Multi-Technique Characterization of a Fine Fraction of CDW and Assessment of Reactivity in a CDW/Lime System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Pozzolanicity Test
2.2.2. Instrumental Techniques
3. Results and Discussion
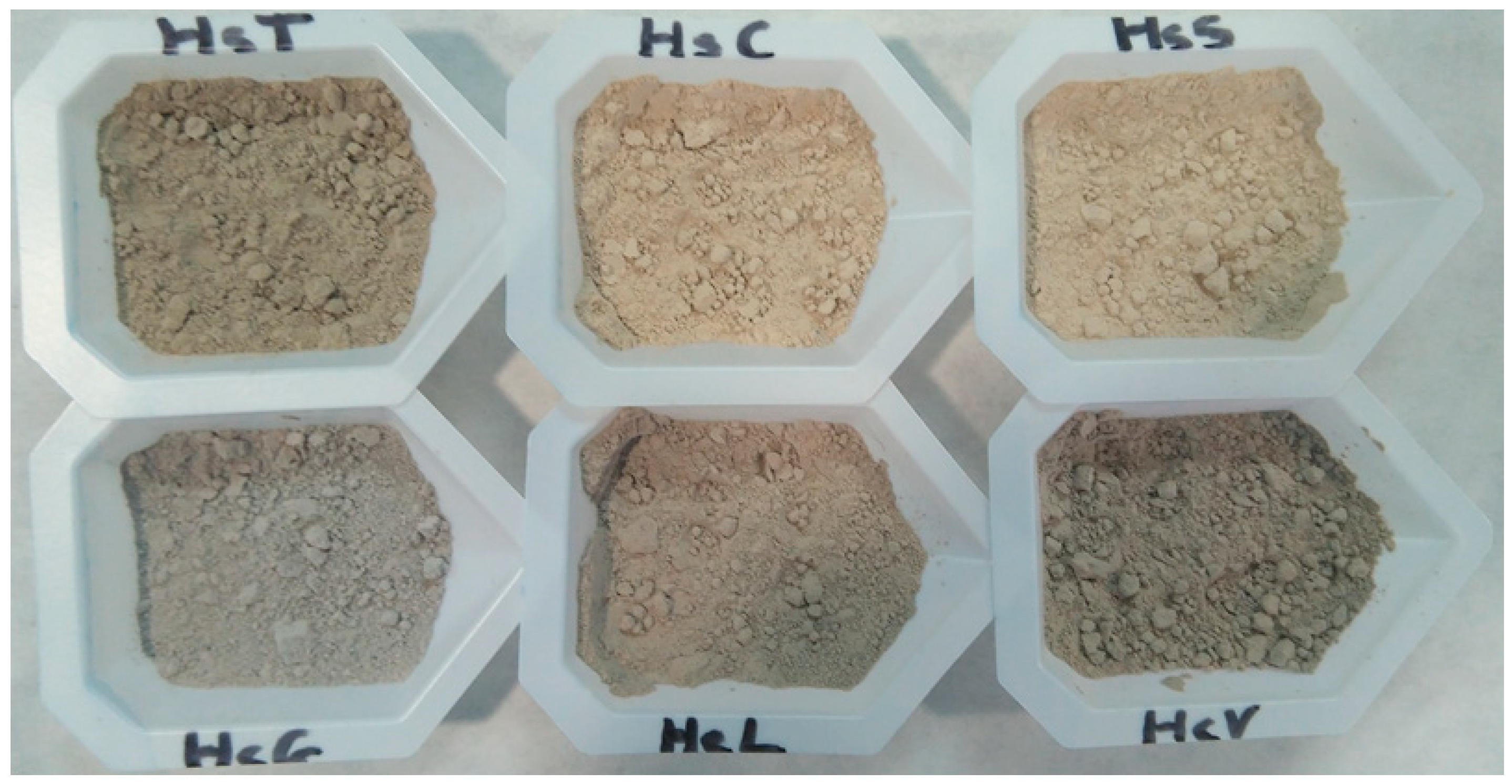
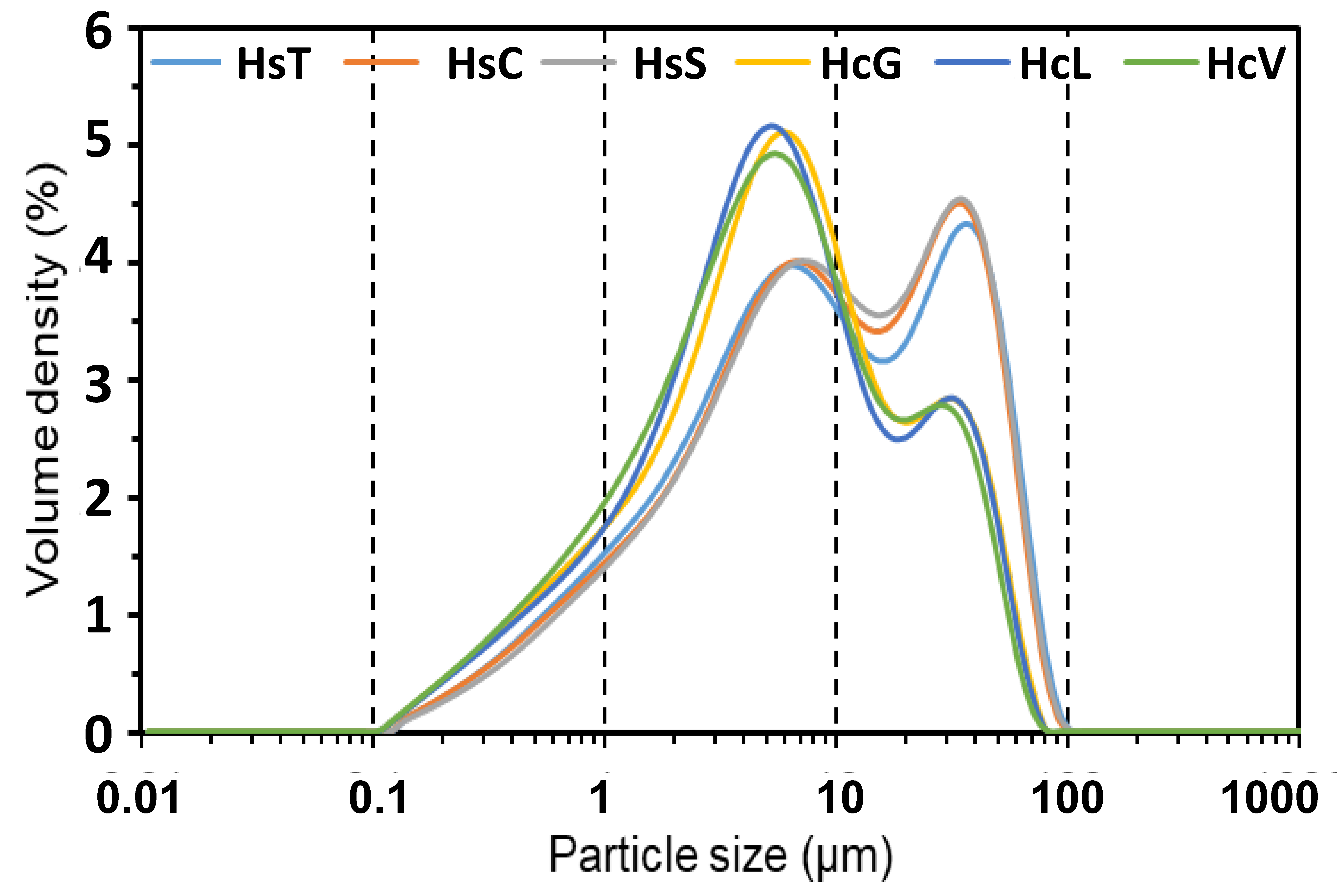
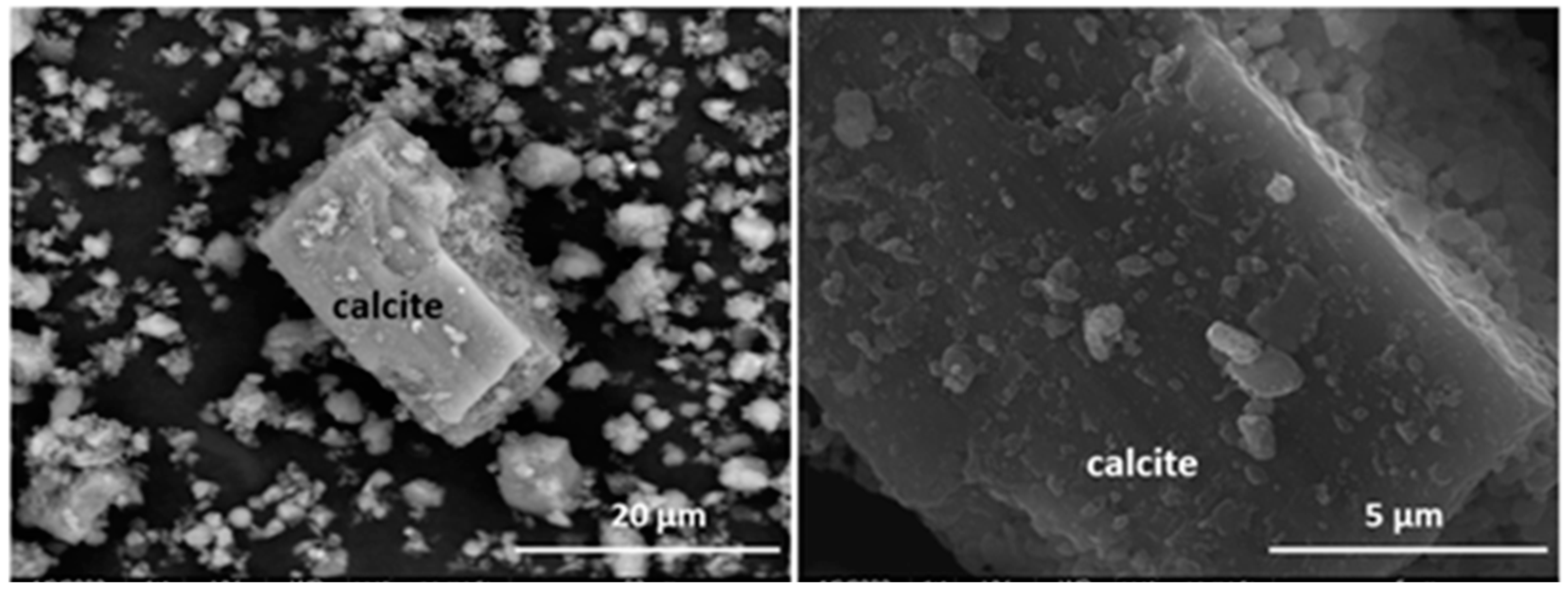
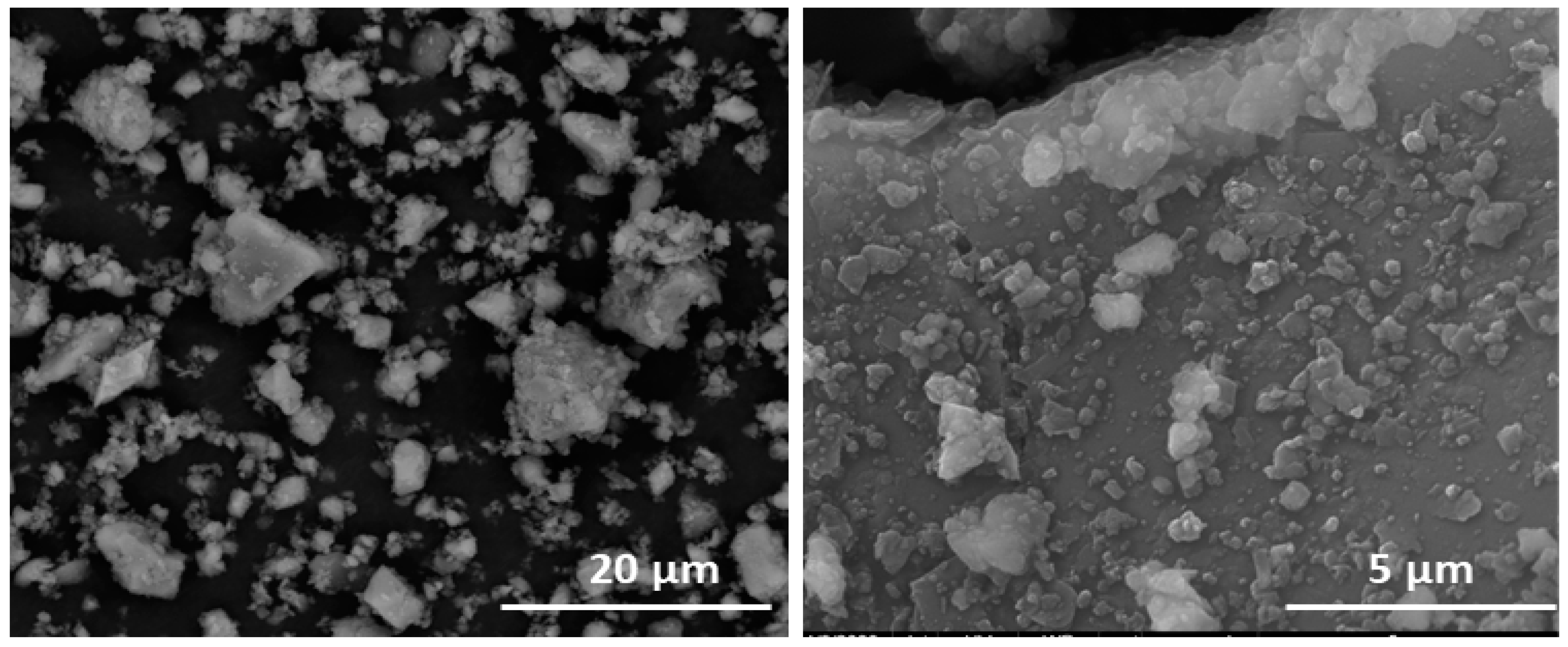
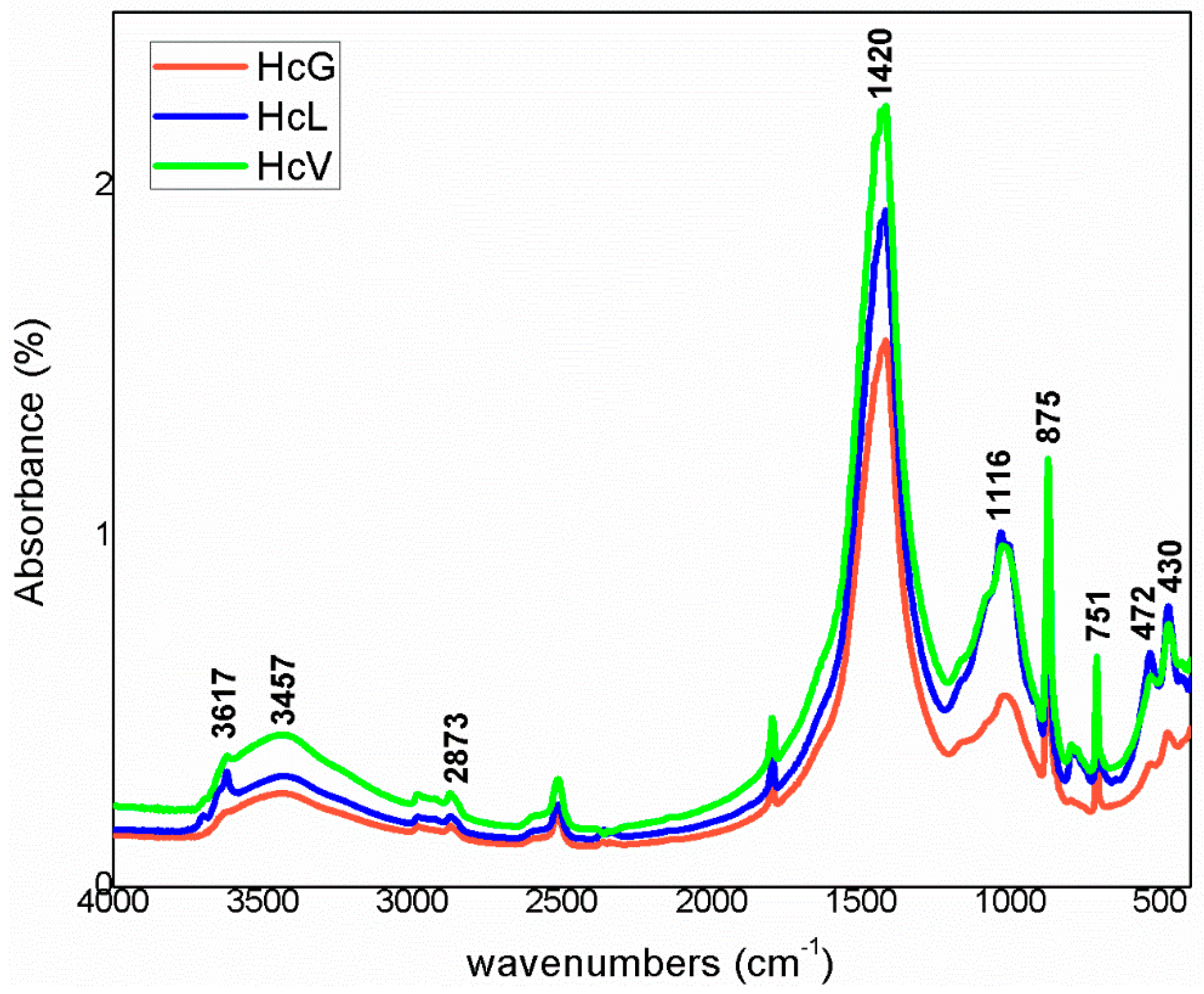
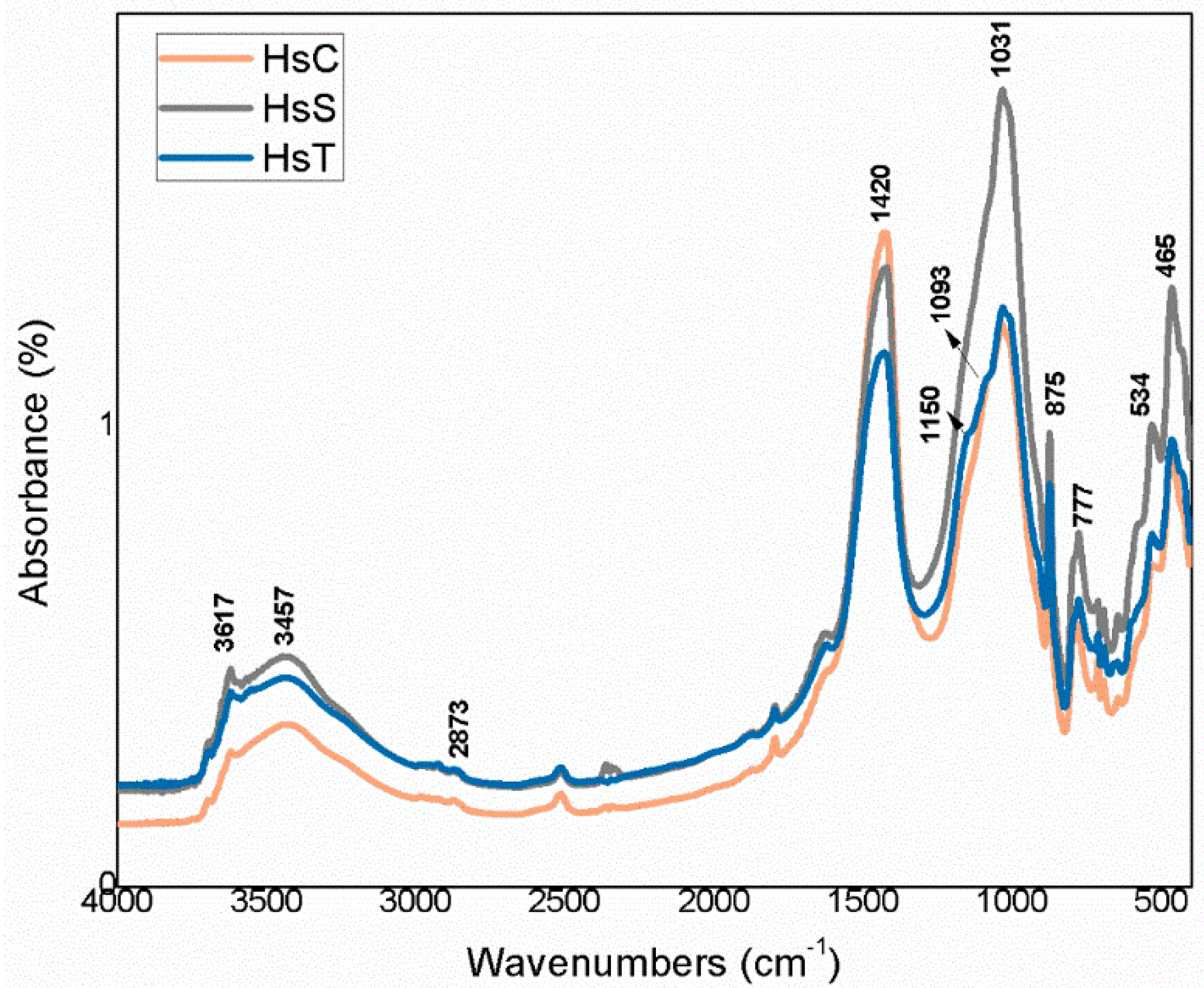
3.1. Starting Material Characterisation
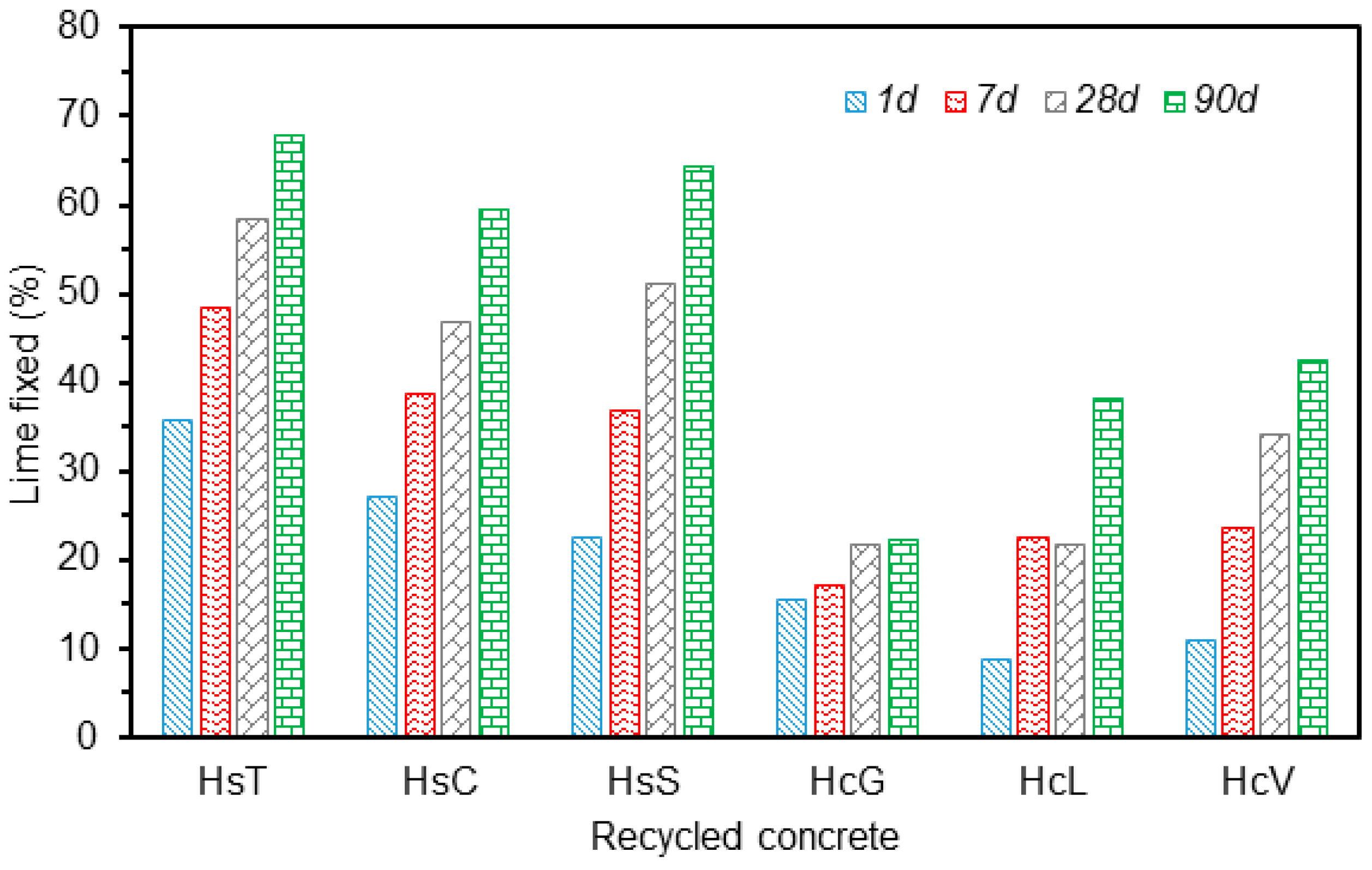
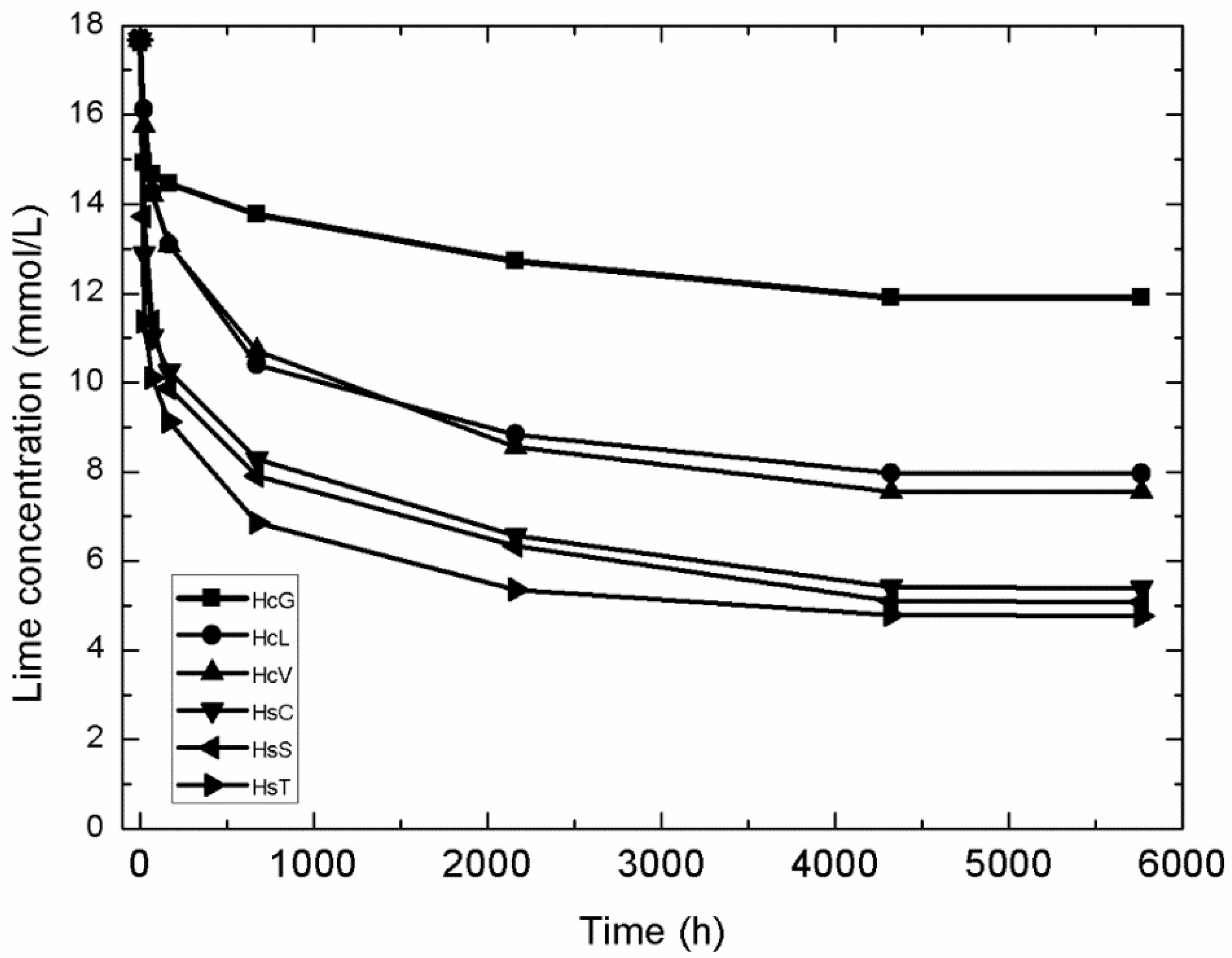
3.2. Variation in Lime Fixed with Time
3.3. Variation in Mineralogical Phases during the Pozzolanic Reaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hafliger, I.F.; John, V.; Passer, A.; Lasvaux, S.; Hoxha, E.; Saade, R.M.; Habert, G. Buildings environmental impacts’ sensitivity related to LCA modelling choices of construction materials. J. Clean. Prod. 2017, 156, 805–816. [Google Scholar] [CrossRef]
- Oliveira, M.L.S.; Izquierdo, M.; Querol, X.; Lieberman, R.N.; Saikia, B.K.; Silva, L.F.O. Nanoparticles from construction wastes: A problem to health and the environment. J. Clean. Prod. 2019, 219, 236–243. [Google Scholar] [CrossRef]
- Chinda, T. Investigation of factors affecting a construction waste recycling decision. Civ. Eng. Environ. Syst. 2016, 33, 214–226. [Google Scholar] [CrossRef]
- EU Construction and Demolition Waste. Protocol and Guidelines. Available online: https://ec.europe.eu/growth/content/eu-construction and demolition protocol-0 (accessed on 12 January 2020).
- Medina, C.; Zhu, W.; Howind, T.; Frías, M.; Sanchez de Rojas, M.I. Effect of the constituents (asphalt, clay materials, floating particles and fines) of construction and demolition waste on the properties of recycled concretes. Constr. Build. Mater. 2015, 79, 22–33. [Google Scholar] [CrossRef]
- Asociación Española de Residuos de Construcción y Demolición. Informe Producción y Gestión de RCDs en España 2011–2015; Asociación Española de Residuos de Construcción y Demolición: Madrid, Spain, 2017. [Google Scholar]
- Khudyakova, L.I.; Kislov, E.V.; Paleev, P.L.; Kotova, I.Y. Nephrite-bearing mining waste as a promising mineral additive in the production of new cement types. Minerals 2020, 10, 394. [Google Scholar] [CrossRef]
- Moreno-Pérez, E.; Hernández-Ávila, J.; Rangel-Martínez, Y.; Cerecedo-Sáenz, E.; Arenas-Flores, A.; Reyes-Valderrama, M.I.; Salinas-Rodríguez, E. Chemical and mineralogical characterization of recycled aggregates from construction and demolition waste from Mexico City. Minerals 2018, 8, 237. [Google Scholar] [CrossRef]
- Circular Economy Action Plan. Available online: https://ec.europa.eu/environment/circular-economy/ (accessed on 15 January 2020).
- Guía Español de Áridos Reciclados Procedentes de RCD; Proyecto Gear; Government of Spain: Madrid, Spain, 2012.
- Gastaldi, D.; Canonico, F.; Capelli, L.; Buzzi, L.; Boccaleri, E.; Irico, S. An investigation on the recycling of hydrated cement from concrete demolition waste. Cem. Concr. Comp. 2015, 61, 29–35. [Google Scholar] [CrossRef]
- Fernández Ledesma, E.; Fernaádez Rodríguez, J.M.; Jiménez, J.R.; Galvin, A.P. Properties of masonry mortars manufactured with fine recycled concrete aggregates. Const. Buil. Mat. 2014, 71, 289–298. [Google Scholar] [CrossRef]
- Florea, M.V.A.; Ning, Z.; Brouwers, H.J.H. Activation of liberated concrete fines and their application in mortars. Const. Buil. Mat. 2014, 50, 1–12. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, J.; Liu, X.; Lin, Y.; Yu, H. Design and performance of emulsified asphalt mixtures containing construction and demolition waste. Constr. Build. Mater. 2020, 239. [Google Scholar] [CrossRef]
- Šljivić-Ivanović, M.; Smičiklas, I. Utilization of C&D waste in radioactive waste treatment—Current knowledge and perspectives. In Advances in Construction and Demolition Waste Recycling; Pacheco-Torgal, F., Ding, Y., Koutamanis, A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; Chapter 23; pp. 475–500. [Google Scholar]
- Asensio, E.A.; Medina, C.; Frías, M.; Sánchez de Rojas, M.I. Clay-based construction and demolition waste as a pozzolanic addition in blended cements. Effect on sulfate resistance. Constr. Build. Mater. 2016, 127, 950–958. [Google Scholar] [CrossRef]
- Asensio, E.; Medina, C.; Frías, M.; Sánchez de Rojas, M.I. Characterization of Ceramic-Based Construction and Demolition Waste: Use as Pozzolan in Cements. J. Am. Ceram. Soc. 2016, 99, 4121–4127. [Google Scholar] [CrossRef]
- Asensio, E.; Medina, C.; Frías, M.; Sánchez de Rojas, M.I. Use of clay-based construction and demolition waste as additions in the design of new low and very low heat of hydration cements. Mater. Struct. 2018, 51, 101–111. [Google Scholar] [CrossRef]
- Medina, C.; Banfill, P.F.G.; Sánchez de Rojas, M.I.; Frías, M. Rheological and calorimetric behaviour of cements blended with containing ceramic sanitary ware and construction/demolition waste. Constr. Build. Mater. 2013, 40, 822–831. [Google Scholar] [CrossRef]
- Krour, H.; Trauchessec, R.; Lecomte, A.; Diliberto, C.; Barnes-Davin, L.; Bolze, B.; Delhay, A. Incorporation rate of recycled aggregates in cement raw meals. Constr. Build. Mater. 2020, 248. [Google Scholar] [CrossRef]
- Contreras, M.; Teixeira, S.R.; Lucas, M.C.; Lima, L.C.N.; Cardoso, D.S.L.; da Silva, G.A.C.; Gregório, G.C.; de Souza, A.E.; dos Santos, A. Recycling of construction and demolition waste for producing new construction material (Brazil case-study). Constr. Build. Mater. 2016, 123, 594–600. [Google Scholar] [CrossRef]
- Omrane, M.; Rabehi, M. Effect of natural pozzolan and recycled concrete aggregates on thermal and physico-mechanical characteristics of self-compacting concrete. Constr. Build. Mater. 2020, 247. [Google Scholar] [CrossRef]
- Ferreira, S.R.L.; Anjos, M.A.S.; Nóbrega, A.K.C.; Pereira, J.E.S.; Ledesma, E.F. The role of powder content of the recycled aggregates of CDW in the behaviour of rendering mortars. Constr. Build. Mater. 2019, 208, 601–612. [Google Scholar] [CrossRef]
- Jesus, S.; Maia, C.; Brazão, C.; de Brito, J.; Veiga, R. Rendering mortars with incorporation of very fine aggregates from construction and demolition waste. Constr. Build. Mater. 2019, 229, 116844. [Google Scholar] [CrossRef]
- Ulsen, C.; Kahn, H.; Hawlitschek, G.; Masini, E.H.; Angulo, S.C.; John, V.M. Production of recycled sand from construction and demolition waste. Constr. Build. Mater. 2013, 40, 1168–1173. [Google Scholar] [CrossRef]
- European Commission. Resource Efficient Use of Mixed Wastes Improving Management of Construction and Demolition Waste. Final Report October 2017. Available online: https://op.europa.eu/en/publication-detail/-/publication/78e42e6c-d8a6-11e7-a506-01aa75ed71a1/language-en/format-PDF/source-118503004 (accessed on 10 October 2019).
- ACI 130R-19: Report on the Role of Materials in Sustainable Concrete Construction; American Concrete Institute: Farmington Hills, MI, USA, 2019.
- Instrucción Española del Hormigón Estructural (EHE-08), 5th ed.; Ministerio de Fomento: Madrid, Spain, 2011.
- NBN B 15-001/ PTV 406. Standaardisatie Vangeprefabriceerde Voorgespannen Betonliggers voor Kunstwerken; FEBEFAST: Belgium, Brussels, 2017. [Google Scholar]
- Standard DIN 4226-101. Recycled Aggregates for Concrete in Accordance with DIN EN 12620—Part 101: Types and Regulated Dangerous Substances; German Institute for Standardization: Berlin, Germany, 2017. [Google Scholar]
- Standard NTC2008. Norme Tecniche per le Costruzioni; Ministry of Infrastructure and Transport: Rome, Italy, 2008. [Google Scholar]
- Standard BS 8500-2:2015 Concrete—Complementary British Standard to BS EN 206. Specification for Constituent Materials and Concrete (+A2:2019); British Standards Institution: London, UK, 2015.
- Moreno-Juez, J.; Vegas, I.; Gebremariam, A.T.; García-Cortés, V.; Di Maio, F. Treatment of end-of-life concrete in an innovative heating-air classification system for circular cement-based products. J. Clean. Prod. 2020. [Google Scholar] [CrossRef]
- Moore, M.; Reynolds, R.C. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystal. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Le Saoût, G.; Kocaba, V.; Scrivener, K.L. Application of the Rietveld method to the analysis of anhydrous cements. Cem. Conc. Res. 2011, 41, 133–148. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Phase Identification from Powder Diffraction, Crystal Impact. 1997. Available online: http://www.crystalimpact.com/match (accessed on 15 June 2019).
- Tsubota, M.; Kitagawa, J. A necessary criterion for obtaining accurate lattice parameters by Rietveld method. Sci. Rep. 2017, 7, 15381. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, J.G.; Harnit, A.B. Microstructure and composition of the hydrated cement paste of an 84 old years old concrete bridge construction. Cem. Concr. Res. 1975, 5, 163–170. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Apply 29Si, 27Al MAS NMR and selective dissolution in identifying the reaction degree of alkali activated slag-fly ash composites. Ceram. Inter. 2017, 43, 12408–12419. [Google Scholar] [CrossRef]
- Hansen, M.R.; Jakobsen, H.J.; Sbisted, J. 29Si chemical shift anisotropies in calcium silicates from high-field 29Si MAS NMR spectroscopy. Inorg. Chem. 2003, 42, 2368–2377. [Google Scholar] [CrossRef]
- Spearing, D.R.; Stebbins, J.F. The 29Si shielding tensor in low quartz. Am. Mineral. 1989, 74, 956–959. [Google Scholar]
- Skibsted, J.; Henderson, E.; Jakobsen, H.J. Characterisation of calcium aluminate phases in cements by 27Al MAS NMR spectroscopy. Inor. Chem. 2003, 32, 1013–1027. [Google Scholar] [CrossRef]
- Müller, D.; Gessner, W.; Samoson, A.; Lippmaa, E.; Scheler, G. Solid-state 27Al NMR studies on polycrystalline aluminates of the system CaO-Al2O3. Polyhedron 1986, 5, 779–785. [Google Scholar] [CrossRef]
- Faucon, P.; Charpentier, T.; Bertrandie, D.; Nonat, A.; Virlet, J.; Petit, J.C. Characterisation of calcium aluminate hydrates and related hydrates of cement pastes by 27Al MQ-MAS NMR. Inorg. Chem. 1998, 37, 3726–3733. [Google Scholar] [CrossRef]
- Plevova, E.; Vaculikova, L.; Valovicova, V. Thermal analysis and FT-IR spectroscopy of synthetic clay mineral mixtures. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Sánchez de Rojas, M.I.; Frías, M. The pozzolanic activity of different materials. Its influence on the hydration heat in mortars. Cem. Concr. Res. 1996, 26, 203–2213. [Google Scholar]
- Frías, M.; Sánchez de Rojas, M.I.; Cristina, C. The influence of SiMn slag on chemical resistance of blended cement pastes. Constr. Build. Mater. 2009, 23, 1472–1475. [Google Scholar] [CrossRef]
- Villar-Cociña, E.; Valencia, E.; González-Rodriguez, R.; Hernández-Ruiz, J. Kinetics of the pozzolanic reaction between lime and sugar cane straw ash by electrical conductivity measurement: A kinetic-diffusive model. Cem. Concr. Res. 2003, 33, 517–524. [Google Scholar] [CrossRef]
- Villar-Cociña, E.; Frías, M.; Valencia, E.; Sánchez de Rojas, M.I. An evaluation of different kinetic models for determining the kinetic coefficients in sugar cane straw-clay ash/lime system. Adv. Cem. Res. 2006, 18, 17–26. [Google Scholar] [CrossRef]
- Frías, M.; Villar-Cociña, E.; Sánchez de Rojas, M.I.; Valencia-Morales, E. The effect of different pozzolanic activity methods on the kinetics constants of the pozzolanic reaction: Application of a kinetic-diffusive model. Cem. Concr. Res. 2005, 35, 2137–2142. [Google Scholar] [CrossRef]
- Villar-Cociña, E.; Frías, M.; Valencia-Morales, E. Sugar cane wastes as pozzolanic materials: Application of mathematical model. ACI Mater. J. 2008, 105, 258–264. [Google Scholar]
- Qoku, E.; Bier, T.A.; Westphal, T. Phase in assemblage in ettringite-forming cement pastes: A X-Ray diffraction and thermal analyses characterization. J. Build. Eng. 2017, 12, 37–50. [Google Scholar] [CrossRef]
- Yu, J.; Qian, J.; Tang, J.; Ji, Z.; Fan, Y. Effect of ettringite seed crystals on the properties sulphoaluminate cement. Constr. Build. Mater. 2019, 207, 249–257. [Google Scholar] [CrossRef]
- Cody, A.M.; Lee, H.; Cody, R.D.; Spry, P.G. The effects of chemical environment on the nucleation, growth, and stability of ettringite (Ca3Al (OH)6) (SO4)3·26H2O. Cem. Concr. Res. 2004, 34, 869–881. [Google Scholar] [CrossRef]
- Shimada, Y.; Young, J.F. Thermal stability of ettringite in alkaline solutions at 80 °C. Cem. Concr. Res. 2004, 34, 2261–2268. [Google Scholar] [CrossRef]
- Glasser, F.P. The role of sulfate mineralogy and cure temperature in delayed ettringite formation. Cem. Concr. Comp. 1996, 18, 187–193. [Google Scholar] [CrossRef]
- Diamond, S. Delayed Ettringite Formation—Processes and Problems. Cem. Concr. Comp. 1996, 18, 205–215. [Google Scholar] [CrossRef]
- Escadeillas, G.; Aubert, J.E.; Segerer, M.; Prince, W. Some factors affecting delayed ettringite formation in heat-cured mortars. Cem. Concr. Res. 2007, 37, 1445–1452. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A practical Guide to Microstructural Analysis of Cementitious Materials, 1st ed.; CCR Press: Boca Raton, FL, USA, 2016. [Google Scholar]
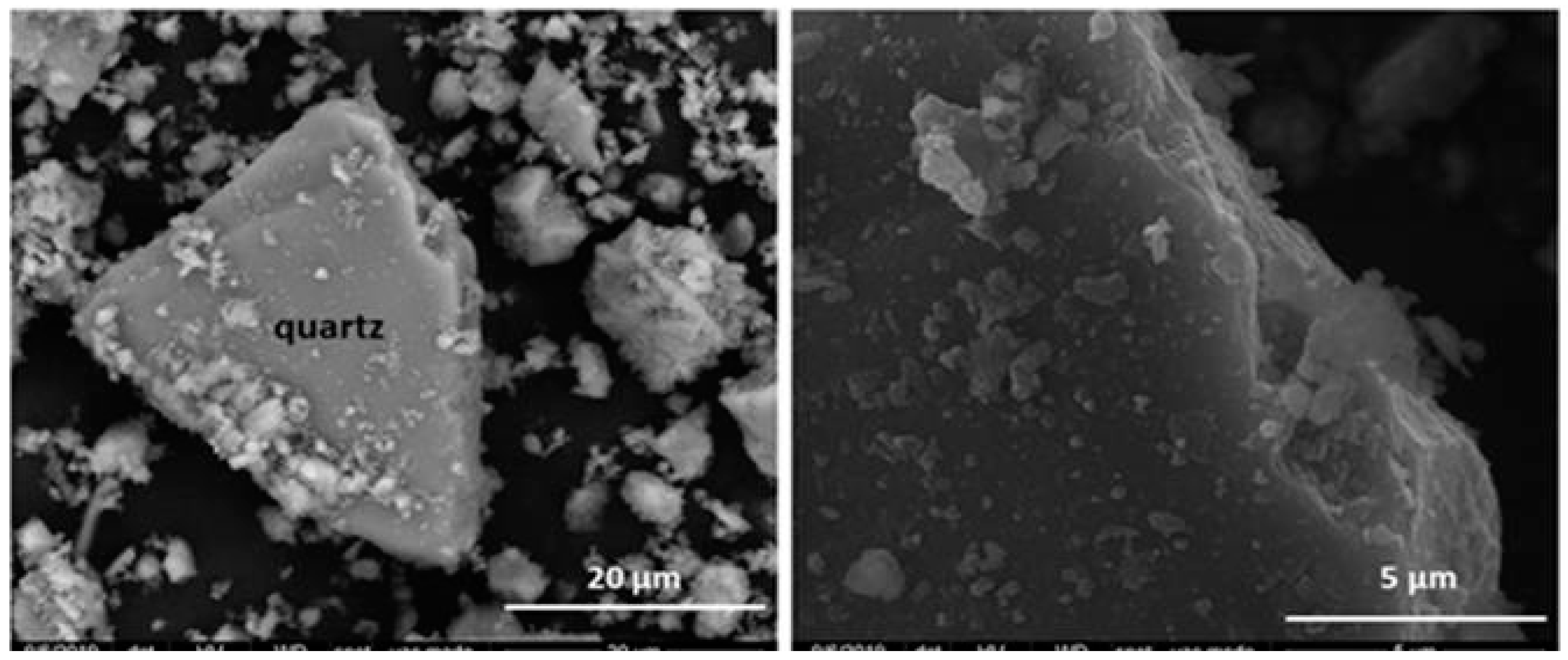
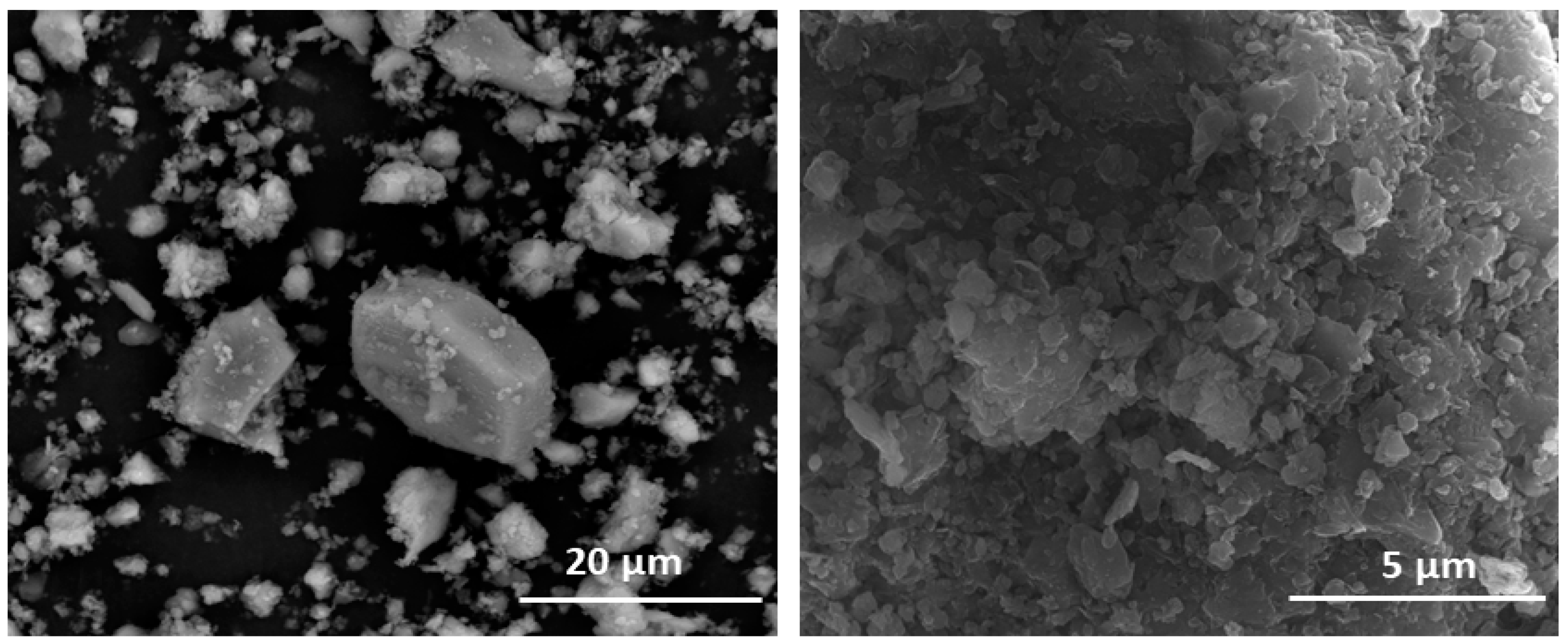
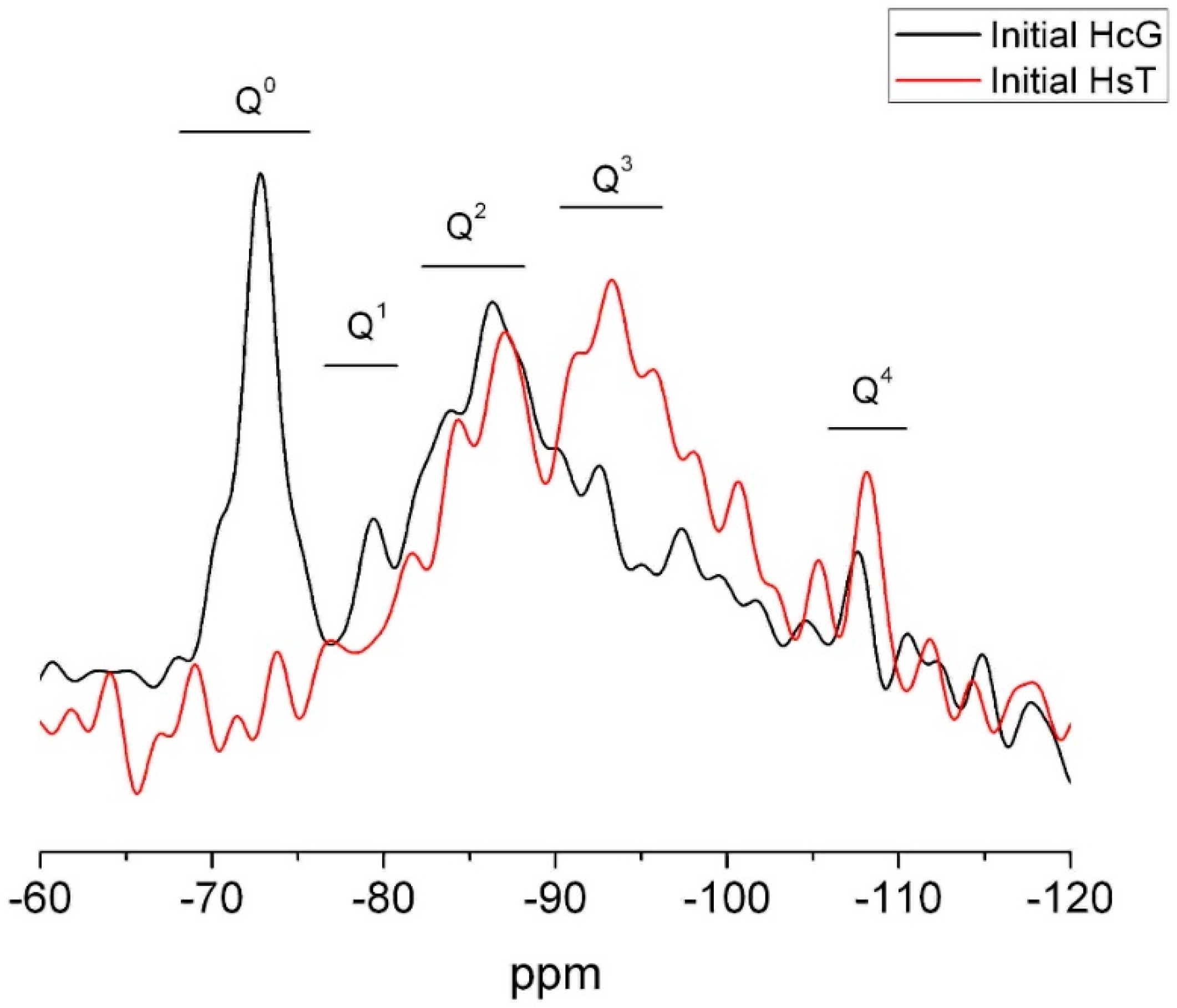
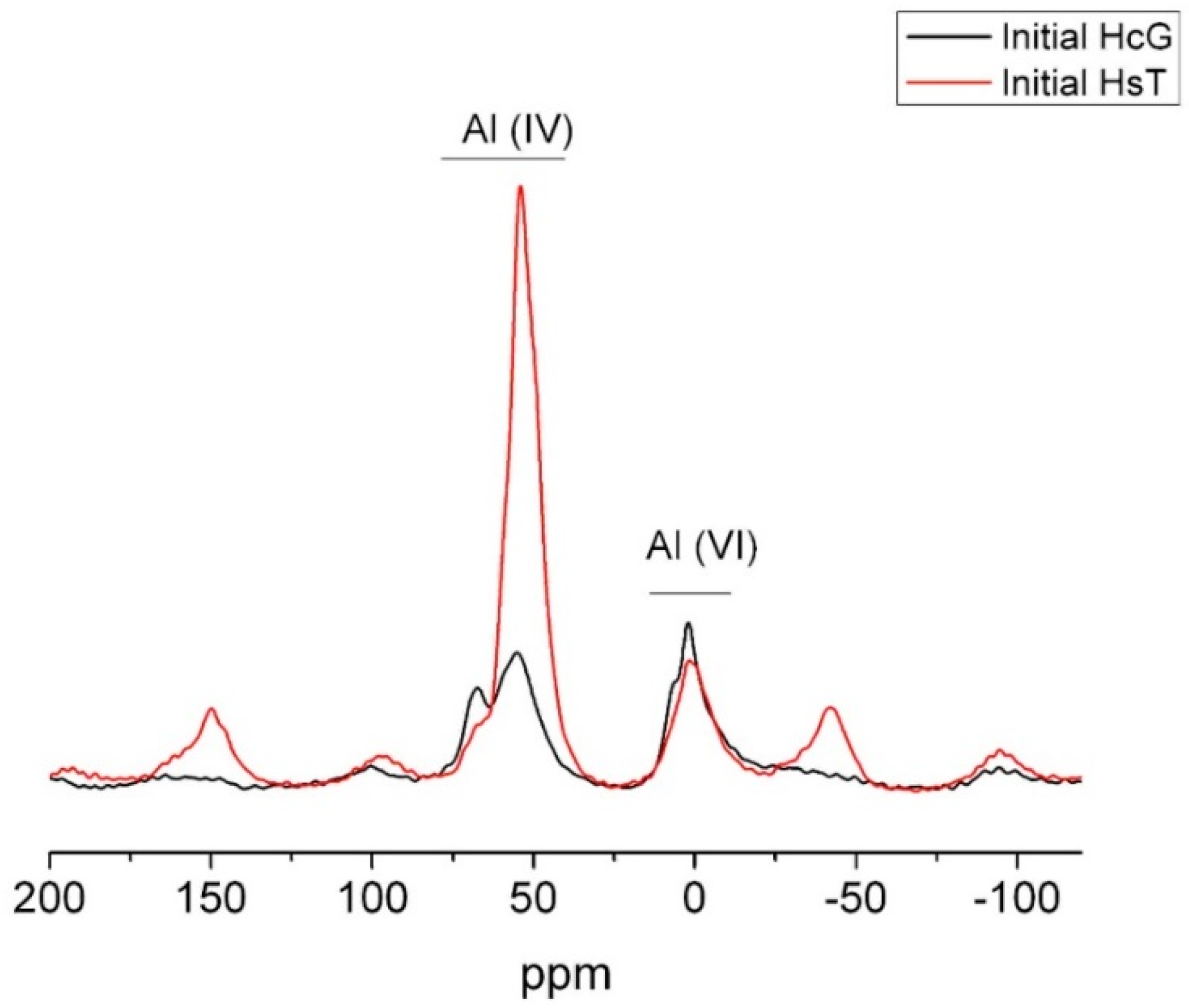
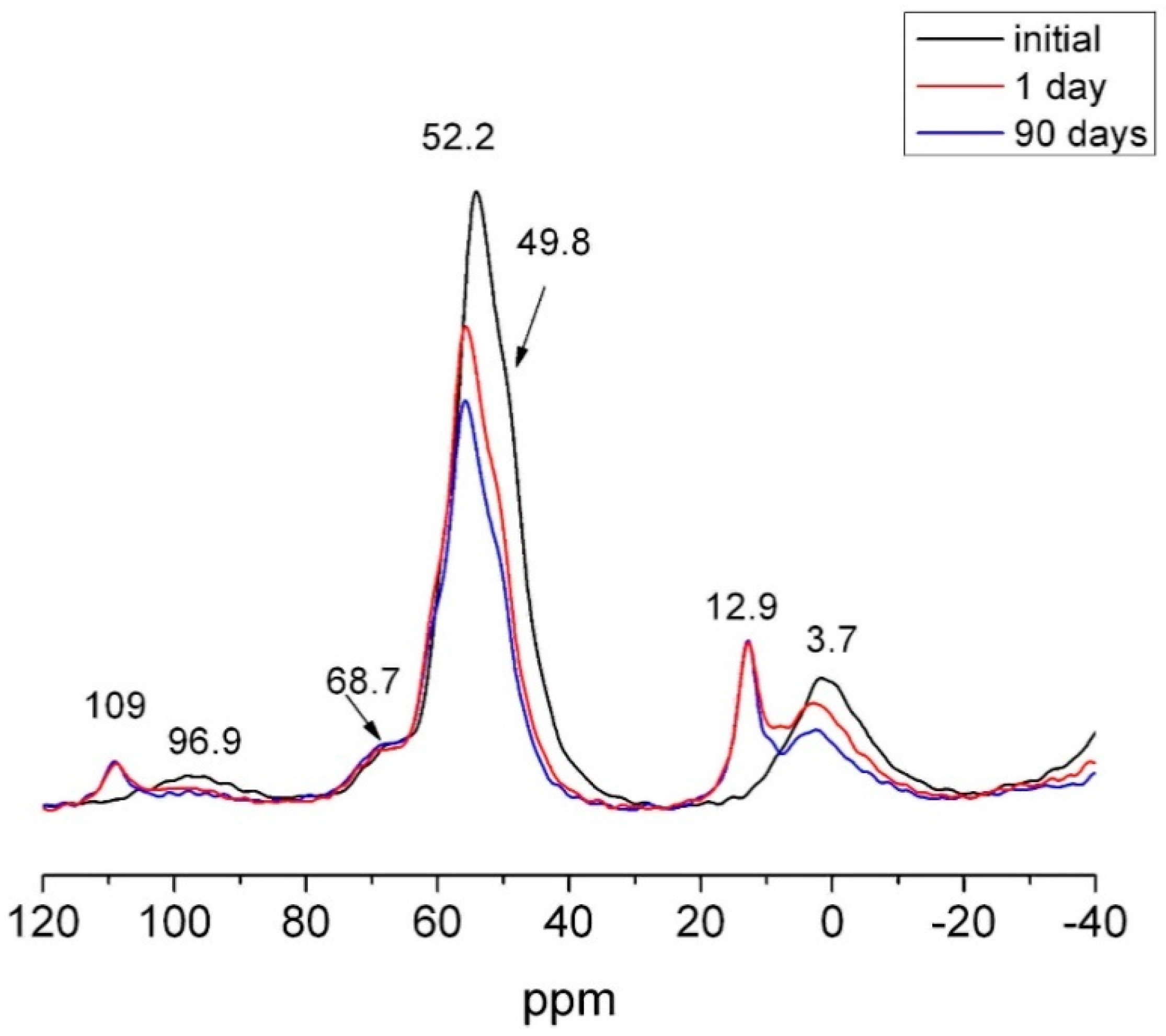
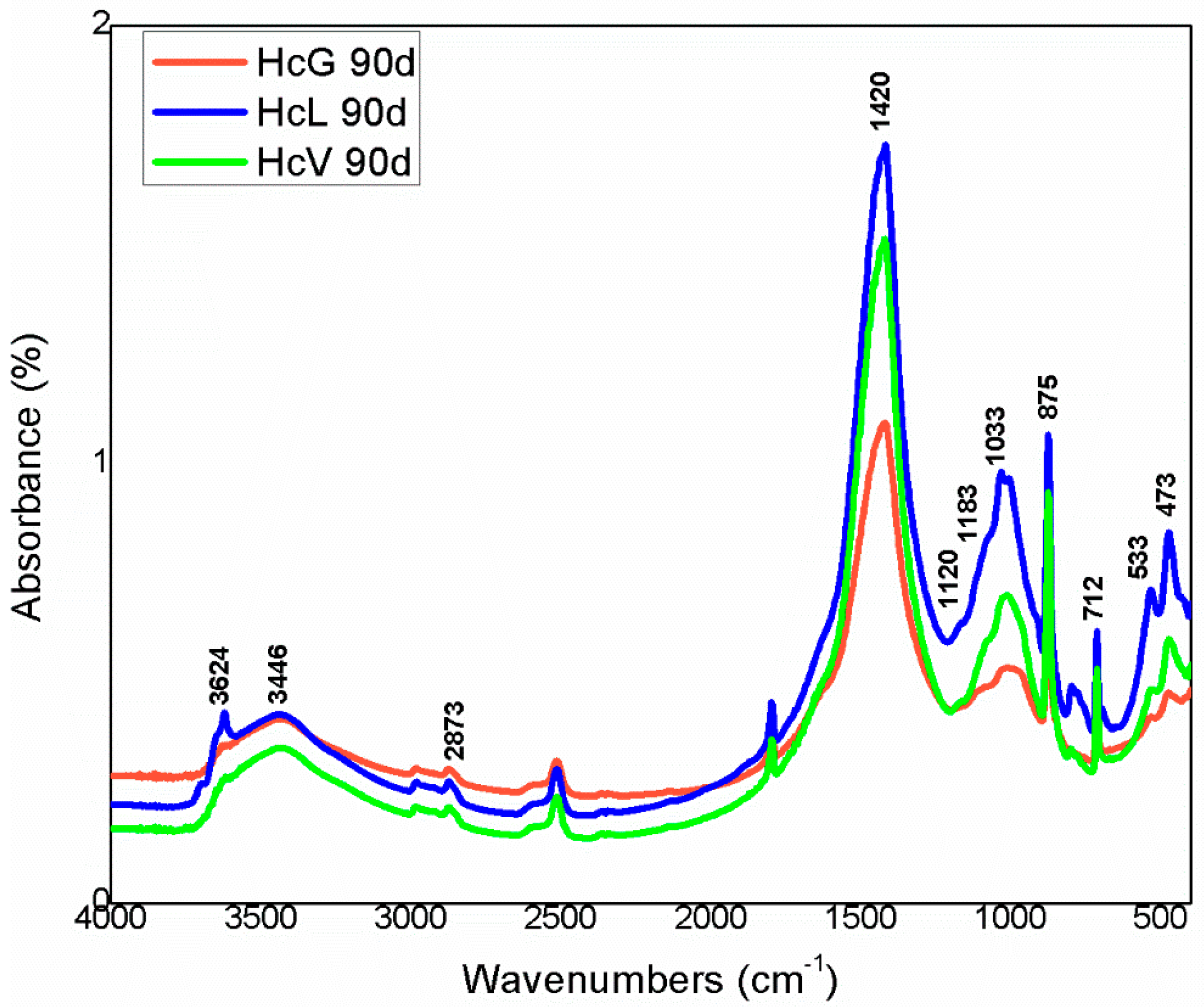
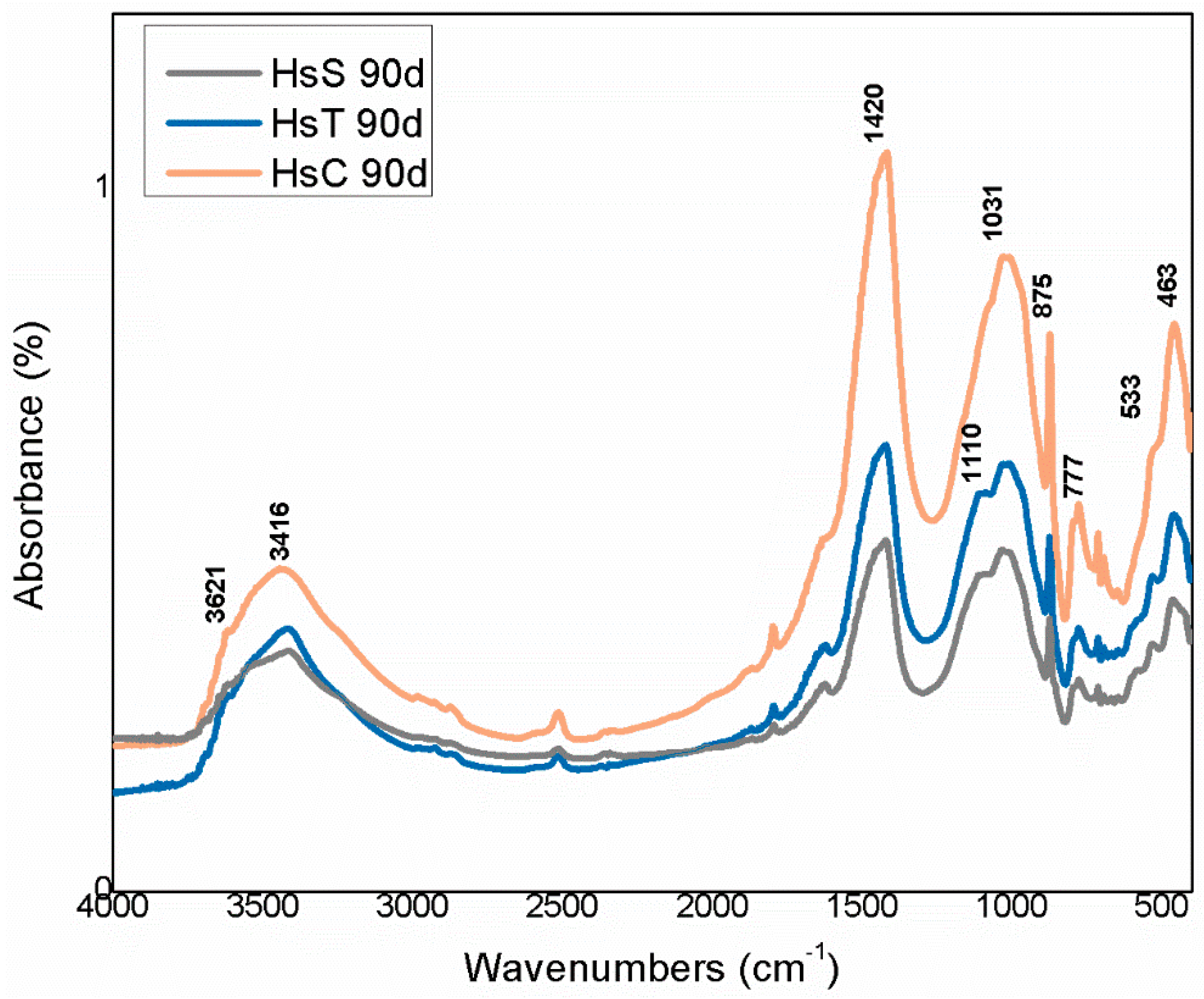
| OPC | HsT | HsC | HsS | HcG | HcL | HcV | |
|---|---|---|---|---|---|---|---|
| SiO2 | 14.22 | 49.97 | 49.22 | 58.00 | 9.34 | 23.27 | 12.10 |
| Al2O3 | 2.89 | 8.98 | 8.01 | 9.56 | 2.88 | 6.58 | 3.78 |
| CaO | 69.81 | 18.65 | 21.38 | 14.48 | 50.32 | 38.66 | 45.93 |
| Fe2O3 | 3.70 | 2.30 | 2.19 | 2.12 | 1.20 | 2.30 | 2.49 |
| MgO | 0.93 | 1.37 | 1.58 | 1.11 | 1.12 | 0.78 | 0.92 |
| SO3 | 3.36 | 2.53 | 0.88 | 0.72 | 0.85 | 0.59 | 0.67 |
| Na2O | 0.33 | 0.80 | 0.63 | 0.90 | 0.18 | 0.41 | 0.25 |
| K2O | 0.76 | 3.35 | 2.61 | 3.83 | 0.47 | 1.07 | 0.72 |
| P2O5 | 0.14 | 0.11 | 0.12 | 0.10 | 0.03 | 0.08 | 0.09 |
| TiO2 | 0.20 | 0.28 | 0.30 | 0.30 | 0.14 | 0.39 | 0.42 |
| MnO | 0.10 | 0.04 | 0.03 | 0.03 | 0.09 | 0.05 | 0.06 |
| LOI | 3.22 | 11.50 | 12.90 | 8.69 | 33.20 | 25.70 | 32.40 |
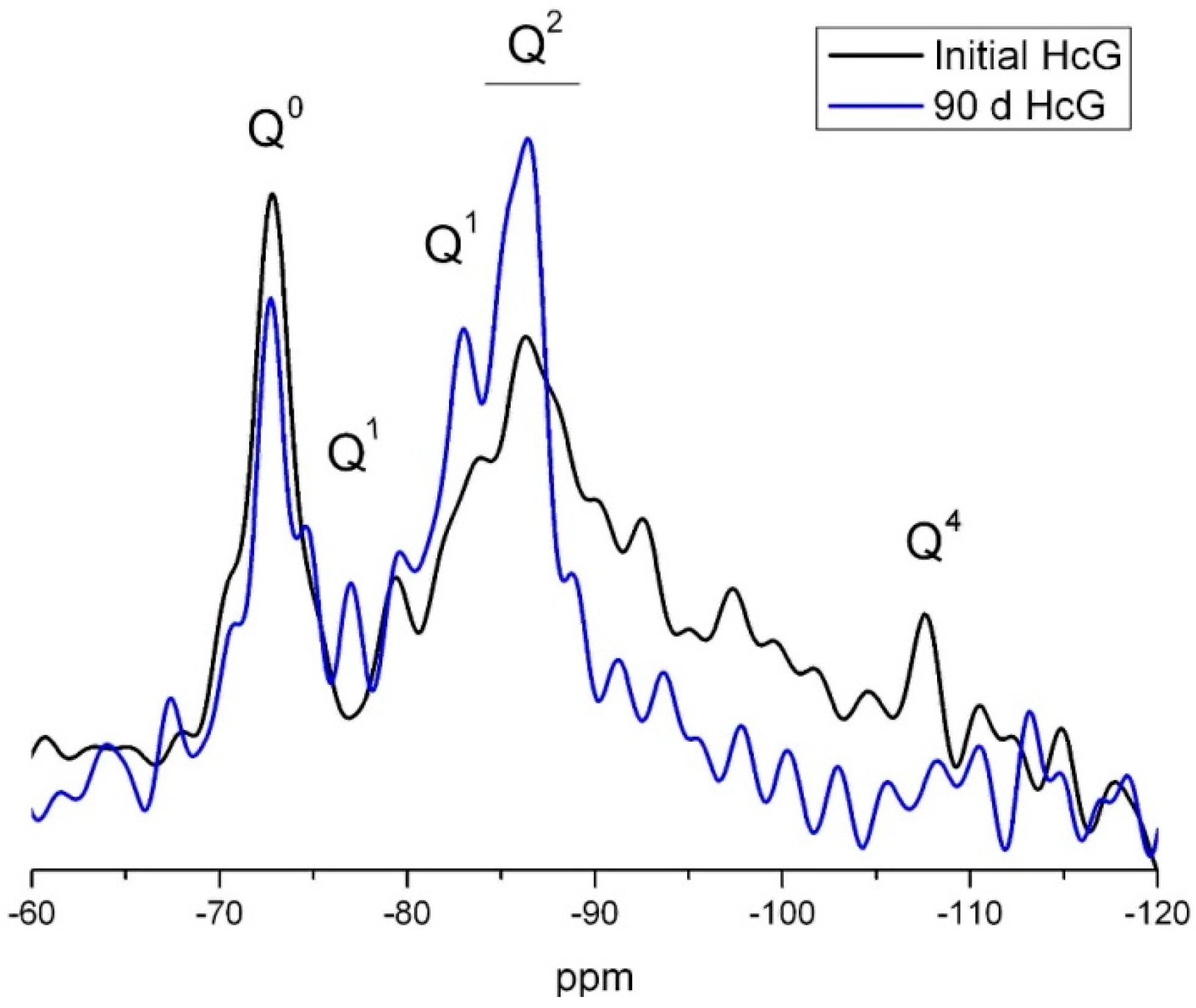
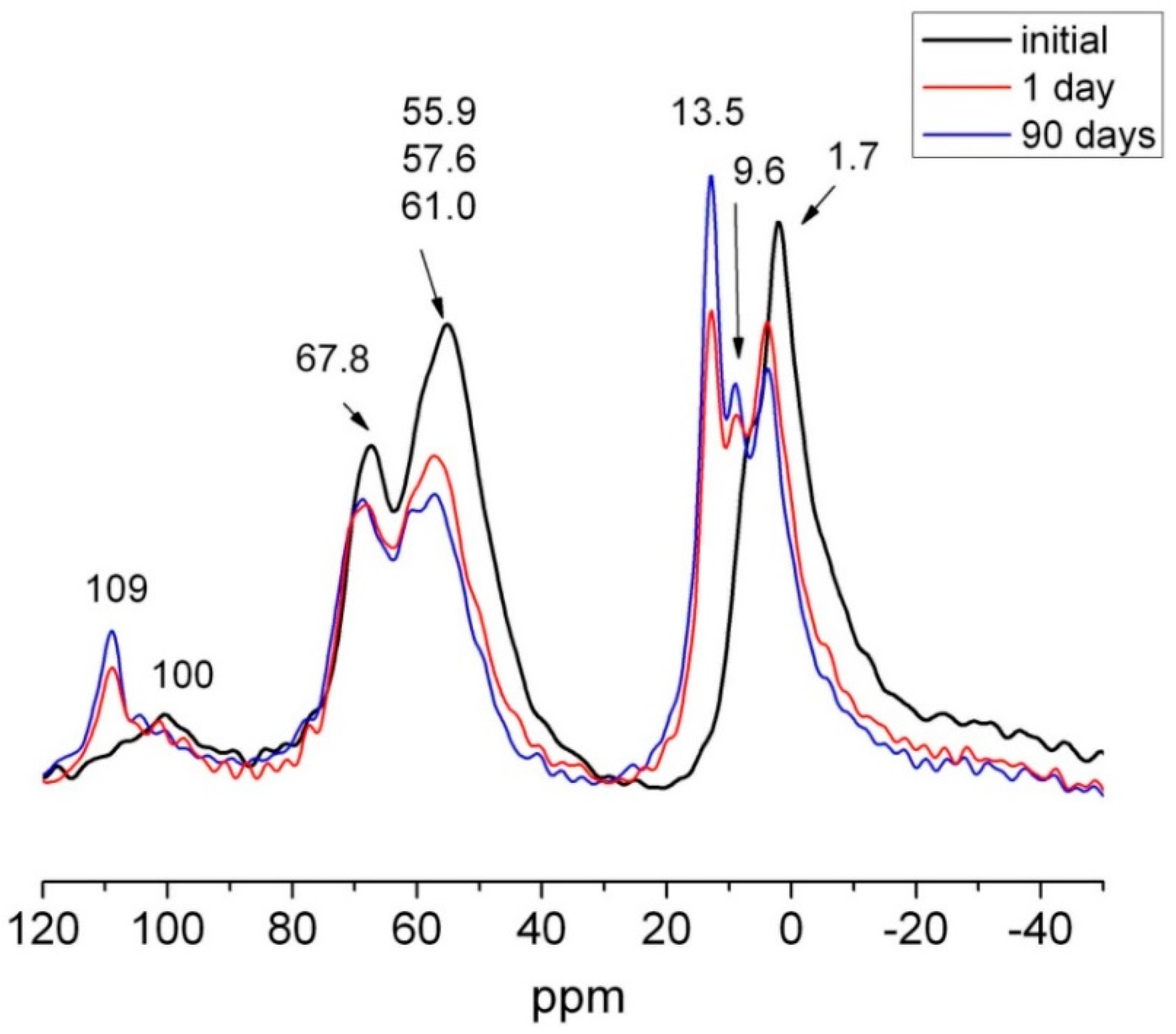
| Waste | Mica (%) | Quartz (%) | Feldspar (%) | Calcite (%) | Kaolinite (%) | Amorphous Material (%) | RB | X2 |
|---|---|---|---|---|---|---|---|---|
| HsT | 4 | 48 | 8 | 24 | n.d. | 16 | 17.6 | 7.3 |
| HsC | 6 | 49 | 6 | 28 | n.d. | 11 | 19.1 | 7.7 |
| HsS | 4 | 58 | 10 | 16 | n.d. | 12 | 22.3 | 8.2 |
| HcG | 10 | 10 | 11 | 52 | n.d. | 17 | 23.9 | 6.9 |
| HcL | 12 | 14 | 13 | 40 | 10 | 11 | 17.9 | 6.2 |
| HcV | 7 | 12 | 10 | 62 | traces | 9 | 16.8 | 5.7 |
| Oxide (%) | Ca Rich Aggregate | Fe Rich Aggregate | Calcite | Deposit on Calcite | Si Rich Aggregate | S Rich Aggregate |
|---|---|---|---|---|---|---|
| Al2O3 | 6.91 ± 2.18 | 1.82 ± 0.27 | n.d. | 3.88 ± 0.95 | 3.61 ± 1.25 | 6.59 ± 2.07 |
| SiO2 | 23.57 ± 4.16 | 2.85 ± 0.48 | 0.63 ± 0.11 | 9.79 ± 2.07 | 8.53 ± 3.26 | 14.31 ± 3.15 |
| MgO | 1.58 ± 0.59 | 7.96 ± 1.36 | n.d. | 0.64 ± 0.29 | 3.64 ± 0.58 | 1.05 ± 0.13 |
| CaO | 63.08 ± 4.32 | 3.56 ± 1.18 | 99.37 ± 2.59 | 83.93 ± 5.62 | 69.91 ± 5.46 | 50.79 ± 2.16 |
| Fe2O3 | 1.66 ± 0.77 | 83.81 ± 4.29 | n.d. | 1.08 ± 0.33 | 5.48 ± 1.33 | 5.98 ± 2.31 |
| SO3 | 2.10 ± 1.07 | n.d. | n.d. | n.d. | 7.83 ± 3.52 | 19.76 ± 3.01 |
| K2O | 0.80 ± 0.11 | n.d. | n.d. | 0.68 ± 0.18 | 0.93 ± 0.07 | 1.53 ± 0.42 |
| Oxide (%) | Ti Rich Aggregate | Fe Rich Aggregate | Quartz | Deposit on Quartz | Si Rich Aggregate |
|---|---|---|---|---|---|
| Al2O3 | 10.11 ± 2.37 | 13.36 ± 1.68 | 0.77 ± 0.21 | 7.82 ± 1.64 | 16.19 ± 2.25 |
| SiO2 | 39.27 ± 4.23 | 33.18 ± 2.94 | 98.81 ± 2.48 | 78.66 ± 3.82 | 51.94 ± 3.19 |
| MgO | 2.42 ± 0.99 | 2.60 ± 1.16 | n.d. | 1.04 ± 0.75 | 4.38 ± 1.24 |
| CaO | 33.43 ± 4.15 | 38.16 ± 3.21 | 0.41 ± 0.09 | 7.51 ± 2.47 | 16.08 ± 2.36 |
| Fe2O3 | 1.11 ± 0.54 | 11.42 ± 2.25 | n.d. | 1.21 ± 0.83 | 2.22 ± 1.48 |
| TiO2 | 11.03 ± 1.03 | 0.76 ± 0.13 | n.d. | n.d. | n.d. |
| Na2O | 1.09 ± 0.12 | n.d. | n.d. | 0.55 ± 0.21 | 2.72 ± 0.86 |
| K2O | 1.54 ± 0.36 | 0.51 ± 0.09 | n.d. | 3.20 ± 1.26 | 3.32 ± 1.73 |
| SO3 | n.d. | n.d. | n.d. | n.d. | 3.15 ± 1.07 |
| Waste | τ (h) | Reaction Rate Constant (K·h−1) | Ccorr | R | R2 |
|---|---|---|---|---|---|
| HcG | 140.3 ± 15.2 | (1.18 ± 0.12) × 10−4 | 12.52 ± 0.4 | 0.8765 | 0.8548 |
| HcL | 141.9 ± 16.1 | (1.62 ± 0.91) × 10−4 | 8.91 ± 0.51 | 0.9364 | 0.9281 |
| HcV | 162.2 ± 18.4 | (1.27 ± 0.95) × 10−4 | 8.69 ± 0.65 | 0.9340 | 0.9231 |
| HsC | 112.5 ± 10.6 | (3.49 ± 0.82) × 10−4 | 6.54 ± 0.79 | 0.9067 | 0.8987 |
| HsS | 124.1 ± 12.2 | (3.06 ± 0.94) × 10−4 | 6.15 ± 0.63 | 0.9225 | 0.9115 |
| HsT | 87.7 ± 8.4 | (6.58 ± 0.98) × 10−4 | 5.68 ± 0.83 | 0.9006 | 0.8943 |
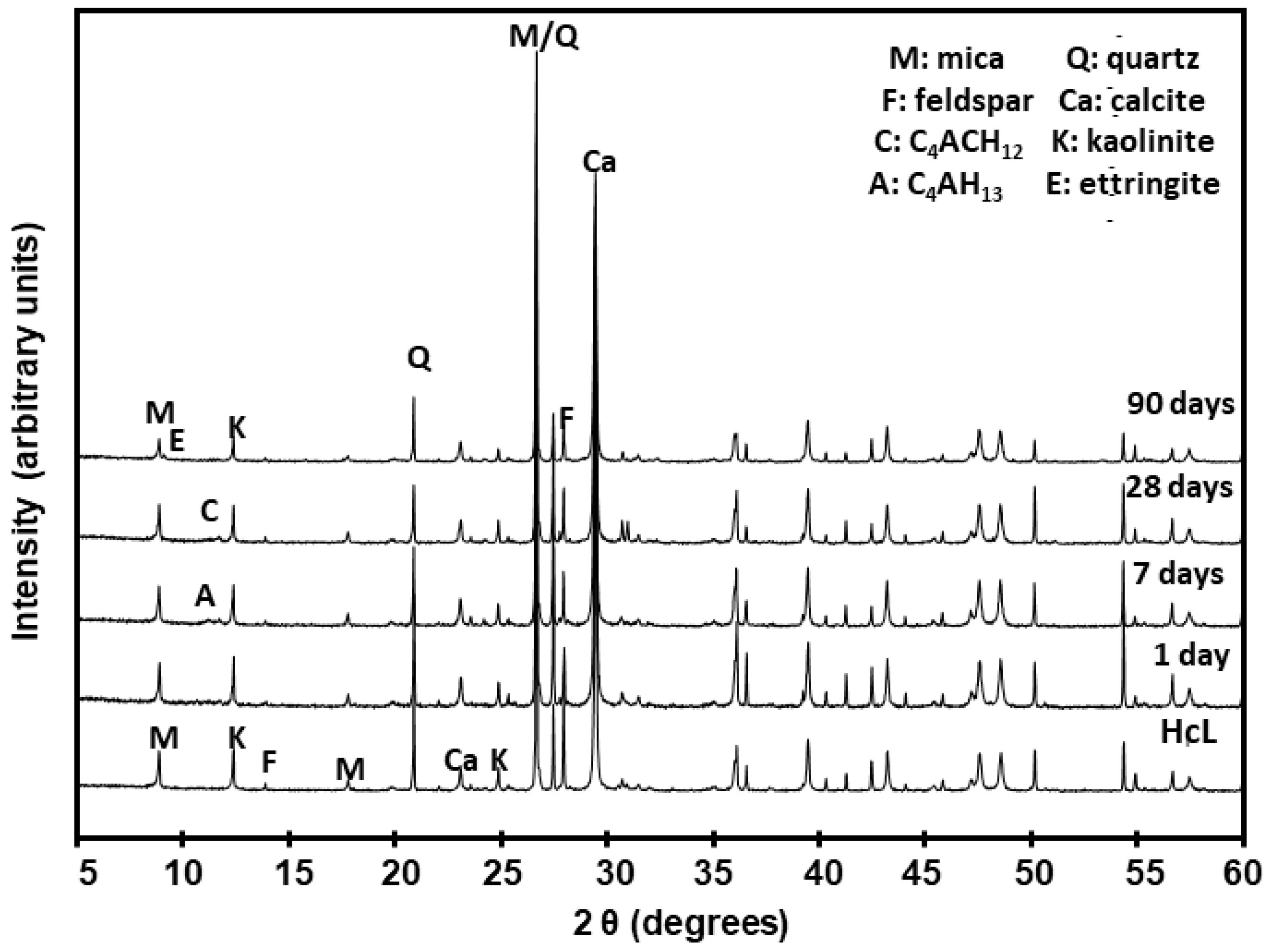
| Waste | Time (Days) | M (%) | Q (%) | F (%) | C (%) | C4AH13 (%) | Ett (%) | Am. Mat. (%) | C4AcH12 (%) | K (%) | RB | X2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HcG | initial | 10 | 9 | 11 | 52 | n.d. | n.d. | 17 | n.d. | n.d. | 23.9 | 6.9 |
| 1 | 10 | 9 | 11 | 52 | n.d. | n.d. | 18 | n.d. | n.d. | 21.6 | 67 | |
| 7 | 10 | 9 | 10 | 51 | t | n.d. | 20 | n.d. | n.d. | 22.5 | 7.2 | |
| 28 | 10 | 9 | 9 | 51 | n.d. | n.d. | 21 | n.d. | n.d. | 21.4 | 6.5 | |
| 90 | 10 | 9 | 9 | 47 | n.d. | t | 25 | n.d. | n.d. | 19.4 | 5.5 | |
| HcL | initial | 12 | 14 | 13 | 40 | n.d. | n.d. | 11 | n.d. | 10 | 17.9 | 6.2 |
| 1 | 12 | 14 | 10 | 42 | n.d. | n.d. | 12 | n.d. | 10 | 20.5 | 5.7 | |
| 7 | 10 | 14 | 8 | 45 | 1 | n.d. | 12 | t | 10 | 21.3 | 6.4 | |
| 28 | 8 | 14 | 8 | 48 | t | n.d. | 13 | 1 | 8 | 19.9 | 5.9 | |
| 90 | 6 | 14 | 6 | 51 | n.d. | t | 17 | n.d. | 6 | 18,7 | 6.9 | |
| HcV | Initial | 7 | 12 | 10 | 62 | n.d. | n.d. | 9 | n.d. | t | 16.8 | 5.7 |
| 1 | 7 | 12 | 10 | 63 | n.d. | n.d. | 8 | n.d. | n.d. | 18.2 | 6.3 | |
| 7 | 7 | 12 | 8 | 59 | t | n.d. | 14 | n.d. | n.d. | 15.5 | 5.7 | |
| 28 | 5 | 10 | 4 | 64 | n.d. | t | 17 | n.d. | n.d. | 17.3 | 6.1 | |
| 90 | 5 | 10 | 2 | 63 | n.d. | t | 20 | n.d. | n.d. | 19.2 | 7.4 | |
| HsC | initial | 6 | 49 | 6 | 28 | n.d. | n.d. | 11 | n.d. | n.d. | 19.1 | 7.7 |
| 1 | 4 | 49 | 4 | 31 | n.d. | n.d. | 12 | n.d. | n.d. | 17.5 | 5.9 | |
| 7 | 4 | 49 | 2 | 38 | n.d. | n.d. | 7 | n.d. | n.d. | 18.5 | 6.2 | |
| 28 | 2 | 49 | 6 | 35 | n.d. | n.d. | 8 | n.d. | n.d. | 15.9 | 5.4 | |
| 90 | 2 | 39 | 10 | 35 | n.d. | t | 4 | n.d. | n.d. | 16.1 | 6.1 | |
| HsS | initial | 4 | 58 | 10 | 16 | n.d. | n.d. | 12 | n.d. | n.d. | 22.3 | 8.2 |
| 1 | 4 | 58 | 8 | 18 | n.d. | n.d. | 11 | n.d. | n.d. | 32.4 | 9.3 | |
| 7 | 3 | 58 | 6 | 24 | n.d. | n.d. | 8 | n.d. | n.d. | 20.9 | 7.5 | |
| 28 | 3 | 58 | 4 | 28 | n.d. | n.d. | 6 | n.d. | n.d. | 16.3 | 5.2 | |
| 90 | 3 | 58 | 4 | 28 | 1 | t | 5 | n.d. | n.d. | 15.8 | 4.9 | |
| HsT | initial | 4 | 48 | 8 | 24 | n.d. | n.d. | 16 | n.d. | n.d. | 17.6 | 7.3 |
| 1 | 3 | 48 | 11 | 24 | n.d. | n.d. | 14 | n.d. | n.d. | 18.5 | 8.1 | |
| 7 | 2 | 48 | 10 | 28 | n.d. | t | 12 | n.d. | n.d. | 19.2 | 6.3 | |
| 28 | 2 | 48 | 8 | 31 | n.d. | t | 11 | n.d. | n.d. | 17.1 | 5.7 | |
| 90 | 2 | 48 | 8 | 31 | n.d. | t | 11 | n.d. | n.d. | 16.4 | 5.8 |
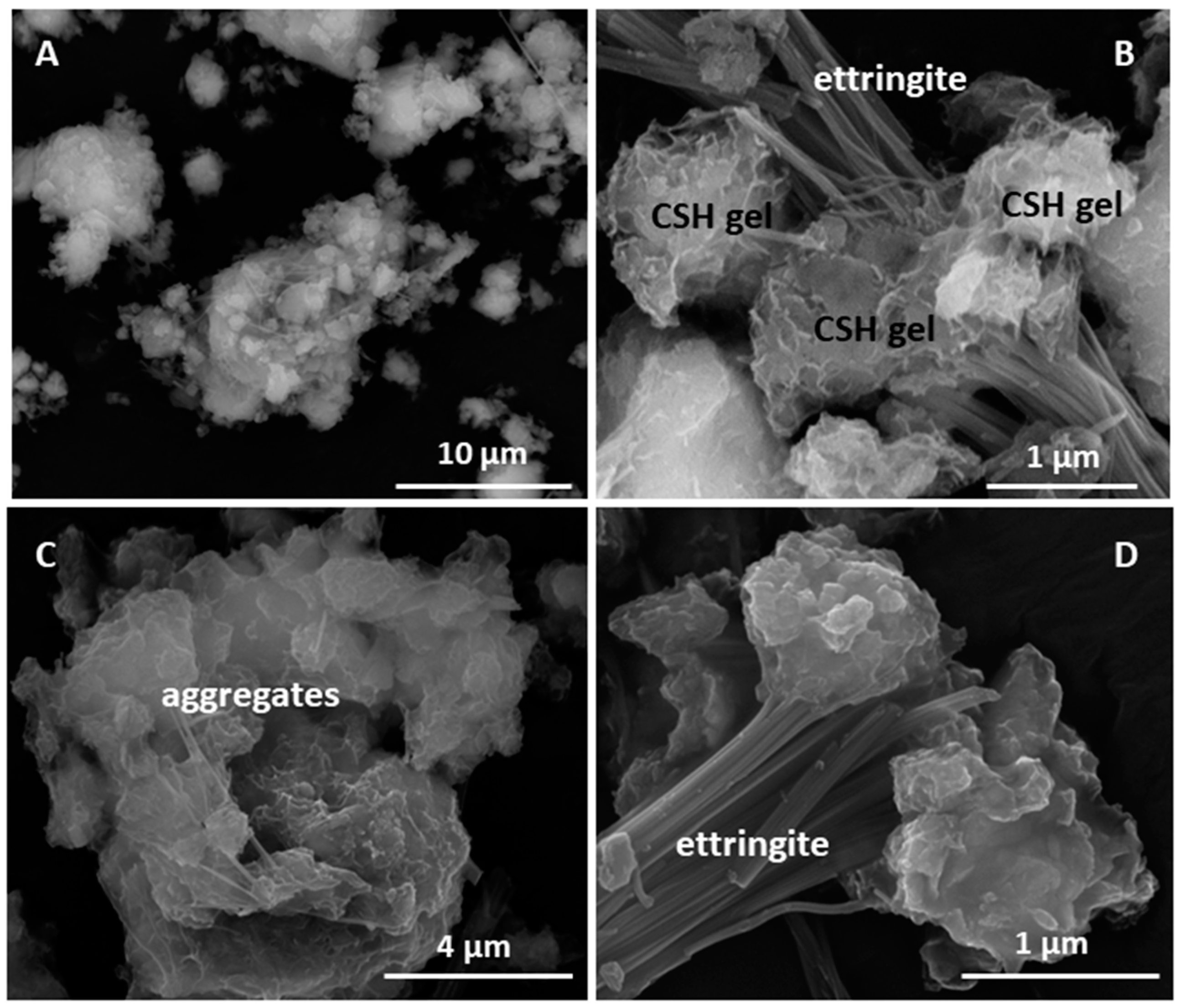
| Oxide (%) | C–S–H Gel | Ettringite | C4AcH11 |
|---|---|---|---|
| Al2O3 | 10.19 ± 0.52 | 27.70 ± 2.12 | 31.08 ± 1.27 |
| SiO2 | 17.31 ± 0.88 | 4.27 ± 0.95 | 3.21 ± 0.92 |
| SO3 | n.d. | 21.16 ± 0.72 | 8.99 ± 0.51 |
| MgO | 1.73 ± 0.26 | n.d. | n.d. |
| CaO | 70.77 ± 2.39 | 46.87 ± 2.28 | 56.72 ± 1.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frías, M.; Vigil de la Villa, R.; Martínez-Ramírez, S.; Fernández-Carrasco, L.; Villar-Cociña, E.; García-Giménez, R. Multi-Technique Characterization of a Fine Fraction of CDW and Assessment of Reactivity in a CDW/Lime System. Minerals 2020, 10, 590. https://doi.org/10.3390/min10070590
Frías M, Vigil de la Villa R, Martínez-Ramírez S, Fernández-Carrasco L, Villar-Cociña E, García-Giménez R. Multi-Technique Characterization of a Fine Fraction of CDW and Assessment of Reactivity in a CDW/Lime System. Minerals. 2020; 10(7):590. https://doi.org/10.3390/min10070590
Chicago/Turabian StyleFrías, Moisés, Raquel Vigil de la Villa, Sagrario Martínez-Ramírez, Lucía Fernández-Carrasco, Ernesto Villar-Cociña, and Rosario García-Giménez. 2020. "Multi-Technique Characterization of a Fine Fraction of CDW and Assessment of Reactivity in a CDW/Lime System" Minerals 10, no. 7: 590. https://doi.org/10.3390/min10070590
APA StyleFrías, M., Vigil de la Villa, R., Martínez-Ramírez, S., Fernández-Carrasco, L., Villar-Cociña, E., & García-Giménez, R. (2020). Multi-Technique Characterization of a Fine Fraction of CDW and Assessment of Reactivity in a CDW/Lime System. Minerals, 10(7), 590. https://doi.org/10.3390/min10070590






