Fedorite from Murun Alkaline Complex (Russia): Spectroscopy and Crystal Chemical Features
Abstract
1. Introduction
2. Sample Description
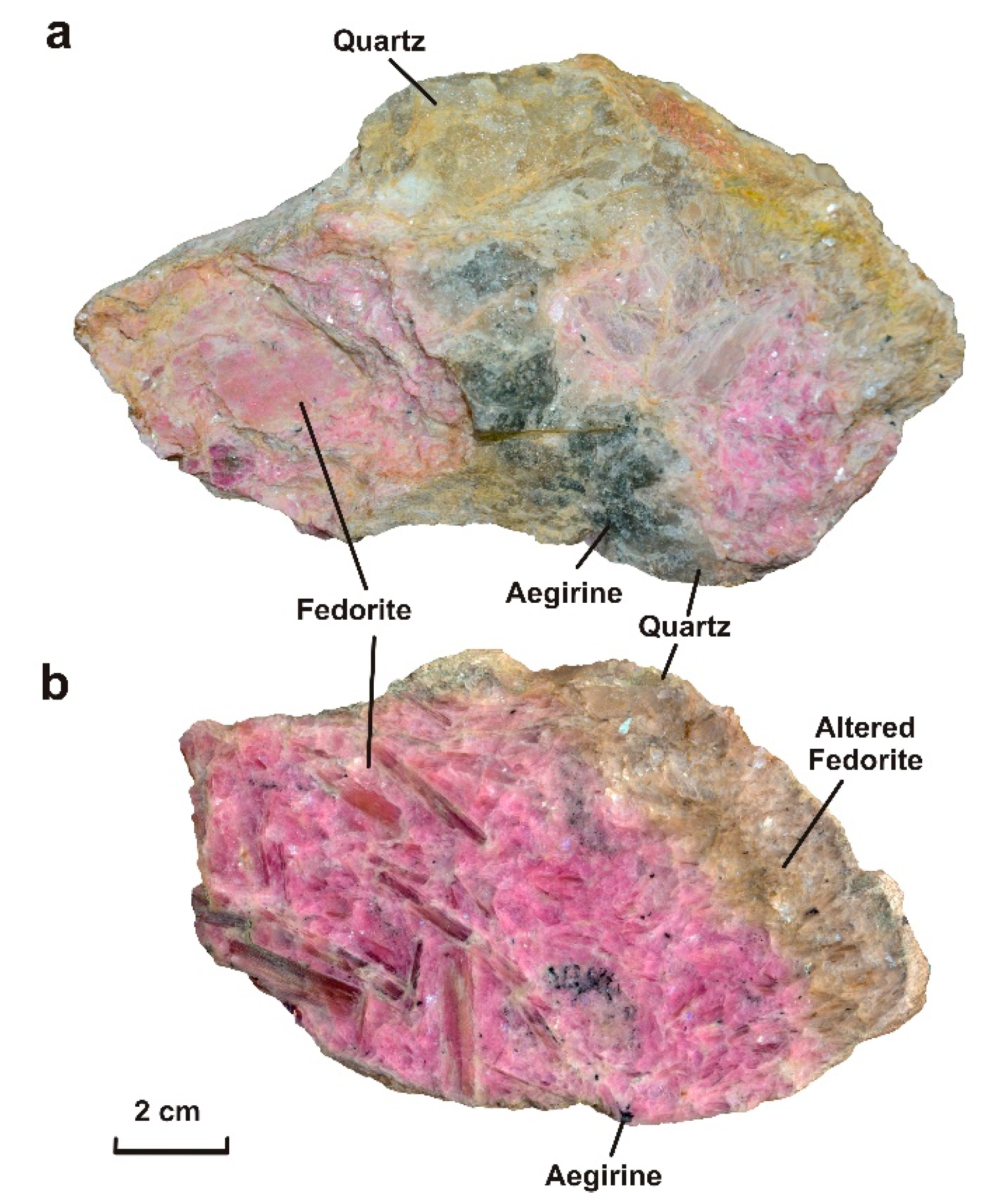
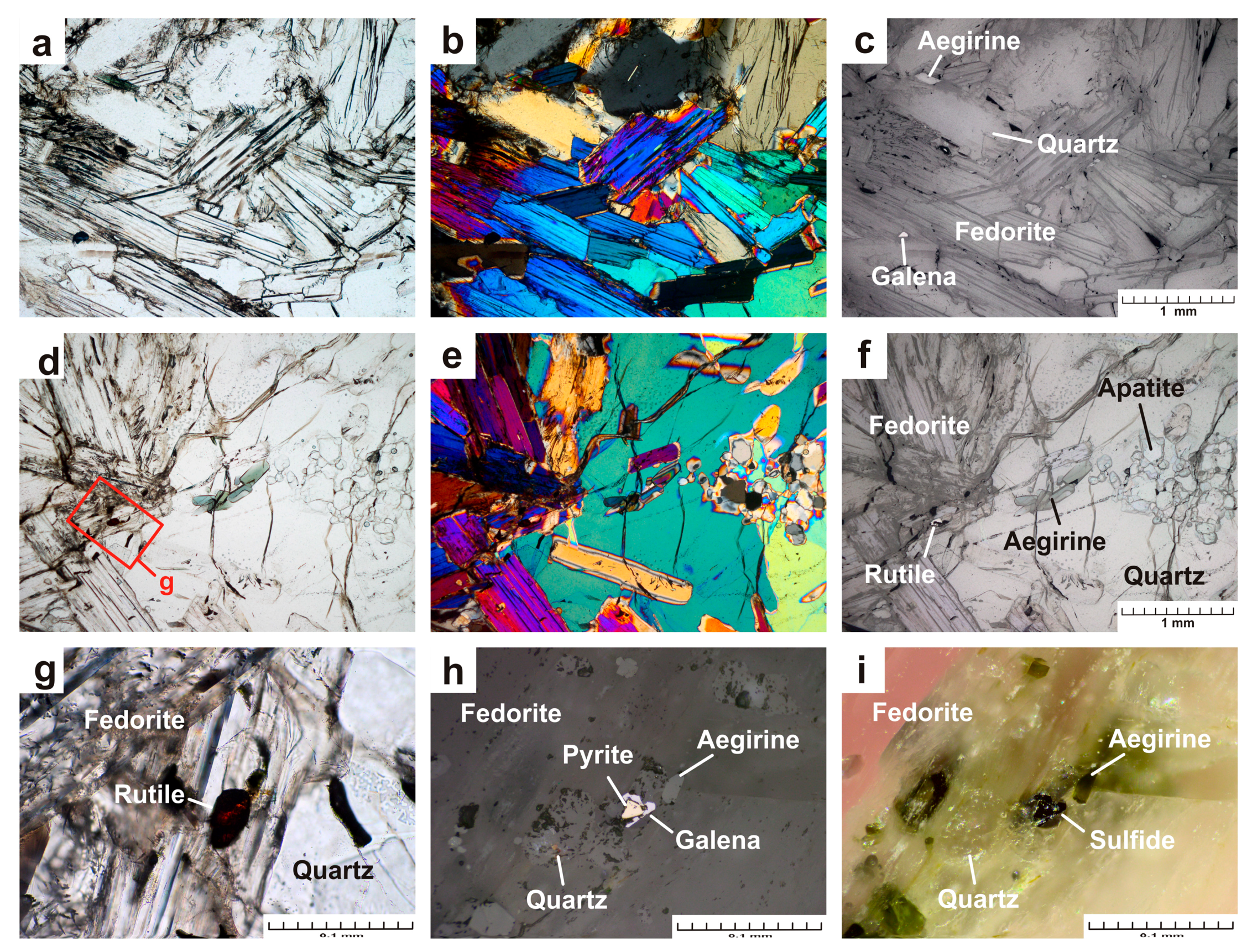
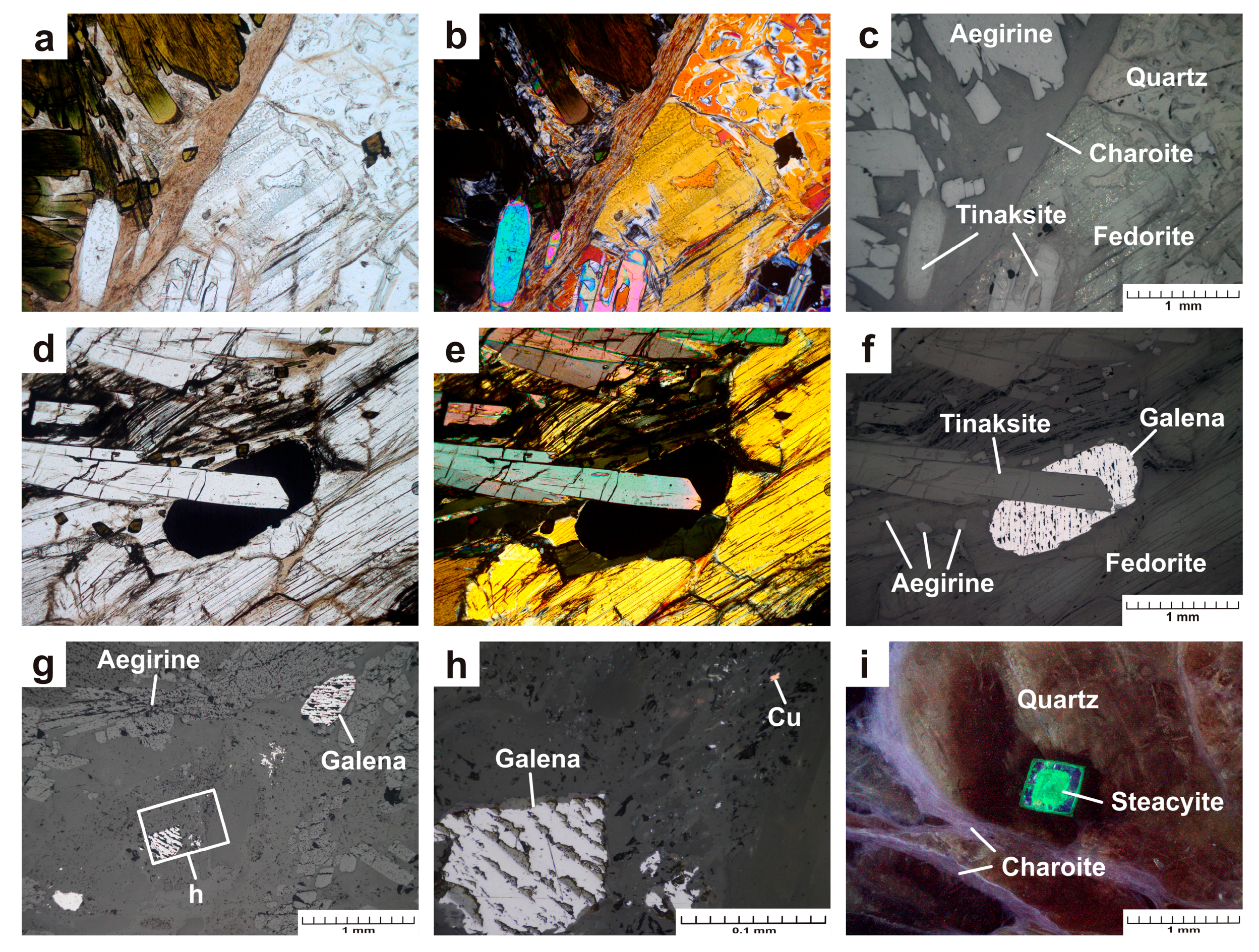
- The sample Gav-43 (Gavrilovskaya zone of Murun complex) is a lenticular fragment (18 × 12 × 9 cm) composed mainly of well-formed large pale raspberry-red to bright mauve crystals of fedorite (70 vol.%), transparent grains of quartz (25 vol.%) and small grains of aegirine (5 vol.%) (Figure 1a,b). Fluorapatite, rutile, galena, pyrite are found in small amounts. Fedorite forms large tabular euhedral and subhedral crystals up to 4.0 × 4.0 × 0.5 cm in size. The mineral exhibits a pearly luster, it is translucent to transparent, with perfect cleavage on {001}. Small inclusions of euhedral crystals of dark green aegirine (up to 0.2 × 0.2 × 0.6 mm in size), fluorapatite (up to 0.07 × 0.07 × 0.15 mm) and rutile (0.02 × 0.02 × 0.03 mm) are found in fedorite (Figure 2 a–g). The single elongated euhedral crystals of aegirine (up to 0.2 × 0.2 × 5 mm in size) are concentrated in transparent quartz grains in the central part of the polished sample. Less commonly, aegirine forms aggregates. (Figure 2 a–c). Quartz is xenomorphic with respect to fedorite. Anhedral quartz grains are transparent. They contain numerous small crystals of aegirine (0.3 × 0.3 × 0.7 mm in size) and fluorapatite (up to 0.1 × 0.1 × 0.25 mm in size). Rarely, sulfide grains (galena and pyrite up to 0.02 mm in size) can be found in quartz (Figure 2h,i).
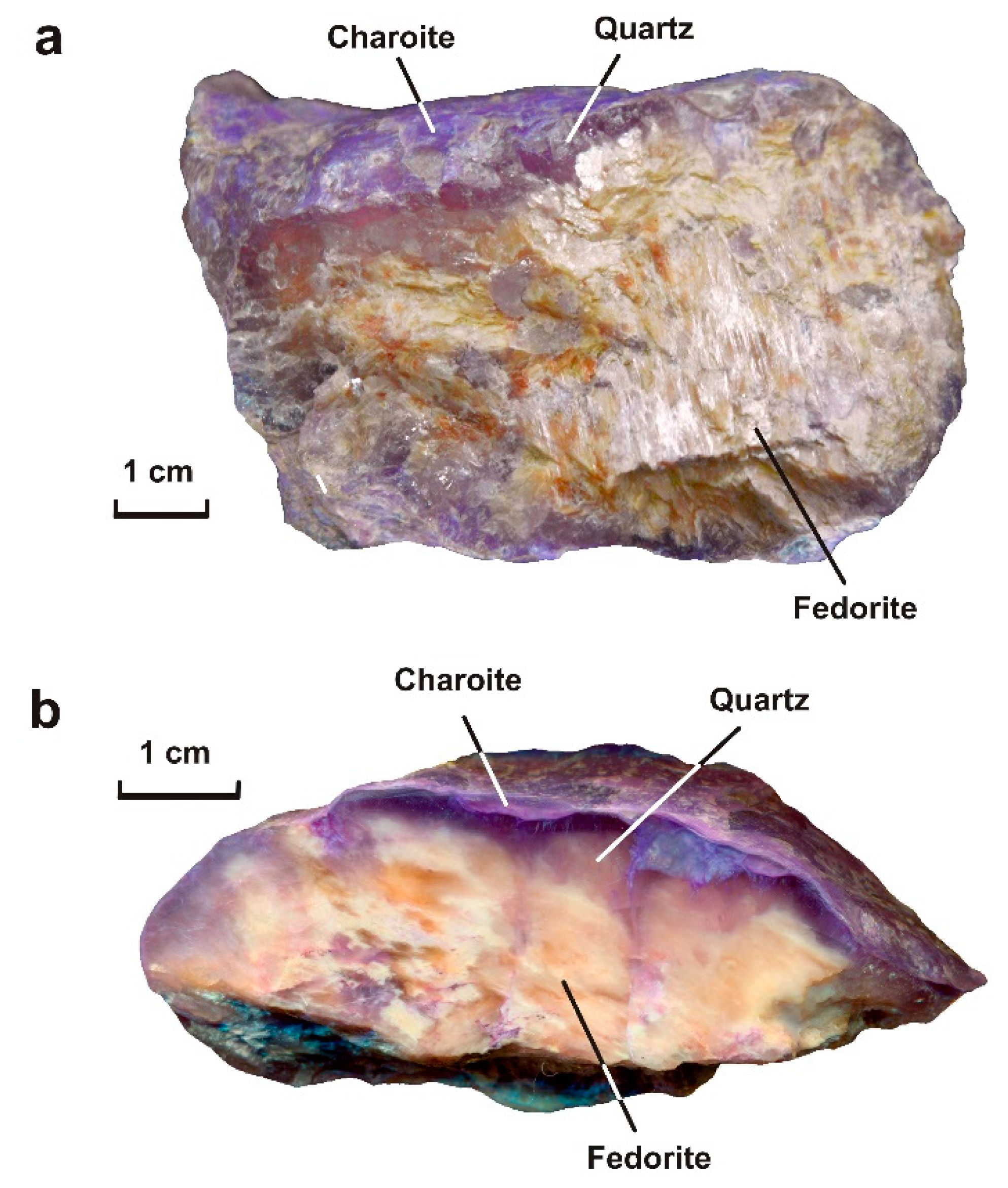
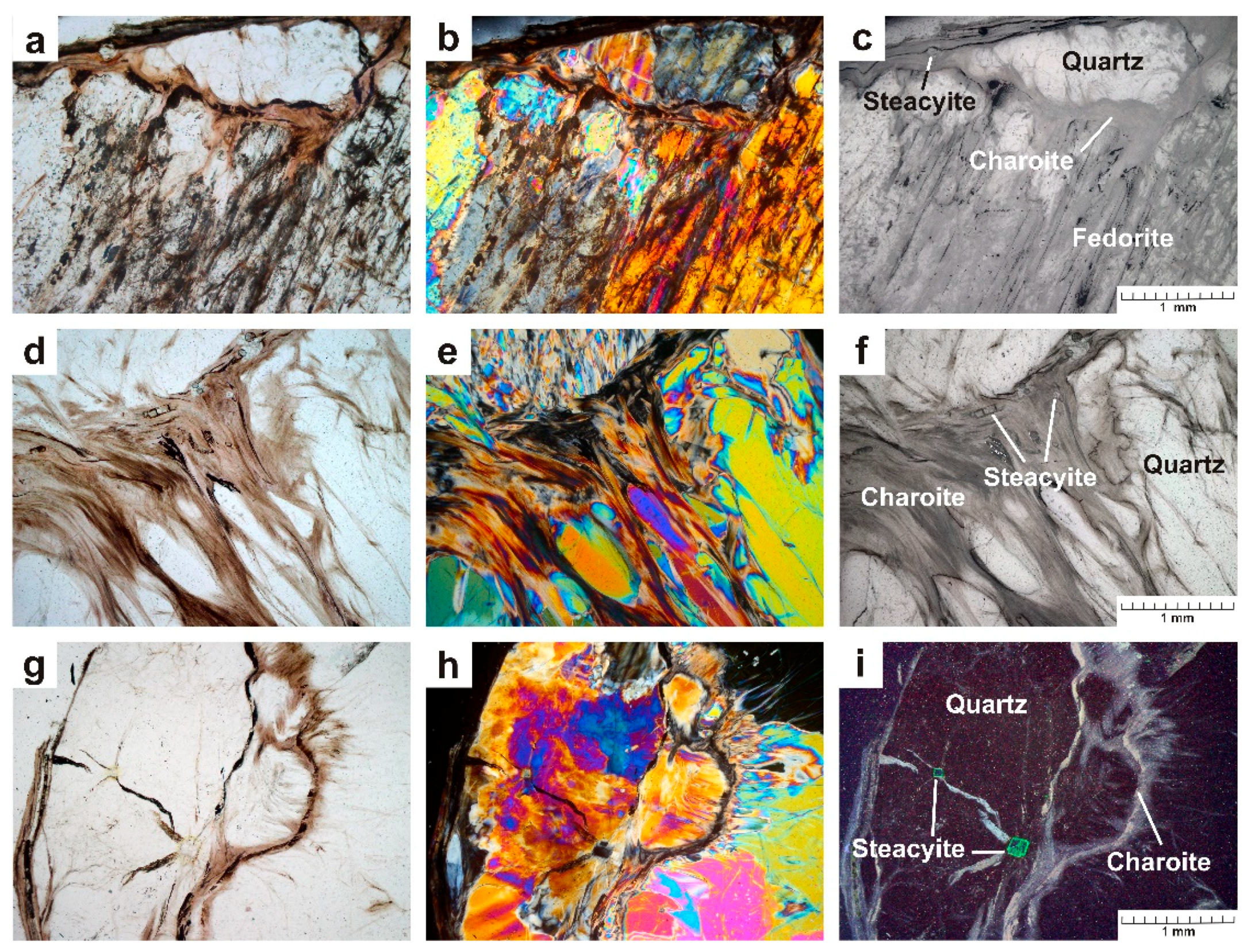
- The sample Yak-5 (Yakutskiy district) is a lenticular fragment (8 × 6 × 3 cm) consisting of fedorite in the central part (60 vol.%) and quartz (20 vol.%), charoite (20 vol.%) and microcline (single grains) on the periphery (Figure 3a,b). A thin layer of charoite covers the microcline–quartz–fedorite aggregate. At the same time, thin veins of charoite are also found in the central part. Steacyite and fluorapatite are present in the sample in small amounts. Fedorite forms tabular subhedral and anhedral crystals (up to 5.0 × 4.5 mm in size) of a pale raspberry to pale brown color with a pearly luster. The mineral is translucent to transparent, cleavage is perfect on {001}. Small inclusions of euhedral fluorapatite crystals (up to 0.3 × 0.3 × 0.8 mm) are found in fedorite. Fedorite is partially altered and replaced by secondary minerals (Figure 4a–c). The microcline is found in the form of single tabular white anhedral grains (up to 1 mm in size). Quartz is observed in the form of transparent lenticular or anhedral grains (up to 10 mm in size). These grains form a rim (wide of 4–10 mm) around fedorite, interrupted by thin veins of charoite (Figure 4a–i). Quartz is xenomorphic with respect to fedorite. Thin fibrous crystals of charoite and euhedral grains of steacyite (up to 0.2 × 0.2 × 0.5 mm in size) were found in quartz. Radial fractures are observed in quartz around the steacyite grains, which were formed as a result of radiation damage (Figure 4g–i). Charoite is a cryptocrystalline aggregate of lilac color with a pinkish tint and a silky luster. The elongated fibrous crystals form a shell that surrounds the quartz-fedorite lens and individual quartz grains. Hypoidiomorphic steacyite grains (up to 0.1 × 0.1 × 0.3 mm in size) are elongated along charoite veins (Figure 4a–f).
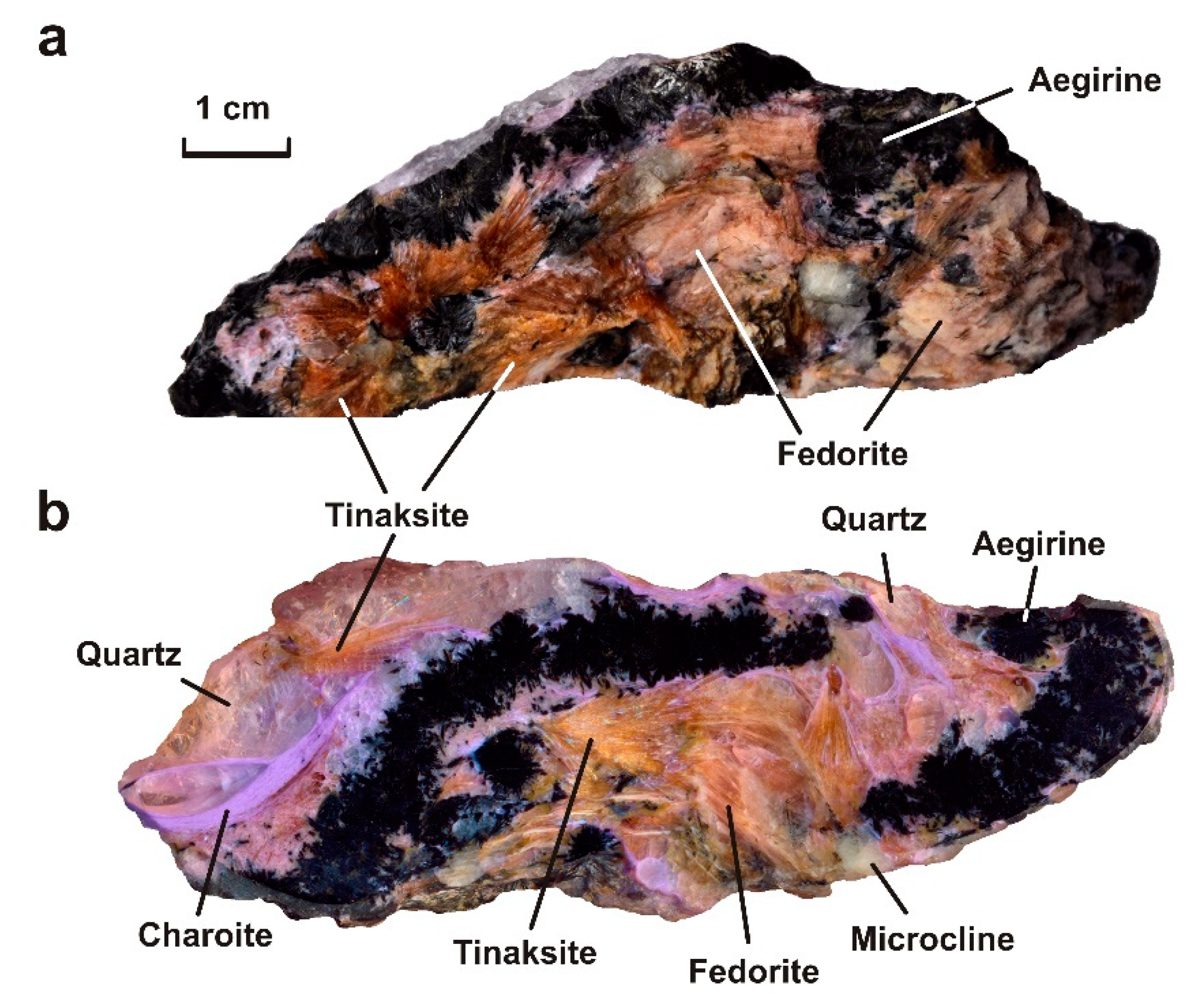
- The sample Irk-53 (Irkutskiy district) is characterized by the greatest mineral diversity. It is a lenticular fragment (10 × 6 × 3.5 cm) consisting of the following minerals (in vol.%): aegirine (25), tinaksite (15), fedorite (10), microcline (2), quartz (33), charoite (15) (Figure 5a,b). Steacyite, galena and native copper are present in the sample in small amounts. Aegirine is represented by dark green elongated euhedral and subhedral crystals (up to 0.2 × 2.5 mm in size) with vitreous luster (Figure 5a–c). The crystals form sheaf-like and radially radiant aggregates with a diameter of up to 5 mm. Tinaksite is represented by elongated euhedral and subhedral light brown crystals (up to 0.3 × 15 mm) with a vitreous luster (Figure 6a–f). Crystals form sheaf-like aggregates up to 15 mm in length. Fedorite forms tabular subhedral pale raspberry-red crystals (up to 0.8 × 1.5 mm) with a pearly luster (Figure 6a–f). The mineral is translucent to transparent with perfect cleavage on {001}. The microcline is found in the form of single tabular anhedral greenish grains (up to 5 mm in size). Quartz is observed in the form of transparent lenticular or anhedral grains (up to 10 mm in size) with a greasy luster. Quartz is xenomorphic with respect to aegirine, tinaksite and fedorite. Charoite is a cryptocrystalline aggregate of lilac color with a silky luster. Elongated fibrous crystals (less than 1 μm thick) form aggregates that surround earlier minerals (Figure 5a,b and Figure 6a–c). Separate charoite fibers were found in quartz in the form of inclusions that indicates a close crystallization time of these minerals. Steacyite is found in paragenesis with charoite and quartz. Euhedral transparent tetragonal yellowish-brownish crystals of steacyite reach 0.5 × 0.5 × 12 mm in size. Moreover, its grains observed in quartz are larger and have a higher degree of euhedrality than steacyite grains in charoite. Galena is found in the form of elongated subhedral and euhedral grains (up to 1.2 × 0.6 mm) in fedorite and quartz (Figure 6d–h). Small grains of native copper (up to 0.01 mm in size) were also found in quartz (Figure 6h).
3. Materials and Methods
3.1. Chemical Analysis
3.2. Single-Crystal X-ray Diffraction Study
3.3. Polyhedra Characteristics Calculation
3.4. Spectroscopy Study
4. Results and Discussion
4.1. Chemical Composition
4.2. Structural Features
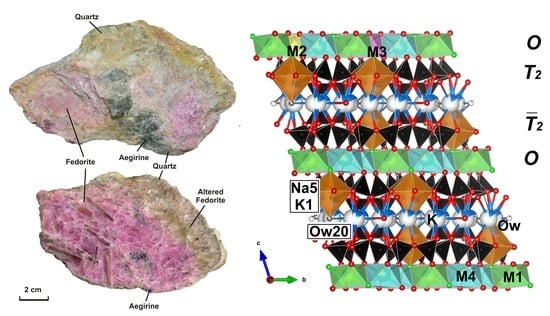
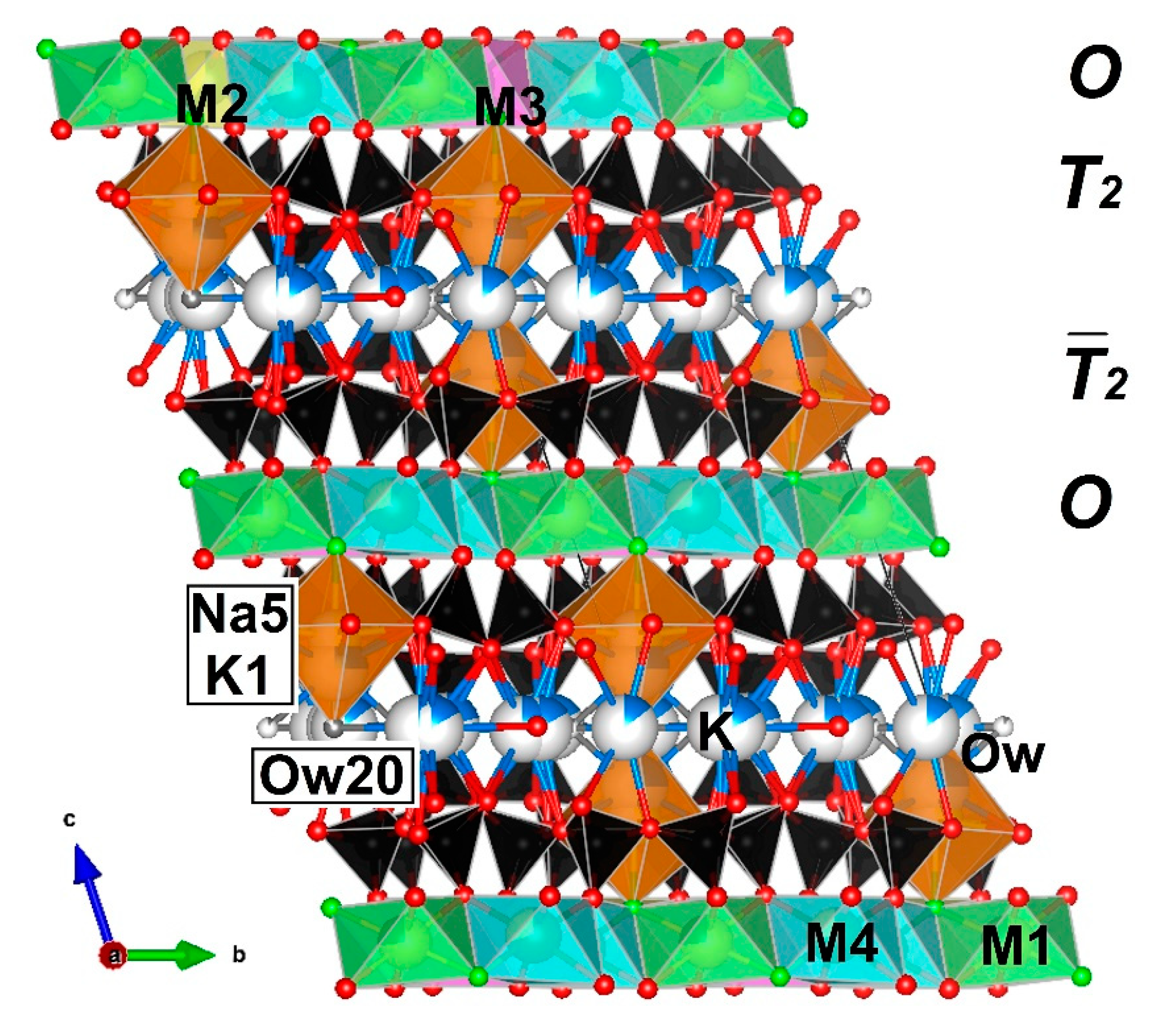
4.2.1. Si-Tetrahedra (T-Sheets)
4.2.2. M-Octahedra (O-Sheets)
4.2.3. Interlayer Cations (A) and H2O Molecules
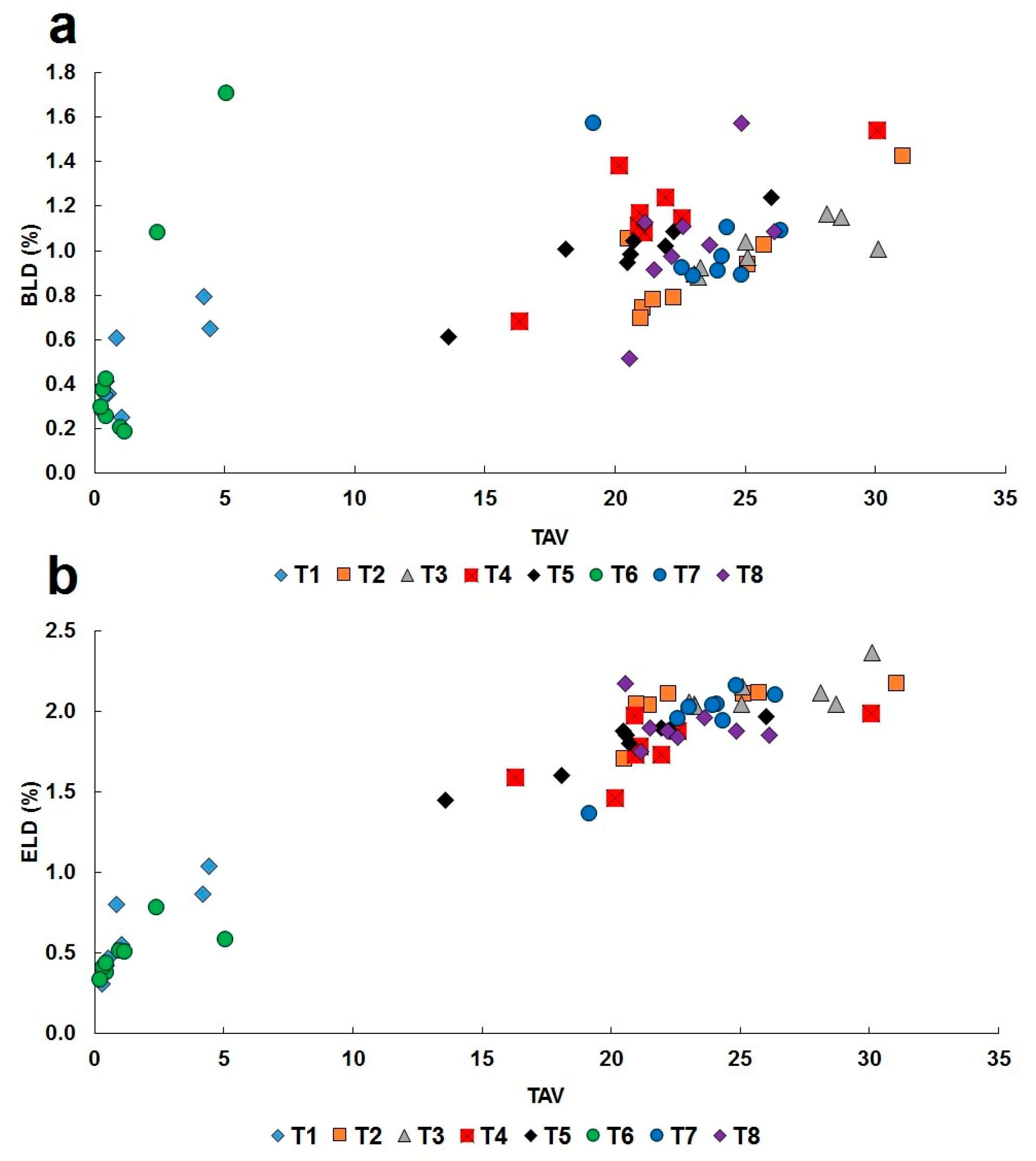
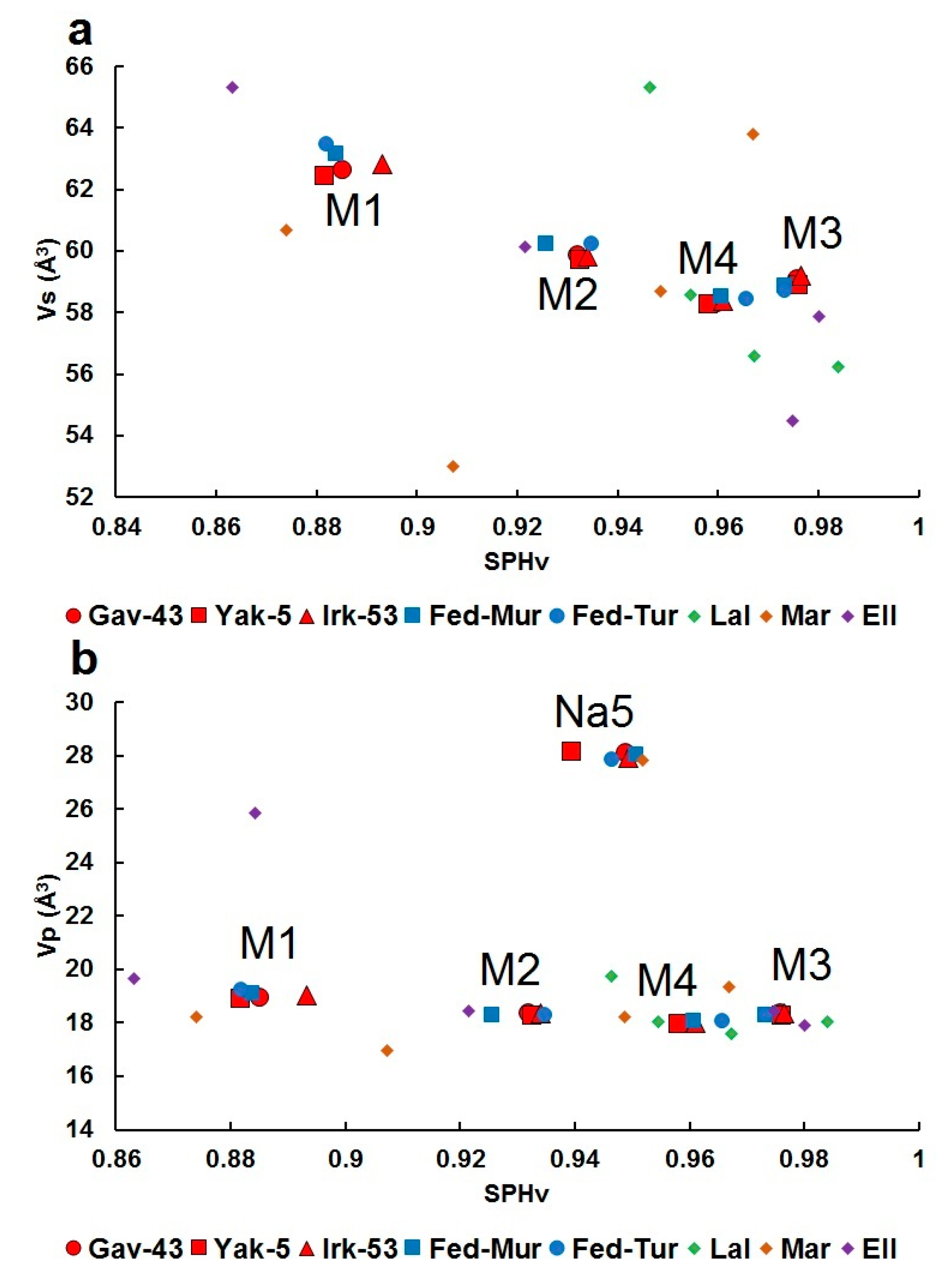
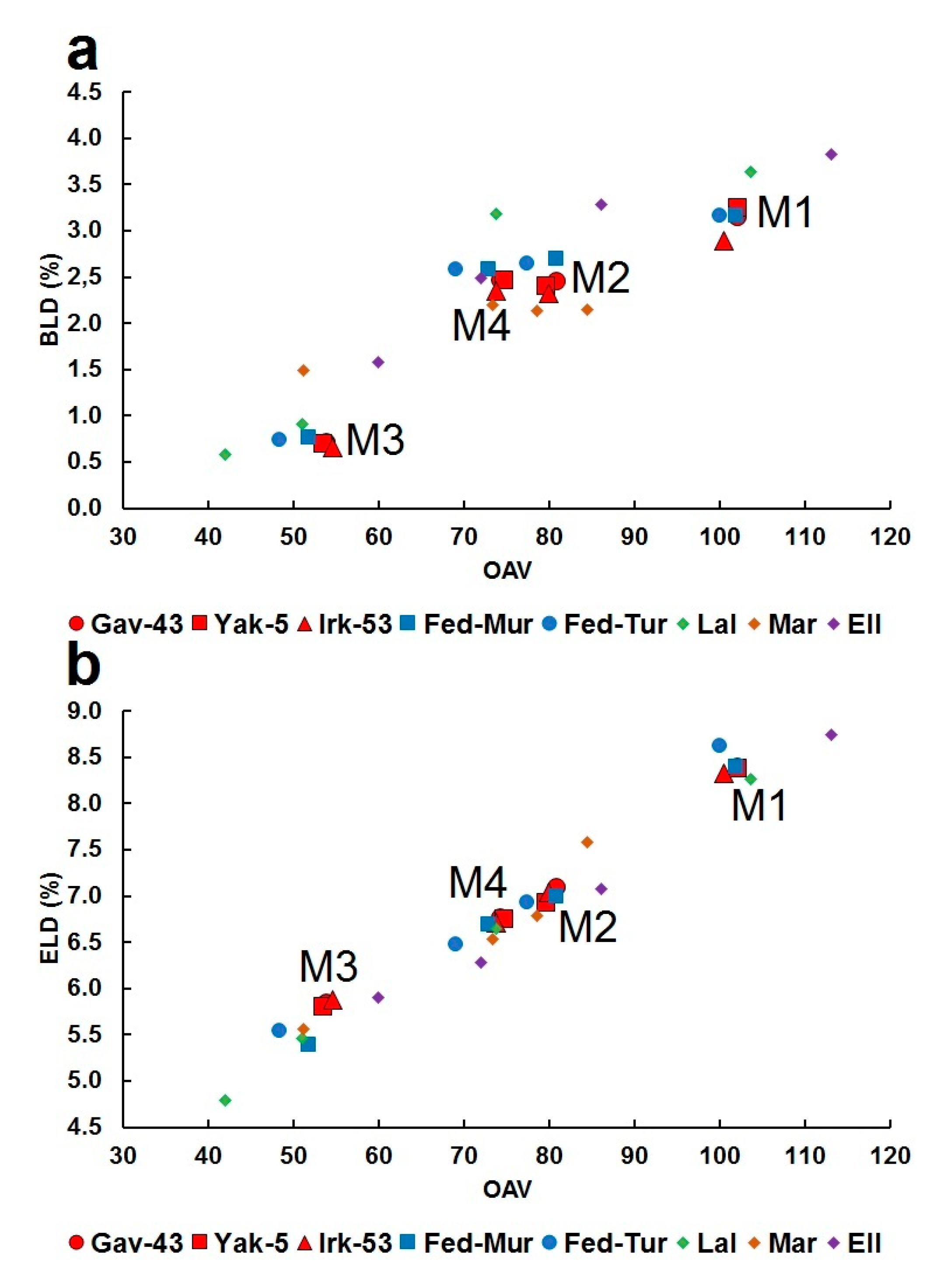
4.3. Structural Relationship between Fedorite, Lalondeite, Martinite and Ellingsenite, Comparison of Polyhedral Characteristics and Deformation of Coordination Polyhedra
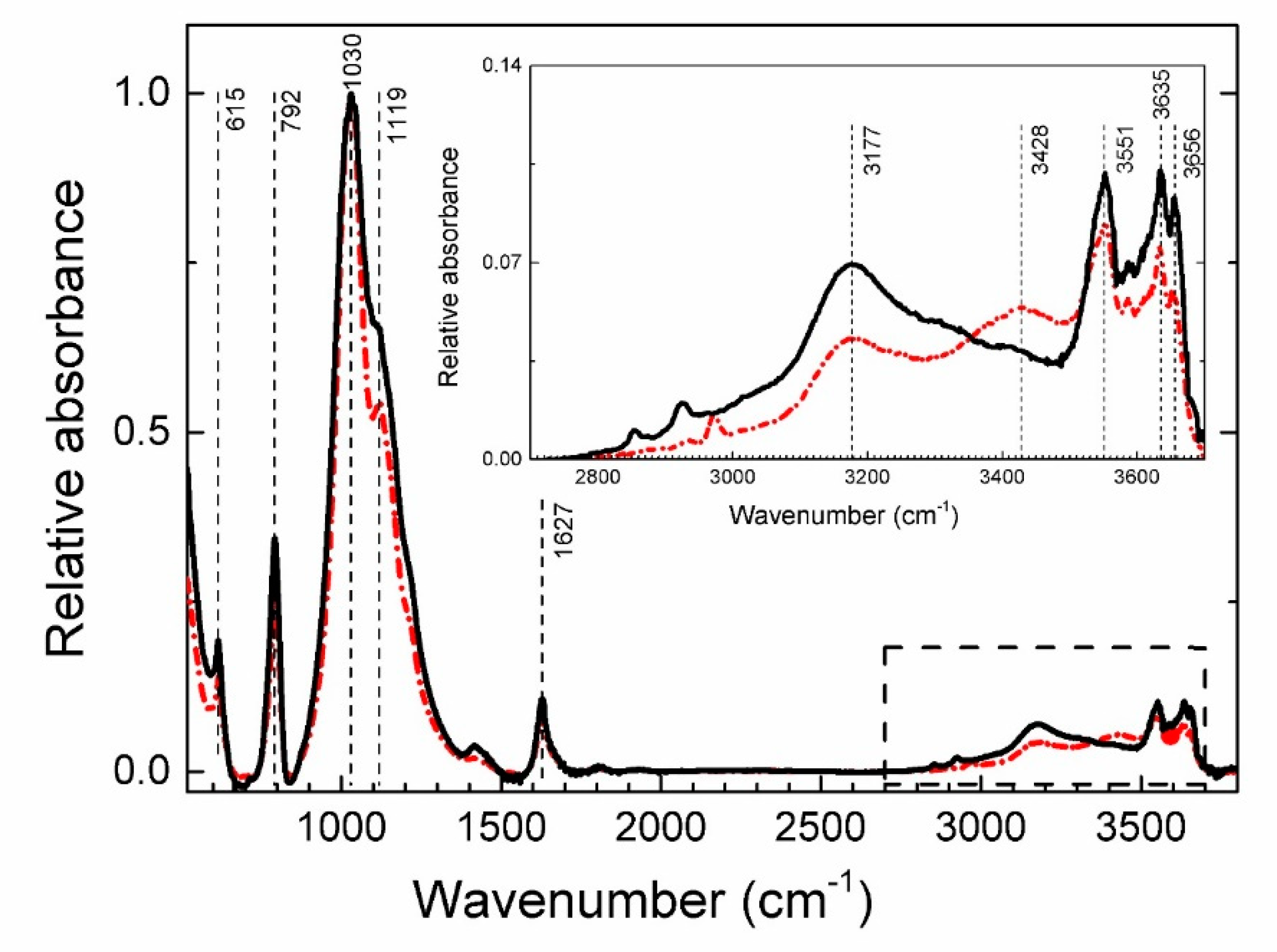
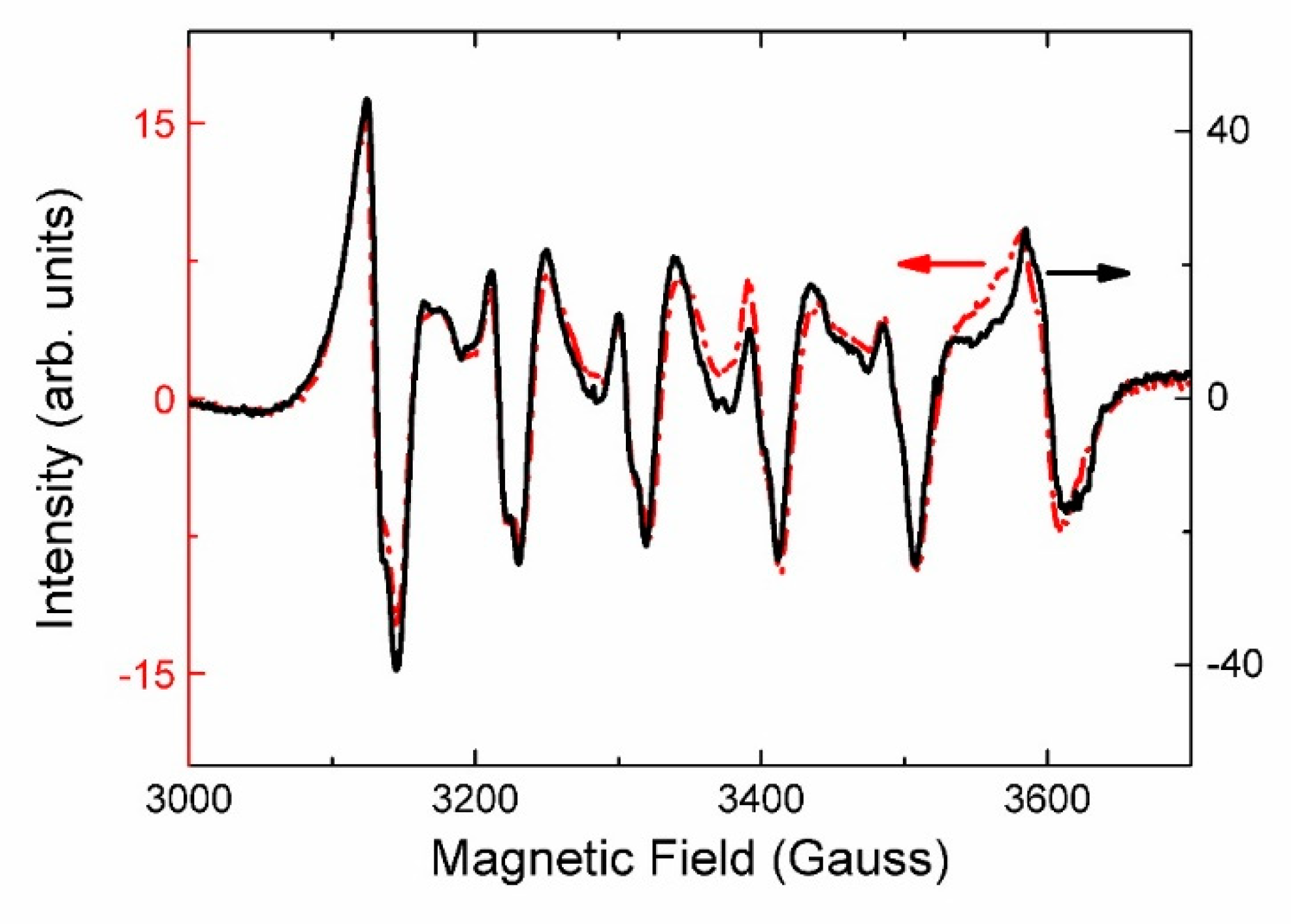
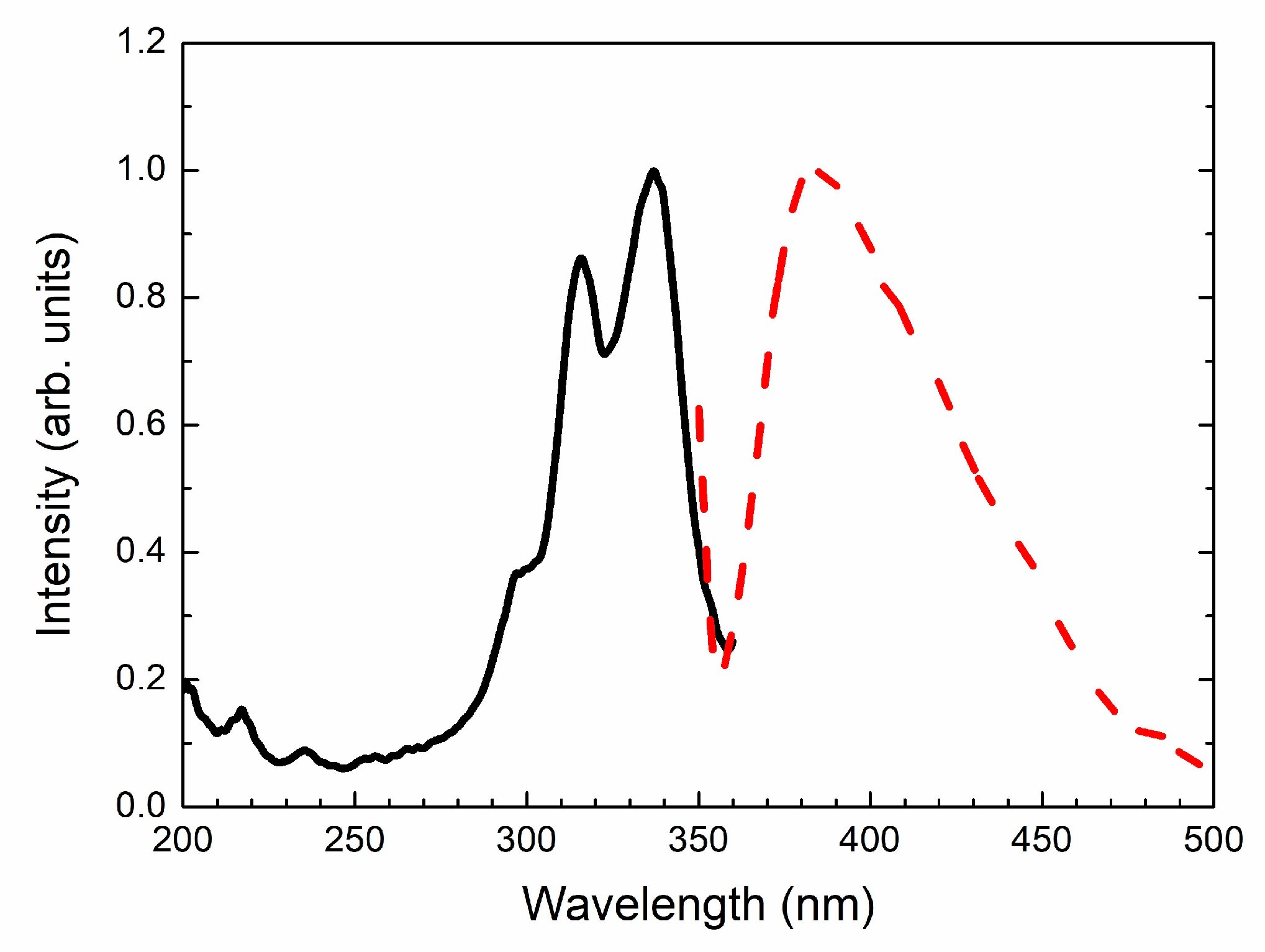
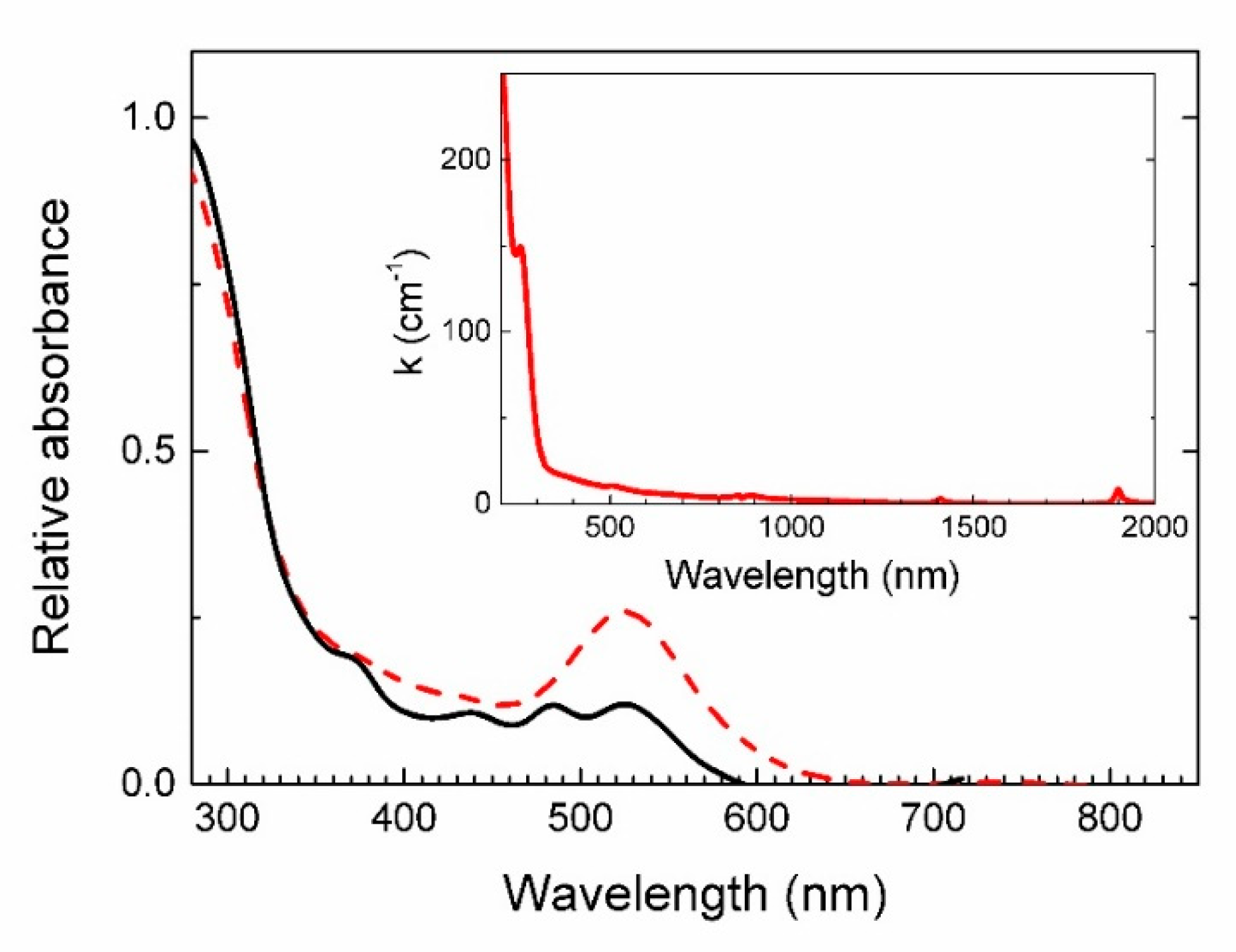
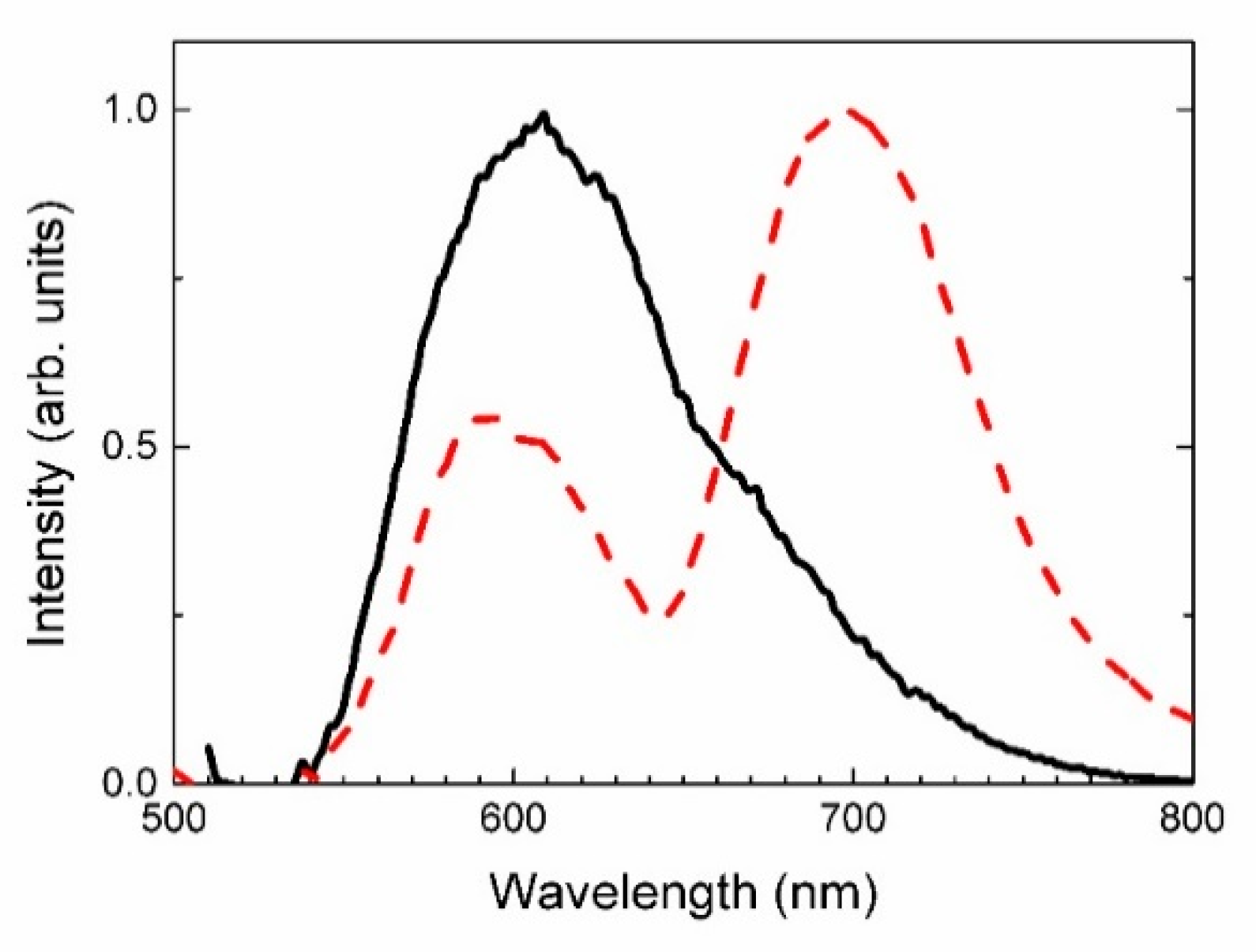
4.4. Spectroscopy
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kukharenko, A.A.; Orlova, M.P.; Bulakh, A.G.; Bagdasarov, E.A.; Rimskaya-Korsakova, O.M.; Nefedov, E.I.; Il’inskii, G.A.; Sergeev, A.S.; Abakumova, N.B. Caledonian Complex of Ultrabasic, Alkaline Rocks and Carbonatites of the Kola Peninsula and North Karelia; Nedra: Moscow, Russia, 1965. [Google Scholar]
- Khomyakov, A.P.; Lapin, A.V.; Kazakov, M.E. New Data on Mineralogy of Deposits of Alkaline Formations; IMGRE: Moscow, Russia, 1979; pp. 3–7. [Google Scholar]
- Sokolova, G.V.; Kashaev, A.A.; Drits, V.A.; Ilyukhin, V.V. The crystal structure of fedorite. Sov. Phys. Cryst. 1983, 28, 95–97. [Google Scholar]
- Joswig, W.; Drits, V.A.; Sokolova, G.V. Refinement of the structure of fedorite. Sov. Phys. Cryst. 1988, 33, 763–765. [Google Scholar]
- Konev, A.A.; Vorobyev, Y.I.; Bulakh, A.G. Charoit–der Schmuckstein aus Sibirien und seine seltenen Begleit-minerale. Lapis 1993, 18, 13–20. [Google Scholar]
- Konev, A.A.; Vorob’ev, E.I.; Lazebnik, K.A. Mineralogy of Murun massif. Sib. Branch Russ. Acad. Sci. 1996, 111–113. [Google Scholar]
- Mitchell, R.H.; Burns, P.C. The structure of fedorite: A re-appraisal. Can. Mineral. 2001, 39, 769–777. [Google Scholar] [CrossRef][Green Version]
- Hawthorne, F.C.; Uvarova, Y.A.; Sokolova, E. A structure hierarchy for silicate minerals: Sheet silicates. Mineral. Mag. 2019, 83, 3–55. [Google Scholar] [CrossRef]
- Bonaccorsi, E.; Merlino, S. Modular microporous minerals: Cancrinite-davine group and C-S-H phases. Rev. Mineral. Geochem. 2005, 57, 241–290. [Google Scholar] [CrossRef]
- Ferraris, G. Polysomatism as a tool for correlating properties and structure. In Modular Aspects of Minerals; Merlino, S., Ed.; Eotvos University Press: Budapest, Hungary, 1997. [Google Scholar]
- Ferraris, G.; Gula, A. Polysomatic aspects of microporous minerals—Heterophyllosilicates, palysepioles and rhodesite-related structures. Rev. Mineral. Geochem. 2005, 57, 69–104. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Aksenov, S.M. Crystal chemistry of silicates with three-layer TOT and HOH modules of layered, chainlike, and mixed types. Cryst. Rep. 2011, 56, 910–934. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Vladykin, N.V.; Demonterova, E.I.; Gorovoy, V.A.; Dokuchits, E.Y. 40Ar/39Ar geochronology of the Malyy (Little) Murun massif, Aldan shield of the Siberian craton: A simple story for an intricate igneous complex. Minerals 2018, 8, 602. [Google Scholar] [CrossRef]
- Bruker APEX2; Version 2014.11-0; Bruker AXS Inc.: Madison, WI, USA, 2014.
- Bruker SAINT; Version V8.34; Bruker AXS Inc.: Madison, WI, USA, 2013.
- CrysAlis, PRO.; Version 1.171.35.21; Agilent Technologies Ltd.: Yarnton, UK, 2018.
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 2003. [Google Scholar]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. Crystals version 12: Software for guided crystal structure analysis. J. App. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Cryst. 1985, B41, 244–247. [Google Scholar] [CrossRef]
- Gagnè, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Cryst. 2015, B71, 562–578. [Google Scholar] [CrossRef]
- Breese, N.E.; O’Keeffe, M. Bond-valence parameters for solid. Acta Cryst. 1991, B47, 192–197. [Google Scholar] [CrossRef]
- Hoppe, R. Effective coordination numbers (ECoN) and mean fictive ionic radii (MEFIR). Z. Krist. Cryst. Mater. 1979, 150, 23–52. [Google Scholar] [CrossRef]
- Hoppe, R.; Voigt, S.; Glaum, H.; Kissel, J.; Müller, H.P.; Bernet, K. A new route to charge distributions in ionic solids. J. Less Common. Met. 1989, 156, 105–122. [Google Scholar] [CrossRef]
- Nespolo, M.; Ferraris, G.; Ohashi, H. Charge distribution as a tool to investigate structural details: Meaning and application to pyroxenes. Acta Cryst. 1999, B55, 902–916. [Google Scholar] [CrossRef]
- Brown, I.D. The Chemical Bond in Inorganic Chemistry: The Bond Valence Model; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Balić-Žunić, T.; Makovicky, E. Determination of the centroid or ‘the best centre’ of a coordination polyhedron. Acta Cryst. 1996, B52, 78–81. [Google Scholar] [CrossRef]
- Balić-Žunić, T.; Vicković, I. IVTON—Program for the calculation of geometrical aspects of crystal structures and some crystal chemical applications. J. Appl. Cryst. 1996, 29, 305–306. [Google Scholar] [CrossRef]
- Makovicky, E.; Balić-Žunić, T. New measure of distortion for coordination polyhedral. Acta Cryst. 1998, B54, 766–773. [Google Scholar] [CrossRef]
- Renner, B.; Lehmann, G. Correlation of angular and bond length distortions in TO4 units in crystals. Z. für Kristallogr. 1986, 175, 43–59. [Google Scholar]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic elongation: A quantitative measure of distortion in coordination polyhedra. Science 1971, 172, 567–570. [Google Scholar] [CrossRef]
- Kaneva, E.; Shendrik, R.; Mesto, E.; Bogdanov, A.; Vladykin, N. Spectroscopy and crystal chemical properties of NaCa2[Si4O10]F natural agrellite with tubular structure. Chem. Phys. Lett. 2020, 136868. [Google Scholar] [CrossRef]
- Sofich, D.; Tushinova, Y.L.; Shendrik, R.; Bazarov, B.G.; Dorzhieva, S.G.; Chimitova, O.D.; Bazarova, J.G. Optical spectroscopy of molybdates with composition Ln2Zr3(MoO4)9 (Ln: Eu, Tb). Opt. Mater. 2018, 81, 71–77. [Google Scholar] [CrossRef]
- Kaneva, E.; Lacalamita, M.; Mesto, E.; Schingaro, E.; Scordari, F.; Vladykin, N. Structure and modeling of disorder in miserite from the Murun (Russia) and Dara-i-Pioz (Tajikistan) massifs. Phys. Chem. Miner. 2014, 41, 49–63. [Google Scholar] [CrossRef]
- Lacalamita, M.; Mesto, E.; Kaneva, E.; Scordari, F.; Pedrazzi, G.; Vladykin, N.; Schingaro, E. Structure refinement and crystal chemistry of tokkoite and tinaksite from the Murun massif (Russia). Mineral. Mag. 2017, 81, 251–272. [Google Scholar] [CrossRef]
- Kaneva, E.; Radomskaya, T.; Suvorova, L.; Sterkhova, I.; Mitichkin, M. Crystal chemistry of fluorcarletonite, a new mineral from the Murun alkaline complex (Russia). Eur. J. Miner. 2020, 32, 137–146. [Google Scholar] [CrossRef]
- Rozhdestvenskaya, I.V.; Nikishova, L.V.; Lazebnik, K.A. The crystal structure of frankamenite. Mineral. Mag. 1996, 60, 897–905. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- McDonald, A.M.; Chao, G.Y. Lalondeite, a new hydrated Na-Ca fluorosilicate species from Mont Saint-Hilaire, Quebec: Description and crystal structure. Can. Mineral. 2009, 47, 181–191. [Google Scholar] [CrossRef]
- McDonald, A.M.; Chao, G.Y. Martinite, a new hydrated sodium calcium fluorborosilicate species from Mont Saint-Hilaire, Quebec: Description, structure determination and genetic implication. Can. Mineral. 2007, 45, 1281–1292. [Google Scholar] [CrossRef]
- Yakovenchuk, V.N.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Selivanova, E.A.; Mikhailova, J.A.; Krivovichev, S.V.; Zolotarev, A.A.; Zalkind, O.A. Ellingsenite, Na5Ca6Si18O38(OH)13·6H2O, a new martinite-related mineral species from phonolite of the Aris alkaline complex, Namibia. Can. Mineral. 2011, 49, 1099–1107. [Google Scholar] [CrossRef]
- Yarovoy, P.N.; Konev, A.A.; Serykh, S.V. Nature of the luminescence of certain minerals from the Murun alkalic massif. Dokl. Earth Sci. 1989, 304, 226–229. [Google Scholar]
- Czaja, M.; Lisiecki, R. Luminescence of agrellite specimen from Kipawa River locality. Minerals 2019, 9, 752. [Google Scholar] [CrossRef]
- Shendrik, R.Y.; Kovalev, I.I.; Rusakov, A.I.; Solol’nikova, Y.V.; Shalaev, A.A. Luminescence of BaBrI crystals doped with Ce3+ ions. Phys. Solid State 2019, 61, 830–834. [Google Scholar] [CrossRef]
- Zhou, O.; Dolgov, L.; Srivastava, A.M.; Zhou, L.; Wang, Z.; Shi, J.; Dramićanin, M.D.; Brik, M.G.; Wu, M. Mn2+ and Mn4+ red phosphors: Synthesis, luminescence and applications in WLEDs. A review. J. Mater. Chem. C 2018, 11, 2652–2671. [Google Scholar] [CrossRef]
- Yarovoy, P.N. Laser-induced Luminescence Identification of Materials. 1996. Available online: https://luminescence.csiro.au/ (accessed on 4 August 2020).
- Yarovoy, P.N.; Badenikov, V.Y. Luminescence and Interaction of Laser Irradiation with Dielectrics; Irkutsk State Technical University: Irkutsk, Russia, 2004. [Google Scholar]
- Yarovoy, P.N. Luminescence and energy conversion in minerals, ceramics and catalysts under laser and X-ray excitation. Doctoral Thesis, Tomsk Polytechnic University, Tomsk, Russia, 2000. [Google Scholar]
- Hasegawa, T.; Nishiwaki, Y.; Fujishiro, F.; Kamei, S.; Ueda, T. Quantitative determination of the effective Mn4+ concentration in a Li2TiO3:Mn4+ phosphor and its effect on the photoluminescence efficiency of deep red emission. ACS Omega 2019, 4, 19856–19862. [Google Scholar] [CrossRef]
- Khaidukov, N.M.; Brekhovskikh, M.N.; Kirikova, N.Y.; Kondratyuk, V.A.; Makhov, V.N. Luminescence of MgAl2O4 and ZnAl2O4 spinel ceramics containing some 3d ions. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Yang, L.; Hu, S.; Wang, Z.; Jing, Z.; Nian, H.; Liu, B.; Zhou, G.; Wang, S. Preparation and luminescence properties of thermally stable Mn4+ doped spinel red-emitted ceramic phosphors. J. Lumin. 2020, 220, 117016. [Google Scholar] [CrossRef]
- Tanabe, Y.; Sugano, S. On the absorption spectra of complex ions II. J. Phys. Soc. Jpn. 1954, 9, 766–779. [Google Scholar] [CrossRef]
- Gaft, M.; Nagli, L.; Panczer, G.; Rossman, G.R.; Reisfeld, R. Laser-induced time-resolved luminescence of orange kyanite Al2SiO5. Opt. Mater. 2011, 33, 1476–1480. [Google Scholar] [CrossRef]
- Vladykin, N.V.; Borokovikov, A.A.; Dokuchits, E.Y.; Thomas, V.G. Genesis of charoite rocks in the Murun massif, Aldan shield, Russia. Geochem. Int. 2018, 56, 1135–1147. [Google Scholar] [CrossRef]
- Borovikov, A.A.; Vladykin, N.V.; Tretiakova, I.G.; Dokuchits, E.Y. Physicochemical conditions of formation of hydrothermal titanium mineralization on the Murunskiy alkaline massif, western Aldan (Russia). Ore Geol. Rev. 2018, 95, 1066–1075. [Google Scholar] [CrossRef]
- Shaw, S.; Henderson, M.B.; Clark, S. In-situ synchrotron study of the kinetics, thermodynamics, and reaction mechanisms of the hydrothermal crystallization of gyrolite, Ca16Si24O60(OH)8·14H2O. Am. Mineral. 2002, 87, 533–541. [Google Scholar] [CrossRef]
| Constituent | Gav-43 | Yak-5 | Irk-53 | Fedorite, Murun [7] | Fedorite, Turiy [7] |
| SiO2 | 66.9(6) | 67.1(4) | 67.4(9) | 66.60(28) | 67.02(63) |
| Al2O3 | 0.07(4) | 0.08(4) | 0.07(5) | 0.10(2) | 0.32(6) |
| Na2O | 6.2(7) | 5.9(3) | 5.9(3) | 9.08(17) | 8.91(22) |
| MgO | 0.02(2) | b.d.l. | b.d.l. | b.d.l. | 0.01(1) |
| K2O | 5.8(5) | 7.4(4) | 6.9(5) | 1.58(8) | 3.01(5) |
| CaO | 16.8(5) | 16.8(3) | 17.0(2) | 17.19(7) | 15.85(21) |
| TiO2 | b.d.l. | b.d.l. | b.d.l. | 0.01(2) | 0.02(4) |
| MnO | 0.11(2) | 0.34(2) | 0.35(4) | 0.19(2) | 0.32(3) |
| FeO | 0.07(4) | 0.05(5) | 0.05(4) | 0.02(2) | 0.11(4) |
| SrO | 0.7(2) | b.d.l. | b.d.l. | n.d. | n.d. |
| Nb2O5 | b.d.l. | b.d.l. | b.d.l. | n.d. | n.d. |
| BaO | 0.1(1) | b.d.l. | 0.2(2) | n.d. | 0.27(9) |
| F | 2.4(3) | 2.1(5) | 2.0(2) | 3.19(16) | 2.69(15) |
| Cl | 0.24(4) | 0.06(1) | 0.05(2) | 0.14(1) | 0.61(1) |
| 99.41 | 99.75 | 99.92 | 98.10 | 99.28 | |
| O=F | 1.01 | 0.88 | 0.84 | 1.34 | 1.13 |
| O=Cl | 0.06 | 0.01 | 0.01 | 0.03 | 0.13 |
| Sum | 98.34 | 98.86 | 99.07 | 96.73 | 98.02 |
| Atoms Per Formula Unit | |||||
| Constituent | Gav-43 | Yak-5 | Irk-53 | Fedorite, Murun [7]* | Fedorite, Turiy [7]* |
| Si | 15.98 | 15.98 | 15.98 | 15.96 | 16.01 |
| Al | 0.02 | 0.02 | 0.02 | 0.03 | 0.09 |
| Na | 2.88 | 2.73 | 2.72 | 4.22 | 4.12 |
| Mg | 0.01 | - | - | - | - |
| K | 1.78 | 2.25 | 2.08 | 0.48 | 0.92 |
| Ca | 4.31 | 4.29 | 4.33 | 4.41 | 4.06 |
| Ti | - | - | - | - | - |
| Mn | 0.02 | 0.07 | 0.07 | 0.04 | 0.07 |
| Fe | 0.01 | 0.01 | 0.01 | - | 0.02 |
| Sr | 0.09 | - | - | - | - |
| Nb | - | - | - | - | - |
| Ba | 0.01 | - | 0.03 | - | 0.03 |
| F | 1.79 | 1.60 | 1.53 | } 2.47 | } 2.27 |
| Cl | 0.10 | 0.03 | 0.02 | ||
| Gav-43 | Yak-5 | Irk-53 | |
|---|---|---|---|
| Crystal Data | |||
| a (Å) | 9.6463(5) | 9.6355(3) | 9.6446(1 |
| b (Å) | 9.6485(5) | 9.6364(3) | 9.6519(1) |
| c (Å) | 12.6189(5) | 12.6153(3) | 12.6177(2) |
| α (°) | 102.448(4) | 102.482(2) | 102.459(1) |
| β (°) | 96.235(4) | 96.237(2) | 96.207(1) |
| γ (°) | 119.927(5) | 119.926(3) | 119.900(2) |
| V (Å3) | 960.96(4) | 958.20(2) | 961.44(1) |
| Z | 2 | 2 | 2 |
| Crystal dimensions (mm) | 0.005 × 0.021 × 0.038 | 0.006 × 0.023 × 0.034 | 0.006 × 0.022 × 0.026 |
| Data Collection | |||
| Independent reflections | 46757 | 46140 | 59565 |
| Rmerging [R(int)] (%) | 6.6 | 2.9 | 3.3 |
| hmin, hmax | –14, 13 | –14, 14 | –16, 16 |
| kmin, kmax | –14, 14 | –14, 14 | –16, 16 |
| lmin, lmax | –19, 19 | –18, 19 | –21, 21 |
| Refinement | |||
| Space group | P | P | P |
| Reflections used in the refinement (I > 3σ(I)) | 4687 | 5693 | 8075 |
| N. of refined parameters | 437 | 427 | 427 |
| Ra [on F] (%) | 3.10 | 2.40 | 2.34 |
| Rwb [on F] (%) | 3.52 | 2.92 | 2.92 |
| Goofc | 1.0437 | 1.0549 | 1.0109 |
| Δρmin/Δρmax (e–/Å3) | –0.70/0.53 | –0.66/0.45 | –1.05/0.71 |
| This Study (Murun Complex) |
|---|
| Gav-43: (K1.78Na0.23Sr0.09Ba0.01)Σ = 2.11(Ca4.31Na2.65Mn0.02Fe0.01Mg0.01)Σ = 7.0(Si15.98Al0.02)Σ = 16.0(O37.54OH0.46)Σ = 38.0 (F1.79Cl0.10OH0. 11) Σ = 2.0·2.5H2O |
| Yak-5: (K2.25Na0.10)Σ = 2.35(Ca4.29Na2.63Mn0.07Fe0.01)Σ = 7.0(Si15.98Al0.02)Σ = 16.0(O37.70OH0.30)Σ = 38.0 (F1.60Cl0.03OH0.37) Σ = 2.0·0.8H2O |
| Irk-53: (K2.08Na0.22Ba0.03)Σ = 2.33(Ca4.33Na2.50Mn0.07Fe0.01)Σ = 7.0(Si15.98Al0.02)Σ = 16.0(O37.66OH0.34)Σ = 38.0 (F1.53Cl0.02OH0.45) Σ = 2.0·0.6H2O |
| Fedorite from Murun complex [7]: (Na1.68K0.48)Σ = 2.16(Ca4.41Na2.54Mn0.04)Σ = 6.99(Si15.96Al0.03)Σ = 15.98O38(F,Cl)2.47·3.47H2O |
| Fedorite from Turiy complex [7]: (Na1.27K0.92Ba0.03)Σ = 2.22(Ca4.06Na2.85Mn0.07Fe0.02)Σ = 7.0(Si16.01Al0.09)Σ = 16.10O38(F,Cl)2.27·3.69H2O |
| Fedorite from Turiy complex [3]: (K1.65Na0.83)Σ = 2.48(Ca4.52Na2.48)Σ = 7.0Si16O38(OH,F)2·H2O |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneva, E.V.; Shendrik, R.Y.; Radomskaya, T.A.; Suvorova, L.F. Fedorite from Murun Alkaline Complex (Russia): Spectroscopy and Crystal Chemical Features. Minerals 2020, 10, 702. https://doi.org/10.3390/min10080702
Kaneva EV, Shendrik RY, Radomskaya TA, Suvorova LF. Fedorite from Murun Alkaline Complex (Russia): Spectroscopy and Crystal Chemical Features. Minerals. 2020; 10(8):702. https://doi.org/10.3390/min10080702
Chicago/Turabian StyleKaneva, Ekaterina V., Roman Yu. Shendrik, Tatiana A. Radomskaya, and Ludmila F. Suvorova. 2020. "Fedorite from Murun Alkaline Complex (Russia): Spectroscopy and Crystal Chemical Features" Minerals 10, no. 8: 702. https://doi.org/10.3390/min10080702
APA StyleKaneva, E. V., Shendrik, R. Y., Radomskaya, T. A., & Suvorova, L. F. (2020). Fedorite from Murun Alkaline Complex (Russia): Spectroscopy and Crystal Chemical Features. Minerals, 10(8), 702. https://doi.org/10.3390/min10080702