A Study on the Formation Environment of the La Cumbre Amber Deposit, from Santiago Province, the Northwestern Part of the Dominican Republic
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Analytical Methods
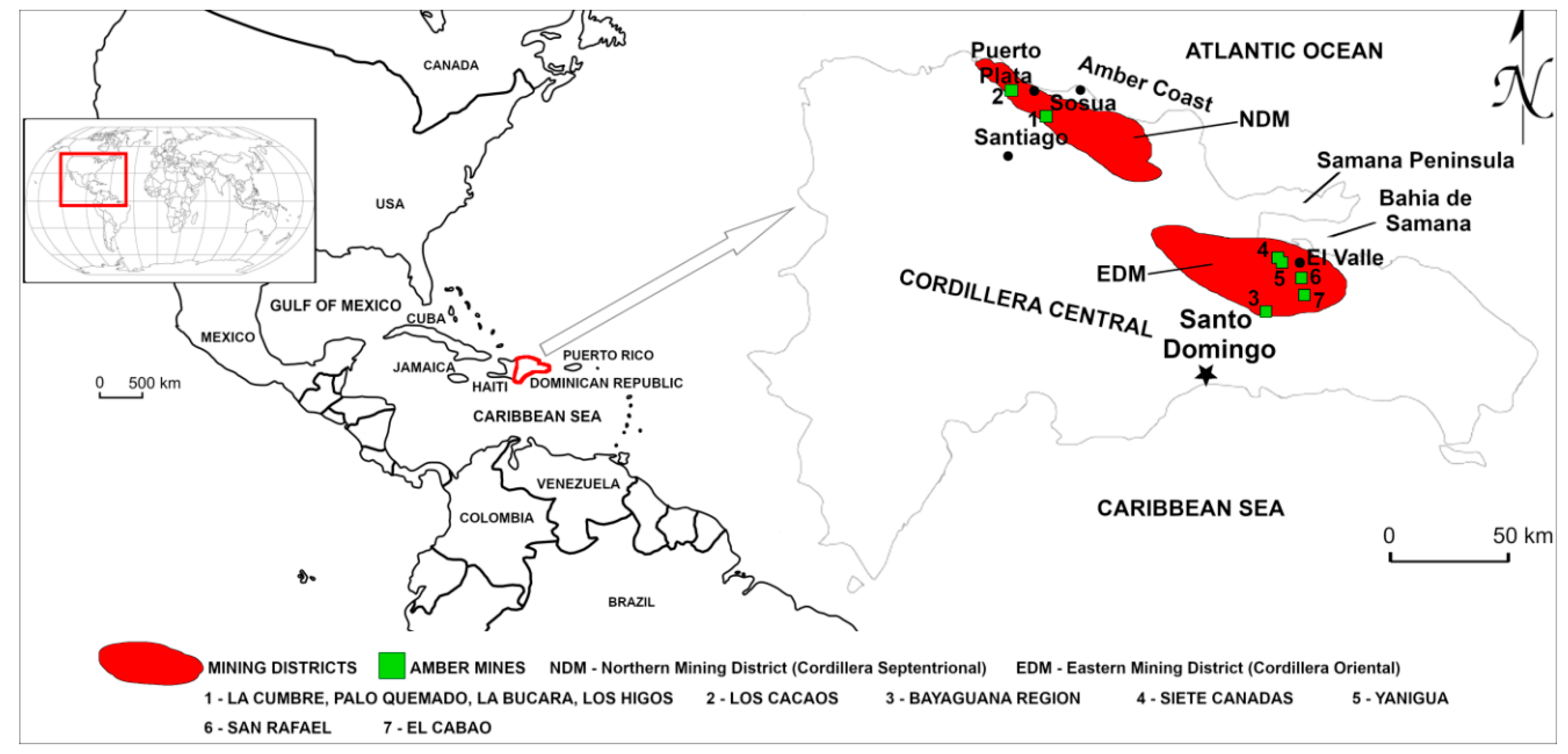
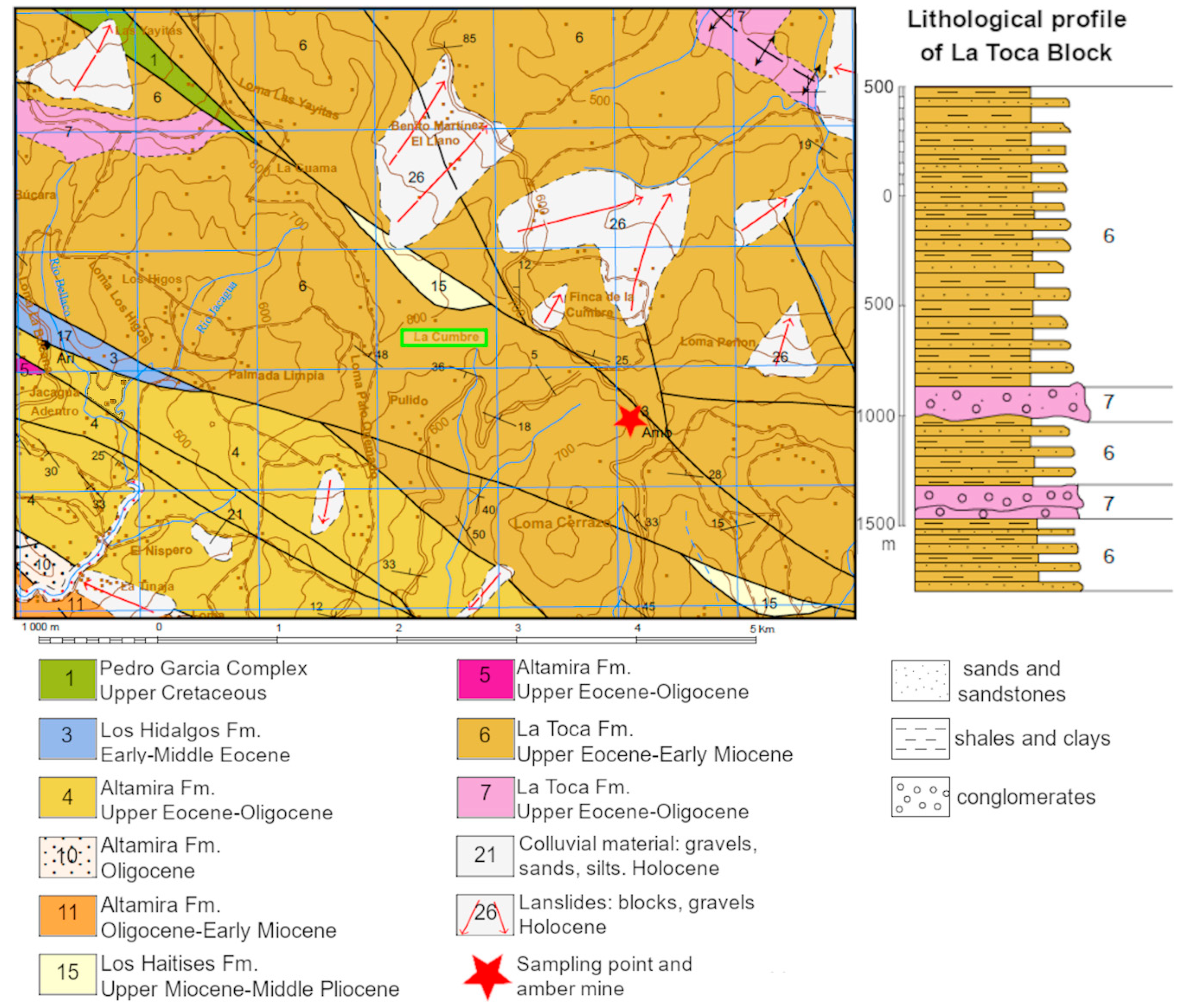
3. Geological Setting
4. Results
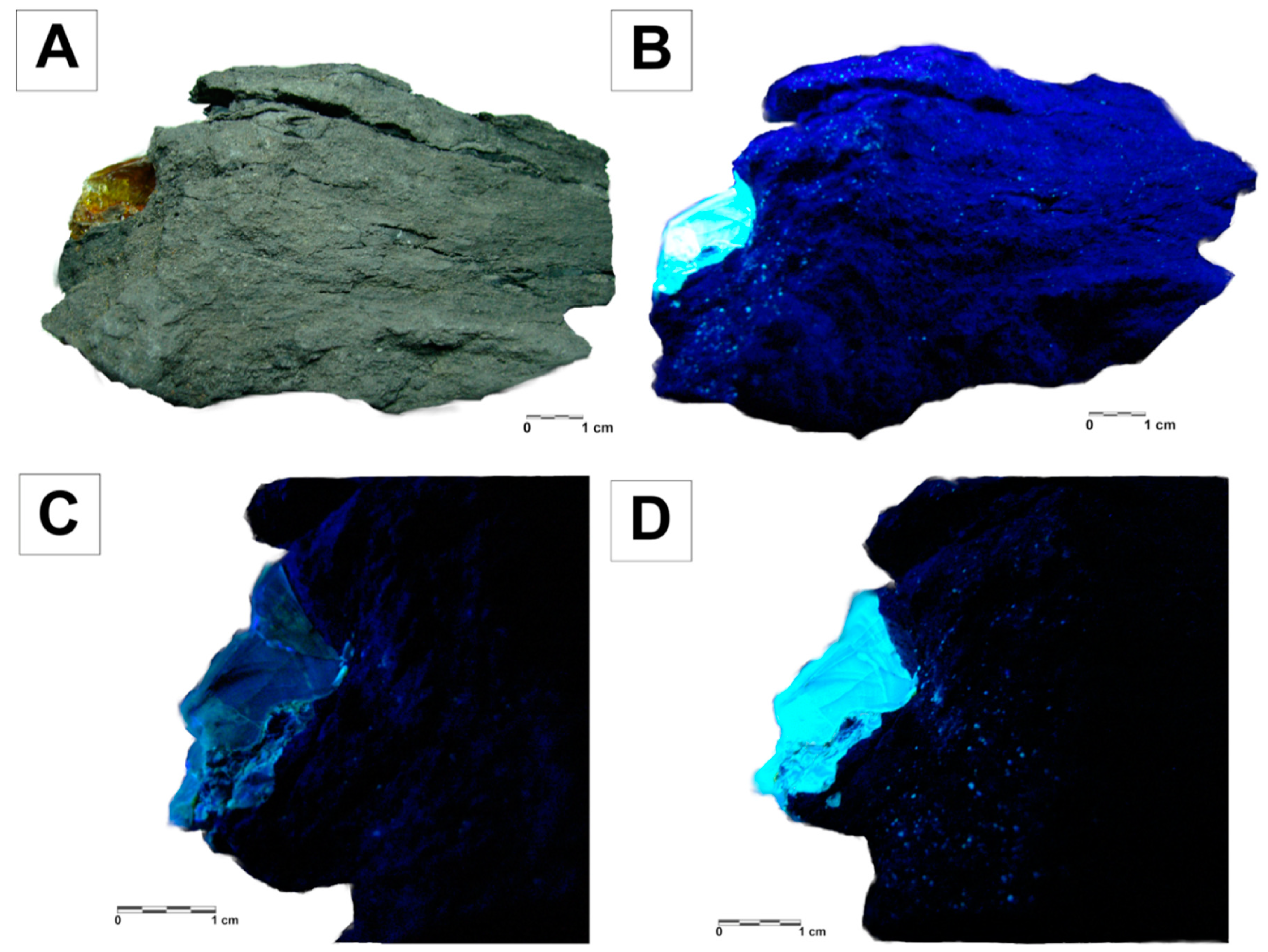
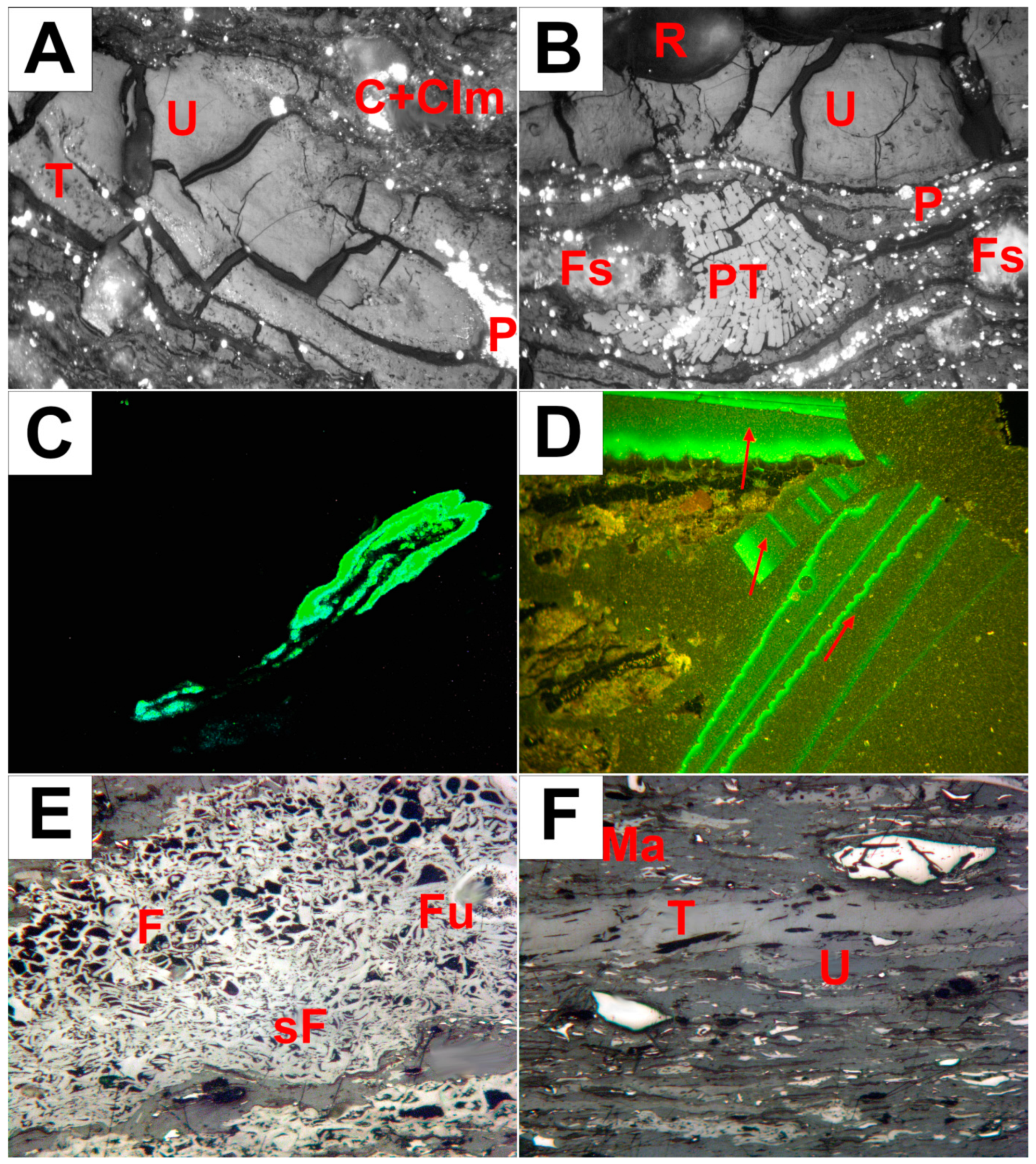
4.1. Macroscopic Characterization
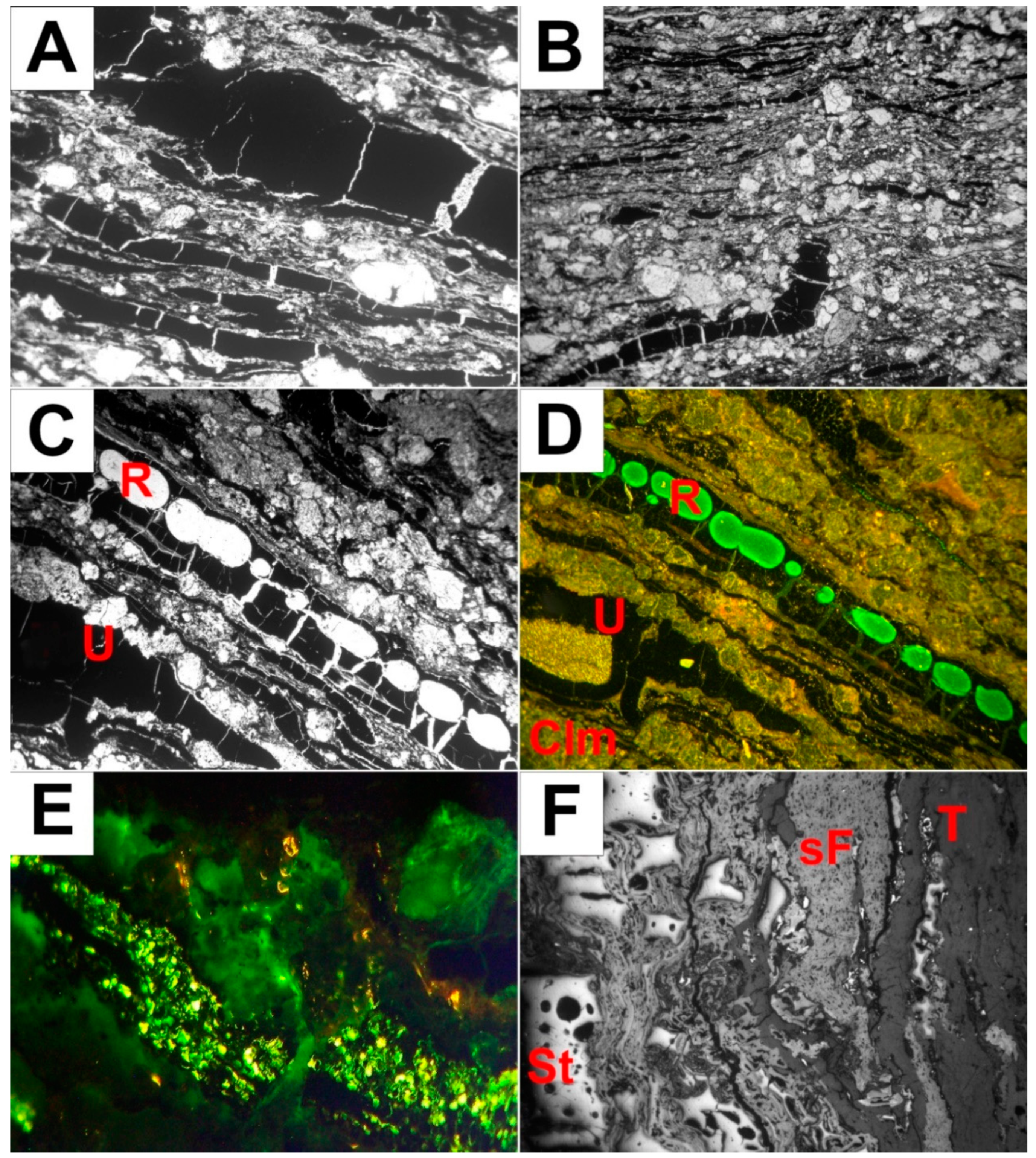
4.2. Microscopic Observations Under Transmitted and Reflected Light
4.3. Quantitative Composition of the Coaly Shales
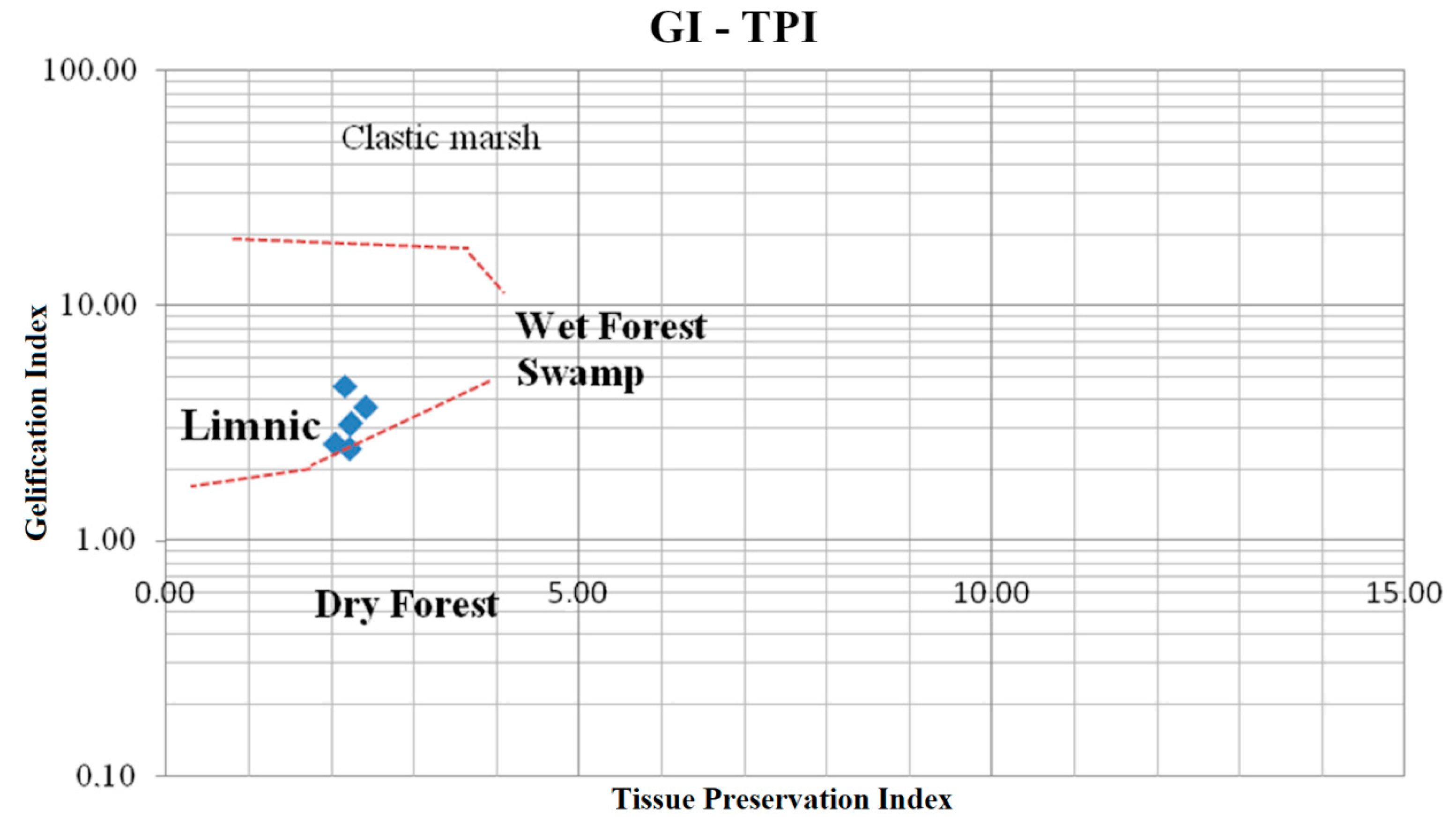
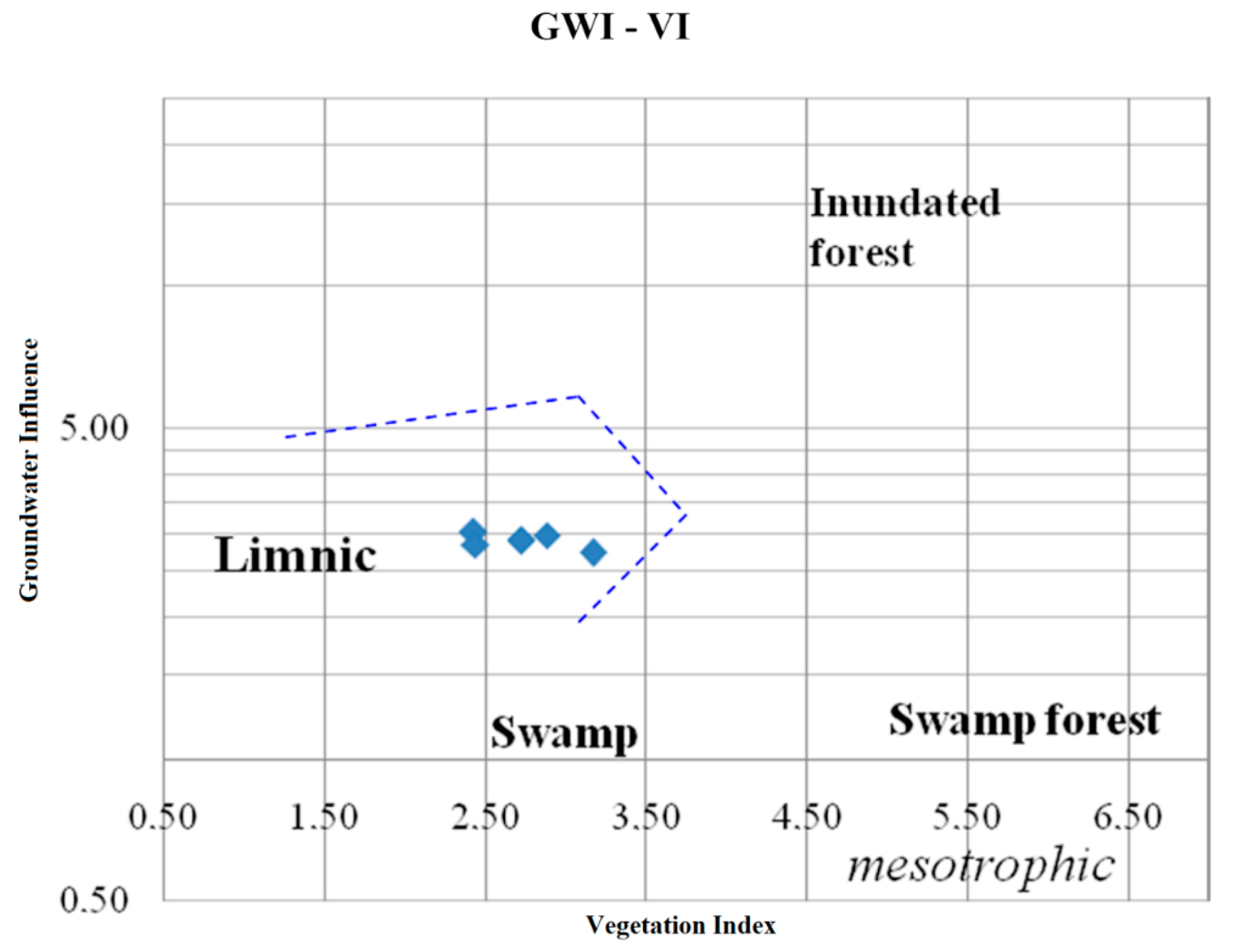
4.4. Facies Analysis
4.5. Dominican Amber Characteristics
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tomkeieff, S.I. Coals and Bitumens and Related Fossil Carbonaceous Substances: Nomenclature and Classification, 1st ed.; Pergamon Press: Oxford, UK, 1954. [Google Scholar]
- Langenheim, J.H. Plant Resins: Chemistry, Evolution, Ecology, and Ethnobotany; Timber Press: Portland, OR, USA, 2003. [Google Scholar]
- Anderson, K.B.; Winans, E.E.; Botto, R.E. The nature and fate of natural resins in the geosphere. II. Identification, classification, and nomenclature of resinites. Org. Geochem. 1992, 18, 829–841. [Google Scholar] [CrossRef]
- Cunningham, A.; West, P.R.; Hammond, G.S.; Langenheim, J.H. The existence and photochemical initiation of free radicals in Hymenaea trunk resins. Phytochemistry 1977, 16, 1442–1443. [Google Scholar] [CrossRef]
- Anderson, K.B. New evidence concerning the structure, composition, and maturation of class I (polylabdanoid) resinites. In Amber, Resinite and Fossil Resins; Anderson, K.B., Crelling, J., Eds.; American Chemical Society Symposium Series: Washington, DC, USA, 1995; Volume 617, pp. 105–129. [Google Scholar] [CrossRef]
- Poinar, G.O., Jr.; Mastalerz, M. Taphonomy of fossilised resins: Determining the biostratinomy of amber. Acta Geol. Hisp. 2000, 35, 171–182. [Google Scholar]
- Winkler, W.; Kirchner, E.C.; Asenbaum, A.; Musso, M. A Raman spectroscopic approach to the maturation process of fossil resins. J. Raman Spectrosc. 2001, 32, 59–63. [Google Scholar] [CrossRef]
- Jehlička, J.; Jorge Villar, S.E.; Edwards, H.G.M. Fourier transform Raman spectra of Czech and Moravian fossil resins from freshwater sediments. J. Raman Spectrosc. 2004, 35, 761–767. [Google Scholar] [CrossRef]
- Naglik, B.; Kosmowska-Ceranowicz, B.; Natkaniec-Nowak, L.; Drzewicz, P.; Dumańska-Słowik, M.; Matusik, J.; Wagner, M.; Milovsky, R.; Stach, P.; Szyszka, A. Evolutionary history of fossil resin from Jambi Province (Sumatra, Indonesia) based on physico-chemical studies. Minerals 2018, 8, 95. [Google Scholar] [CrossRef]
- Stach, P.; Martinkutė, G.; Šinkūnas, P.; Natkaniec-Nowak, L.; Drzewicz, P.; Naglik, B.; Bogdasarov, M. An attempt to correlate the physical properties of fossil and subfossil resins with their age and geographic location. J. Polym. Eng. 2019, 39, 716–728. [Google Scholar] [CrossRef]
- Kosmowska-Ceranowicz, B. Succinite and some other fossil resins in Poland and Europe (deposits, finds, features and differences in IRS). Estud. Mus. Cienc. Nat. Alava 1999, 14, 73–117. [Google Scholar]
- Zherikhin, V.V.; Eskov, K.Y. Mesozoic and Lower Tertiary resins in former USSR. Estud. Mus. Cienc. Nat. Álava 1999, 14, 119–131. [Google Scholar]
- Iturralde-Vinent, M.A. Geology of the amber-bearing deposits of the Greater Antilles. Caribb. J. Sci. 2001, 37, 141–167. [Google Scholar]
- Roghi, G.; Ragazzi, E.; Gianolla, P. Triassic amber of the southern Alps (Italy). Palaios 2006, 21, 143–154. [Google Scholar] [CrossRef]
- Bogdasarov, M.A. Mineralogy of fossil resins in Northern Eurasia. Geol. Ore Depos. 2007, 49, 630–637. [Google Scholar] [CrossRef]
- Bogdasarov, M.A. Amber and Others Fossil Resins of Eurasia. Ph.D. Thesis, Brest State A.S. Pushkin University, Pushkin, Russia, 2010. (In Russian). [Google Scholar]
- Penney, D. Dominican amber. In Biodiversity of Fossils in Amber from the Major World Deposits; Penney, D., Ed.; Siri Scientific Press: Manchester, UK, 2010; pp. 23–42. [Google Scholar]
- Ragazzi, E.; Schmidt, A.R. Amber. In Encyclopedia of Geobiology; Reitner, J., Thiel, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 24–36. [Google Scholar] [CrossRef]
- Anderson, K.B. The nature and fate of natural resins in the geosphere–VII. A radiocarbon (14C) age scale for description of immature natural resins: An invitation to scientific debate. Org. Geochem. 1997, 25, 251–253. [Google Scholar] [CrossRef]
- Kosmowska-Ceranowicz, B. Bursztyn i inne żywice kopalne. Żywice kopalne Ameryki Środkowej: Bursztyn meksykański i bursztyn dominikański/Amber and Other Fossil Resins. Fossil resins of Central America: Mexican amber and Dominican amber. Polski Jubiler 2000, 1, 18–20. (In Polish) [Google Scholar]
- Kosmowska-Ceranowicz, B. Bursztyn w Polsce i na Świecie/Amber in Poland and in the World, 1st ed.; Warsaw University Press: Warsaw, Poland, 2012; ISBN 978-83-235-0774-1. [Google Scholar]
- Poinar, G.O., Jr.; Poinar, R. The Amber Forest: A Reconstruction of a Vanished World; Princeton University Press: Princeton, NJ, USA, 1999; ISBN 0-691-02888-5. [Google Scholar]
- Poinar, G.O., Jr. Palaeoecological perspectives in Dominican amber. Ann. Soc. Entomol. Fr. 2010, 46, 23–52. [Google Scholar] [CrossRef]
- Matuszewska, A.; Gołąb, A.; Salomon, A. Mikrotwardość bursztynu i jego imitacji/Microhardness of amber and its imitations. Polski Jubiler 2002, 1, 26–29. (In Polish) [Google Scholar]
- Matuszewska, A.; Gołąb, A. Próba wykorzystania parametru mikrotwardości żywic kopalnych i sztucznych, jako cechy klasyfikacyjnej/An attempt at using the parameter of the microhardness of fossil and artificial resins as a classification feature. Bursztynisko 2008, 31, 56–61, (In Polish and in English). [Google Scholar]
- Matuszewska, A. Bursztyn (Sukcynit), Inne Żywice Kopalne, Subfosylne i Współczesne/Amber (Succinite) and Other Modern, Subfossil and Fossil Resins, 1st ed.; Oficyna Wydawnicza Wacław Walasek: Katowice, Poland, 2010; ISBN 978-83-60743-34-8. (In Polish) [Google Scholar]
- Iturralde-Vinent, M.A.; MacPhee, R.D.E. Age and palaeogeographical origin of Dominican amber. Science 1996, 273, 1850–1852. [Google Scholar] [CrossRef]
- Bachmann, R. The Caribbean Plate and the Question of its Formation; University of Mining and Technology, Institute of Geology, Department of Tectonophysics: Freiberg, Germany, 2001; Available online: http://www.geo.tu-freiberg.de/hydro/oberseminar/pdf/Raik%20Bachmann.pdf (accessed on 15 March 2020).
- Kalkreuth, W.; Kotis, T.; Papanicolaou, C.; Kokkinalis, P. The geology and coal petrology of a Miocene lignite profile at Meliadi Mine, Katerini, Greece. Int. J. Coal Geol. 1991, 17, 51–67. [Google Scholar] [CrossRef]
- Gruber, W.; Sachsenhofer, R. Coal deposition in the Noric Depression (Eastern Alps): Raised and low-lying mires in Miocene pull-apart basins. Int. J. Coal Geol. 2001, 48, 89–114. [Google Scholar] [CrossRef]
- Diessel, C.F.K. On the correlation between coal facies and depositional environments. In Advances in the Study of the Sydney Basin, Proceedings of the Twentieth Symposium Department of Geology; University of Newcastle (N.S.W.): Newcastle, Australia, 1986; pp. 11–22. [Google Scholar]
- Calder, J.; Gibling, M.R.; Mukhopadhyay, P.K. Peat formation in a Westphalian B piedmont setting, Cumberland Basin, Nova Scotia: Implication for the maceral-base interpretation or rheotrophic and raised paleomires. Bull. Soc. Géol. Fr. 1991, 162, 283–298. [Google Scholar]
- Stach, E.; Mackowsky, M.-T.; Teichmüller, M.; Taylor, G.H.; Chandra, D.; Teichmüller, R. Stach’s Textbook of Coal Petrology, 3rd ed.; Gebrüder Borntraeger: Berlin/Stuttgart, Germany, 1982. [Google Scholar]
- Kalkreuth, W.; Leckie, D. Sedimentological and petrographical characteristic of Cretaceous strandplain coals; a model for coal accumulation from the North American Western Interior Seaway. Int. J. Coal Geol. 1989, 12, 381–424. [Google Scholar] [CrossRef]
- Diessel, C.F.K.; Boyd, R.; Wadsworth, J.; Leckie, D.; Chalmers, G. On balanced and unbalanced accummulation/peat accumulation ratios in the Cretaceous coals from Gates Formation, Western Canada, and their sequence-stratigraphic significance. Int. J. Coal Geol. 2000, 43, 143–186. [Google Scholar] [CrossRef]
- Buillit, N.; Lalier-Verges, E.; Pradier, B.; Nicolas, G. Coal petrographic genetic units in deltaic-plain deposits of the Campanian Mesa Verde Group (New Mexica, USA). Int. J. Coal Geol. 2002, 52, 93–110. [Google Scholar] [CrossRef][Green Version]
- Potter, J.; McIlreath, I.; Natras, T. Lithotypes, Macerals and Coal Facies Studies of Lower Cretaceous Medicine River Coals in South Central Alberta: Applications in CBM Exploration, Depositional Environments and Tectonic History Studies. In Proceedings of the 2008 CSPG-CSEG-CWLS Convention: Back to exploration, Calgary, Canada, 12–15 May 2008; pp. 777–780. [Google Scholar]
- Potter, J.; Beaton, A.; McDougall, W.; Namburdiri, E.M.V.; Vigrass, L.W. Depositional environments of the Hart coal zone (Paleocene), Willow Bunch Coalfield, southern Saskatchewan, Canada from petrographic, palynological, palaeobotanical, mineral and trace elemements studies. Int. J. Coal Geol. 1991, 19, 253–281. [Google Scholar] [CrossRef]
- Wagner, M.; Drobniak, A. Petrology of the so-called cuboidal clays from the Belchatow lignite deposit. Prace Geol. PAN 2000, 147, 73–97, (In Polish, English summary). [Google Scholar]
- Flores, D. Organic facies and depositional palaeonvironment of lignites from Rio Maior Basin (Portugal). Int. J. Coal Geol. 2002, 48, 181–195. [Google Scholar] [CrossRef]
- Wilkins, R.W.T.; Sherwood, N.; Li, Z. RaMM (Raman maturity method) study of samples used in an interlaboratory exercise on a standard test method for determination of vitrinite reflectance on dispersed organic matter in rocks. Mar. Pet. Geol. 2018, 91, 236–250. [Google Scholar] [CrossRef]
- ISO 7404-5. Methods for the Petrographic Analysis of Coals—Part 5: Method of Determining Microscopically the Reflectance of Vitrinite; International Organization for Standardization: Geneva, Switzerland, 2009; p. 14. [Google Scholar]
- ISO 7404–3. Methods for the Petrographic Analysis of Coals—Part 3: Method of Determining Maceral Group Composition; International Organization for Standardization: Geneva, Switzerland, 2009; p. 7. [Google Scholar]
- ISO 6507-1. Metallic Materials—Vickers Hardness Test—Part 1: Test Method; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- De Zoeten, R.; Mann, P. Structural geology and Cenozoic tectonic history of the Central Cordillera Septentrional, Dominican Republic. In Geologic and Tectonic Development of the North America-Caribbean Plate Boundary in Hispaniola; Mann, P., Draper, G., Lewis, J.F., Eds.; Geological Society of America Special Paper: Boulder, CO, USA, 1991; Volume 262, pp. 265–279. [Google Scholar] [CrossRef]
- Dolan, J.; Mann, P.; De Zoeten, R.; Heubeck, C.; Shiroma, J.; Monechi, S. Sedimentologic, stratigraphy, and tectonic synthesis of Eocene-Miocene sedimentary basins, Hispaniola and Puerto Rico. In Geologic and Tectonic Development of the North America-Caribbean Plate Boundary in Hispaniola; Mann, P., Draper, G., Lewis, J.F., Eds.; Geological Society of America Special Paper: Boulder, CO, USA, 1991; Volume 262, pp. 217–240. [Google Scholar]
- Merino, J.I.R.; Valera, F.P.; de Los Santos, M.A.; Braga, J.C.; Granados, L.; Zarza, A.A.; Fernandez, M.J.; Viruete, J.E.; Hernaiz, P.P.; Solé, J.E.; et al. Geological Map of Dominican Republic at 1:50,000 scale San Francisco Above (sheet 6074-I). In Geotematic Cartography Program of the Dominican Republic, Project 1B; General Directorate of Mining: Santo Domingo, Dominican Republic, 2010. [Google Scholar]
- Eberle, W.; Hirdes, W.; Muff, R.; Pelaez, M. The geology of the Cordillera Septentrional (Dominican Republic). In Proceedings of the Transactions of the 9th Caribbean Geological Conference, Santo Domingo, Dominican Republic, 15–26 August 1980. [Google Scholar]
- Redmond, B. The Tertiary of the Central Cordillera Septentrional. In Proceedings of the Transactions of the 9th Caribbean Geological Conference, Santo Domingo, Dominican Republic, 15–26 August 1982. [Google Scholar]
- Widera, M. Depoisitional environments of overbank sedimentation in the lignite-bearing Gray Clays Member: New evidence from Middle Miocene deposits of Central Poland. Sedim. Geol. 2016, 335, 150–165. [Google Scholar] [CrossRef]
- Wagner, M. Petrologic studium of terrestrial organic matter in Carpathians flysch sediments, southern Poland. Int. J. Coal Geol. 1996, 29, 259–272. [Google Scholar] [CrossRef]
- Kwiecińska, B.; Wagner, M. Pseudowitrynit i pseudohuminit—Mało poznane składniki kopalnego węgla/Pseudovitrinite and pseudohuminite—Not well known components of hard and brown coals. Zesz. Nauk. Politech. Śląskiej 2001, 249, 75–81, (In Polish, English summary). [Google Scholar]
- Kwiecińska, B.; Wagner, M. Atlas Petrograficzny Węgla Brunatnego: Litotypy i Macerały/A Petrographic Atlas of Brown Coals (Lignites) from the Polish Deposits, 1st ed.; Wydawnictwo JAK Andrzej Choczewski; AGH University of Science and Technology: Kraków, Poland, 2001; ISBN 10: 83-914795-4-4. (In Polish, English summary). [Google Scholar]
- Mernagh, T.P.; Trudu, A.G. A laser Raman microprobe study of some geologically important sulphide minerals. Chem. Geol. 1993, 103, 113–127. [Google Scholar] [CrossRef]
- Wart, H.E.; Lewis, A.; Scherga, H.A.; Saeva, F.D. Disulfide bond dihedral angles for Raman Spectroscopy. Proc. Natl. Acad. Sci. USA 1973, 70, 2619–2623. [Google Scholar] [CrossRef] [PubMed]
- Brody, R.H.; Edwards, H.G.M.; Pollard, A.M. A study of amber and copal samples using FT-Raman spectroscopy. Spectrochim. Acta A 2001, 57, 1325–1338. [Google Scholar] [CrossRef]
- Downs, R.T. The RRUFF Project: An integrated study of the chemistry, crystallography, Raman and infrared spectroscopy of minerals. In Proceedings of the Program and Abstracts of the 19th General Meeting of the International Mineralogical Association, Kobe, Japan, 23–28 July 2006; Commision on Ore Mineralogy; Report of the Sulfofosalt Subcommittee. pp. 3–13. [Google Scholar]
- Montoro, O.R.; Taravillo, M.; San Andrés, M.; De La Roja, J.M.; Barrero, A.F.; Arteaga, P.; Baonza, V.G. Raman spectroscopic study of the formation of fossil resin analogues. J. Raman Spectrosc. 2014, 45, 1230–1235. [Google Scholar] [CrossRef]
- Barone, G.; Capitani, D.; Mazzoleni, P.; Proietti, N.; Raneri, S.; Longobardo, U.; Di Tullio, V. 13C Solid State Nuclear Magnetic Resonance and μ-Raman Spectroscopic Characterization of Sicilian Amber. J. Appl. Spectrosc. 2016, 70, 1346–1355. [Google Scholar] [CrossRef]
- Nims, C.; Cron, B.; Wetherington, M.; Macalady, J.; Cosmidis, J. Low frequency Raman Spectroscopy for micron-scale and in vivo characterisation of elemental sulphur in microbial samples. Sci. Rep. 2019, 9, 7971–7982. [Google Scholar] [CrossRef]
- Wesełucha-Birczyńska, A.; Toboła, T.; Natkaniec-Nowak, L. Raman microscopy of inclusions in blue halites. Vib. Spectrosc. 2008, 48, 302–307. [Google Scholar] [CrossRef]
- Wesełucha-Birczyńska, A.; Natkaniec-Nowak, L. A Raman microspectroscopic study of organic inclusions in “watermelon” tourmaline from the Paprok mine (Nuristan, Afghanistan). Vib. Spectrosc. 2011, 57, 248–253. [Google Scholar] [CrossRef]
- Wilkins, R.W.T.; Boudou, R.; Sherwood, N.; Xiao, X. Thermal Maturity Evaluation from Inertinites by Raman Spectroscopy: The ‘RaMM’ Technique. Int. J. Coal Geol. 2014, 128–129, 143–152. [Google Scholar] [CrossRef]
- Wilkins, R.W.T.; Wang, M.; Gan, H.; Li, Z. A RaMM Study of Thermal Maturity of Dispersed Organic Matter in Marine Source Rocks. Int. J. Coal Geol. 2015, 150–151, 252–264. [Google Scholar] [CrossRef]
- Wesełucha-Birczyńska, A.; Toboła, T. Hydrocarbon alteration in the bituminous salt of the Kłodawa Salt Dome (Central Poland). Mar. Pet. Geol. 2016, 75, 325–340. [Google Scholar] [CrossRef]
- Hardcastle, F.D. Raman Spectroscopy of Titania (TiO2) Nanotubular Water-Splitting Catalysts. J. Ark. Acad. Sci. (JAAS) 2011, 65, 43–48. [Google Scholar]
- Naglik, B.; Mroczkowska-Szerszeń, M.; Dumańska-Słowik, M.; Natkaniec-Nowak, L.; Drzewicz, P.; Stach, P.; Żukowska, G. Fossil Resins–Constraints from Portable and Laboratory Near-infrared Raman Spectrometers. Minerals 2020, 10, 104. [Google Scholar] [CrossRef]
- Seyfullah, L.J.; Beimforde, C.; Dal Corso, J.; Perrichot, V.; Rikkinen, J.; Schmidt, A.R. Production and preservation of resins—Past and present. Biol. Rev. 2018, 93, 1684–1714. [Google Scholar] [CrossRef]
- UN; ECE. Committee on Sustainable Energy. In International Codification System for Low-Rank Coal Utilization; United Nations: New York, NY, USA; Geneva, Switzerland, 2002. [Google Scholar]
- ISO 11760-2007. Coal Classification/Klasyfikacja Węgla; National Standards Authority in Poland (Polski Komitet Normalizacyjny): Warsaw, Poland, 2007; p. 16. ISBN 978-83-251-2358-1. [Google Scholar]
- Kosmowska-Ceranowicz, B.; Sachanbiński, M.; Łydżba-Kopczyńska, B. Analytical characterization of "Indonesian amber" deposits: Evidence of formation from volcanic activity. Baltica 2017, 30, 55–60. [Google Scholar] [CrossRef]
- Yeates, D.; Grimaldi, D. A new Metatrichia window fly (Diptera: Scenopinidae) in Dominican amber, with a review of the systematics and biogeography of the genus. Am. Mus. Novit. 1993, 3078, 1–8. [Google Scholar]
- Zuparko, R.L.; Poinar, G.O., Jr. Aivalykus dominicanus (Hymenoptera: Braconidae), a new species from Dominican amber. Proc. Entomol. Soc. Wash. 1997, 99, 744–747. [Google Scholar]
- Zavortink, T.J.; Poinar, G.O., Jr. Anopheles (Nyssorhynchus) dominicanus sp. n. (Diptera: Culicidae) from Dominican amber. Ann. Entomol. Soc. Am. 2000, 93, 1230–1235. [Google Scholar] [CrossRef]
- Sinninghe Damste, J.S.; Eglinton, T.I.; de Leeuw, J.W.; Schenck, P.A. Organic sulphur in macromolecular sedimentary organic matter. I. Structure and origin of sulphur-containing moieties in kerogen, asphaltenes and coal as revealed by flash pyrolysis. Geochim. Cosmochim. Acta 1989, 53, 873–889. [Google Scholar] [CrossRef]
- Sinninghe Damste, J.S.; De Leeuw, J.W. Analysis, structure and geochemical significance of organically-bound sulphur in the geosphere: State of the art and future research. Org. Geochem. 1990, 16, 1077–1101. [Google Scholar] [CrossRef]
- Stankiewicz, B.A.; Poinar, H.N.; Briggs, D.E.; Evershed, R.P.; Poinar, G.O., Jr. Chemical preservation of plants and insects in natural resins. Proc. R. Soc. Lond. Part B 1998, 265, 641–647. [Google Scholar] [CrossRef]
- Wittenberg, G.; Danon, A. Disulfide bond formation in chloroplasts. Formation of disulfide bonds in signaling chloroplast proteins. Plant Sci. 2008, 175, 459–466. [Google Scholar] [CrossRef]
- Callegaro, S.; Baker, D.R.; De Min, A.; Marzoli, A.; Geraki, K.; Bertrand, H.; Viti, C.; Nestola, F. Microanalyses link sulphur from large igneous provinces and Mesozoic mass extinctions. Geology 2014, 42, 895–898. [Google Scholar] [CrossRef]
- Kucha, H. Precious metal alloys and organic matter in the Zechstein copper deposits, Poland. Tschermaks Mineral. Petrogr. Mitt. 1981, 28, 1–16. [Google Scholar] [CrossRef]
- Brouwer, S.B.; Brouwer, P.A. Geologia de la region ambarifera oriental de la Republica Dominicana. In Proceedings of the Transactions of the 9th Caribbean Geological Conference, Santo Domingo, Dominican Republic, 15–26 August 1982. [Google Scholar]
- Martín-González, A.; Wierzchos, J.; Gutiérrez, J.C.; Alonso, J.; Ascaso, C. Double fossilization in eukaryotic microorganisms from Lower Cretaceous amber. BMC Biol. 2009, 7, 9. [Google Scholar] [CrossRef]
| Index | Index Significance |
|---|---|
| Gelification index (GI) | macerals gelified to inert tissues |
| Vegetation index (VI) | tissue macerals with resinite to detrital ones with sporite, alginite and cutinite |
| Goundwater horizon location index (GWI) | gelified macerals and minerals to the huminite group macerals |
| Preserved plant tissue index (TPI) | tissue macerals (from the group of huminite and inertinite) to detrital with gelinite |
| Sample | Rro (%) | n | stdv (%) | A | Ast | K | Kst | Rteor (%) | Remp (%) | Z (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Coal from La Cumbre deposit | 0.39 | 110 | 0.03 | 0.4486 | 0.748 | −0.265 | −0.463 | 0.19 | 0.10 | 6.1 |
| Macerals (Code) | 1 | 2 | 3 | 4 | 5 | Average |
|---|---|---|---|---|---|---|
| textinite (T) | 5.6 | 7.3 | 5.2 | 5.4 | 6.0 | 5.9 |
| ulminite (U) | 14.2 | 14.8 | 16.2 | 13.0 | 13.4 | 14.3 |
| corpogelinite (CG) | 1.0 | 0.8 | 0.5 | 1.5 | 1.0 | 1.0 |
| densinite (D) | 8.4 | 8.6 | 9.5 | 7.1 | 8.2 | 8.4 |
| ∑ huminite (H) | 29.2 | 31.5 | 31.4 | 27.0 | 28.6 | 29.5 |
| sporinite (Sp) | rare | rare | rare | rare | rare | rare |
| alginite (Al) | 0.0 | 0.0 | 0.0 | rare | 0.0 | rare |
| resinite (R) | 3.5 | 4.0 | 3.1 | 3.7 | 3.0 | 3.5 |
| fluorinite (Fl) | 1.0 | 0.0 | 0.7 | 1.2 | 2.0 | 1.0 |
| cutinite (K) | 0.5 | 0.5 | 1.0 | 0.0 | 0.5 | 0.5 |
| fluorinite (Fl) | 1.7 | 1.5 | 2.1 | 1.5 | 1.5 | 1.7 |
| liptodetrinite (Ld) | 1.3 | 1.3 | 0.5 | 2.3 | 1.1 | 1.3 |
| ∑ liptinite (L) | 8.0 | 7.3 | 7.4 | 8.7 | 8.1 | 7.9 |
| fusinite (F) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| semifusinite (Sf) | 3.5 | 3.5 | 3.1 | 3.9 | 3.5 | 3.5 |
| funginite+secretinite (Fu+S) | 3.5 | 2.8 | 2.1 | 4.1 | 4.4 | 3.4 |
| macrinite (Ma) | 0.5 | 0.5 | 0.7 | 0.5 | 0.5 | 0.5 |
| inertodetrinite (Id) | 1.1 | 1.0 | 0.8 | 1.5 | 1.2 | 1.1 |
| ∑ inertinite (I) | 8.6 | 7.8 | 6.7 | 10.0 | 9.6 | 8.5 |
| ∑ organic matter (OM) | 45.8 | 46.6 | 45.5 | 45.7 | 46.3 | 46.0 |
| quartz (Q) | 5.2 | 4.9 | 4.7 | 5.3 | 5.7 | 5.2 |
| feldspars (Fs) | 3.8 | 3.5 | 3.9 | 3.0 | 4.5 | 3.7 |
| clay minerals (Clm) | 35.0 | 34.4 | 35.3 | 36.4 | 33.3 | 34.9 |
| pyrite (P) | 8.7 | 9.0 | 9.0 | 8.5 | 8.7 | 8.8 |
| others minerals (Om) | 1.5 | 1.6 | 1.6 | 1.1 | 1.5 | 1.5 |
| ∑ minerals (MM) | 54.2 | 53.4 | 54.5 | 54.3 | 53.7 | 54.0 |
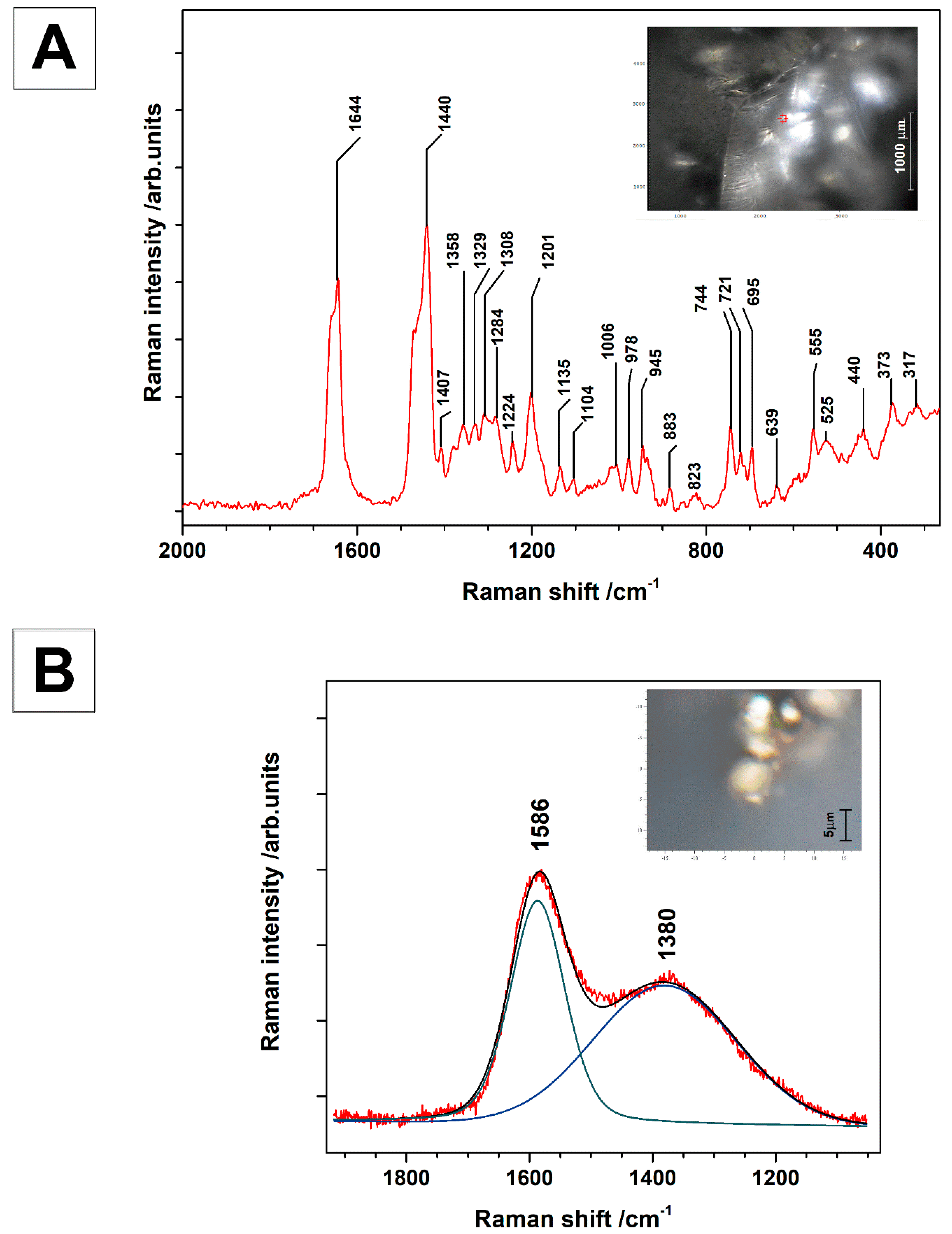
| Raman Bands [cm−1] | Interpretation |
|---|---|
| 1644 | νsym C=C non conjugated |
| 1440 | δ (CH2), δ (CH3) scissors |
| 1407 | δ (CH2), δ (CH3) |
| 1358 | δ (CH2), δ (CH3) |
| 1329 | δ (CH2), δ (CH3) |
| 1308 | δ (CH2), δ (CH3) twisting |
| 1284 | δ (CH2), δ (CH3) |
| 1201 | δ (CCH), δ(C-O) |
| 1135 | ν(CC) ring breathing |
| 1104 | ν(C-C) |
| 1006 | ν(CC) aromatic |
| 978 | ρ(CH2), ρ (CH3) |
| 945 | ρ(CH2), ρ (CH3) |
| 883 | ρ(CH2), ν(COC) |
| 823 | ρ(CH2) |
| 744 | ν(CC) isolated |
| 721 | ν(CC) isolated |
| 695 | ν(CC) isolated |
| 639 | ν(C-S), TiO2 (anatase) |
| 555 | δ (CCO) |
| 525 | δ (COC) in plane deformation, sulfur compounds |
| 440 | sulfur compounds, TiO2 |
| 373 | sulfur compounds, TiO2 |
| 317 | sulfur compounds |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stach, P.; Natkaniec-Nowak, L.; Wagner, M.; Dumańska-Słowik, M.; Mroczkowska-Szerszeń, M.; Wesełucha-Birczyńska, A.; Drzewicz, P.; George, C.; Garcia, E. A Study on the Formation Environment of the La Cumbre Amber Deposit, from Santiago Province, the Northwestern Part of the Dominican Republic. Minerals 2020, 10, 736. https://doi.org/10.3390/min10090736
Stach P, Natkaniec-Nowak L, Wagner M, Dumańska-Słowik M, Mroczkowska-Szerszeń M, Wesełucha-Birczyńska A, Drzewicz P, George C, Garcia E. A Study on the Formation Environment of the La Cumbre Amber Deposit, from Santiago Province, the Northwestern Part of the Dominican Republic. Minerals. 2020; 10(9):736. https://doi.org/10.3390/min10090736
Chicago/Turabian StyleStach, Paweł, Lucyna Natkaniec-Nowak, Marian Wagner, Magdalena Dumańska-Słowik, Maja Mroczkowska-Szerszeń, Aleksandra Wesełucha-Birczyńska, Przemysław Drzewicz, Carlos George, and Edwin Garcia. 2020. "A Study on the Formation Environment of the La Cumbre Amber Deposit, from Santiago Province, the Northwestern Part of the Dominican Republic" Minerals 10, no. 9: 736. https://doi.org/10.3390/min10090736
APA StyleStach, P., Natkaniec-Nowak, L., Wagner, M., Dumańska-Słowik, M., Mroczkowska-Szerszeń, M., Wesełucha-Birczyńska, A., Drzewicz, P., George, C., & Garcia, E. (2020). A Study on the Formation Environment of the La Cumbre Amber Deposit, from Santiago Province, the Northwestern Part of the Dominican Republic. Minerals, 10(9), 736. https://doi.org/10.3390/min10090736







