Chromium Members of the Pumpellyite Group: Shuiskite-(Cr), Ca2CrCr2[SiO4][Si2O6(OH)](OH)2O, a New Mineral, and Shuiskite-(Mg), a New Species Name for Shuiskite
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrence and General Appearance
2.2. Analytical Methods
3. Results
3.1. Physical Properties and Optical Data
3.2. Chemical Data
3.3. Infrared Spectroscopy
- The bands in the range of O–H-stretching vibrations (in the range from 2900 to 3520 cm–1) in the IR spectrum of shuiskite-(Cr) are shifted towards lower frequencies as compared to shuiskite-(Mg), which corresponds to stronger hydrogen bonds formed by the OH groups in the former mineral;
- The strong absorption maximum at 929 cm–1 observed in the IR spectrum of shuiskite-(Cr) is absent in the spectrum of shuiskite-(Mg). Consequently, this band can be hypothetically assigned to Si–O-stretching vibrations of XCr···O(9)–Si(3) or XCr···O(2)–Si(2);
- The low-frequency shifts in the bands of shuiskite-(Cr) relative to those of shuiskite-(Mg) in the range 360–490 cm–1 (mixed modes involving (Cr,Mg)–O stretching vibrations) are due to the fact that Cr3+ cation is heavier than Mg2+.
3.4. Powder X-Ray Diffraction Data
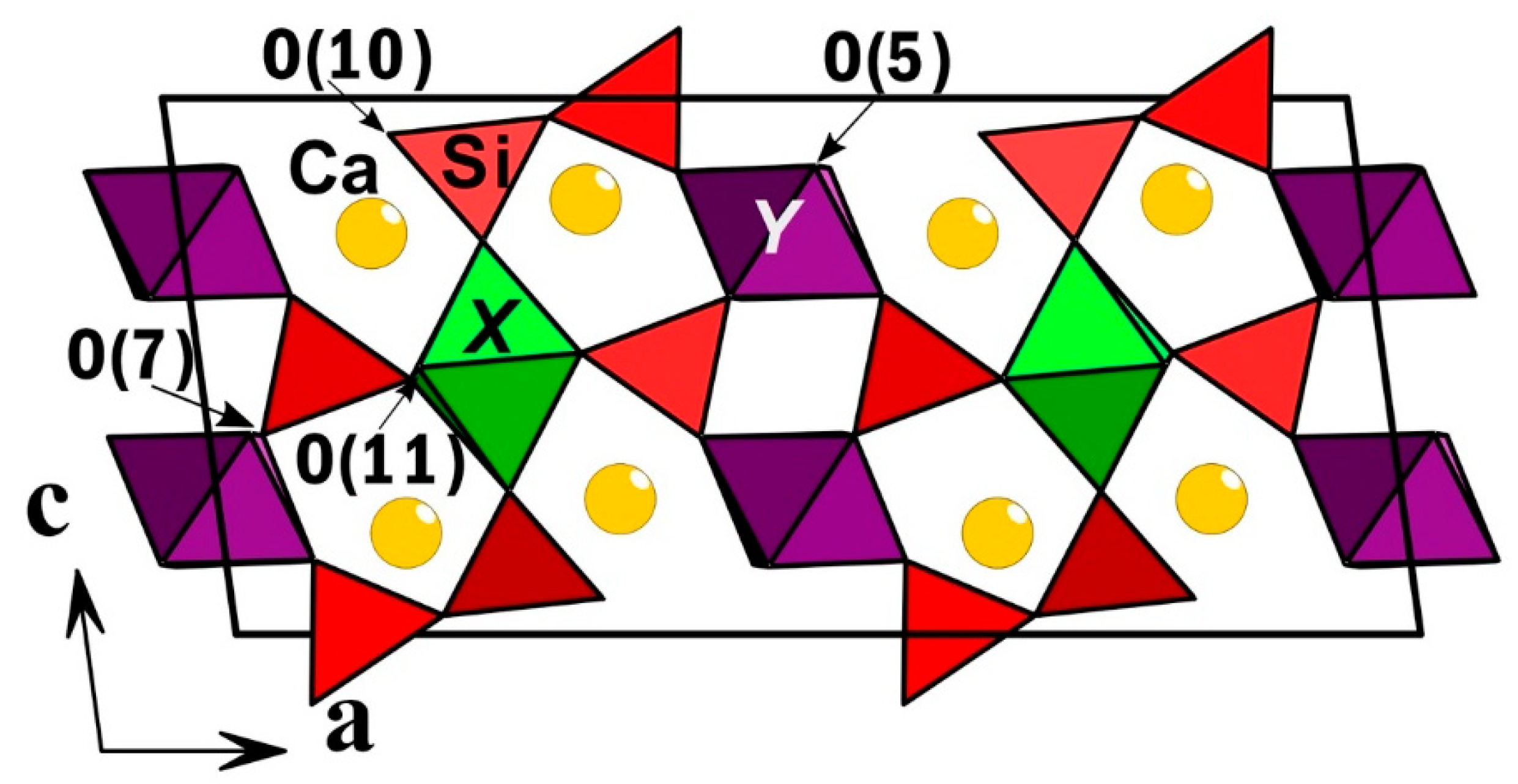
3.5. Single-Crystal X-Ray Diffraction Data and Description of The Crystal Structure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Passaglia, E.; Gottardi, G. Crystal chemistry and nomenclature of pumpellyites and julgoldites. Can. Mineral. 1973, 12, 219–223. [Google Scholar]
- Palache, C.; Vassar, H.E. Some minerals of the Keweenawan copper deposits: Pumpellyite a new mineral; sericite; saponite. Am. Mineral. 1925, 10, 412–418. [Google Scholar]
- Moore, P.B. Julgoldite, the Fe2+-Fe3+ dominant pumpellyite. Lithos 1971, 4, 93–99. [Google Scholar] [CrossRef]
- Togari, K.; Akasaka, M. Okhotskite, a new mineral, an Mn3+-dominant member of the pumpellyite group, from the Kokuriki mine, Hokkaido, Japan. Mineral. Mag. 1987, 51, 611–614. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Caprilli, E.; Marchensini, M. Poppiite, the V3+ end-member of the pumpellyite group: Description and crystal structure. Am. Mineral. 2006, 91, 584–588. [Google Scholar] [CrossRef]
- Ivanov, O.K.; Arkhangel’skaya, V.A.; Miroshnikova, L.O.; Shilova, T.A. Shuiskite, the chromium analogue of pumpellyite, from the Bisersk deposit, Urals. Zapiski VMO 1981, 110, 508–512. (In Russian) [Google Scholar]
- Lykova, I.S.; Varlamov, D.A.; Chukanov, N.V.; Pekov, I.V.; Zubkova, N.V. Crystal chemistry of shuiskite and chromian pumpellyite-(Mg). Eur. J. Mineral. 2018, 30, 1133–1139. [Google Scholar] [CrossRef]
- Ivanov, O.K.; Kainov, V.I.; Malinovskii, Y.A. A variety of shuiskite with a low water content from the Sarany chromite mine. Zapiski VMO 1985, 114, 49–55. (In Russian) [Google Scholar]
- Ivanov, O.K. Mineralogy of Saranovskoe chromite deposit (Middle Urals). Mineral. Alm. 2016, 21, 128. [Google Scholar]
- Coombs, D.S. The pumpellyite mineral series. Mineral. Mag. 1953, 30, 113–135. [Google Scholar] [CrossRef]
- Britvin, S.N.; Dolivo-Dobrovolsky, D.V.; Krzhizhanovskaya, M.G. Software for processing the X-ray powder diffraction data obtained from the curved image plate detector of Rigaku RAXIS Rapid II diffractometer. Zapiski RMO 2017, 146, 104–107. (In Russian) [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Chervonnyi, A.D. Infrared Spectroscopy of Minerals and Related Compounds; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–1109. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Crystallogr. 2015, B71, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Akasaka, M. The distribution of chromium in chromian pumpellyite from Sarani, Urals, Russia: A TOF neutron and X-ray Rietveld study. Can. Mineral. 2007, 45, 837–846. [Google Scholar] [CrossRef]
- Hamada, M.; Akasaka, M.; Seto, S.; Makino, K. Crystal chemistry of chromian pumpellyite from Osayama, Okayama Prefecture, Japan. Am. Mineral. 2010, 95, 1294–1304. [Google Scholar] [CrossRef]
- Nagashima, M.; Akasaka, M.; Ikeda, K.; Kyono, A.; Makino, K. X-ray single-crystal and optical spectroscopic study of chromian pumpellyite from Sarany, Urals, Russia. J. Mineral. Petrol. Sci. 2010, 105, 187–193. [Google Scholar] [CrossRef][Green Version]
- Yoshiasa, A.; Matsumoto, T. Crystal structure refinement and crystal chemistry of pumpellyite. Am. Mineral. 1985, 70, 1011–1019. [Google Scholar]
- Mevel, C.; Kienast, J.R. Chromian jadeite, phengite, pumpellyite, and lawsonite in a high-pressure metamorphosed gabbro from the French Alps. Mineral. Mag. 1980, 43, 979–984. [Google Scholar] [CrossRef]
- The Official IMA-CNMNC List of Mineral Names. Updated list of IMA-approved minerals (March 2020). 2020. Available online: http://cnmnc.main.jp (accessed on 18 April 2020).
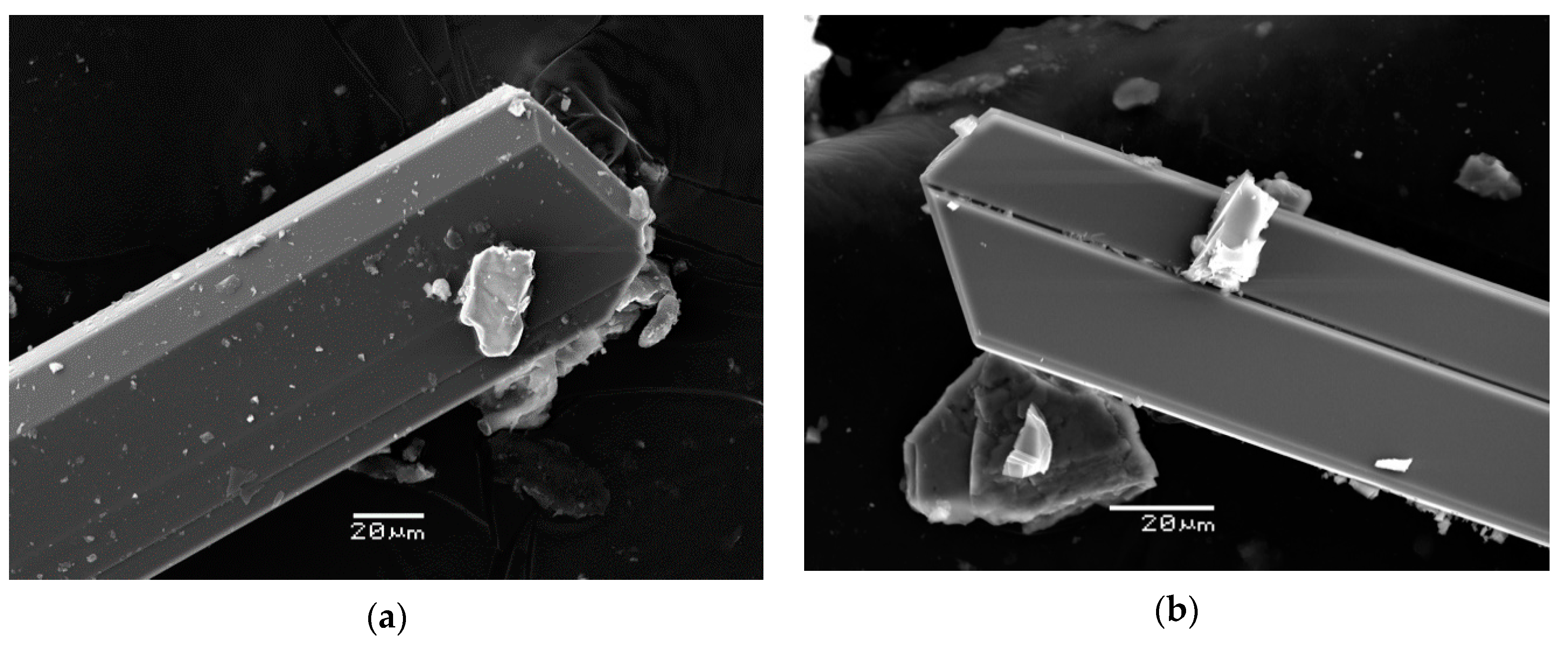

| Constituent | Mean | Range | Standard Deviation |
|---|---|---|---|
| CaO | 21.33 | 20.98–21.62 | 0.26 |
| MgO | 3.17 | 2.32–3.78 | 0.54 |
| Al2O3 | 5.41 | 4.78–5.93 | 0.46 |
| Cr2O3 | 28.50 | 27.61–29.89 | 0.99 |
| TiO2 | 0.18 | 0.00–0.68 | 0.29 |
| SiO2 | 33.86 | 33.44–34.24 | 0.33 |
| H2O | 5.82 1 | ||
| Total | 98.27 |
| Imeas | dmeas | Icalc1 | dcalc2 | hkl |
|---|---|---|---|---|
| 16 | 9.53 | 18 | 9.53 | 200 |
| 8 | 8.74 | 7 | 8.75 | 001 |
| 4 | 6.93 | 4 | 6.93 | 20–1 |
| 7 | 6.05 | 9 | 6.05 | 201 |
| 9 | 4.883 | 9 | 4.882 | 11–1 |
| 34 | 4.759 | 39 | 4.766 | 400 |
| 36 | 4.707 | 45 | 4.702 | 111 |
| 6 | 4.442 | 7 | 4.448 | 40–1 |
| 18 | 4.375 | 23 | 4.375 | 002 |
| 5 | 4.059 | 6 | 4.059 | 31–1 |
| 3 | 3.960 | 4 | 3.965 | 401 |
| 75 | 3.783 | 69 | 3.785 | 202 |
| 3 | 3.545 | 2 | 3.546 | 11–2 |
| 14 | 3.468 | 16 | 3.467 | 40–2 |
| 4 | 3.409 | 4 | 3.409 | 112 |
| 1 | 3.239 | 1 | 3.245 | 31–2 |
| 4 | 3.138 | 4 | 3.140 | 51–1 |
| 18 | 3.023 | 18 | 3.024 | 402 |
| 12 | 3.000 | 10 | 3.000 | 020 |
| 100 | 2.913 | 18, 100, 19 | 2.916, 2.914, 2.902 | 003, 511, 20–3 |
| 14 | 2.861 | 2, 18 | 2.864, 2.862 | 601, 220 |
| 12 | 2.839 | 15 | 2.838 | 021 |
| 52 | 2.755 | 61, 8, 5 | 2.756, 2.753, 2.747 | 60–2, 22–1, 51–2 |
| 10 | 2.688 | 10 | 2.688 | 221 |
| 31 | 2.643 | 41 | 2.643 | 11–3 |
| 48 | 2.539 | 70 | 2.539 | 420 |
| 39 | 2.470 | 6, 21, 30 | 2.487, 2.474, 2.468 | 42–1, 022, 71–1 |
| 8 | 2.442 | 13 | 2.441 | 22–2 |
| 4 | 2.384 | 4, 2 | 2.383, 2.383 | 80–1, 800 |
| 31 | 2.352 | 41 | 2.351 | 222 |
| 12 | 2.327 | 15 | 2.326 | 313 |
| 6 | 2.298 | 8 | 2.296 | 51–3 |
| 3 | 2.270 | 2 | 2.269 | 42–2 |
| 27 | 2.225 | 31 | 2.224 | 80–2 |
| 8 | 2.198 | 6 | 2.198 | 20–4 |
| 14 | 2.186 | 10, 8 | 2.187, 2.181 | 004, 620 |
| 4 | 2.164 | 4 | 2.165 | 62–1 |
| 23 | 2.13 | 31 | 2.130 | 422 |
| 12 | 2.099 | 13 | 2.099 | 40–4 |
| 8 | 2.073 | 4, 3, 2 | 2.072, 2.072, 2.071 | 11–4, 621, 204 |
| 2 | 2.049 | 2 | 2.047 | 513 |
| 4 | 2.030 | 1, 3 | 2.033, 2.029 | 31–4, 62–2 |
| 4 | 2.019 | 5 | 2.017 | 71–3 |
| 8 | 1.9309 | 4, 8 | 1.9336, 1.9286 | 131, 60–4 |
| 14 | 1.8842 | 5, 14 | 1.8859, 1.883 | 314, 622 |
| 20 | 1.8667 | 4, 25 | 1.866, 1.8659 | 82–1, 820 |
| 4 | 1.8499 | 4 | 1.8496 | 423 |
| 2 | 1.8320 | 3 | 1.8309 | 62–3 |
| 3 | 1.7693 | 3 | 1.7674 | 024 |
| 5 | 1.7528 | 6 | 1.7518 | 71–4 |
| 19 | 1.7194 | 16, 6 | 1.7200, 1.7194 | 42–4, 514 |
| 6 | 1.6990 | 6 | 1.6969 | 604 |
| 10 | 1.6783 | 4, 8 | 1.6793, 1.6765 | 31–5, 111–1 |
| 9 | 1.6551 | 13 | 1.6542 | 13–3 |
| 35 | 1.6017 | 5, 3, 56 | 1.6090, 1.6086, 1.6007 | 1020, 73–1, 424 |
| 16 | 1.5897 | 19 | 1.5887 | 1200 |
| 20 | 1.5705 | 27 | 1.5697 | 102–2 |
| 5 | 1.5524 | 5 | 1.551 | 1021 |
| 3 | 1.5232 | 3 | 1.5208 | 22–5 |
| 10 | 1.5124 | 11 | 1.5120 | 804 |
| 42 | 1.5013 | 29, 38 | 1.5009, 1.5000 | 82–4, 040 |
| Formula Derived from the Structure Refinement | Ca2(Cr0.52Mg0.48)(Cr1.40Al0.60)[SiO4][Si2O6(OH)](OH)2.48O0.52 |
|---|---|
| Crystal system, space group, Z | Monoclinic, C2/m, 4 |
| a (Å) | 19.2436(6) |
| b (Å) | 5.9999(2) |
| c (Å) | 8.8316(3) |
| β (°) | 97.833(3) |
| V (Å3) | 1010.17(6) |
| λ (MoKα) (Å), T (K) | 0.71073, 293 |
| Diffractometer | Xcalibur S CCD |
| θ range (°) | 2.94 – 34.79 |
| Crystal size (mm3) | 0.049 × 0.053 × 0.377 |
| Absorption coefficient µ (mm−1) | 3.618 |
| F000 | 1028 |
| h, k, l range | −30 ≤ h ≤ 29, −9 ≤ k ≤ 9, −13≤ l ≤ 14 |
| Reflections collected | 3579 |
| Unique reflections [I > 2σ(I)] | 2793 |
| Number of refined parameters | 127 |
| Weighting scheme | 1/[σ2(Fo2) + (0.0434P)2 + 5.7592], P = [(Fo)2 + 2(Fc)2]/3 |
| R1 | 0.0469 |
| wR2all(F2) | 0.1088 |
| GoF | 1.076 |
| Δρmax/Δρmin (e/Å3) | 1.342/−1.706 |
| Site | x | y | z | Ueq | s.o.f. |
|---|---|---|---|---|---|
| Ca(1) | 0.33945(4) | 1/2 | 0.25177(10) | 0.00834(17) | 1 |
| Ca(2) | 0.15486(5) | 1/2 | 0.18808(11) | 0.01106(18) | 1 |
| X | 1/4 | 1/4 | 1/2 | 0.0054(3) | Cr0.516(8)Mg0.484(9) |
| Y | 0.49532(3) | 0.24730(9) | 0.25394(6) | 0.00457(16) | Cr0.700(7)Al0.300(8) |
| Si(1) | 0.09159(6) | 0 | 0.05128(13) | 0.0050(2) | 1 |
| Si(2) | 0.24812(6) | 0 | 0.16523(13) | 0.0060(2) | 1 |
| Si(3) | 0.40195(6) | 0 | 0.46535(13) | 0.0048(2) | 1 |
| O(1) | 0.07386(11) | 0.2230(4) | 0.1373(2) | 0.0083(4) | 1 |
| O(2) | 0.24643(11) | 0.2293(4) | 0.2657(2) | 0.0080(4) | 1 |
| O(3) | 0.41468(11) | 0.2209(4) | 0.3671(3) | 0.0081(4) | 1 |
| O(4) | 0.44434(16) | 1/2 | 0.1303(3) | 0.0064(5) | 1 |
| O(5) = OH | 0.45671(16) | 0 | 0.1265(4) | 0.0086(5) | 1 |
| O(6) | 0.04507(16) | 1/2 | 0.3709(3) | 0.0074(5) | 1 |
| O(7) = OH | 0.03450(18) | 0 | 0.3737(4) | 0.0105(6) | 1 |
| O(8) | 0.17686(15) | 0 | 0.0349(4) | 0.0083(5) | 1 |
| O(9) | 0.17653(16) | 1/2 | 0.4751(4) | 0.0090(5) | 1 |
| O(10) = OH,O | 0.31327(17) | 0 | 0.0665(4) | 0.0123(6) | 1 |
| O(11) = OH,O | 0.18416(16) | 0 | 0.4971(3) | 0.0077(5) | 1 |
| Ca(1) | O(11) | 2.324(3) | X | O(11) | 1.961(2) | Si(1) | O(1) | 1.598(2) × 2 |
|---|---|---|---|---|---|---|---|---|
| O(3) | 2.353(2) × 2 | O(11) | 1.9613(19) | O(4) | 1.657(3) | |||
| O(4) | 2.412(3) | O(9) | 2.052(2) × 2 | O(8) | 1.667(3) | |||
| O(2) | 2.433(2) × 2 | O(2) | 2.065(2) × 2 | <Si(1)-O> | 1.630 | |||
| O(8) | 2.508(3) | <X-O> | 2.026 | |||||
| <Ca(1)-O> | 2.402 | Si(2) | O(10) | 1.622(3) | ||||
| Y | O(7) | 1.941(2) | O(2) | 1.640(2) × 2 | ||||
| Ca(2) | O(1) | 2.281(2) × 2 | O(5) | 1.947(2) | O(8) | 1.666(3) | ||
| O(10) | 2.410(3) | O(1) | 1.950(2) | <Si(2)-O> | 1.642 | |||
| O(2) | 2.426(2) × 2 | O(3) | 1.963(2) | |||||
| O(9) | 2.512(3) | O(6) | 1.979(2) | Si(3) | O(3) | 1.621(2) × 2 | ||
| O(6) | 2.828(3) | O(4) | 2.040(2) | O(6) | 1.652(3) | |||
| <Ca(2)-O> | 2.452 | <Y-O> | 1.970 | O(9) | 1.666(3) | |||
| <Si(3)-O> | 1.640 | |||||||
| Ca(1) | Ca(2) | X | Y | Si(1) | Si(2) | Si(3) | Σ | |
|---|---|---|---|---|---|---|---|---|
| O(1) | 0.36 × 2↓ | 0.51 | 1.07 × 2↓ | 1.94 | ||||
| O(2) | 0.24 × 2↓ | 0.25 × 2↓ | 0.35 × 2↓ | 0.96 × 2↓ | 1.80 | |||
| O(3) | 0.30 × 2↓ | 0.49 | 1.01 × 2↓ | 1.80 | ||||
| O(4) | 0.26 | 0.40 × 2→ | 0.91 | 1.97 | ||||
| O(5) | 0.51 × 2→ | 1.02 | ||||||
| O(6) | 0.08 | 0.47 × 2→ | 0.93 | 1.95 | ||||
| O(7) | 0.52 × 2→ | 1.04 | ||||||
| O(8) | 0.20 | 0.89 | 0.89 | 1.98 | ||||
| O(9) | 0.19 | 0.36 × 2↓ x2→ | 0.89 | 1.80 | ||||
| O(10) | 0.26 | 1.01 | 1.27 | |||||
| O(11) | 0.32 | 0.46 × 2↓ x2→ | 1.24 | |||||
| Σ | 1.86 | 1.75 | 2.34 | 2.90 | 3.94 | 3.82 | 3.84 |
| Mineral | Shuiskite-(Cr) | Shuiskite-(Mg) |
|---|---|---|
| Formula | Ca2CrCr2[SiO4][Si2O6(OH)](OH)2O | Ca2MgCr2[SiO4][Si2O6(OH)](OH)3 |
| Crystal system Space group | Monoclinic C2/m | Monoclinic C2/m |
| a, Å b, Å c, Å β, º V, Å3 Z | 19.2436(6) 5.9999(2) 8.8316(3) 97.833(3) 1010.17(6) 4 | 19.2156(7) 5.9779(2) 8.8268(3) 97.785(3) 1004.59(6) 4 |
| D, g/cm3 | 3.432 (calc) | 3.24 (meas) 3.238 (calc) |
| Strongest reflections of the powder X-ray diffraction pattern: d, Å (I) | 4.707 (36) 3.783 (75) 2.913 (100) 2.755 (52) 2.539 (48) 2.470 (39) 1.5013 (42) | 2.90 (90) 2.73 (70) 2.64 (50) 2.46 (50) 2.22 (40) 2.12 (40) 1.593 (100) 1.487 (80) |
| References | This work | [6,7] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lykova, I.; Varlamov, D.; Chukanov, N.; Pekov, I.; Belakovskiy, D.; Ivanov, O.; Zubkova, N.; Britvin, S. Chromium Members of the Pumpellyite Group: Shuiskite-(Cr), Ca2CrCr2[SiO4][Si2O6(OH)](OH)2O, a New Mineral, and Shuiskite-(Mg), a New Species Name for Shuiskite. Minerals 2020, 10, 390. https://doi.org/10.3390/min10050390
Lykova I, Varlamov D, Chukanov N, Pekov I, Belakovskiy D, Ivanov O, Zubkova N, Britvin S. Chromium Members of the Pumpellyite Group: Shuiskite-(Cr), Ca2CrCr2[SiO4][Si2O6(OH)](OH)2O, a New Mineral, and Shuiskite-(Mg), a New Species Name for Shuiskite. Minerals. 2020; 10(5):390. https://doi.org/10.3390/min10050390
Chicago/Turabian StyleLykova, Inna, Dmitry Varlamov, Nikita Chukanov, Igor Pekov, Dmitry Belakovskiy, Oleg Ivanov, Natalia Zubkova, and Sergey Britvin. 2020. "Chromium Members of the Pumpellyite Group: Shuiskite-(Cr), Ca2CrCr2[SiO4][Si2O6(OH)](OH)2O, a New Mineral, and Shuiskite-(Mg), a New Species Name for Shuiskite" Minerals 10, no. 5: 390. https://doi.org/10.3390/min10050390
APA StyleLykova, I., Varlamov, D., Chukanov, N., Pekov, I., Belakovskiy, D., Ivanov, O., Zubkova, N., & Britvin, S. (2020). Chromium Members of the Pumpellyite Group: Shuiskite-(Cr), Ca2CrCr2[SiO4][Si2O6(OH)](OH)2O, a New Mineral, and Shuiskite-(Mg), a New Species Name for Shuiskite. Minerals, 10(5), 390. https://doi.org/10.3390/min10050390







