Grain Size Distribution and Clay Mineral Distinction of Rare Earth Ore through Different Methods
Abstract
1. Introduction
2. Background
2.1. Study Site
2.2. Regional Stratigraphy and Lithology
3. Materials and Methods
3.1. Sample Collection
3.2. Simulated Leaching Experiment
3.3. Particle size Analysis
3.4. Clay Mineral Analysis
4. Results
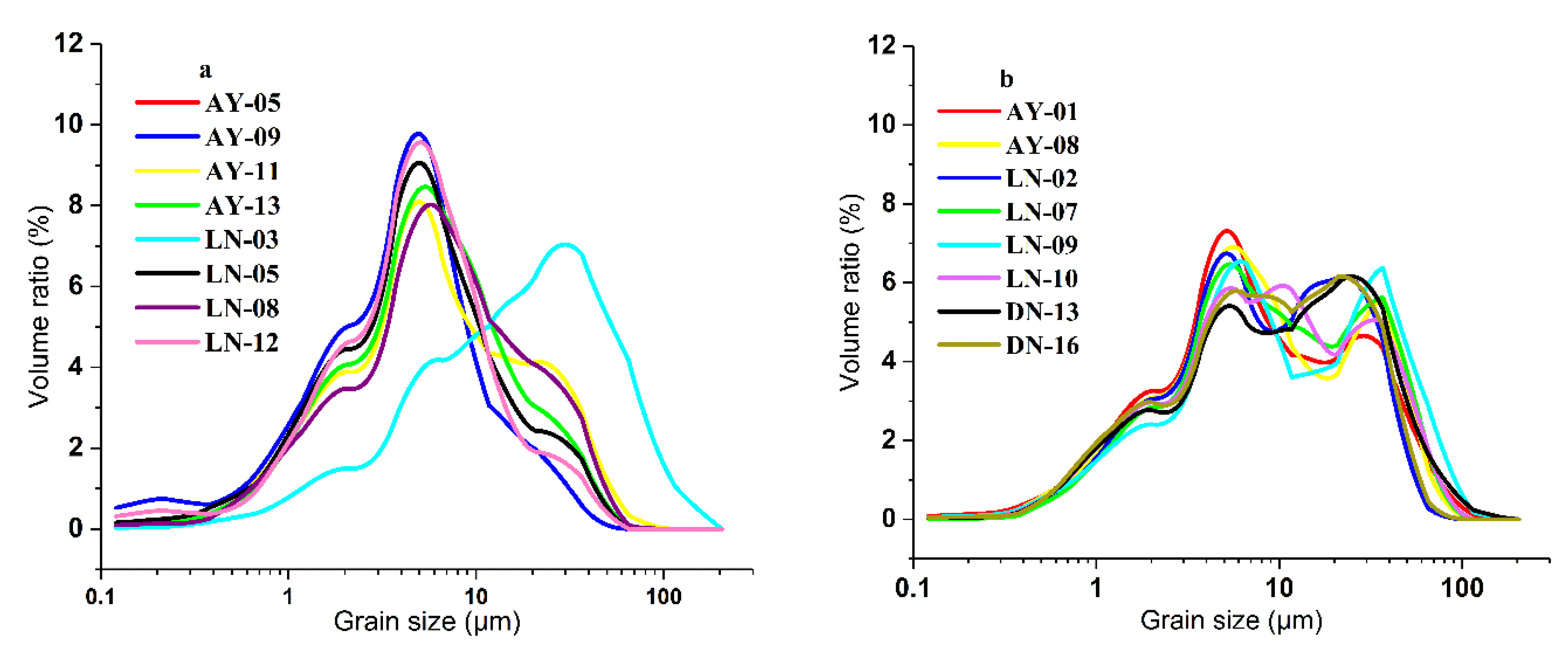
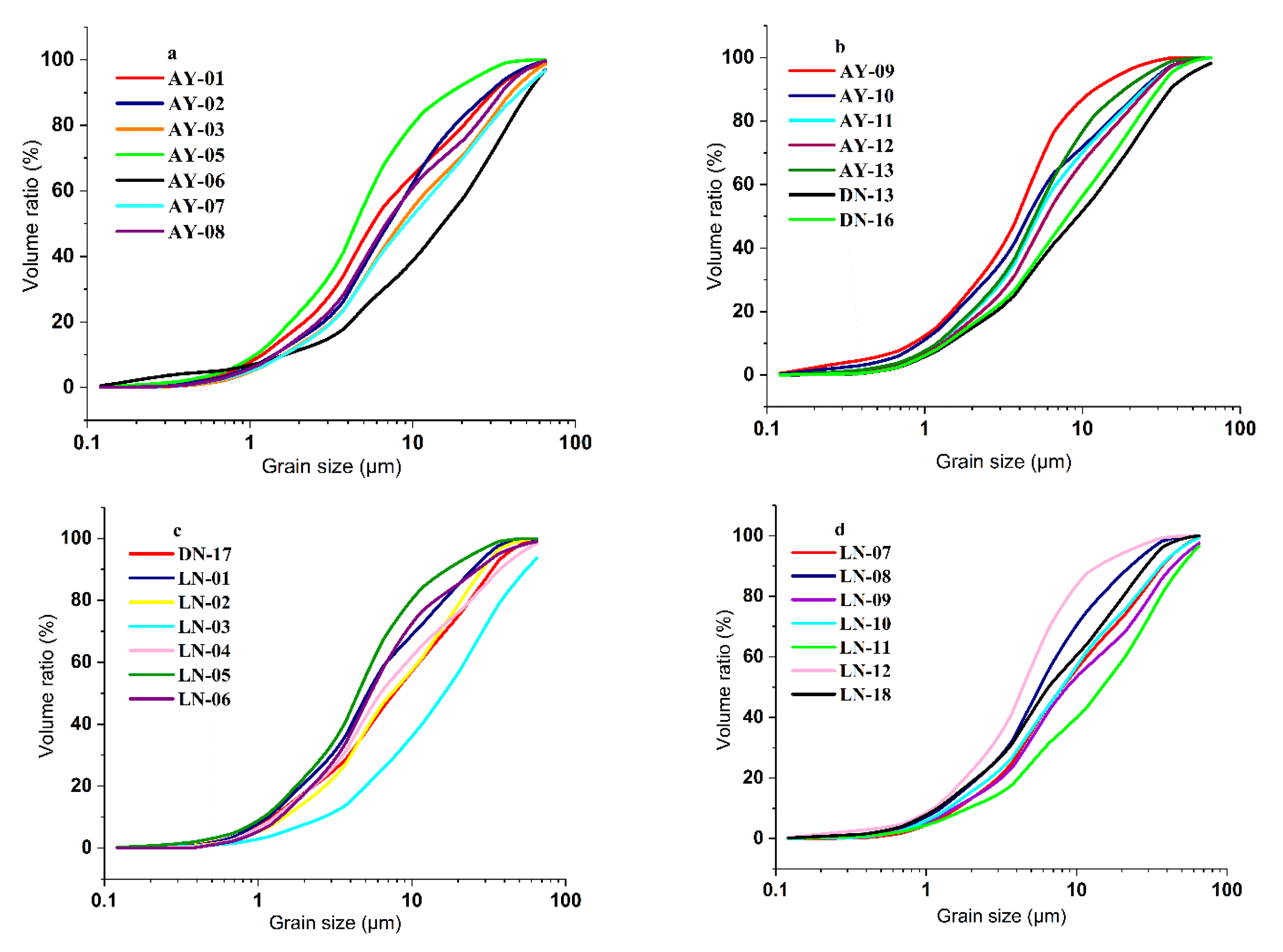
4.1. Particle Size Analysis
4.2. Regional Clay Mineral Analysis
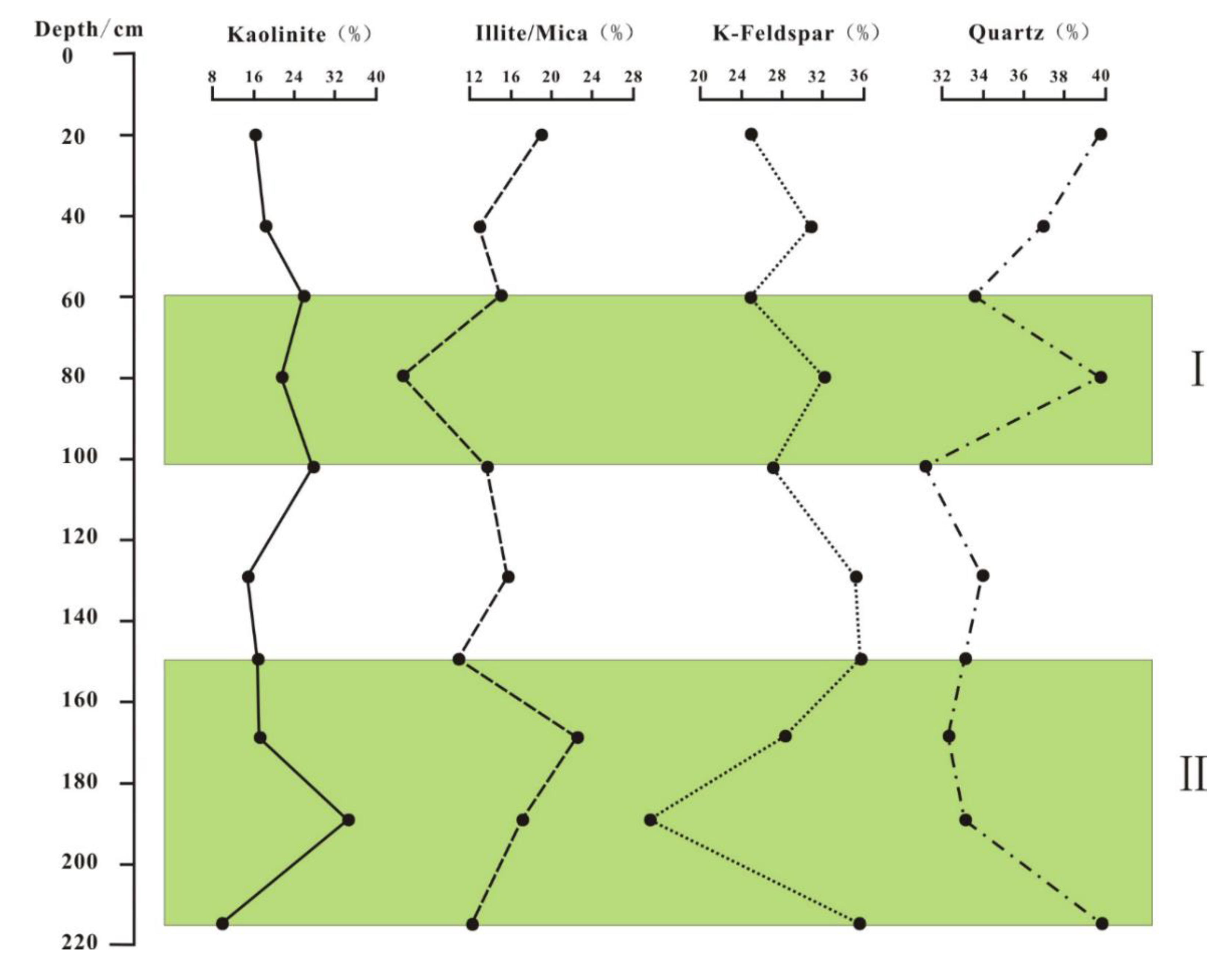
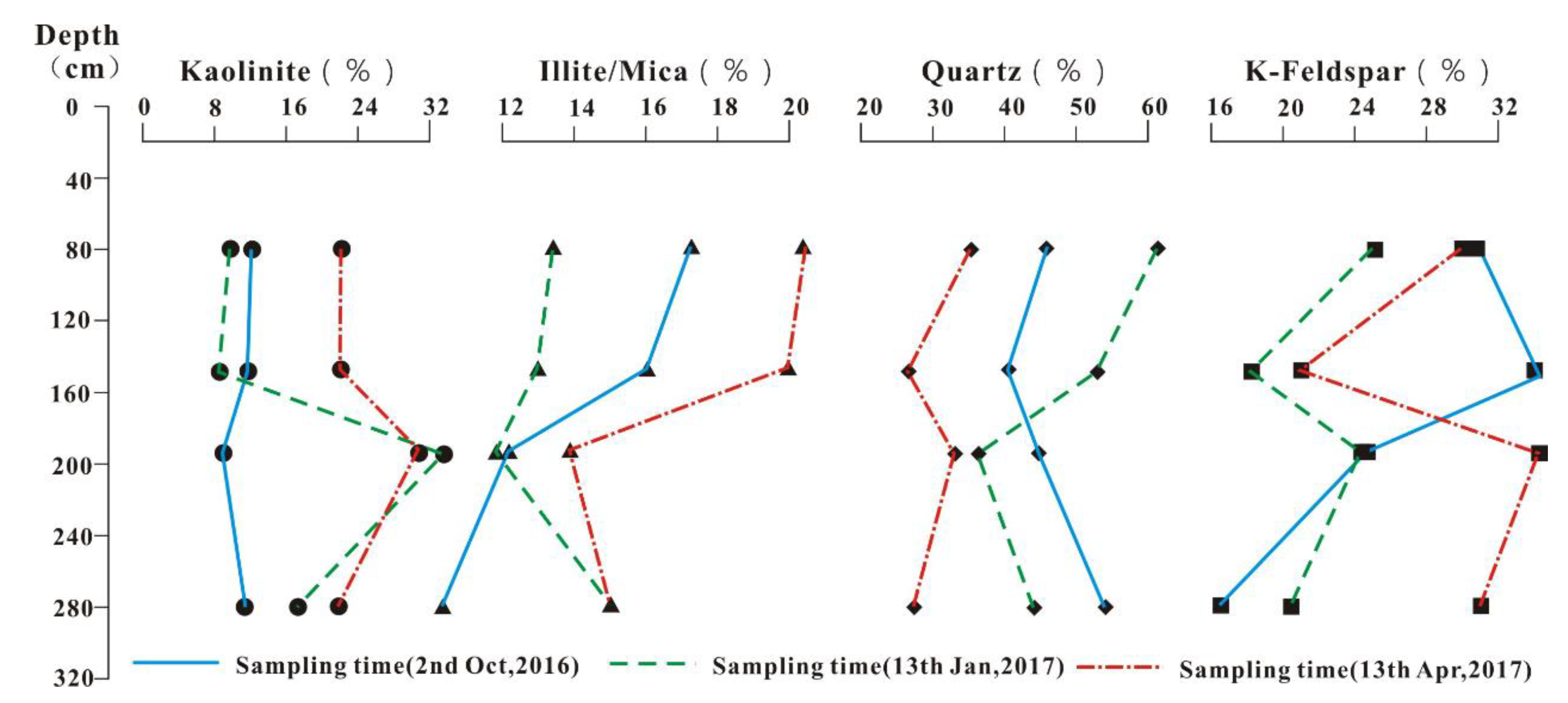
4.3. Analysis of Clay Minerals in the in Situ Leaching Profile
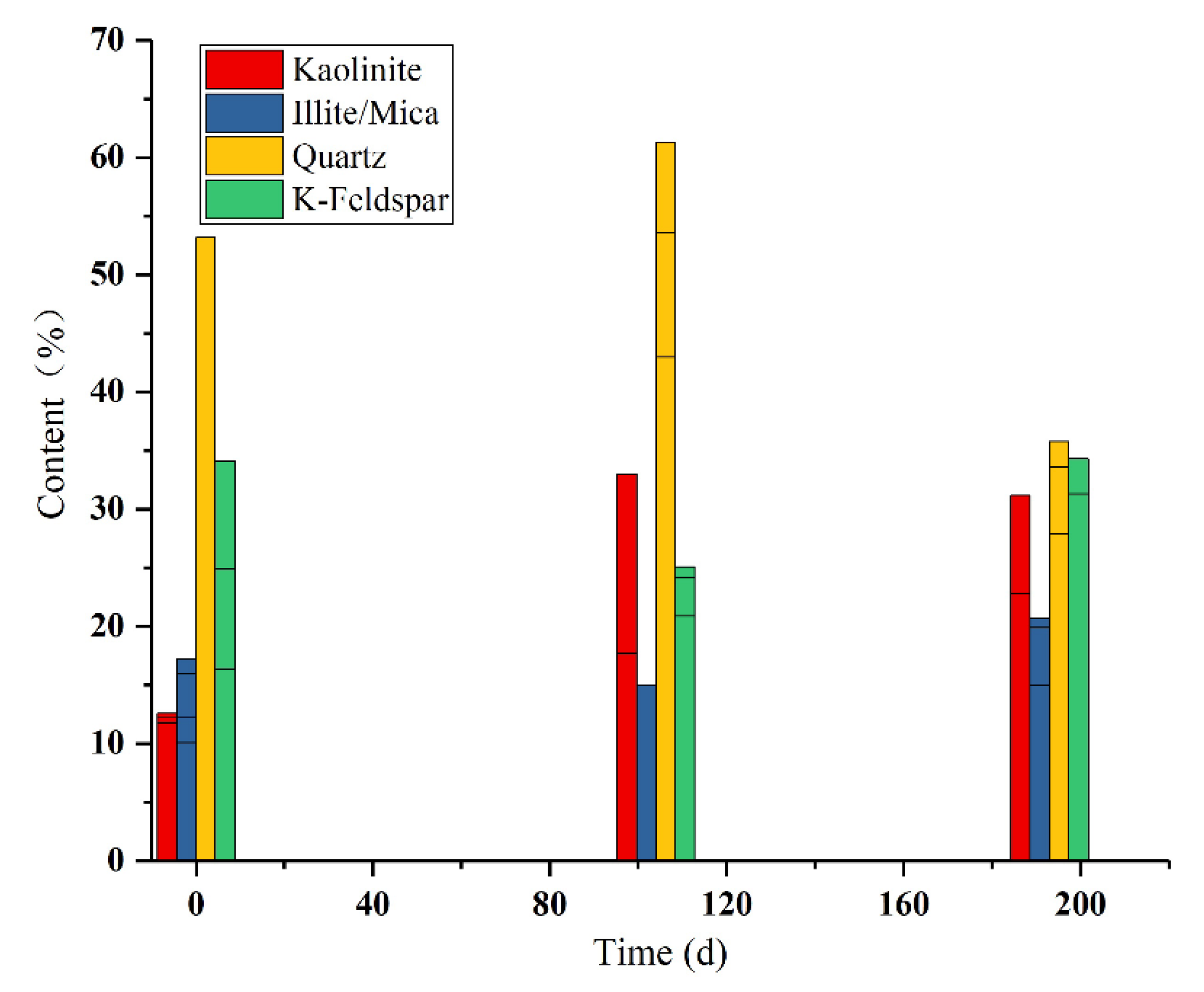
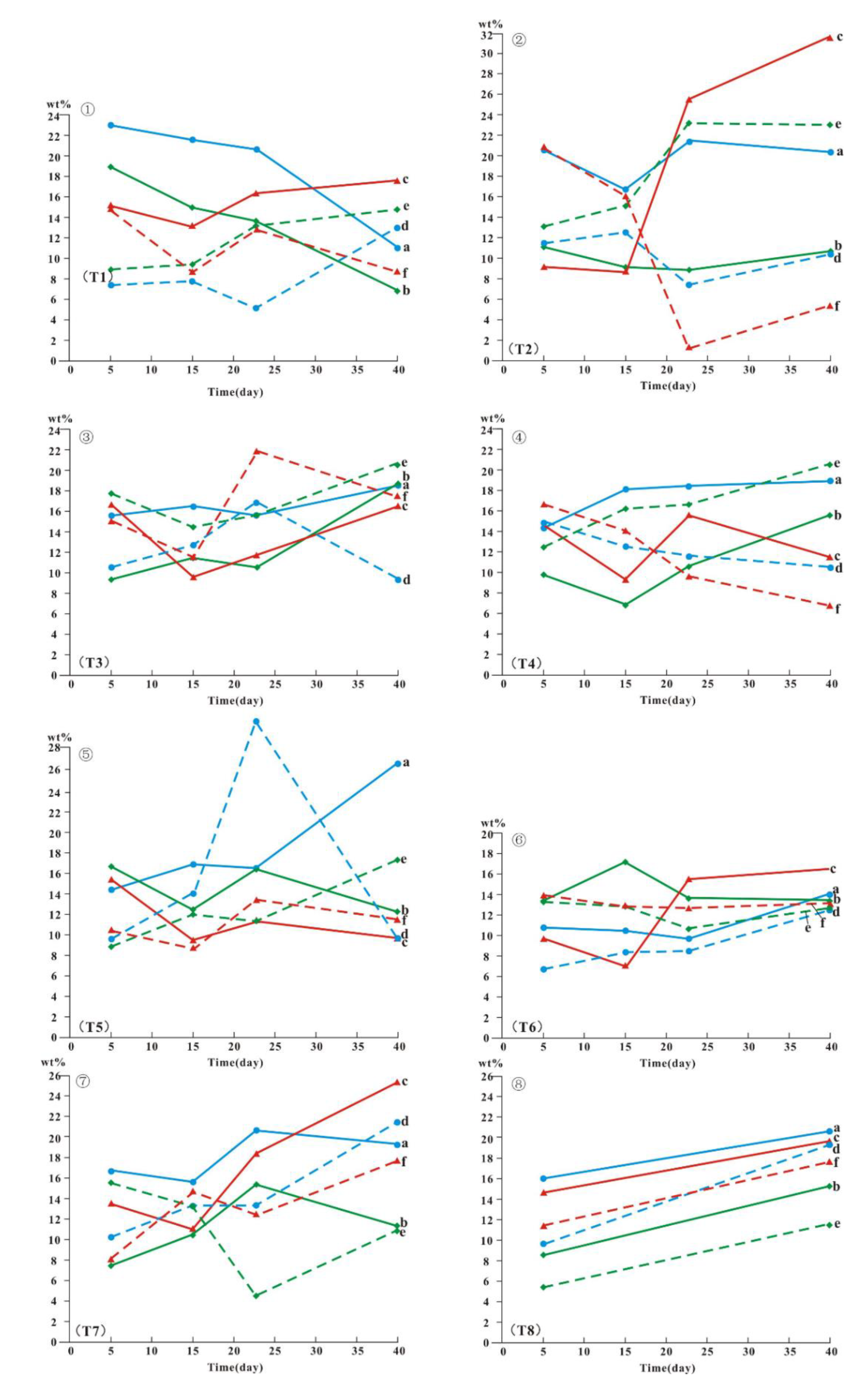
4.4. Clay Minerals in the Simulated Leaching Profile
5. Discussion
5.1. Soil Particle Size and Distribution of Clay Minerals in REE Mining Areas
5.2. Vertical Variation of Clay Minerals in REE Ores
5.3. Simulating Migration of Clay Minerals during Leaching
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Y.Q.; Hu, C.S.; Luo, Z.M. Geological characteristic of mineralization of rare earth deposit of the ion-absorption type and their prospecting direction. Bull. Chin. Acad. Geol. Sci. 1981, 2, 102–118, (In Chinese with English abstract). [Google Scholar]
- Chen, D.Q.; Wu, J.S. The mineralization mechanism of ion-adsorbed REE deposit. J. Chin. Rare Earth Soc. 1990, 8, 175–179, (In Chinese with English abstract). [Google Scholar]
- Chi, R.A.; Tian, J. Review of weathered crust rare earth ore. J. Chin. Rare Earth Soc. 2007, 25, 641–650, (In Chinese with English abstract). [Google Scholar]
- Harlavan, Y.; Erel, Y. The release of Pb and REE from granitoids by the dissolution of accessory phases. Geochim. Cosmochim. Acta. 2002, 66, 837–848. [Google Scholar] [CrossRef]
- Galan, E.; Fernandez-Caliani, J.C.; Miras, A.; Aparicio, P.; Marquez, M.G. Residence and fractionation of rare earth elements during kaolinization of alkaline peraluminous granites in NW Spain. Clay Miner. 2007, 42, 341–352. [Google Scholar] [CrossRef]
- Sanematsu, K.; Murakami, H.; Watanabe, Y.; Duangsurigna, S.; Vilayhack, S. Enrichment of rare earth elements (REE) in granitic rocksand their weathered crusts in central and southern Laos. Bull. Geol. Surv. Jpn. 2009, 60, 527–558. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, D.H.; Chen, Z.H.; Chen, Z.Y. Progress of Research on Metallogenic Regularity of Ion-adsorption Type REE Deposit in the Nanling Range. Acta Geol. Sin. 2017, 91, 2814–2827. [Google Scholar]
- Bao, Z.W.; Zhao, Z.H. Geochemistry of Mineralization with Exchangeable REY in the Weathering Crusts of Granitic Rocks in South China. Ore Geol. Rev. 2008, 33, 519–535. [Google Scholar] [CrossRef]
- Fan, C.Z.; Zhang, Y.; Chen, Z.H.; Zhu, Y.; Fan, X.T. The study of clay minerals from weathered crust rare earth ores in southern Jiangxi Province. Acta Petrol Mineral. 2015, 34, 803–810, (In Chinese with English abstract). [Google Scholar]
- Coppin, F.; Berger, G.; Bauer, A.; Castet, S.; Loubet, M. Sorption of lanthanides on smectite and kaolinite. Chem. Geol. 2002, 182, 57–68. [Google Scholar] [CrossRef]
- Brasbury, M.H.; Baeyens, B. Sorption of Eu on Na- and Ca-montmorillonites: Experimental investigations and modelling with cation exchange and surface complexation. Geochim. Cosmochim. Acta 2002, 66, 2325–2334. [Google Scholar] [CrossRef]
- Chi, R.A.; Tian, J.; Luo, X.P.; Xu, Z.G.; He, Z.Y. The basic research on the weathered crust elution-deposited rare earth ores. Nonferrous Met. Sci. Eng. 2012, 3, 1–13, (In Chinese with English abstract). [Google Scholar]
- Wilson, M.J. The origin and formation of clay minerals in soils pat, present and future perspectives. Clay Miner. 1999, 34, 7–25. [Google Scholar] [CrossRef]
- Meunier, A.; Velde, B. The Geology of Illite; Verlag Berlin/Heidelberg, Springer: Berlin/Heidelberg, Germany, 2004; pp. 63–143. [Google Scholar]
- Zhang, Z.Y.; Huang, L.; Liu, F.; Wang, M.K.; Fu, Q.L.; Zhu, J. Characteristics of clay minerals in soil particles of two Alfisols in China. Appl. Clay Sci. 2016, 120, 51–60. [Google Scholar] [CrossRef]
- Cudahy, T.; Caccett, M.; Thomas, M.; Hewson, R.; Abrams, M.; Kato, M.; Kashimura, O.; Ninomiya, Y.; Yamaguchi, Y.; Collings, S.; et al. Satellite-derived mineral mapping and monitoring of weathering, deposition and erosion. Sci. Rep. 2016, 6, 23702. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, G.A.; Papangelakis, V.G. An overview of rare-earth recovery by ion-exchange leaching from ion-adsorption clays of various origins. Mineral. Mag. 2016, 80, 63–76. [Google Scholar] [CrossRef]
- Ishihara, S.; Hua, R.; Hoshino, M.; Murakami, H. REE abundance and REE minerals in granitic rocks in the Nanling range, Jiangxi province, Southern China, and generation of the REE-rich weathered crust deposits. Resour. Geol. 2008, 58, 355–372. [Google Scholar] [CrossRef]
- Wang, X.; Lei, Y.; Ge, J.; Wu, S. Production forecast of China’s rare earths based on the generalized Weng model and policy recommendations. Resour. Pol. 2015, 43, 11–18. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Russell, M.; Orbell, G.E. Weathering sequence of soils from volcanic ash involving allophane and halloysite, New Zealand. Geoderma 1983, 29, 41–57. [Google Scholar] [CrossRef]
- Botero, Y.L.; López-Rendón, J.E.; Ramírez, D.; Zapata, D.M.; Jaramillo, F. From Clay Minerals to Al2O3 Nanoparticles: Synthesis and Colloidal Stabilization for Optoelectronic Applications. Minerals 2020, 10, 118. [Google Scholar] [CrossRef]
- Tian, J.; Yin, J.Q.; Chi, R.A.; Rao, G.H.; Jiang, M.T.; Ouyang, K.X. Kinetics on leaching rare earth from the weathered crust elution-deposited rare earth ores with ammonium sulfate solution. Hydrometallurgy 2010, 101, 166–170. [Google Scholar]
- Wu, D.R.; Wu, A.Q.; He, X.Y.; Li, M. Trends of Extreme Climate in South Jiangxi from 1956 to 2013. J. Yangtze River Sci. Res. Inst. 2017, 34, 24–29. [Google Scholar]
- Jiangxi Bureau of Geological and Mineral Resources. Regional Geology of Jiangxi Province; Geological Publishing House: Beijing, China, 1984; pp. 1–308.
- Jiangxi Bureau of Geological and Mineral Exploration and Development. Drawing Instructions of Digital Geological Map of Jiangxi Province; Geological Publishing House: Beijing, China, 1998; pp. 1–80.
- Zhao, Z.; Wang, D.H.; Chen, Z.Y.; Chen, Z.H.; Zheng, G.D.; Liu, X.X. Zircon U-Pb Age, Endogenic Mineralization and Petrogenesis of Rare Earth Ore-bearing Granite in Longnan, Jiangxi Province. Acta Geosci. Sin. 2014, 35, 719–725, (In Chinese with English abstract). [Google Scholar]
- Liu, C.Y.; Liu, J.H.; Zhong, L.X.; Xu, S.; Chen, L.K. Soil particle size distribution characteristics of ionic rare earth: A case study in rare earth mine of Jiangwozi in Gan County. Nonferrous Met. Sci. Eng. 2017, 8, 125–130, (In Chinese with English abstract). [Google Scholar]
- Hu, X.P.; Shi, Y.X.; Dai, X.R.; Wang, J.T.; Liu, B.; Wang, N.Y. Characteristics and source of city wall’s earth in the Neolithic Liangzhu City based on XRD analyses of clay minerals. Acta. Petrol. Mineral. 2013, 32, 373–382. [Google Scholar]
- Mukasa-Tebandeke, I.Z.; Ssebuwufu, P.J.M.; Nyanzi, S.A.; Schumann, A.; Nyakairu, G.W.A.; Ntale, M.; Lugolobi, F. The Elemental, Mineralogical, IR, DTA and XRD Analyses Characterized Clays and Clay Minerals of Central and Eastern Uganda. Adv. Mater. Phys. Chem. 2015, 5, 67–86. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Gi, Y.J. Formation of Vermicular Kaolinite from Halloysite Aggregates in the Weathering of Plagioclase. Clays Clay Miner. 1998, 46, 270–279. [Google Scholar]
- Banfield, J.F. The mineralogy and chemistry of granite weathering. Master’s Thesis, Australian National University, Canberra, Australia, 1985; 130p. [Google Scholar]
- Banfield, J.E.; Eggleton, R.A. Analytical transmission electron microscope studies of plagiodase, muscovite, and Potassium feldspar weathering. Clays Clay Miner. 1990, 38, 77–89. [Google Scholar] [CrossRef]
- Yang, Z.M. A study on clay minerals from the REE-rich weathered crust developed on the Longnan granite in Jiangxi. Sci. Geo. Sin. 1987, 1, 70–81, (In Chinese with English abstract). [Google Scholar]
- Righi, D.; Meunier, A. Origin of Clays by Rock Weathering and Soil Formation; Springer: Berlin/Heidelberg, Germany, 1995; pp. 1–119. [Google Scholar]
- Wu, K.X.; Zang, L.; Zhu, P.; Chen, L.K.; Tian, Z.F.; Xin, X.Y. Research on Particle Size Distribution and Its Variation of Ion-adsorption Type Rare Earth Ore. Chin. Rare Earths. 2016, 37, 67–74, (In Chinese with English abstract). [Google Scholar]
- Aberg, B. Void ratio of noncohesive soils and similar materials. J. Geotech Eng. 1992, 118, 1315–1334. [Google Scholar] [CrossRef]
- Yan, J.B.; Wu, K.X.; Liu, H.; Liao, C.L.; Jing, X.W.; Zhu, P.; Ouyang, H. Patterns of particle size distribution and genesis of Ion-adsorption REE Ore-exemplified by Dabu REE Ore Deposit, Ganxian County, Southern Jiangxi Province. Chin. Rare Earths 2018, 36, 372–384, (In Chinese with English abstract). [Google Scholar]
- Wang, Z.; Zhao, Z.; Zou, X.Y.; Chen, Z.Y.; Tu, X.J. Petrogeochemical Characteristics and Metallogenetic Potential of Epimetamorphic Rocks in South Jiangxi Province. Rock Miner. Anal. 2018, 37, 96–107, (In Chinese with English abstract). [Google Scholar]
- Zhang, L.; Wu, K.X.; Chen, L.K.; Zhu, P.; Ouyang, H. Overview of Metallogenic Features of Ion-adsorption Type REE Deposits in Southern Jiangxi Province. J. Chin. Soc. Rare Earth. 2015, 33, 10–17, (In Chinese with English abstract). [Google Scholar]
- Zhang, Z.H. A Study on weathering crust ion adsorption type REE deposits, south China. Contrib. Geol. Miner. Resour. Res. 1990, 5, 57–71, (In Chinese with English abstract). [Google Scholar]
- Goldberg, K.; Humayun, M. The applicability of the Chemical Index of Alteration as a paleoclimatic indicator: An example from the Permian of the Paraná Basin, Brazil. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 293, 175–183. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar] [CrossRef]
- Samotoin, N.D.; Bortnikov, N.S. Formation of Kaolinite Nanoand Microcrystals by Weathering of Phyllosilicates. Geol. Ore Depos. 2011, 53, 340–352. [Google Scholar] [CrossRef]
- Oliveira, D.P.; Sartor, L.R.; Souza Júnior, V.S.; Corrêa, M.M.; Romero, R.E.; Andrade, G.R.P.; Ferreira, T.O. Weathering and clay formation in semi-arid calcareous soils from Northeastern Brazil. Catena 2018, 162, 325–332. [Google Scholar] [CrossRef]
- Uzarowicz, Ł.; Skiba, S.; Skiba, M.; Segvic, B. Clay-mineral Formation in Soils Developed in the Weathering Zone of Pyrite-Bearing Schists: A Case Study from the Abandoned Pyrite Mine in Wieściszowice, Lower Silesia, SW Poland. Clays Clay Miner. 2011, 6, 581–594. [Google Scholar] [CrossRef]
- Liu, S.K.; Han, C.; Liu, J.M.; Li, H. Hydrothermal decomposition of potassium feldspar under alkaline conditions. RSC Adv. 2015, 5, 93301–93309. [Google Scholar] [CrossRef]
- Dixon, J.B.; Weed, S.B.; Parpitt, R.L. Minerals in Soil Environments. Soil Sci. 1989, 150, 283–304. [Google Scholar]
- Yuan, G.H.; Cao, Y.C.; Schulz, H.M.; Hao, F.; Gluyas, J.; Liu, K.; Yang, T.; Wang, Y.Z.; Xi, K.L.; Li, F.L. A review of feldspar alteration and its geological significance in sedimentary basins: From shallow aquifers to deep hydrocarbon reservoirs. Earth-Sci. Rev. 2019, 191, 114–140. [Google Scholar] [CrossRef]
- Liu, J.H.; Chen, L.K.; Liu, C.Y.; Qiu, L.R.; He, S. Pb speciation in rare earth minerals and use of entropy and fuzzy clustering methods to assess the migration capacity of Pb during mining activities. Ecotox. Environ. Safe. 2018, 165, 334–342. [Google Scholar]
- Cuevas, J.; Leguey, S.; Garralón, A.; Rastrero, M.R.; Procopio, J.R.; Sevilla, M.T.; Jiménez, N.S.; Abad, R.R.; Garrido, A. Behavior of kaolinite and illite-based clays as landfill barriers. Appl. Clay Sci. 2009, 42, 497–509. [Google Scholar] [CrossRef]
- Tian, J.; Yin, J.Q.; Chen, K.H.; Rao, G.H.; Jiang, M.T.; Chi, R.A. Extraction of rare earths from the leach liquor of the weathered crust elution-deposited rare earth ore with non-precipitation. Int. J. Miner. Process. 2011, 98, 125–131. [Google Scholar]
- Xiao, Y.; Feng, Z.; Huang, X.; Huang, L.; Chen, Y.; Wang, L.; Long, Z. Recovery of rare earths from weathered crust elution-deposited rare earth ore without ammonia-nitrogen pollution: I. Leaching with magnesium sulfate. Hydrometallurgy 2015, 153, 58–65. [Google Scholar]
- Tan, P.; Oberhardt, N.; Dypvik, H.; Riber, L. Weathering profiles and clay mineralogical developments, Bornholm. Denmark. Mar. Pet. Geol. 2017, 80, 32–48. [Google Scholar] [CrossRef]
- Wan, Y.X.; Liu, C.Q. Study on Adsorption of Rare Earth Elements by Kaolinite. J. Rare Earth 2005, 23, 337–341. [Google Scholar]
- He, Z.Y.; Zhang, Z.Y.; Yu, J.X.; Zhou, F.; Xu, Y.L.; Xu, Z.G.; Chen, Z.; Chi, R.A. Kinetics of column leaching of rare earth and aluminum from weathered crust elution-deposited rare earth ore with ammonium salt solutions. Hydrometallurgy 2016, 163, 33–39. [Google Scholar]






| Soil Column No. | Leaching Solution | pH | Content of (NH4)2SO4 (wt%) | Leaching Solution Flow Rate (mL/min) |
|---|---|---|---|---|
| T1 | (NH4)2SO4 | 5 | 8 | 3 |
| T2 | (NH4)2SO4 | 3 | 8 | 3 |
| T3 | (NH4)2SO4 | 4 | 8 | 3 |
| T4 | (NH4)2SO4 | 5 | 12 | 3 |
| T5 | (NH4)2SO4 | 5 | 4 | 3 |
| T6 | (NH4)2SO4 | 5 | 8 | 1 |
| T7 | (NH4)2SO4 | 5 | 8 | 5 |
| T8 (Simulated rainfall) | Deionized water | 6.8~7.2 | 0 | 3 |
| Serial Number | Sample | Clay (%) | Other Minerals (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Vermiculite | Chlorite | Kaolinite | Illite-Mica | Quartz | Potassium Feldspar | Plagioclase | ||
| 1 | AY-01 * | - | 1.8 | 37 | 9.4 | 42.8 | 8.3 | - |
| 2 | AY-02 | - | - | 40.3 | 10.1 | 39.8 | 9.8 | - |
| 3 | AY-03 * | - | - | 37.5 | 13.5 | 40.8 | 6.7 | - |
| 4 | AY-05 * | - | 2.8 | 37.3 | 3.4 | 38 | 13.4 | - |
| 5 | AY-06 * | - | 2.7 | 38.1 | 6.3 | 30.8 | 15.9 | - |
| 6 | AY-07 | - | - | 62.1 | 13.9 | 20.3 | 3.7 | - |
| 7 | AY-08 | - | - | 56 | 16.4 | 22.8 | 4.7 | - |
| 8 | AY-09 * | - | 2.5 | 31.5 | 5.9 | 42.2 | 10.5 | - |
| 9 | AY-10 | - | - | 36.9 | 9.5 | 42.2 | 11.3 | - |
| 10 | AY-11 | - | - | 36.2 | 4.9 | 55.7 | 3.2 | - |
| 11 | AY-12 * | - | 3.4 | 29.2 | 0 | 43.8 | 6 | - |
| 12 | AY-13 * | 6.6 | 1 | 45.8 | 3.1 | 23.3 | 23.1 | - |
| 13 | DN-13 * | - | 2.6 | 28.6 | 1.8 | 31.4 | 31.8 | - |
| 14 | DN-16 * | 1.5 | 1.5 | 24.5 | 1.3 | 34.1 | 35.3 | - |
| 15 | LN-01 * | 1.9 | 0.9 | 32.8 | - | 48.7 | 6.8 | - |
| 16 | LN-02 | 3.8 | - | 25.4 | 21 | 35 | 18.7 | - |
| 17 | LN-03 | - | - | 8.8 | 28.6 | 54.4 | 8.2 | - |
| 18 | LN-04 * | - | - | 30.3 | 2 | 47.8 | 18.6 | - |
| 19 | LN-05 | 0.1 | - | 23 | 19.1 | 44.1 | 13.8 | - |
| 20 | LN-06 * | - | 11.9 | 0.4 | 31.5 | 29.3 | 10 | |
| 21 | LN-07 | 1.5 | - | 12.4 | 13 | 59.6 | 15 | - |
| 22 | LN-08 * | - | - | 30 | 0.3 | 47.8 | 13.2 | 0.4 |
| 23 | LN-09 * | 1.4 | - | 33.7 | 0.6 | 43.7 | 16.9 | - |
| 24 | LN-10 * | 0.6 | - | 37.9 | 1.3 | 39.3 | 15.4 | 1.2 |
| 25 | LN-11 | 0.8 | - | 13.5 | 6.4 | 53.9 | 26.2 | - |
| 26 | LN-12 * | - | - | 30.1 | 0.3 | 38.3 | 25 | 1 |
| 27 | LN-18 * | - | - | 30.9 | 3.2 | 49.8 | 6.5 | 1.5 |
| Serial Number | Sample | Kaolinite | Illite-Mica | Quartz | Potassium Feldspar | Depth (cm) |
|---|---|---|---|---|---|---|
| 1 | GX-01 | 16.2 | 19.2 | 39.7 | 24.8 | 20 |
| 2 | GX-02 | 18.7 | 13.5 | 37 | 30.8 | 45 |
| 3 | GX-03-2 | 25.9 | 15.3 | 33.6 | 25.2 | 60 |
| 4 | GX-03-1 | 20.3 | 7.3 | 39.7 | 32.7 | 80 |
| 5 | GX-04-5 | 28.2 | 13.6 | 31 | 27.2 | 105 |
| 6 | GX-04-4 | 14.9 | 15.9 | 34 | 35.2 | 130 |
| 7 | GX-04-3 | 16.4 | 11.3 | 33.3 | 39 | 150 |
| 8 | GX-04-2 | 16.5 | 22.5 | 32.3 | 28.6 | 170 |
| 9 | GX-04-1 | 35 | 17.2 | 33.1 | 14.7 | 190 |
| 10 | GX-05 | 9.8 | 12.4 | 39.9 | 37.9 | 215 |
| 11 | P01-1-1 | 12.6 | 17.2 | 46.2 | 31.3 | 80 |
| 12 | P01-2-1 | 12.3 | 16 | 40.5 | 34.1 | 150 |
| 13 | P01-3-1 | 9.5 | 12.3 | 44 | 24.9 | 195 |
| 14 | P01-4-1 | 11.8 | 10.1 | 53.2 | 16.4 | 280 |
| 15 | P01-1-2 | 8.9 | 13.4 | 61.3 | 25.1 | 80 |
| 16 | P01-2-2 | 8.3 | 13 | 53.6 | 18.3 | 150 |
| 17 | P01-3-2 | 33 | 11.9 | 36.8 | 24.2 | 195 |
| 18 | P01-4-2 | 17.7 | 15 | 43 | 20.9 | 280 |
| 19 | P01-1-3 | 22.5 | 20.7 | 35.8 | 30.6 | 80 |
| 20 | P01-2-3 | 22.8 | 19.9 | 26.7 | 21.4 | 150 |
| 21 | P01-3-3 | 31.2 | 13.8 | 33.6 | 34.3 | 195 |
| 22 | P01-4-3 | 22.8 | 15 | 27.9 | 31.3 | 280 |
| Serial Number | Sample | Kaolinite | Illite-Mica | Quartz | Potassium Feldspar | Plagioclase |
|---|---|---|---|---|---|---|
| 1 | T1-1-1 | 22.3 | 7.4 | 45.7 | 24.6 | - |
| 2 | T1-1-2 | 21.5 | 7.8 | 48.1 | 22.6 | - |
| 3 | T1-1-3 | 20.2 | 5.1 | 45.2 | 29.6 | - |
| 4 | T1-1-5 | 11.1 | 12.9 | 45.9 | 30.1 | - |
| 5 | T1-3-1 | 18.9 | 8.8 | 55.6 | 16.7 | - |
| 6 | T1-3-2 | 14.9 | 9.4 | 39.4 | 36.2 | - |
| 7 | T1-3-3 | 13.8 | 13.2 | 51.7 | 21.3 | - |
| 8 | T1-3-5 | 6.8 | 14.7 | 54.6 | 23.9 | - |
| 9 | T1-5-1 | 15.2 | 14.6 | 36.6 | 33.6 | - |
| 10 | T1-5-2 | 13.1 | 8.6 | 48.1 | 30.2 | - |
| 11 | T1-5-3 | 16.1 | 12.8 | 37.2 | 33.9 | - |
| 12 | T1-5-5 | 17.5 | 8.2 | 39.2 | 35.1 | - |
| 13 | T2-1-1 | 20.5 | 11.4 | 42.5 | 25.5 | - |
| 14 | T2-1-2 | 16.6 | 12.5 | 37.7 | 33.2 | - |
| 15 | T2-1-3 | 21.7 | 7.2 | 45.3 | 25.8 | - |
| 16 | T2-1-5 | 20.2 | 10.2 | 46.3 | 23.2 | - |
| 17 | T2-3-1 | 11.2 | 12.5 | 44.8 | 35.1 | - |
| 18 | T2-3-2 | 9.2 | 15.0 | 43.0 | 32.8 | - |
| 19 | T2-3-3 | 8.8 | 23.2 | 35.9 | 32.1 | - |
| 20 | T2-3-5 | 10.5 | 23.0 | 43.1 | 23.5 | - |
| 21 | T2-5-1 | 8.9 | 20.8 | 44.4 | 25.8 | - |
| 22 | T2-5-2 | 8.5 | 16.0 | 42.2 | 33.4 | - |
| 23 | T2-5-3 | 25.3 | 1.2 | 45.5 | 28.0 | - |
| 24 | T2-5-5 | 31.5 | 5.3 | 31.0 | 32.2 | - |
| 25 | T3-1-1 | 15.3 | 10.5 | 55.5 | 18.7 | - |
| 26 | T3-1-2 | 16.2 | 12.8 | 40.2 | 30.8 | - |
| 27 | T3-1-3 | 15.7 | 16.9 | 39.6 | 27.8 | - |
| 28 | T3-1-5 | 18.4 | 9.7 | 48.4 | 23.5 | - |
| 29 | T3-3-1 | 9.2 | 17.9 | 43.3 | 29.6 | - |
| 30 | T3-3-2 | 11.2 | 14.2 | 45.7 | 28.9 | - |
| 31 | T3-3-3 | 10.5 | 15.8 | 43.5 | 30.3 | - |
| 32 | T3-3-5 | 18.6 | 20.2 | 38.0 | 23.2 | - |
| 33 | T3-5-1 | 16.5 | 15.0 | 28.5 | 40.1 | - |
| 34 | T3-5-2 | 9.2 | 11.4 | 44.8 | 34.6 | - |
| 35 | T3-5-3 | 11.8 | 21.9 | 28.3 | 38.1 | - |
| 36 | T3-5-5 | 16.2 | 17.5 | 25.9 | 40.5 | - |
| 37 | T4-1-1 | 14.1 | 14.9 | 43.9 | 27.2 | - |
| 38 | T4-1-2 | 18.1 | 12.3 | 48.5 | 21.1 | - |
| 39 | T4-1-3 | 18.2 | 11.8 | 42.8 | 27.2 | - |
| 40 | T4-1-5 | 18.5 | 10.6 | 34.0 | 36.9 | - |
| 41 | T4-3-1 | 9.9 | 12.2 | 46.2 | 31.7 | - |
| 42 | T4-3-2 | 6.9 | 16.1 | 41.8 | 35.2 | - |
| 43 | T4-3-3 | 10.4 | 16.7 | 29.8 | 43.1 | - |
| 44 | T4-3-5 | 15.7 | 20.4 | 37.3 | 26.6 | - |
| 45 | T4-5-1 | 14.3 | 16.5 | 34.0 | 35.2 | - |
| 46 | T4-5-2 | 9.1 | 14.0 | 42.5 | 34.4 | - |
| 47 | T4-5-3 | 15.3 | 9.5 | 50.1 | 25.1 | - |
| 48 | T4-5-5 | 11.2 | 6.8 | 51.4 | 27.4 | 3.1 |
| 49 | T5-1-1 | 14.2 | 9.6 | 48.3 | 27.9 | - |
| 50 | T5-1-2 | 16.9 | 14.0 | 43.6 | 25.5 | - |
| 51 | T5-1-3 | 16.4 | 30.6 | 35.4 | 17.6 | - |
| 52 | T5-1-5 | 26.7 | 9.6 | 34.2 | 29.5 | - |
| 53 | T5-3-1 | 16.6 | 8.9 | 49.4 | 25.2 | - |
| 54 | T5-3-2 | 12.4 | 12.1 | 47.9 | 27.5 | - |
| 55 | T5-3-3 | 16.2 | 11.1 | 41.5 | 31.2 | - |
| 56 | T5-3-5 | 12.1 | 17.1 | 39.8 | 31.0 | - |
| 57 | T5-5-1 | 15.2 | 10.2 | 29.0 | 37.8 | 7.9 |
| 58 | T5-5-2 | 9.4 | 8.8 | 31.8 | 32.4 | 17.6 |
| 59 | T5-5-3 | 11.4 | 13.2 | 36.7 | 29.7 | 9.1 |
| 60 | T5-5-5 | 9.6 | 11.5 | 54.2 | 24.6 | - |
| 61 | T6-1-1 | 10.9 | 6.9 | 52.9 | 23.9 | 5.4 |
| 62 | T6-1-2 | 10.5 | 8.4 | 57.7 | 19 | 4.4 |
| 63 | T6-1-3 | 9.8 | 8.6 | 57.7 | 23.9 | - |
| 64 | T6-1-5 | 14.0 | 12.5 | 46.9 | 26.6 | - |
| 65 | T6-3-1 | 13.3 | 13.2 | 40.6 | 30.4 | 2.4 |
| 66 | T6-3-2 | 15.1 | 12.9 | 49.7 | 22.3 | - |
| 67 | T6-3-3 | 13.7 | 10.3 | 47.0 | 29.0 | - |
| 68 | T6-3-5 | 13.3 | 12.8 | 60.6 | 13.2 | - |
| 69 | T6-5-1 | 9.8 | 13.9 | 47.8 | 20.8 | 7.7 |
| 70 | T6-5-2 | 7.0 | 11.6 | 59.1 | 22.3 | - |
| 71 | T6-5-3 | 15.4 | 12.9 | 40.8 | 26.1 | 4.8 |
| 72 | T6-5-5 | 16.4 | 12.3 | 38.0 | 29.8 | - |
| 73 | T7-1-1 | 16.8 | 10.2 | 43.0 | 22.5 | 7.4 |
| 74 | T7-1-2 | 15.7 | 13.2 | 40.1 | 31.0 | - |
| 75 | T7-1-3 | 20.6 | 13.3 | 39.9 | 26.2 | - |
| 76 | T7-1-5 | 19.1 | 21.3 | 38.3 | 21.3 | - |
| 77 | T7-3-1 | 7.5 | 15.5 | 40.5 | 30.5 | 6.1 |
| 78 | T7-3-2 | 10.3 | 13.1 | 51.3 | 22.4 | 2.9 |
| 79 | T7-3-3 | 15.3 | 4.5 | 38.5 | 31.1 | 10.6 |
| 80 | T7-3-5 | 11.3 | 10.9 | 53.3 | 19.6 | 4.9 |
| 81 | T7-5-1 | 13.5 | 8.0 | 34.1 | 38 | 6.4 |
| 82 | T7-5-2 | 11.0 | 14.6 | 32.8 | 41.6 | - |
| 83 | T7-5-3 | 18.3 | 12.4 | 28.0 | 41.3 | - |
| 84 | T7-5-5 | 25.3 | 17.7 | 41.5 | 15.5 | - |
| 85 | T8-1-1 | 16.0 | 9.6 | 46.4 | 28.0 | - |
| 86 | T8-1-5 | 20.6 | 19.3 | 30.7 | 29.4 | - |
| 87 | T8-3-1 | 8.5 | 5.4 | 34.7 | 51.4 | - |
| 88 | T8-3-5 | 15.2 | 11.5 | 35.7 | 37.6 | - |
| 89 | T8-5-1 | 14.6 | 11.3 | 49.9 | 24.2 | - |
| 90 | T8-5-5 | 19.6 | 17.7 | 33.8 | 28.9 | - |
| Sample No. | D10 (μm) | D50 (μm) | D90 (μm) | Dav (μm) | D [3,2] (μm) | D [4,3] (μm) |
|---|---|---|---|---|---|---|
| AY-01 | 1.32 | 6.25 | 33.88 | 12.88 | 3.09 | 12.88 |
| AY-02 | 1.43 | 7.28 | 29.54 | 11.65 | 3.36 | 11.65 |
| AY-03 | 1.64 | 8.47 | 40.28 | 15.64 | 4.09 | 15.64 |
| AY-05 | 1.10 | 4.50 | 17.10 | 7.02 | 2.30 | 7.02 |
| AY-06 | 1.79 | 15.51 | 50.28 | 21.57 | 3.67 | 21.57 |
| AY-07 | 2.21 | 12.76 | 40.04 | 17.22 | 4.75 | 17.22 |
| AY-08 | 1.44 | 6.93 | 35.50 | 13.21 | 3.48 | 13.21 |
| AY-09 | 0.82 | 3.88 | 11.99 | 5.40 | 1.77 | 5.40 |
| AY-10 | 0.96 | 4.57 | 23.70 | 8.76 | 2.21 | 8.76 |
| AY-11 | 1.34 | 5.38 | 20.86 | 8.42 | 2.89 | 8.42 |
| AY-12 | 1.13 | 6.39 | 28.22 | 11.06 | 2.61 | 11.06 |
| AY-13 | 1.20 | 5.02 | 18.49 | 7.70 | 2.58 | 7.70 |
| DN-13 | 1.64 | 12.75 | 44.85 | 18.90 | 4.37 | 18.90 |
| DN-16 | 1.37 | 8.07 | 30.07 | 12.42 | 3.50 | 12.42 |
| LN-01 | 1.16 | 5.14 | 24.69 | 9.41 | 2.84 | 6.10 |
| LN-02 | 1.50 | 7.45 | 28.71 | 11.85 | 3.59 | 11.85 |
| LN-03 | 5.03 | 20.86 | 61.00 | 27.80 | 8.19 | 27.80 |
| LN-04 | 1.49 | 8.39 | 38.06 | 14.92 | 3.67 | 14.92 |
| LN-05 | 1.37 | 6.42 | 33.75 | 13.00 | 3.53 | 13.00 |
| LN-06 | 1.41 | 5.47 | 26.90 | 10.20 | 3.31 | 10.20 |
| LN-07 | 1.62 | 8.17 | 37.10 | 14.42 | 4.07 | 14.42 |
| LN-08 | 1.26 | 5.68 | 22.95 | 9.08 | 2.89 | 9.08 |
| LN-09 | 1.55 | 8.63 | 43.42 | 17.11 | 3.74 | 17.11 |
| LN-10 | 1.41 | 8.08 | 36.38 | 13.98 | 3.58 | 13.98 |
| LN-11 | 1.91 | 15.00 | 49.19 | 21.02 | 4.51 | 21.02 |
| LN-12 | 1.12 | 4.41 | 13.85 | 6.45 | 2.08 | 6.45 |
| LN-18 | 1.21 | 6.68 | 28.74 | 11.42 | 2.79 | 11.42 |
| Sample No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| SiO2 | 70.34 | 75.55 | 76.14 | 74.88 | 72.48 | 74.58 |
| TiO2 | 0.44 | 0.25 | 0.03 | 0.05 | 0.13 | 0.15 |
| Al2O3 | 14.59 | 12.13 | 12.97 | 13.44 | 13.4 | 13.34 |
| Fe2O3 | 2.03 | 1.18 | 0.09 | 0.21 | 0.51 | 0.56 |
| FeO | 0.64 | 1.01 | 1.07 | 1.42 | 1.28 | 1.25 |
| MnO | 0.25 | 0.07 | 0.03 | 0.08 | 0.11 | 0.05 |
| MgO | 0.57 | 0.34 | 0.07 | 0.19 | 0.17 | 0.18 |
| CaO | 0.55 | 0.42 | 0.62 | 0.58 | 1.51 | 0.73 |
| Na2O | 3.37 | 2.74 | 4.25 | 3.97 | 3.62 | 3.65 |
| K2O | 5.7 | 5.36 | 4.52 | 4.61 | 5.37 | 4.8 |
| P2O5 | 0.05 | 0.04 | 0.02 | 0.06 | 0.04 | 0.05 |
| LOI | 0.92 | 0.7 | 0.96 | 0.57 | 1.62 | 0.6 |
| Total | 100.33 | 99.85 | 100.77 | 100.28 | 100.24 | 100.03 |
| CIA | 64 | 63 | 63 | 65 | 61 | 64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Jin, X.; Chen, H.; He, Z.; Qiu, L.; Duan, H. Grain Size Distribution and Clay Mineral Distinction of Rare Earth Ore through Different Methods. Minerals 2020, 10, 353. https://doi.org/10.3390/min10040353
Chen L, Jin X, Chen H, He Z, Qiu L, Duan H. Grain Size Distribution and Clay Mineral Distinction of Rare Earth Ore through Different Methods. Minerals. 2020; 10(4):353. https://doi.org/10.3390/min10040353
Chicago/Turabian StyleChen, Lingkang, Xiongwei Jin, Haixia Chen, Zhengwei He, Lanrong Qiu, and Hurong Duan. 2020. "Grain Size Distribution and Clay Mineral Distinction of Rare Earth Ore through Different Methods" Minerals 10, no. 4: 353. https://doi.org/10.3390/min10040353
APA StyleChen, L., Jin, X., Chen, H., He, Z., Qiu, L., & Duan, H. (2020). Grain Size Distribution and Clay Mineral Distinction of Rare Earth Ore through Different Methods. Minerals, 10(4), 353. https://doi.org/10.3390/min10040353




