Comprehensive Characterization of the Structure and Gel Property of Organo-Montmorillonite: Effect of Layer Charge Density of Montmorillonite and Carbon Chain Length of Alkyl Ammonium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Organo-MT
2.3. Analytical Methods
3. Results and Discussion
3.1. XRD Analysis
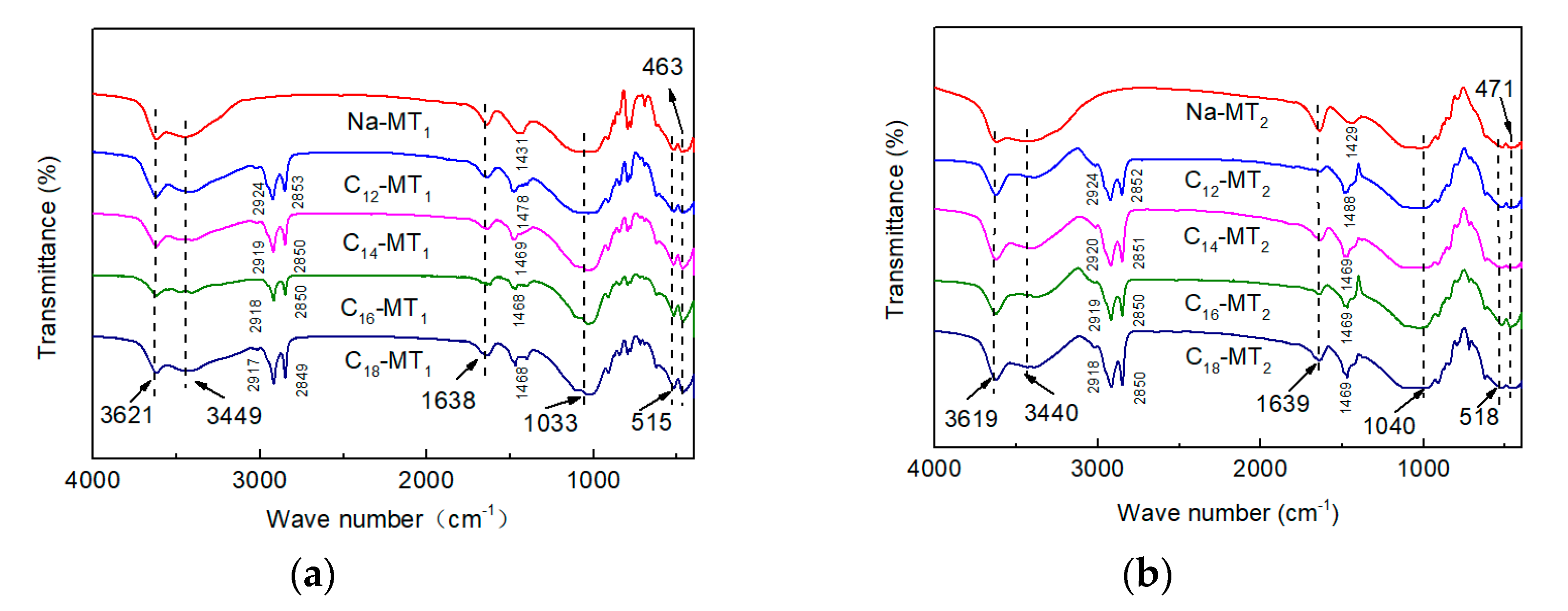
3.2. FTIR Measurement
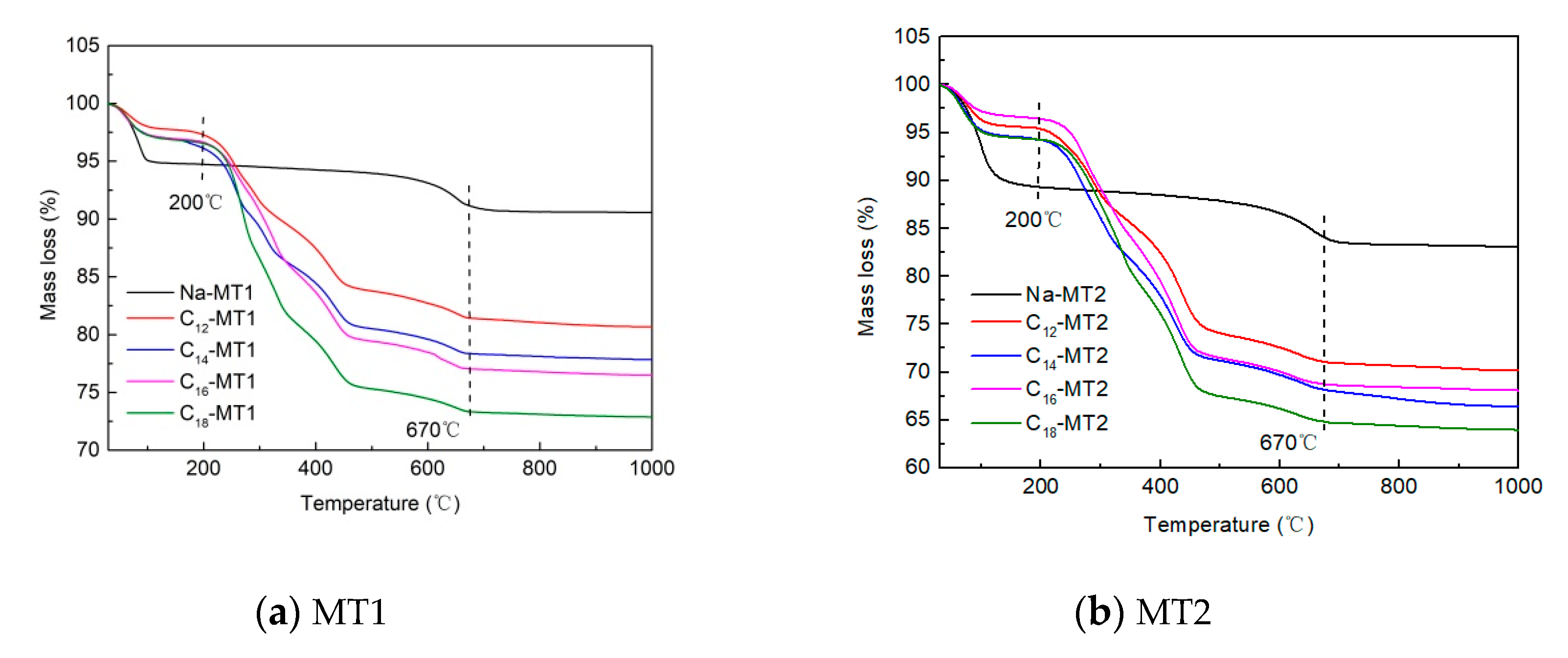
3.3. TG Experiment
3.4. Contact Angle Test
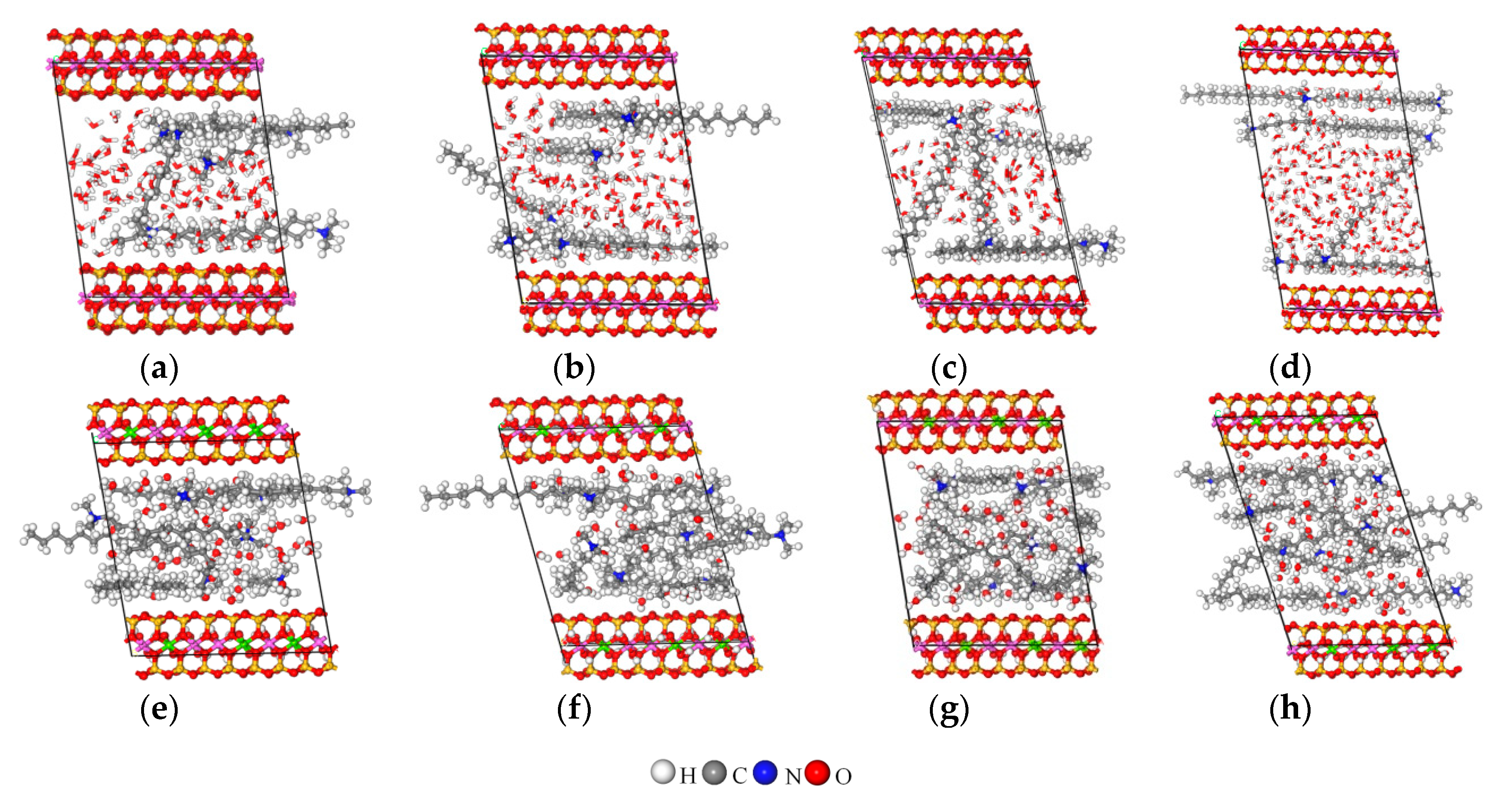
3.5. MD Simulation
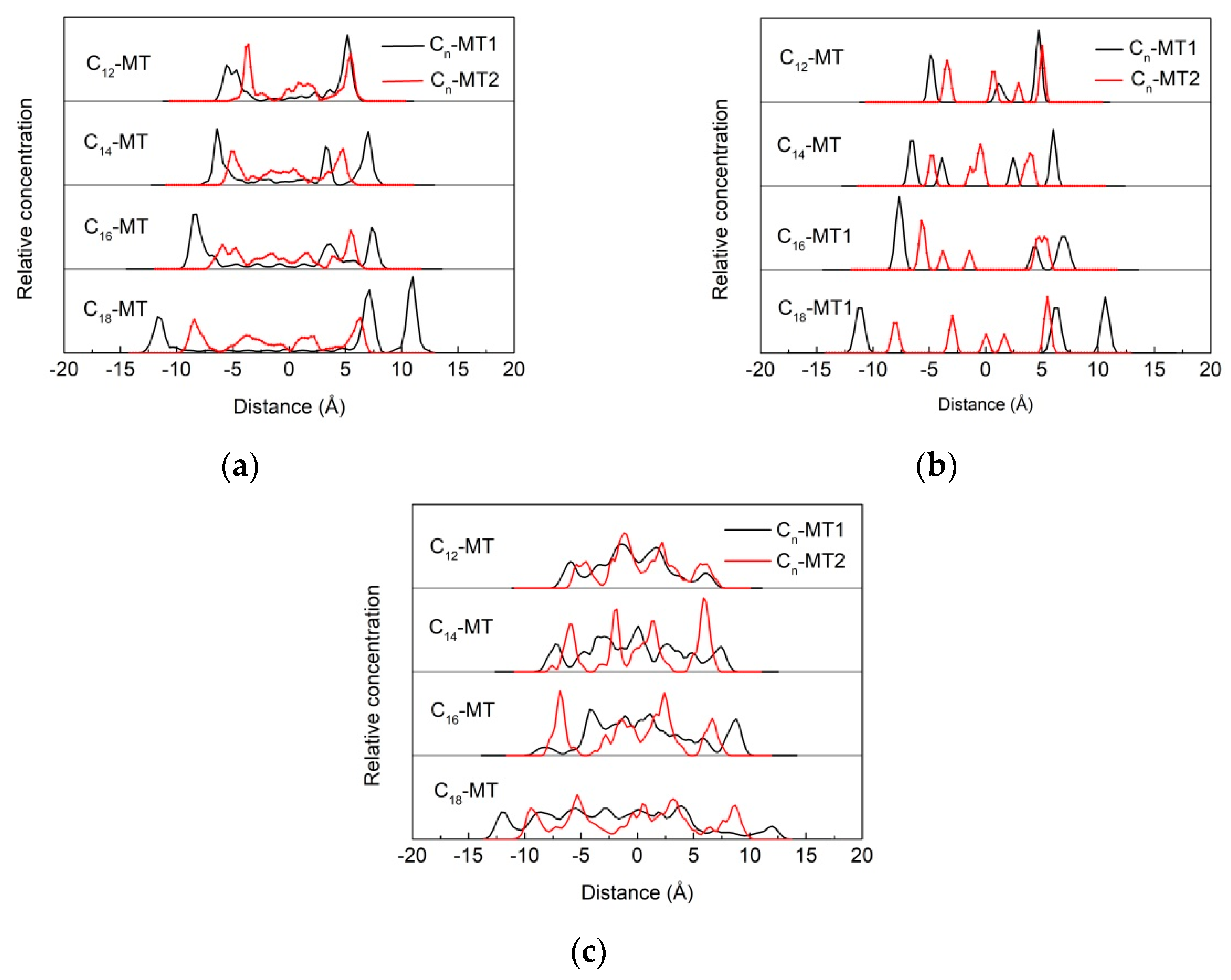
3.5.1. Z-Axis Concentration Distribution
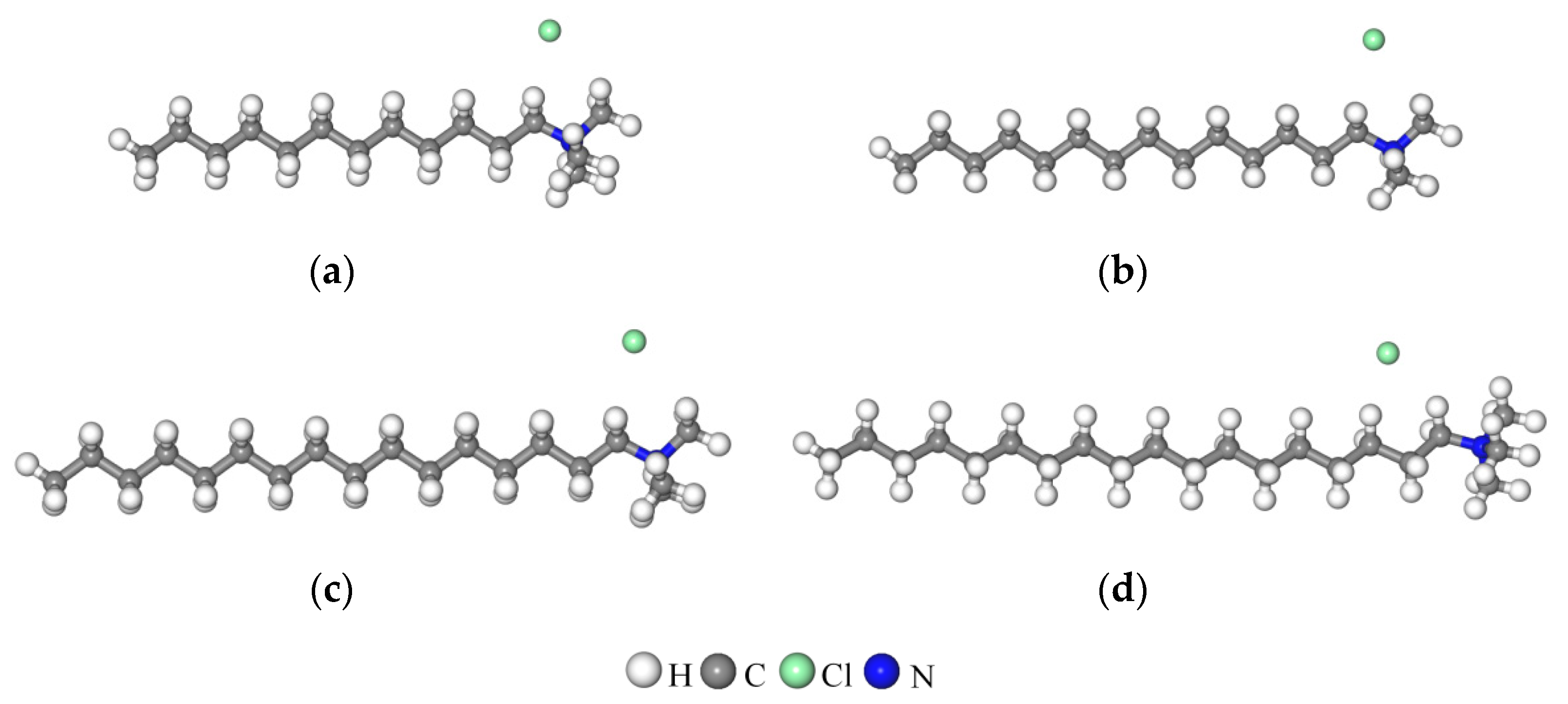
3.5.2. Arrangement of Alkyl Ammonium Cation within Organo-MT Interlayer
3.6. Gel Apparent Viscosity of Organo-MT
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ding, F.; Gao, M.; Shen, T.; Zeng, H.; Xiang, Y. Comparative study of organo-vermiculite, organo-montmorillonite and organo-silica nanosheets functionalized by an ether-spacer-containing Gemini surfactant: Congo red adsorption and wettability. Chem. Eng. J. 2018, 349, 388–396. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galán, E.; Theng, B.K.G. Structure and mineralogy of clay minerals. In Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 21–81. [Google Scholar]
- Yang, S.; Gao, M.; Luo, Z. Adsorption of 2-Naphthol on the organo-montmorillonites modified by Gemini surfactants with different spacers. Chem. Eng. J. 2014, 256, 39–50. [Google Scholar] [CrossRef]
- Jankovič, Ľ.; Madejová, J.; Komadel, P.; Jochec-Mošková, D.; Chodák, I. Characterization of systematically selected organo-montmorillonites for polymer nanocomposites. Appl. Clay Sci. 2011, 51, 438–444. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Q.; Zhu, J.; Xi, Y.; He, H.; Zhu, R.; Tao, Q.; Ayoko, G.A. Adsorption of phenol and Cu(II) onto cationic and zwitterionic surfactant modified montmorillonite in single and binary systems. Chem. Eng. J. 2016, 283, 880–888. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Yang, B.; Sun, R. Rapid modification of montmorillonite with novel cationic Gemini surfactants and its adsorption for methyl orange. Mater. Chem. Phys. 2011, 130, 1220–1226. [Google Scholar] [CrossRef]
- Xi, Y.; Ding, Z.; He, H.; Frost, R.L. Structure of organoclays—An X-ray diffraction and thermogravimetric analysis study. J. Colloid Interface Sci. 2004, 277, 116–120. [Google Scholar] [CrossRef]
- Qiu, J.; Li, G.; Liu, D.; Jiang, S.; Wang, G.; Chen, P.; Zhu, X.-N.; Cao, X.-Q.; Lyu, X.-J. Effect of layer charge characteristics on the distribution characteristics of H2O and Ca2+ in Ca-montmorillonites interlayer space: Molecular dynamics simulation. Materials 2019, 12, 2318. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, D.; Jiang, S.; Chen, G.; Wang, Y.; Li, G.; Yao, G.; Wu, P.; Zhu, X.-N.; Lyu, X.; et al. Effect of crystal chemistry properties on the distribution characteristics of H2O and Na+ in Na-montmorillonite interlayer space: Molecular dynamics simulation study. Minerals 2020, 10, 162. [Google Scholar] [CrossRef]
- Xu, Y.; Khan, M.A.; Wang, F.; Xia, M.; Lei, W. Novel multi amine-containing Gemini surfactant modified montmorillonite as adsorbents for removal of phenols. Appl. Clay Sci. 2018, 162, 204–213. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Zhou, Y.; Wang, S.; Zheng, J.; He, J. Self-assembly of alkylammonium chains on montmorillonite: Effect of interlayer cations, CEC, and chain length. Russ. J. Phys. Chem. A 2018, 91, 2621–2628. [Google Scholar] [CrossRef]
- Ma, J.; Cui, B.; Dai, J.; Li, D. Mechanism of adsorption of anionic dye from aqueous solutions onto organobentonite. J. Hazard. Mater. 2011, 186, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Ke, Y.; Deng, Q.; Lu, S.; Ji, J.; Hu, X.; Zhao, Y. Synthesis and characterization of polystyrene-montmorillonite nanocomposite particles using an anionic-surfactant-modified clay and their friction performance. Appl. Sci. 2018, 8, 964. [Google Scholar] [CrossRef]
- Jian, X.; Xuebing, W.; Bingyao, D.; Qingsheng, L. Modification of montmorillonite by different surfactants and its use for the preparation of polyphenylene sulfide nanocomposites. High Perform. Polym. 2015, 28, 618–629. [Google Scholar] [CrossRef]
- Zhang, Q.; Meng, Z.; Zhang, Y.; Lv, G.; Lv, F.; Wu, L. Modification of a Na-montmorillonite with quaternary ammonium salts and its application for organics removal from TNT red water. Water Sci. Technol. 2014, 69, 1798–1804. [Google Scholar] [CrossRef][Green Version]
- Yang, S.; Gao, M.; Luo, Z.; Yang, Q. The characterization of organo-montmorillonite modified with a novel aromatic-containing gemini surfactant and its comparative adsorption for 2-naphthol and phenol. Chem. Eng. J. 2015, 268, 125–134. [Google Scholar] [CrossRef]
- Zhuang, G.; Zhang, H.; Wu, H.; Zhang, Z.; Liao, L. Influence of the surfactants’ nature on the structure and rheology of organo-montmorillonite in oil-based drilling fluids. Appl. Clay Sci. 2017, 135, 244–252. [Google Scholar] [CrossRef]
- Osman, M.A.; Ploetze, M.; Skrabal, P. Structure and Properties of Alkylammonium Monolayers Self-Assembled on Montmorillonite Platelets. J. Phys. Chem. 2004, 108, 2580–2588. [Google Scholar] [CrossRef]
- Ozola, R.; Krauklis, A.; Burlakovs, J.; Klavins, M.; Vincevica-Gaile, Z.; Hogland, W. Surfactant-modified clay sorbents for the removal of p-nitrophenol. Clays Clay Miner. 2019, 67, 132–142. [Google Scholar] [CrossRef]
- Ouellet-Plamondon, C.M.; Stasiak, J.; Al-Tabbaa, A. The effect of cationic, non-ionic and amphiphilic surfactants on the intercalation of bentonite. Colloids Surf. Physicochem. Eng. Asp. 2014, 444, 330–337. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, Z.; Wu, S.; Tan, J.; Meng, K. Preparation and characterization of novel cationic–nonionic organo-montmorillonite. Mater. Express. 2015, 5, 180–190. [Google Scholar] [CrossRef]
- Sun, J.; Zhuang, G.; Wu, S.; Zhang, Z. Structure and performance of anionic–cationic-organo-montmorillonite in different organic solvents. Rsc. Adv. 2016, 6, 54747–54753. [Google Scholar] [CrossRef]
- Taleb, K.; Pillin, I.; Grohens, Y.; Saidi-Besbes, S. Gemini surfactant modified clays: Effect of surfactant loading and spacer length. Appl. Clay Sci. 2018, 161, 48–56. [Google Scholar] [CrossRef]
- Yang, J.; Yu, K.; Liu, C. Chromium immobilization in soil using quaternary ammonium cations modified montmorillonite: Characterization and mechanism. J. Hazard. Mater. 2017, 321, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Slaný, M.; Jankovič, Ľ.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Bianchi, A.E.; Fernández, M.; Pantanetti, M.; Viña, R.; Torriani, I.; Sánchez, R.M.T.; Punte, G. ODTMA+ and HDTMA+ organo-montmorillonites characterization: New insight by WAXS, SAXS and surface charge. Appl. Clay Sci. 2013, 83–84, 280–285. [Google Scholar] [CrossRef]
- Wu, L.M.; Liao, L.B.; Lv, G.C. Influence of interlayer cations on organic intercalation of montmorillonite. J. Colloid Interface Sci. 2015, 454, 1–7. [Google Scholar] [CrossRef]
- Peng, C.; Min, F.; Liu, L. Effect of pH on the adsorption of dodecylamine on montmorillonite: Insights from experiments and molecular dynamics simulations. Appl. Surf. Sci. 2017, 425, 996–1005. [Google Scholar] [CrossRef]
- Qiu, J.; Li, G.-Q.; Jiang, S.; Liu, D.-L.; Chen, P.; Wang, G.-F. Effect of layer charge on adsorption properties of octadecyl trimethyl ammonium chloride by montmorillonite. Sci. Adv. Mater. 2019, 11, 299–305. [Google Scholar] [CrossRef]
- Açışlı, Ö.; Karaca, S.; Gürses, A. Investigation of the alkyl chain lengths of surfactants on their adsorption by montmorillonite (Mt) from aqueous solutions. Appl. Clay Sci. 2017, 142, 90–99. [Google Scholar] [CrossRef]
- Schampera, B.; Tunega, D.; Solc, R.; Woche, S.K.; Mikutta, R.; Wirth, R.; Dultz, S.; Guggenberger, G. External surface structure of organoclays analysed by transmission electron microscopy and X-ray photoelectron spectroscopy in combination with molecular dynamics simulations. J. Colloid Interface Sci. 2016, 478, 188–200. [Google Scholar] [CrossRef]
- Brito, D.F.; da Silva Filho, E.C.; Fonseca, M.G.; Jaber, M. Organophilic bentonites obtained by microwave heating as adsorbents for anionic dyes. J. Environ. Chem. Eng. 2018, 6, 7080–7090. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, J.; He, H.; Yuan, P.; Shen, W.; Liu, D. Infrared investigation of organo-montmorillonites prepared from different surfactants. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2010, 76, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Ding, Z.; He, H.; Frost, R.L. Infrared spectroscopy of organoclays synthesized with the surfactant octadecyltrimethylammonium bromide. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2005, 61, 515–525. [Google Scholar] [CrossRef]
- Wang, G.; Wang, S.; Sun, Z.; Zheng, S.; Xi, Y. Structures of nonionic surfactant modified montmorillonites and their enhanced adsorption capacities towards a cationic organic dye. Appl. Clay Sci. 2017, 148, 1–10. [Google Scholar] [CrossRef]
- Zhuang, G.; Zhang, Z.; Jaber, M.; Gao, J.; Peng, S. Comparative study on the structures and properties of organo-montmorillonite and organo-palygorskite in oil-based drilling fluids. J. Ind. Eng. Chem. 2017, 56, 248–257. [Google Scholar] [CrossRef]



| Type | SiO2 | Al2O3 | MgO | Na2O | Fe2O3 | K2O | CaO | TiO2 |
|---|---|---|---|---|---|---|---|---|
| Ca-MT1 | 68.65 | 16.04 | 4.15 | 1.99 | 2.98 | 2.86 | 3.33 | 0 |
| Ca-MT2 | 66.83 | 18.79 | 6.44 | 0.34 | 2.80 | 0.41 | 3.98 | 0.41 |
| Types | SSA (m2/g) | PV (cm3/g) | APD (nm) | CEC (mmol/100g) |
|---|---|---|---|---|
| Na-MT1 | 32.6945 | 0.0575 | 7.0370 | 90.69 |
| Na-MT2 | 44.9558 | 0.0632 | 5.6280 | 108.23 |
| Type | C12-MT1 | C14-MT1 | C16-MT1 | C18-MT1 |
| H2O | 110 | 140 | 170 | 219 |
| alkyl ammonium cation | 6 | 6 | 6 | 6 |
| Type | C12-MT2 | C14-MT2 | C16-MT2 | C18-MT2 |
| H2O | 30 | 40 | 50 | 70 |
| alkyl ammonium cation | 11 | 11 | 11 | 11 |
| Type | C12-MT1 | C14-MT1 | C16-MT1 | C18-MT1 |
| Volume/Å3 | 7391.34 | 8455.92 | 8784.63 | 13224.80 |
| c/Å | 22.6606 | 25.7746 | 29.2132 | 35.5689 |
| β/° | 97.8919 | 99.8504 | 103.7590 | 101.2330 |
| Type | C12-MT2 | C14-MT2 | C16-MT2 | C18-MT2 |
| Volume/Å3 | 7153.53 | 7036.72 | 7849.23 | 8609.17 |
| c/Å | 21.3819 | 22.8186 | 24.0655 | 28.6229 |
| β/° | 98.8988 | 104.471 | 99.0000 | 107.4760 |
| T (°C) | C12-MT1 | C14-MT1 | C16-MT1 | C18-MT1 |
| 0–200 | 2.4 | 3.3 | 3.3 | 3.3 |
| 200–670 | 16.0 | 18.3 | 19.5 | 23.4 |
| T (°C) | C12-MT2 | C14-MT2 | C16-MT2 | C18-MT2 |
| 0–200 | 4.1 | 5.2 | 6.4 | 5.2 |
| 200–670 | 24.8 | 26.5 | 26.6 | 30.0 |
| Type | C12-MT1 | C14-MT1 | C16-MT1 | C18-MT1 |
| Gel apparent viscosity | 1.87 | 2.28 | 3.71 | 13.31 |
| Type | C12-MT2 | C14-MT2 | C16-MT2 | C18-MT2 |
| Gel apparent viscosity | 1.20 | 1.92 | 1.60 | 3.49 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Liu, D.; Wang, Y.; Chen, G.; Jiang, S.; Li, G.; Wang, Y.; Wang, W.; Wu, P.; Liu, X.; et al. Comprehensive Characterization of the Structure and Gel Property of Organo-Montmorillonite: Effect of Layer Charge Density of Montmorillonite and Carbon Chain Length of Alkyl Ammonium. Minerals 2020, 10, 378. https://doi.org/10.3390/min10040378
Qiu J, Liu D, Wang Y, Chen G, Jiang S, Li G, Wang Y, Wang W, Wu P, Liu X, et al. Comprehensive Characterization of the Structure and Gel Property of Organo-Montmorillonite: Effect of Layer Charge Density of Montmorillonite and Carbon Chain Length of Alkyl Ammonium. Minerals. 2020; 10(4):378. https://doi.org/10.3390/min10040378
Chicago/Turabian StyleQiu, Jun, Dongliang Liu, Yueting Wang, Guowei Chen, Shan Jiang, Guoqing Li, Yaqi Wang, Wenxin Wang, Peng Wu, Xiaodong Liu, and et al. 2020. "Comprehensive Characterization of the Structure and Gel Property of Organo-Montmorillonite: Effect of Layer Charge Density of Montmorillonite and Carbon Chain Length of Alkyl Ammonium" Minerals 10, no. 4: 378. https://doi.org/10.3390/min10040378
APA StyleQiu, J., Liu, D., Wang, Y., Chen, G., Jiang, S., Li, G., Wang, Y., Wang, W., Wu, P., Liu, X., Wang, G., & Lyu, X. (2020). Comprehensive Characterization of the Structure and Gel Property of Organo-Montmorillonite: Effect of Layer Charge Density of Montmorillonite and Carbon Chain Length of Alkyl Ammonium. Minerals, 10(4), 378. https://doi.org/10.3390/min10040378




