Effects of Composition, Pressure, and Temperature on the Elastic Properties of SiO2–TiO2 Glasses: An Integrated Ultrasonic and Brillouin Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Density and Ultrasonic Measurements
2.2. Brillouin Scattering Measurements
3. Results and Discussion
3.1. Elastic Parameters at 1 bar and 25 °C
3.2. Densification
3.3. Comparison between Ultrasonic and Brillouin Measurements
3.4. Mode Grüneisen Parameters γHT and γLT
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, D.L. Solid solution of TiO2 in SiO2. J. Am. Ceram. Soc. 1970, 53, 418–419. [Google Scholar] [CrossRef]
- Nordberg, M.E. Glass Having an Expansion Lower than That of Silica. U.S. Patent 2,326,059, 3 August 1943. [Google Scholar]
- Corning Glass Works. Low Expansion Materials Bulletin; Corning Glass Works: Corning, NY, USA, 1969. [Google Scholar]
- Ricker, R.W.; Hummel, F.A. Reactions in the system TiO2-SiO2; Revision of the phase diagram. J. Am. Ceram. Soc. 1951, 34, 271–279. [Google Scholar] [CrossRef]
- DeVries, R.C.; Roy, R.; Osborn, E.F. The system TiO2-SiO2. Trans. Br. Ceram. Soc. 1954, 53, 525–540. [Google Scholar]
- Schultz, P.; Smyth, G.T. Ultra-low expansion glasses and their structure in the SiO2-TiO2 system. In Amorphous Materials; Douglas, R.W., Ellis, B., Eds.; Wiley-Interscience: New York, NY, USA, 1972; pp. 453–461. [Google Scholar]
- Schultz, P.C. Method for Producing TiO2-SiO2 Glasses. U.S. Patent 3,690,855, 12 September 1972. [Google Scholar]
- Schultz, P.C. Binary titania-silica glasses containing 10–20 wt% TiO2. J. Am. Ceram. Soc. 1976, 58, 214–219. [Google Scholar] [CrossRef]
- Arndt, J. Irreversible compression of glasses of the system TiO2-SiO2 by high static pressures. In Proceedings of the 4th International Conference on High Pressure, Kyoto, Japan, 25–29 November 1974; The Physico-Chemical Society of Japan: Tokyo, Japan, 1975; pp. 317–320. [Google Scholar]
- Hirao, K.; Tanaka, K.; Furukawa, S.; Soga, N. Anomalous temperature dependence of the sound velocities of SiO2-TiO2 glasses. J. Mater. Sci. Lett. 1995, 14, 697–699. [Google Scholar] [CrossRef]
- Kushibiki, J.-I.; Arakawa, M.; Ueda, T.; Fujinoki, A. Homogeneous TiO2-SiO2 glass for extreme ultraviolet lithography evaluated by the line-focus-beam ultrasonic material characterization system. Appl. Phys. Express 2008, 1, 087002. [Google Scholar] [CrossRef]
- Carson, D.S.; Maurer, R.D. Optical attenuation in titania-silica glasses. J. Non-Cryst. Solids 1973, 11, 368–380. [Google Scholar] [CrossRef]
- Copley, G.J.; Redmond, A.D.; Yates, B. Influence of titania upon thermal expansion of vitreous silica. Phys. Chem. Glasses 1973, 14, 73–76. [Google Scholar]
- Plummer, W.A.; Hagy, H.E. Precision thermal expansion measurements on low expansion optical materials. Appl. Opt. 1968, 7, 825–831. [Google Scholar] [CrossRef]
- Kurkjian, C.R.; Peterson, G.E. An EPR study of Ti3+-Ti4+ in TiO2-SiO2 glasses. Phys. Chem. Glasses 1974, 15, 12–17. [Google Scholar]
- Turnbull, R.C.; Lawrence, W.G. The role of titania in silica glasses. J. Am. Ceram. Soc. 1952, 35, 48–53. [Google Scholar] [CrossRef]
- Trap, H.J.L.; Stevels, J.M. Conventional and inverted glasses containing titania, Part I. Phys. Chem. Glasses 1960, 1, 107–118. [Google Scholar]
- Hirayama, C.; Berg, D. Dielectric properties of glasses in the system TiO2-Na2O-SiO2. Phys. Chem. Glasses 1961, 2, 145–151. [Google Scholar]
- Manghnani, M.H. Pressure and temperature dependence of the elastic moduli of Na2O-TiO2-SiO2 glasses. J. Am. Ceram. Soc. 1972, 55, 360–365. [Google Scholar] [CrossRef]
- McSkimin, J.H. Pulse superposition method for measuring ultrasonic wave velocities in solids. J. Acoust. Soc. Am. 1961, 33, 12–16. [Google Scholar] [CrossRef]
- Anderson, O.L.; Dienes, G.J. The anomalous properties of vitreous silica. Chap. 18. In Non-Crystalline Solids; Frechette, V.D., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1960; pp. 449–490. [Google Scholar]
- Barron, T.H.K. On the thermal expansion of solids at low temperatures. Phil. Mag. 1955, 46, 720–734. [Google Scholar] [CrossRef]
- Scannell, G.; Koike, A.; Huang, L. Structure and thermo-mechanical response of TiO2-SiO2 glasses to temperature. J. Non-Cryst. Solids 2016, 447, 238–247. [Google Scholar] [CrossRef]
- Williams, Q.; Manghnani, M.H.; Matsui, T. The effect of coordination changes on the bulk moduli of amorphous silicates: The SiO2-TiO2 system as a test case. Am. Miner. 2019, 104, 679–685. [Google Scholar] [CrossRef]
- Chmel, A.; Eranosyan, G.M.; Karshak, A.A. Vibrational spectroscopic study of Ti-substituted SiO2. J. Non-Cryst. Solids 1992, 136, 213–217. [Google Scholar] [CrossRef]
- Chandrasekhar, H.R.; Chandrasekhar, M.; Manghnani, M.H. Phonons in TiO2-SiO2 glasses. J. Non-Cryst. Solids 1980, 40, 567–575. [Google Scholar] [CrossRef]
- Cook, R.K. Variation of elastic constants and static strains with hydrostatic pressure: A method for calculation from ultrasonic measurements. J. Acoust. Soc. Am. 1957, 29, 445–449. [Google Scholar] [CrossRef]
- Tkachev, S.N.; Manghnani, M.H.; Williams, Q.; Ming, L.C. Compressibility of hydrated and anhydrous Na2O-2SiO2 liquid and also glass to 8 GPa using Brillouin scattering. J. Geophys. Res. 2005, 110, B07201. [Google Scholar] [CrossRef]
- Andreatch, P.; McSkimin, H.J. Pressure dependence of ultrasonic wave velocities and elastic stiffness moduli for a TiO2-SiO2 glass (Corning 7971). J. Appl. Phys. 1976, 47, 1299–1301. [Google Scholar] [CrossRef]
- Gerlich, D.; Wolf, M.; Yaacov, I.; Nissenson, B. Thermoelastic properties of ULE® titanium silicate glass. J. Non-Cryst. Solids 1976, 21, 243–249. [Google Scholar] [CrossRef]
- Vacher, R.; Pelous, J.; Plicque, F.; Zarembowitch, A. Ultrasonic and Brillouin scattering study of the elastic properties of vitreous silica between 10 and 300 K. J. Non-Cryst. Solids 1981, 45, 397–410. [Google Scholar] [CrossRef]
- Tielburger, D.; Merz, R.; Enhrenfels, R.; Hunklinger, S. Thermally activated relaxation processes in vitreous silica: An investigation by Brillouin scattering at high pressures. Phys. Rev. B 1992, 45, 2750–2760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Bass, J.D.; Taniguchi, T.; Goncharov, A.F.; Chang, Y.-Y.; Jacobsen, S.D. Elasticity of cubic boron nitride under ambient conditions. J. Appl. Phys. 2011, 109, 063521. [Google Scholar] [CrossRef]
- Le Parc, R.; Levelut, C.; Pelous, J.; Martinez, V.; Champagnon, B. Influence of fictive temperature and composition of silica glass on anomalous elastic behavior. J. Phys. Cond. Matter 2006, 18, 7507–7527. [Google Scholar] [CrossRef]
- Birch, F. Elasticity and constitution of the Earth’s interior. J. Geophys. Res. 1952, 57, 227–286. [Google Scholar] [CrossRef]
- Sonneville, C.; De Ligny, D.; Mermet, A.; Champagnon, B.; Martinet, C.; Henderson, G.H.; Deschamps, T.; Margueritat, J.; Barthel, E. In situ Brillouin study of sodium alumino silicate glasses under pressure. J. Chem. Phys. 2013, 139, 074501. [Google Scholar] [CrossRef]
- Xu, J.-A.; Manghnani, M.H.; Ming, L.C.; Wang, S.-Y. High-pressure Raman study of TiO2-SiO2 glasses: Evidence of the structural change. In High Pressure Research: Application to Earth and Planetary Sciences; Syono, Y., Manghnani, M.H., Eds.; Terra Publishing: Tokyo, Japan, 1992; pp. 519–525. [Google Scholar]
- Vacher, R.; Courtens, E.; Foret, M. Anharmonic versus relaxational sound damping in glasses. II. Vitreous silica. Phys. Rev. B 2005, 72, 214205. [Google Scholar] [CrossRef]
- Rau, S.; Enss, C.; Hunklinger, S.; Neu, P.; Wurger, A. Acoustic properties of oxide glasses at low temperatures. Phys. Rev. B 1995, 52, 7179–7194. [Google Scholar] [CrossRef]
- Rouxel, T.; Ji, H.; Hammouda, T.; Moreac, A. Poisson’s ratio and the densification of glass under high pressure. Phys. Rev. Lett. 2008, 100, 225501. [Google Scholar] [CrossRef] [PubMed]
- Guerette, M.; Ackerson, M.R.; Thomas, J.; Yuan, F.; Watson, E.B.; Walker, D.; Huang, L. Structure and properties of silica glass densified in cold compression and hot compression. Sci. Rep. 2015, 5, 15343. [Google Scholar] [CrossRef] [PubMed]
- Schuele, D.E.; Smith, C.S. Low temperature thermal expansion of RbI. J. Phys. Chem. Solids 1964, 25, 801–814. [Google Scholar] [CrossRef]


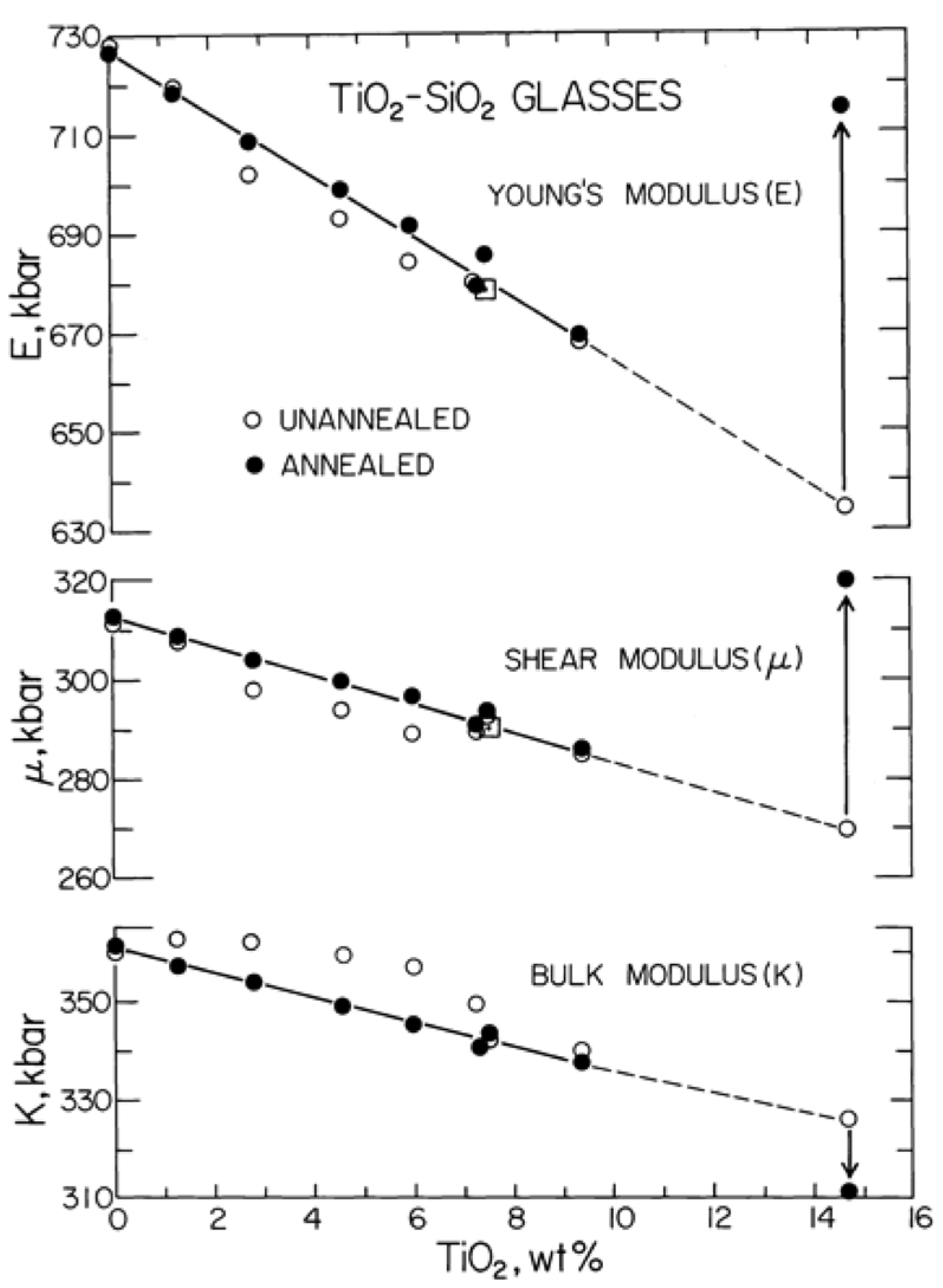
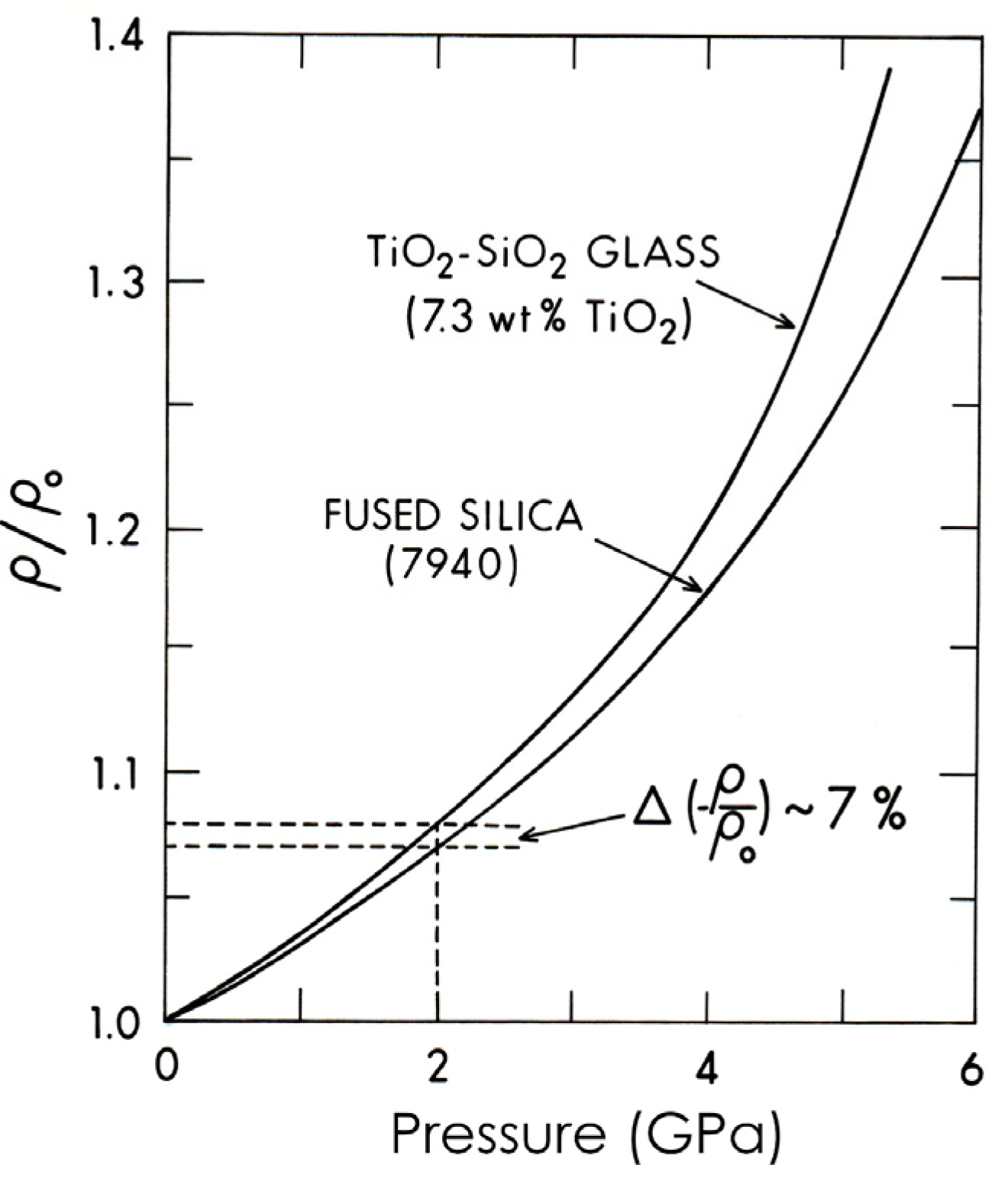

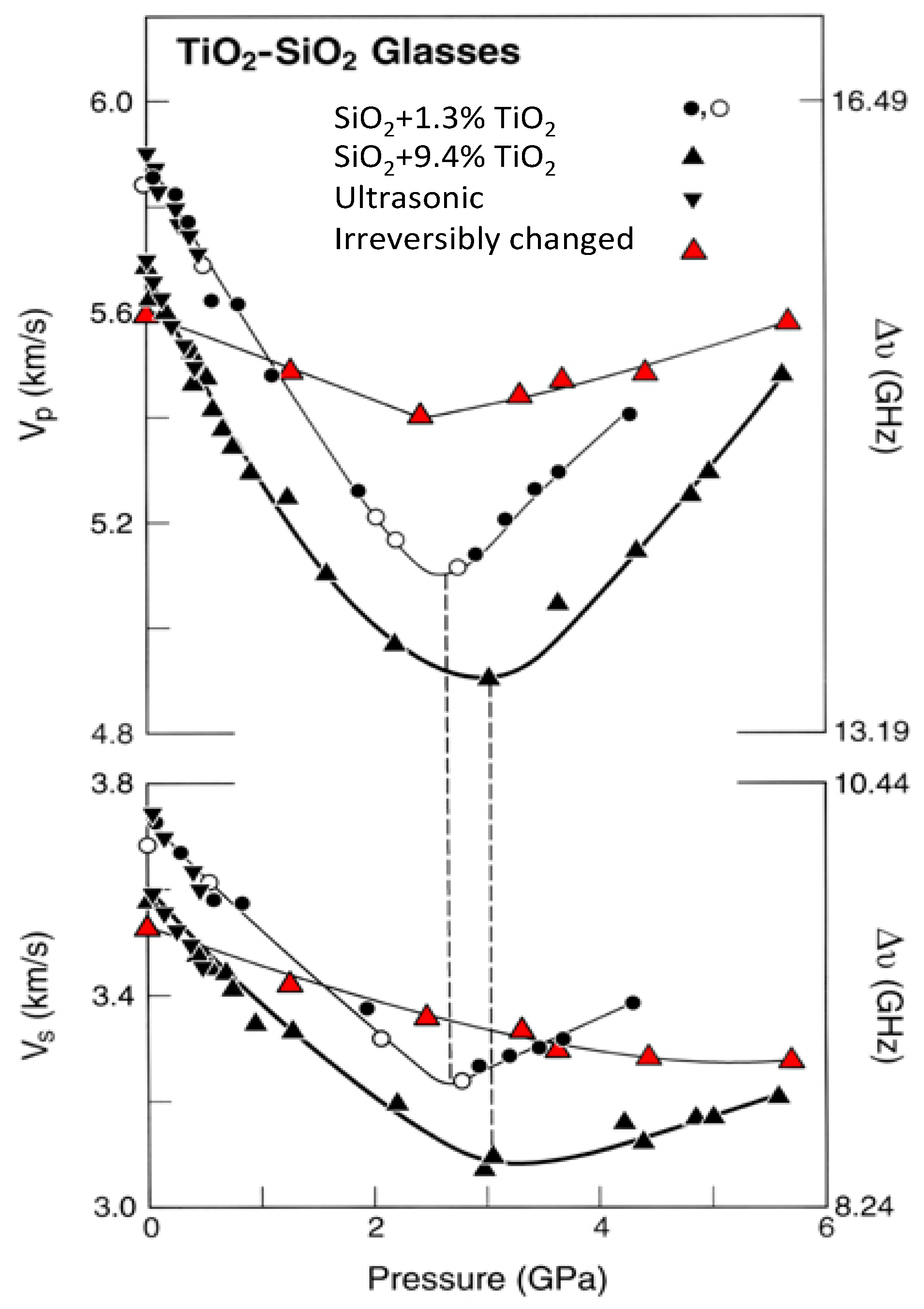
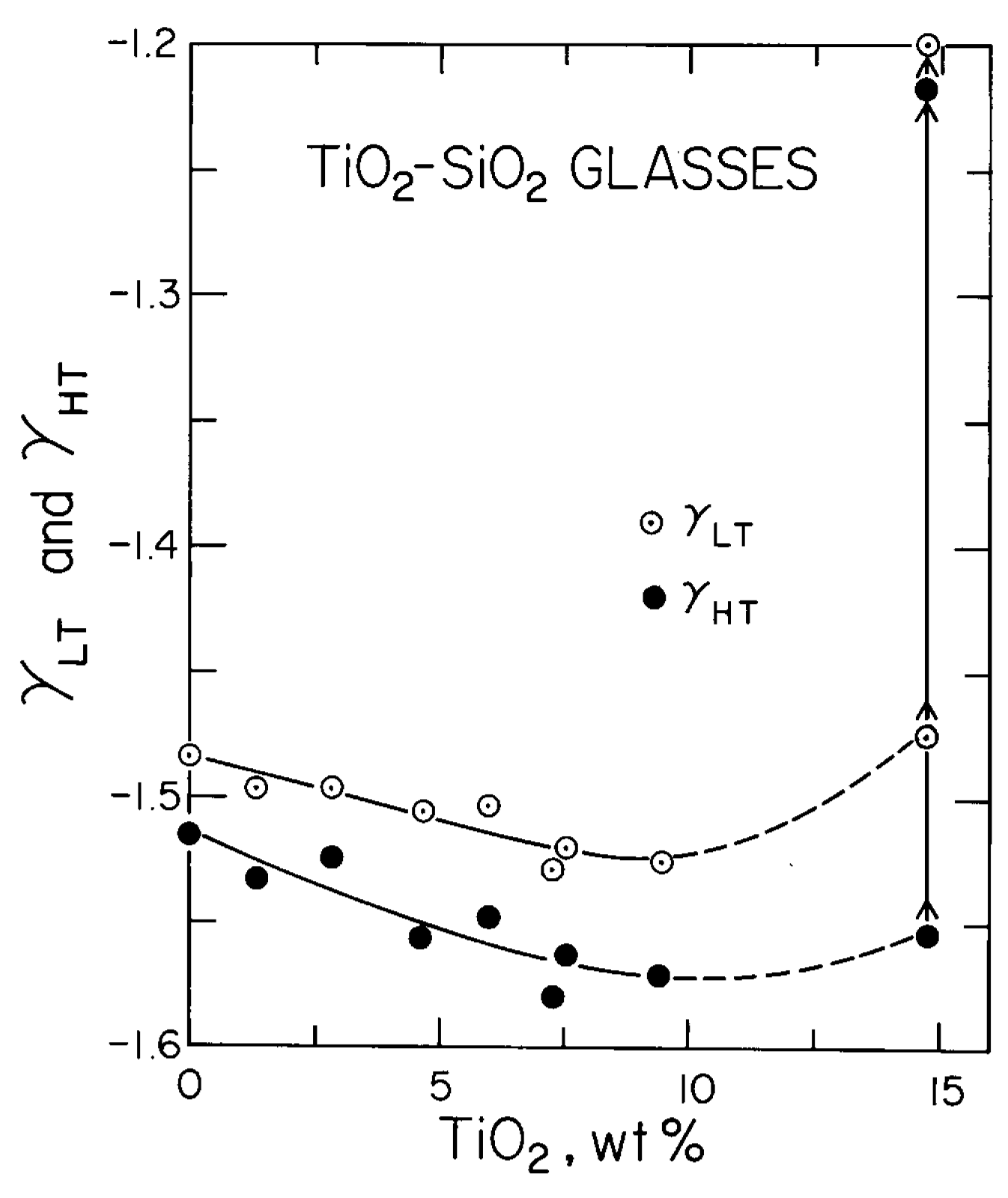
| Glass Number | Composition in wt% | ρ (g/cm3) | Vp (km/s) | Vs (km/s) | K (GPa) | μ(GPa) | E (GPa) | σ Poisson’s Ratio | |
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | TiO2 | ||||||||
| 7940A (fused silica) | 100 | 0 | 2.2007 | 5.947 | 3.769 | 36.16 | 31.26 | 728.0 | 0.165 |
| T4A | 98.7 | 1.3 | 2.2005 | 5.911 | 3.746 | 35.71 | 30.88 | 79.11 | 0.164 |
| T1A | 97.2 | 2.8 | 2.2001 | 5.872 | 3.717 | 35.32 | 30.40 | 70.88 | 0.166 |
| T5A | 95.4 | 4.6 | 2.1995 | 5.833 | 3.692 | 34.87 | 29.98 | 69.70 | 0.166 |
| T6A | 94.0 | 6.0 | 2.1992 | 5.803 | 3.672 | 34.53 | 29.65 | 69.16 | 0.166 |
| T2A | 92.7 | 7.3 | 2.1986 | 5.758 | 3.635 | 34.15 | 29.0.5 | 67.90 | 0.169 |
| ULE 7971A | 92.5 | 7.5 | 2.1993 | 5.783 | 3.653 | 34.42 | 29.35 | 62.56 | 0.168 |
| T3A | 90.6 | 9.4 | 2.1985 | 5.719 | 3.607 | 33.77 | 28.60 | 66.91 | 0.170 |
| Glass Number | Composition in wt% | dK/dP | dρ/dP g/cm3 Mbar−1 | dμ/dP | dE/dP | dσ/dP Mbar−1 | |
|---|---|---|---|---|---|---|---|
| SiO2 | TiO2 | ||||||
| 7940A (fused silica) | 100 | 0 | −5.37 | 6.68 | −3.53 | −8.82 | −1.01 |
| T4A | 98.7 | 1.3 | −5.68 | 6.78 | −3.56 | −9.00 | −1.24 |
| T1A | 97.2 | 2.8 | −5.42 | 6.80 | −3.56 | −8.87 | −1.00 |
| T5A | 95.4 | 4.6 | −5.97 | 7.01 | −3.55 | −9.13 | −1.52 |
| T6A | 94.0 | 6.0 | −5.85 | 7.06 | −3.54 | −9.05 | −1.42 |
| T2A | 92. | 7.3 | −5.96 | 7.20 | −3.60 | −9.20 | −1.47 |
| ULE 7971A | 92.5 | 7.5 | −5.88 | 7.10 | −3.60 | −9.16 | −1.36 |
| T3A | 90.6 | 9.4 | −5.77 | 7.26 | −3.59 | −9.09 | −1.30 |
| Composition TiO2, wt% | g/cm3 | Brillouin Vp (km/s) | Scattering Vs (km/s) | Ultrasonic Vp (km/s) | Ultrasonic Vs (km/s) | ΔVp; % | ΔVs; % |
|---|---|---|---|---|---|---|---|
| 1.3 | 2.2005 | 5.933 | 3.743 | 5.911 | 3.746 | 0.4 | −0.08 |
| 2.8 | 2.2001 | 5.890 | 3.732 | 5.872 | 3.717 | 0.3 | 0.4 |
| 4.6 | 2.1995 | 5.828 | 3.683 | 5.833 | 3.692 | −0.09 | −0.2 |
| 6.0 | 2.1992 | 5.800 | 3.656 | 5.803 | 3.672 | −0.05 | −0.4 |
| 7.3 | 2.1986 | 5.751 | 3.622 | 5.758 | 3.635 | −0.1 | −0.3 |
| 9.4 | 2.1985 | 5.746 | 3.616 | 5.719 | 3.607 | 0.5 | 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manghnani, M.H.; Williams, Q.; Matsui, T.; Schultz, P.C.; Kurkjian, C.R. Effects of Composition, Pressure, and Temperature on the Elastic Properties of SiO2–TiO2 Glasses: An Integrated Ultrasonic and Brillouin Study. Minerals 2020, 10, 481. https://doi.org/10.3390/min10050481
Manghnani MH, Williams Q, Matsui T, Schultz PC, Kurkjian CR. Effects of Composition, Pressure, and Temperature on the Elastic Properties of SiO2–TiO2 Glasses: An Integrated Ultrasonic and Brillouin Study. Minerals. 2020; 10(5):481. https://doi.org/10.3390/min10050481
Chicago/Turabian StyleManghnani, Murli H., Quentin Williams, Teruyuki Matsui, Peter C. Schultz, and Charles R. Kurkjian. 2020. "Effects of Composition, Pressure, and Temperature on the Elastic Properties of SiO2–TiO2 Glasses: An Integrated Ultrasonic and Brillouin Study" Minerals 10, no. 5: 481. https://doi.org/10.3390/min10050481
APA StyleManghnani, M. H., Williams, Q., Matsui, T., Schultz, P. C., & Kurkjian, C. R. (2020). Effects of Composition, Pressure, and Temperature on the Elastic Properties of SiO2–TiO2 Glasses: An Integrated Ultrasonic and Brillouin Study. Minerals, 10(5), 481. https://doi.org/10.3390/min10050481





