Improved Understanding of the Sulfidization Mechanism in Amine Flotation of Smithsonite: An XPS, AFM and UV–Vis DRS Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Flotation Tests
2.3. Characterization
3. Results and Discussion
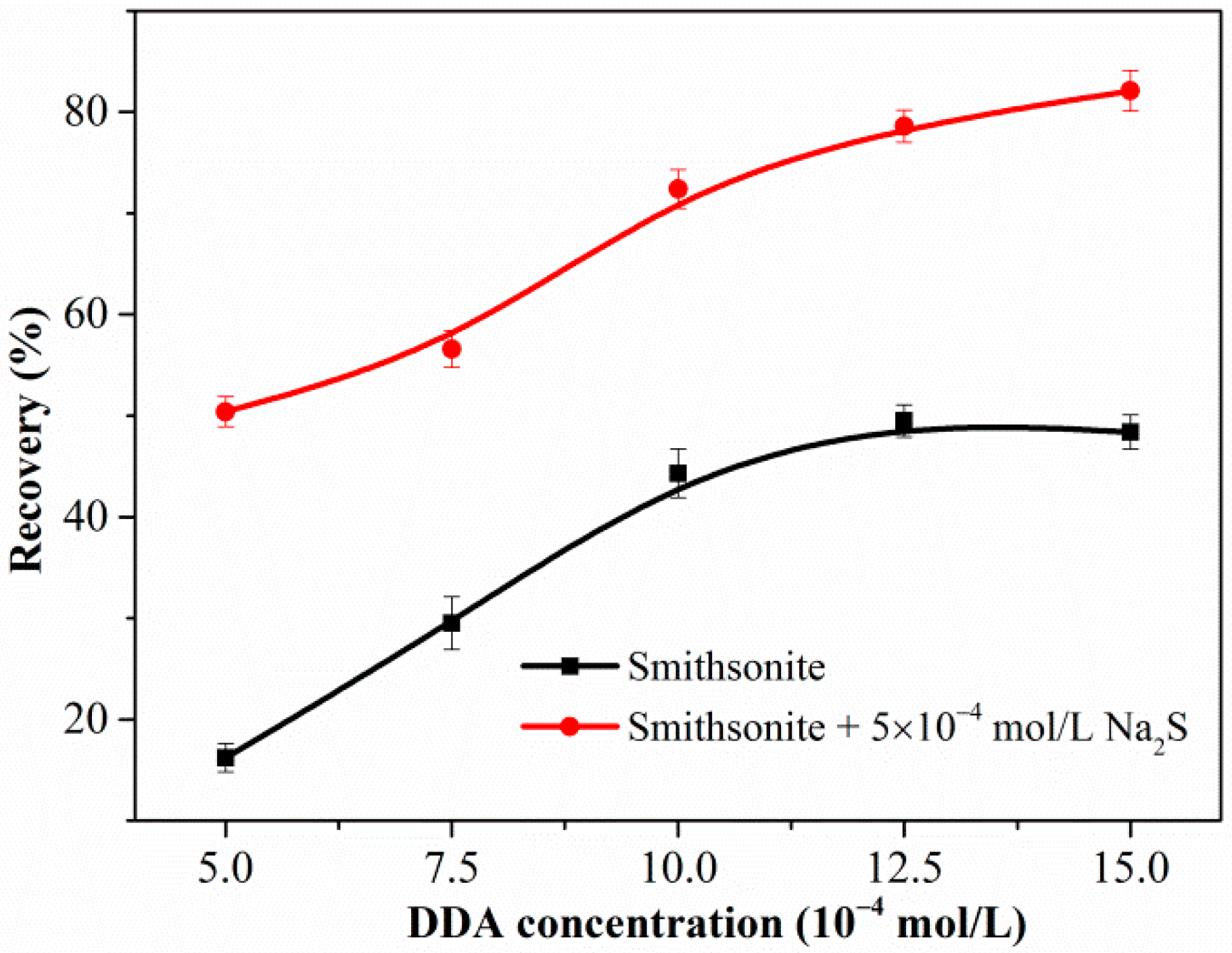
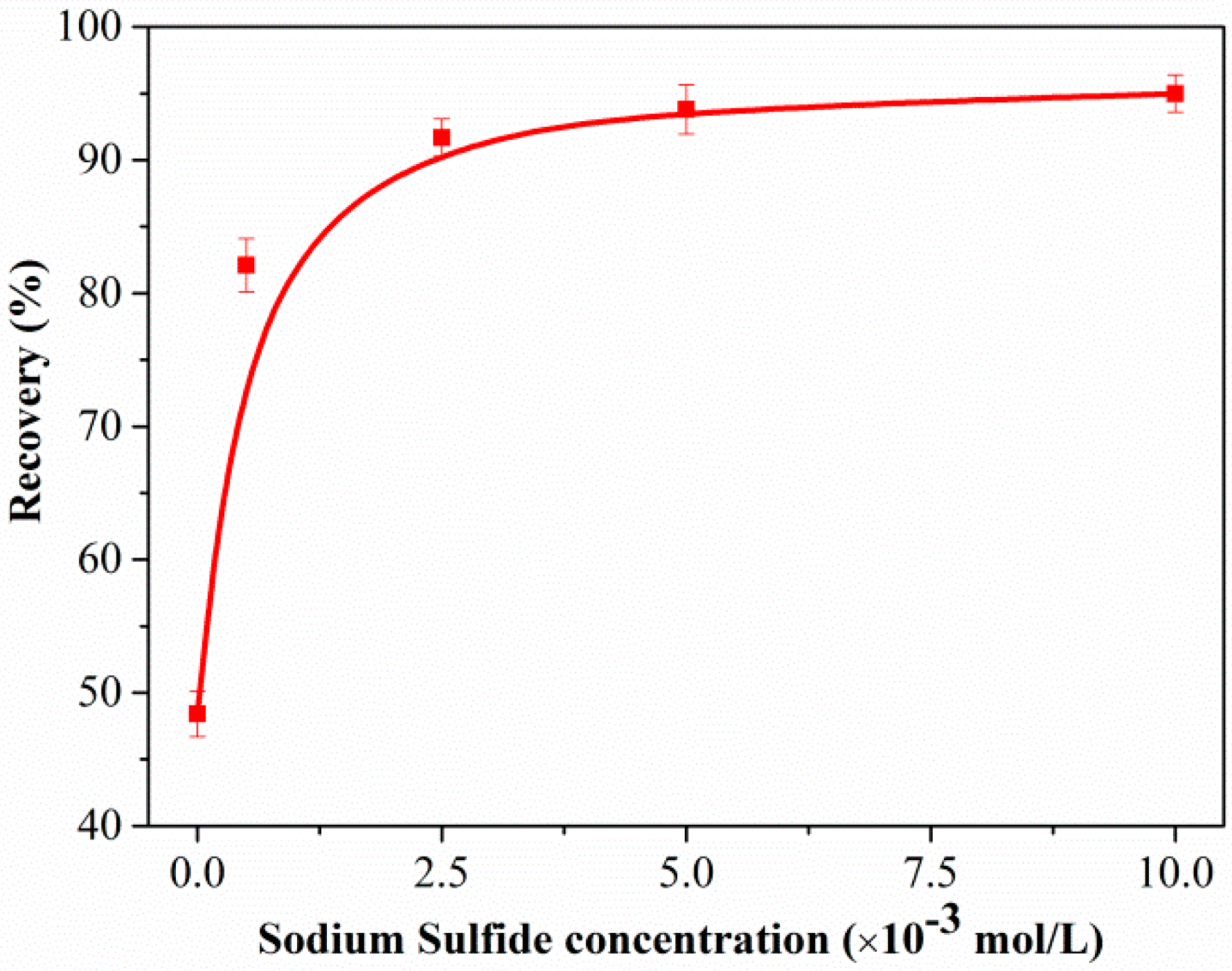
3.1. Flotation Test
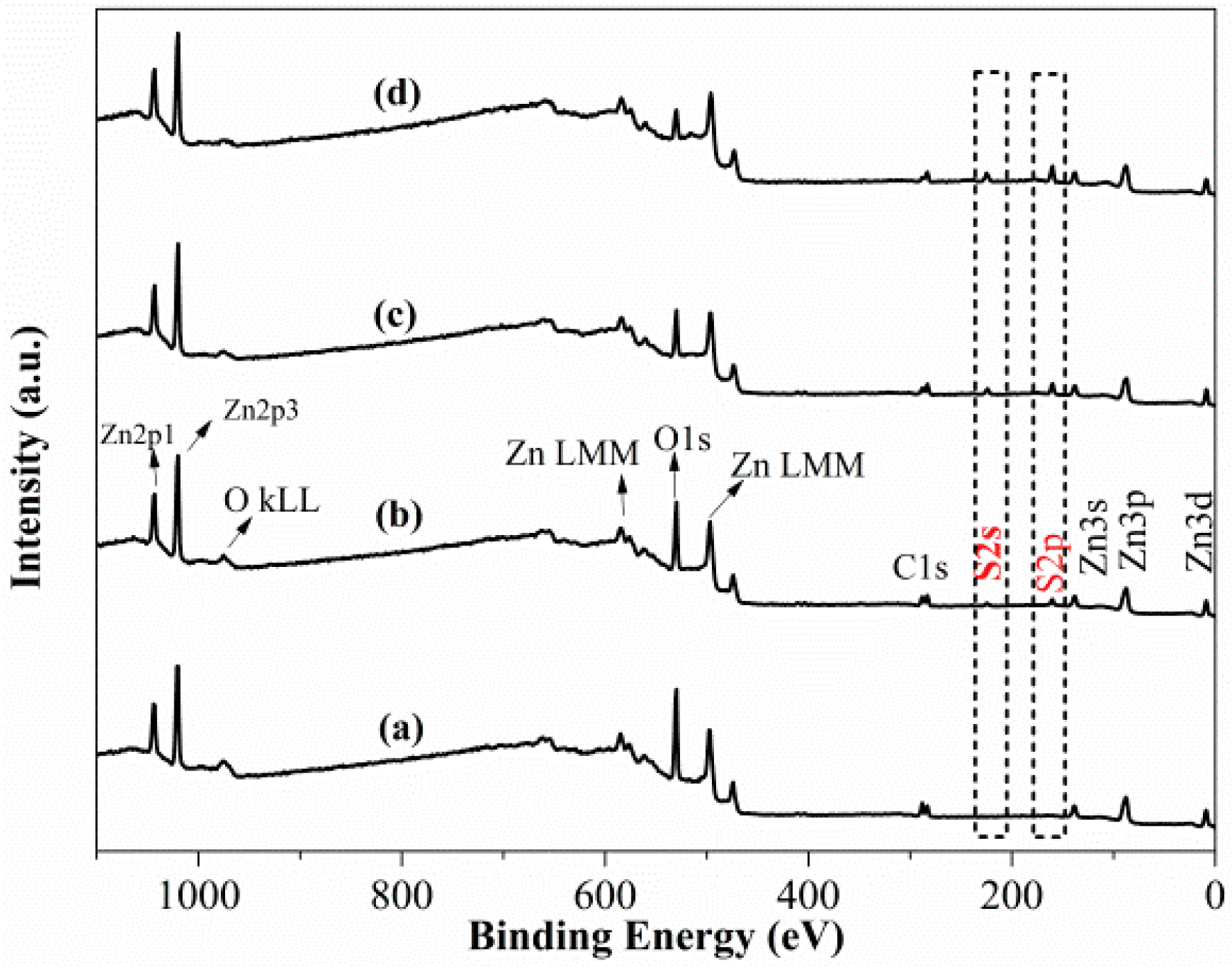
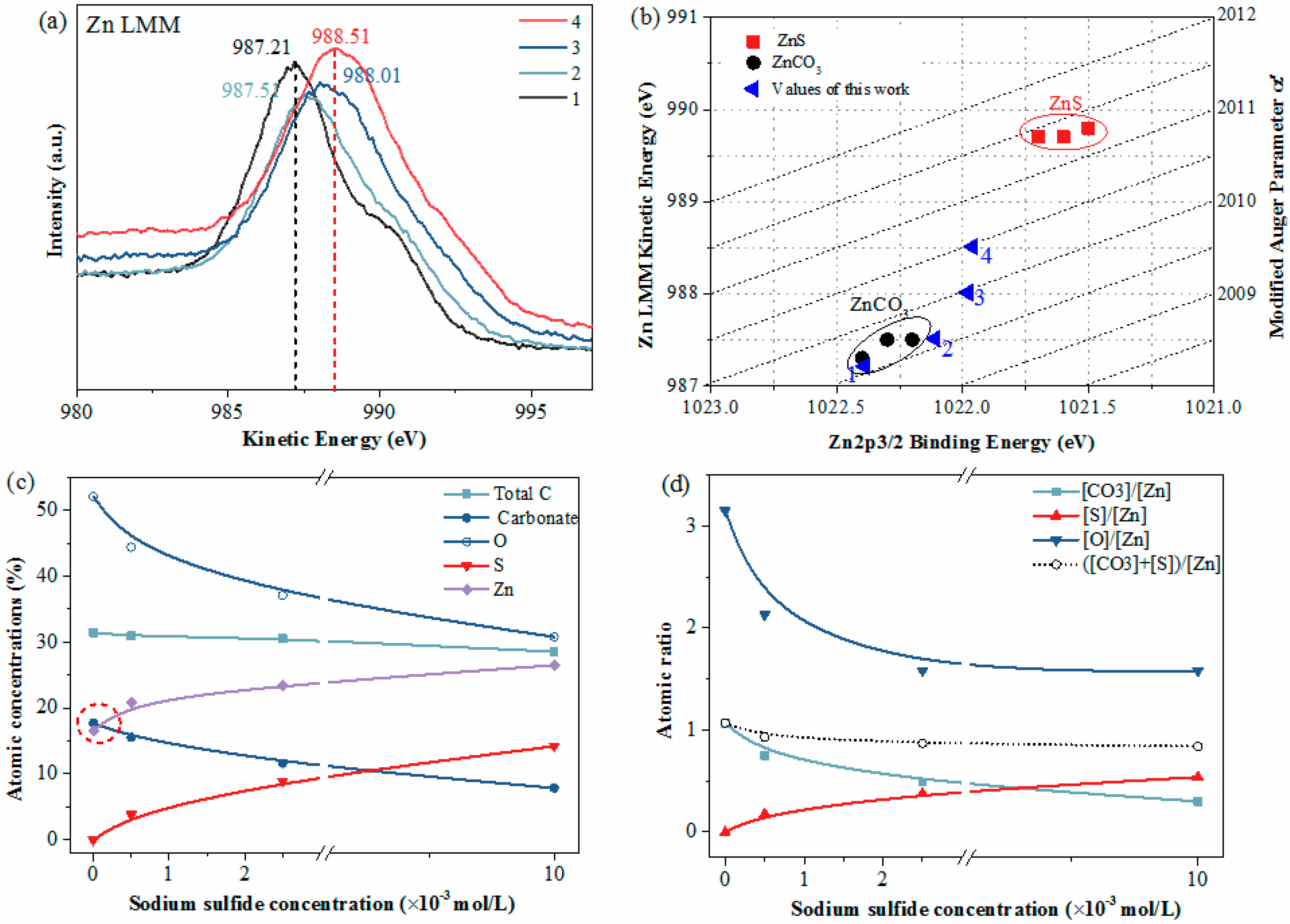
3.2. XPS Measurement
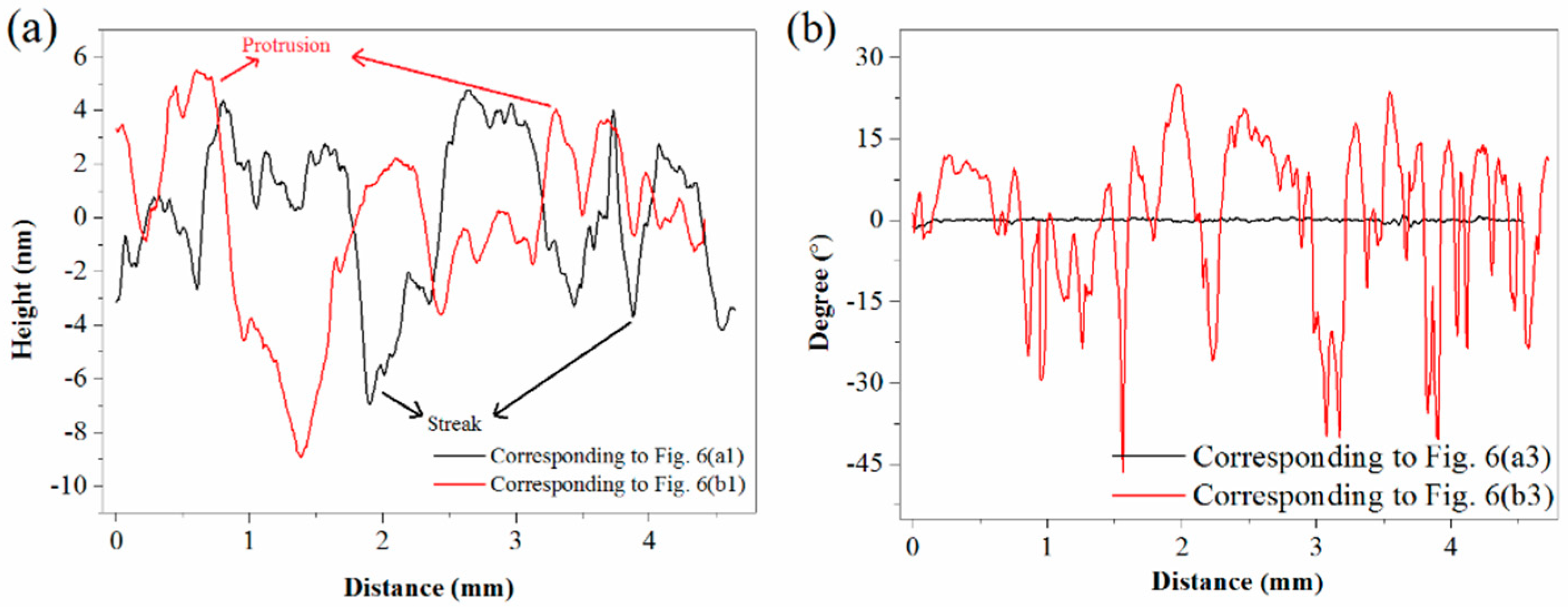
3.3. AFM Observation
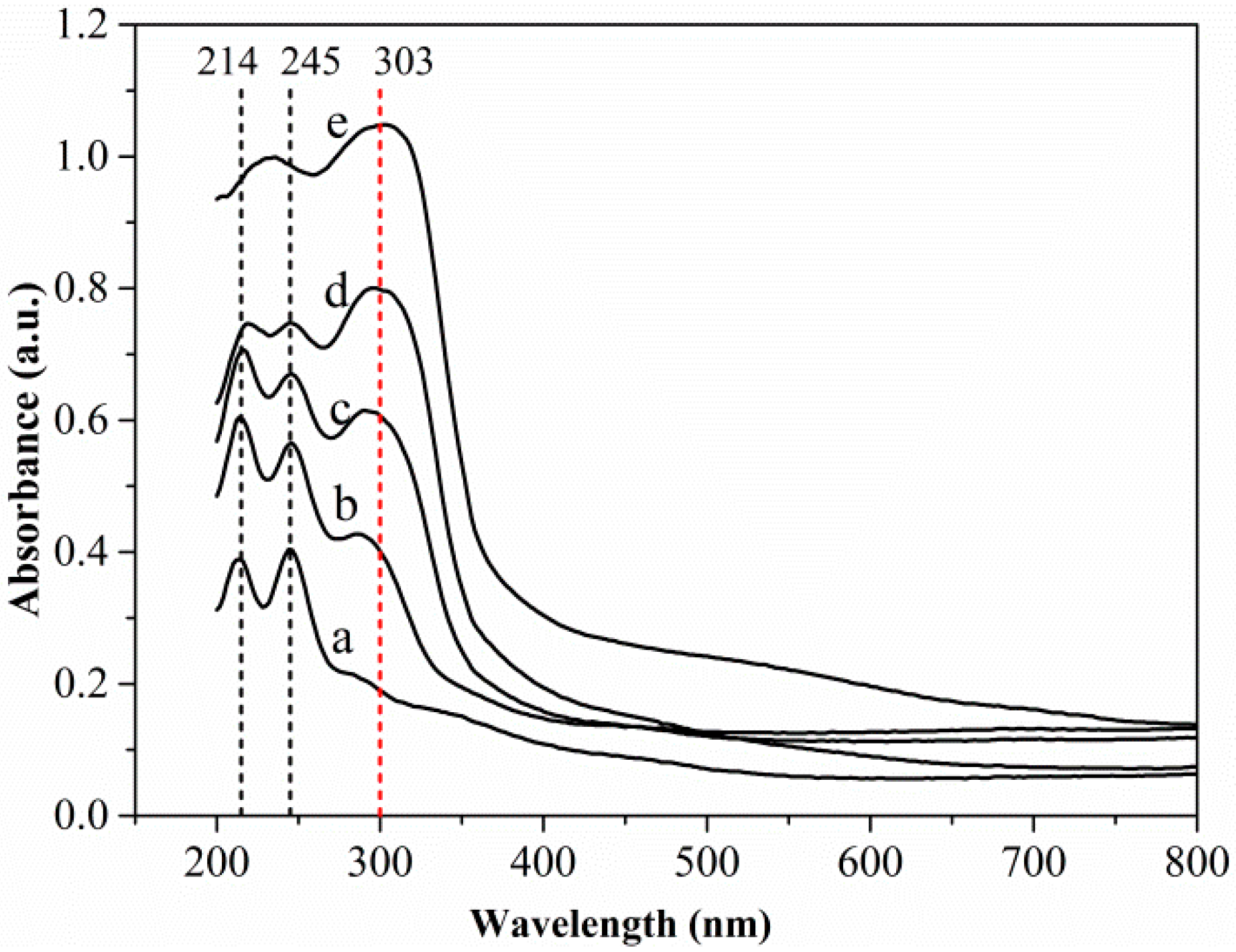
3.4. UV–Vis DRS Analysis
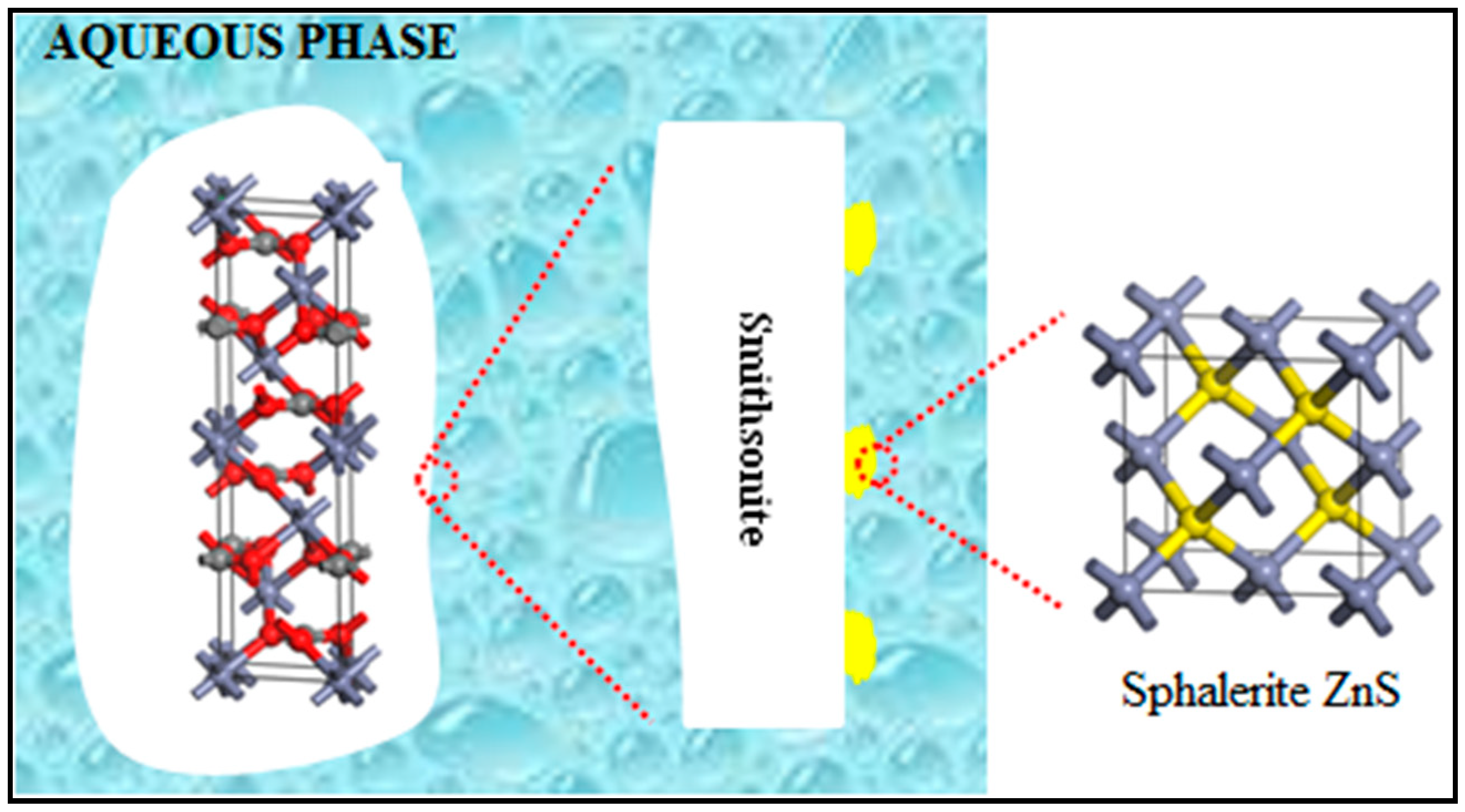
3.5. Sulfidization Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boni, M.; Mondillo, N. The “Calamines” and the “Others”: The great family of supergene nonsulfide zinc ores. Ore Geol. Rev. 2015, 67, 208–233. [Google Scholar] [CrossRef]
- Feng, Q.C.; Wen, S.M. Formation of zinc sulfide species on smithsonite surfaces and itsresponse to flotation performance. J. Alloys Compd. 2017, 709, 602–608. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Song, S.; Li, H.; Liu, Y. Flotation separation of smithsonite from calcite using 2-phosphonobutane-1,2,4-tricarboxylic acid as a depressant. Powder Technol. 2019, 352, 11–15. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, G.; Song, S.; Li, H. Flotation separation of smithsonite from quartz using calcium lignosulphonate as a depressant and sodium oleate as a collector. Miner. Eng. 2019, 131, 385–391. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef]
- Fa, K.Q.; Miller, J.D.; Jiang, T.; Li, G.H. Sulphidization flotation for recovery of lead and zinc from oxide-sulfide ores. Trans. Nonferr. Met. Soc. China 2005, 15, 1138–1144. [Google Scholar]
- Luo, B.; Liu, Q.; Deng, J.; Li, S.; Yu, L.; Lai, H. Determining the lead-sulfur species formed on smithsonite surfaces during lead-ion enhanced sulfidation processing. Appl. Surf. Sci. 2020, 506, 144628. [Google Scholar] [CrossRef]
- Jia, K.; Feng, Q.; Zhang, G.; Ji, W.; Zhang, G.-F.; Yang, B. The role of S(II) and Pb(II) in xanthate flotation of smithsonite: Surface properties and mechanism. Appl. Surf. Sci. 2018, 442, 92–100. [Google Scholar] [CrossRef]
- Deng, R.; Hu, Y.; Ku, J.; Zuo, W.; Yang, Z. Adsorption of Fe(III) on smithsonite surfaces and implications for flotation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 308–315. [Google Scholar] [CrossRef]
- Lai, H.; Deng, J.; Wen, S. Application of ToF-SIMS and PCA to study interaction mechanism of dodecylamine and smithsonite. Appl. Surf. Sci. 2019, 496, 143698. [Google Scholar] [CrossRef]
- Luo, B.; Liu, Q.; Deng, J.; Yu, L.; Lai, H.; Song, C.; Li, S. Characterization of sulfide film on smithsonite surface during sulfidation processing and its response to flotation performance. Powder Technol. 2019, 351, 144–152. [Google Scholar] [CrossRef]
- Garbassi, F.; Marabini, A.M. Spectroscopic investigation of sulphidation of zinc and lead carbonates. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1986, 82, 2043. [Google Scholar] [CrossRef]
- Wu, D.; Wen, S.; Deng, J.; Liu, J.; Mao, Y. Study on the sulfidation behavior of smithsonite. Appl. Surf. Sci. 2015, 329, 315–320. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, D.; Feng, Q.; Wen, S.; Chang, W. DFT insights into the electronic properties and adsorption mechanism of HS− on smithsonite (1 0 1) surface. Miner. Eng. 2019, 141, 105846. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, M.; Chen, J.; Li, Y.; Zhao, C.; Mu, X. A density functional based tight binding (DFTB+) study on the sulfidization-amine flotation mechanism of smithsonite. Appl. Surf. Sci. 2018, 458, 454–463. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, Y.; Ejtemaei, M.; Nguyen, A.V.; Wang, Y.; Wen, S. DFT simulation of S-species interaction with smithsonite (0 0 1) surface: Effect of water molecule adsorption position. Results Phys. 2019, 15, 102575. [Google Scholar] [CrossRef]
- Liu, R.Z.; Liu, D.W.; Li, J.L.; Liu, S.Y.; Liu, Z.C.; Gao, L.Q.; Jia, X.D.; Ao, S.F. Improved understanding of the sulfidization mechanism in cerussite flotation: An XPS, ToF-SIMS and FESEM investigation. Colloid. Surf. A 2020, 595, 124508. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, S.; Qin, L.; Wang, W.; Zhang, T.; Song, S.; Komarneni, S. Self-assembled gels of Fe-chitosan/montmorillonite nanosheets: Dye degradation by the synergistic effect of adsorption and photo-Fenton reaction. Chem. Eng. J. 2020, 379, 122322. [Google Scholar] [CrossRef]
- Frateur, I.; Lecoeur, J.; Zanna, S.; Olsson, C.; Landolt, D.; Marcus, P. Adsorption of BSA on passivated chromium studied by a flow-cell EQCM and XPS. Electrochim. Acta 2007, 52, 7660–7669. [Google Scholar] [CrossRef]
- Peng, W.; Han, G.; Cao, Y.; Sun, K.; Song, S. Efficiently removing Pb(II) from wastewater by graphene oxide using foam flotation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 266–272. [Google Scholar] [CrossRef]
- Available online: https://xpssimplified.com/elements/zinc.php (accessed on 29 March 2020).
- Buckley, A.; Wouterlood, H.; Woods, R. The surface composition of natural sphalerites under oxidative leaching conditions. Hydrometallurgy 1989, 22, 39–56. [Google Scholar] [CrossRef]
- Deroubaix, G.; Marcus, P. X-ray photoelectron spectroscopy analysis of copper and zinc oxides and sulphides. Surf. Interface Anal. 1992, 18, 39–46. [Google Scholar] [CrossRef]
- Laajalehto, K.; Kartio, I.; Nowak, P. XPS study of clean metal sulfide surfaces. Appl. Surf. Sci. 1994, 81, 11–15. [Google Scholar] [CrossRef]
- Lai, H.; Deng, J.; Wen, S.; Liu, Q. Elucidation of lead ions adsorption mechanism on marmatite surface by PCA-assisted ToF-SIMS, XPS and zeta potential. Miner. Eng. 2019, 144, 106035. [Google Scholar] [CrossRef]
- Dake, L.S.; Baer, D.R.; Zachara, J.M. Auger parameter measurements of zinc compounds relevant to zinc transport in the environment. Surf. Interface Anal. 1989, 14, 71–75. [Google Scholar] [CrossRef]
- Jovanov, V.; Yumnam, N.; Müller, A.; Gruber, M.; Wagner, V. Determining Material-Specific Morphology of Bulk-Heterojunction Organic Solar Cells Using AFM Phase Imaging. J. Phys. Chem. C 2017, 121, 9173–9180. [Google Scholar] [CrossRef]
- O’Nei, K.D.; Semenikhin, O.A.; O’Neil, K. AFM Phase Imaging of Electropolymerized Polybithiophene Films at Different Stages of Their Growth. J. Phys. Chem. C 2007, 111, 14823–14832. [Google Scholar] [CrossRef]
- Rossel, R.A.V.; McGlynn, R.; McBratney, A.B. Determining the composition of mineral-organic mixes using UV–vis–NIR diffuse reflectance spectroscopy. Geoderma 2006, 137, 70–82. [Google Scholar] [CrossRef]
- Zanjanchi, M.A.; Noei, H.; Moghimi, M. Rapid determination of aluminum by UV–vis diffuse reflectance spectroscopy with application of suitable adsorbents. Talanta 2006, 70, 933–939. [Google Scholar] [CrossRef]
- Banerjee, I.A.; Yu, L.; Matsui, H. Room-Temperature Wurtzite ZnS Nanocrystal Growth on Zn Finger-like Peptide Nanotubes by Controlling Their Unfolding Peptide Structures. J. Am. Chem. Soc. 2005, 127, 16002–16003. [Google Scholar] [CrossRef]
- Liang, X.; Xing, L.; Xiang, J.; Zhang, F.; Jiao, J.; Cui, L.; Song, B.; Chen, S.; Zhao, C.; Sai, H. The Role of the Liquid–Liquid Interface in the Synthesis of Nonequilibrium Crystalline Wurtzite ZnS at Room Temperature. Cryst. Growth Des. 2012, 12, 1173–1179. [Google Scholar] [CrossRef]
- Badawi, M.; Cristol, S.; Paul, J.-F.; Payen, E. DFT study of furan adsorption over stable molybdenum sulfide catalyst under HDO conditions. ComptesRendusChim. 2009, 12, 754–761. [Google Scholar] [CrossRef]
- Badawi, M.; Paul, J.; Cristol, S.; Payen, E.; Romero, Y.; Richard, F.; Brunet, S.; Lambert, D.; Portier, X.; Popov, A.; et al. Effect of water on the stability of Mo and CoMo hydrodeoxygenation catalysts: A combined experimental and DFT study. J. Catal. 2011, 282, 155–164. [Google Scholar] [CrossRef]
- Foucaud, Y.; Badawi, M.; Filippov, L.O.; Filippova, I.V.; Lébegue, S. Surface Properties of Fluorite in Presence of Water: An Atomistic Investigation. J. Phys. Chem. B 2018, 122, 6829–6836. [Google Scholar] [CrossRef]
- Bandura, A.V.; Kubicki, J.D.; Sofo, J. Periodic Density Functional Theory Study of Water Adsorption on the α-Quartz (101) Surface. J. Phys. Chem. C 2011, 115, 5756–5766. [Google Scholar] [CrossRef]
- Souvi, S.M.O.; Badawi, M.; Virot, F.; Cristol, S.; Cantrel, L.; Paul, J.-F. Influence of water, dihydrogen and dioxygen on the stability of the Cr2O3 surface: A first-principles investigation. Surf. Sci. 2017, 666, 44–52. [Google Scholar] [CrossRef]
- Foucaud, Y.; Badawi, M.; Filippov, L.; Filippova, I.; Lébegue, S. A review of atomistic simulation methods for surface physical-chemistry phenomena applied to froth flotation. Miner. Eng. 2019, 143, 106020. [Google Scholar] [CrossRef]
- Foucaud, Y.; Lebègue, S.; Filippov, L.O.; Filippova, I.V.; Badawi, M. Molecular Insight into Fatty Acid Adsorption on Bare and Hydrated (111) Fluorite Surface. J. Phys. Chem. B 2018, 122, 12403–12410. [Google Scholar] [CrossRef]
- Foucaud, Y.; Badawi, M.; Filippov, L.O.; Barres, O.; Filippova, I.V.; Lébegue, S. Synergistic adsorptions of Na2CO3 and Na2SiO3 on calcium minerals revealed by spectroscopic and ab initio molecular dynamics studies. Chem. Sci. 2019, 10, 9928–9940. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Pei, B.; Liu, Z.; Wang, Y.; Li, J.; Liu, D. Improved Understanding of the Sulfidization Mechanism in Amine Flotation of Smithsonite: An XPS, AFM and UV–Vis DRS Study. Minerals 2020, 10, 370. https://doi.org/10.3390/min10040370
Liu R, Pei B, Liu Z, Wang Y, Li J, Liu D. Improved Understanding of the Sulfidization Mechanism in Amine Flotation of Smithsonite: An XPS, AFM and UV–Vis DRS Study. Minerals. 2020; 10(4):370. https://doi.org/10.3390/min10040370
Chicago/Turabian StyleLiu, Ruizeng, Bin Pei, Zhicheng Liu, Yunwei Wang, Jialei Li, and Dianwen Liu. 2020. "Improved Understanding of the Sulfidization Mechanism in Amine Flotation of Smithsonite: An XPS, AFM and UV–Vis DRS Study" Minerals 10, no. 4: 370. https://doi.org/10.3390/min10040370
APA StyleLiu, R., Pei, B., Liu, Z., Wang, Y., Li, J., & Liu, D. (2020). Improved Understanding of the Sulfidization Mechanism in Amine Flotation of Smithsonite: An XPS, AFM and UV–Vis DRS Study. Minerals, 10(4), 370. https://doi.org/10.3390/min10040370





