4 | |
| P3 | 5.0 | n.d. | 95.0 | |
| P4 | 3.3 | <0.5 | 96.5 | |
| P5 | 4.7 | <0.5 | 95.0 | |
| Average EDX | 5.8 | 94.1 | ||
| Average mass balance | 4.7 | 0.03 | 95.3 | |
| 664 | P1 | 4.1 | <0.5 | 95.7 |
| P1 | 4.2 | <0.5 | 95.7 | |
| P2 | 3.6 | <0.5 | 96.2 | |
| P2 | 4.4 | <0.5 | 95.2 | |
| P3 | 5.7 | <0.5 | 93.9 | |
| P3 | 7.0 | <0.5 | 92.8 | |
| P4 | 3.8 | <0.5 | 95.9 | |
| P4 | 4.1 | <0.5 | 95.6 | |
| P5 | 5.0 | <0.5 | 94.7 | |
| P5 | 4.5 | 0.7 | 94.9 | |
| P5 | 2.8 | 1.2 | 95.8 | |
| P6 | 5.5 | <0.5 | 93.8 | |
| P6 | 5.8 | <0.5 | 93.9 | |
| P7 | 3.4 | <0.5 | 96.4 | |
| P7 | 4.1 | <0.5 | 95.7 | |
| P7 | 2.0 | 0.5 | 97.5 | |
| P8 | 0.4 | 0.7 | 98.9 | |
| P8 | 1.3 | 1.2 | 97.5 | |
| P8 | 1.2 | 2.7 | 95.3 | |
| P9 | 1.3 | 0.8 | 97.9 | |
| P9 | 6.6 | 0.7 | 93.4 | |
| Average EDX | 3.8 | 95.6 | ||
| Average mass balance | 3.6 | 0.03 | 96.1 | |
| Calculated equilibrium | 0.3 | 0.02 | 99.7 | |
| Day | Particle | XSrSO4 | XRaSO4 | XBaSO4 |
|---|---|---|---|---|
| (%) | (%) | (%) | ||
| (Ba0.71Sr0.29)SO4_0.5 g/kg_RT | ||||
| 1 | P1 | 27.4 | n.d. | 72.6 |
| P1 | 5.8 | <0.5 | 93.8 | |
| P1 | 20.8 | <0.5 | 78.9 | |
| P2 | 9.2 | <0.5 | 90.7 | |
| P2 | 8.1 | 0.5 | 91.5 | |
| P3 | 30.5 | <0.5 | 69.3 | |
| P3 | 27.4 | n.d. | 72.6 | |
| P3 | 22.3 | <0.5 | 77.4 | |
| P4 | 9.0 | <0.5 | 90.5 | |
| P4 | 15.8 | 1.59 | 82.6 | |
| P5 | 23.9 | <0.5 | 75.8 | |
| P6 | 4.3 | <0.5 | 95.4 | |
| Average EDX | 17.0 | 82.6 | ||
| Average mass balance | 23.2 | 0.1 | 76.6 | |
| 42 | P1 | 6.5 | 1.1 | 92.8 |
| P1 | 21.6 | <0.5 | 78.0 | |
| P2 | 8.4 | <0.5 | 91.1 | |
| P3 | 7.3 | 2.8 | 89.9 | |
| P3 | 5.4 | 2.4 | 92.2 | |
| P4 | 11.2 | 1.1 | 87.8 | |
| P4 | 27.9 | <0.5 | 71.7 | |
| P5 | 23.1 | <0.5 | 76.7 | |
| P5 | 9.1 | 0.6 | 90.3 | |
| Average EDX | 13.4 | 85.6 | ||
| Average mass balance | 22.3 | 0.3 | 77.4 | |
| 98 | P1 | 7.9 | 0.9 | 91.2 |
| P2 | 22.7 | <0.5 | 76.9 | |
| P2 | 17.6 | <0.5 | 82.2 | |
| P2 | 6.9 | <0.5 | 92.8 | |
| P3 | 11.8 | <0.5 | 87.8 | |
| P3 | 15.4 | <0.5 | 84.5 | |
| P4 | 17.3 | 0.7 | 82.0 | |
| P5 | 12.9 | 1.0 | 86.1 | |
| P6 | 11.9 | 0.9 | 87.3 | |
| P6 | 24.3 | <0.5 | 75.3 | |
| P6 | 14.8 | <0.5 | 84.8 | |
| Average EDX | 14.1 | 85.4 | ||
| Average mass balance | 16.9 | 0.3 | 82.8 | |
| 664 | P1 | 9.9 | 0.6 | 89.6 |
| P1 | 9.8 | 0.5 | 89.7 | |
| P2 | 11.6 | 0.5 | 87.9 | |
| P2 | 10.5 | <0.5 | 89.3 | |
| P3 | 7.8 | <0.5 | 91.9 | |
| P4 | 7.2 | <0.5 | 92.5 | |
| P4 | 8.1 | 0.5 | 91.4 | |
| P4 | 8.9 | n.d. | 91.1 | |
| P5 | 21.4 | <0.5 | 78.4 | |
| P5 | 5.0 | <0.5 | 94.6 | |
| P5 | 9.0 | <0.5 | 90.9 | |
| P5 | 11.7 | 0.7 | 87.6 | |
| P6 | 9.0 | <0.5 | 90.7 | |
| P6 | 5.2 | <0.5 | 94.5 | |
| P6 | 8.4 | <0.5 | 91.3 | |
| P7 | 5.3 | 0.5 | 94.2 | |
| P7 | 10.3 | <0.5 | 89.4 | |
| P7 | 4.7 | <0.5 | 95.0 | |
| P8 | 8.2 | <0.5 | 91.5 | |
| P8 | 10.0 | 1.4 | 88.6 | |
| P8 | 7.4 | <0.5 | 92.2 | |
| P9 | 21.3 | <0.5 | 78.3 | |
| P9 | 10.0 | 0.5 | 89.5 | |
| P10 | 9.6 | 0.8 | 89.6 | |
| P10 | 17.4 | <0.5 | 82.3 | |
| Average EDX | 9.9 | 89.7 | ||
| Average mass balance | 9.5 | 0.4 | 90.2 | |
| Calculated equilibrium | 0.6 | 0.3 | 99.1 | |
| Day | Particle | XSrSO4 | XRaSO4 | XBaSO4 |
|---|---|---|---|---|
| (%) | (%) | (%) | ||
| (Ba0.95Sr0.05)SO4_5 g/kg_90 | ||||
| 1 | P1 | 10.3 | <0.5 | 89.5 |
| P1 | 12.8 | n.d | 87.2 | |
| P1 | 13.6 | <0.5 | 86.5 | |
| P2 | 10.1 | n.d | 89.9 | |
| P2 | 3.9 | 1.7 | 94.4 | |
| P3 | 4.0 | <0.5 | 95.6 | |
| P4 | 4.9 | <0.5 | 94.6 | |
| Average EDX | 8.5 | 91.1 | ||
| Average mass balance | 4.6 | 0.03 | 95.4 | |
| 42 | P1 | 4.1 | 0.5 | 95.4 |
| P1 | 1.5 | 0.7 | 97.1 | |
| P2 | 2.0 | <0.5 | 97.8 | |
| P3 | 4.0 | <0.5 | 95.9 | |
| P4 | 1.0 | <0.5 | 98.8 | |
| Average EDX | 2.5 | 97.0 | ||
| Average mass balance | 4.1 | 0.03 | 95.9 | |
| 98 | P1 | 2.6 | <0.5 | 97.2 |
| P2 | 1.6 | 0.98 | 97.4 | |
| P3 | 1.5 | <0.5 | 98.2 | |
| P4 | 7.0 | <0.5 | 91.8 | |
| P5 | 0.8 | <0.5 | 99.0 | |
| P6 | 4.4 | <0.5 | 95.5 | |
| Average EDX | 3.0 | 96.5 | ||
| Average mass balance | 4.0 | 0.03 | 96.0 | |
| 664 | P1 | 3.4 | <0.5 | 96.3 |
| P2 | 6.3 | n.d | 93.7 | |
| P2 | 6.0 | <0.5 | 93.8 | |
| P3 | 3.1 | <0.5 | 96.5 | |
| P3 | 2.3 | 0.6 | 97.1 | |
| P4 | 4.4 | <0.5 | 95.2 | |
| P4 | 5.0 | <0.5 | 94.9 | |
| P5 | 1.6 | <0.5 | 98.0 | |
| P5 | 2.7 | <0.5 | 97.2 | |
| P6 | 5.8 | <0.5 | 94.0 | |
| P6 | 3.8 | <0.5 | 95.9 | |
| P7 | 2.2 | 0.5 | 97.3 | |
| P8 | 4.8 | <0.5 | 95.0 | |
| P9 | 3.2 | 0.6 | 96.2 | |
| Average EDX | 3.9 | 95.8 | ||
| Average mass balance | 3.4 | 0.03 | 96.6 | |
| Calculated equilibrium | 2.2 | 0.02 | 97.8 | |
References
- Rutherford, P.M.; Dudas, M.J.; Arocena, J.M. Heterogeneous distribution of radionuclides, barium and strontium in phosphogypsum by-product. Sci. Total Environ. 1996, 180, 201–209. [Google Scholar] [CrossRef]
- Fisher, R.S. Geologic and Geochemical Controls on Naturally Occurring Radioactive Materials (NORM) in Produced Water from Oil, Gas, and Geothermal Operations. Environ. Geosci. 1998, 5, 139–150. [Google Scholar] [CrossRef]
- Burnett, W.C.; Elzerman, A.W. Nuclide migration and the environmental radiochemistry of Florida phosphogypsum. J. Environ. Radioact. 2001, 54, 27–51. [Google Scholar] [CrossRef]
- Martin, A.J.; Crusius, J.; McNee, J.J.; Yanful, E.K. The mobility of radium-226 and trace metals in pre-oxidized subaqueous uranium mill tailings. Appl. Geochem. 2003, 18, 1095–1110. [Google Scholar] [CrossRef]
- Liu, D.J.; Hendry, M.J. Controls on 226Ra during raffinate neutralization at the Key Lake uranium mill, Saskatchewan, Canada. Appl. Geochem. 2011, 26, 2113–2120. [Google Scholar] [CrossRef]
- Zhang, T.; Gregory, K.; Hammack, R.W.; Vidic, R.D. Co-precipitation of Radium with Barium and Strontium Sulfate and Its Impact on the Fate of Radium during Treatment of Produced Water from Unconventional Gas Extraction. Environ. Sci. Technol. 2014, 48, 4596–4603. [Google Scholar] [CrossRef]
- Norrby, S.; Andersson, J.; Dverstorp, B.; Kautsky, F.; Lilja, C.; Sjöblom, R.; Sundström, B.; Toverud, Ö.; Wingefors, S. SKI SITE-94 Saekerhetsanalys foer Djupfoervar iett Kristallint berg; Svensk Kärnbränslehantering AB (SKB): Stockholm, Sweden, 1997. [Google Scholar]
- Grandia, F.; Merino, J.; Bruno, J. Assessment of the Radium-Barium Co-Precipitation and Its Potential Influence on the Solubility of Ra in the Near-Field; SKB Technical Report TR-08-07; SKB: Stockholm, Sweden, 2008. [Google Scholar]
- NAGRA. Technischer Bericht NTW 14-03. In Charakteristische Dosisintervalle und Unterlagen zur Bewertung der Barrierensysteme; NAGRA: Wettingen, Switzerland, 2014. [Google Scholar]
- Curti, E.; Xto, J.; Borca, C.N.; Henzler, K.; Huthwelker, T.; Prasianakis, N.I. Modelling Ra-bearing baryte nucleation/precipitation kinetics at the pore scale: Application to radioactive waste disposal. Eur. J. Mineral. 2019, 247–262. [Google Scholar] [CrossRef]
- Zhu, C. Coprecipitation in the barite isostructural family: 1. binary mixing properties. Geochim. Cosmochim. Acta 2004, 68, 3327–3337. [Google Scholar] [CrossRef]
- Rosenberg, Y.O.; Metz, V.; Ganor, J. Co-precipitation of radium in high ionic strength systems: 1. Thermodynamic properties of the Na-Ra-Cl-SO4-H2O system—Estimating Pitzer parameters for RaCl2. Geochim. Cosmochim. Acta 2011, 75, 5389–5402. [Google Scholar] [CrossRef]
- Rosenberg, Y.O.; Sadeh, Y.; Metz, V.; Pina, C.M.; Ganor, J. Nucleation and growth kinetics of RaxBa1−xSO4 solid solution in NaCl aqueous solutions. Geochim. Cosmochim. Acta 2014, 125, 290–307. [Google Scholar] [CrossRef]
- Curti, E.; Fujiwara, K.; Iijima, K.; Tits, J.; Cuesta, C.; Kitamura, A.; Glaus, M.A.; Müller, W. Radium uptake during barite recrystallization at 23 ± 2 °C as a function of solution composition: An experimental 133Ba and 226Ra tracer study. Geochim. Cosmochim. Acta 2010, 74, 3553–3570. [Google Scholar] [CrossRef]
- Klinkenberg, M.; Brandt, F.; Breuer, U.; Bosbach, D. Uptake of Ra during the recrystallization of barite: A microscopic and time of flight-secondary ion mass spectrometry study. Environ. Sci. Technol. 2014, 48, 6620–6627. [Google Scholar] [CrossRef] [PubMed]
- Torapava, N.; Ramebäck, H.; Curti, E.; Lagerkvist, P.; Ekberg, C. Recrystallization of 223Ra with barium sulfate. J. Radioanal. Nucl. Chem. 2014, 301, 545–553. [Google Scholar] [CrossRef]
- Brandt, F.; Curti, E.; Klinkenberg, M.; Rozov, K.; Bosbach, D. Replacement of barite by a (Ba,Ra)SO4 solid solution at close-to-equilibrium conditions: A combined experimental and theoretical study. Geochim. Cosmochim. Acta 2015. [Google Scholar] [CrossRef]
- Prieto, M.; Heberling, F.; Rodríguez-Galán, R.M.; Brandt, F. Crystallization behavior of solid solutions from aqueous solutions: An environmental perspective. Prog. Cryst. Growth Charact. Mater. 2016, 62, 29–68. [Google Scholar] [CrossRef]
- Heberling, F.; Metz, V.; Böttle, M.; Curti, E.; Geckeis, H. Barite recrystallization in the presence of 226Ra and 133Ba. Geochim. Cosmochim. Acta 2018. [Google Scholar] [CrossRef]
- Rollog, M.; Cook, N.J.; Guagliardo, P.; Ehrig, K.J.; Kilburn, M. Radionuclide-bearing minerals in Olympic Dam copper concentrates. Hydrometallurgy 2019, 190, 105153. [Google Scholar] [CrossRef]
- Schmandt, D.S.; Cook, N.J.; Ehrig, K.; Gilbert, S.; Wade, B.P.; Rollog, M.; Ciobanu, C.L.; Kamenetsky, V.S. Uptake of trace elements by baryte during copper ore processing: A case study from Olympic Dam, South Australia. Miner. Eng. 2019, 135, 83–94. [Google Scholar] [CrossRef]
- Vinograd, V.L.; Brandt, F.; Rozov, K.; Klinkenberg, M.; Refson, K.; Winkler, B.; Bosbach, D. Solid-aqueous equilibrium in the BaSO4-RaSO4-H2O system: First-principles calculations and a thermodynamic assessment. Geochim. Cosmochim. Acta 2013, 122, 398–417. [Google Scholar] [CrossRef]
- Vinograd, V.L.; Kulik, D.A.; Brandt, F.; Klinkenberg, M.; Weber, J.; Winkler, B.; Bosbach, D. Thermodynamics of the solid solution—Aqueous solution system (Ba,Sr,Ra)SO4 + H2O: I. The effect of strontium content on radium uptake by barite. Appl. Geochem. 2018, 89, 59–74. [Google Scholar] [CrossRef]
- Vinograd, V.L.; Kulik, D.A.; Brandt, F.; Klinkenberg, M.; Weber, J.; Winkler, B.; Bosbach, D. Thermodynamics of the solid solution—Aqueous solution system (Ba,Sr,Ra)SO4 + H2O: II. Radium retention in barite-type minerals at elevated temperatures. Appl. Geochem. 2018, 93, 190–208. [Google Scholar] [CrossRef]
- Klinkenberg, M.; Weber, J.; Barthel, J.; Vinograd, V.; Poonoosamy, J.; Kruth, M.; Bosbach, D.; Brandt, F. The solid solution–aqueous solution system (Sr,Ba,Ra)SO4 + H2O: A combined experimental and theoretical study of phase equilibria at Sr-rich compositions. Chem. Geol. 2018, 497, 1–17. [Google Scholar] [CrossRef]
- Patel, A.R.; Bhat, H.L. Growth of strontium sulphate single crystals by chemically reacted flux and their dislocaton configuration. J. Cryst. Growth 1971, 8, 153–156. [Google Scholar] [CrossRef]
- Patel, A.R.; Koshy, J. Growth of barium sulphate single crystals by chemically reacted flux. J. Cryst. Growth 1968, 2, 128–130. [Google Scholar] [CrossRef]
- Kulik, D.A.; Wagner, T.; Dmytrieva, S.V.; Kosakowski, G.; Hingerl, F.F.; Chudnenko, K.V.; Berner, U.R. GEM-Selektor geochemical modeling package: Revised algorithm and GEMS3K numerical kernel for coupled simulation codes. Comput. Geosci. 2013, 17, 1–24. [Google Scholar] [CrossRef]
- Helgeson, H.C.; Kirkham, D.H.; Flowers, G.C. Theoretical prediction of the thermodynamic behavior of aqueous electrolytes by high pressures and temperatures; IV, Calculation of activity coefficients, osmotic coefficients, and apparent molal and standard and relative partial molal properties to 600 d. Am. J. Sci. 1981, 281, 1249–1516. [Google Scholar] [CrossRef]
- Thoenen, T.; Hummel, W.; Berner, U.; Curti, E. The PSI/Nagra Chemical Thermodynamic Database 12/07; Nuclear Energy and Safety Research Department Laboratory for Waste Management (LES): Villigen, Switzerland, 2014. [Google Scholar]
- Weber, J.; Barthel, J.; Klinkenberg, M.; Bosbach, D.; Kruth, M.; Brandt, F. Retention of 226Ra by barite: The role of internal porosity. Chem. Geol. 2017, 466, 722–732. [Google Scholar] [CrossRef]
- Brandt, F.; Klinkenberg, M.; Poonoosamy, J.; Weber, J.; Bosbach, D. The Effect of Ionic Strength and Sraq upon the Uptake of Ra during the Recrystallization of Barite. Minerals 2018, 8, 502. [Google Scholar] [CrossRef]
- Poonoosamy, J.; Klinkenberg, M.; Deissmann, G.; Brandt, F.; Bosbach, D.; Mäder, U.; Kosakowski, G. Effects of solution supersaturation on barite precipitation in porous media and consequences on permeability: Experiments and modelling. Geochim. Cosmochim. Acta 2020, 270, 43–60. [Google Scholar] [CrossRef]
| Solid Solution | XBaSO4 | XSrSO4 |
|---|---|---|
| (Ba0.95Sr0.05)SO4 | 0.95 | 0.05 ± 20% |
| (Ba0.83Sr0.17)SO4 | 0.83 | 0.17 ± 20% |
| (Ba0.71Sr0.29)SO4 | 0.71 | 0.29 ± 20% |
| Name | Solid/Liquid | c(Ra) | Temperature |
|---|---|---|---|
| (g/kg) | (10−6 mol/kg) | (°C) | |
| (Ba0.95Sr0.05)SO4_0.5 g/kg_RT | 0.5 | 5 | 23 ± 2 |
| (Ba0.83Sr0.17)SO4_0.5 g/kg_RT | 0.5 | 5 | 23 ± 2 |
| (Ba0.71Sr0.29)SO4_0.5 g/kg_RT | 0.5 | 5 | 23 ± 2 |
| Reference (Ba0.95Sr0.05)SO4_0.5 g/kg_RT | 0.5 | 0 | 23 ± 2 |
| Reference (Ba0.83Sr0.17)SO4_0.5 g/kg_RT | 0.5 | 0 | 23 ± 2 |
| Reference (Ba0.71Sr0.29)SO4_0.5 g/kg_RT | 0.5 | 0 | 23 ± 2 |
| (Ba0.95Sr0.05)SO4_0.5 g/kg_70 | 0.5 | 5 | 70 |
| (Ba0.83Sr0.17)SO4_0.5 g/kg_70 | 0.5 | 5 | 70 |
| (Ba0.71Sr0.29)SO4_0.5 g/kg_70 | 0.5 | 5 | 70 |
| Reference (Ba0.95Sr0.05)SO4_0.5 g/kg_70 | 0.5 | 0 | 70 |
| Reference (Ba0.83Sr0.17)SO4_0.5 g/kg_70 | 0.5 | 0 | 70 |
| Reference (Ba0.71Sr0.29)SO4_0.5 g/L_70 | 0.5 | 0 | 70 |
| (Ba0.95Sr0.05)SO4_0.5 g/kg_90 | 0.5 | 5 | 90 |
| (Ba0.83Sr0.17)SO4_0.5 g/kg_90 | 0.5 | 5 | 90 |
| (Ba0.71Sr0.29)SO4_0.5 g/kg_90 | 0.5 | 5 | 90 |
| Reference (Ba0.95Sr0.05)SO4_0.5 g/kg_90 | 0.5 | 0 | 90 |
| Reference (Ba0.83Sr0.17)SO4_0.5 g/kg_90 | 0.5 | 0 | 90 |
| Reference (Ba0.71Sr0.29)SO4_0.5 g/kg_90 | 0.5 | 0 | 90 |
| (Ba0.95Sr0.05)SO4_5 g/kg_90 | 5.0 | 5 | 90 |
| (Ba0.83Sr0.17)SO4_5 g/kg_90 | 5.0 | 5 | 90 |
| (Ba0.71Sr0.29)SO4_5 g/kg_90 | 5.0 | 5 | 90 |
| Reference (Ba0.95Sr0.05)SO4_5 g/kg_90 | 5.0 | 0 | 90 |
| Reference (Ba0.83Sr0.17)SO4_5 g/kg_90 | 5.0 | 0 | 90 |
| Reference (Ba0.71Sr0.29)SO4_5 g/kg_90 | 5.0 | 0 | 90 |
| Experiment | Composition of Solid Solution Present at Equilibrium | ||
|---|---|---|---|
| XBaSO4 | XRaSO4 (%) | XSrSO4 | |
| (Ba0.95Sr0.05)SO4_0.5 g/kg_RT | 99.74 | 0.24 | 0.02 |
| (Ba0.83Sr0.17)SO4_0.5 g/kg_RT | 99.52 | 0.27 | 0.21 |
| (Ba0.71Sr0.29)SO4_0.5 g/kg_RT | 99.06 | 0.31 | 0.63 |
| Reference (Ba0.95Sr0.05)SO4_0.5 g/kg_RT | 99.99 | - | 0.01 |
| Reference (Ba0.83Sr0.17)SO4_0.5 g/kg_RT | 99.83 | - | 0.17 |
| Reference (Ba0.71Sr0.29)SO4_0.5 g/kg_RT | 99.49 | - | 0.51 |
| (Ba0.95Sr0.05)SO4_0.5 g/kg_70 | 99.72 | 0.24 | 0.04 |
| (Ba0.83Sr0.17)SO4_0.5 g/kg_70 | 99.29 | 0.27 | 0.44 |
| (Ba0.71Sr0.29)SO4_0.5 g/kg_70 | 98.34 | 0.30 | 1.36 |
| Reference (Ba0.95Sr0.05)SO4_0.5 g/kg_70 | 99.96 | - | 0.04 |
| Reference (Ba0.83Sr0.17)SO4_0.5 g/kg_70 | 99.63 | - | 0.37 |
| Reference (Ba0.71Sr0.29)SO4_0.5 g/L_70 | 98.88 | - | 1.12 |
| (Ba0.95Sr0.05)SO4_0.5 g/kg_90 | 99.69 | 0.23 | 0.07 |
| (Ba0.83Sr0.17)SO4_0.5 g/kg_90 | 99.17 | 0.26 | 0.57 |
| (Ba0.71Sr0.29)SO4_0.5 g/kg_90 | 97.60 | 0.30 | 2.10 |
| Reference (Ba0.95Sr0.05)SO4_0.5 g/kg_90 | 99.94 | - | 0.06 |
| Reference (Ba0.83Sr0.17)SO4_0.5 g/kg_90 | 99.42 | - | 0.58 |
| Reference (Ba0.71Sr0.29)SO4_0.5 g/kg_90 | 98.24 | - | 1.76 |
| (Ba0.95Sr0.05)SO4_5 g/kg_90 | 97.83 | 0.02 | 2.15 |
| (Ba0.83Sr0.17)SO4_5 g/kg_90 | 90.21 | 0.02 | 9.77 |
| (Ba0.71Sr0.29)SO4_5 g/kg_90 | 90.20 | 0.03 | 9.77 |
| Reference (Ba0.95Sr0.05)SO4_5 g/kg_90 | 98.02 | - | 1.98 |
| Reference (Ba0.83Sr0.17)SO4_5 g/kg_90 | 92.55 | - | 7.45 |
| Reference (Ba0.71Sr0.29)SO4_5 g/kg_90 | 92.55 | - | 7.45 |
| Day | Method | Number of Particles EDS-Analyses | XSrSO4 Minimum | XSrSO4 Maximum | XSrSO4 Average | |
|---|---|---|---|---|---|---|
| (%) | (%) | (%) | ||||
| (Ba0.95Sr0.05)SO4_0.5 g/kg_RT | ||||||
| 1 | EDX | 5 | 5 | 3.5 | 9.8 | 6.2 |
| Mass balance | 4.6 | |||||
| 42 | EDX | 5 | 7 | 2.9 | 8.2 | 5.0 |
| Mass balance | 4.7 | |||||
| 98 | EDX | 5 | 7 | 3.2 | 10.3 | 5.8 |
| Mass balance | 4.7 | |||||
| 664 | EDX | 9 | 21 | 0.4 | 9.8 | 4.1 |
| Mass balance | 3.6 | |||||
| Calculated equilibrium | 0.3 | |||||
| (Ba0.71Sr0.29)SO4_0.5 g/kg_RT | ||||||
| 1 | EDX | 6 | 12 | 4.3 | 30.5 | 17.0 |
| Mass balance | 23.2 | |||||
| 42 | EDX | 5 | 9 | 5.4 | 27.9 | 13.4 |
| Mass balance | 22.3 | |||||
| 98 | EDX | 6 | 11 | 6.9 | 24.3 | 14.1 |
| Mass balance | 16.9 | |||||
| 664 | EDX | 10 | 25 | 4.7 | 21.4 | 9.9 |
| Mass balance | 9.5 | |||||
| Calculated equilibrium | 0.6 | |||||
| (Ba0.95Sr0.05)SO4_5 g/kg_90 | ||||||
| 1 | EDX | 4 | 7 | 3.9 | 13.6 | 8.5 |
| Mass balance | 4.6 | |||||
| 42 | EDX | 4 | 5 | 1 | 4.1 | 2.5 |
| Mass balance | 4.1 | |||||
| 98 | EDX | 6 | 6 | 0.8 | 7 | 3.0 |
| Mass balance | 4.0 | |||||
| 664 | EDX | 9 | 14 | 1.6 | 6.3 | 3.9 |
| Mass balance | 3.4 | |||||
| Calculated equilibrium | 2.2 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandt, F.; Klinkenberg, M.; Poonoosamy, J.; Bosbach, D. Recrystallization and Uptake of 226Ra into Ba-Rich (Ba,Sr)SO4 Solid Solutions. Minerals 2020, 10, 812. https://doi.org/10.3390/min10090812
Brandt F, Klinkenberg M, Poonoosamy J, Bosbach D. Recrystallization and Uptake of 226Ra into Ba-Rich (Ba,Sr)SO4 Solid Solutions. Minerals. 2020; 10(9):812. https://doi.org/10.3390/min10090812
Chicago/Turabian StyleBrandt, Felix, Martina Klinkenberg, Jenna Poonoosamy, and Dirk Bosbach. 2020. "Recrystallization and Uptake of 226Ra into Ba-Rich (Ba,Sr)SO4 Solid Solutions" Minerals 10, no. 9: 812. https://doi.org/10.3390/min10090812
APA StyleBrandt, F., Klinkenberg, M., Poonoosamy, J., & Bosbach, D. (2020). Recrystallization and Uptake of 226Ra into Ba-Rich (Ba,Sr)SO4 Solid Solutions. Minerals, 10(9), 812. https://doi.org/10.3390/min10090812






