Toward an Improved Understanding of the Marine Barium Cycle and the Application of Marine Barite as a Paleoproductivity Proxy
Abstract
1. Introduction
2. Barium Cycling: Processes in the Modern Ocean
2.1. Sources of Dissolved Barium to the Ocean
2.2. Barium Uptake and Release from Biogenic Material
2.3. Barite Formation in the Water Column
2.4. Barite Dissolution in Deep Water
2.5. Barite Preservation in Sediments
2.6. New Insights from Barium Isotopes
3. Marine Barite Accumulation in the Present and Past and the Global C Cycle
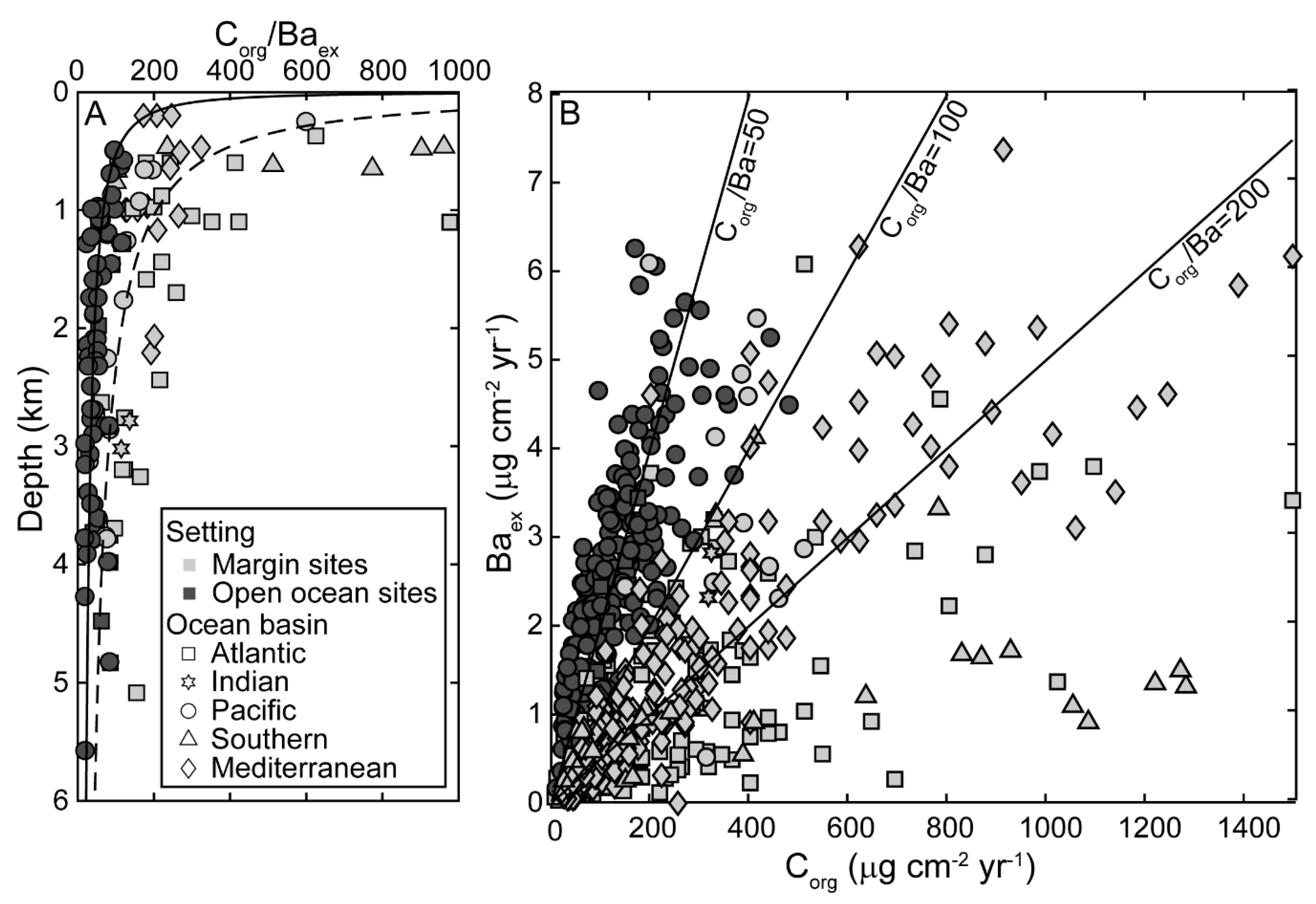
3.1. Relationship between Excess Barium Flux and C Export in the Water Column
3.2. Barite Accumulation in the Modern Ocean
3.3. Temporal Variability of Barite Accumulation and Implications for Carbon Export
4. Modeling the Marine Ba Cycle and Implications for Barite as a Paleoproductivity Proxy
4.1. Existing Models and Their Findings
4.2. A Reevaluation of the Box Model
Sensitivity to Changes in Ba Inputs
4.3. Implications, Limitations, and Future Works
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berner, R.A.; Lasaga, A.C.; Garrels, R.M. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am. J. Sci. 1983, 283, 641–683. [Google Scholar] [CrossRef]
- Sarmiento, J.L.; Toggweiler, J.R. A new model for the role of the oceans in determining atmospheric pCO2. Nature 1984, 308, 621–624. [Google Scholar] [CrossRef]
- Falkowski, M.; Barber, B.J.; Smetacek, A.L.; Engelhardt, K.A.M.; Ruesink, J.L.; Srivastava, D.S. Biogeochemical controls and feedbacks on ocean primary production. Science 1998, 281, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; Buesseler, K.O.; Doney, S.C.; Sailley, S.F.; Behrenfeld, M.J.; Boyd, P.W. Global assessment of ocean carbon export by combining satellite observations and food-web models. Glob. Biogeochem. Cycles 2014, 28, 181–196. [Google Scholar] [CrossRef]
- Volk, T.; Hoffert, M.I. Ocean carbon pumps: Analysis of relative strengths and efficiencies in ocean-driven atmospheric CO2 changes. Geophys. Monogr. Ser. 1985, 32, 99–110. [Google Scholar]
- Arndt, S.; Jørgensen, B.B.; LaRowe, D.E.; Middelburg, J.J.; Pancost, R.D.; Regnier, P. Quantifying the degradation of organic matter in marine sediments: A review and synthesis. Earth-Sci. Rev. 2013, 123, 53–86. [Google Scholar] [CrossRef]
- Paytan, A. Ocean paleoproductivity. In Encyclopedia of Paleoclimatology and Ancient Environments; Gornitz, V., Ed.; Kluwer Academic Publishers: Berlin Germany, 2008; pp. 643–651. ISBN 978-1-4020-4551-6. [Google Scholar]
- Wolgemuth, K.; Broecker, W.S. Barium in sea water. Earth Planet. Sci. Lett. 1970, 8, 372–378. [Google Scholar] [CrossRef]
- Paytan, A.; Kastner, M. Benthic Ba fluxes in the central Equatorial Pacific, implications for the oceanic Ba cycle. Earth Planet. Sci. Lett. 1996, 142, 439–450. [Google Scholar] [CrossRef]
- Collier, R.W.; Edmond, J.M. The trace elements geochemistry of marine biogenic particulate matter. Prog. Ocean. 1984, 13. [Google Scholar] [CrossRef]
- Dymond, J.; Suess, E.; Lyle, M. Barium in deep-sea sediment: A geochemical proxy for paleoproductivity. Paleoceanography 1992, 7, 163–181. [Google Scholar] [CrossRef]
- Bishop, J.K.B. The barite-opal-organic carbon association in oceanic particulate matter. Nature 1988, 332, 341–343. [Google Scholar] [CrossRef]
- Faure, G. Principles and Applications of Geochemistry; Prentice-Hall: Upper Saddle River, NJ, USA, 1998. [Google Scholar]
- Bacon, M.P.; Edmond, J.M. Barium at Geosecs III in the southwest Pacific. Earth Planet. Sci. Lett. 1972, 16, 66–74. [Google Scholar] [CrossRef]
- Church, T.M.; Wolgemuth, K. Marine barite saturation. Earth Planet. Sci. Lett. 1972, 15, 35–44. [Google Scholar] [CrossRef]
- Li, Y.H.; Ku, T.L.; Mathieu, G.; Wolgemuth, K. Ba in the antarctic ocean and its implications regarding the marine geochemistry of Ba and 226Ra*. Earth Planet. Sci. Lett. 1973, 19, 352–358. [Google Scholar] [CrossRef]
- Chan, L.H.; Edmond, J.M.; Stallard, R.F.; Broecker, W.S.; Chung, Y.C.; Weiss, R.F.; Ku, T.L. Radium and barium at GEOSECS stations in the Atlantic and Pacific. Earth Planet. Sci. Lett. 1976, 32, 258–267. [Google Scholar] [CrossRef]
- Chan, L.H.; Drummond, D.; Edmond, J.M.; Grant, B. On the barium data from the Atlantic GEOSECS expedition. Deep. Res. 1977, 24, 613–649. [Google Scholar] [CrossRef]
- Bridgestock, L.; Hsieh, Y.-T.; Porcelli, D.; Homoky, W.B.; Bryan, A.; Henderson, G.M. Controls on the barium isotope compositions of marine sediments. Earth Planet. Sci. Lett. 2018, 481, 101–110. [Google Scholar] [CrossRef]
- Hsieh, Y.T.; Henderson, G.M. Barium stable isotopes in the global ocean: Tracer of Ba inputs and utilization. Earth Planet. Sci. Lett. 2017, 473, 269–278. [Google Scholar] [CrossRef]
- Bates, S.L.; Hendry, K.R.; Pryer, H.V.; Kinsley, C.W.; Pyle, K.M.; Woodward, E.M.S.; Horner, T.J. Barium isotopes reveal role of ocean circulation on barium cycling in the Atlantic. Geochim. Cosmochim. Acta 2017, 204, 286–299. [Google Scholar] [CrossRef]
- Horner, T.J.; Kinsley, C.W.; Nielsen, S.G. Barium-isotopic fractionation in seawater mediated by barite cycling and oceanic circulation. Earth Planet. Sci. Lett. 2015, 430, 511–522. [Google Scholar] [CrossRef]
- Hemsing, F.; Hsieh, Y.-T.; Bridgestock, L.; Spooner, P.T.; Robinson, L.F.; Frank, N.; Henderson, G.M. Barium isotopes in cold-water corals. Earth Planet. Sci. Lett. 2018, 491, 183–192. [Google Scholar] [CrossRef]
- Elderfield, H.; Schultz, A. Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean. Annu. Rev. Earth Planet. Sci. 1996, 24, 191–224. [Google Scholar] [CrossRef]
- Monnin, C.; Wheat, C.G.; Dupre, B.; Elderfield, H.; Mottl, M.M. Barium geochemistry in sediment pore waters and formation waters of the oceanic crust on the eastern flank of the Juan de Fuca Ridge (ODP Leg 168). Geochem., Geophys. Geosystems 2001, 2. [Google Scholar] [CrossRef]
- Torres, M.E.; Bohrmann, G.; Suess, E. Authigenic barites and fluxes of barium associated with fluid seeps in the Peru subduction zone. Earth Planet. Sci. Lett. 1996, 144, 469–481. [Google Scholar] [CrossRef]
- Dickens, G.R.; Fewless, T.; Thomas, E.; Bralower, T.J. Excess barite accumulation during the Paleocene-Eocene Thermal Maximum: Massive input of dissolved barium from seafloor gas hydrate reservoirs. In Causes and Consequences of Globally Warm Climates in the Early Paleogene; Geological Society of America: Boulder, CO, USA, 2003; Volume 369, pp. 11–23. [Google Scholar]
- Dehairs, F.; Chesselet, R.; Jedwab, J. Discrete suspended particles of barite and the barium cycle in the open ocean. Earth Planet. Sci. Lett. 1980, 49, 528–550. [Google Scholar] [CrossRef]
- Ganeshram, R.S.; François, R.; Commeau, J.; Brown-Leger, S.L. An experimental investigation of barite formation in seawater. Geochim. Cosmochim. Acta 2003, 67, 2599–2605. [Google Scholar] [CrossRef]
- Van Beek, P.; François, R.; Conte, M.; Reyss, J.L.; Souhaut, M.; Charette, M. 228Ra/226Ra and 226Ra/Ba ratios to track barite formation and transport in the water column. Geochim. Cosmochim. Acta 2007, 71, 71–86. [Google Scholar] [CrossRef]
- Martinez-Ruiz, F.; Jroundi, F.; Paytan, A.; Guerra-Tschuschke, I.; Abad, M.D.M.; González-Muñoz, M.T. Barium bioaccumulation by bacterial biofilms and implications for Ba cycling and use of Ba proxies. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ruiz, F.; Paytan, A.; Gonzalez-Muñoz, M.T.; Jroundi, F.; Abad, M.M.; Lam, P.J.; Bishop, J.K.B.; Horner, T.J.; Morton, P.L.; Kastner, M. Barite formation in the ocean: Origin of amorphous and crystalline precipitates. Chem. Geol. 2019, 511, 441–451. [Google Scholar] [CrossRef]
- Bertram, M.A.; Cowen, J.P. Morphological and compositional evidence for biotic precipitation of marine barite. J. Mar. Res. 1997, 55, 577–593. [Google Scholar] [CrossRef]
- Dehairs, F.; Jacquet, S.; Savoye, N.; Van Mooy, B.A.S.; Buesseler, K.O.; Bishop, J.K.B.; Lamborg, C.H.; Elskens, M.; Baeyens, W.; Boyd, P.W.; et al. Barium in twilight zone suspended matter as a potential proxy for particulate organic carbon remineralization: Results for the North Pacific. Deep. Res. Part II Top. Stud. Oceanogr. 2008, 55, 1673–1683. [Google Scholar] [CrossRef]
- Fisher, N.S.; Guillard, R.R.L.; Bankston, D.C. The accumulation of barium by marine phytoplankton grown in culture. J. Mar. Res. 1991, 49, 339–354. [Google Scholar] [CrossRef]
- Goldberg, E.D.; Arrhenius, G.O.S. Chemistry of Pacific pelagic sediments. Geochim. Cosmochim. Acta 1958, 13, 153–212. [Google Scholar] [CrossRef]
- Bernstein, R.E.; Byrne, R.H.; Betzer, P.R.; Greco, A.M. Morphologies and transformations of celestite in seawater: The role of acantharians in strontium and barium geochemistry. Geochim. Cosmochim. Acta 1992, 56, 3273–3279. [Google Scholar] [CrossRef]
- Bernstein, R.E.; Byrne, R.H.; Schijf, J. Acantharians: A missing link in the oceanic biogeochemistry of barium. Deep. Res. Part I Oceanogr. Res. Pap. 1998, 45, 491–505. [Google Scholar] [CrossRef]
- Chow, T.J.; Goldberg, E.D. On the marine geochemistry of barium. Geochim. Cosmochim. Acta 1960, 20, 192–198. [Google Scholar] [CrossRef]
- Francois, R.; Honjo, S.; Manganini, S.J.; Ravizza, G.E. Biogenic barium fluxes to the deep sea: Implications for paleoproductivity reconstruction. Glob. Biogeochem. Cycles 1995, 9, 289–303. [Google Scholar] [CrossRef]
- McManus, J.; Berelson, W.M.; Klinkhammer, G.P.; Johnson, K.S.; Coale, K.H.; Anderson, R.F.; Kumar, N.; Burdige, D.J.; Hammond, D.E.; Brumsack, H.J.; et al. Geochemistry of barium in marine sediments: Implications for its use as a paleoproxy. Geochim. Cosmochim. Acta 1998, 62, 3453–3473. [Google Scholar] [CrossRef]
- Klump, J.; Hebbeln, D.; Wefer, G. High concentrations of biogenic barium in Pacific sediments after termination I—A signal of changes in productivity and deep water chemistry. Mar. Geol. 2001, 177, 1–11. [Google Scholar] [CrossRef]
- McManus, J.; Berelson, W.M.; Hammond, D.E.; Klinkhammer, G.P. Barium cycling in the North Pacific: Implications for the utility of Ba as a paleoproductivity and paleoalkalinity proxy. Paleoceanography 1999, 14, 53–61. [Google Scholar] [CrossRef]
- Paytan, A.; Kastner, M.; Chavez, F.P. Glacial to interglacial fluctuations in productivity in the equatorial Pacific as indicated by marine barite. Science 1996, 274, 1355–1357. [Google Scholar] [CrossRef] [PubMed]
- Monnin, C.; Jeandel, C.; Cattaldo, T.; Dehairs, F. The marine barite saturation state of the world’s oceans. Mar. Chem. 1999, 65, 253–261. [Google Scholar] [CrossRef]
- Hanor, J.S.; Chan, L.-H. Non-conservative behavior of barium during mixing of Mississippi River and Gulf of Mexico waters. Earth Planet. Sci. Lett. 1977, 37, 242–250. [Google Scholar] [CrossRef]
- Cao, Z.; Siebert, C.; Hathorne, E.C.; Dai, M.; Frank, M. Corrigendum to “Constraining the oceanic barium cycle with stable barium isotopes” [Earth Planet. Sci. Lett. 434 (2016) 1–9]. Earth Planet. Sci. Lett. 2020, 530, 116003. [Google Scholar] [CrossRef]
- Coffey, M.; Dehairs, F.; Collette, O.; Luther, G.; Church, T.; Jickells, T. The behavior of dissolved barium in estuaries. Estuar. Coast. Shelf Sci. 1997, 45, 113–121. [Google Scholar] [CrossRef]
- Joung, D.; Shiller, A.M. Dissolved barium behavior in Louisiana Shelf waters affected by the Mississippi/Atchafalaya River mixing zone. Geochim. Cosmochim. Acta 2014, 141, 303–313. [Google Scholar] [CrossRef]
- Santos, I.R.; Burnett, W.C.; Misra, S.; Suryaputra, I.G.N.A.; Chanton, J.P.; Dittmar, T.; Peterson, R.N.; Swarzenski, P.W. Uranium and barium cycling in a salt wedge subterranean estuary: The influence of tidal pumping. Chem. Geol. 2011, 287, 114–123. [Google Scholar] [CrossRef]
- Schlitzer, R.; Anderson, R.F.; Dodas, E.M.; Lohan, M.; Geibert, W.; Tagliabue, A.; Bowie, A.; Jeandel, C.; Maldonado, M.T.; Landing, W.M.; et al. The GEOTRACES Intermediate Data Product 2017. Chem. Geol. 2018, 493, 210–223. [Google Scholar] [CrossRef]
- Schlitzer, R. eGEOTRACES-Electronic Atlas of GEOTRACES Sections and Animated 3D Scenes. Available online: http://www.egeotraces.org (accessed on 9 May 2020).
- Broecker, W.S.; Peng, T.-H. Tracers in the Sea; Lamont-Doherty Geologic Observatory: Palisades, NY, USA, 1982. [Google Scholar]
- Dymond, J.; Collier, R. Particulate barium fluxes and their relationships to biological productivity. Deep. Res. Part II Top. Stud. Oceanogr. 1996, 43, 1283–1308. [Google Scholar] [CrossRef]
- Von Damm, K.; Edmond, J.; Grant, B.; Measures, C.; Walden, B.; Weiss, R. Chemistry of submarine hydrothermal solutions at 21° N, East Pacific Rise. Geochim. Cosmochim. Acta 1985, 49, 2197–2220. [Google Scholar] [CrossRef]
- Edmond, J.M.; Boyle, E.D.; Drummond, D.; Grant, G. Desorption of Ba in the plume of the Zaire River. Neth. J. Sea Res. 1978, 12, 324–328. [Google Scholar] [CrossRef]
- Das, A.; Krishnaswami, S. Barium in Deccan Basalt Rivers: Its Abundance, Relative Mobility and Flux. Aquat. Geochemistry 2006, 12, 221–238. [Google Scholar] [CrossRef]
- Mayfield, K.K.; Eisenhauer, A.; Santiago Ramos, D.P.; Higgins, J.A.; Horner, T.J.; Auro, M.; Magna, T.; Moosdorf, N.; Charette, M.A.; Gonneea, M.E.; et al. The importance of groundwater discharge in marine isotope budgets. Nat. Commun. accepted.
- Edmond, J.M.; Spivack, A.; Grant, B.C.; Ming-Hui, H.; Zexiam, C.; Sung, C.; Xiushau, Z. Chemical dynamics of the Changjiang estuary. Cont. Shelf Res. 1985, 4, 17–36. [Google Scholar] [CrossRef]
- Roy-Barman, M.; Pons-Branchu, E.; Levier, M.; Bordier, L.; Foliot, L.; Gdaniec, S.; Ayrault, S.; Garcia-Orellana, J.; Masque, P.; Castrillejo, M. Barium during the GEOTRACES GA-04S MedSeA cruise: The Mediterranean Sea Ba budget revisited. Chem. Geol. 2019, 511, 431–440. [Google Scholar] [CrossRef]
- Edmond, J.M.; Measures, C.; McDuff, R.E.; Chan, L.H.; Collier, R.; Grant, B.; Gordon, L.I.; Corliss, J.B. Ridge crest hydrothermal activity and the balances of the major and minor elements in the ocean: The Galapagos data. Earth Planet. Sci. Lett. 1979, 46, 1–18. [Google Scholar] [CrossRef]
- Shaw, T.J.; Moore, W.S.; Kloepfer, J.; Sochaski, M.A. The flux of barium to the coastal waters of the southeastern USA: The importance of submarine groundwater discharge. Geochim. Cosmochim. Acta 1998, 62, 3047–3054. [Google Scholar] [CrossRef]
- Hanor, J.S. Barite-Celestine Geochemistry and Environments of Formation. Rev. Mineral. Geochem. 2000, 40, 193–275. [Google Scholar] [CrossRef]
- Stecher, H.A.; Kogut, M.B. Rapid barium removal in the Delaware estuary. Geochim. Cosmochim. Acta 1999, 63, 1003–1012. [Google Scholar] [CrossRef]
- Moore, W.S. High fluxes of radium and barium from the mouth of the Ganges-Brahmaputra River during low river discharge suggest a large groundwater source. Earth Planet. Sci. Lett. 1997, 150, 141–150. [Google Scholar] [CrossRef]
- Gonneea, M.E.; Mulligan, A.E.; Charette, M.A. Seasonal cycles in radium and barium within a subterranean estuary: Implications for groundwater derived chemical fluxes to surface waters. Geochim. Cosmochim. Acta 2013, 119, 164–177. [Google Scholar] [CrossRef]
- Santos, R.V.; Sondag, F.; Cochonneau, G.; Lagane, C.; Brunet, P.; Hattingh, K.; Chaves, J.G.S. Source area and seasonal 87Sr/86Sr variations in rivers of the Amazon basin. Hydrol. Process. 2015, 29, 187–197. [Google Scholar] [CrossRef]
- Lin, I.-T.; Wang, C.-H.; You, C.-F.; Lin, S.; Huang, K.-F.; Chen, Y.-G. Deep submarine groundwater discharge indicated by tracers of oxygen, strontium isotopes and barium content in the Pingtung coastal zone, southern Taiwan. Mar. Chem. 2010, 122, 51–58. [Google Scholar] [CrossRef]
- Torres, M.E.; McManus, J.; Huh, C.-A. Fluid seepage along the San Clemente Fault scarp: Basin-wide impact on barium cycling. Earth Planet. Sci. Lett. 2002, 203, 181–194. [Google Scholar] [CrossRef]
- McQuay, E.L.; Torres, M.E.; Collier, R.W.; Huh, C.-A.; McManus, J. Contribution of cold seep barite to the barium geochemical budget of a marginal basin. Deep Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 801–811. [Google Scholar] [CrossRef]
- Torres, M.E.; Bohrmann, G.; Dubé, T.E.; Poole, F.G. Formation of modern and Paleozoic stratiform barite at cold methane seeps on continental margins. Geology 2003, 31, 897–900. [Google Scholar] [CrossRef]
- Aloisi, G.; Wallmann, K.; Bollwerk, S.M.; Derkachev, A.; Bohrmann, G.; Suess, E. The effect of dissolved barium on biogeochemical processes at cold seeps. Geochim. Cosmochim. Acta 2004, 68, 1735–1748. [Google Scholar] [CrossRef]
- Sternberg, E.; Tang, D.; Ho, T.Y.; Jeandel, C.; Morel, F.M.M. Barium uptake and adsorption in diatoms. Geochim. Cosmochim. Acta 2005, 69, 2745–2752. [Google Scholar] [CrossRef]
- Stroobants, N.; Dehairs, F.; Goeyens, L.; Vanderheijden, N.; Van Grieken, R. Barite formation in the Southern Ocean water column. Mar. Chem. 1991, 35, 411–421. [Google Scholar] [CrossRef]
- Dehairs, F.; Stroobants, N.; Goeyens, L. Suspended barite as a tracer of biological activity in the Southern Ocean. Mar. Chem. 1991, 35, 399–410. [Google Scholar] [CrossRef]
- Dehairs, F.; Baeyens, W.; Goeyens, L. Accumulation of suspended barite at mesopelagic depths and export production in the Southern Ocean. Science 1992, 258, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, S.H.M.; Dehairs, F.; Dumont, I.; Becquevort, S.; Cavagna, A.J.; Cardinal, D. Twilight zone organic carbon remineralization in the Polar Front Zone and Subantarctic Zone south of Tasmania. Deep. Res. Part II Top. Stud. Oceanogr. 2011, 58, 2222–2234. [Google Scholar] [CrossRef]
- Planchon, F.; Cavagna, A.J.; Cardinal, D.; André, L.; Dehairs, F. Late summer particulate organic carbon export and twilight zone remineralisation in the Atlantic sector of the Southern Ocean. Biogeosciences 2013, 10, 803–820. [Google Scholar] [CrossRef]
- Gonzalez-Muñoz, M.T.; Martinez-Ruiz, F.; Morcillo, F.; Martin-Ramos, J.D.; Paytan, A. Precipitation of barite by marine bacteria: A possible mechanism for marine barite formation. Geology 2012, 40, 675–678. [Google Scholar] [CrossRef]
- Torres-Crespo, N.; Martínez-Ruiz, F.; González-Muñoz, M.T.; Bedmar, E.J.; De Lange, G.J.; Jroundi, F. Role of bacteria in marine barite precipitation: A case study using Mediterranean seawater. Sci. Total Environ. 2015, 512–513, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Muñoz, M.T.; Fernandez-Luque, B.; Martinez-Ruiz, F.; Chekroun, K.B.; Arias, J.M.; Rodriguez-Gallego, M.; Martinez-Canamero, M.; de Linares, C.; Paytan, A. Precipitation of barite by Myxococcus xanthus: Possible implications for the biogeocehmical cycle of barium. Appl. Environ. Microbiol. 2003, 69, 5722–5725. [Google Scholar] [CrossRef]
- Bernstein, R.E.; Byrne, R.H. Acantharians and marine barite. Mar. Chem. 2004, 86, 45–50. [Google Scholar] [CrossRef]
- Arrhenius, G.; Bonatti, E. Neptunism and vulcanism in the ocean. Prog. Oceanogr. 1965, 3, 7–22. [Google Scholar] [CrossRef]
- Tendal, O.S. A monograph of the Xenophyophoria (Rhizopodea, Protozoa). Galathea Rep. 1972, 12, 7–103. [Google Scholar]
- Gooday, A.J.; Nott, J.A. Intracellular Barite Crystals in Two Xenophyophores, Aschemonella Ramuliformis and Galatheammina Sp. (Protozoa: Rhizopoda) With Comments on the Taxonomy of A. Ramuliformis. J. Mar. Biol. Assoc. UK 1982, 62, 595–605. [Google Scholar] [CrossRef]
- Swinbanks, D.D.; Shirayama, Y. High levels of natural radionuclides in a deep-sea infaunal xenophyophore. Nature 1986, 320, 354–358. [Google Scholar] [CrossRef]
- Paytan, A.; Griffith, E.M. Marine barite: Recorder of variations in ocean export productivity. Deep. Res. Part II Top. Stud. Oceanogr. 2007, 54, 687–705. [Google Scholar] [CrossRef]
- Falkner, K.K.; Klinkhammer, G.P.; Bowers, T.S.; Todd, J.F.; Lewis, B.L.; Landing, W.M.; Edmond, J.M. The behavior of barium in anoxic marine waters. Geochim. Cosmochim. Acta 1993, 57, 537–554. [Google Scholar] [CrossRef]
- Blount, C.W. Barite solubilities and thermodynamic quantities up to 300 C and 1400 bars. Am. Mineral. 1977, 62, 942–957. [Google Scholar]
- Brumsack, H.J.; Gieskes, J.M. Interstitial water trace-metal chemistry of laminated sediments from the Gulf of California, Mexico. Mar. Chem. 1983, 14, 89–106. [Google Scholar] [CrossRef]
- Von Breymann, M.T.; Emeis, K.C.; Camerlenghi, A. Geochemistry of sediments from the Peru upwelling area: Results from Sites 680, 682, 685 and 688. Geology 1990, 491–503. [Google Scholar] [CrossRef]
- Von Breymann, M.T.; Brumsack, H.; Emeis, K.C. Depositional and Diagenetic Behavior of Barium in the Japan Sea. In Proceedings of the Ocean Drilling Program, 127/128 Scientific Results; Ocean Drilling Program: College Station, TX, USA, 1992; Volume 127, pp. 651–665. [Google Scholar]
- Gingele, F.; Dahmke, A. Discrete barite particles and barium as tracers of paleoproductivity in south Atlantic sediments. Paleoceanography 1994, 9, 151–168. [Google Scholar] [CrossRef]
- Torres, M.E.; Brumsack, H.J.; Bohrmann, G.; Emeis, K.C. Barite fronts in continental margin sediments: A new look at barium remobilization in the zone of sulfate reduction and formation of heavy barites in diagenetic fronts. Chem. Geol. 1996, 127, 125–139. [Google Scholar] [CrossRef]
- Dickens, G.R. Sulfate profiles and barium fronts in sediment on the Blake Ridge: Present and past methane fluxes through a large as hydrate reservoir. Geochim. Cosmochim. Acta 2001, 65, 529–543. [Google Scholar] [CrossRef]
- Griffith, E.M.; Paytan, A. Barite in the ocean—occurrence, geochemistry and palaeoceanographic applications. Sedimentology 2012, 59, 1817–1835. [Google Scholar] [CrossRef]
- Paytan, A.; Mearon, S.; Cobb, K.; Kastner, M. Origin of marine barite deposits: Sr and S isotope characterization. Geology 2002, 30, 747–750. [Google Scholar] [CrossRef]
- Griffith, E.M.; Paytan, A.; Wortmann, U.G.; Eisenhauer, A.; Scher, H.D. Combining metal and nonmetal isotopic measurements in barite to identify mode of formation. Chem. Geol. 2018, 500, 148–158. [Google Scholar] [CrossRef]
- Von Allmen, K.; Böttcher, M.E.; Samankassou, E.; Nägler, T.F. Barium isotope fractionation in the global barium cycle: First evidence from barium minerals and precipitation experiments. Chem. Geol. 2010, 277, 70–77. [Google Scholar] [CrossRef]
- Eagle, M.; Paytan, A.; Arrigo, K.R.; van Dijken, G.; Murray, R.W. A comparison between excess barium and barite as indicators of carbon export. Paleoceanography 2003, 18. [Google Scholar] [CrossRef]
- Bains, S.; Norris, R.D.; Corfield, R.M.; Faul, K.L. Termination of global warmth at the Palaeocene/Eocene boundary through productivity feedback. Nature 2000, 407, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, B. Barium, equatorial high productivity, and the northward wandering of the Indian continent. Paleoceanography 1987, 2, 63–77. [Google Scholar] [CrossRef]
- Torfstein, A.; Winckler, G.; Tripati, A. Productivity feedback did not terminate the Paleocene-Eocene Thermal Maximum (PETM). Clim. Past 2010, 6, 265–272. [Google Scholar] [CrossRef]
- Nurnberg, C.C.; Bohrmann, G.; Schluter, M.; Frank, M. Barium accumulation in the Atlantic sector of the Southern Ocean: Results from 190,000 year records. Paleoceanography 1997, 12, 594–603. [Google Scholar] [CrossRef]
- Ma, Z.; Ravelo, A.C.; Liu, Z.; Zhou, L.; Paytan, A. Export production fluctuations in the eastern equatorial Pacific during the Pliocene-Pleistocene: Reconstruction using barite accumulation rates. Paleoceanography 2015, 30, 1455–1469. [Google Scholar] [CrossRef]
- Ma, Z.; Gray, E.; Thomas, E.; Murphy, B.; Zachos, J.; Paytan, A. Carbon sequestration during the Palaeocene-Eocene Thermal Maximum by an efficient biological pump. Nat. Geosci. 2014, 7, 382–388. [Google Scholar] [CrossRef]
- Carter, S.C.; Griffith, E.M.; Penman, D.E. Peak intervals of equatorial Pacific export production during the middle Miocene climate transition. Geology 2016, 44, 923–926. [Google Scholar] [CrossRef]
- Erhardt, A.M.; Pälike, H.; Paytan, A. High-resolution record of export production in the eastern equatorial Pacific across the Eocene-Oligocene transition and relationships to global climatic records. Paleoceanography 2013, 28, 130–142. [Google Scholar] [CrossRef]
- Griffith, E.M.; Calhoun, M.; Thomas, E.; Averyt, K.; Erhardt, A.; Bralower, T.; Lyle, M.; Olivarez-Lyle, A.; Paytan, A. Export productivity and carbonate accumulation in the Pacific Basin at the transition from a greenhouse to icehouse climate (late Eocene to early Oligocene). Paleoceanography 2010, 25, 1–15. [Google Scholar] [CrossRef]
- Moore, J.C.; Wade, B.S.; Westerhold, T.; Erhardt, A.M.; Coxall, H.K.; Baldauf, J.; Wagner, M. Equatorial Pacific productivity changes near the Eocene-Oligocene boundary. Paleoceanography 2014, 29, 825–844. [Google Scholar] [CrossRef]
- Fagel, N.; Dehairs, F.; Peinert, R.; Antia, A.; André, L. Reconstructing export production at the NE Atlantic margin: Potential and limits of the Ba proxy. Mar. Geol. 2004, 204, 11–25. [Google Scholar] [CrossRef]
- McManus, J.; Dymond, J.; Dunbar, R.B.; Collier, R.W. Particulate barium fluxes in the Ross Sea. Mar. Geol. 2002, 184, 1–15. [Google Scholar] [CrossRef]
- Sun, W.P.; Han, Z.B.; Hu, C.Y.; Pan, J.M. Particulate barium flux and its relationship with export production on the continental shelf of Prydz Bay, east Antarctica. Mar. Chem. 2013, 157, 86–92. [Google Scholar] [CrossRef]
- Sanchez-Vidal, A.; Collier, R.W.; Calafat, A.; Fabres, J.; Canals, M. Particulate barium fluxes on the continental margin: A study from the Alboran Sea (Western Mediterranean). Mar. Chem. 2005, 93, 105–117. [Google Scholar] [CrossRef]
- Sternberg, E.; Jeandel, C.; Miquel, J.-C.; Gasser, B.; Souhaut, M.; Arraes-Mescoff, R.; Francois, R. Particulate barium fluxes and export production in the northwestern Mediterranean. Mar. Chem. 2007, 105, 281–295. [Google Scholar] [CrossRef]
- Dehairs, F.; Fagel, N.; Antia, A.N.; Peinert, R.; Elskens, M.; Goeyens, L. Export production in the Bay of Biscay as estimated from barium-barite in settling material: A comparison with new production. Deep. Res. Part I Oceanogr. Res. Pap. 2000, 47, 583–601. [Google Scholar] [CrossRef]
- Martin, J.H.; Knauer, G.A.; Karl, D.M.; Broenkow, W.W. VERTEX: Carbon cycling in the northeast Pacific. Deep. Res. 1987, 34, 267–285. [Google Scholar] [CrossRef]
- Francois, R.; Honjo, S.; Krishfield, R.; Manganini, S. Factors controlling the flux of organic carbon to the bathypelagic zone of the ocean. Glob. Biogeochem. Cycles 2002, 16, 34-1–34-20. [Google Scholar] [CrossRef]
- Klaas, C.; Archer, D.E. Association of sinking organic matter with various types of mineral ballast in the deep sea: Implications for the rain ratio. Glob. Biogeochem. Cycles 2002, 16, 63-1–63-14. [Google Scholar] [CrossRef]
- Jeandel, C.; Tachikawa, K.; Bory, A.; Dehairs, F. Biogenic barium in suspended and trapped material as a tracer of export production in the tropical NE Atlantic (EUMELI sites). Mar. Chem. 2000, 71, 125–142. [Google Scholar] [CrossRef]
- Berger, W.H. Cenozoic sedimentation in the eastern tropical pacific. Bull. Geol. Soc. Am. 1973, 84, 1941–1954. [Google Scholar] [CrossRef]
- Moore, T.C.; Backman, J.; Raffi, I.; Nigrini, C.; Sanfilippo, A.; Pälike, H.; Lyle, M. Paleogene tropical Pacific: Clues to circulation, productivity, and plate motion. Paleoceanography 2004, 19. [Google Scholar] [CrossRef]
- Chavez, F.P.; Barber, R.T. An estimate of new production in the equatorial Pacific. Deep. Res. 1987, 34, 1229–1242. [Google Scholar] [CrossRef]
- Schenau, S.J.; Prins, M.A.; De Lange, G.J.; Monnin, C. Barium accumulation in the Arabian Sea: Controls on barite preservation in marine sediments. Geochim. Cosmochim. Acta 2001, 65, 1545–1556. [Google Scholar] [CrossRef]
- Lyle, M.; Baldauf, J. Biogenic sediment regimes in the Neogene equatorial Pacific, IODP Site U1338: Burial, production, and diatom community. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 433, 106–128. [Google Scholar] [CrossRef]
- Röhl, U.; Bralower, T.J.; Norris, R.D.; Wefer, G. New chronology for the late Paleocene thermal maximum and its environmental implications. Geology 2000, 28, 927. [Google Scholar] [CrossRef]
- Dickens, G.R.; O’Neil, J.R.; Rea, D.K.; Owen, R.M. Dissociation of oceanic methane hydrate as a cause of the carbon isotope excursion at the end of the Paleocene. Paleoceanography 1995, 10, 965–971. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Bridgestock, L.; Hsieh, Y.-T.; Porcelli, D.; Henderson, G.M. Increased export production during recovery from the Paleocene–Eocene thermal maximum constrained by sedimentary Ba isotopes. Earth Planet. Sci. Lett. 2019, 510, 53–63. [Google Scholar] [CrossRef]
- Bralower, T.J. Evidence of surface water oligotrophy during the Paleocene-Eocene thermal maximum: Nannofossil assemblage data from Ocean Drilling Program Site 690, Maud Rise, Weddell Sea. Paleoceanography 2002, 17, 13-1–13-12. [Google Scholar] [CrossRef]
- Kelly, D.C.; Zachos, J.C.; Bralower, T.J.; Schellenberg, S.A. Enhanced terrestrial weathering/runoff and surface ocean carbonate production during the recovery stages of the Paleocene-Eocene thermal maximum. Paleoceanography 2005, 20. [Google Scholar] [CrossRef]
- Gibbs, S.J.; Sheward, R.M.; Bown, P.R.; Poulton, A.J.; Alvarez, S.A. Warm plankton soup and red herrings: Calcareous nannoplankton cellular communities and the Palaeocene–Eocene Thermal Maximum. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376. [Google Scholar] [CrossRef]
- Gibbs, S.J.; Stoll, H.M.; Bown, P.R.; Bralower, T.J. Ocean acidification and surface water carbonate production across the Paleocene–Eocene thermal maximum. Earth Planet. Sci. Lett. 2010, 295, 583–592. [Google Scholar] [CrossRef]
- Naehr, T.H.; Stakes, D.S.; Moore, W.S. Mass wasting, ephemeral fluid flow, and barite deposition on the California continental margin. Geology 2000, 28, 315. [Google Scholar] [CrossRef]
- Paytan, A.; Averyt, K.; Faul, K.; Gray, E.; Thomas, E. Barite accumulation, ocean productivity, and Sr/Ba in barite across the Paleocene–Eocene Thermal Maximum. Geology 2007, 35, 1139. [Google Scholar] [CrossRef]

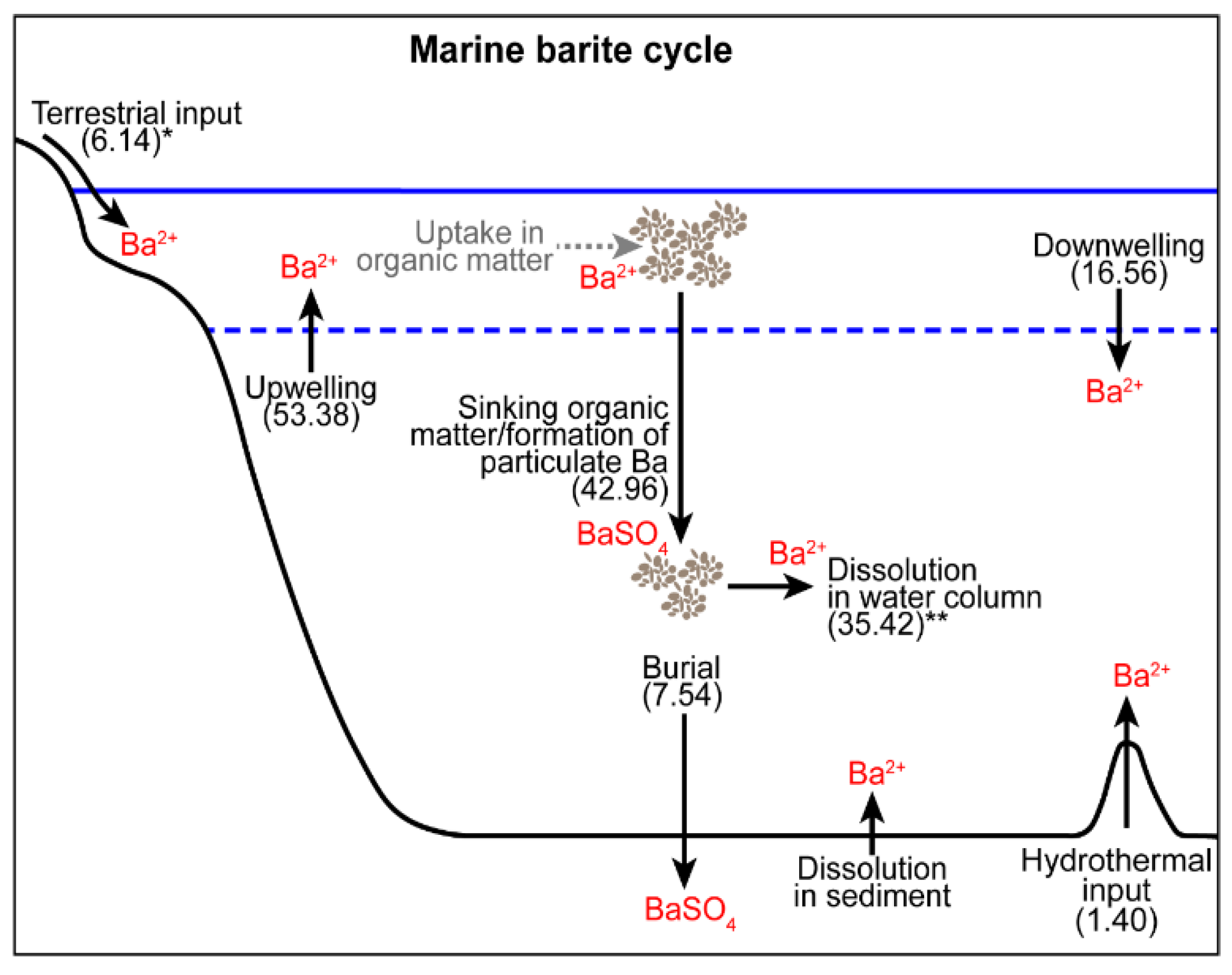




| Description | Equation |
|---|---|
| Changes in surface water Ba standing stock | |
| Changes in deep water Ba standing stock | |
| Ba concentration in surface water | |
| Ba concentration in deep water | |
| Downwelling flux | |
| Upwelling flux | |
| Particulate rain (Dickens et al. [27]) | |
| Particulate rain (this study) | OR |
| Dissolution flux | |
| Burial flux |
| Parameter | Description | Units | Standard Conditions |
|---|---|---|---|
| BaSh | Standing stock of Ba in surface water | Gmol | 3731 |
| BaDp | Standing stock of Ba in deep water | Gmol | 140,148 |
| FRiv | River/groundwater Ba flux | Gmol/yr | 14.75 |
| FUp | Upwelling Ba flux | Gmol/yr | 128.15 |
| FDw | Downwelling Ba flux | Gmol/yr | 39.80 |
| FPR | Particulate rain Ba flux | Gmol/yr | 103.10 |
| FDis | Benthic dissolution Ba flux | Gmol/yr | 85.00 |
| FHyd | Hydrothermal Ba flux | Gmol/yr | 3.35 |
| FCold | Cold seep Ba flux | Gmol/yr | 0.00 |
| FBur | Burial Ba flux | Gmol/yr | 18.10 |
| [Ba]Sh | Dissolved Ba in surface water | nmol/kgsw | 36 |
| [Ba]Dp | Dissolved Ba in deep water | nmol/kgsw | 116 |
| [Ba]Sat | Dissolved Ba at saturation | mol/kgsw | 330 |
| C | Exchange of surface and deep water | kgsw/yr | 1.105 × 1018 |
| D | Coefficient between dissolved Ba in shallow water and solid Ba in organic matter | kgsw/mol (carbon) | 3.8157 × 105 |
| b | (this study) Linear constant relating FPR to POrg | unitless | 0.0137 |
| e | (this study) Exponential constant relating FPR to POrg | unitless | 0.8554 |
| Porg | Sinking organic carbon flux | Gmol/yr | 7500 |
| f | Fraction of FPR buried (0–1) | unitless | 0.17556 |
| k | “Time factor” for barite preservation (0–1) | unitless | 0.4994 |
| MSh | Mass of surface water | kg | 1.04 × 1020 |
| MDp | Mass of deep-water | kg | 1.21 × 1021 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carter, S.C.; Paytan, A.; Griffith, E.M. Toward an Improved Understanding of the Marine Barium Cycle and the Application of Marine Barite as a Paleoproductivity Proxy. Minerals 2020, 10, 421. https://doi.org/10.3390/min10050421
Carter SC, Paytan A, Griffith EM. Toward an Improved Understanding of the Marine Barium Cycle and the Application of Marine Barite as a Paleoproductivity Proxy. Minerals. 2020; 10(5):421. https://doi.org/10.3390/min10050421
Chicago/Turabian StyleCarter, Samantha C., Adina Paytan, and Elizabeth M. Griffith. 2020. "Toward an Improved Understanding of the Marine Barium Cycle and the Application of Marine Barite as a Paleoproductivity Proxy" Minerals 10, no. 5: 421. https://doi.org/10.3390/min10050421
APA StyleCarter, S. C., Paytan, A., & Griffith, E. M. (2020). Toward an Improved Understanding of the Marine Barium Cycle and the Application of Marine Barite as a Paleoproductivity Proxy. Minerals, 10(5), 421. https://doi.org/10.3390/min10050421





