Organically Templated Layered Uranyl Molybdate [C3H9NH+]4[(UO2)3(MoO4)5] Structurally Based on Mineral-Related Modular Units
Abstract
:1. Introduction
2. Materials and Methods
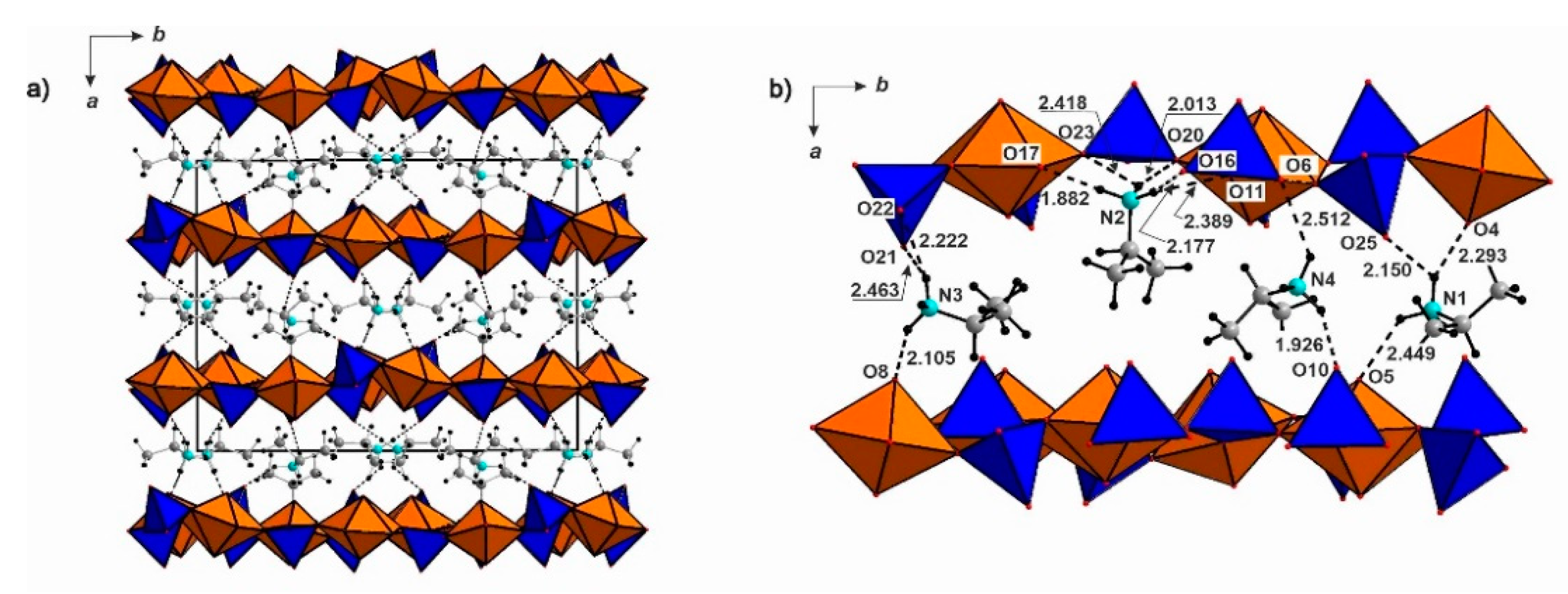
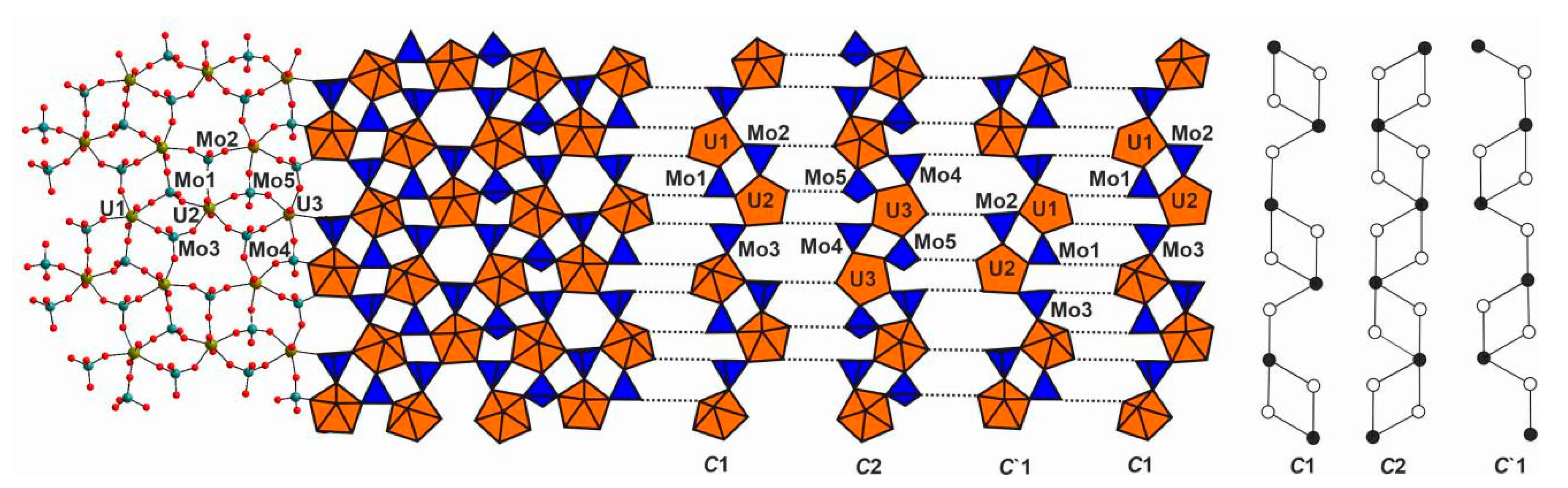
3. Results
4. Discussion
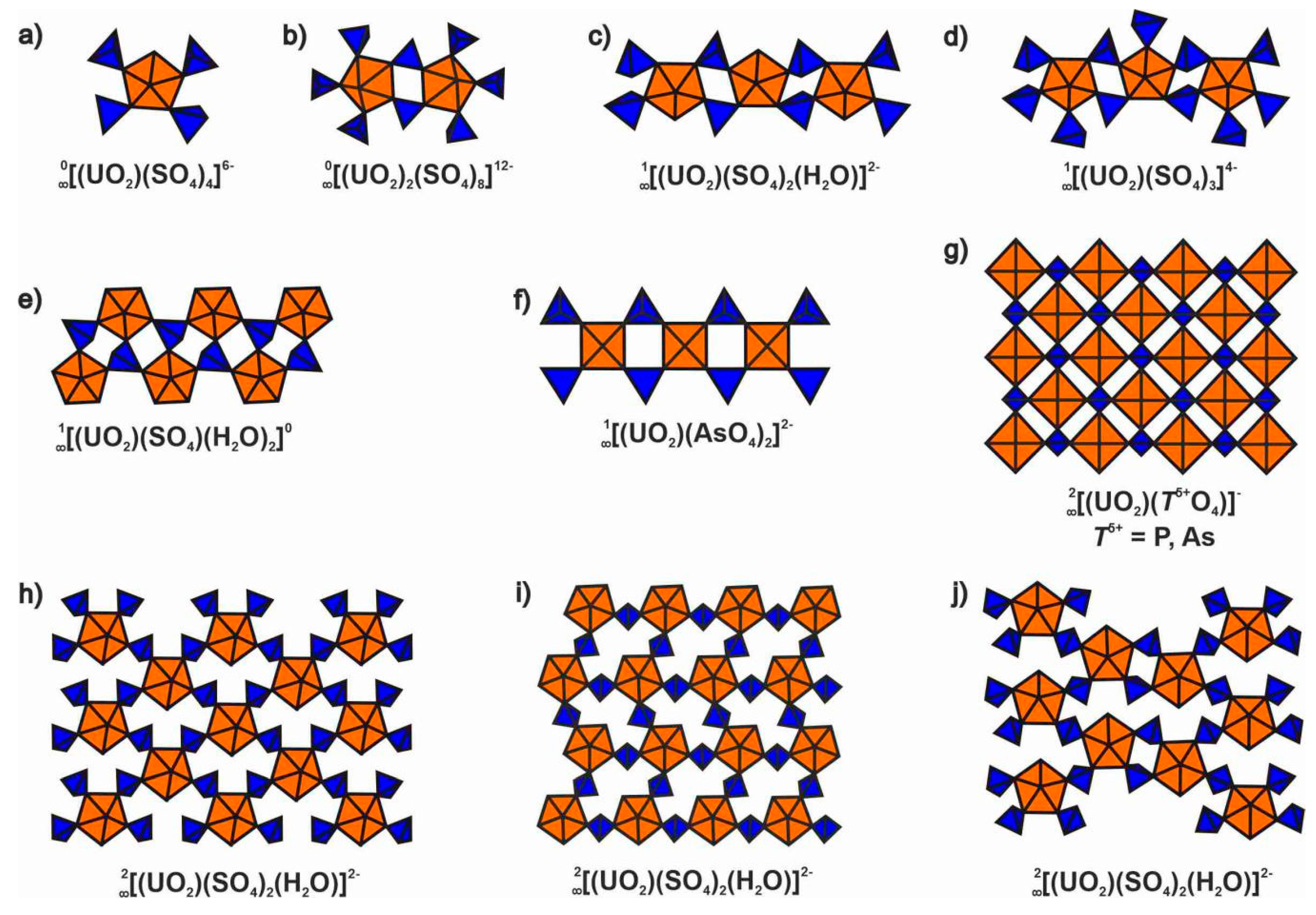
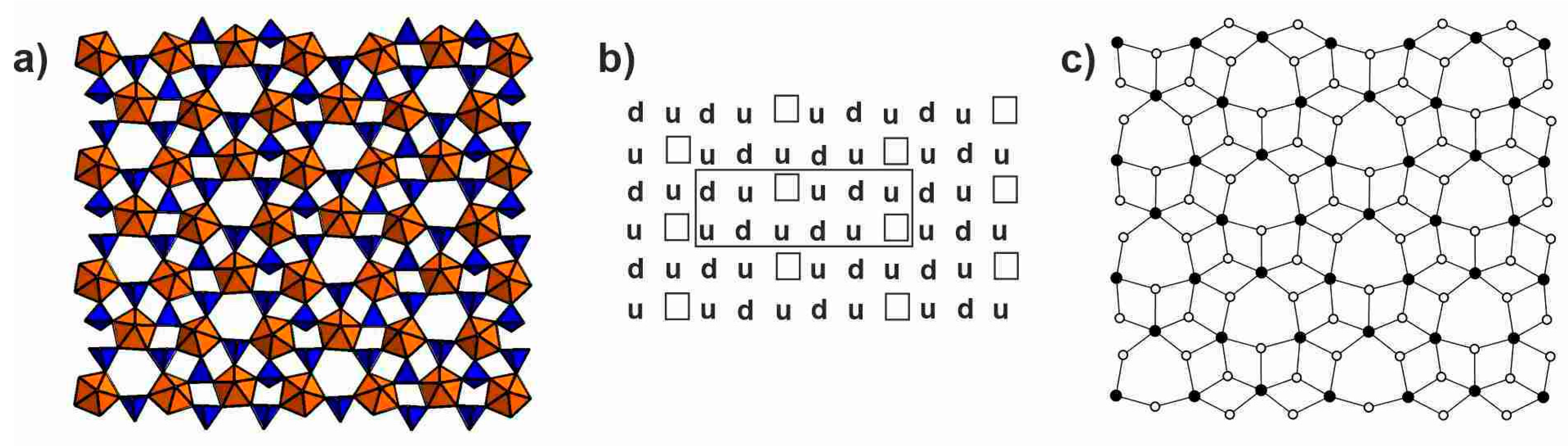
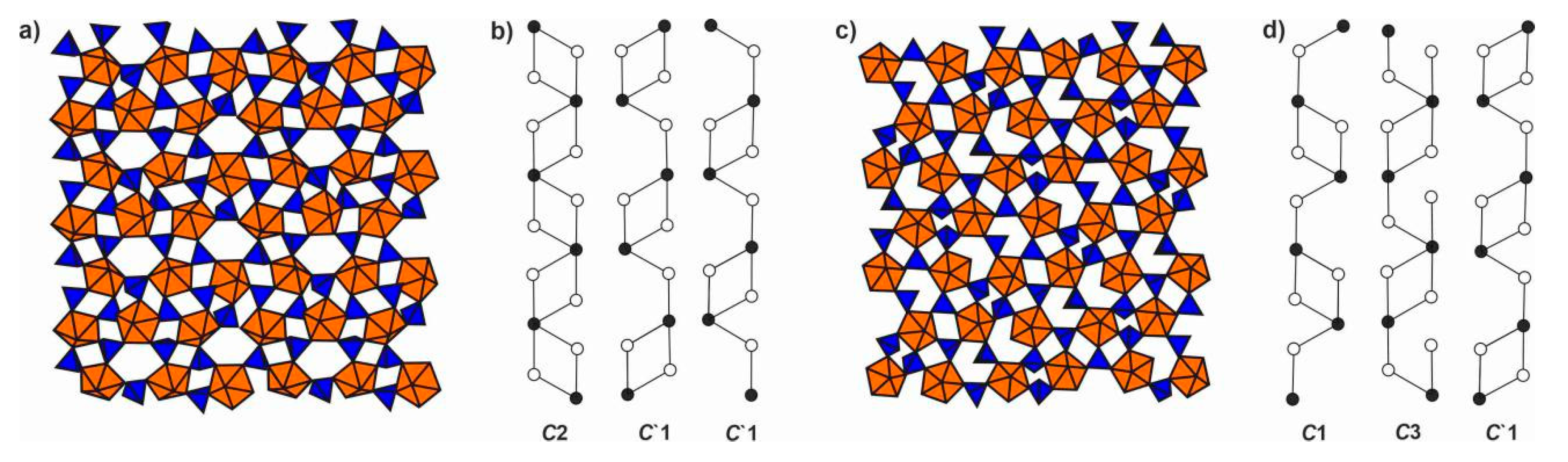
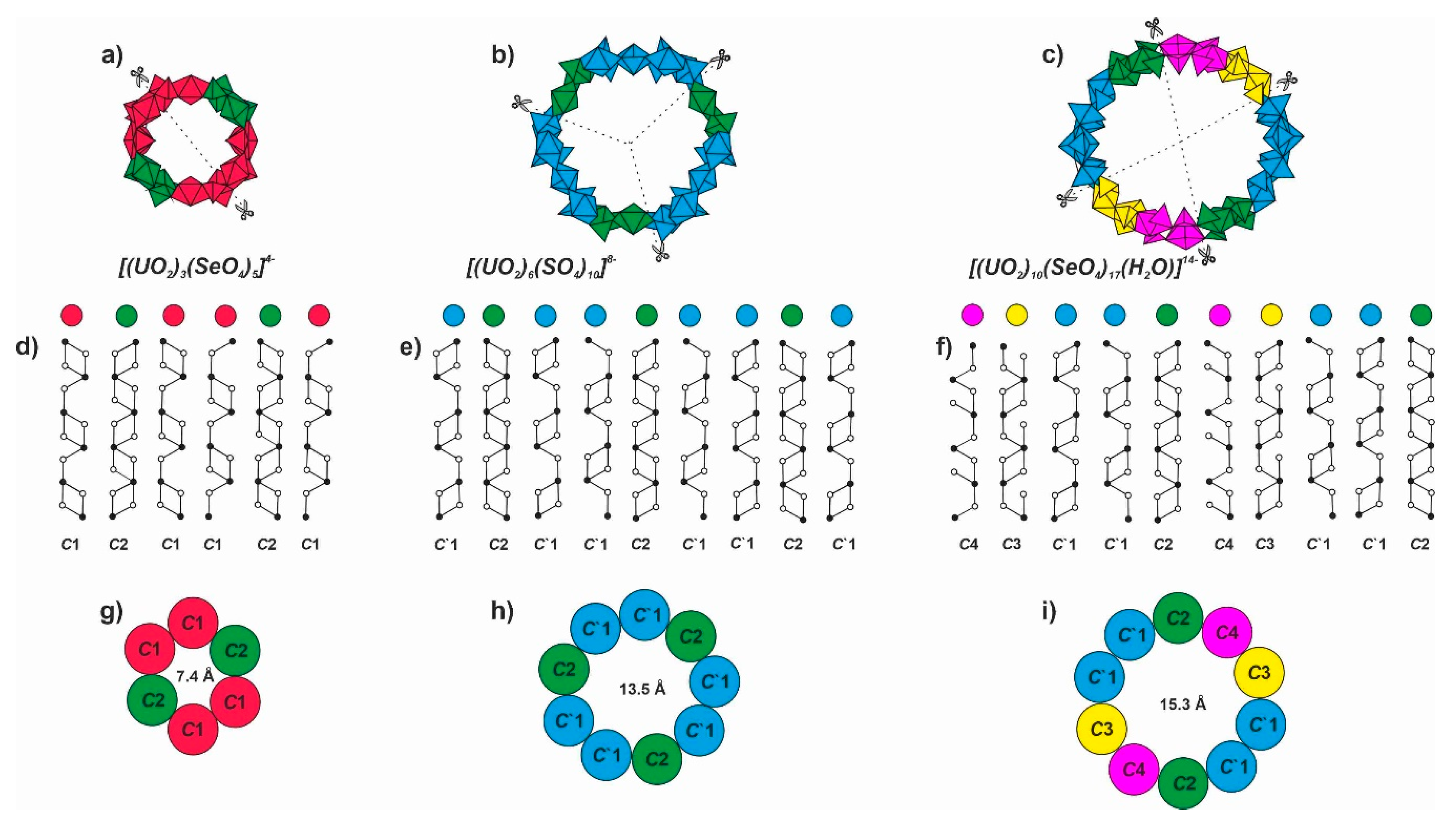
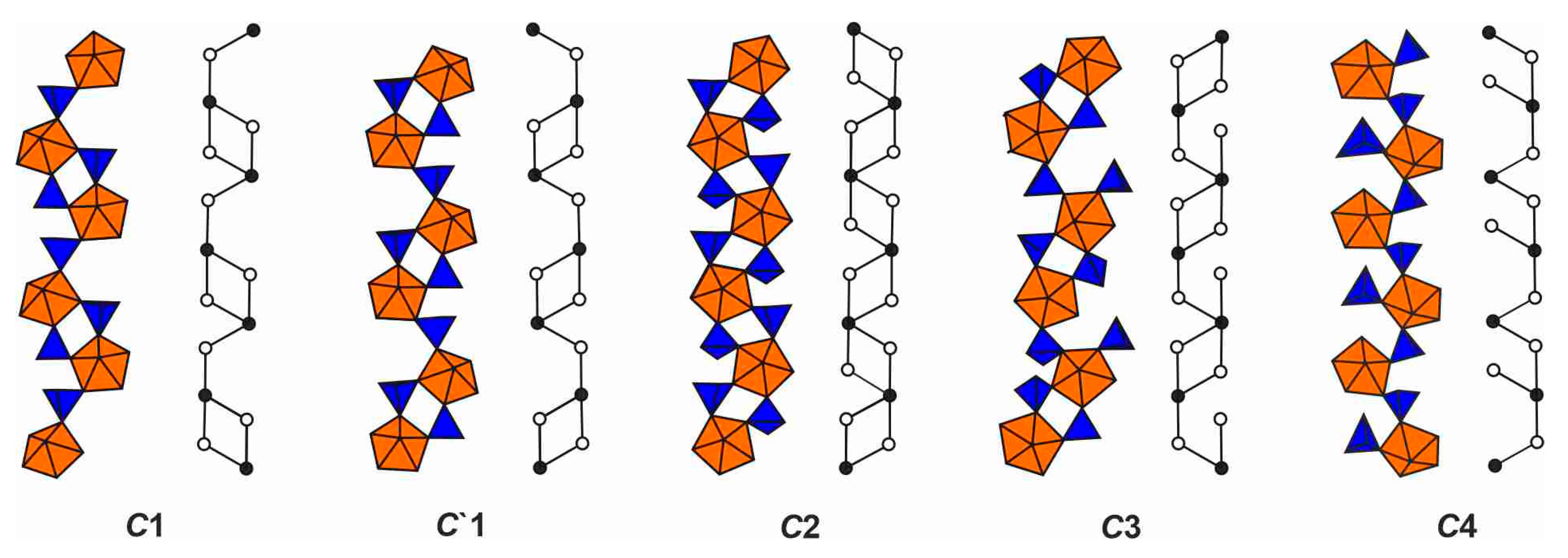
4.1. Structural Topology of Nanotubules Is Uranyl Oxysalts
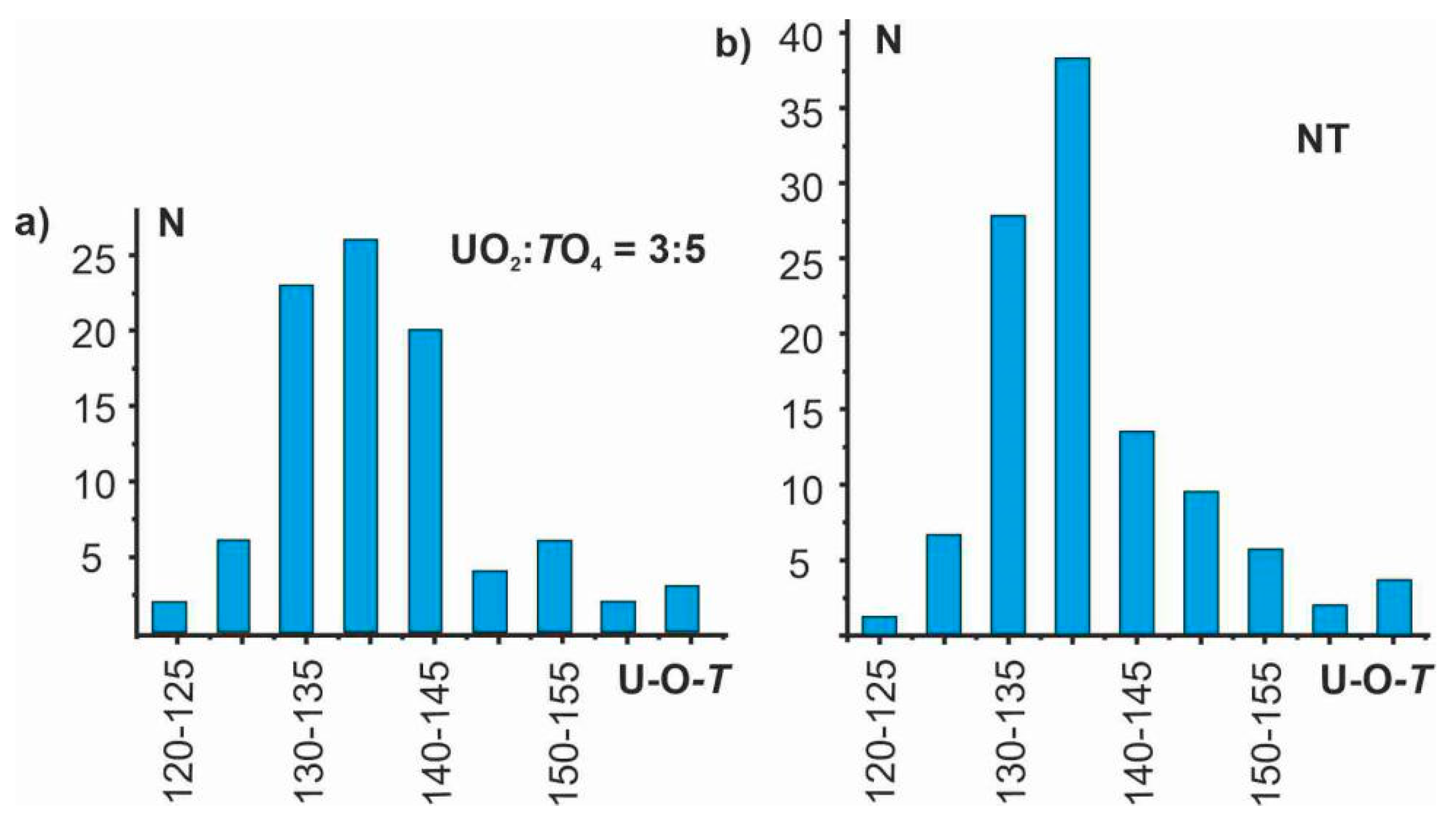
4.2. The Flexibility of U-O-T Bridges
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belova, L.N. Zones of Oxidation of Hydrothermal Deposits of Uranium; Nedra: Moscow, Russia, 1975; p. 158. [Google Scholar]
- Hazen, R.M.; Ewing, R.C.; Sverjensky, D.A. Evolution of uranium and thorium minerals. Am. Mineral. 2009, 94, 1293–1311. [Google Scholar] [CrossRef]
- Finch, R.J.; Murakami, T. Systematics and paragenesis of uranium minerals. In Uranium: Mineralogy, Geochemistry and the Environment; Burns, P.C., Finch, R.J., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 1999; Reviews in Mineralogy; Volume 38, pp. 91–180. [Google Scholar]
- Baker, R.J. Uranium minerals and their relevance to long term storage of nuclear fuels. Coord. Chem. Rev. 2014, 266–267, 123–136. [Google Scholar] [CrossRef]
- Andrews, M.B.; Cahill, C.L. Uranyl bearing hybridmaterials: Synthesis, speciation, and solid-state structures. Chem. Rev. 2013, 113, 1121–1136. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, T.; Mihalcea, I.; Henry, N.; Volkringer, C. The crystal chemistry of uranium carboxylates. Coord. Chem. Rev. 2014, 266–267, 69–109. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural crystallography of inorganic oxysalts. Crystallogr. Rev. 2009, 15, 279–281. [Google Scholar]
- Krivovichev, S.V.; Plášil, J. Mineralogy and crystallography of uranium. In Uranium: Cradle to Grave; Mineralogical Association of Canada Short Course Series; Burns, P.C., Sigmon, G.E., Eds.; Mineralogical Association of Canada: Quebec, QC, Canada, 2013; pp. 15–120. [Google Scholar]
- Kampf, A.R.; Plášil, J.; Kasatkin, A.V.; Marty, J. Belakovskiite, Na7(UO2)(SO4)4(SO3OH)(H2O)3, a new uranyl sulfate mineral from the Blue Lizard mine, San Juan County, Utah, USA. Mineral. Mag. 2014, 78, 639–649. [Google Scholar] [CrossRef]
- Plášil, J.; Kampf, A.R.; Kasatkin, A.V.; Marty, J. Bluelizardite, Na7(UO2)(SO4)4Cl(H2O)2, a new uranyl sulfate mineral from the Blue Lizard mine, San Juan County, Utah, USA. J. Geosci. Czech. 2014, 59, 145–158. [Google Scholar] [CrossRef]
- Gurzhiy, V.V.; Plášil, J. Structural complexity of natural uranyl sulfates. Acta Crystallogr. Sect. B 2018, 75, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Kampf, A.R.; Plášil, J.; Kasatkin, A.V.; Marty, J. Bobcookite, NaAl(UO2)2(SO4)4·18H2O and wetherillite, Na2Mg(UO2)2(SO4)4×18H2O, two new uranyl sulfate minerals from the Blue Lizard mine, San Juan County, Utah, USA. Mineral. Mag. 2015, 79, 695–714. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Kasatkin, A.V.; Marty, J.; Čejka, J. Fermiite, Na4(UO2)(SO4)3∙3H2O and oppeneimerite, Na2(UO2)(SO4)2·3H2O, two new uranyl sulfate minerals from the Blue Lizard mine, San Juan County, Utah, USA. Mineral. Mag. 2015, 79, 1123–1142. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Burns, P.C.; Tananaev, I.G. (Eds.) Structural Chemistry of Inorganic Actinide Compounds; Elsevier: Amsterdam, The Netherlands, 2007; p. 494. [Google Scholar]
- Kampf, A.R.; Sejkora, J.; Witzke, T.; Plášil, J.; Čejka, J.; Nash, B.P.; Marty, J. Rietveldite, Fe(UO2)(SO4)2(H2O)5, a new uranyl sulfate mineral from Giveaway-Simplot mine (Utah, USA), Willi Agatz mine (Saxony, Germany) and Jáchymov (Czech Republic). J. Geosci. Czech. 2017, 62, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Plášil, J.; Hloušek, J.; Kasatkin, A.V.; Novak, M.; Čejka, J.; Lapčák, L. Svornostite, K2Mg[(UO2)(SO4)2]2 8H2O, a new uranyl sulfate mineral from Jáchymov, Czech Republic. J. Geosci. Czech. 2015, 60, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Plášil, J.; Kampf, A.R.; Kasatkin, A.V.; Marty, J.; Skoda, R.; Silva, S.; Cejka, K. Meisserite, Na5(UO2)(SO4)3(SO3OH)(H2O), a new uranyl sulfate mineral from the Blue Lizard mine, San Juan County, Utah, USA. Mineral. Mag. 2013, 77, 2975–2988. [Google Scholar] [CrossRef]
- Nazarchuk, E.V.; Charkin, D.O.; Siidra, O.I.; Gurzhiy, V.V. Crystal-chemical features of U(VI) compounds with inorganic complexes derived from [(UO2)(TO4)(H2O)n], T = S, Cr, Se: Synthesis and crystal structures of two new uranyl sulfates. Radiochemistry 2018, 60, 345–351. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Kasatkin, A.V.; Marty, J.; Čejka, J.; Lapčák, L. Shumwayite, [(UO2)(SO4)(H2O)2]2 H2O, a new uranyl sulfate mineral from Red Canyon, San Juan County, Utah, USA. Mineral. Mag. 2017, 81, 273–285. [Google Scholar] [CrossRef]
- Pushcharovskii, D.Y.; Rastsvetaeva, R.K.; Sarp, H. Crystal structure of deloryite, Cu4(UO2)(Mo2O8)(OH)6. J. Alloys Compd. 1996, 239, 23–26. [Google Scholar] [CrossRef]
- Mereiter, K. The crystal structure of walpurgite, (UO2)Bi4O4(AsO4)2·2H2O. Tschermaks Mineral. Petrogr. Mitt. 1982, 30, 129–139. [Google Scholar] [CrossRef]
- Krause, W.; Effenberger, H.; Brandstaetter, F. Orthowalpurgite, (UO2)Bi4O4(AsO4)2·2(H2O), a new mineral from the Black Forest, Germany. Eur. J. Inorg. Mineral. 1995, 7, 1313–1324. [Google Scholar]
- Locock, A.J.; Burns, P.C. The crystal structure of synthetic autunite, Ca((UO2)(PO4))2(H2O)11. Am. Mineral. 2003, 88, 240–244. [Google Scholar] [CrossRef]
- Kampf, A.R.; Plášil, J.; Kasatkin, A.V.; Nash, B.P.; Marty, J. Magnesioleydetite and straβmannite, two new uranyl sulfate minerals with sheet structures from Red Canyon, Utah. Mineral. Mag. 2019, 83, 349–360. [Google Scholar] [CrossRef]
- Alekseev, E.V.; Suleimanov, E.V.; Chuprunov, E.V.; Marychev, M.O.; Ivanov, V.; Fukin, G.K. Crystal structures and nonlinear optical properties of K2UO2(SO4)2∙2H2O compound al 293K. Crystallogr. Rep. 2006, 51, 29–33. [Google Scholar] [CrossRef]
- Plášil, J.; Kasatkin, A.V.; Skoda, R.; Novak, M.; Kallistova, A.; Dusek, M.; Skala, R.; Fejfarova, K.; Cejka, J.; Meisser, N.; et al. Leydetite, Fe(UO2)(SO4)2(H2O)11, a new uranyl sulfate mineral from Mas d’Alary, Lodeve, France. Mineral. Mag. 2013, 77, 429–441. [Google Scholar] [CrossRef]
- Pekov, I.V.; Krivovichev, S.V.; Yapaskurt, V.O.; Chukanov, N.V.; Belakovskiy, D.I. Beshtauite, (NH4)2(UO2)(SO4)2·2(H2O), a new mineral from Mount Beshtau, Northern Caucasus, Russia. Am. Mineral. 2014, 99, 1783–1787. [Google Scholar] [CrossRef] [Green Version]
- Márquez-Zavalía, M.F.; Galliski, M.A. Goldichite of fumarolic origin from the Santa 1062 Bárbara mine, Jujuy, Northwestern Argentina. Can. Mineral. 1995, 33, 1059–1062. [Google Scholar]
- Ling, J.; Sigmon, G.E.; Ward, M.; Roback, N.; Burns, P.C. Syntheses, structures, and IR spectroscopic characterization of new uranyl sulfate/selenate 1D-chain, 2D-sheet and 3D-framework. Z. Kristallogr. 2010, 225, 230–239. [Google Scholar] [CrossRef]
- Doran, M.B.; Norquist, A.J.; O’Hare, D. Exploration of composition space in templated uranium sulfates. Inorg. Chem. 2003, 42, 6989–6995. [Google Scholar] [CrossRef]
- Norquist, A.J.; Doran, M.B.; Thomas, P.M.; O’Hare, D. Structural diversity in organically templated uranium sulfates. Dalton Trans. 2003, 6, 1168–1175. [Google Scholar] [CrossRef]
- Siidra, O.I.; Nazarchuk, E.V.; Suknotova, A.N.; Kayukov, R.A.; Krivovichev, S.V. Cr(VI) trioxide as a starting material for the synthesis of novel zero-, one-, and two-dimensional uranyl dichromates and chromate-dichromates. Inorg. Chem. 2013, 52, 4729–4735. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Cryst. 2015, B71, 561–578. [Google Scholar] [CrossRef] [Green Version]
- Halasyamani, P.S.; Francis, R.J.; Walker, S.M.; O’Hare, D. New layered uranium(VI) molybdates: syntheses and structures of (NH3(CH2)3NH3)(H3O)2(UO2)3(MoO4)5, C(NH2)3(UO2)(OH)(MoO4), (C4H12N2)(UO2)(MoO4)2 and (C5H14N2)(UO2)(MoO4)2H2O. Inorg. Chem. 1999, 38, 271–279. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Tananaev, I.G.; Myasoedov, B.F. Geometric isomerism of layered complexes of uranyl selenates: Synthesis and structure of (H3O)[C5H14N]2[(UO2)3(SeO4)4(HSeO4)(H2O)] and (H3O)[C5H14N]2[(UO2)3(SeO4)4(HSeO4)(H2O)](H2O). Radiochemistry 2006, 48, 552–560. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Burns, P.C. Crystal chemistry of K uranyl chromates: Crystal structures of K5[(UO2)(CrO4)3](NO3)(H2O)3, K4[(UO2)3(CrO4)5](H2O)7 and K2[(UO2)2(CrO4)3](H2O)6. Z. Kristallogr. Cryst. Mater. 2003, 218, 725–730. [Google Scholar]
- Moore, P.B. Laueite, pseudolaueite, stewartite and metavauxite: A study in combinatorial polymorphism. Neu. Jb. Mineral. Abh. 1975, 123, 148–159. [Google Scholar]
- Krivovichev, S.V.; Tananaev, I.G. Microscopic model of crystal genesis from aqueous solution of uranyl selenates. J. Sib. Fed. Uni. Chem. 2009, 2, 133–149. [Google Scholar]
- Krivovichev, S.V.; Burns, P.C. The modular structure of the novel uranyl sulfate sheet in [Co(H2O)6]3[(UO2)5(SO4)8(H2O)](H2O)5. J. Geosci. Czech. 2014, 59, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Nazarchuk, E.V.; Siidra, O.I.; Charkin, D.O. Specific features of the crystal chemistry of layered uranyl compounds with the ratio UO2:TO4 = 5:8 (T = S6+, Cr6+, Se6+, Mo6+). Radiochemistry 2018, 60, 352–361. [Google Scholar] [CrossRef]
- Nazarchuk, E.V.; Charkin, D.O.; Kozlov, D.V.; Siidra, O.I.; Kalmykov, S.N. Topological analysis of the layered uranyl compounds bearing slabs with UO2:TO4 ratio of 2:3. Radiochim. Acta. 2020, 108, 249–260. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Tananaev, I.G.; Kahlenberg, V.; Kaindl, R.; Myasoedov, B.F. Synthesis, structure, and properties of inorganic nanotubes based on uranyl selenates. Radiochemistry 2005, 47, 525–536. [Google Scholar] [CrossRef]
- Alekseev, E.V.; Krivovichev, S.V.; Depmeier, W. A crown ether as template for microporous and nanostructured uranium compounds. Angew. Chem. Int. Ed. 2008, 47, 549–551. [Google Scholar] [CrossRef]
- Siidra, O.I.; Nazarchuk, E.V.; Charkin, D.O.; Chukanov, N.V.; Depmeier, W.; Bocharov, S.N.; Sharikov, M.I. Uranyl sulfate nanotubules templated by N-phenylglycine. Nanomaterials 2018, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Tenne, R.; Margulis, L.; Genut, M.; Hodes, G. Polyhedral and cylindrical structures of tungsten disulphide. Nature 1992, 360, 444–446. [Google Scholar] [CrossRef]
- Imai, H.; Matsuta, M.; Shimizu, K.; Hirashima, H.; Negishi, N. Preparation of TiO2 fibers with well-organized structures. J. Mater. Chem. 2000, 10, 2005–2006. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Burns, P.C. Geometrical isomerism in uranyl chromates II. Crystal structures of Mg2[(UO2)3(CrO4)5] 17(H2O) and Ca2[(UO2)3(CrO4)5] 19(H2O). Z. Crystallogr. 2003, 218, 683–690. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Kahlenberg, V. Structural diversity of sheets in rubidium uranyl oxoselenates: Synthesis and crystal structures of Rb2((UO2)(SeO4)2(H2O))(H2O), Rb2((UO2)2(SeO4)3(H2O)2)(H2O)4 and Rb4((UO2)3(SeO4)5(H2O)). Z. Anorg. Allg. Chem. 2005, 631, 739–744. [Google Scholar] [CrossRef]
- Nazarchuk, E.V.; Krivovichev, S.V.; Filatov, S.K. Phase transitions and high-temperature crystal chemistry of polymorphous modifications of Cs2(UO2)2(MoO4). Radiochemistry 2004, 46, 438–440. [Google Scholar] [CrossRef]
- Siidra, O.I.; Nazarchuk, E.V.; Kayukov, R.A.; Bubnova, R.S.; Krivovichev, S.V. CrVI–CrV transition in Uranyl Chromium compounds: Synthesis and high-temperature X-ray diffraction study of Cs2[(UO2)2(CrO4)3]. Z. Anorg. Allg. Chem. 2013, 639, 2302–2306. [Google Scholar] [CrossRef]
| Space Group | Cc |
|---|---|
| a(Å) | 16.768 (6) |
| b(Å) | 20.553 (8) |
| c(Å) | 11.897 (4) |
| β, ° | 108.195 (7) |
| V(Å3) | 3895 (2) |
| Dx | 3.155 |
| θ max (°). | 1.62–28.00 |
| No. of measured, independent reflections | 9176/7160 |
| Rint | 0.0694 |
| Rsigma | 0.1213 |
| wR1 | 0.1182 |
| R1 | 0.0520 |
| S | 0.944 |
| CCDC | 2014144 |
| U1-O3 | 1.765 (13) | U2-O1 | 1.724 (15) | U3-O4 | 1.729 (14) |
| U1-O5 | 1.796 (12) | U2-O2 | 1.767 (15) | U3-O8 | 1.791 (13) |
| <Ur1-O> | 1.780 | <Ur2-O> | 1.745 | <Ur3-O> | 1.760 |
| U1-O12 | 2.299 (12) | U2-O13 | 2.304 (11) | U3-O26 | 2.287 (14) |
| U1-O18 | 2.36 7(11) | U2-O9 | 2.320 (10) | U3-O24 | 2.332 (12) |
| U1-O22 | 2.371 (11) | U2-O23 | 2.372 (11) | U3-O20 | 2.369 (10) |
| U1-O16 | 2.408 (10) | U2-O17 | 2.375 (11) | U3-O11 | 2.391 (11) |
| U1-O7 | 2.437 (11) | U2-O6 | 2.583 (12) | U3-O19 | 2.497 (12) |
| <U1-Oeq> | 2.376 | <U2-Oeq> | 2.391 | <U3-Oeq> | 2.375 |
| Mo1-O21 | 1.688 (12) | Mo2-O10 | 1.691 (15) | Mo3-O15 | 1.698 (13) |
| Mo1-O22 | 1.735 (11) | Mo2-O6 | 1.742 (13) | Mo3-O17 | 1.774 (10) |
| Mo1-O9 | 1.770 (11) | Mo2-O16 | 1.766 (10) | Mo3-O18 | 1.779 (11) |
| Mo1-O12 | 1.776 (12) | Mo2-O11 | 1.798 (11) | Mo3-O7 | 1.789 (11) |
| <Mo1-O> | 1.742 | <Mo2-O> | 1.749 | <Mo3-O> | 1.760 |
| Mo4-O14 | 1.703 (13) | Mo5-O25 | 1.666 (16) | ||
| Mo4-O19 | 1.761 (12) | Mo5-O26 | 1.702 (14) | ||
| Mo4-O23 | 1.761 (11) | Mo5-O24 | 1.765 (13) | ||
| Mo4-O20 | 1.795 (11) | Mo5-O13 | 1.767 (12) | ||
| <Mo4-O> | 1.755 | <Mo5-O> | 1.725 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarchuk, E.; Charkin, D.; Siidra, O.; Kalmykov, S. Organically Templated Layered Uranyl Molybdate [C3H9NH+]4[(UO2)3(MoO4)5] Structurally Based on Mineral-Related Modular Units. Minerals 2020, 10, 659. https://doi.org/10.3390/min10080659
Nazarchuk E, Charkin D, Siidra O, Kalmykov S. Organically Templated Layered Uranyl Molybdate [C3H9NH+]4[(UO2)3(MoO4)5] Structurally Based on Mineral-Related Modular Units. Minerals. 2020; 10(8):659. https://doi.org/10.3390/min10080659
Chicago/Turabian StyleNazarchuk, Evgeny, Dmitri Charkin, Oleg Siidra, and Stepan Kalmykov. 2020. "Organically Templated Layered Uranyl Molybdate [C3H9NH+]4[(UO2)3(MoO4)5] Structurally Based on Mineral-Related Modular Units" Minerals 10, no. 8: 659. https://doi.org/10.3390/min10080659
APA StyleNazarchuk, E., Charkin, D., Siidra, O., & Kalmykov, S. (2020). Organically Templated Layered Uranyl Molybdate [C3H9NH+]4[(UO2)3(MoO4)5] Structurally Based on Mineral-Related Modular Units. Minerals, 10(8), 659. https://doi.org/10.3390/min10080659







