Abstract
-AlOOH has been shown to be stable at the pressure–temperature conditions of the lower mantle. However, its stability remains uncertain at the conditions expected for the lowermost mantle where temperature is expected to rise quickly with increasing depth. Our laser-heated diamond-anvil cell experiments show that -AlOOH undergoes dehydration at ∼2000 K above 90 GPa. This dehydration temperature is lower than geotherm temperatures expected at the bottom ∼700 km of the mantle and suggests that -AlOOH in warm slabs would dehydrate in this region. Our experiments also show that the released HO from dehydration of -AlOOH can react with metallic iron, forming iron oxide, iron hydroxide, and possibly iron hydride. Our observations suggest that HO from the dehydration of subducting slabs, if it occurs, could alter the chemical composition of the surrounding mantle and core regions.
1. Introduction
Hydrogen is known to significantly affect the chemical and physical properties of silicates and iron alloys at high pressure–temperature - [,]. Therefore, knowing how it cycles through Earth’s interior and how it can be stored in different regions is key for understanding the global dynamics of the planet. Hydrous minerals in subducting slabs have the potential to carry HO from the surface of Earth to its core-mantle boundary (CMB). The stability of hydrous minerals at conditions relevant to the lower mantle has, hence, been extensively studied using laboratory experiments and theoretical calculations [,].
Dense hydrous magnesium silicates (DHMS) composing subducting slabs undergo different phase transitions with increasing depth. Under geotherm conditions, many will dehydrate into a mixture of HO and anhydrous minerals. Phase H, for example, the DHMS with the highest pressure stability so far, dehydrates into bridgmanite and HO above 50 GPa and 1300 K [,,]. However, recent high-pressure experiments have suggested that -AlOOH, which is isostructural with phase H and forms a solid solution with it [], could be stable in the lower mantle [,]. It has also been shown that Al could separate from bridgmanite and CaSiO perovskite and form -AlOOH in the presence of HO [,]. -AlOOH is, therefore, a strong candidate as a major carrier of HO in the deep mantle.
In addition, the potential stability of -AlOOH in the lowermost mantle makes it an interesting phase to study together with metallic iron at the P-T conditions of the CMB to understand its possible interactions with the outer core. Terasaki et al. [] studied the reaction between AlOOH and FeNi alloy at high P-T. They reported the appearance of metal hydride at 500–700 K below the dehydration temperature of -AlOOH []. Because untransformed metastable diaspore starting material persists at high temperature in their experiments, it is unclear if this low-temperature appearance of metal hydride could also occur with the dehydration of the more stable form of AlOOH, i.e., phase. Also, given the fact that the dehydration product of -AlOOH (i.e., AlO) undergoes a phase transition around 100 GPa from corundum (hereafter cor-AlO) to the RhO-II type structure (hereafter rs-AlO) [], the phase behavior of -AlOOH could be complicated in the lower mantle.
In this paper, we investigate the dehydration of -AlOOH at pressures and temperatures relevant to the lower mantle in the laser-heated diamond anvil cell (LHDAC). We also explore the potential reaction between the released HO from the dehydration of -AlOOH and metallic iron. We then discuss the implications of our results for the Earth’s lower mantle and the core-mantle boundary (CMB).
2. Experimental Methods
For starting materials, we synthesized two samples of -AlOOH using a 1100 ton multi-anvil press apparatus at Arizona State University (ASU) following the experimental procedure of Suzuki et al. []. One sample (BB1409JT) was synthesized from natural diaspore, and the other (BB1412JT) from synthetic reagent grade Al(OH) from Sigma-Aldrich. Both were placed in platinum capsules and pressurized to 20 GPa using 10/5 assemblies []. We then equilibrated at 1273 K for 1 h, after which, the sample was quenched to room temperature and slowly decompressed to room pressure for recovery. Raman spectroscopy showed purity of the samples [], and electron probe micro analysis (EPMA) confirmed the composition []. Both samples exhibit fluorescence signal at 680–700 nm in laser spectroscopy and the signal is likely from a small amount of Cr detected in our EPMA analysis (∼0.6–0.7 wt.%). X-ray diffraction shown in Figure 1a, also confirms the purity (i.e., single phase besides Ar and Au also used in the experiment), as well as structure of the sample. In some runs, we also used natural diaspore as a starting material (Table 1).
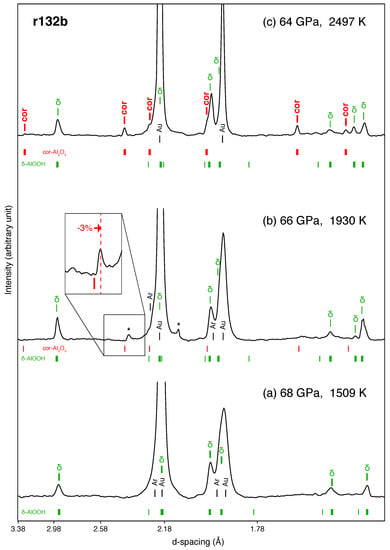
Figure 1.
Dehydration of -AlOOH detected in XRD patterns for run #132b. (a) At 1509 K, only precursor materials (i.e., -AlOOH, Ar and Au) are present; (b) At 1930 K, two unknown peaks appear, one of which could belong to cor-AlO. However, as the inset shows, the unit-cell volume of cor-AlO would have to be 3% smaller than that expected at the P-T conditions. (c) At 2497 K, where dehydration of -AlOOH to cor-AlO occurs, peaks belonging to the later can be clearly seen. The stars (*) denote unidentified lines appearing during heating. The calculated peak positions for the phases are shown as vertical ticks with different colors. The thick ticks refer to identified peaks, while thin ticks are calculated peak positions for which identification was less robust (likely due to their intrinsically low diffraction intensities). X-ray energy was 30 keV.

Table 1.
Experimental runs conducted in this study. SM: starting material, P: pressure, T: temperature, Medium: pressure transmitting medium, and Coupler: laser coupler for heating. Uncertainties on P and average uncertainties on T ranges are given in parenthesis. Runs in which we observed unknown peaks are denoted by stars (*).
We compressed the starting materials using diamond anvils with culet sizes ranging between 400 and 150 m mounted in symmetric diamond anvil cells. For laser coupling, we mixed the starting -AlOOH with 25 wt.% Fe or 10 wt.% Au. These metal powders are both high purity (99.98%) grade metal-basis with grain size below 3 m and were purchased from Alfa Aesar. Iron metal was dried for 24 h before mixing with the starting material. We then pressed the mixture into a foil, which was loaded in the drilled chamber of a pre-indented rhenium gasket. To reduce deviatoric stress in the sample chamber and ensure thermal insulation of the sample from the diamond anvils during laser heating, the foil was sandwiched between two foils of NaCl (or KCl for P < 35 GPa) or in an Ar medium either cryogenically or gas loaded at ASU. For Ar loading, the sample foil was equally spaced from both sides of the anvils by propping the foil with pure AlOOH (i.e., without coupler) grains less than a few microns in size.
Diffraction patterns were recorded at in-situ high pressure–temperature - in the LHDAC at the 13IDD beamline in the GSECARS sector of Advanced Photon Source [] using an X-ray monochromatic beam of 30 keV energy. The 3 × 4 X-ray beam was co-axially aligned with double-sided, near-infrared laser-heating, providing a 20–25 m heating spot. We first compressed the sample to target pressures at 300 K. Pressures were calculated from measured unit-cell volumes and the equations of state of Au [], NaCl [], and KCl []. We laser heated the sample while collecting diffraction patterns using a Pilatus detector to monitor the appearance of dehydration product (AlO), or other reaction products. We progressively increased temperature past the conditions for AlO appearance or until laser coupling changed, upon which we quenched the sample. Temperatures were calculated by fitting a Planck equation to the thermal radiation spectra from both sides of the DAC. We laser heated different spots in the same sample to examine the reproducibility (or refine our estimate) of the temperatures of the -AlOOH dehydration. Two types of laser coupling changes occurred at the dehydration temperature: (1) fading coupling likely driven by the migration of the metallic coupler outwards from the heated area, thus preventing further temperature increase; or (2) sudden coupling enhancement likely because of optical property changes in the sample by appearance of new phases through dehydration or chemical reaction. For the latter case, temperature measurement often became unreliable after dehydration. We provide information on the experimental runs in Table 1.
We integrated 2D diffraction images to 1D diffraction patterns in the Dioptas software package []. We used a LaB standard to calibrate and then correct distortions and the detector distance (≈200 mm) during the integration to 1D patterns. Peak identification, unit-cell fitting and peak fitting were done using the PeakPo software package []. Further refinements of the unit-cell parameters for some of the synthesized phases were conducted using the UnitCell software package [].
3. Dehydration of -AlOOH at High -
We performed X-ray diffraction at in-situ high pressure and high temperature following the method described in the previous section. Pressures ranged between 28 and 110 GPa, and temperatures ranged between 1010 and 3858 K, thus covering P-T conditions for most of the lower mantle. In most of our heating runs, we observed diffraction lines from AlO polymorphs at sufficiently high temperatures, providing evidence for dehydration.
Figure 1 shows diffraction patterns for run #132B at in-situ high temperature during heating. In this run, the sample was loaded with Au in an Ar medium and initially compressed to 68 GPa. We started heating the sample at low temperatures (∼1100 K), where peaks of -AlOOH started sharpening (Figure 1a). As we increased temperature, from 1930 K we observed the appearance of two additional peaks that we could not index with any known phases (see Figure 1b and Appendix A). While one of these two peaks (with a Å) is close to the calculated position of corundum, for it to fit would require a unit-cell volume smaller (see inset in Figure 1b). The 2D diffraction images show that this peak results from a couple of spots only (Figure A1B), suggesting strong granularity and preferred orientation of this phase during its formation, which could explain the anomalous volume if the phase was cor-AlO. In addition, no other peaks belonging to cor-AlO could be identified in the pattern. One possibility would be that such a decrease in volume would shift these other peaks to regions where much stronger -AlOOH and Ar peaks exist (Figure 1b), thus preventing the detection of a potential cor-AlO phase with anomalous volume. Both unidentified peaks persist up to 2300 K and suddenly disappear at 2497 ± 150 K, where several peaks appear in the diffraction pattern that can be matched well with cor-AlO (Figure 1c). We could not observe peaks for HO, because of its weak X-ray scattering cross section. Therefore, because we estimate our dehydration temperatures of -AlOOH into cor-AlO and HO on the sole criterion of the observation of AlO, these temperatures likely represent upper bound values.
Four more runs at pressures between 28 and 45 GPa using the same approach produced cor-AlO (dehydration product) peaks at temperatures around 1850 K. For the runs at 60 and 86 GPa, however, we did not observe cor-AlO peaks up to the maximum temperature attained, suggesting that dehydration occurs above 1900 and 2497 K, respectively. Because of larger thermal gradients due to the absence of medium (i.e., no NaCl nor Ar) in the run at 86 GPa, the degree of dehydration could also have been less and therefore difficult to detect. Other unidentified peaks were sometimes also observed in these runs but no trend in d-spacing as a function of pressure, temperature, or pre-loaded material appears (see Appendix A for more details).
Above 100 GPa, we carried out a total of four experimental runs. In run #131 starting at 105 GPa, we progressively increased the sample temperature through a gradual increase of laser power (Figure 2a). At 1959 K and 103 GPa we detected the main diffraction lines of rs-AlO (indicated by thicker ticks in Figure 2b), the high-pressure polymorph of cor-AlO reported at similar pressure conditions [,,], which marks the onset of -AlOOH dehydration. Upon further heating, we identify more diffraction lines belonging to rs-AlO (Figure 2c). At 1994 K and 103 GPa, numerous other peaks appeared in the pattern (Figure 2c). Such appearance likely results from reactions between metallic iron (mixed with the starting material in some runs) and HO from the dehydration of -AlOOH, as we discuss in the next section. Three other runs carried at 110 GPa used diaspore as a starting material, unlike run #131, which used synthesized -AlOOH. This set of experiments aims at allowing a direct comparison with Duan et al. [] (who only used diaspore as a starting material) where the discrepancy in dehydration temperature above 100 GPa is significant. Unlike runs using synthesized -AlOOH as a starting material, we observed very spotty diffraction lines from -AlOOH. The strong preferred orientation of -AlOOH synthesized in this way made the detection of dehydration product rs-AlO more difficult. However, although the appearance of rs-AlO in these runs was harder to spot, dehydration temperatures were similar, albeit slightly higher than in run #131.
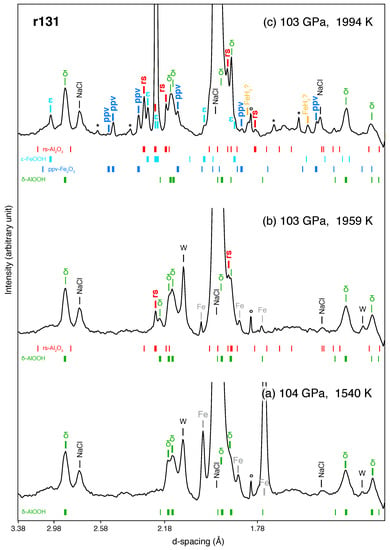
Figure 2.
Dehydration of -AlOOH detected in XRD patterns for run #131. (a) At 1540 K, precursor materials (i.e., -AlOOH, Ar and Au) as well as W (see Text for details) are present; (b) at 1959 K, new peaks appear, which can be indexed as rs-AlO; (c) at 1994 K, metallic iron contained in the starting material is found to react with released HO and produce -FeOOH and ppv-FeO. Some peaks might also be attributed to FeH. The circles (∘) denote unidentified lines present before heating. The stars (*) denote unidentified lines appearing during heating. The calculated peak positions for the phases are shown as vertical ticks with different colors. The thick ticks refer to identified peaks, while the thin ticks are calculated peak positions for which identification was less robust. X-ray energy was 30 keV.
Our P-T conditions for the dehydration of -AlOOH into AlO are shown in Figure 3 along with the data points reported by Duan et al. []. We observed the appearance of AlO (a product of the dehydration of -AlOOH) at two different pressure ranges: 28–68 GPa (cor-AlO) and 100–110 GPa (rs-AlO). As discussed above, because of the lack of thermal insulation, the run at 86 GPa is less reliable and a dehydration temperature could not be estimated. Also, in the run at 68 GPa, the few unidentified peaks observed over a broad temperature range leave some ambiguity in determining the dehydration temperature between 69 and 86 GPa. For example, if the lines are from cor-AlO or a previously unknown Al-oxide phase, the dehydration temperature should be 1929 K. If the new lines are from a previously unknown hydrous phase, the dehydration temperature can be as high as 2497 K. Granularity and/or preferred orientation of the dehydration product, AlO, could introduce uncertainties. Lastly, in the runs between 68 and 86 GPa, our starting material was not mixed with metallic iron, and we could not use the appearance of iron-bearing phases as another criterion for dehydration, as we describe in the next section.
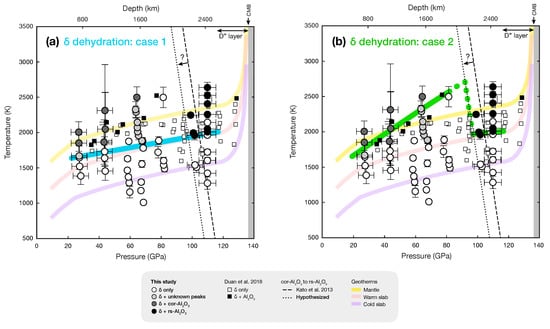
Figure 3.
Pressure and temperature conditions of -AlOOH dehydration from XRD experiments. The solid circles are our data points (white: -AlOOH only, dark gray: cor-AlO in -AlOOH, black: rs-AlO in -AlOOH with Fe hydroxides, light gray: appearance of unknown peaks near dehydration). Our proposed dehydration lines are shown by the colored lines for (a) case 1 and (b) case 2 (see the text for detail). Above 100 GPa, a single dehydration line is proposed. The phase boundary between cor-AlO and rs-AlO reported by Kato et al. [] is shown as a thin black dashed line. The dotted line shows hypothesized boundary for the phase transition to match with our interpretation. The open and the solid black squares are observations of -AlOOH and -AlOOH + AlO, respectively, by Duan et al. []. The cold and warm slab temperature profiles, as well as the mantle geotherm [], are also shown by different thick colored curves.
Considering these factors, we provide two alternate interpretations for the dehydration of -AlOOH in the mid-lower mantle (i.e., at depths between 1000 and 2100 km). In the first case, we invoke a single trend to fit the P-T conditions of the dehydration of -AlOOH between 28 and 110 GPa (blue line in Figure 3a). In this case, we hypothesize that the unidentified peaks at 68 GPa above 1929 K (light gray circles in Figure 3) arise from a previous-unknown aluminum oxide phase, which could be a sign of the destabilization of -AlOOH at the onset of dehydration.
For our second hypothesized dehydration curve, we assign the dehydration of -AlOOH only by the appearance of known AlO polymorphs (dark gray and black circles, and green line in Figure 3b). The inferred steep increase of the dehydration temperature between 60 and 80 GPa and our direct observation of rs-AlO at 1959 K and 102 GPa suggest a potentially large decrease in the dehydration temperature at 80–100 GPa. However, the lack of data points at the key pressure range in our dataset prevents us from estimating the temperature decrease reliably. It is possible that the dehydration curve is highly non-linear and the slope could strongly decrease with pressure. In this case, the temperature decrease observed at 80–100 GPa could be gradual. This pressure range coincides with where AlO, the dehydration product of -AlOOH, transitions from corundum to RhO-II-type structure. A similar case with a large decrease in dehydration temperature upon phase transition of the dehydration product was reported by Daniel et al. []. In their study, the dehydration of lawsonite in the MORB + HO system was observed to change depending on the stable SiO polymorph at given conditions. Therefore, a similar change in crystal structure of AlO could result in a change in energetics between the reactant and the products, which could also explain the decrease in temperature of -AlOOH dehydration across the cor–rs phase boundary.
Our dehydration temperature is in good agreement with those of Duan et al. [] below 50 GPa and within K. It is difficult to access agreement at 60–70 GPa because of the uncertainty around the estimated temperature of dehydration in our experiment. At ∼80 GPa, while there are large temperature gaps between data points in both datasets, these are in general agreement. Duan et al. [] did not report the observation of dehydration product AlO between 80 and 120 GPa. Instead, they reported only -AlOOH to be stable at the temperatures where we, on the other hand, observed the formation of AlO (Figure 3). In some of our experiments using diaspore as a starting material, the preferred orientation of rs-AlO was much more severe than in experiments starting with -AlOOH. Therefore, it was more challenging to accurately determine the dehydration temperature from the sole appearance of rs-AlO. However, the diffraction peaks from the HO + metallic iron reaction products, such as FeOOH and FeO, allowed us to confirm our dehydration temperatures. Duan et al. [] used diaspore as a starting material without metallic iron. Despite these differences, both datasets agree that -AlOOH would remain stable in a cold slab and would therefore retain HO fluid down to at least 2000 km (Figure 3). However, for warmer slabs, our data suggest that subducted -AlOOH would undergo dehydration at ∼2200 km depth and release HO in the lowermost mantle.
4. Reaction between Iron Metal and HO Released from -AlOOH Dehydration
In some runs, we mixed the starting material together with metallic iron (see Table 1) to study the reaction of HO (from -AlOOH dehydration) delivered to the mantle with either local metallic iron from the charge disproportionation of Fe in bridgmanite, or iron at the core-mantle boundary (the outer core side). In the runs performed in the 28–60 GPa range, no new phases were observed after dehydration occurred (i.e., cor-AlO had formed). In the runs performed above 100 GPa, however, the diffraction patterns became increasingly complicated once dehydration occurred (Figure 2 and Figure A2). New peaks appeared after the formation of rs-AlO and persisted during the rest of the heating cycle, even after temperature was quenched. We found that these peaks mostly result from small but intense diffraction dots in 2D images (Figure A2), suggesting that the phases giving rise to these peaks consist of a few large grains of single crystals. This, and the fact that the appearance of new lines is concomitant with the decrease in intensity of metallic iron, is consistent with the fact that the new phases might have been grown in the presence of HO dehydrated from -AlOOH. While the spottiness and high number of diffraction lines in 2D images makes phase identification challenging, some of the peaks can robustly be assigned to -FeOOH and ppv-FeO (see Figure 2 and Figure A2).
-FeOOH was observed in all runs above 100 GPa (Table 1; Figure 2 and Figure A2). Our measured volumes are in good agreement with data reported by Gleason et al. [] and close to that of Fe end-member (Figure 4). Previous studies of metallic iron in a HO medium [,] reported the formation of FeOOH in the pyrite structure (the high-pressure polymorph of -FeOOH) above ∼78 GPa and 1400 K. Therefore, we interpret -FeOOH as a reaction product between metallic iron (pre-loaded in the sample) and HO released from the dehydration of -AlOOH. We also note that the pressure conditions where we observed -FeOOH (between 100 and 110 GPa) are higher than previous reports for pure Fe end-member (below 80 GPa) []. We therefore hypothesize that the occurrence of -FeOOH above 100 GPa in our experiments might be caused by the presence of Al, which could stabilize the structure at higher pressures than its Al-free counterpart []. Although our measured unit-cell volumes of the phase is close to that of reported Al-free -FeOOH (Figure 4), we cannot rule out the possibility of a small amount of Al in the phase. Chemical analysis of the run products could have provided additional information. However, samples from our experiments conducted over 100 GPa could not be recovered because of significant fracturing upon decompression.
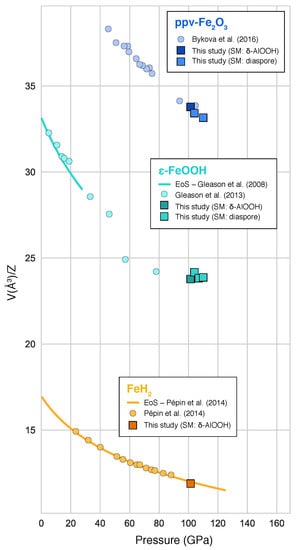
Figure 4.
Measured unit-cell volumes normalized to Z (number of chemical formula in a unit cell) of phases formed from reaction of HO (released from dehydration of -AlOOH) and metallic iron, along with data from the literature [,,]. SM stands for Starting Material (See Table 1).
Together with -FeOOH, peaks belonging to another phase appear in three out of four runs performed above 100 GPa (Table 1). A doublet between Å and Å (Figure 2) can be assigned well to the characteristic doublet of ppv-FeO. These peaks are well resolved in our patterns and the angular range does not have any other lines from other phases (Figure 2 and Figure A2). Other peaks are less well resolved but can also be explained by ppv-FeO. In addition, our measured volumes for temperature-quenched ppv-FeO at in-situ high pressure matches that of Bykova et al. [] at similar pressures (Figure 4). Ppv-FeO has been reported as a dehydration product of FeOOH in the FeO–HO system at 95 GPa above 2400 K []. The fact that we observed both ppv-FeO and -FeOOH may mean that our temperature conditions have been very close to those of the dehydration of FeOOH at this pressure.
Finally, two new peaks appeared after the dehydration of -AlOOH and were exclusively observed in run #131 (Table 1; Figure 2 and Figure A2). These peaks match the structure of iron-polyhydride FeH [], albeit with a slightly smaller unit-cell volume (by 1.6%) than that predicted at this pressure (Figure 4). Previous experiments performed on iron metal in an HO medium reported the formation of iron hydride [,,], which is consistent with our observation after the dehydration of -AlOOH. A difference is that, if these two peaks are indeed from FeH, we found it in a more hydrogenated form than previous studies.
5. Implications
Previous high-pressure experiments have shown that when Al-bearing bridgmanite reacts with HO, it can reduce the amount of Al in bridgmanite and form a separate AlOOH–MgSiO(OH) phase [], also called phase H. These experiments also found that the presence of Al in the structure of phase H increases its thermal stability. Similarly, -FeOOH can form at least a partial solid solution with -AlOOH []. Last but not least, Chen et al. [] found that Al is separated from CaSiO perovskite in the presence of HO and forms a separate -AlOOH phase at high pressures. Therefore, -AlOOH is important when considering the HO cycle in the deep mantle.
Depending on the temperature and degree of hydration (therefore mineral compositions) of subducting slabs, dehydration of hydrous minerals can occur at different depths []. According to our experimental results, -AlOOH in cold slabs would not experience dehydration until it reaches the CMB (Figure 3). Because of the large heat flow from the outer core at the CMB, and therefore a steep increase in temperature at these depths, -AlOOH in the slabs would likely undergo dehydration and release HO. HO released from the dehydration of -AlOOH (or other hydrous minerals in subducting slabs) could therefore react with metallic iron from the outer core. For the FeOOH system, Nishi et al. [] found that FeOOH dehydrates to FeO + HO above ∼2400 K at CMB related pressures. Because the temperature at the CMB could reach 3500–4000 K [,], significant dehydration of hydrous minerals transported to the CMB could induce the oxidation of the core and then supply iron oxide to the mantle, FeO, which would dissolve in bridgmanite or ppv-MgSiO in the reaction zone. The dissolution of FeO could enrich mantle materials with Fe and reduce the melting temperatures of mantle silicates. Such dense and partially molten structures locally formed could also be related to the seismic observations of ultra low velocity zones (ULVZs) []. In this scenario, ULVZs could be the reaction zones resulting from dehydration of deeply subducted hydrous materials.
Our experiments also suggest that -AlOOH in warmer slabs could be dehydrated at shallower depths (Figure 3A,B) and released HO could react with local metallic iron. The lower mantle is thought to contain 1 wt.% of metallic iron produced by the charge disproportionation of Fe in bridgmanite [,,], which has been regarded as an important redox buffering agent in the lower mantle []. Based on our observations, we proposed two cases of dehydration of -AlOOH in the lower mantle. In the first of two proposed cases, dehydration could occur from 1000 km depths (Figure 3A). We did not see evidence of reaction between HO and metallic iron up to 45 GPa. However, if HO released from the dehydration of -AlOOH above 2100 km was to react with metallic iron, the process could oxidize metallic iron to form FeOOH as a separate phase or as a component in phase H []. If such a reaction indeed occurs, it would reduce the redox buffering capability in the dehydration zone at the mid mantle. In the second proposed case of dehydration (Figure 3B), the dehydration could occur at depths as great as 2100 km. Here, we found that HO released from the dehydration of -AlOOH reacts with metallic iron above 100 GPa and 1959 K, forming iron oxide (FeO) and iron hydroxide (FeOOH). While its formation in our experiments remains inconclusive, iron hydride may also form as a reaction product. Such a reaction between metallic iron and HO released from the dehydration of hydrous minerals could therefore be important for understanding the oxygen and hydrogen cycles in the deep mantle and the core-mantle boundary.
Author Contributions
Conceptualization, H.P. and S.-H.S.; methodology, J.T., V.B.P., and E.G.; software, S.-H.S.; validation, H.P., K.D.L. and S.-H.S.; formal analysis, H.P., J.T., and S.-H.S.; investigation, H.P.; resources, S.-H.S. and P.R.B.; data curation, H.P.; writing—original draft preparation, H.P.; writing—review and editing, H.P., K.D.L., P.R.B., and S.-H.S.; visualization, H.P.; supervision, S.-H.S.; project administration, S.-H.S.; funding acquisition, P.R.B., K.D.L., and S.-H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the research grant “Water from the Heavens: The Origins of Earth’s Hydrogen” sponsored by the W. M. Keck Foundation (P.I. Peter Buseck). S.-H.S. and H.P. were also supported by NASA (80NSSC18K0353). S.-H.S. was supported by NSF (EAR1338810). Portions of this work were performed at GSECARS (University of Chicago, Sector 13). GSECARS is supported by the NSF (EAR-1634415) and DOE (DE-FG02-94ER14466). APS is the U.S. DOE Office of Science User Facilities operated for the DOE Office of Science by ANL (DE-AC02-06CH11357). All the data are either presented in the main text or in the supporting information.
Acknowledgments
We thank three anonymous reviewers and the editor for discussion, which improved the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| LHDAC | laser-heated diamond anvil cell |
| P | pressure |
| T | temperature |
| CMB | core-mantle boundary |
| DHMSD | dense hydrous magnesium silicates |
| ppv | post-perovskite |
| ASU | Arizona State University |
| ULVZs | ultra low velocity zones |
Appendix A. Unidentified Peaks
Some peaks that could not be indexed with any known phases to our knowledge appeared in different runs. In run #402 at 28 GPa, one unidentified peak with Å appeared at 1669 K and disappeared at 1851 K when cor-AlO appeared. Neither W (from the needle used to load the samples or the thermocouple used for the synthesis of -AlOOH), Re (from the gasket or the thermocouple used for the synthesis of -AlOOH), Pt (from the capsule material used for the synthesis of -AlOOH), nor diamond (anvils) can explain these peaks. Similarly, in run #132b at 68 GPa, two unidentified peaks with Å and Å appeared at 1929 K (Figure 1b) and remained in the patterns with further heating. These unidentified peaks subsequently disappeared at 2497 K, when peaks belonging to cor-AlO appeared. Neither W, Re, Pt, diamond nor hcp-Ar can explain these peaks. In runs #232 and #432 at 44 GPa, one unidentified peak with Å was observed to appear at, respectively, 1865 and 2048 K. Unlike runs #402 and #132b, this peak appears upon or after the appearance of cor-AlO. The peak remains through the entire heating cycles and is present in quenched patterns. No W, Re or Pt was observed in these runs, and diamond does not match.
In run #131 at 105 GPa, four unidentified peaks with Å, Å, Å, and Å appear in the pattern together with the appearance of iron-bearing phases (i.e., -FeOOH and ppv-FeO) (Figure 2c). W was present in the run; however, neither W, Pt, Re nor diamond could be matched to these unknown lines. Finally, in runs #203B and #303B at 110 GPa, one unidentified peak with Å can be seen in both runs and appears around 2300 K after the appearance of rs-AlO and iron-bearing phases. No unknown peaks were observed in runs #118B at 60 GPa, #103 at 86 GPa and #403B at 110 GPa.
The potential reactions of metals with HO further complicates our assessment of unidentified peaks since metals are known to react readily with HO to form hydrides [,]. While we cannot rule out the existence of impurities in the sample, unknown phase transformations of -AlOOH or intermediate dehydration reactions are also possible explanations for these unidentified peaks. Given the spottiness of these rings in our 2D patterns (see Figure A1 and Figure A2), it is also difficult to infer their existence in 1D patterns presented by Duan et al. []. We could not identify the phase(s) belonging to these peaks, nor a trend in their appearance with different pressures (see Figure A3), different couplers (Au and Fe) or different pressure transmitting materials (KCl and NaCl) used in this study. However, most of these unidentified peaks appeared near or upon dehydration of -AlOOH, suggesting that in these cases, they might arise from reactions between released HO and media in the sample chamber, such as KCl and NaCl.
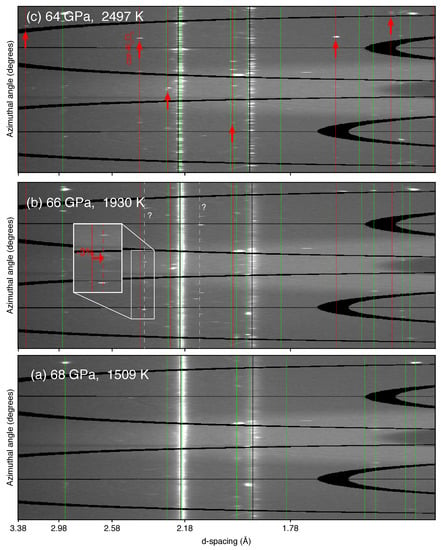
Figure A1.
X-ray diffraction images for run #132B, where dehydration of -AlOOH occurs at 2497 K showing the formation of cor-AlO. X-ray energy was 30 keV. (a) At 1509 K, only precursor materials (i.e., -AlOOH, Ar and Au) are present; (b) at 1930 K, two unknown lines appear, one of which could belong to cor-AlO. However, as the inset shows, the unit-cell volume of cor-AlO would have to be 3% smaller than that expected at the P-T conditions; (c) at 2497 K, where dehydration of -AlOOH to cor-AlO occurs, lines belonging to the later can be clearly seen.
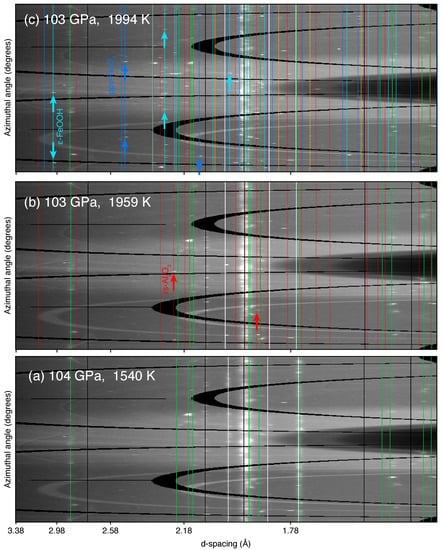
Figure A2.
X-ray diffraction images for run #131, where dehydration of -AlOOH occurs at 1954 K showing the formation of rs-AlO. X-ray energy was 30 keV. (a) At 1540 K, precursor materials (i.e., -AlOOH, Ar and Au) as well as W (see Text for details) are present; (b) at 1959 K, new lines appear, which can be indexed as rs-AlO; (c) at 1994 K, metallic iron contained in the starting material is found to react with released HO and produce -FeOOH and ppv-FeO.
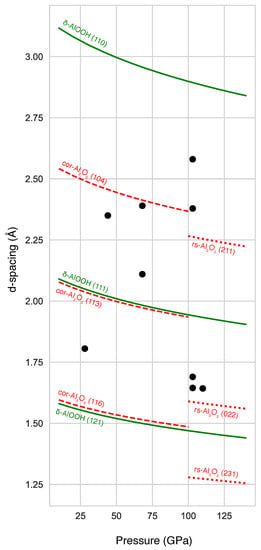
Figure A3.
of unknown peaks observed in experimental runs as a function of pressure (black symbols). For reference, of -AlOOH, cor-AlO and rs-AlO for three different peaks are shown as well.
References
- Badding, J.V.; Hemley, R.; Mao, H. High-pressure chemistry of hydrogen in metals: In situ study of iron hydride. Science 1991, 253, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Sakamaki, K.; Takahashi, E.; Nakajima, Y.; Nishihara, Y.; Funakoshi, K.; Suzuki, T.; Fukai, Y. Melting phase relation of FeHx up to 20 GPa: Implication for the temperature of the Earth’s core. Phys. Earth Planet. Inter. 2009, 174, 192–201. [Google Scholar] [CrossRef]
- Williams, Q.; Hemley, R.J. Hydrogen in the deep Earth. Annu. Rev. Earth Planet. Sci. 2001, 29, 365–418. [Google Scholar] [CrossRef]
- Demouchy, S.; Bolfan-Casanova, N. Distribution and transport of hydrogen in the lithospheric mantle: A review. Lithos 2016, 240, 402–425. [Google Scholar] [CrossRef]
- Tsuchiya, J. First principles prediction of a new high-pressure phase of dense hydrous magnesium silicates in the lower mantle. Geophys. Res. Lett. 2013, 40, 4570–4573. [Google Scholar] [CrossRef]
- Nishi, M.; Irifune, T.; Tsuchiya, J.; Tange, Y.; Nishihara, Y.; Fujino, K.; Higo, Y. Stability of hydrous silicate at high pressures and water transport to the deep lower mantle. Nat. Geosci. 2014, 7, 224. [Google Scholar] [CrossRef]
- Ohtani, E.; Amaike, Y.; Kamada, S.; Sakamaki, T.; Hirao, N. Stability of hydrous phase H MgSiO4H2 under lower mantle conditions. Geophys. Res. Lett. 2014, 41, 8283–8287. [Google Scholar] [CrossRef]
- Ohira, I.; Ohtani, E.; Sakai, T.; Miyahara, M.; Hirao, N.; Ohishi, Y.; Nishijima, M. Stability of a hydrous δ-phase, AlOOH–MgSiO2(OH)2, and a mechanism for water transport into the base of lower mantle. Earth Planet. Sci. Lett. 2014, 401, 12–17. [Google Scholar] [CrossRef]
- Sano, A.; Ohtani, E.; Kondo, T.; Hirao, N.; Sakai, T.; Sata, N.; Ohishi, Y.; Kikegawa, T. Aluminous hydrous mineral δ-AlOOH as a carrier of hydrogen into the core-mantle boundary. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, N.; Wang, S.; Li, X.; Guo, X.; Ni, H.; Prakapenka, V.B.; Mao, Z. Phase stability and thermal equation of state of δ-AlOOH: Implication for water transportation to the Deep Lower Mantle. Earth Planet. Sci. Lett. 2018, 494, 92–98. [Google Scholar] [CrossRef]
- Chen, H.; Leinenweber, K.; Prakapenka, V.; Prescher, C.; Meng, Y.; Bechtel, H.; Kunz, M.; Shim, S.H. Possible H2O storage in the crystal structure of CaSiO3 perovskite. Phys. Earth Planet. Inter. 2020, 299, 106412. [Google Scholar] [CrossRef]
- Terasaki, H.; Ohtani, E.; Sakai, T.; Kamada, S.; Asanuma, H.; Shibazaki, Y.; Hirao, N.; Sata, N.; Ohishi, Y.; Sakamaki, T.; et al. Stability of Fe–Ni hydride after the reaction between Fe–Ni alloy and hydrous phase (δ-AlOOH) up to 1.2 Mbar: Possibility of H contribution to the core density deficit. Phys. Earth Planet. Inter. 2012, 194, 18–24. [Google Scholar] [CrossRef]
- Kato, J.; Hirose, K.; Ozawa, H.; Ohishi, Y. High-pressure experiments on phase transition boundaries between corundum, Rh2O3 (II)-and CaIrO3-type structures in Al2O3. Am. Miner. 2013, 98, 335–339. [Google Scholar] [CrossRef]
- Suzuki, A.; Ohtani, E.; Kamada, T. A new hydrous phase δ-AlOOH synthesized at 21 GPa and 1000 °C. Phys. Chem. Miner. 2000, 27, 689–693. [Google Scholar] [CrossRef]
- Leinenweber, K.D.; Tyburczy, J.A.; Sharp, T.G.; Soignard, E.; Diedrich, T.; Petuskey, W.B.; Wang, Y.; Mosenfelder, J.L. Cell assemblies for reproducible multi-anvil experiments (the COMPRES assemblies). Am. Miner. 2012, 97, 353–368. [Google Scholar] [CrossRef]
- Ohtani, E.; Litasov, K.; Suzuki, A.; Kondo, T. Stability field of new hydrous phase, δ-AlOOH, with implications for water transport into the deep mantle. Geophys. Res. Lett. 2001, 28, 3991–3993. [Google Scholar] [CrossRef]
- Prakapenka, V.; Kubo, A.; Kuznetsov, A.; Laskin, A.; Shkurikhin, O.; Dera, P.; Rivers, M.; Sutton, S. Advanced flat top laser heating system for high pressure research at GSECARS: Application to the melting behavior of germanium. High Press. Res. 2008, 28, 225–235. [Google Scholar] [CrossRef]
- Ye, Y.; Prakapenka, V.; Meng, Y.; Shim, S.H. Intercomparison of the gold, platinum, and MgO pressure scales up to 140 GPa and 2500 K. J. Geophys. Res. Solid Earth 2017, 122, 3450–3464. [Google Scholar] [CrossRef]
- Ye, Y.; Shim, S.H.; Prakapenka, V.; Meng, Y. Equation of state of solid Ne inter-calibrated with the MgO, Au, Pt, NaCl-B2, and ruby pressure scales up to 130 GPa. High Press. Res. 2018, 38, 377–395. [Google Scholar] [CrossRef]
- Dewaele, A.; Belonoshko, A.B.; Garbarino, G.; Occelli, F.; Bouvier, P.; Hanfland, M.; Mezouar, M. High-pressure–high-temperature equation of state of KCl and KBr. Phys. Rev. B 2012, 85, 214105. [Google Scholar] [CrossRef]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Shim, S.H. PeakPo-A Python Software for X-ray Diffraction Analysis at High Pressure and High Temperature; Zenodo: Meyrin, Switzerland, 2017. [Google Scholar]
- Holland, T.; Redfern, S. Unit cell refinement from powder diffraction data: The use of regression diagnostics. Miner. Mag. 1997, 61, 65–77. [Google Scholar] [CrossRef]
- Thomson, K.T.; Wentzcovitch, R.M.; Bukowinski, M.S. Polymorphs of alumina predicted by first principles: Putting pressure on the ruby pressure scale. Science 1996, 274, 1880–1882. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.F.; Degtyareva, O.; Prewitt, C.T.; Dera, P.; Sata, N.; Gregoryanz, E.; Mao, H.k.; Hemley, R.J. Crystal structure of a high-pressure/high-temperature phase of alumina by in situ X-ray diffraction. Nat. Mater. 2004, 3, 389. [Google Scholar] [CrossRef] [PubMed]
- Daniel, I.; Fiquet, G.; Gillet, P.; Schmidt, M.; Hanfland, M. PVT equation of state of lawsonite. Phys. Chem. Miner. 1999, 26, 406–414. [Google Scholar] [CrossRef]
- Brown, J.; Shankland, T. Thermodynamic parameters in the Earth as determined from seismic profiles. Geophys. J. Int. 1981, 66, 579–596. [Google Scholar] [CrossRef]
- Gleason, A.; Quiroga, C.; Suzuki, A.; Pentcheva, R.; Mao, W. Symmetrization driven spin transition in ε-FeOOH at high pressure. Earth Planet. Sci. Lett. 2013, 379, 49–55. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Q.; Kim, D.Y.; Wu, Z.; Wang, W.; Xiao, Y.; Chow, P.; Meng, Y.; Prakapenka, V.B.; Mao, H.K.; et al. Hydrogen-bearing iron peroxide and the origin of ultralow-velocity zones. Nature 2017, 551, 494. [Google Scholar] [CrossRef]
- Yuan, L.; Ohtani, E.; Ikuta, D.; Kamada, S.; Tsuchiya, J.; Naohisa, H.; Ohishi, Y.; Suzuki, A. Chemical reactions between Fe and H2O up to megabar pressures and implications for water storage in the Earth’s mantle and core. Geophys. Res. Lett. 2018, 45, 1330–1338. [Google Scholar] [CrossRef]
- Gleason, A.E.; Jeanloz, R.; Kunz, M. Pressure-temperature stability studies of FeOOH using X-ray diffraction. Am. Miner. 2008, 93, 1882–1885. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, L.; Ohtani, E.; Meng, Y.; Greenberg, E.; Prakapenka, V.B. Stability of Fe-bearing hydrous phases and element partitioning in the system MgO–Al2O3–Fe2O3–SiO2–H2O in Earth’s lowermost mantle. Earth Planet. Sci. Lett. 2019, 524, 115714. [Google Scholar] [CrossRef]
- Bykova, E.; Dubrovinsky, L.; Dubrovinskaia, N.; Bykov, M.; McCammon, C.; Ovsyannikov, S.V.; Liermann, H.P.; Kupenko, I.; Chumakov, A.I.; Rüffer, R.; et al. Structural complexity of simple Fe2O3 at high pressures and temperatures. Nat. Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pépin, C.M.; Dewaele, A.; Geneste, G.; Loubeyre, P.; Mezouar, M. New iron hydrides under high pressure. Phys. Rev. Lett. 2014, 113, 265504. [Google Scholar] [CrossRef] [PubMed]
- Nishi, M.; Kuwayama, Y.; Tsuchiya, J.; Tsuchiya, T. The pyrite-type high-pressure form of FeOOH. Nature 2017, 547, 205. [Google Scholar] [CrossRef]
- Pépin, C.; Geneste, G.; Dewaele, A.; Mezouar, M.; Loubeyre, P. Synthesis of FeH5: A layered structure with atomic hydrogen slabs. Science 2017, 357, 382–385. [Google Scholar] [CrossRef]
- Ohtani, E.; Hirao, N.; Kondo, T.; Ito, M.; Kikegawa, T. Iron-water reaction at high pressure and temperature, and hydrogen transport into the core. Phys. Chem. Miner. 2005, 32, 77–82. [Google Scholar] [CrossRef]
- Van Keken, P.E.; Kiefer, B.; Peacock, S.M. High-resolution models of subduction zones: Implications for mineral dehydration reactions and the transport of water into the deep mantle. Geochem. Geophys. Geosyst. 2002, 3. [Google Scholar] [CrossRef]
- Fiquet, G.; Auzende, A.; Siebert, J.; Corgne, A.; Bureau, H.; Ozawa, H.; Garbarino, G. Melting of peridotite to 140 gigapascals. Science 2010, 329, 1516–1518. [Google Scholar] [CrossRef] [PubMed]
- Andrault, D.; Bolfan-Casanova, N.; Nigro, G.L.; Bouhifd, M.A.; Garbarino, G.; Mezouar, M. Solidus and liquidus profiles of chondritic mantle: Implication for melting of the Earth across its history. Earth Planet. Sci. Lett. 2011, 304, 251–259. [Google Scholar] [CrossRef]
- Rost, S.; Garnero, E.J.; Williams, Q.; Manga, M. Seismological constraints on a possible plume root at the core–mantle boundary. Nature 2005, 435, 666. [Google Scholar] [CrossRef]
- Frost, D.J.; Liebske, C.; Langenhorst, F.; McCammon, C.A.; Trønnes, R.G.; Rubie, D.C. Experimental evidence for the existence of iron-rich metal in the Earth’s lower mantle. Nature 2004, 428, 409. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Grocholski, B.; Ye, Y.; Alp, E.E.; Xu, S.; Morgan, D.; Meng, Y.; Prakapenka, V.B. Stability of ferrous-iron-rich bridgmanite under reducing midmantle conditions. Proc. Natl. Acad. Sci. USA 2017, 114, 6468–6473. [Google Scholar] [CrossRef] [PubMed]
- Bindi, L.; Shim, S.H.; Sharp, T.G.; Xie, X. Evidence for the charge disproportionation of iron in extraterrestrial bridgmanite. Sci. Adv. 2020, 6, eaay7893. [Google Scholar] [CrossRef]
- Rohrbach, A.; Schmidt, M.W. Redox freezing and melting in the Earth’s deep mantle resulting from carbon–iron redox coupling. Nature 2011, 472, 209. [Google Scholar] [CrossRef] [PubMed]
- Hirao, N.; Kondo, T.; Ohtani, E.; Takemura, K.; Kikegawa, T. Compression of iron hydride to 80 GPa and hydrogen in the Earth’s inner core. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).