Surface Weathering of Tuffs: Compositional and Microstructural Changes in the Building Stones of the Medieval Castles of Hungary
Abstract
1. Introduction
2. Materials and Methods
3. Environmental Setting
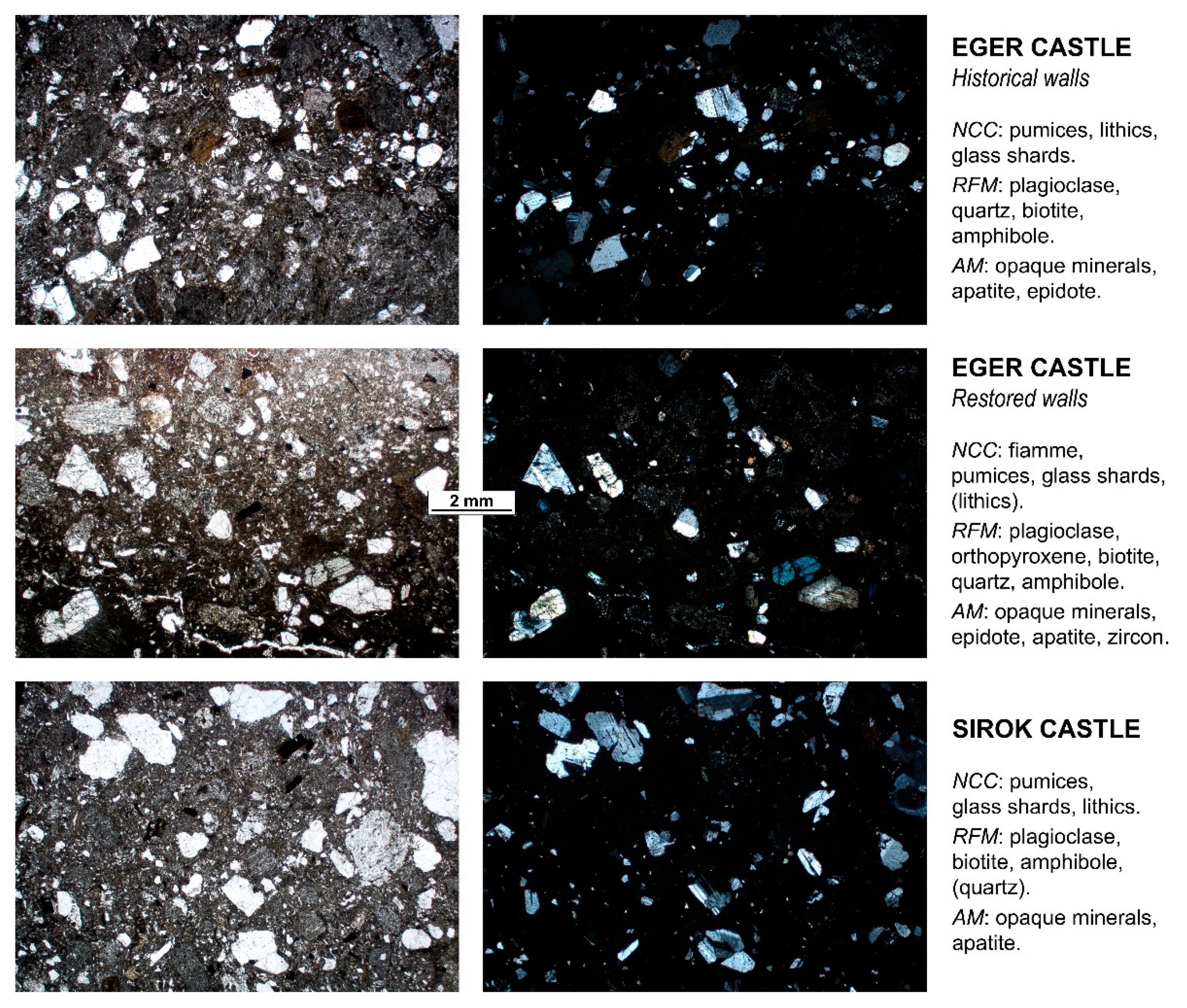
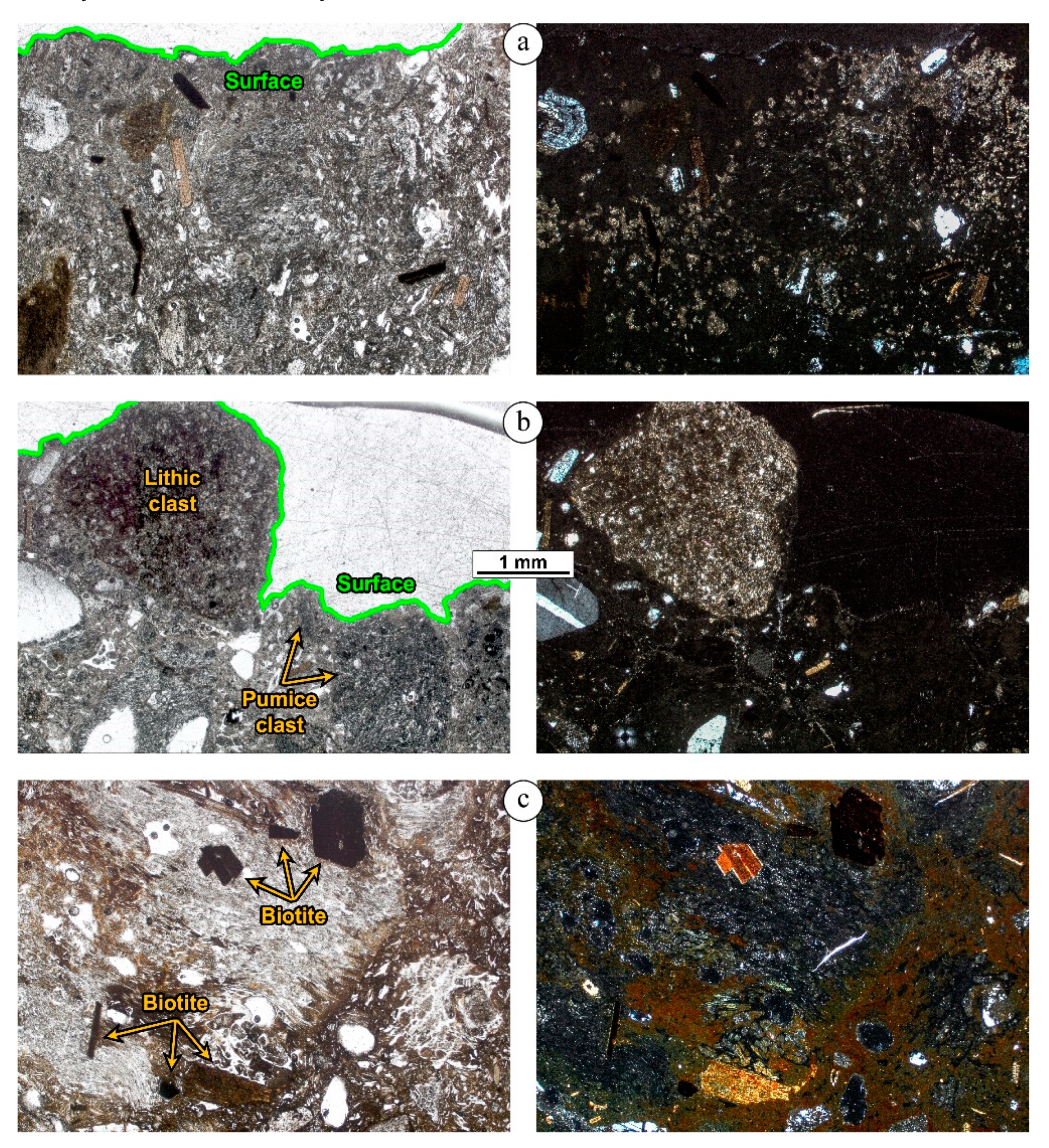
4. Petrographic Study
5. Surface Weathering
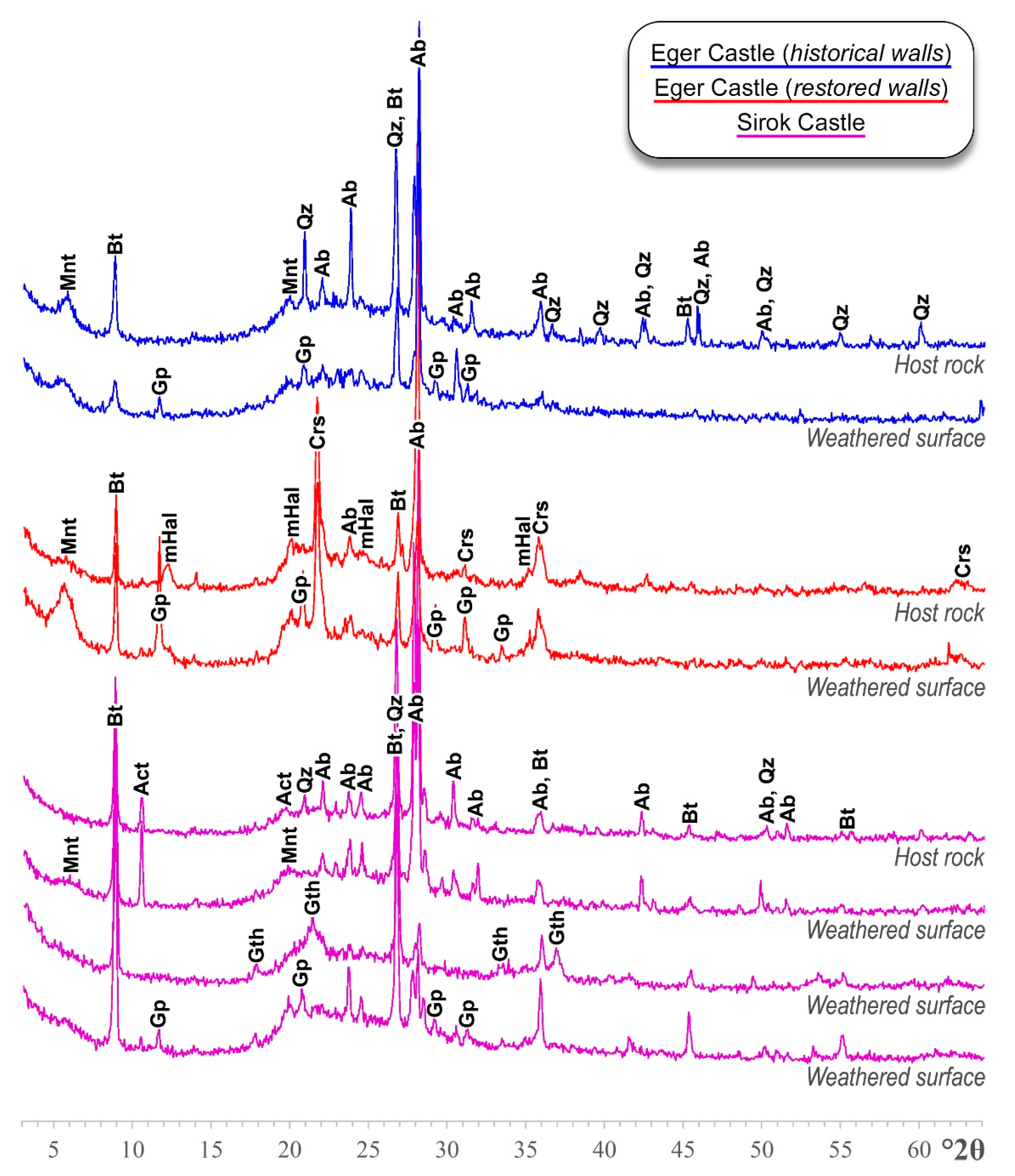
5.1. Mineralogy and Microstructure
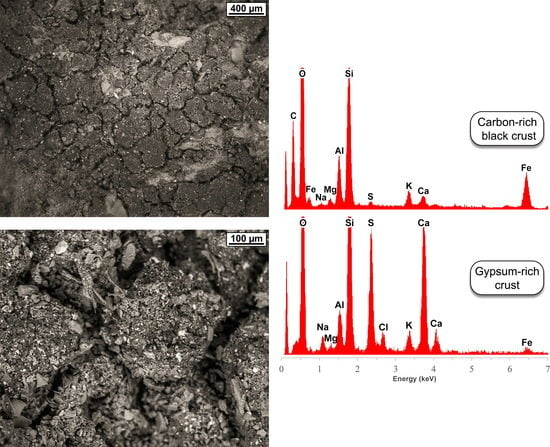
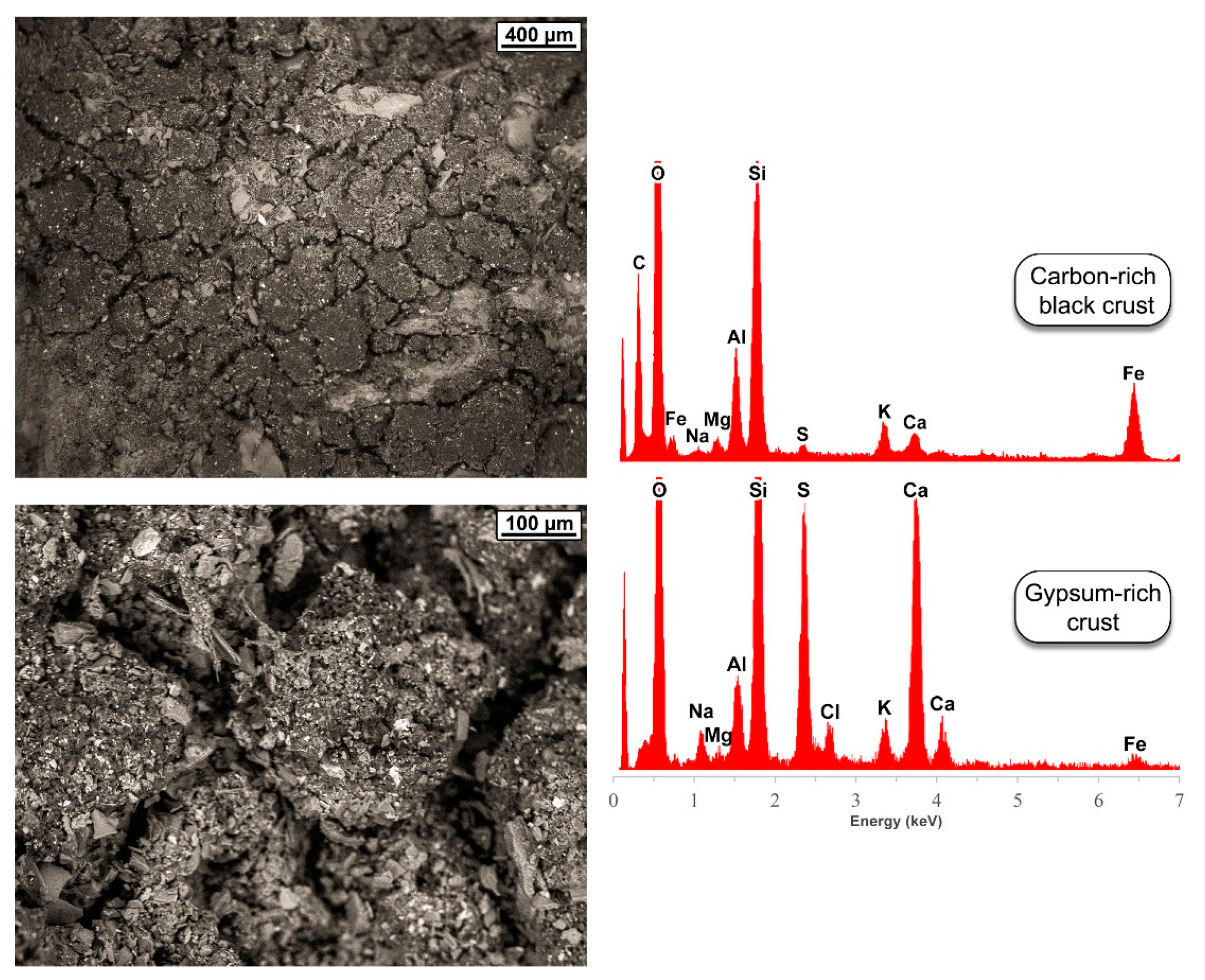
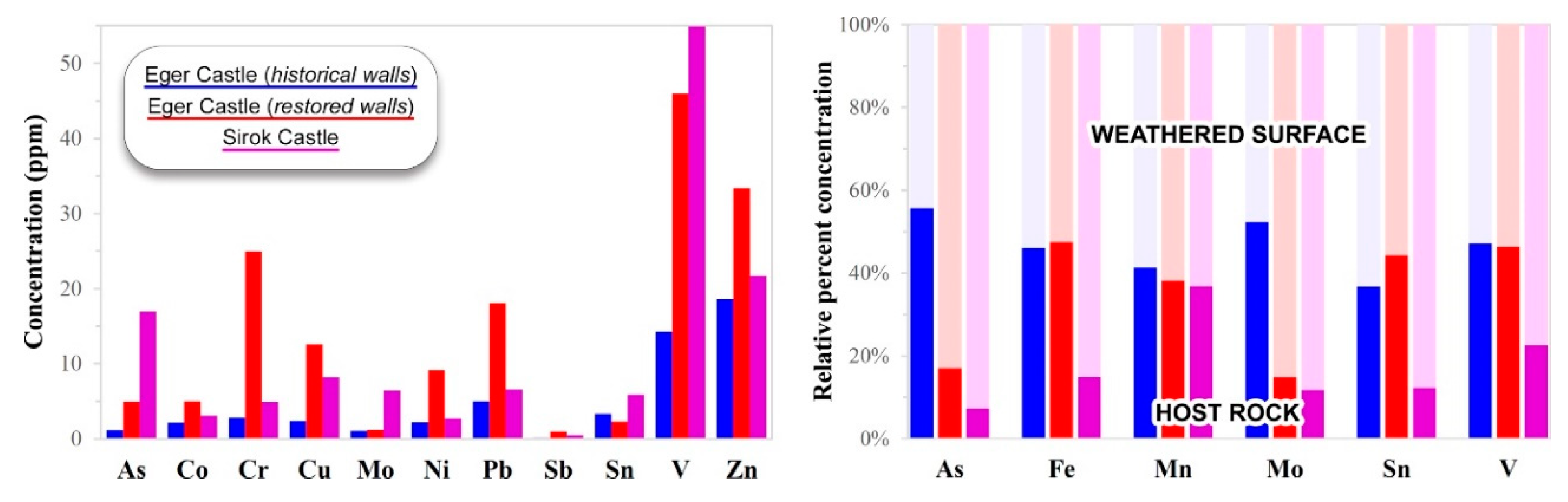
5.2. Trace-Element Chemical Composition
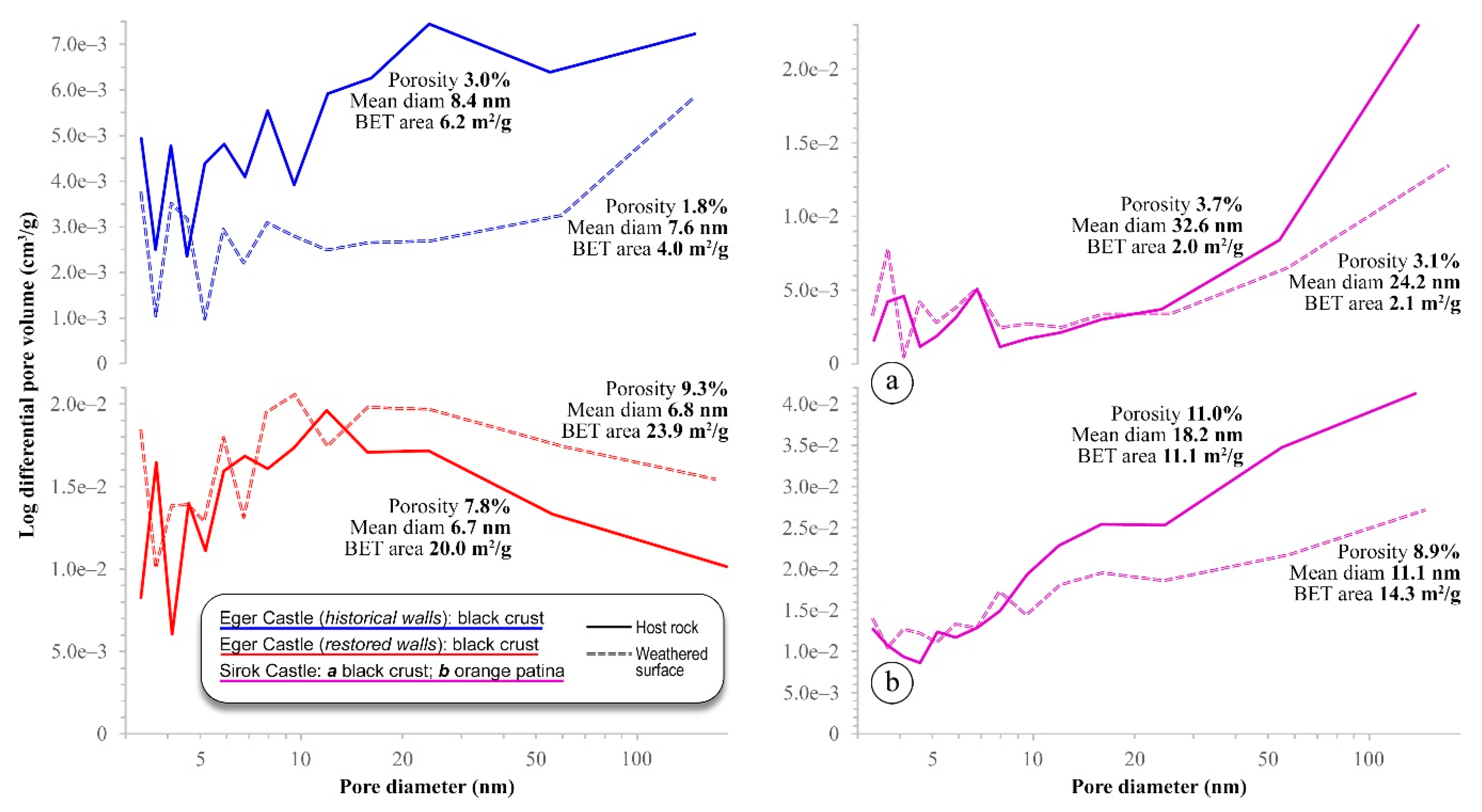
5.3. Microporosity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Török, Á. Hungarian dimensional stones: An overview. Z. Der Dtsch. Ges. Geowiss. 2007, 158, 361–374. [Google Scholar] [CrossRef]
- Kleb, B. Eger Múltja a Jelenben–The Past in the Present Life of Eger; Eger Városi Tanács VB Műszaki Osztálya: Közdok, Budapest, 1978. [Google Scholar]
- Kleb, B. Engineering-geological test on settlements with cellar difficulties. Period. Polytech. Civ. Eng. 1988, 32, 99–129. [Google Scholar]
- Baráz, C.; Kiss, G. Marked Stones and Places of Fable in the Mátra Forest (NE-Hungary); Bükk National Park Directorate: Eger, Hungry, 2010. [Google Scholar]
- Harangi, S. Volcanic heritage of the Carpathian-Pannonian Region in eastern-central Europe. In Volcanic Tourist Destinations; Erfurt-Cooper, P., Ed.; Geoheritage, Geoparks and Geotourism; Springer: Berlin, Germany, 2014; pp. 103–123. [Google Scholar]
- Forgó, L.Z.; Török, Á. Influence of petrophysical and petrographical properties on the behaviour of rhyolite tuff, example from Eger, Hungary. In International PhD Symposium in Civil Engineering, 5th ed.; Walraven, J., Blaauwendraad, J., Scarpas, T., Snijder, B., Eds.; Special Publications 271; Taylor & Francis Group: London, UK, 2004; pp. 589–597. [Google Scholar]
- Török, Á.; Forgó, L.Z.; Vogt, T.; Löbens, S.; Siegesmund, S.; Weiss, T. The influence of lithology and pore-size distribution on the durability of acid volcanic tuffs, Hungary. In Building Stone Decay: From Diagnosis to Conservation; Přikryl, R., Smith, B.J., Eds.; The Geological Society of London: London, UK, 2007; pp. 251–260. [Google Scholar]
- Stück, H.; Forgó, L.Z.; Rüdrich, J.; Siegesmund, S.; Török, Á. The behaviour of consolidated volcanic tuffs: Weathering mechanisms under simulated laboratory conditions. Environ. Geol. 2008, 56, 699–713. [Google Scholar] [CrossRef]
- Németh, G.; Mlinárik, L.; Török, Á. Adsorption and chemical precipitation of lead and zinc from contaminated solutions in porous rocks: Possible application in environmental protection. J. Afr. Earth Sci. 2016, 122, 98–106. [Google Scholar] [CrossRef]
- Török, Á.; Barsi, Á.; Bögöly, G.; Lovas, T.; Somogyi, Á.; Görög, P. Slope stability and rockfall assessment of volcanic tuffs using RPAS with 2-D FEM slope modelling. Nat. Hazards Earth Syst. Sci. 2018, 18, 583–597. [Google Scholar] [CrossRef]
- Germinario, L.; Török, Á. Variability of technical properties and durability in volcanic tuffs from the same quarry region–examples from Northern Hungary. Eng. Geol. 2019, 262, 105319. [Google Scholar] [CrossRef]
- Bianchetti, P.L.; Lombardi, G.; Marini, S.; Meucci, C. The volcanic rocks of the monuments of the Forum and Palatine (Rome): Characterization, alterations, and results of chemical treatments. In Lavas and Volcanic Tuffs; Charola, A.E., Koestler, R.J., Lombardi, G., Eds.; ICCROM: Rome, Italy, 1994; pp. 83–105. [Google Scholar]
- De Casa, G.; Giglio, G.; Lombardi, G.; Mariottini, M. Characterization and state of decay of the volcanic tuff of the Tabularium in the Roman Forum, Italy. In Lavas and Volcanic Tuffs; Charola, A.E., Koestler, R.J., Lombardi, G., Eds.; ICCROM: Rome, Italy, 1994; pp. 107–127. [Google Scholar]
- Paterno, M.C.P. A Study of the Weathering of Volcanic tuffs In a Tropical Environment, Including the Evaluation of a Consolidant. Master’s Thesis, University of Pennsylvania, Philadelphia, PA, USA, 1999. [Google Scholar]
- Ostroumov, M.; Garduño-Monroy, V.H.; Carreón-Nieto, H.; Lozano-Santa Cruz, R. Mineralogía y geoquímica de los procesos de degradación en monumentos históricos: Primer acercamiento a un caso mexicano (Morelia, Michoacán). Rev. Mex. Cienc. Geológicas 2003, 20, 223–232. [Google Scholar]
- Topal, T.; Sözmen, B. Deterioration mechanisms of tuffs in Midas monument. Eng. Geol. 2003, 68, 201–223. [Google Scholar] [CrossRef]
- Nijland, T.G.; Brendle, S.; van Hees, R.P.J.; de Haas, G.J.L.M. Decay of Rhenish tuff in Dutch monuments. Part 1: Use, composition and weathering. Heron 2004, 48, 149–166. [Google Scholar]
- Doehne, E.; Simon, S.; Mueller, U.; Carson, D.; Ormsbee, A. Characterization of carved rhyolite tuff–The Hieroglyphic Stairway of Copán, Honduras. Restor. Build. Monum. 2005, 11, 247–254. [Google Scholar] [CrossRef]
- Zedef, V.; Kocak, K.; Doyen, A.; Ozsen, H.; Kekec, B. Effect of salt crystallization on stones of historical buildings and monuments, Konya, Central Turkey. Build. Environ. 2007, 42, 1453–1457. [Google Scholar] [CrossRef]
- Siedel, H. Salt-induced alveolar weathering of rhyolite tuff on a building: Causes and processes. In Proceedings of the International Conference “Salt Weathering on Buildings and Stone Sculptures”, Copenhagen, Denmark, 22–24 October 2008; pp. 79–88. [Google Scholar]
- Erguler, Z.A. Field-based experimental determination of the weathering rates of the Cappadocian tuffs. Eng. Geol. 2009, 105, 186–199. [Google Scholar] [CrossRef]
- Ciccioli, P.; Cattuto, C.; Plescia, P.; Valentini, V.; Negrotti, R. Geochemical and engineering geological properties of the volcanic tuffs used in the Etruscan tombs of Norchia (northern Latium, Italy) and a study of the factors responsible for their rapid surface and structural decay. Archaeometry 2010, 52, 229–251. [Google Scholar] [CrossRef]
- Oguchi, C.; Takaya, Y.; Yamazaki, M.; Ohnishi, R.; Thidar, A.; Hatta, T. High acidic sulphate salt production on the cave wall in the Yoshimi Hyaku-Ana historic site, central Japan. In Proceedings of the XIX CBGA Congress, Thessaloniki, Greece, 23–26 September 2010; pp. 413–419. [Google Scholar]
- Oguchi, C.T.; Yuasa, H. Simultaneous wetting/drying, freeze/thaw and salt crystallization experiments of three types of Oya tuff. In Natural Stone Resources for Historical Monuments; Přikryl, R., Török, Á., Eds.; Special Publications 333; The Geological Society of London: London, UK, 2010; pp. 59–72. [Google Scholar]
- Reyes-Zamudio, V.; Angeles-Chávez, C.; Cervantes, J. Clay minerals in historic buildings. J. Therm. Anal. Calorim. 2011, 104, 405–413. [Google Scholar] [CrossRef]
- Yavuz, A.B. Durability assessment of the Alaçatı tuff (Izmir) in western Turkey. Environ. Earth Sci. 2012, 67, 1909–1925. [Google Scholar] [CrossRef]
- Mosonyi, E.; Cobârzan, N. Weathering, conservation state and compatibility studies on the construction materials used for renovation of the historical Dej reformed church fortifying walls. Rom. J. Mater. 2016, 46, 542–551. [Google Scholar]
- Lee, C.H.; Araki, N. Evaluation of nondestructive diagnosis and material characteristics of stone lantern at Damyang Gaeseonsaji temple site in Korea. J. Conserv. Sci. 2019, 35, 279–293. [Google Scholar] [CrossRef]
- Harangi, S. Neogene to Quaternary volcanism of the Carpathian-Pannonian Region–A review. Acta Geol. Hung. 2001, 44, 223–258. [Google Scholar]
- Harangi, S.; Lenkey, L. Genesis of the Neogene to Quaternary volcanism in the Carpathian-Pannonian region: Role of subduction, extension, and mantle plume. In Cenozoic Volcanism in the Mediterranean Area; Beccaluva, L., Bianchini, G., Wilson, M., Eds.; Special Paper 418; Geological Society of America: Boulder, CO, USA, 2007; pp. 67–92. [Google Scholar]
- Seghedi, I.; Downes, H. Geochemistry and tectonic development of Cenozoic magmatism in the Carpathian-Pannonian region. Gondwana Researh 2011, 20, 655–672. [Google Scholar] [CrossRef]
- Horváth, F.; Musitz, B.; Balázs, A.; Végh, A.; Uhrin, A.; Nádor, A.; Koroknai, B.; Pap, N.; Tóth, T.; Wórum, G. Evolution of the Pannonian basin and its geothermal resources. Geothermics 2015, 53, 328–352. [Google Scholar] [CrossRef]
- Lukács, R.; Harangi, S.; Guillong, M.; Bachmann, O.; Fodor, L.; Buret, Y.; Dunkl, I.; Sliwinski, J.; von Quadt, A.; Peytcheva, I.; et al. Early to Mid-Miocene syn-extensional massive silicic volcanism in the Pannonian Basin (East-Central Europe): Eruption chronology, correlation potential and geodynamic implications. Earth-Sci. Rev. 2018, 179, 1–19. [Google Scholar]
- Péczely, G. Éghajlattan; Nemzeti Tankönyvkiadó: Budapest, Hungry, 2002. [Google Scholar]
- Climate-Data.org Hungary Climate. 2018. Available online: https://en.climate-data.org/europe/hungary-20 (accessed on 7 June 2018).
- EEA. Air Quality in Europe–2019 Report; European Environment Agency: Copenhagen, Denmark, 2009.
- EEA Air Pollutant Emissions Data Viewer (Gothenburg Protocol, LRTAP Convention). 2017. Available online: https://www.eea.europa.eu/data-and-maps/dashboards/air-pollutant-emissions-data-viewer (accessed on 8 June 2018).
- OLM. Hungarian Air Quality Network–Automatic Monitoring Network. 2020. Available online: http://www.levegominoseg.hu/automatic-monitoring-network (accessed on 21 March 2020).
- Lantai, K.; Wopera, Á.; Nagy, G. Air quality in the Northern Hungarian region. Mater. Sci. Eng. 2016, 41, 79–88. [Google Scholar]
- Tiner, T. Far from the core–regions and industrial parks in economic shadow in Hungary. Part one. Hung. Geogr. Bull. 2010, 59, 89–106. [Google Scholar]
- Massey, S.W. The effects of ozone and NOx on the deterioration of calcareous stone. Sci. Total Environ. 1999, 227, 109–121. [Google Scholar] [CrossRef]
- Sharma, M.; Agarwal, A.K.; Bharathi, K.V.L. Characterization of exhaust particulates from diesel engine. Atmos. Environ. 2005, 39, 3023–3028. [Google Scholar] [CrossRef]
- Germinario, L.; Siegesmund, S.; Maritan, L.; Simon, K.; Mazzoli, C. Trachyte weathering in the urban built environment related to air quality. Herit. Sci. 2017, 5, 44. [Google Scholar] [CrossRef]
- Charola, A.E.; Ware, R. Acid deposition and the deterioration of stone: A brief review of a broad topic. In Natural Stone, Weathering Phenomena, Conservation Strategies and Case Studies; Siegesmund, S., Weiss, T., Vollbrecht, A., Eds.; Special Publications 205; The Geological Society of London: London, UK, 2002; pp. 393–406. [Google Scholar]
- Graue, B.; Siegesmund, S.; Oyhantcabal, P.; Naumann, R.; Licha, T.; Simon, K. The effect of air pollution on stone decay: The decay of the Drachenfels trachyte in industrial, urban, and rural environments–a case study of the Cologne, Altenberg and Xanten cathedrals. Environ. Earth Sci. 2013, 69, 1095–1124. [Google Scholar] [CrossRef]
- Colella, C.; de’ Gennaro, M.; Aiello, R. Use of zeolitic tuff in the building industry. Rev. Mineral. Geochem. 2001, 45, 551–587. [Google Scholar] [CrossRef]
- Fitzner, B. Volcanic tuffs: The description and quantitative recording of their weathered state. In Lavas and Volcanic Tuffs; Charola, A.E., Koestler, R.J., Lombardi, G., Eds.; ICCROM: Rome, Italy, 1994; pp. 33–51. [Google Scholar]
- Smith, B.J.; Török, Á.; McAlister, J.J.; Megarry, J. Observations on the factors influencing stability of building stones following contour scaling: A case study of the oolitic limestones from Budapest, Hungary. Build. Environ. 2003, 38, 1173–1183. [Google Scholar] [CrossRef]
- Farkas, O.; Siegesmund, S.; Licha, T.; Török, Á. Geochemical and mineralogical composition of black weathering crusts on limestones from seven different European countries. Environ. Earth Sci. 2018, 77, 211. [Google Scholar] [CrossRef]
- Yu, S.; Oguchi, C.T. Role of pore size distribution in salt uptake, damage, and predicting salt susceptibility of eight types of Japanese building stones. Eng. Geol. 2010, 115, 226–236. [Google Scholar] [CrossRef]
- Wedekind, W.; Ruedrich, J.; Siegesmund, S. Natural building stones of Mexico–Tenochtitlán: Their use, weathering and rock properties at the Templo Mayor, Palace Heras Soto and the Metropolitan Cathedral. Environ. Earth Sci. 2011, 63, 1787–1798. [Google Scholar] [CrossRef]
- López-Doncel, R.; Wedekind, W.; Leiser, T.; Molina-Maldonado, S.; Velasco-Sánchez, A.; Dohrmann, R.; Kral, A.; Wittenborn, A.; Aguillón-Robles, A.; Siegesmund, S. Salt bursting tests on volcanic tuff rocks from Mexico. Environ. Earth Sci. 2016, 75, 212. [Google Scholar] [CrossRef]
- Akın, M.; Özvan, A.; Dinçer, İ.; Topal, T. Evaluation of the physico-mechanical parameters affecting the deterioration rate of Ahlat ignimbrites (Bitlis, Turkey). Environ. Earth Sci. 2017, 76, 827. [Google Scholar] [CrossRef]
- Fisher, R.V.; Schmincke, H.U. Pyroclastic Rocks; Springer: Berlin, Germany, 1984. [Google Scholar]
- Steindlberger, E. Volcanic tuffs from Hesse (Germany) and their weathering behaviour. Environ. Geol. 2004, 46, 378–390. [Google Scholar] [CrossRef]
- Wedekind, W.; López-Doncel, R.; Dohrmann, R.; Kocher, M.; Siegesmund, S. Weathering of volcanic tuff rocks caused by moisture expansion. Environ. Earth Sci. 2013, 69, 1203–1224. [Google Scholar] [CrossRef]
- Heap, M.J.; Farquharson, J.I.; Kushnir, A.R.; Lavallée, Y.; Baud, P.; Gilg, H.A.; Reuschlé, T. The influence of water on the strength of Neapolitan Yellow Tuff, the most widely used building stone in Naples (Italy). Bull. Volcanol. 2018, 80, 51. [Google Scholar] [CrossRef]
- Pötzl, C.; Siegesmund, S.; Dohrmann, R.; Koning, J.M.; Wedekind, W. Deterioration of volcanic tuff rocks from Armenia: Constraints on salt crystallization and hydric expansion. Environ. Earth Sci. 2018, 77, 660. [Google Scholar] [CrossRef]
- Harmens, H.; Norris, D. Spatial and Temporal Trends in Heavy Metal Accumulation in Mosses in Europe (1990–2005); Centre for Ecology and Hydrology: Bangor, UK, 2008. [Google Scholar]
- Winther, M.; Slentø, E. Heavy Metal Emissions for Danish Road Transport; NERI Technical Report No. 780; National Environmental Research Institute: Aarhus, Danmark, 2010. [Google Scholar]
- Comite, V.; Álvarez de Buergo, M.; Barca, D.; Belfiore, C.M.; Bonazza, A.; La Russa, M.F.; Pezzino, A.; Randazzo, L.; Ruffolo, S.A. Damage monitoring on carbonate stones: Field exposure tests contributing to pollution impact evaluation in two Italian sites. Constr. Build. Mater. 2017, 152, 907–922. [Google Scholar] [CrossRef]
- Ma, J.H.; Chu, C.J.; Li, J.; Song, B. Heavy metal pollution in soils on railroad side of Zhengzhou-Putian section of Longxi-Haizhou Railroad, China. Pedosphere 2009, 19, 121–128. [Google Scholar] [CrossRef]
- Salma, I.; Pósfai, M.; Kovács, K.; Kuzmann, E.; Homonnay, Z.; Posta, J. Properties and sources of individual particles and some chemical species in the aerosol of a metropolitan underground railway station. Atmos. Environ. 2009, 43, 3460–3466. [Google Scholar] [CrossRef]
- Wiłkomirski, B.; Sudnik-Wójcikowska, B.; Galera, H.; Wierzbicka, M.; Malawska, M. Railway transportation as a serious source of organic and inorganic pollution. WaterAir Soil Pollut. 2011, 218, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Dzierżanowski, K.; Gawroński, S.W. Heavy metal concentration in plants growing on the vicinity of railroad tracks: A pilot study. Chall. Mod. Technol. 2012, 3, 42–45. [Google Scholar]
- Chen, Z.; Wang, K.; Ai, Y.W.; Li, W.; Gao, H.; Fang, C. The effects of railway transportation on the enrichment of heavy metals in the artificial soil on railway cut slopes. Environ. Monit. Assess. 2014, 186, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Grossi, C.M.; Murray, M. Characteristics of carbonate building stones that influence the dry deposition of acidic gases. Constr. Build. Mater. 1999, 13, 101–108. [Google Scholar] [CrossRef]
- Steiger, M.; Charola, A.E.; Sterflinger, K. Weathering and deterioration. In Stone in Architecture. Properties, Durability, 5th ed.; Siegesmund, S., Snethlage, R., Eds.; Springer: Berlin, Germany, 2014; pp. 225–316. [Google Scholar]
- Germinario, L.; Andriani, G.F.; Laviano, R. Decay of calcareous building stone under the combined action of thermoclastism and cryoclastism: A laboratory simulation. Constr. Build. Mater. 2015, 75, 385–394. [Google Scholar] [CrossRef]
- Germinario, L.; Siegesmund, S.; Maritan, L.; Mazzoli, C. Petrophysical and mechanical properties of Euganean trachyte and implications for dimension stone decay and durability performance. Environ. Earth Sci. 2017, 76, 739. [Google Scholar] [CrossRef]


| Location | SO2 | NO2 | CO | O3 | NOX | NO | C6H6 | PM10 |
|---|---|---|---|---|---|---|---|---|
| Eger | 7.1 | 18.6 | 486.6 | 45.3 | 27.8 | 6.0 | 1.2 | 24.7 |
| Budapest (3 stations avg.) | 5.7 | 41.0 | 621.2 | 31.5 | 80.1 | 25.8 | 0.9 | 32.2 |
| Location | Ab | Bt | Qz | Mnt | mHal | Act | Crs |
|---|---|---|---|---|---|---|---|
| Eger Castle (historical walls) | 60% | 10% | 15% | 15% | - | - | - |
| Eger Castle (restored walls) | 40% | 15% | - | 5% | 20% | - | 20% |
| Sirok Castle | 60% | 25% | 5% | - | - | 10% | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germinario, L.; Török, Á. Surface Weathering of Tuffs: Compositional and Microstructural Changes in the Building Stones of the Medieval Castles of Hungary. Minerals 2020, 10, 376. https://doi.org/10.3390/min10040376
Germinario L, Török Á. Surface Weathering of Tuffs: Compositional and Microstructural Changes in the Building Stones of the Medieval Castles of Hungary. Minerals. 2020; 10(4):376. https://doi.org/10.3390/min10040376
Chicago/Turabian StyleGerminario, Luigi, and Ákos Török. 2020. "Surface Weathering of Tuffs: Compositional and Microstructural Changes in the Building Stones of the Medieval Castles of Hungary" Minerals 10, no. 4: 376. https://doi.org/10.3390/min10040376
APA StyleGerminario, L., & Török, Á. (2020). Surface Weathering of Tuffs: Compositional and Microstructural Changes in the Building Stones of the Medieval Castles of Hungary. Minerals, 10(4), 376. https://doi.org/10.3390/min10040376