Quantitative Analysis of Asbestos-Containing Materials Using Various Test Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. X-ray Diffractometer
2.3. Scanning and Transmission Electron Microscopy (SEM and TEM)
2.4. Homogeneity Evaluation
3. Results and Discussion
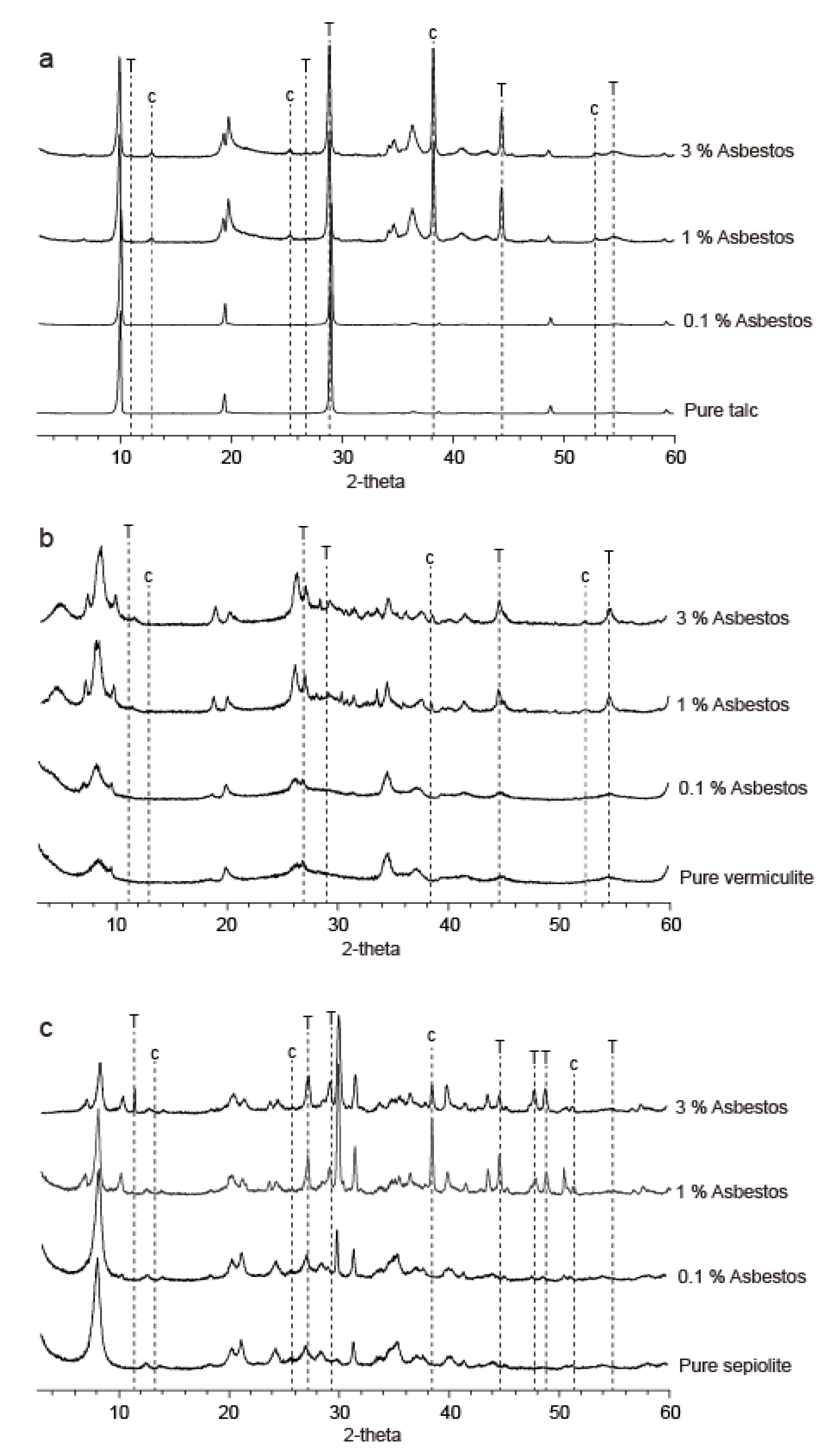
3.1. XRPD Analysis and Homogeneity Evaluation
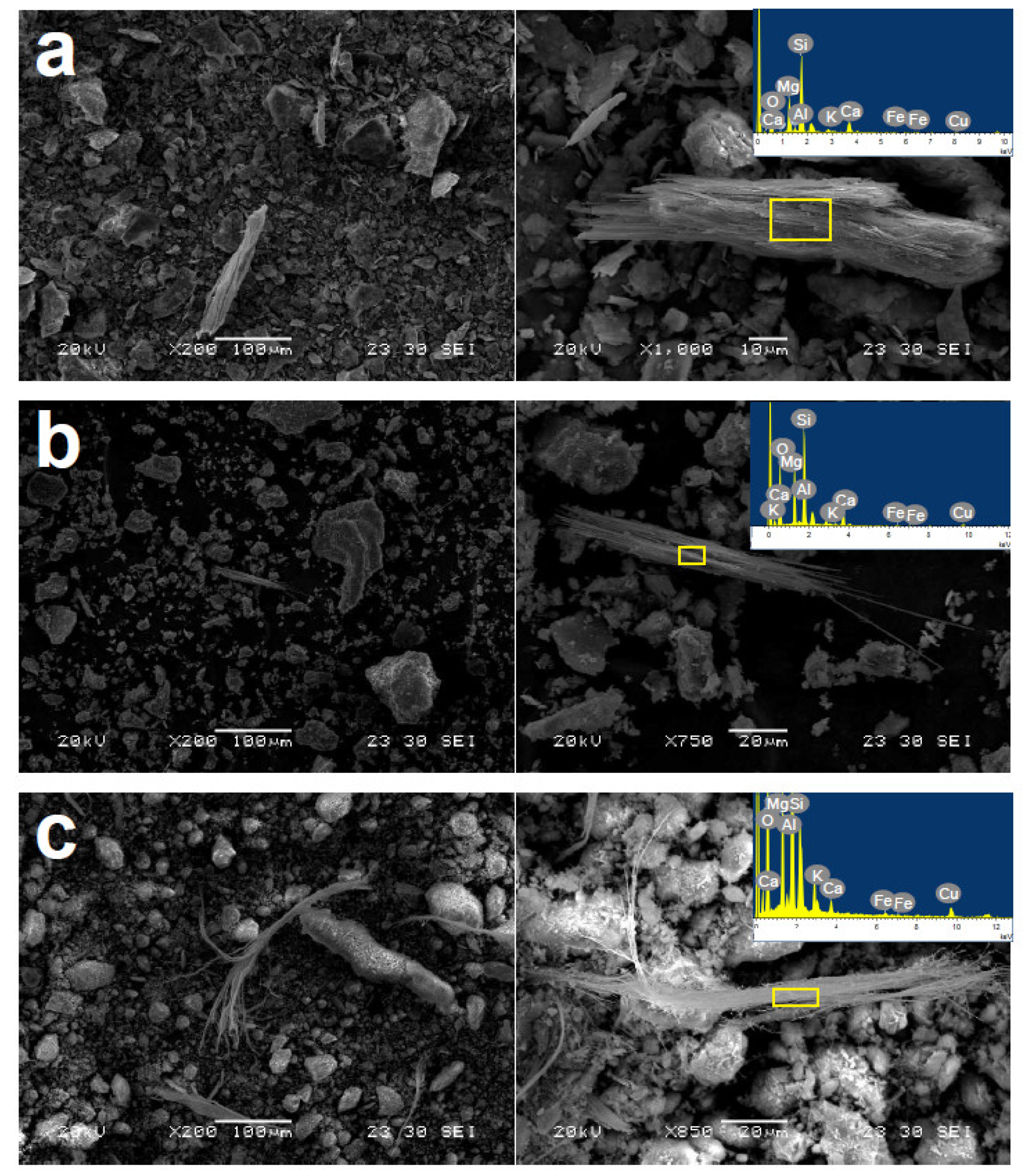
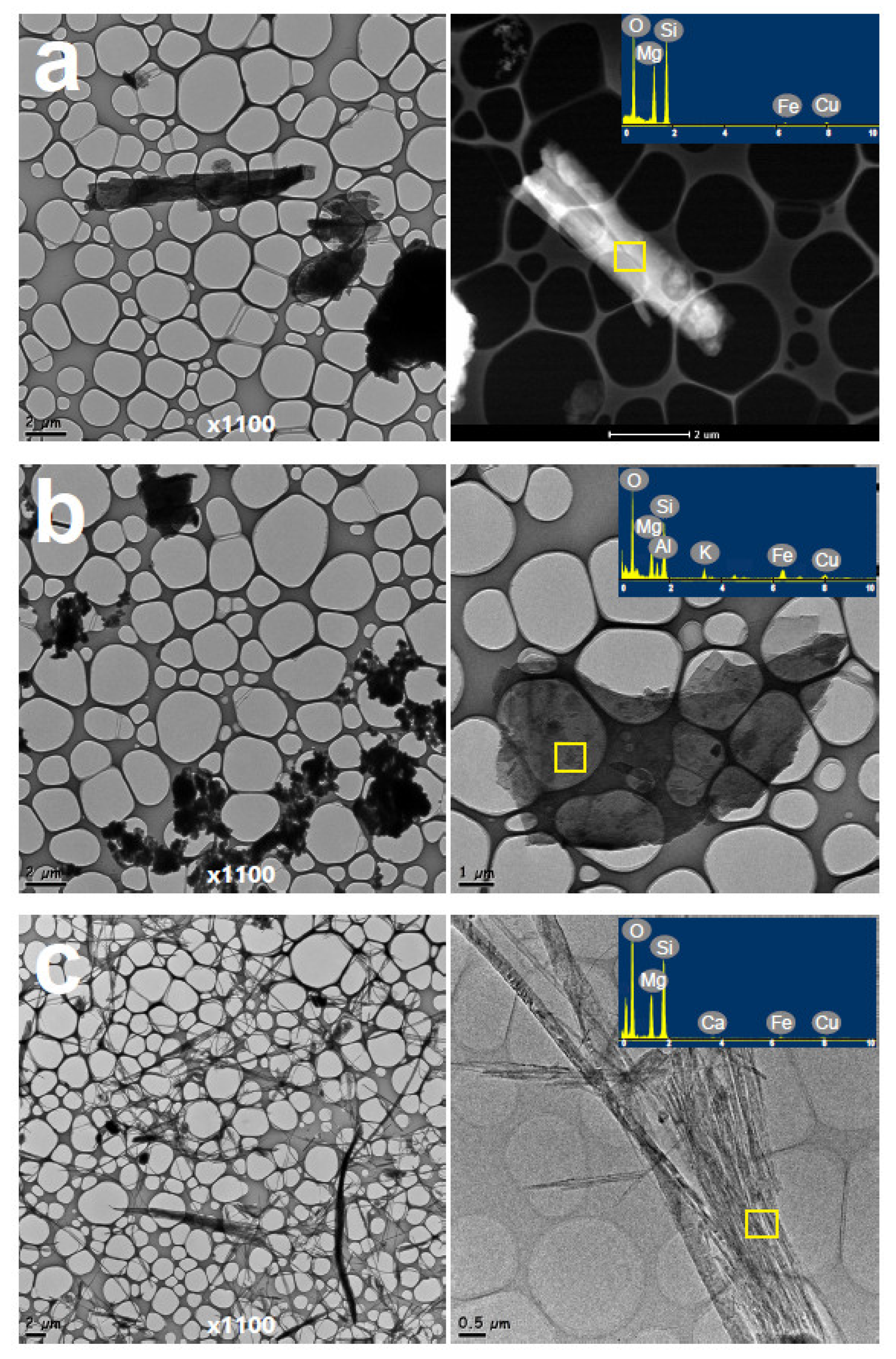
3.2. Electron Microscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mossman, B.T.; Bignon, J.; Corn, M.; Seaton, A.; Gee, J.B. Asbestos: Scientific developments and implications for public policy. Science 1990, 247, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Dell, L.; Adams, R.; Rose, T.; Van Orden, D. State-of-the-science assessment of non-asbestos amphibole exposure: Is there a cancer risk? Environ. Geochem. Health 2013, 35, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.J. Selected Silicate Minerals and Their Asbestiform Varieties: Mineralogical Definition Sand Identification-Characterization; Department of the Interior, Bureau of Mines: Washington, DC, USA, 1977; Volume 8751. [Google Scholar]
- Punturo, R.; Ricchiuti, C.; Mengel, K.; Apollaro, C.; De Rosa, R.; Bloise, A. Serpentinite-derived soils in southern Italy: Potential for hazardous exposure. J. Mediterr. Earth Sci. 2018, 10, 51–61. [Google Scholar]
- Punturo, R.; Ricchiuti, C.; Bloise, A. Assessment of Serpentine Group Minerals in Soils: A Case Study from the Village of San Severino Lucano (Basilicata, Southern Italy). Fibers 2019, 7, 18. [Google Scholar] [CrossRef]
- Kim, H.-W.; Park, G.-Y.; Han, J.-G.; Han, Y.-S.; Hwang, B.-G.; Lee, J.-H. Releasing of asbestos fibers from the weathered asbestos cement slate roofing. J. Korean Soc. Occup. Environ. Hyg. 2010, 20, 88–93. [Google Scholar]
- Murr, L.E.; Soto, K. TEM comparison of chrysotile (asbestos) nanotubes and carbon nanotubes. J. Mater. Sci. 2004, 39, 4941–4947. [Google Scholar] [CrossRef]
- Wagner, J.C.; Sleggs, C.A.; Marchand, P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Occup. Environ. Med. 1960, 17, 260–271. [Google Scholar] [CrossRef]
- World Health Organization. Asbestos and other natural mineral fibres. International programme on chemical safety. Environ. Health Criteria 1986, 53, 194. [Google Scholar]
- Skinner, H.C.W.; Ross, M.; Frondel, C. Asbestos and Other Fibrous Materials: Mineralogy, Crystal Chemistry, and Health Effects; Oxford University Press: Oxford, UK, 1988. [Google Scholar]
- Mossman, B.T.; Gee, J.B.L. Asbestos-related diseases. New Engl. J. Med. 1989, 320, 1721–1730. [Google Scholar] [CrossRef]
- Jablonski, R.P.; Kim, S.; Cheresh, P.; Liu, G.; Kamp, D.W. Insights into mineral fibre-induced lung epithelial cell toxicity and pulmonary fibrosis. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Northwestern University: Evanston, IL, USA, 2017; pp. 447–500. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Becklake, M.R. Asbestos-related diseases of the lung and other organs: Their epidemiology and implications for clinical practice. Am. Rev. Respir. Dis. 1976, 114, 187–227. [Google Scholar]
- International Agency for Research on Cancer. Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). In Metals, Arsenic, Dusts and Fibres; A review of human carcinogens; World Health Organization: Geneva, Switzerland, 2012; pp. 219–309. [Google Scholar]
- Samet, J.M.; Dominici, F.; Curriero, F.C.; Coursac, I.; Zeger, S.L. Fine particulate air pollution and mortality in 20 US cities, 1987–1994. New Engl. J. Med. 2000, 343, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Europe, W. Air Quality Guidelines, 2nd ed.; Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- LaDou, J.; Castleman, B.; Frank, A.; Gochfeld, M.; Greenberg, M.; Huff, J.; Joshi, T.K.; Landrigan, P.J.; Lemen, R.; Myers, J. The case for a global ban on asbestos. Environ. Health Perspect. 2010, 118, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Della Ventura, G.; Vigliaturo, R.; Gieré, R.; Pollastri, S.; Gualtieri, A.F.; Iezzi, G. Infra Red Spectroscopy of the Regulated Asbestos Amphiboles. Minerals 2018, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Asahi, T.; Kobayashi, S.; Nakayama, K.; Konya, T.; Fujinawa, G.; Nakamura, T. Preparation and evaluation of a chrysotile asbestos-containing standard material for validating x-ray diffractometric quantitation. Anal. Sci. 2011, 27, 1217. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Jaurand, M.; Magne, L.; Kheuang, L.; Pinchon, M.; Bignon, J. The interactions between asbestos fibers and metaphase chromosomes of rat pleural mesothelial cells in culture. A scanning and transmission electron microscopic study. Am. J. Pathol. 1987, 126, 343. [Google Scholar]
- Bloise, A.; Miriello, D. Multi-analytical approach for identifying asbestos minerals in situ. Geosciences 2018, 8, 133. [Google Scholar] [CrossRef]
- Januch, J.; Brattin, W.; Woodbury, L.; Berry, D. Evaluation of a fluidized bed asbestos segregator preparation method for the analysis of low-levels of asbestos in soil and other solid media. Anal. Methods 2013, 5, 1658–1668. [Google Scholar] [CrossRef]
- Borchert, H.; Shevchenko, E.V.; Robert, A.; Mekis, I.; Kornowski, A.; Grübel, G.; Weller, H. Determination of nanocrystal sizes: A comparison of TEM, SAXS, and XRD studies of highly monodisperse CoPt3 particles. Langmuir 2005, 21, 1931–1936. [Google Scholar] [CrossRef]
- Compton, S.P. TEM Quantification of Amphibole in Asbestos Containing Materials: A Summary of Data 20 Years in the Making. Microsc. Microanal. 2018, 24, 1184–1185. [Google Scholar] [CrossRef]
- Vigliaturo, R.; Pollastri, S.; Gieré, R.; Gualtieri, A.F.; Dražić, G. Experimental quantification of the Fe-valence state at amosite-asbestos boundaries using acSTEM dual-electron energy-loss spectroscopy. Am. Mineral. 2019, 104, 1820–1828. [Google Scholar] [CrossRef]
- Engelmann, H.-J. Advantages and Disadvantages of TEM Sample Preparation Using the FIB Technique. Prakt. Metallogr. 2003, 40, 163–174. [Google Scholar]
- Vigliaturo, R.; Della Ventura, G.; Choi, J.K.; Marengo, A.; Lucci, F.; O’Shea, M.J.; Pérez-Rodríguez, I.; Gieré, R. Mineralogical characterization and dissolution experiments in Gamble’s solution of Tremolitic amphibole from Passo di Caldenno (Sondrio, Italy). Minerals 2018, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F. Naturally Occurring Asbestos: A Global Health Concern? State of the Art and Open Issues. Environ. Eng. Geosci. 2020, 26, 3–8. [Google Scholar] [CrossRef]
- Hirsch, R.F. Analysis of variance in analytical chemistry. Anal. Chem. 1977, 49, 691A–700A. [Google Scholar] [CrossRef]
- Guide, I. General Requirements for the Competence of Reference Material Producers; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Guide, I. 35 Reference Materials—General and Statistical Principles for Certification; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Schulte, P.S.; Trout, D.; Zumwalde, R.D. Asbestos Fibers and Other Elongate Mineral Particles: State of the Science and Roadmap for Research; United States Department of Health and Human Services: Washington, DC, USA, 2011. [Google Scholar]
- Davis, J.M. In Vivo Assays to Evaluate the Pathogenic Effects of Minerals in Rodents; Mineralogical Society of America: Washington, DC, USA, 1993. [Google Scholar]
- Militello, G.M.; Sanguineti, E.; Yus González, A.; Mantovani, F.; Gaggero, L. The Concentration of Asbestos Fibers in Bulk Samples and Its Variation with Grain Size. Minerals 2019, 9, 539. [Google Scholar] [CrossRef]
- Yang, H.; Xiao, Y.; Liu, K.; Yang, Y.; Feng, Q. Physicochemical dispersion of chrysotile. Colloids Surf. A Physicochem. Eng. Asp. 2007, 301, 341–345. [Google Scholar] [CrossRef]
- Bloise, A.; Ricchiuti, C.; Lanzafame, G.; Punturo, R. X-ray synchrotron microtomography: A new technique for characterizing chrysotile asbestos. Sci. Total Environ. 2020, 703, 135675. [Google Scholar] [CrossRef]
| No. | Integral Intensity | No. | Integral Intensity | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | ||
| T1 | 6266 | 4789 | 7236 | T9 | 20,512 | 17,567 | 18,319 |
| T2 | 5399 | 5385 | 2458 | T10 | 21,744 | 16,513 | 19,647 |
| T3 | 6874 | 5860 | 6624 | T11 | 20,793 | 21,933 | 22,993 |
| T4 | 5828 | 6230 | 7330 | T12 | 24,704 | 21,838 | 20,618 |
| T5 | 6845 | 6257 | 5552 | T13 | 18,121 | 21,015 | 23,730 |
| T6 | 5828 | 7410 | 5714 | T14 | 22,182 | 18,722 | 23,055 |
| T7 | 6136 | 7013 | 8095 | T15 | 18,627 | 23,218 | 22,257 |
| T8 | 5565 | 5346 | 6427 | T16 | 22,617 | 20,363 | 18,707 |
| Avg. | 6093 | 6036 | 6180 | Avg. | 21,163 | 20,146 | 21,166 |
| SD | 549 | 880 | 1725 | SD | 2147 | 2335 | 2119 |
| No. | Integral Intensity | No. | Integral Intensity | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | ||
| V1 | 1976 | 1140 | 773 | V9 | 7794 | 9723 | 6376 |
| V2 | 1712 | 2455 | 956 | V10 | 15,107 | 12,506 | 7065 |
| V3 | 3303 | 3702 | 722 | V11 | 7089 | 9855 | 6619 |
| V4 | 647 | 2363 | 915 | V12 | 6373 | 15122 | 12880 |
| V5 | 2487 | 2384 | 7027 | V13 | 6775 | 6729 | 12584 |
| V6 | 4540 | 5355 | 5896 | V14 | 14,093 | 7823 | 11,234 |
| V7 | 4203 | 5905 | 2175 | V15 | 8342 | 11499 | 16851 |
| V8 | 5177 | 4073 | 7749 | V16 | 9689 | 8670 | 18,849 |
| Avg. | 3006 | 3422 | 3277 | Avg. | 9408 | 10,241 | 11,557 |
| SD | 1566 | 1636 | 3068 | SD | 3376 | 2715 | 4713 |
| No. | Integral Intensity | No. | Integral Intensity | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | ||
| S1 | 7670 | 9292 | 10,224 | S9 | 23,627 | 15,868 | 23,029 |
| S2 | 11,133 | 13,466 | 12,441 | S10 | 17,171 | 18,428 | 22,624 |
| S3 | 11,132 | 8976 | 13,323 | S11 | 20,297 | 16,305 | 20,722 |
| S4 | 7977 | 11,097 | 10,260 | S12 | 19,141 | 21,844 | 19,389 |
| S5 | 11,385 | 11,343 | 12,337 | S13 | 18,244 | 22,307 | 18,799 |
| S6 | 10,431 | 9783 | 9008 | S14 | 22,293 | 24,458 | 24,180 |
| S7 | 9436 | 6678 | 11,391 | S15 | 18,188 | 20,574 | 22,984 |
| S8 | 6804 | 12,749 | 9156 | S16 | 21,169 | 22,869 | 23,549 |
| Avg. | 9496 | 10,423 | 11,018 | Avg. | 20,016 | 20,332 | 21,910 |
| SD | 1800 | 2197 | 1600 | SD | 2235 | 3150 | 2007 |
| Sample No. T1–T8 | ||||||
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Squares | F Ratio | P-Value | F-Crit |
| Between groups | 12,676,331 | 7 | 1,810,904 | 1.8388 | 0.1482 | 2.6572 |
| Among groups | 15,757,699 | 16 | 984,856 | |||
| Total | 28,434,030 | 23 | ||||
| Sample No. T9–T16 | ||||||
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Squares | F Ratio | P-Value | F-Crit |
| Between groups | 31,973,313 | 7 | 4,597,616 | 0.9688 | 0.4854 | 2.6572 |
| Among groups | 75,432,023 | 16 | 5,714,501 | |||
| Total | 107,405,336 | 23 | ||||
| Sample No. V1–V8 | ||||||
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Squares | F Ratio | P-Value | F-Crit |
| Between groups | 64,612,875 | 7 | 9230411 | 3.8964 | 0.0115 | 2.6572 |
| Among groups | 37,903,497 | 16 | 2368969 | |||
| Total | 102,516,372 | 23 | ||||
| Sample No. V9–V16 | ||||||
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Squares | F Ratio | P-Value | F-Crit |
| Between groups | 76,695,341 | 7 | 10,956,477 | 0.7656 | 0.6237 | 2.6572 |
| Among groups | 229,000,008 | 16 | ||||
| Total | 305,695,349 | 23 | ||||
| Sample No. S1–S8 | ||||||
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Squares | F Ratio | P-Value | F-Crit |
| Between groups | 32,276,823 | 7 | 4,610,975 | 1.4317 | 0.2600 | 2.6572 |
| Among groups | 51,528,728 | 16 | 3,220,546 | |||
| Total | 83,805,551 | |||||
| Sample No. S9–S16 | ||||||
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Squares | F Ratio | P-Value | F-Crit |
| Between groups | 52,197,523 | 7 | 7,456,360 | 1.2310 | 0.3426 | 2.6572 |
| Among groups | 96,916,481 | 16 | 6,057,280 | |||
| Total | 149,114,004 | 23 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Yoo, K.-C.; Jung, J. Quantitative Analysis of Asbestos-Containing Materials Using Various Test Methods. Minerals 2020, 10, 568. https://doi.org/10.3390/min10060568
Yang K, Yoo K-C, Jung J. Quantitative Analysis of Asbestos-Containing Materials Using Various Test Methods. Minerals. 2020; 10(6):568. https://doi.org/10.3390/min10060568
Chicago/Turabian StyleYang, Kiho, Kyu-Cheul Yoo, and Jaewoo Jung. 2020. "Quantitative Analysis of Asbestos-Containing Materials Using Various Test Methods" Minerals 10, no. 6: 568. https://doi.org/10.3390/min10060568
APA StyleYang, K., Yoo, K.-C., & Jung, J. (2020). Quantitative Analysis of Asbestos-Containing Materials Using Various Test Methods. Minerals, 10(6), 568. https://doi.org/10.3390/min10060568







