First-Principles Study of the Elastic Properties of Nickel Sulfide Minerals under High Pressure
Abstract
1. Introduction
2. Methods
3. Results
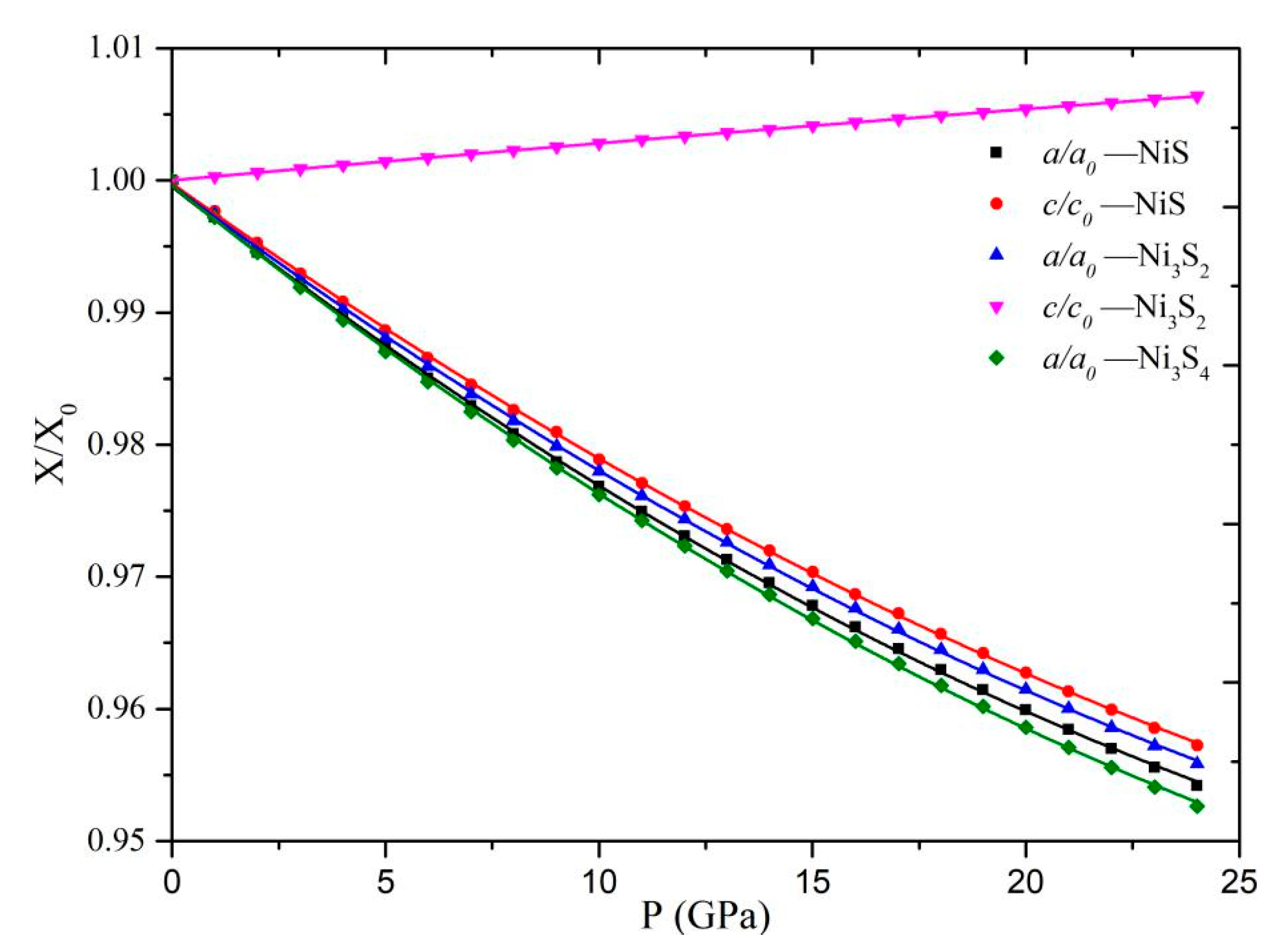
3.1. Crystal Structure
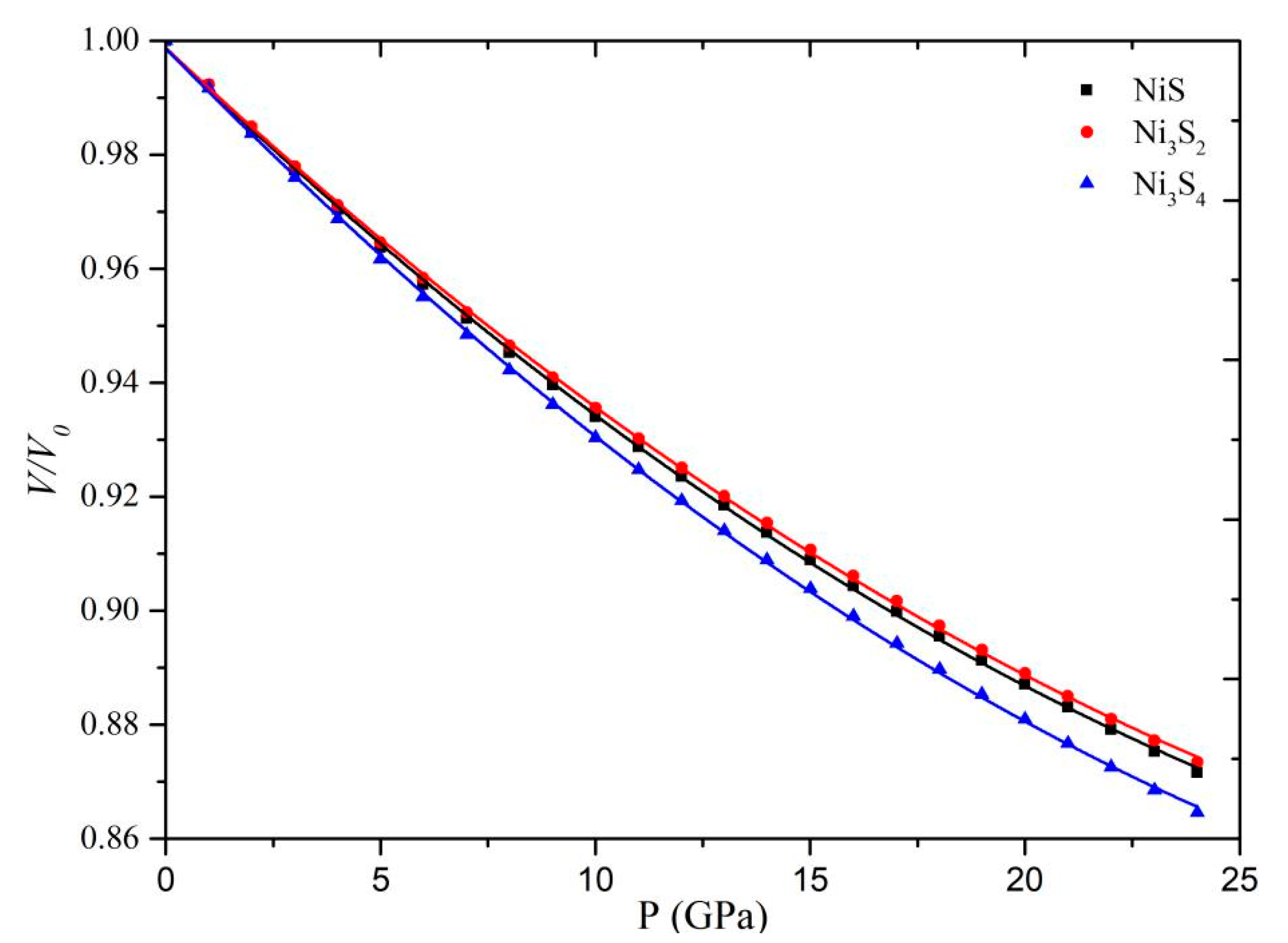
3.2. Equation of State
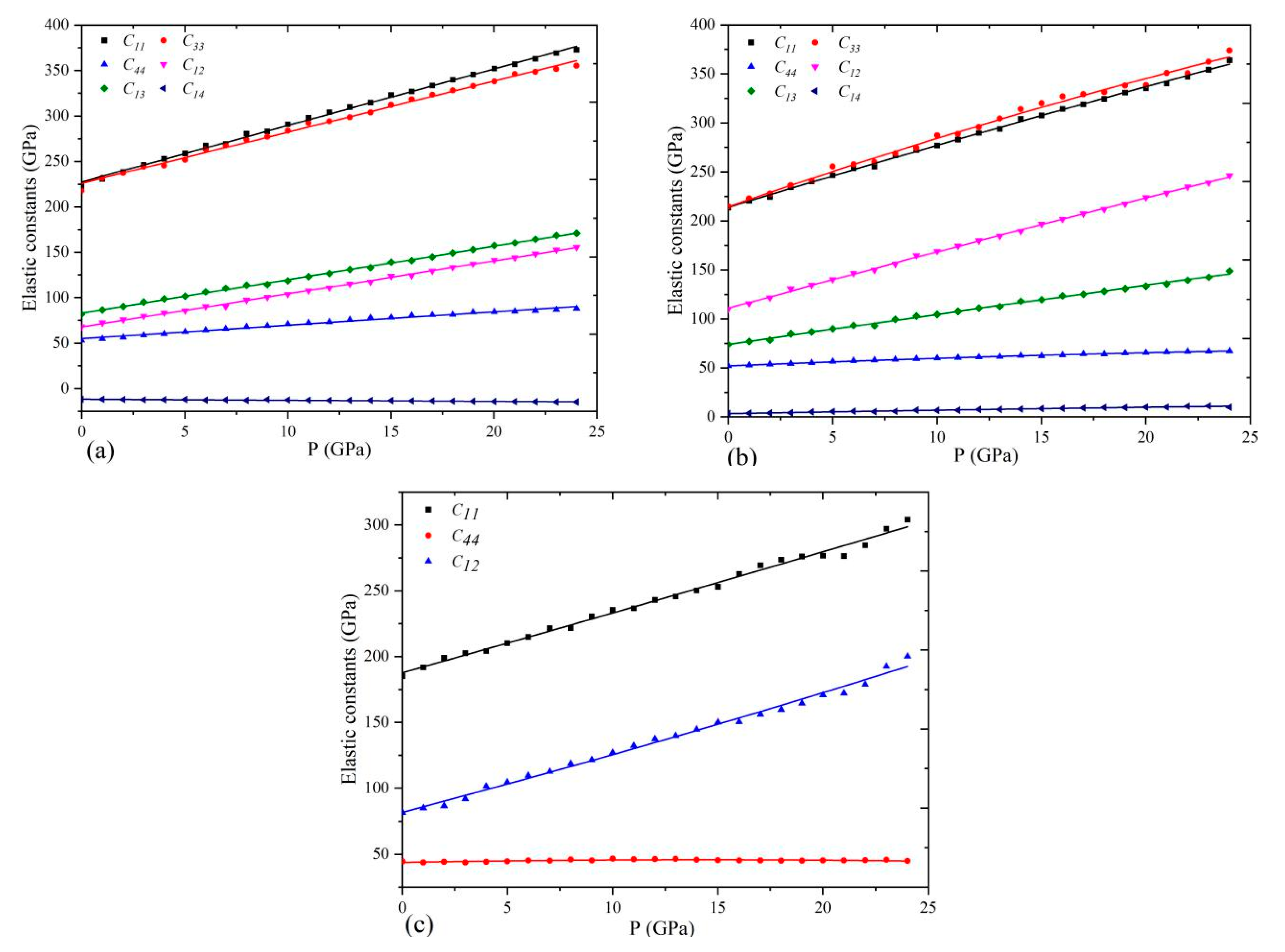
3.3. Elastic Constant
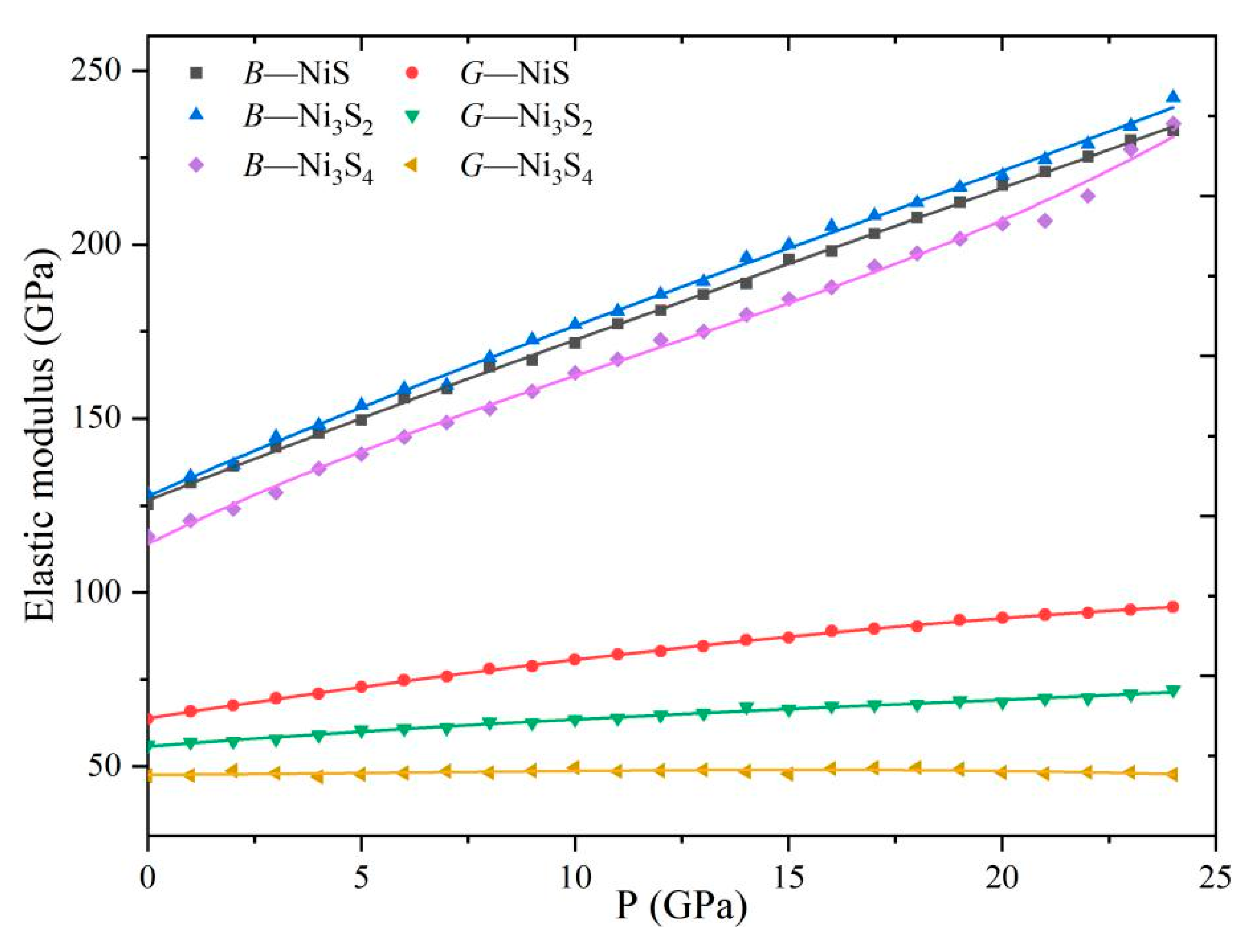
3.4. Elasticity Modulus
4. Discussion
4.1. Mechanical Stability of Ni Sulfides under High Pressure
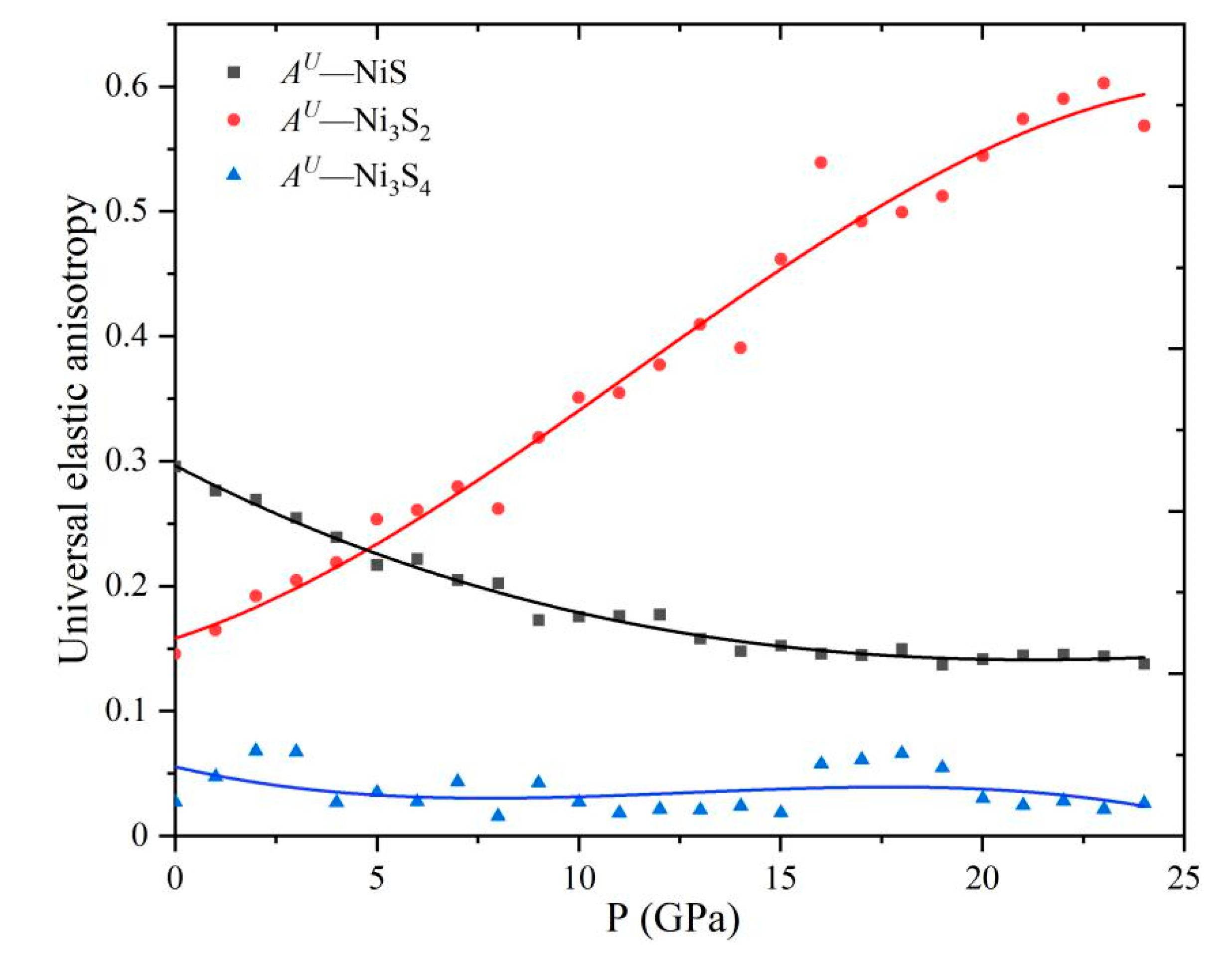
4.2. Elastic Anisotropy of Ni Sulfides
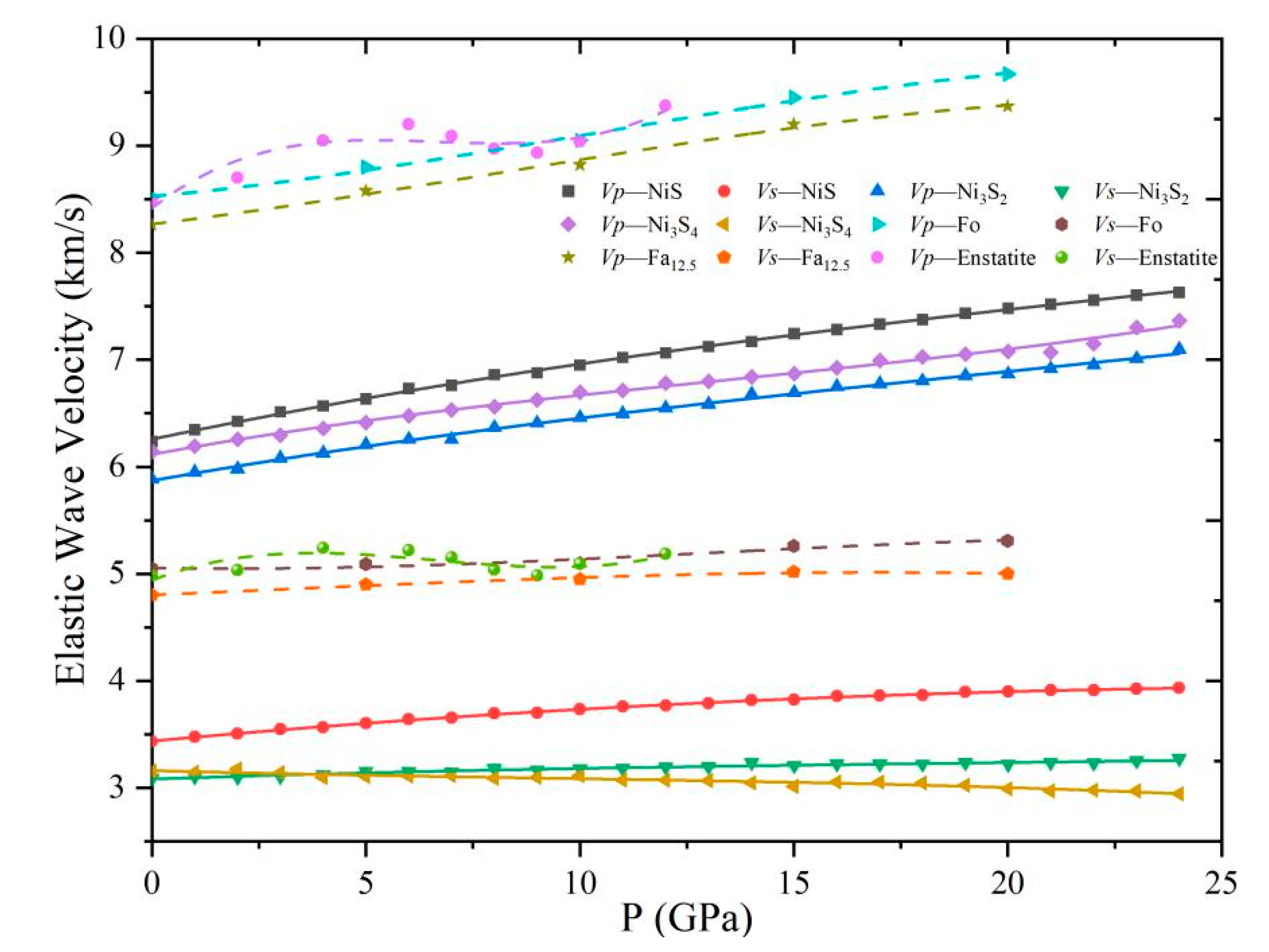
4.3. Elastic Wave Velocity of Ni Sulfides
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mudd, G.M. Global trends and environmental issues in nickel mining: Sulfides versus laterites. Ore. Geol. Rev. 2010, 38, 9–26. [Google Scholar] [CrossRef]
- Kuck, P.H. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2012.
- Kuck, P.H. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2006.
- Guillaume, F.; Huang, S.; Harris, K.D.M.; Couzi, M.; Talaga, D. Optical phonons in millerite (NiS) from single-crystal polarized Raman spectroscopy. J. Raman. Spectrosc. 2008, 39, 1419–1422. [Google Scholar] [CrossRef]
- Kerestedjian, T.; Gervilla, F.; Gonzalez-Jimenez, J.M.; Proenza, J. Godlevskite Ni9S8 from Dobromirtsi, Central Rhodopes, Bulgaria: Fifirst report for the country and genetic implications. Geochem. Min. Pet. Sofifia. 2007, 45, 19–28. [Google Scholar]
- Wang, J.Z.; Chew, S.Y.; Wexler, D.; Wang, G.X.; Ng, S.H.; Zhong, S.; Liu, H.K. Nanostructured nickel sulfide synthesized via a polyol route as a cathode material for the rechargeable lithium battery. Electrochem. Commun. 2007, 9, 1877–1880. [Google Scholar] [CrossRef]
- Du, W.M.; Wang, Z.Y.; Zhu, Z.Q.; Hu, S.; Zhu, X.Y.; Shi, Y.F.; Huan, P.; Qian, X.F. Facile synthesis and superior electrochemical performances of CoNi2S4/graphene nanocomposite suitable for supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 9613–9619. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Welters, W.J.J.; Booy, E.; De Beer, V.H.J.; Van Santen, R.A.; Geus, J.W.; Jansen, J.B.H. Catalytic activity of nickel sulfide catalysts supported on Al-pillared montmorillonite for thiophene hydrodesulfurization. Appl. Catal. A Gen. 1993, 97, 77–85. [Google Scholar] [CrossRef]
- Meng, J.L.; Yu, Z.M.; Li, Y.; Li, Y.D. PdS-modified CdS/NiS composite as an efficient photocatalyst for H2 evolution in visible light. Catal. Today 2014, 225, 136–141. [Google Scholar] [CrossRef]
- Ripley, E.M.; Dong, S.F.; Li, C.S.; Wasylenki, L.E. Cu isotope variations between conduit and sheet-style Ni-Cu-PGE sulfide mineralization in the Midcontinent Rift System, North America. Chem. Geol. 2015, 414, 59–68. [Google Scholar] [CrossRef]
- Szabó, C.; Bodnar, R.J. Chemistry and origin of mantle sulfides in spinel peridotite xenoliths from alkaline basaltic lavas, Nógraád-Gömör Volcanic Field, northern Hungary and southern Slovakia. Geochim. Cosmochim. Acta 1995, 59, 3917–3927. [Google Scholar] [CrossRef]
- Shaw, C.S.J. Origin of sulfide blebs in variably metasomatized mantle xenoliths, Quaternary West Eifel volcanic field, Germany. Can. Miner. 1997, 35, 1453–1463. [Google Scholar]
- Zhang, Z.; Hirschmann, M.M. Experimental constraints on mantle sulfide melting up to 8 GPa. Am. Miner. 2016, 101, 181–192. [Google Scholar] [CrossRef]
- Helffrich, G.; Kendall, J.M.; Hammond, J.O.S.; Carroll, M.R. Sulfide melts and long-term low seismic wavespeeds in lithospheric and asthenospheric mantle. Geophys. Res. Lett. 2011, 38, L11301. [Google Scholar] [CrossRef]
- VanDecar, J.C.; James, D.E.; Assumpção, M. Seismic evidence for a fossil mantle plume beneath South America and implications for plate driving forces. Nature 1995, 378, 25–31. [Google Scholar] [CrossRef]
- Arrowsmith, S.J.; Kendall, M.; White, N.; VanDecar, J.C.; Booth, D.C. Seismic imaging of a hot upwelling beneath the British Isles. Geology 2005, 33, 345–348. [Google Scholar] [CrossRef]
- Hammond, J.O.; Collier, J.S.; Kendall, J.M.; Helffrich, G.; Rümpker, G. Plume scar in the mantle lithosphere beneath the Seychelles revealed by seismic imaging. Earth Planet Sci. Lett. 2012, 355, 20–31. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Wang, W.Z. Advances in first-principles calculation of mineral elasticity under high temperature and high pressure. Sci. China Earth Sci. 2016, 46, 582–621. [Google Scholar]
- Xie, H.S. Deep earth exploration and high pressure research. Physics 2001, 30, 145–148. [Google Scholar]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.; Probert, M.I.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Krist.-Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Vanderbilt, D.; Wickham, L.K. Elastic energies of coherent germanium islands on silicon. MRS Proc. 1990, 202, 555. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. 1976, B13, 5188. [Google Scholar] [CrossRef]
- Nye, J.F. Physical Properties of Crystals; Oxford University Press: Oxford, UK, 1985. [Google Scholar]
- Voigt, W. Handbook of Crystal Physics; Taubner: Leipzig, Germany, 1928. [Google Scholar]
- Reuss, A. Calculation of the flow limits of mixed crystals on the basis of the plasticity of monocrystals. Z. Angew. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Hill, R. The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. 1952, A65, 349. [Google Scholar] [CrossRef]
- Rajamani, V.T.; Prewitt, C.T. The crystal structure of millerite. Can. Miner. 1974, 12, 253–257. [Google Scholar]
- Fleet, M.E. The crystal structure of heazlewoodite, and metallic bonds in sulfide minerals. Am. Miner. 1977, 62, 341–345. [Google Scholar]
- Lundqvist, D. X-ray studies on the ternary system Fe-Ni-S. Arkiv for Kemi. Miner. Och Geol. 1947, 24, 12. [Google Scholar]
- Liu, S.Q.; Li, Y.B.; Liu, J.; Ju, Y.W.; Liu, J.M.; Yang, Z.M.; Shi, Y.L. Equilibrium lithium isotope fractionation in Li-bearing minerals. Geochim. Cosmochim. Acta 2018, 235, 360–375. [Google Scholar] [CrossRef]
- Wang, J.H.; Cheng, Z.; Brédas, J.L.; Liu, M. Electronic and vibrational properties of nickel sulfides from first principles. J. Chem. Phys. 2007, 127, 214. [Google Scholar] [CrossRef]
- Yu, Y.G.; Ross, N.L. Vibrational and thermodynamic properties of Ni3S2 polymorphs from first-principles calculations. Phys. Chem. Miner. 2011, 38, 241–249. [Google Scholar] [CrossRef]
- Wu, Z.J.; Zhao, E.J.; Xiang, H.P.; Hao, X.F.; Liu, X.J.; Meng, J. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys. Rev. 2007, B76, 054115. [Google Scholar]
- Salje, E. Phase transitions in ferroelastic and co-elastic crystals. Ferroelectrics 1990, 104, 111–120. [Google Scholar] [CrossRef]
- Fedorov, F.I. Theory of Elastic Waves in Crystals; Plenum: New York, NY, USA, 1968. [Google Scholar]
- Sin’ko, G.V.; Smirnov, N.A. Ab initio calculations of elastic constants and thermodynamic properties of bcc, fcc, and hcp Al crystals under pressure. Phys. Condens. Matter. 2002, 14, 6989–7005. [Google Scholar]
- Silver, P.G.; Chan, W.W. Implications for continental structure and evolution from seismic anisotropy. Nature 1998, 335, 34–39. [Google Scholar] [CrossRef]
- Kaneshima, S. Seismic anisotropy: A review of studies by Japanese researchers. J. Phys. Earth 1995, 43, 301–319. [Google Scholar] [CrossRef]
- Ranganathan, S.I.; Ostoja-Starzewski, M. Universal elastic anisotropy index. Phys. Rev. Lett. 2008, 101, 055504. [Google Scholar] [CrossRef]
- Ducea, M.N.; Park, S.K. Enhanced Mantle Conductivity from Sulfide Minerals, Southern Sierra Nevada, California. Geophys. Res. Lett. 2000, 27, 2405–2408. [Google Scholar] [CrossRef]
- Liu, L.; Du, J.G.; Liu, W.; Zhao, J.J. Elastic behavior of (MgxFe1−x)2SiO4 olivine at high pressure from first-principles simulations. J. Phys. Chem. Solids 2010, 71, 1094–1097. [Google Scholar] [CrossRef]
- Padilha, A.L.; Vitorello, I.; Antunes, C.E.; Padua, M.B. Imaging three-dimensional crustal conductivity structures reflecting continental flood basalt effects hidden beneath thick intracratonic sedimentary basin. J. Geophys. Res. 2015, 120, 4702–4719. [Google Scholar] [CrossRef]
| Millerite | This Study | Calc. [31] | Calc. [32] | Expt. [28] | Error (%) |
|---|---|---|---|---|---|
| a (Å) | 9.5955 | 9.5917 | 9.62 | 9.6190 | 0.15 |
| c (Å) | 3.1498 | 3.1434 | 3.15 | 3.1499 | 0.06 |
| V (Å3) | 251.161 | 250.450 | 252.399 | 0.10 | |
| Heazlewoodite | This Study | Calc. [31] | Calc. [32] | Expt. [29] | Error (%) |
| a (Å) | 4.0820 | 4.0765 | 4.09 | 4.0821 | 0.1 |
| α (°) | 89.3177 | 89.367 | 89.4 | 89.475 | 0 |
| V (Å3) | 68.0029 | 67.730 | 68.014 | 0.4 | |
| Polydymite | This Study | Calc. [31] | Calc. [32] | Expt. [30] | Error (%) |
| a (Å) | 9.4757 | 9.4702 | 9.49 | 9.457 | 0.1 |
| V (Å3) | 849.813 | 849.332 | 845.785 | 0.4 |
| Mineral | V0 (Å3) | B0 (GPa) | B’0 |
|---|---|---|---|
| NiS | 251.15 | 123.79 | 5.00 |
| Ni3S2 | 67.99 | 127.93 | 4.83 |
| Ni3S2 [34] | 68.74 | 119.2 | 4.7 |
| Ni3S4 | 850.83 | 116.65 | 4.74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Tian, Y.; Liu, S.; Yang, P.; Li, Y. First-Principles Study of the Elastic Properties of Nickel Sulfide Minerals under High Pressure. Minerals 2020, 10, 737. https://doi.org/10.3390/min10090737
Zhang Q, Tian Y, Liu S, Yang P, Li Y. First-Principles Study of the Elastic Properties of Nickel Sulfide Minerals under High Pressure. Minerals. 2020; 10(9):737. https://doi.org/10.3390/min10090737
Chicago/Turabian StyleZhang, Qiuyuan, Ye Tian, Shanqi Liu, Peipei Yang, and Yongbing Li. 2020. "First-Principles Study of the Elastic Properties of Nickel Sulfide Minerals under High Pressure" Minerals 10, no. 9: 737. https://doi.org/10.3390/min10090737
APA StyleZhang, Q., Tian, Y., Liu, S., Yang, P., & Li, Y. (2020). First-Principles Study of the Elastic Properties of Nickel Sulfide Minerals under High Pressure. Minerals, 10(9), 737. https://doi.org/10.3390/min10090737





