Abstract
The surface charge distribution of clay particles governs the interparticle forces and their arrangement in clay-water systems. The plasticity properties are the consequences of the interaction at the microscopic scale, even if they are traditionally linked to the mechanical properties of fine-grained soils. In the paper, the plasticity modifications induced by the addition of lime were experimentally investigated for two different clays (namely kaolinite and bentonite) in order to gain microstructural insights of the mechanisms affecting their plastic behavior as a function of the lime content and curing time. Zeta potential and dynamic light scattering measurements, as well as thermogravimetric analyses, highlighted the mechanisms responsible for the plastic changes at a small scale. The increase of the interparticle attraction forces due to the addition of lime increased the liquid and plastic limits of kaolinite in the short term, without significant changes in the long term due to the low reactivity of the clay in terms of pozzolanic reactions. The addition of lime to bentonite resulted in a decrease of interparticle repulsion double layer interactions. Rearrangement of the clay particles determined a reduction of the liquid limit and an increase of the plastic limit of the treated clays in the very short term. Precipitation of the bonding compounds due to pozzolanic reactions increased both the liquid and plastic limits over the time.
1. Introduction
Plasticity limits, quantitatively defined by Atterberg in 1911 [1], determine the range of water content in which a clayey soil exhibits a plastic behavior. As evidenced by Haigh et al. (2013) [2], it is widely accepted that both liquid and plastic limits can be found by measuring soil strength. The liquid limit test procedure developed by Casagrande (1932b) [3], based on the relationship between the number of blows required to close a standard groove cut into the sample and its water content, identifies the moisture content at which the sample has an undrained shear strength of approximately 2.5 kPa. The fall cone test for liquid limit determination [4] can be interpreted as a measurement of soil strength, as shown by Houlsby (1982) [5]. In the rolling test, the water content at which a soil thread breaks apart at a diameter of 3 mm is assumed as the plastic limit of the soil. During the test, a suction increase owing to the evaporation of the pore fluid can prevent the tensile failure of the soil specimen subjected to null total stresses along the longitudinal and horizontal direction. At suctions greater than the air entry value, above which the soil starts to desaturate, the soil progressively shows a transition to brittle behavior [2], governed by the water menisci at particle contacts. Failure of the soil specimen occurs by crumbling of the thread, as a consequence of either air entry or cavitation of the pore water [2].
Microstructural insights in the different mechanisms governing the plastic limits of fine-grained soils have been proposed by several authors. The plasticity properties of clays as a function of the pH, ionic strength, and type of electrolytes have been widely investigated with reference to particle-particle interactions [6,7,8,9,10,11]. According to Warkentin (1961) [12], the liquid limit of clayey soil is controlled by interparticle forces and can be regarded as the distance between particles or structural units of particles at which interaction forces become sufficiently weak to allow relative displacements upon loading. Nagaraj and Jayadeva (1981) [13] suggested that the liquid limit of soils is directly linked to the surface charge of particles, mainly influenced by soil-water chemo-physical interactions.
The addition of lime to a clayey soil has a strong impact on the plasticity properties of treated soil as a result of chemical interactions, which take place after the treatment [14,15,16,17,18,19,20]. The addition of lime to a soil can induce an increase or decrease of its liquid limit depending on the mineralogy of clay particles and associated exchangeable cations, as reported by many studies [21,22,23,24,25]. Conversely, the plastic limit generally shows an increasing trend after lime treatment [23,24,25,26,27,28]. Despite several data on the modification of plastic limits of lime-treated soils, no extensive microstructural studies on the mechanisms linking the chemo-physical evolution of a clay-lime-water system and plasticity properties have been found in the literature.
In the present experimental study, modification of the plasticity properties of two clays (namely kaolinite and calcium bentonite) induced by the addition of lime were investigated as a function of the lime contents and curing time. The liquid and plastic limits of treated clay determined with standard tests were interpreted by taking in account the chemo-physical evolution of a clay-lime-water system over the curing time. At the microscopic scale, modifications of the surface charge properties of clay minerals and the following clay particle arrangements were investigated. The mechanisms linking the chemo-physical evolution of treated clays at the micro scale and macroscopic plasticity properties are herein discussed.
2. Materials and Methods
2.1. Materials
Speswhite kaolin was one of the two clays chosen for the present investigation. It was supplied by Imerys Minerals, Plymouth, UK. Its specific gravity is Gs = 2.6, the specific surface area is 14 m2/g, resulting from nitrogen adsorption (BET), and the pH value of the clay is 4.6. The X-ray diffraction pattern of kaolin is shown in Figure 1a. The mineralogical composition of the clay is mainly formed by kaolinite with a small amount of quartz and muscovite. The ethylene glycol treatment did not detect any swelling clay phases.
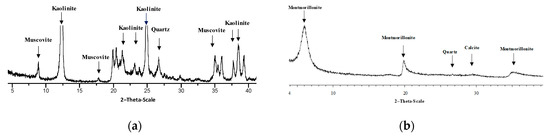
Figure 1.
X-ray diffraction patterns: (a) Spwt kaolin; (b) Bentonite.
Natural calcium bentonite, the second clay investigated, was supplied by SSB Ltd. (Santa Giusta, Italy). Its specific gravity is Gs = 2.54, the specific surface area is 99.38 m2/g as results from nitrogen adsorption (BET), and the pH value of the clay is 7.55. The X-ray diffraction pattern of bentonite is shown in Figure 1b. Its mineralogical composition is mainly formed by Ca montmorillonite, with small parts of quartz and calcite.
The chemical composition and Atterberg limits of the two clays are respectively reported in Table 1 and Table 2.

Table 1.
Chemical composition of clays.

Table 2.
Atterberg limits.
The quicklime added to the clays was composed of more than 90% of free calcium oxide, 1% Ca(OH)2, and 4% calcite.
The evolution of the pH measurements of the treated clays as function of the lime contents and curing time is shown in Figure 2. The presence of a high pH environment, namely a pH value of 12.4 (the pH of a saturated lime solution), is essential to guarantee the effectiveness of lime treatment. For kaolin, 3% CaO is the amount of lime required to promote the most effective arrangement of particles in the short term and to guarantee ongoing pozzolanic reactions in the long term. For bentonite, the amount of lime required to provide a stable and high pH for a long time is 5% CaO.
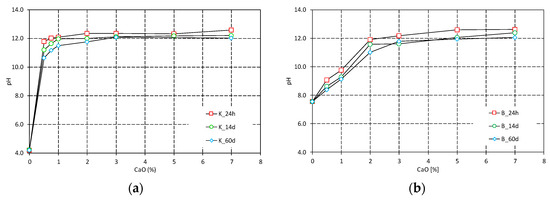
Figure 2.
pH measurements of treated clays: (a) Spwt kaolin; (b) Bentonite.
2.2. Methods
2.2.1. Zeta Potential and Dynamic Light Scattering (DLS) Measurements
The surface charges of clay particles were determined by means of Zeta potential measurements. Zeta potential was evaluated from the electrophoretic mobility using the Smoluchowski formula [29] using a Malvern Nano Zetasizer apparatus (Malvern Instruments, Malvern, UK) at a constant temperature T = 25 °C. The measurements were performed on clay particle suspensions prepared at different pH values ranging between 2 and 12.4. The increase of pH was ruled by the addition of HCl, KOH, or Ca(OH)2.
The evolution of the average size of the particle agglomerates by the DLS technique was also determined by a Malvern Nano Zetasizer apparatus. DLS measurements were made at T = 25 °C on clay suspension in de-ionized water and in Ca(OH)2 solutions at pH 12.4.
2.2.2. Thermogravimetric Analysis
Thermogravimetric analyses were performed with a Netzsch STA 449F3 Jupiter (Netzsch, Selb, Germany). In total, 50 to 100 mg of finely ground sample mass under an argon atmosphere was heated from ambient temperature to 1000 °C with a rate of 10 °C min−1. Tests were performed on samples prepared at increasing lime contents (1%, 3%, and 5% for kaolin and 3%, 5%, and 7% for bentonite). Specimens were sealed in hermetic plastic bag and were cured at room temperature (T = 20 °C) for increasing time intervals, namely 1, 3, 7, 14, 28, 60, 90, 210, and 270 days for kaolin and 1, 7, and 28 days for bentonite. Treated samples were freeze-dried before testing.
2.2.3. Atterberg Limits
Liquid and plastic limits were measured according to standard methods described by ASTM D4318-10 [30]. The water content at the limit liquid (wL) was determined as the 25-blow point on a line drawn through five measured points on a plot of the water content vs. logarithm of the number of blows. Plastic limit (wP) was determined as the water content at which a soil thread of 3 mm diameter started to crumble when hand rolled. The difference between the liquid limit and plastic limit was defined as the plasticity index (PI = wL − wP). Tests were performed on samples prepared at increasing lime contents (1%, 3%, and 5% for kaolin and 1.5%, 3%, and 5% for bentonite) and increasing curing times (1, 3, and 14 days for kaolin and 1, 7, and 14 days for bentonite).
3. Results
3.1. Kaolin
The changes of the liquid limit, plastic limit, and plasticity index of lime-treated kaolin as a function of the lime content and curing time are represented in Figure 3. The addition of lime increases the liquid limit of the raw clay for each lime content (Figure 3a). The increase is relevant as highlighted by comparing the liquid limits of the raw and treated samples. A further increase in the lime content results in a progressively lower increase of the liquid limit towards a threshold value. The role of the curing time is marginal, especially for higher lime contents. An increase of the plastic limit was detected for an increasing lime content as well (Figure 3b). Very slight differences were detected for the plastic limits at an increasing curing time, which is particularly evident for the plastic limit at 5% CaO. The resulting increasing trend of the plasticity index is shown in Figure 3c.
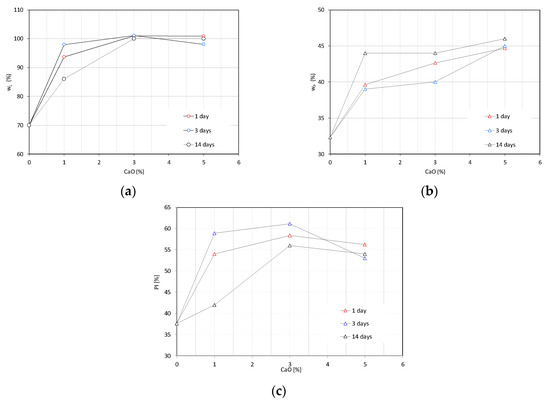
Figure 3.
Changes of the plasticity properties of lime-treated kaolin as a function of the lime content and curing time: (a) liquid limit (wL), (b) plastic limit (wP), and (c) plasticity index (PI).
An insight into the effects of the chemical environment induced by the addition of lime on the chemo-physical interactions of kaolinite particles was allowed by the microstructural analyses results. The evolution of the zeta potential of raw and lime-treated kaolin suspensions as a function of pH is reported in Figure 4a. Raw kaolin suspensions show an increase of the magnitude of the zeta potential with increasing pH as a result of deprotonation reactions on the lateral and octahedral basal faces of kaolinite particles. A progressive decrease of the zeta potential was observed at increasing pH after the addition of increasing calcium hydroxide concentrations. The reduction is sharp for pH higher than 9. Calcium undergoes hydrolysis at higher pH (pH > 10), leading to the formation of monovalent species [27,28]. As observed by Chemeda et al. (2015) [29], the detected behavior can be related to the formation and specific adsorption of the first hydrolysis product of calcium (i.e., CaOH+).
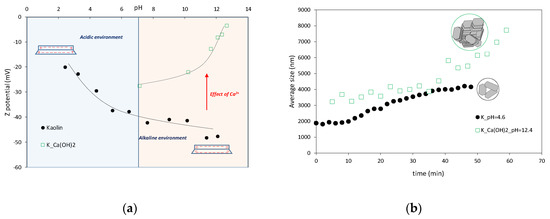
Figure 4.
(a) Effects of pH and Ca(OH)2 on the surface charge of kaolin suspensions (after [14]); (b) Evolution of the average floc size of kaolin suspensions (after [14]).
Particle aggregation over time was monitored by means of DLS measurements. An increase of the average size of particle aggregates was observed for the kaolin suspension in deionized water (pH = 4.6) (Figure 4b). The positively charged edge and negatively charged basal face of clay platelets promote an edge to face mode of particle aggregation [31,32,33,34]. The addition of calcium (Ca(OH)2) and the higher pH (pH = 12.4) lead to a further increase of the average size of kaolinite aggregates over time, highlighting a flocculated structure of the kaolinite suspension.
3.2. Bentonite
The evolution of the liquid limit, plastic limit, and plasticity index of bentonite as a function of the lime content is reported in Figure 5. A decrease of the liquid limit from 143% in de-ionized water to 115% with the addition of 5% CaO was observed for a short curing time (i.e., 1 day of curing). At an increasing curing time, the reduction of the liquid limit at an increasing lime content is less relevant; by inspecting the 14-day curve, for each lime content, the liquid limit remains unchanged if compared to the value pertaining to raw bentonite. The addition of an increasing amount of lime provides an increase of the plastic limit of the clay (Figure 5b). The increase is more relevant at higher lime contents and curing times. The reduction of the plasticity index, detected for each lime content and curing time, is reported in Figure 5c.
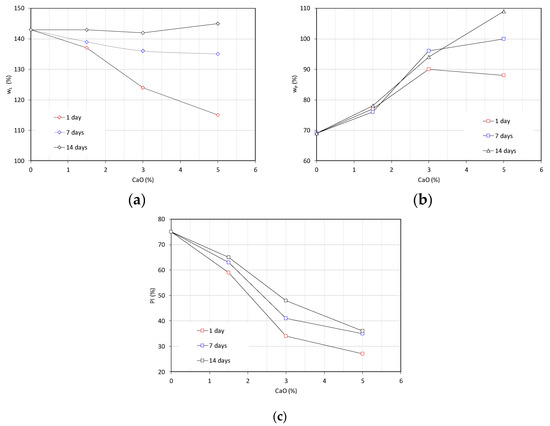
Figure 5.
Changes of the plasticity properties of lime-treated bentonite as a function of the lime content and curing time: (a) liquid limit (wL), (b) plastic limit (wP), and (c) plasticity index (PI).
An insight into the role of pore water chemistry on the chemo-physical interaction of montmorillonite particles was allowed by the microstructural investigations (Figure 6). The evolution of the zeta potential of raw and lime-treated bentonite suspensions as a function of pH is reported in Figure 6a. The magnitude of the zeta potential is slightly affected by the pore water chemistry pH. Conversely, a decrease of the zeta potential was observed at increasing pH after the addition of calcium hydroxide at increasing concentrations.
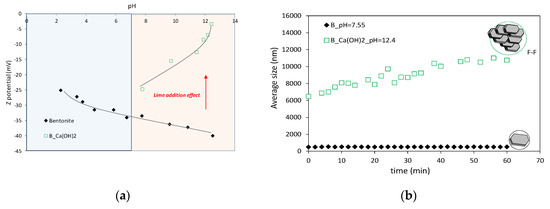
Figure 6.
(a) Effects of pH and Ca(OH)2 on the surface charge of bentonite suspensions; (b) Evolution of the average floc size of bentonite suspensions.
The evolution of the particle aggregation process induced by the pore water chemistry was monitored over time by DLS measurements. For the bentonite suspension in de-ionized water (pH = 7.55) (Figure 6b), the average size of aggregates remains unchanged over time, highlighting a dispersed arrangement of montmorillonite particles, whereas the presence of calcium ions in the highly alkaline environment (pH = 12.4) promotes an increase of the overall size of the particle aggregates, suggesting the formation of an aggregated structure.
4. Discussion
4.1. Kaolin
The surface charge of kaolin suspensions strongly depends on the pore water pH as evidenced by the zeta potential measurements. The entity of this parameter is directly related to the particle repulsions [35] and therefore depends on the pore water chemistry. In an acidic environment, and edge to face particle arrangement is due to the Coulombic attractions between positive and negative charges present on the edges and faces of kaolinite platelets, respectively. In an alkaline environment, the higher negative surface charge on kaolinite particles due to deprotonation reactions is responsible for the increase of the absolute value of the zeta potential. When lime is added to the clay water system, the pore water chemistry is changed due to an increase of the pH and a high concentration of calcium ions. Particle attraction is caused by the presence of CaOH+ between kaolinite platelets counteracting the repulsive effect imposed by the modification of pH, and promoting particle collisions and aggregations. As a consequence, repulsive forces reduce, and the magnitude of attractive forces increases, inducing a flocculated fabric of the treated soil as confirmed by the DLS measurements (Figure 4b). An increase of the undrained shear strength is then expected depending on the fabric changes induced by lime under the prevailing attractive interactions. The evidence at the microstructure level seem to be directly linked to the experimental results at the volume scale of the sample. In the presence of lime, the liquid limit value is higher than the liquid limit of untreated kaolin. Accordingly, with the widely accepted mechanical interpretation, the liquid limit corresponds approximately to the water content at which a soil has an undrained shear strength of about 2.5 kPa [3]. The particle arrangement induced by the pore water chemistry can explain the increase of the liquid limit [6], i.e., more water is needed to lower the undrained shear strength to the target value.
Aggregation of the kaolinite particles induced by lime affects the plastic limit of the clay. The flocculated arrangement of multiple face-to-face stacked kaolinite particles induces the occurrence of larger inter-aggregate pores, lowering the air entry value of the treated clay. As a consequence, the treated kaolin will start to desaturate at a lower suction value, increasing the plastic limit (i.e., the water content at which air entry or pore water cavitation occurs) as measured for each lime content and curing time.
The evolution of the plasticity properties of lime-treated kaolin is slightly affected by the increase of the curing time. Thermogravimetric analyses performed on 3% CaO-treated samples showed a low reactivity of kaolin to promote the development of pozzolanic reactions. In Figure 7, the mass losses of hydrated lime (temperature range between 390–460 °C) and cementitious compounds (temperature range between 110–350 °C) are represented as a function of the time. The precipitation of amorphous secondary phases appeared to form only after a long curing time (>28 days). The evolution of plasticity properties within the considered time intervals is then ruled mainly by the rearrangement of clay particles induced by the addition of lime and not by bonding induced by the ongoing pozzolanic reactions.
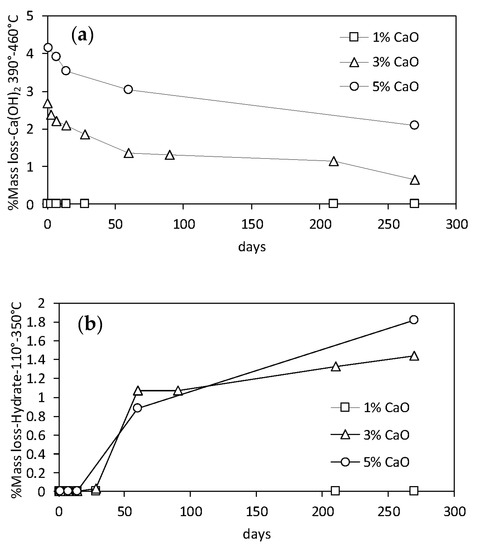
Figure 7.
Quantitative interpretation of the thermogravimetric analyses on treated kaolin at increasing lime contents: (a) portlandite consumption; (b) development of hydrated phases.
4.2. Bentonite
For bentonite suspensions, a slight pH-dependent surface charge behavior was detected from the zeta potential measurements. The surface charge of montmorillonite particles is mainly due to the permanent negative structural charge formed by isomorphic substitutions. As a result, the zeta potential of montmorillonite particles is less sensitive to pH than 1:1 clay minerals (i.e., kaolinite) [36,37]. In de-ionized water, the bentonite suspension (pH = 7.55) exhibits a dispersed arrangement of montmorillonite particles. Clay particles remain suspended over time, not evolving into aggregates of an increasing dimension, as shown by the DLS measurements (Figure 6b). Electrostatic repulsions arising from double-layer interactions of montmorillonite particles prevent aggregations, keeping particles in a fixed configuration with respect to each other and preventing free movement [12]. A change of the pore water chemistry induced by lime affects the dominant interparticle forces in the bentonite suspension. The addition of increasing concentrations of Ca(OH)2 leads to a relevant decrease of the absolute value of the zeta potential (Figure 6) as a consequence of the strong tendency of montmorillonite particles to adsorb divalent cations to neutralize the high negative particle charge [38]. A reduction of the double layer repulsions occurs, favoring particle association as shown by the DLS measurements (Figure 6b). The decrease of the repulsive energy among particles with the addition of increasing calcium ion concentrations can explain the reduction of the liquid limit of the treated bentonite. According to Warkentin (1961) [12], the liquid limit can be interpreted as the distance between particles at which the forces of interaction between clay particles become sufficiently weak to allow easy movement of particles among each other. As the range of repulsion after lime addition is reduced, particles become less constrained and therefore the liquid limit of treated samples decreases. At increasing curing times, the reverse trend of the liquid limit can be considered as being affected by ongoing pozzolanic reactions as shown by the thermogravimetric analyses results (Figure 8). The high reactivity of bentonite is evidenced by the complete consumption of the added lime as well as the fast precipitation of hydrated phases since the very short term. The bonding effect due to the precipitation of cementitious compounds (amorphous C–A–S–H) can explain the increase of the liquid limit over time. The increase is more relevant at high lime contents since higher amounts of hydrated phases formed. As a consequence, higher water contents are needed to lower the undrained shear strength at about 2.5 kPa [3]. In the short term (i.e., 1 day of curing time), the increase of the plastic limit of the treated clay is consistent with the particle association induced by cation exchange reactions. The formation of an aggregated structure, as evidenced by the DLS measurements, is consistent with the decrease of the air-entry value of the treated clays and in turn with the increase of the plastic limit. The relevant and rapid precipitation of hydrates contributes to stiffen the soil skeleton and the transition to a brittle failure behavior, as expected at a water content close to the plastic limit. This effect is amplified for higher lime contents, due to the higher amount of cementitious compounds precipitated in the system, and longer curing times.
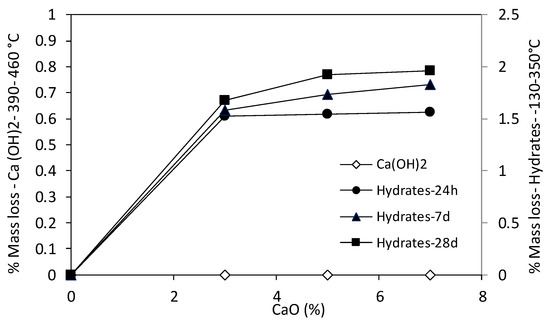
Figure 8.
Quantitative interpretation of thermogravimetric analyses of lime-treated bentonite at increasing lime contents.
5. Conclusions
In this paper, the plasticity modifications induced by the addition of lime were experimentally investigated for two different clays (namely kaolinite and bentonite) in order to gain microstructural insights into the mechanisms affecting their plastic behavior as a function of the lime content and curing time.
The following conclusion can be drawn:
- The results showed a high dependency of the plasticity properties on the clay particle arrangement induced by the pore water chemistry and Ca2+ concentration.
- For lime-treated kaolin, the increase of the liquid and plastic limits as a function of the lime content is consistent with the increase of interparticle attraction forces and the consequent aggregation of kaolinite particles.
- The evolution of the plasticity properties of lime-treated kaolin is slightly affected by the curing time due to the limited reactivity of the clay.
- Lime-treated bentonite samples showed in the short term a decrease of the liquid limit, as a result of modification of the double layer interactions (decrease of repulsion forces), and an increase of the plastic limit, consistent with the aggregated structure detected after the addition of lime.
- The high reactivity of bentonite induces a subsequent increase of the liquid limit over the curing time, and a further increase of the plastic limit, as consequence of the precipitation of hydrates responsible for the bonding between aggregates; the effect is particularly evident at higher lime contents.
Author Contributions
Methodology, G.R., E.V. and D.D.; experimental tests, E.V.; data interpretation, G.R., E.V. and D.D.; writing—original draft preparation, G.R. and E.V.; writing—review and editing, G.R. and E.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Atterberg, A. Lerornas forhallande till vatten, deras plasticitetsgranser och plasticitesgrader. Kungl. Lantbr. Akad. Hadlingar Tidskr. 2011, 2, 132–158. [Google Scholar]
- Haigh, S.K.; Vardanega, P.J.; Bolton, M.D. The plastic limit of clays. Géotechnique 2013, 63, 435–440. [Google Scholar] [CrossRef]
- Casagrande, A. Research on the Atterberg limits of soils. Public Roads 1932, 13, 121–136. [Google Scholar]
- BS 1377: 1990—Methods of Test for Soils for Civil Engineering Purposes; British Standards Institute: Milton Keynes, UK, 1990.
- Houlsby, G.T. Theoretical analysis of the fall cone test. Géotechnique 1982, 32, 111–118. [Google Scholar] [CrossRef]
- Sridharan, A. Engineering behaviour of clays: Influence of mineralogy. In Chemo-Mechanical Coupling of Clays: From Nano-Scale to Engineering Applications; Di Maio, H., Loret, L., Eds.; Swets & Zeitlinger: Lisse, The Netherlands, 2002. [Google Scholar]
- Sridharan, A.; Rao, G.V. Mechanisms controlling the liquid limit of clays. Proc. Int. Conf. Soil Mech. Istanb. 1975, 1, 75–84. [Google Scholar]
- Sridharan, A.; Rao, S.M.; Murthy, N.S. Liquid limit of montmorillonitic soils. Geotech. Test. J. 1986, 9, 156–159. [Google Scholar]
- Sridharan, A.; Rao, S.M.; Murthy, N.S. Liquid limit of kaolinitic soils. Geotechnique 1988, 38, 191–198. [Google Scholar] [CrossRef]
- Sridharan, A.; Prakash, K. Mechanisms controlling the undrained shear strength behaviour of clays. Can. Geotech. J. 1999, 36, 1030–1038. [Google Scholar] [CrossRef]
- Wang, Y.H.; Siu, W.K. Structure characteristics and mechanical properties of kaolinite soils. I. Surface charge and structural characterizations. Can. Geotech. J. 2006, 43, 587–600. [Google Scholar] [CrossRef]
- Warkentin, B.P. Interpretation of the upper plastic limit of clays. Nature 1961, 190, 287–288. [Google Scholar] [CrossRef]
- Nagaraj, T.S.; Jayadeva, M.S. Re-examination of one-point methods of liquid limit determination. Géotechnique 1981, 31, 413–425. [Google Scholar] [CrossRef]
- Vitale, E.; Deneele, D.; Russo, G.; Ouvrard, G. Short term effects on physical properties of lime treated kaolin. Appl. Clay Sci. 2016, 132, 223–231. [Google Scholar] [CrossRef]
- Guidobaldi, G.; Cambi, C.; Cecconi, M.; Comodi, P.; Deneele, D.; Paris, M.; Russo, G.; Vitale, E.; Zucchini, A. Chemo-mineralogical evolution and microstructural modifications of a lime treated pyroclastic soil. Eng. Geol. 2018, 245, 333–343. [Google Scholar] [CrossRef]
- Vitale, E.; Deneele, D.; Paris, M.; Russo, G. Multi-scale analysis and time evolution of pozzolanic activity of lime treated clays. Appl. Clay Sci. 2017, 141, 36–45. [Google Scholar] [CrossRef]
- Vitale, E.; Deneele, D.; Russo, G.; De Sarno, D.; Nicotera, M.V.; Papa, R.; Urciuoli, G. Chemo-mechanical behaviour of lightweight cemented soils. Acta Geotech. 2019, 15, 933–945. [Google Scholar] [CrossRef]
- Vitale, E.; Cecconi, M.; Croce, P.; Deneele, D.; Pane, V.; Russo, G.; Vecchietti, A. Influence of pore water chemistry on hydraulic conductivity of kaolinite suspensions. VI Italian Conference of Researchers in Geotechnical Engineering—Geotechnical Engineering in Multidisciplinary Research: From Microscale to Regional Scale, CNRIG2016. Procedia Eng. 2016, 158, 81–86. [Google Scholar] [CrossRef]
- Vitale, E.; Deneele, D.; Russo, G. Multiscale analysis on the behaviour of a lime treated bentonite. VI Italian Conference of Researchers in Geotechnical Engineering—Geotechnical Engineering in Multidisciplinary Research: From Microscale to Regional Scale, CNRIG2016. Procedia Eng. 2016, 158, 87–91. [Google Scholar] [CrossRef][Green Version]
- Russo, G. Chemo-physical evolution and microstructure features of lime treated soils. In Proceedings of the 3rd European Conference on Unsaturated Soils, E-UNSAT 2016, E3S Web of Conferences, Paris, France, 12 September 2016; Volume 9. [Google Scholar] [CrossRef]
- Clarey, K.E.; Cruchley, A.E. Laboratory experiments in the lime stabilisation of clays with hydrated lime. Géotechnique 1957, 7, 97–111. [Google Scholar] [CrossRef]
- Lund, O.L.; Ramsey, W.J. Experimental lime stabilisation in Nebraska. High Res. Board Bull. 1959, 231, 24–59. [Google Scholar]
- Bell, F.G. Lime stabilization of clay minerals and soils. Eng. Geol. 1996, 42, 223–237. [Google Scholar] [CrossRef]
- Boardman, D.I.; Glendinning, S.; Rogers, C.D. Development of stabilisation and solidification in lime-clay mixes. Géotechnique 2001, 50, 533–543. [Google Scholar] [CrossRef]
- Dash, S.K.; Hussain, M. Lime stabilisation of soils: Reappraisal. J. Mater. Civ. Eng. 2012, 24, 707–714. [Google Scholar] [CrossRef]
- Herrin, M.; Mitchell, H. Lime-Soil Mixtures; Bulletin No. 304, Highway Research Board Bulletin, 1961; Highway Research Board: Washington, DC, USA, 1961; pp. 99–138. [Google Scholar]
- Hilt, G.H.; Davidson, D.T. Lime Fixation on Clayey Soils; Bulletin No. 262, Highway Research Board Bulletin, 1960; Highway Research Board: Washington, DC, USA, 1960; pp. 20–32. [Google Scholar]
- Barker, J.E.; Rogers, C.D.F.; Boardman, D.I. Physiochemical changes in clay caused by ion migration from lime piles. J. Mater. Civ. Eng. 2006, 18, 182–189. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- ASTM D4318-10 Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils; ASTM International: West Conshohocken, PA, USA, 2010. [CrossRef]
- Mpofu, P.; Addai-Mensah, J.; Ralston, J. Influence of hydrolyzable metal ions on the interfacial chemistry, particle interactions, and dewatering behavior of kaolinite dispersions. J. Colloid Interface Sci. 2003, 261, 349–359. [Google Scholar] [CrossRef]
- Mpofu, P.; Addai-Mensah, J.; Ralston, J. Interfacial chemistry, particle interactions and improved dewatering behaviour of smectite clay dispersions. Int. J. Miner. Process. 2005, 75, 155–171. [Google Scholar] [CrossRef]
- Chemeda, Y.C.; Deneele, D.; Christidis, G.E.; Ouvrard, G. Influence of hydrated lime on the surface properties and interaction of kaolinite particles. Appl. Clay Sci. 2015, 107, 1–13. [Google Scholar] [CrossRef]
- Tombacz, E.; Szekeres, M. Surface charge heterogeneity of kaolinite in aqueous suspension in comparison with montmorillonite. Appl. Clay Sci. 2006, 34, 105–124. [Google Scholar] [CrossRef]
- Van Olphen, H. An Introduction to Clay Colloid Chemistry; Wiley Interscience: New York, NY, USA, 1977. [Google Scholar]
- Saka, E.E.; Guler, C. The effects of electrolyte concentration, ion species and pH on the zeta potential and electrokinetic charge density of montmotillonite. Clay Miner. 2006, 41, 853–861. [Google Scholar] [CrossRef]
- Vane, L.M.; Zang, G.M. Effect of aqueous phase properties on clay particle zeta potential and electro-osmotic permeability: Implications for electro-kinetic soil remediation processes. J. Hazard. Mater. 1997, 55, 1–22. [Google Scholar] [CrossRef]
- Ho, C.; Handy, R.L. Electrokinetic properties of lime treated bentonites. Clays Clay Miner. 1963, 12, 267–280. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).