Physico-Chemical, Mineralogical, and Chemical Characterisation of Cretaceous–Paleogene/Neogene Kaolins within Eastern Dahomey and Niger Delta Basins from Nigeria: Possible Industrial Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Sampling
2.3. Analyses
3. Results
3.1. Physico-Chemical Characteristics
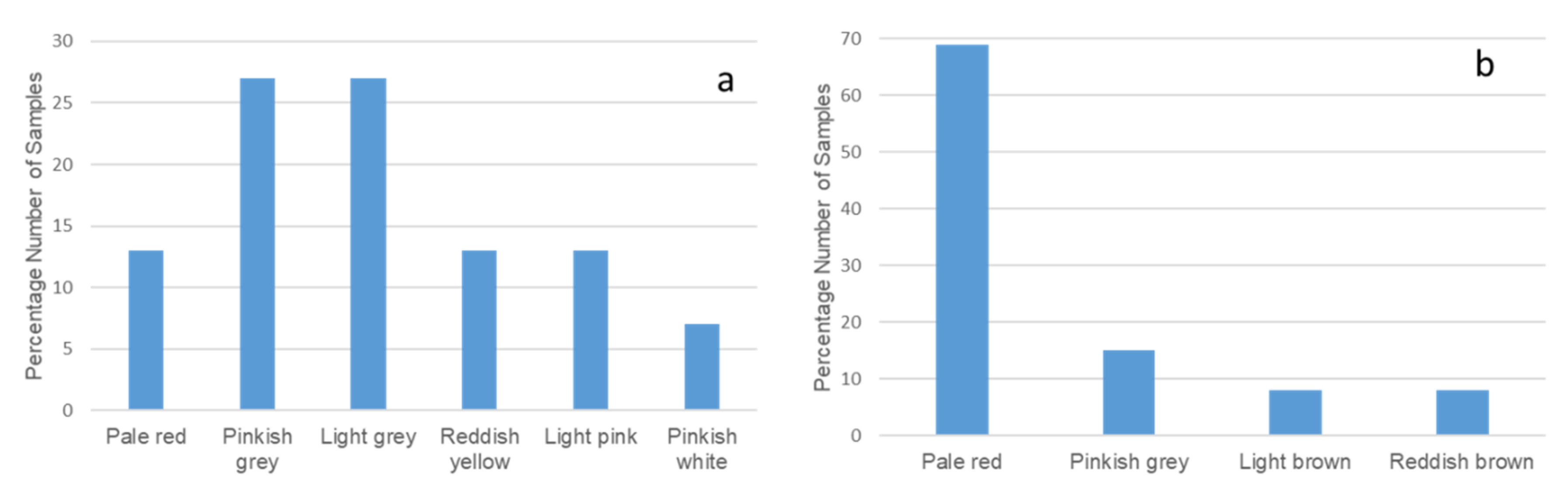
3.1.1. Colour
3.1.2. Hydrogen Ion Concentration (pH) and Electrical Conductivity (EC)
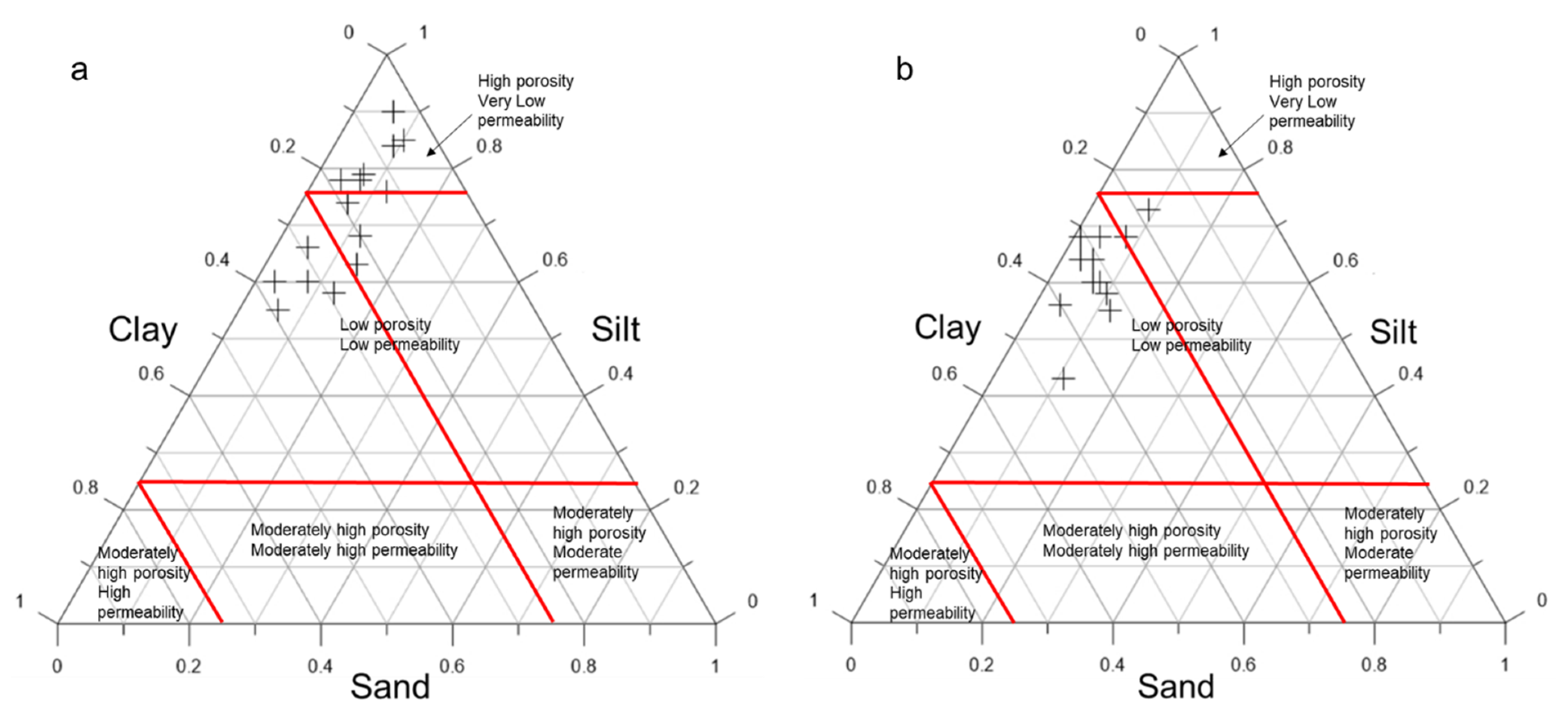
3.1.3. Particle Size Distribution
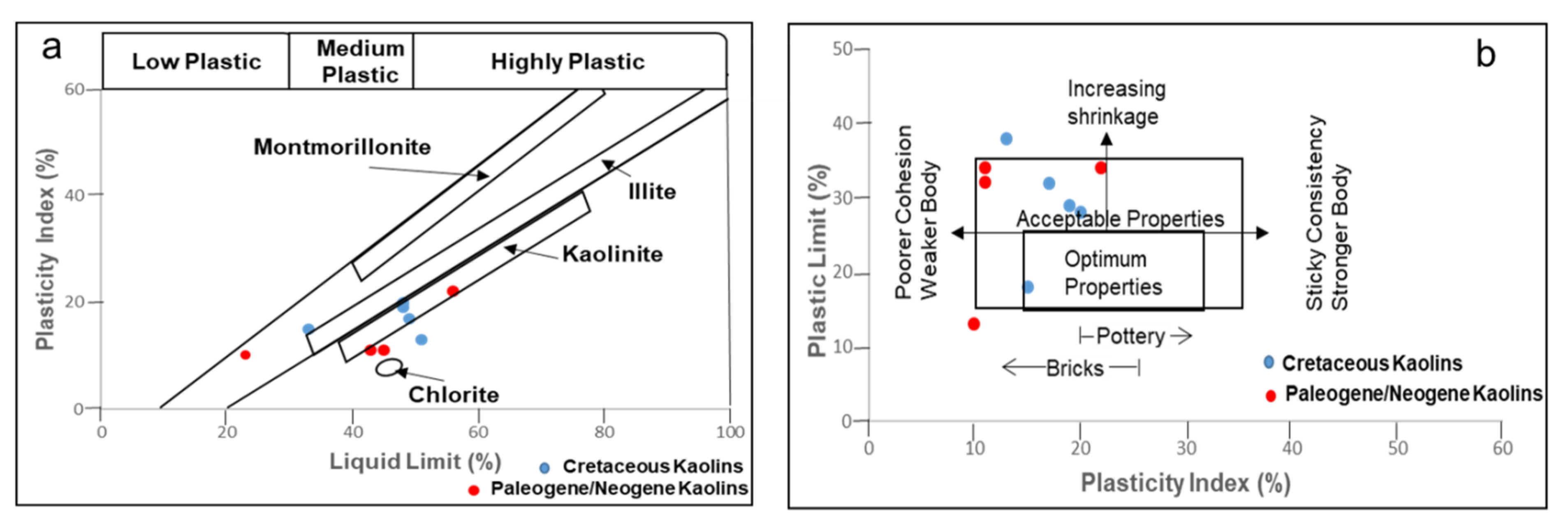
3.1.4. Plasticity
3.2. Mineralogical Characteristics
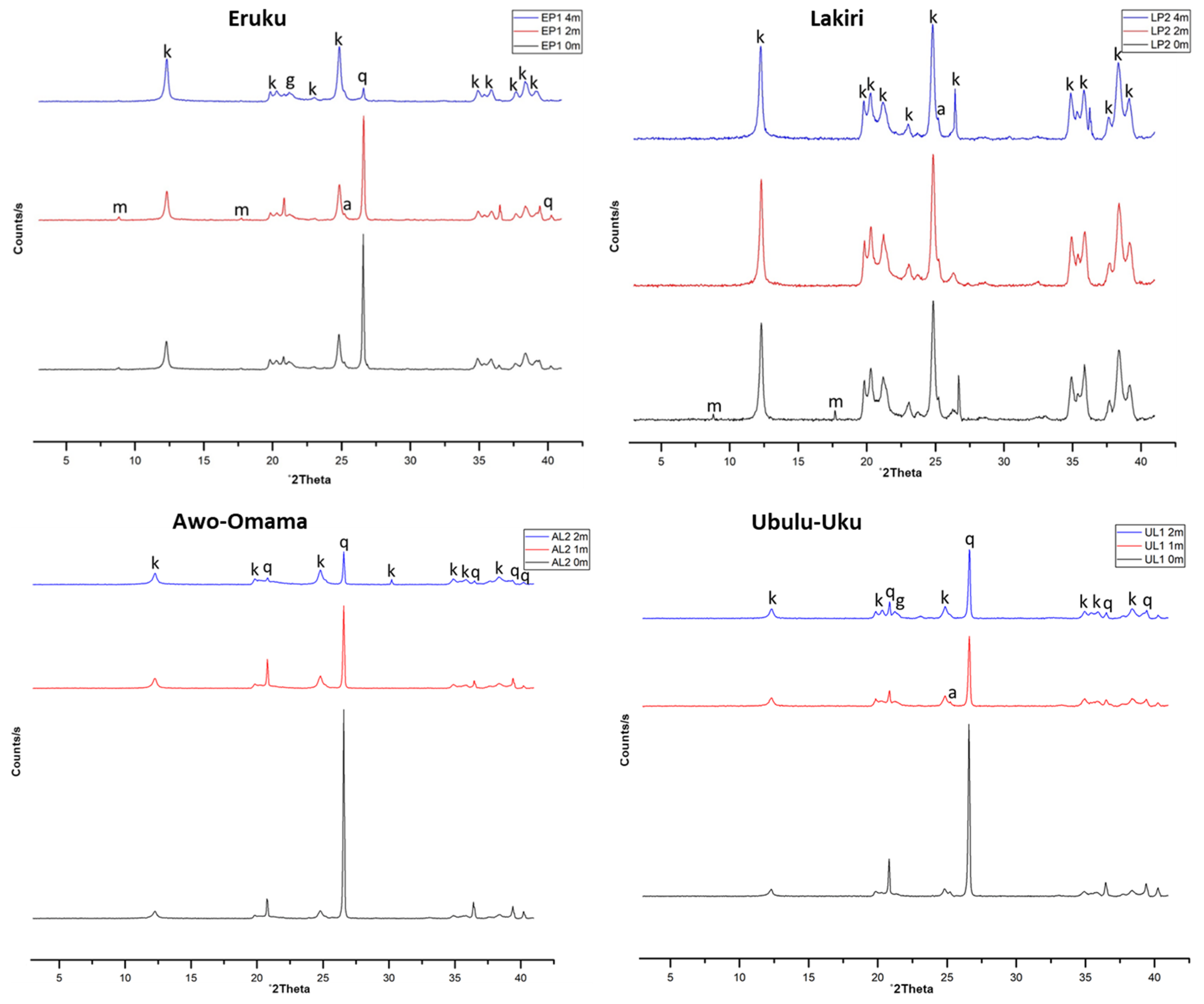
3.2.1. Mineral Compositions
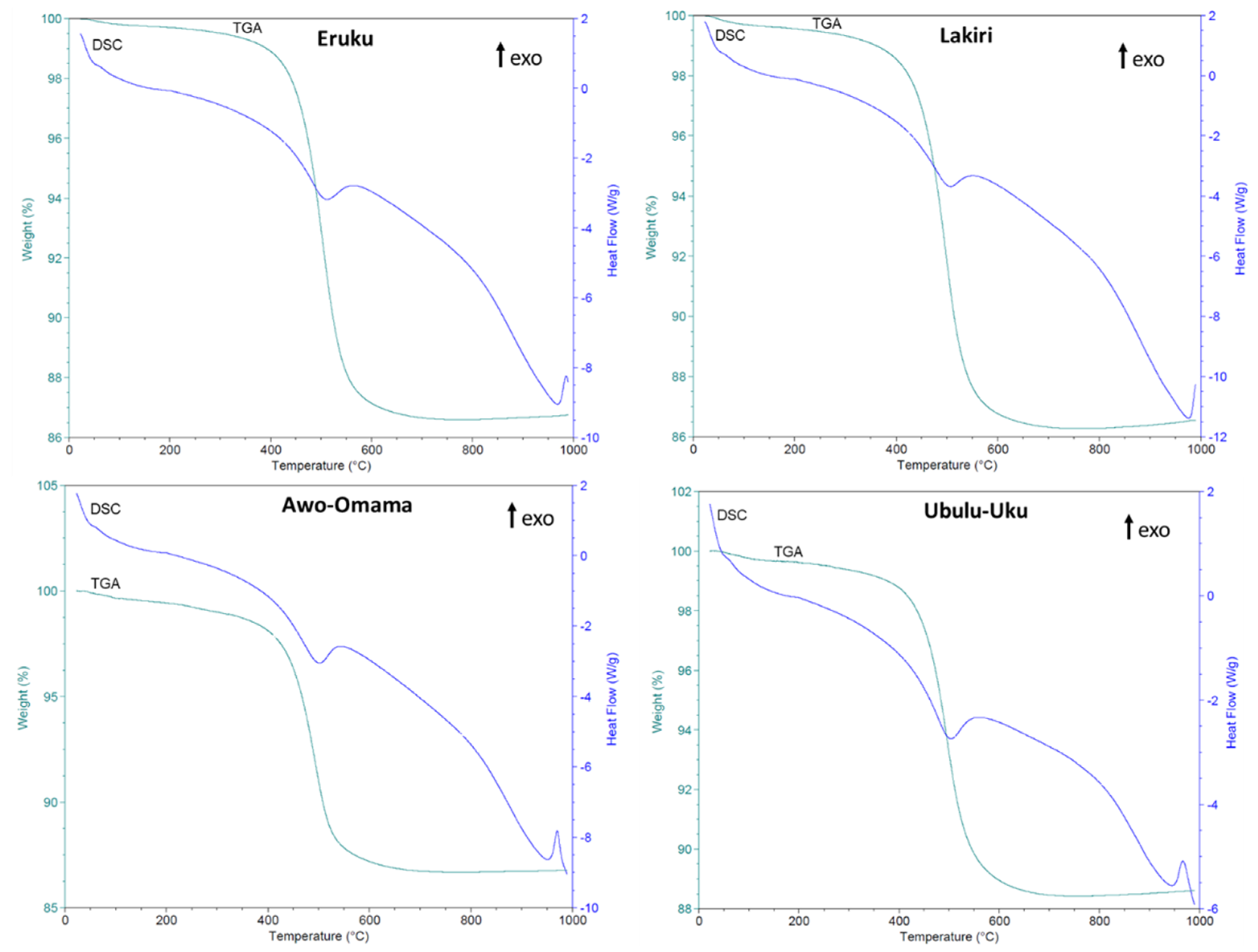
3.2.2. Kaolinite Thermal Evolution
3.2.3. Kaolinite Morphology
3.3. Chemical Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Onyemaobi, O.O. Mineral Resources Exploitation, Processing and Utilization—A Sine Qua Non for Nigeria’s Metallurgical Industrial Development Inaugural Lecture Series 5 of FUTO; FUTO Press: Owerri, Nigeria, 2002; p. 48. [Google Scholar]
- Ekosse, G.-I.E. Kaolin deposits and Occurrences in Africa: Geology, Mineralogy and Utilization. Appl. Clay Sci. 2010, 50, 212–236. [Google Scholar] [CrossRef]
- Oyebanjo, O.M. Paleo-Environmental Conditions and Tectonic Settings of Cretaceous-Tertiary Kaolins in the Eastern Dahomey and Niger Delta Basins in Nigeria. Ph.D. Thesis, University of Venda, Thohoyandou, South Africa, May 2018. [Google Scholar]
- Virta, R.L. Kaolin Industrial Mineral. Rev. Min. Eng. 2004, 56, 28–29. [Google Scholar]
- Murray, H.H. Applied Clay Mineralogy. Occurrences, Processing and Application of Kaolins, Bentonites, Palygorskite–Sepiolite, and Common Clays, 1st ed.; Elsevier: Oxford, UK, 2007; p. 189. [Google Scholar]
- Awad, M.E.; López-Galindo, A.; Medarević, D.; Đuriš, J.; El-Rahmany, M.M.; Ibrić, S.; Viseras, C. Flow and Tableting Behaviors of Some Egyptian Kaolin Powders as Potential Pharmaceutical Excipients. Minerals 2020, 10, 23. [Google Scholar] [CrossRef]
- IndustryArc. Kaolin Market—Industry Analysis, Market Size, Share, Trends, Application Analysis, Growth and Forecast 2019–2025. Available online: https://www.industryarc.com/Research/Kaolin-Market-Research-500193 (accessed on 2 June 2020).
- Murray, H.H.; Kogel, J.E. Engineered clay products for the paper industry. Appl. Clay Sci. 2005, 19, 199–206. [Google Scholar] [CrossRef]
- Premium Times. Nigeria: The Travails of Nigeria’s Artisanal Kaolin Miners Who Toil Daily to Earn Little. Available online: https://allafrica.com/stories/201908160047.html (accessed on 29 May 2020).
- Badmus, B.S.; Olatinsu, O.B. Geophysical evaluation and chemical analysis of kaolin clay deposit of Lakiri village, southwestern Nigeria. Int. J. Phys. Sci. 2009, 4, 592–606. [Google Scholar]
- Emofurieta, W.O.; Kayode, A.A.; Coker, S.A. Mineralogy, Geochemistry and Economic Evaluation of the Kaolin Deposits near Ubulu-Uku, Awo-Omama and Buan in Southern Nigeria. J. Min. Geol. 1992, 28, 211–220. [Google Scholar]
- Oyebanjo, O.M.; Ekosse, G.E.; Odiyo, J.O. Mineral constituents and kaolinite crystallinity of the <2 µm fraction of Cretaceous-Paleogene/Neogene Kaolins from Eastern Dahomey and Niger Delta Basins, Nigeria. Open Geosci. 2018, 10, 157–166. [Google Scholar]
- Nwajide, C.S. Geology of Nigeria’s Sedimentary Basins; CSS Press: Lagos, Nigeria, 2013; p. 565. [Google Scholar]
- Omatsola, M.E.; Adegoke, O.S. Tectonic evolution and Cretaceous stratigraphy of Dahomey Basin. J. Min. Geol. 1981, 18, 130–137. [Google Scholar]
- Van Reeuwijk, L.P. Procedures for Soil Analysis; Tech. Paper 9; International Soil Reference and Information Centre: Wageningen, The Netherlands, 2002; p. 100. [Google Scholar]
- Ruck, L.; Brown, C.T. Quantitative analysis of Munsell color data from archeological ceramics. J. Archaeol. Sci. Rep. 2015, 3, 549–557. [Google Scholar] [CrossRef]
- Casagrande, A. Plasticity chart for the classification of cohesive soils. Trans. Am. Soc. Civ. Eng. 1948, 113, 901. [Google Scholar]
- Ekosse, G.-I.E. Physico-Chemical Studies of Some Clay Deposits in Anambra State, Nigeria. Ph.D. Thesis, Anambra State University of Science and Technology, Uli, Nigeria, 1990; p. 112. [Google Scholar]
- Ekosse, G. Thermoanalytical characterisation, stable isotope and paleoenvironmental considerations of kaolinite from two genetic sources. Fresenius Environ. Bull. 2008, 17, 29–42. [Google Scholar]
- Siddiqui, M.A.; Ahmed, Z.; Saleemi, A.A. Evaluation of Swat kaolin deposits of Pakistan for industrial uses. Appl. Clay Sci. 2005, 29, 55–72. [Google Scholar] [CrossRef]
- Lopez-Galindo, A.; Viseras, C.; Cerezo, P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl. Clay Sci. 2007, 36, 51–63. [Google Scholar] [CrossRef]
- Semiz, B. Characteristics of clay-rich raw materials for ceramic applications in Denizli region (Western Anatolia). Appl. Clay Sci. 2017, 137, 83–93. [Google Scholar] [CrossRef]
- Bloodworth, A.J.; Highley, D.E.; Mitchell, C.J. Industrial Minerals Laboratory Manual—Kaolin; Techical Report British Geological Survey WG/93/1; Natural Environment Research Council: Nottingham, UK, 1993; pp. 1–76. [Google Scholar]
- Ekosse, G.; Mulaba-Bafubiandi, A.; Nkoma, J.S. Physico-Chemistry of Continental Bentonites and Kaolin for Ceramic Applications. Afr. J. Sci. Technol. AJST Sci. Eng. Ser. 2007, 8, 107–115. [Google Scholar]
- Kuscu, M.; Yildiz, A. The mineralogy, geochemistry, and suitability for ceramic applications of Akharm (Afyonkarahisar, W. Turkey) kaolinitic clay. Arab. J. Geosci. 2016, 9, 510. [Google Scholar] [CrossRef]
- Murray, H.H. Clays. In Ullman’s Encyclopedia of Industrial Chemistry, 5th ed.; John Wiley & Sons: New York, NY, USA, 1986; pp. 109–136. [Google Scholar]
- El Ouahabi, M.; Daoudi, L.; de Vleeschouwer, F.; Bindler, R.; Fagel, N. Potentiality of Clay Raw Materials from Northern Morocco in Ceramic Industry: Tetouan and Meknes Areas. J. Miner. Mater. Charact. Eng. 2014, 2, 145–159. [Google Scholar] [CrossRef]
- McManus, J. Grain size distribution and interpretation. In Techniques in Sedimentology; Tucker, M.E., Ed.; Blackwell Scientific Publications: Oxford, UK, 1988; pp. 63–85. [Google Scholar]
- Strazzera, B.; Dondi, M.; Marsigli, M. Composition and ceramic properties of tertiary clays from southern Sardinia (Italy). Appl. Clay Sci. 1997, 12, 247–266. [Google Scholar] [CrossRef]
- Daoudi, L.; Elboudour, E.H.; Saadi, L.; Albizane, A.; Bennazha, J.; Waqif, M.; El Ouahabi, M.; Fagel, N. Characteristics and ceramic properties of clayey materials from Amezmiz region (Western High Atlas, Morocco). Appl. Clay Sci. 2014, 102, 139–147. [Google Scholar]
- Holtz, R.D.; Kovacs, W.D. An Introduction to Geotechnical Engineering; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1981. [Google Scholar]
- Daniel, D.E. Seminar Publication: Design and Construction of RCRA/CERCLA Final Covers; Environmental Protection Agency: Washington, DC, USA, 1991.
- Abajo, M. Manual Sobre Fabricación de Baldosas. Tejas y Ladrillos; Beralmar S.A.: Barcelona, Spain, 2000. [Google Scholar]
- Vieira, C.M.; Sa’nchez, R.; Monteiro, S.N. Characteristics of clays and properties of building ceramics in the state of Rio de Janeiro, Brazil. Constr. Build. Mater. 2008, 22, 781–787. [Google Scholar] [CrossRef]
- Bain, J.A. A plasticity chart as an aid to the identification and assessment of industrial clays. Clay Miner. 1971, 9, 1–17. [Google Scholar] [CrossRef]
- Dondi, M.; Fabbri, B.; Guarini, G. Grain-Size Distribution of Italian Raw Materials for Building Clay Products: A Reappraisal of the Winkler Diagram. Clay Miner. 1998, 33, 435–442. [Google Scholar] [CrossRef]
- Keller, W.D. Flint clay and flint-clay facies. Clays Clay Miner. 1968, 16, 113–128. [Google Scholar] [CrossRef]
- Pruett, R.J.; Alves, P.A. Compositions Comprising Fine Sedimentary Kaolin and Methods for Preparing Same. U.S. Patent 8465583, 18 June 2013. [Google Scholar]
- Wilson, M.J. Rock-Forming Minerals, Sheet Silicates–Clay Minerals, 2nd ed.; The Geological Society: London, UK, 2013; Volume 3c, p. 724. [Google Scholar]
- Gilkes, R.J.; Prakongkep, N. How the unique properties of soil kaolin affect the fertility of tropical soils. Appl. Clay Sci. 2016, 131, 100–106. [Google Scholar] [CrossRef]
- Luz, A.B.; Middea, A. Purification of kaolin by selective flocculation. In Proceedings of the 43rd Annual Conference of Metallurgist of CIM, Hamilton, ON, Canada, 22–25 August 2004; pp. 243–253. [Google Scholar]
- Baioumy, H.M. Geochemistry and origin of the Cretaceous sedimentary kaolin deposits, Red Sea, Egypt. Chem. Erde 2014, 74, 195–203. [Google Scholar] [CrossRef]
- Schroeder, P.A.; Pruett, R.J.; Melear, N.D. Crystal-chemical changes in an oxidative weathering front in a Georgia kaolin deposit. Clays Clay Miner. 2004, 52, 211–220. [Google Scholar] [CrossRef]
- Pruett, R.J. Kaolin deposits and their uses: Northern Brazil and Georgia, USA. Appl. Clay Sci. 2016, 131, 3–13. [Google Scholar] [CrossRef]
- Teklay, A.; Yin, C.; Rosendahl, L.; Bøjer, M. Calcination of kaolinite clay particles for cement production: A modeling study. Cem. Concr. Res. 2014, 61–62, 11–19. [Google Scholar] [CrossRef]
- Vaculíková, L.; Plevová, E.; Vallová, S.; Koutník, I. Characterization and differentiation of kaolinites from selected Czech deposits using infrared spectroscopy and differential thermal analysis. Acta Geodyn. Geomater. 2011, 8, 59–67. [Google Scholar]
- Garcia-Valles, M.; Pi, T.; Alfonso, P.; Canet, C.; Martinez, S.; Jimenez-Franco, A.; Tarrago, M.; Hernández-Cruz, B. Kaolin from Acoculco (Puebla, Mexico) as raw material: Mineralogical and thermal characterization. Clay Miner. 2015, 50, 405–416. [Google Scholar] [CrossRef]
- El Ouahabi, M.; Daoudi, L.; Fagel, N. Mineralogical and geotechnical characterization of clays from northern Morocco for their potential use in the ceramic industry. Clay Miner. 2014, 49, 35–51. [Google Scholar] [CrossRef]
- Garcia-Valles, M.; Alfonso, P.; Martinez, S.; Roca, N. Mineralogical and Thermal Characterization of Kaolinitic Clays from Terra Alta (Catalonia, Spain). Minerals 2020, 10, 142. [Google Scholar] [CrossRef]
- Keller, W.D. Scan electron micrographs of kaolins collected from diverse environments of origin–I. Clays Clay Miner. 1976, 24, 107–113. [Google Scholar] [CrossRef]
- Keller, W.D. Classification of kaolins exemplified by their textures in scan electron micrographs. Clays Clay Miner. 1978, 26, 1–20. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Ekosse, G. Provenance of the Kgwakgwe kaolin deposit, southeastern Botswana and its possible utilization. Appl. Clay Sci. 2001, 20, 137–152. [Google Scholar] [CrossRef]
- Gougazeh, M. Removal of iron and titanium contaminants from Jordanian Kaolins by using chemical leaching. J. Taibah Univ. Sci. 2018, 12, 247–254. [Google Scholar] [CrossRef]
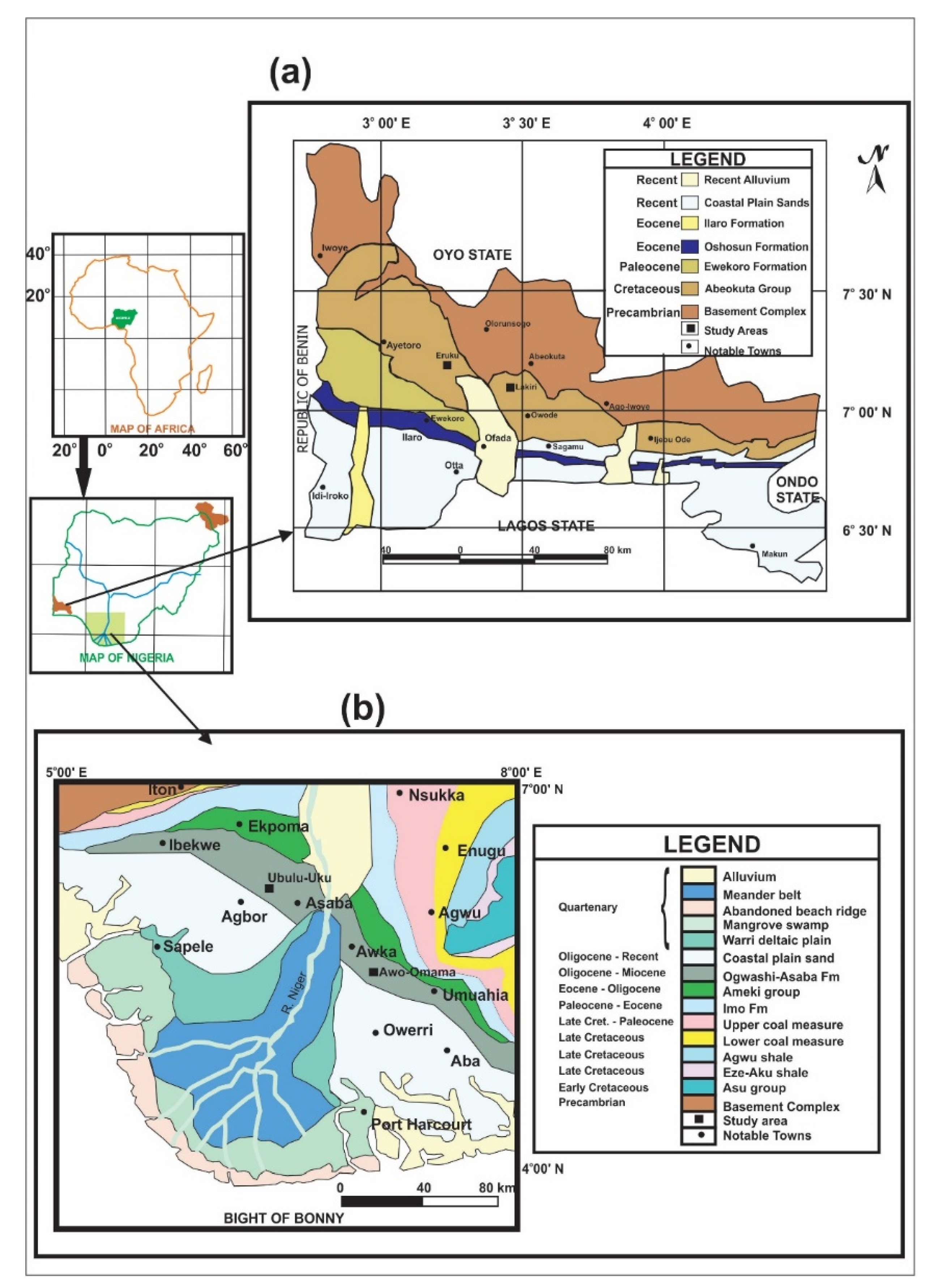
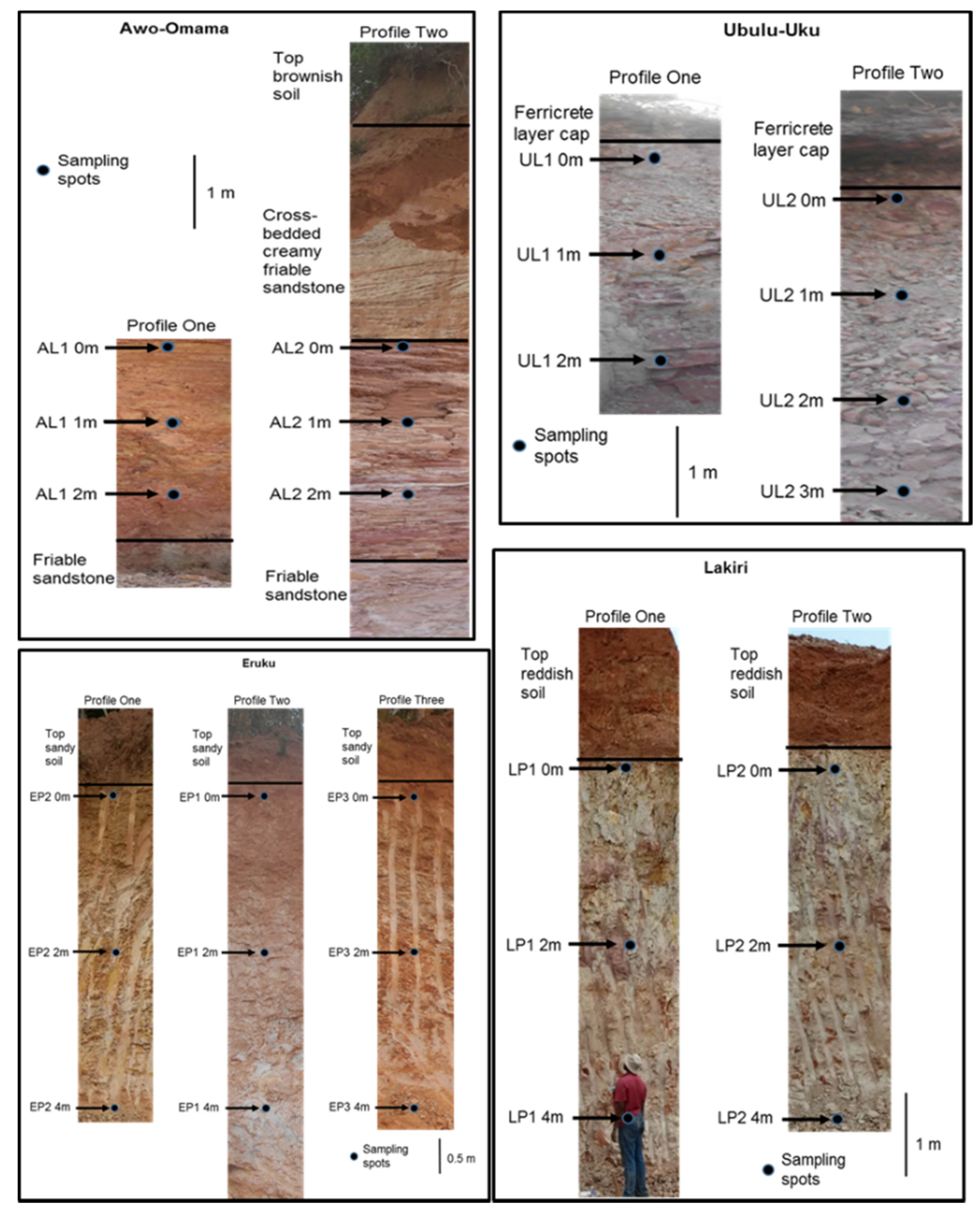

| S/N | Basin | Formation | Age 1 | Deposit | Coordinates | Number of Samples |
|---|---|---|---|---|---|---|
| 1 | Eastern Dahomey | Abeokuta Group | Cretaceous (Valanginian–Barremian) | Eruku (EP) Lakiri (LP) | 7°10′20″ N 3°15′0″ E 7°5′21″ N 3°27′26″ E | 9 samples from 3 profiles at 2 m intervals 6 samples from 2 profiles at 2 m intervals |
| 2 | Niger Delta | Ogwashi-Asaba | Paleogene/Neogene (Oligocene–Miocene) | Awo-Omama (AL) Ubulu-Uku (UL) | 5°39′23″ N 6°56′4″ E 6°24′0″ N 6°25′18″ E | 6 samples from 2 profiles at 1 m intervals 7 samples from 2 profiles at 1 m intervals |
| Age | Deposit | Sample ID | Colour | pH | EC (μm/cm) | Clay (%) | Silt (%) | Sand (%) |
|---|---|---|---|---|---|---|---|---|
| Cretaceous | Eruku | EP1 0 m | Light pink | 4.56 | 5.00 | 58 | 13 | 29 |
| 2 m | Light pink | 4.80 | 0.70 | 60 | 3 | 37 | ||
| 4 m | Light grey | 4.74 | 1.20 | 90 | 6 | 4 | ||
| EP2 0 m | Reddish yellow | 4.73 | 8.10 | 60 | 8 | 32 | ||
| 2 m | Pinkish grey | 4.70 | 2.20 | 66 | 5 | 29 | ||
| 4 m | Reddish yellow | 4.68 | 0.70 | 74 | 7 | 19 | ||
| EP3 0 m | Pinkish grey | 4.87 | 0.60 | 55 | 6 | 39 | ||
| 2 m | Pinkish grey | 4.27 | 0.20 | 63 | 14 | 23 | ||
| 4 m | Light grey | 4.50 | 3.30 | 78 | 7 | 15 | ||
| Lakiri | LP1 0 m | Pinkish white | 5.21 | 2.20 | 68 | 12 | 20 | |
| 2 m | Pale red | 4.70 | 8.40 | 78 | 4 | 18 | ||
| 4 m | Pinkish grey | 4.73 | 2.90 | 85 | 10 | 5 | ||
| LP2 0 m | Pale red | 4.78 | 9.10 | 76 | 12 | 12 | ||
| 2 m | Light grey | 4.94 | 0.50 | 79 | 7 | 14 | ||
| 4 m | Light grey | 4.35 | 1.10 | 84 | 9 | 7 | ||
| Paleogene/Neogene | Awo-Omama | AL1 0 m | Pale red | 4.63 | 0.30 | 60 | 7 | 33 |
| 1 m | Pale red | 4.88 | 13.1 | 58 | 10 | 32 | ||
| 2 m | Pale red | 4.68 | 4.30 | 55 | 12 | 33 | ||
| AL2 0 m | Pale red | 4.69 | 0.20 | 56 | 4 | 40 | ||
| 1 m | Pinkish grey | 4.92 | 1.50 | 64 | 5 | 31 | ||
| 2 m | Pale red | 4.96 | 0.40 | 68 | 4 | 28 | ||
| Ubulu-Uku | UL1 0 m | Pale red | 4.50 | 1.10 | 68 | 8 | 24 | |
| 1 m | Light brown | 4.62 | 1.30 | 73 | 9 | 18 | ||
| 2 m | Pale red | 4.27 | 4.40 | 64 | 3 | 33 | ||
| UL2 0 m | Pale red | 4.84 | 0.70 | 68 | 1 | 31 | ||
| 1 m | Pinkish grey | 5.29 | 3.90 | 68 | 8 | 24 | ||
| 2 m | Pale red | 5.15 | 9.70 | 43 | 11 | 46 | ||
| 3 m | Reddish brown | 4.70 | 1.10 | 60 | 8 | 32 |
| Age | Deposit | Sample ID | LL | PL | PI |
|---|---|---|---|---|---|
| Cretaceous | Eruku | EP1 | 48 | 29 | 19 |
| EP2 | 51 | 38 | 13 | ||
| EP3 | 49 | 32 | 17 | ||
| Lakiri | LP1 | 33 | 18 | 15 | |
| LP2 | 48 | 28 | 20 | ||
| Paleogene/Neogene | Awo-Omama | AL1 | 43 | 32 | 11 |
| AL2 | 56 | 34 | 22 | ||
| Ubulu-Uku | UL1 | 23 | 13 | 10 | |
| UL2 | 45 | 34 | 11 |
| Age | XRD (wt%) | DTA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kao | Qtz | Ana | Hem | Mus | Goe | Dht (°C) | MFt (°C) | TWL (%) | ||
| Cretaceous | Average | 83 | 21 | 1 | - | 5 | 1 | 511 | 981.9 | 13.6 |
| (n = 15) | Max | 99 | 57 | 2 | - | 9 | 1 | 517 | 985 | 14.4 |
| Min | 49 | 1 | 1 | tr | 1 | 1 | 488 | 980 | 13.1 | |
| Paleogene/Neogene | Average | 62 | 37 | 1 | - | - | 1 | 505 | 964.3 | 12.2 |
| (n = 13) | Max | 93 | 74 | 1 | - | - | 1 | 514 | 970 | 13.8 |
| Min | 25 | 6 | 1 | - | - | 1 | 500 | 951 | 10.7 | |
| Deposit | Al2O3 | CaO | Fe2O3 | K2O | MgO | Na2O | SiO2 | TiO2 | LOI | SiO2/Al2O3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eruku | Min | 11.44 | 0.01 | 1.48 | 0.27 | 0.04 | 0.02 | 47.22 | 1.07 | 4.03 | 1.29 |
| (n = 9) | Max | 36.64 | 0.03 | 5.04 | 0.65 | 0.05 | 0.05 | 80.30 | 2.84 | 12.89 | 7.02 |
| Average | 28.25 | 0.02 | 2.56 | 0.41 | 0.04 | 0.02 | 56.82 | 2.10 | 10.00 | 2.33 | |
| Lakiri | Min | 21.40 | 0.01 | 0.59 | 0.06 | bdl | 0.01 | 44.63 | 1.14 | 7.42 | 1.13 |
| (n = 6) | Max | 39.50 | 0.02 | 2.70 | 0.56 | bdl | 0.04 | 69.60 | 2.00 | 13.92 | 3.25 |
| Average | 35.55 | 0.02 | 1.09 | 0.20 | bdl | 0.02 | 49.64 | 1.60 | 12.57 | 1.53 | |
| Awo-Omama | Min | 7.16 | 0.02 | 0.85 | 0.08 | 0.04 | 0.02 | 50.11 | 0.83 | 2.83 | 1.56 |
| (n = 6) | Max | 32.09 | 0.04 | 4.24 | 0.27 | 0.06 | 0.02 | 87.99 | 1.99 | 11.97 | 12.29 |
| Average | 18.02 | 0.03 | 2.42 | 0.16 | 0.04 | 0.02 | 71.04 | 1.35 | 6.87 | 5.69 | |
| Ubulu-Uku | Min | 17.80 | 0.01 | 4.63 | 0.33 | 0.01 | 0.01 | 54.63 | 1.67 | 6.81 | 2.08 |
| (n = 7) | Max | 26.83 | 0.02 | 8.30 | 0.60 | 0.17 | 0.03 | 67.63 | 2.38 | 10.02 | 3.80 |
| Average | 22.65 | 0.02 | 6.34 | 0.46 | 0.06 | 0.02 | 59.95 | 2.03 | 8.71 | 2.76 | |
| SCGK 1 | Average | 38.38 | 0.05 | 0.30 | 0.04 | 0.25 | 0.27 | 45.30 | 1.44 | 13.97 | 1.18 |
| HPNGK 1 | Average | 39.50 | 0.03 | 1.13 | 0.06 | 0.03 | 0.08 | 44.00 | 2.43 | 13.90 | 1.11 |
| SLCEPNCK 1 | Average | 38.03 | 0.01 | 0.59 | 0.02 | 0.01 | 0.03 | 46.56 | 0.78 | 13.80 | 1.22 |
| Paper Coating 2 | Min | 36 | - | 0.5 | 0.5 | - | - | 45 | 0.5 | - | - |
| Max | 38 | - | 1.00 | 1.50 | - | - | 49.00 | 1.30 | - | - | |
| Paper Filler 2 | Min | 37 | - | 0.5 | 0.5 | - | - | 46 | 0.5 | - | - |
| Max | 38 | - | 1.00 | 1.50 | - | - | 48.00 | 1.50 | - | - | |
| Ceramics 2 | Min | 36 | - | 0.6 | 1.2 | - | - | 45 | 0.02 | 11.2 | - |
| Max | 38 | - | 1.00 | 2.70 | - | - | 50.00 | 0.10 | 12.50 | - | |
| Pharm. & | Min | 38.1 | 0.1 | 0.1 | 0 | 0.1 | 0 | 44.6 | 0 | 13.8 | - |
| Cosmetics 2 | Max | 39.5 | 0.2 | 0.20 | 0.20 | 0.2 | 0.1 | 46.40 | 1.40 | 13.90 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyebanjo, O.; Ekosse, G.-I.; Odiyo, J. Physico-Chemical, Mineralogical, and Chemical Characterisation of Cretaceous–Paleogene/Neogene Kaolins within Eastern Dahomey and Niger Delta Basins from Nigeria: Possible Industrial Applications. Minerals 2020, 10, 670. https://doi.org/10.3390/min10080670
Oyebanjo O, Ekosse G-I, Odiyo J. Physico-Chemical, Mineralogical, and Chemical Characterisation of Cretaceous–Paleogene/Neogene Kaolins within Eastern Dahomey and Niger Delta Basins from Nigeria: Possible Industrial Applications. Minerals. 2020; 10(8):670. https://doi.org/10.3390/min10080670
Chicago/Turabian StyleOyebanjo, Olaonipekun, Georges-Ivo Ekosse, and John Odiyo. 2020. "Physico-Chemical, Mineralogical, and Chemical Characterisation of Cretaceous–Paleogene/Neogene Kaolins within Eastern Dahomey and Niger Delta Basins from Nigeria: Possible Industrial Applications" Minerals 10, no. 8: 670. https://doi.org/10.3390/min10080670
APA StyleOyebanjo, O., Ekosse, G.-I., & Odiyo, J. (2020). Physico-Chemical, Mineralogical, and Chemical Characterisation of Cretaceous–Paleogene/Neogene Kaolins within Eastern Dahomey and Niger Delta Basins from Nigeria: Possible Industrial Applications. Minerals, 10(8), 670. https://doi.org/10.3390/min10080670





