Raman Microspectroscopy of Garnets from S-Fibulae from the Archaeological Site Lajh (Slovenia)
Abstract
1. Introduction
2. Materials and Methods
2.1. Artefacts
2.2. Study of Garnets.
2.3. Study of Inclusions
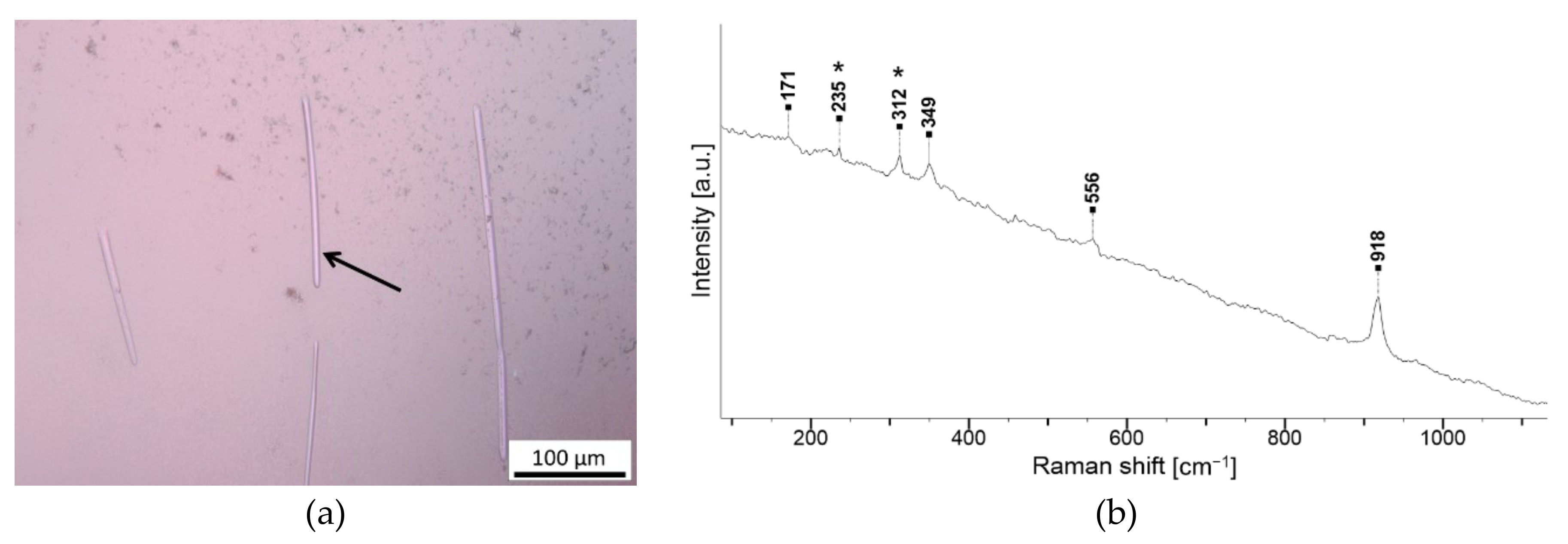
3. Results
3.1. Garnets
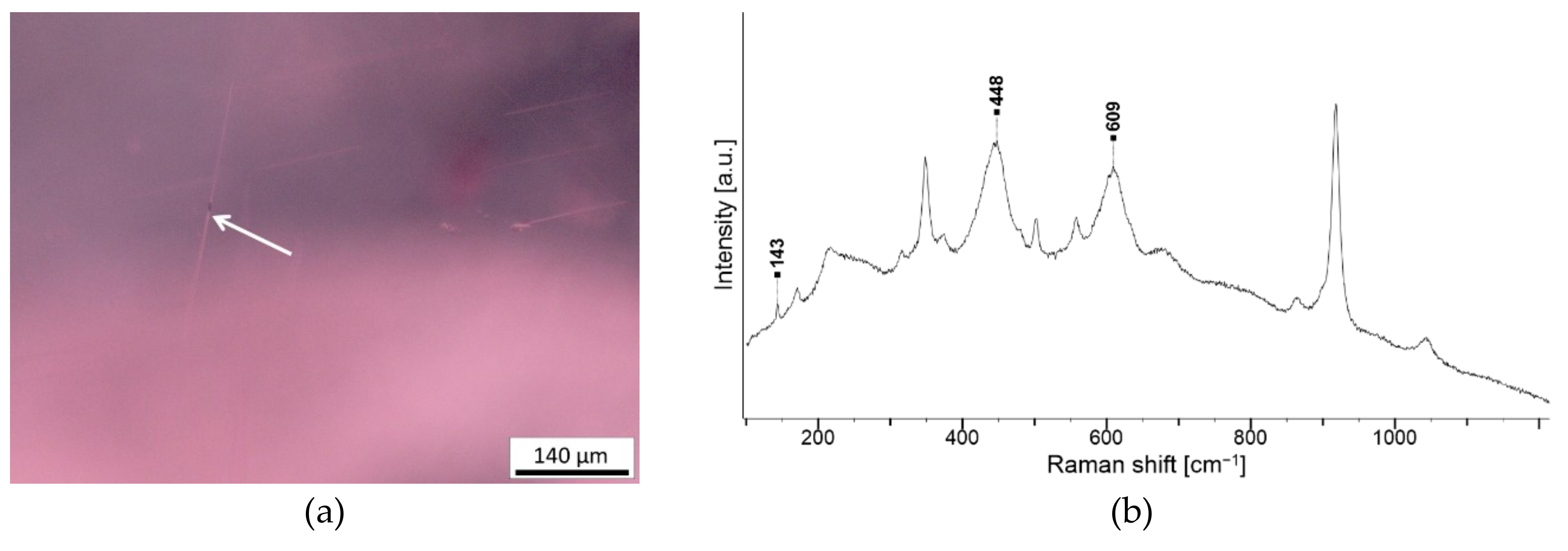
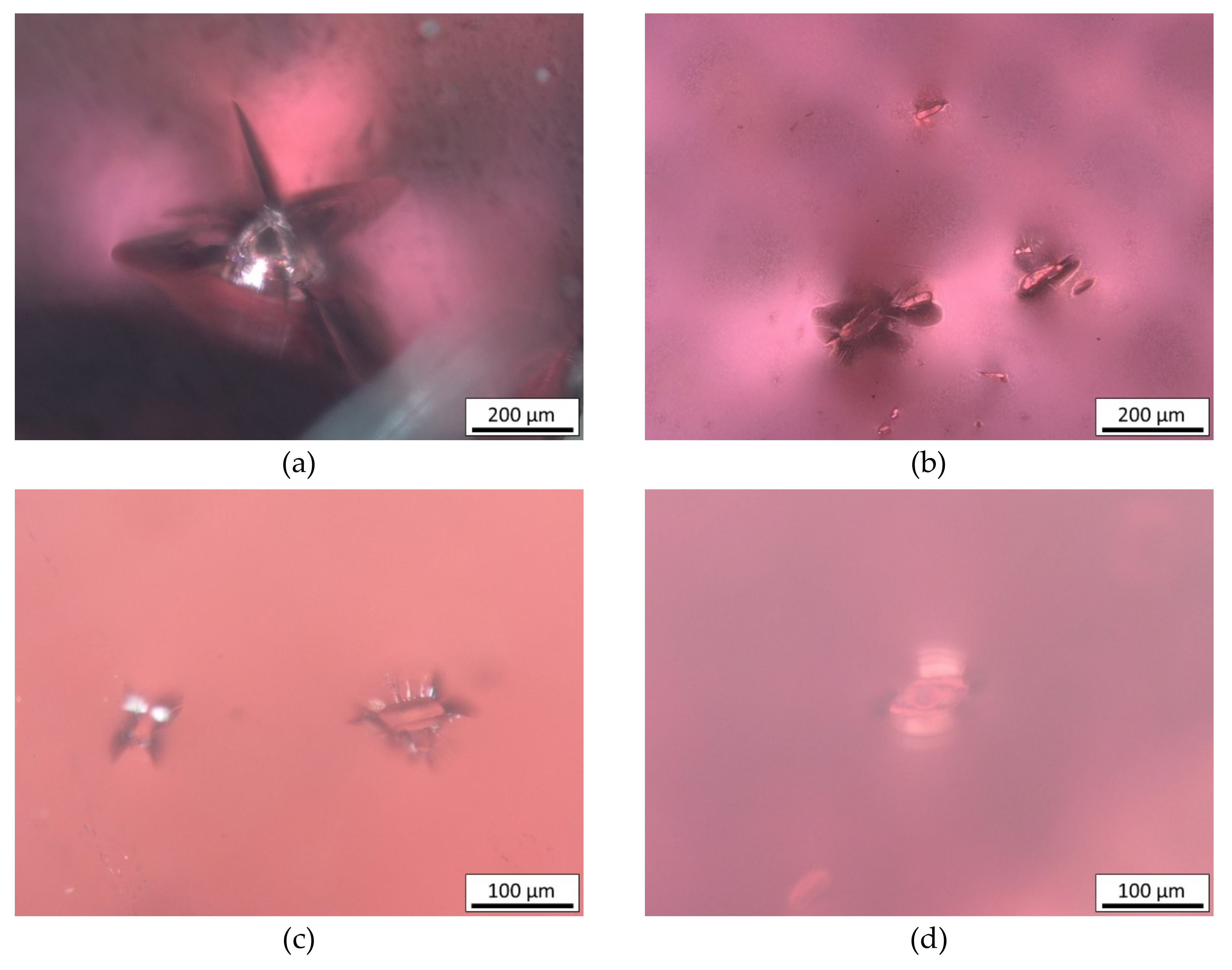
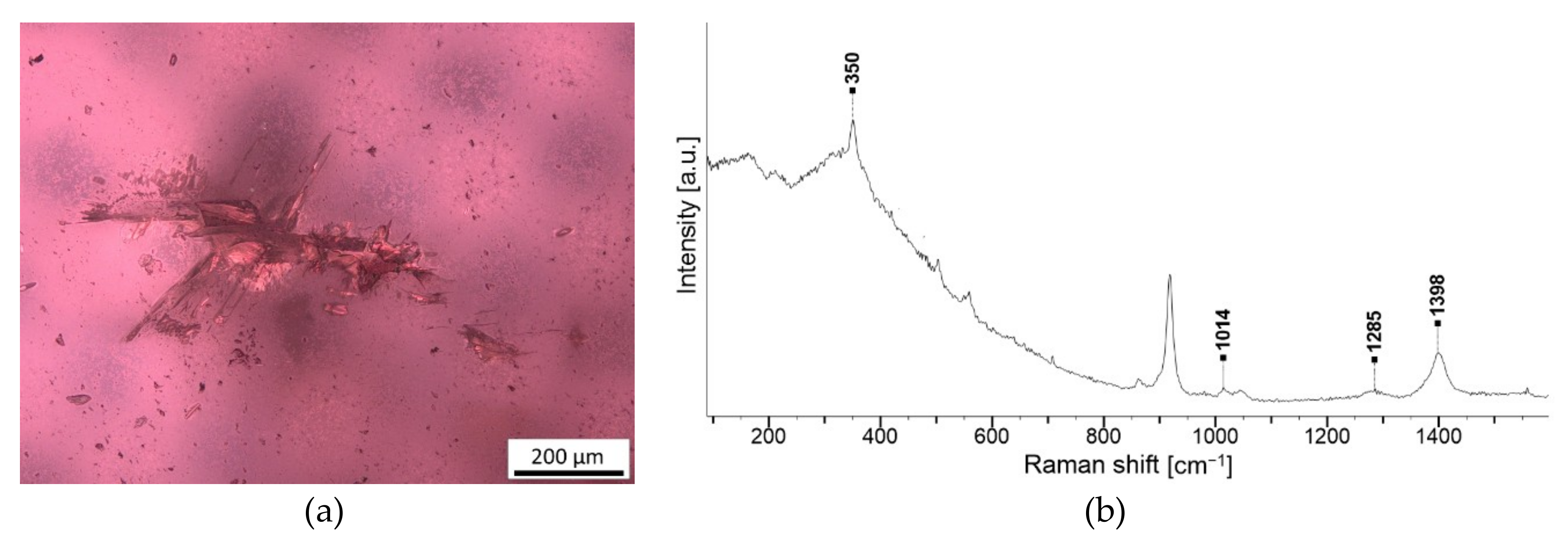
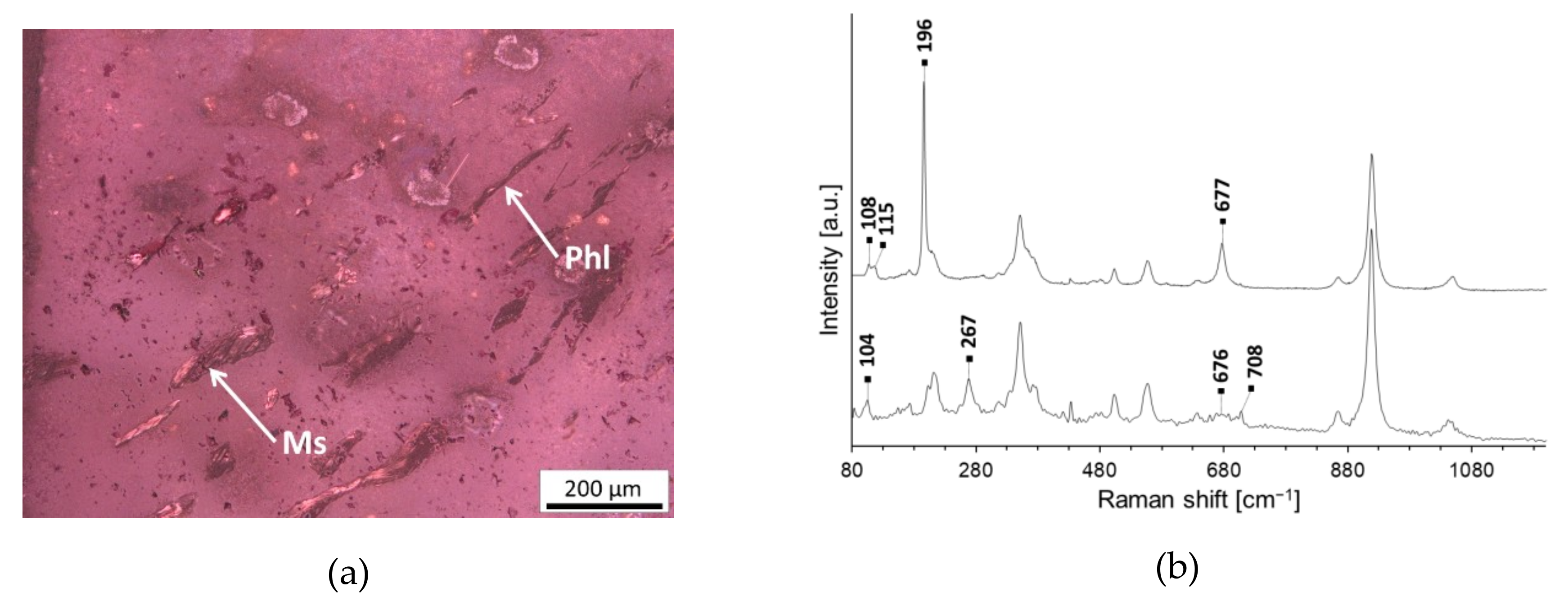
3.2. Inclusions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adams, N. The Garnet Millennium: The Role of Seal Stones in Garnet Studies. In Gems of Heaven: Recent Research on Engraved Gemstones in Late Antiquity c. AD 200-600. Research Publication; Adams, N., Entwistle, C., Eds.; British Museum: London, UK, 2011; Volume 177, pp. 10–24. [Google Scholar]
- Scukin, M.; Bazan, I. L’origine du style cloisonné de l’époque des Grandes Migrations. In La Noblesse et Les Chefs Barbares du IIIe au VIIe Siècle: Mémoires Publiés par l’Association Française d’Archéologie Mérovingienne V; Vallet, F., Kazinski, M., Eds.; Association Française D’archéologie Mérovingienne; Société des amis du Musée des Antiquités Nationales: Rouen, France, 1993; pp. 63–69. [Google Scholar]
- Mathis, F.; Vrielynck, O.; Laclavetine, K.; Chêne, G.; Strivay, D. Study of the provenance of Belgian Merovingian garnets by PIXE at IPNAS cyclotron. Nucl. Instrum. Methods Phys. Res. B 2008, 266, 2348–2352. [Google Scholar] [CrossRef]
- Calligaro, T.; Colinart, S.; Poirot, J.-P.; Sudres, C. Combined external-beam PIXE and μ-Raman characterisation of garnets used in Merovingian jewellery. Nucl. Instrum. Methods Phys. Res. B 2002, 189, 320–327. [Google Scholar] [CrossRef]
- Farges, F. Mineralogy of Louvres Merovingian garnet cloisonné jewelry: Origins of the gems of the first kings of France. Am. Mineral. 1998, 83, 323–330. [Google Scholar] [CrossRef]
- Bimson, M.; La Neice, S.; Leese, M. The characterisation of mounted garnets. Archaeometry 1982, 24, 51–58. [Google Scholar] [CrossRef]
- Quast, D.; Schüssler, U. Mineralogische Untersuchungen zur Herkunft der Granate merowingerzeitlicher Cloisonnéarbeiten. Germania 2000, 78, 75–96. [Google Scholar]
- Gilg, H.A.; Gast, N.; Calligaro, T. Vom Karfunkelstein. In Karfunkelstein und Seide: Neue Schätze aus Bayerns Frühzeit; Wamser, L., Ed.; Friedrich Pustet Verlag: München, Germany, 2010; pp. 87–100. [Google Scholar]
- Bugoi, R.; Oanta-Marghitu, R.; Calligaro, T. IBA investigations of loose garnets from Pietroasa, Apahida and Cluj-Someseni treasures (5th century AD). Nucl. Inst. Methods Phys. Res. Sect. B 2015, 371, 401–406. [Google Scholar] [CrossRef]
- Thoresen, L. Archaeogemmology and Ancient Literary Sources on Gems and their Origins. In Gemstones in the First Millennium AD: Mines, Trade, Workshops and Symbolism; Greiff, S., Hilgner, A., Quast, D., Eds.; Römisch Germanisches Zentralmuseum: Mainz, Germany, 2017; pp. 155–218. [Google Scholar]
- Henderson, R.R. Determining Chemical Composition of the Silicate Garnets Using Raman Spectroscopy. Master’s Thesis, The Univeristy of Arizona, Tuscon, AZ, USA, 2009. [Google Scholar]
- Dubessy, J.; Caumon, M.-C.; Rull Pérez, F. Raman Spectroscopy Applied to Earth Sciences and Cultural Heritage: University Textbook; Dubessy, J., Caumon, M.-C., Rull Pérez, F., Eds.; European Mineralogical Union: London, UK, 2012. [Google Scholar]
- Smith, G.D.; Clark, J.H.R. Raman microscopy in archaeological science. J. Archaeol. Sci. 2004, 31, 1137–1160. [Google Scholar] [CrossRef]
- Stockton, C.M.; Mason, D.V. A Proposed New Classification for Gem-Quality Garnets. Gems. Gemol. 1985, 21, 205–218. [Google Scholar] [CrossRef]
- Will, T.M. Thermodynamics of solid solutions. In Phase Equilibria in Metamorphic Rocks: Thermodynamic Background and Petrological Applications; Springer: Berlin, Germany, 1998; pp. 5–17. [Google Scholar]
- Merli, M.; Callegari, A.; Cannllo, E.; Caucia, F.; Leona, M.; Oberti, R.; Ungaretti, L. Crystal-chemical complexity in natural garnets: Structural constraints on chemical variability. Eur. J. Mineral. 1995, 7, 1239–1249. [Google Scholar] [CrossRef]
- Grew, E.; Locock, A.; Mills, S.J.; Galuskina, I.; Galuskin, E.; Hålenius, U. IMA Report Nomenclature of the garnet supergroup. Am. Mineral. 2013, 98, 785–811. [Google Scholar] [CrossRef]
- Kloprogge, J.T. Infrared and Raman spectroscopy of minerals and inorganic materials. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 274–276. [Google Scholar]
- Krippner, A.; Meinhold, G.; Morton, A.C.; Eynatten, H. Evaluation of garnet discrimination diagrams using geochemical data of garnets derived from various host rocks. Sediment. Geol. 2014, 306, 36–52. [Google Scholar] [CrossRef]
- Mingsheng, P.; Mao, H.K.; Dien, L.; Chao, E.C.T. Raman spectroscopy of garnet-group minerals. Chin. J. Geochem. 1994, 13, 176–183. [Google Scholar] [CrossRef]
- Pinet, M.; Smith, D.C. Raman microspectrometry ofgarnets X3Y2Si3O12: 1. The natural calcic series uvarovite–grossular–andradite. Schweiz. Mineral. Petrogr. Mitt. 1993, 73, 21–40. [Google Scholar]
- Pinet, M.; Smith, D.C. Raman microspectrometry of garnets X3Y2Z3O12 2. The natural aluminium series pyrope-almandine-spessartine. Schweiz. Mineral. Petrogr. Mitt. 1994, 74, 161–179. [Google Scholar]
- Smith, D.C. The RAMANITA© method for non-destructive and in situ semi-quantitative chemical analysis of mineral solid-solutions by multidimensional calibration of Raman wavenumber shifts. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 2299–2314. [Google Scholar] [CrossRef]
- Gilg, H.A.; Gast, N. Determination of titanium content in pyrope by Raman spectroscopy. J. Raman Spectrosc. 2016, 47, 486–491. [Google Scholar] [CrossRef]
- Geiger, C.A. A tale of two garnets: The role of solid solution in the development toward a modern mineralogy. Am. Mineral. 2016, 101, 1735–1749. [Google Scholar] [CrossRef]
- Kuang, Y.; Xu, J.; Li, B.; Ye, Z.; Huang, S.; Chen, W.; Zhang, D.; Zhou, W.; Ma, M. Crystal-Chemical Properties of Synthetic Almandine-Pyrope Solid Solution by X-Ray Single-Crystal Diffraction and Raman Spectroscopy. Crystals 2019, 9, 541. [Google Scholar] [CrossRef]
- Ferrero, S.; Angel, R.J. Micropetrology: Are Inclusions Grains of Truth? J. Petrol. 2018, 59, 1671–1700. [Google Scholar] [CrossRef]
- Gems-inclusions all about inclusions in gemstones. Inclusions types—By time of entrapment. Available online: https://www.gems-inclusions.com/inclusions-types/by-type-of-trapping/for-solid-inclusions-2/ (accessed on 20 August 2019).
- Yardley, B.W.D.; Mackenzie, W.S.; Guilford, C. Atlas of metamorphic rocks and their textures. Terra Nova 1991, 3, 217–218. [Google Scholar] [CrossRef]
- Gem-A, The Gemmological Association of Great Britain. News & Publication: The Source of Garnets Found at the Arikamedu Archaeological Site in South India. Available online: https://gem-a.com/news-publications/news-blogs/journal-digests/journal/the-source-of-garnets-found-at-the-arikamedu-archaeological-site-in-south-india (accessed on 20 August 2019).
- Schmetzer, K.; Gilg, H.A.; Schussler, U.; Panjikar, J.; Calligaro, T.; Perin, P. The Linkage between garnets found in India at the Arikamedu archaeological site and their source at the Garibpet deposit. J. Gemm. 2017, 35, 598–627. [Google Scholar] [CrossRef]
- Horváth, E.; Bendő, Z. Provenance study on a collection of loose garnets from a gepidic period grave in Northeast Hungary. Archeometriai Muh. 2011, 8, 17–32. [Google Scholar]
- Kataria, P. Book review: Geology of Rajasthan: Status and perspective. J. Geol. Soc. India 2000, 55, 452–453. [Google Scholar]
- Rameshchandra Phani, P. Mineral Resources of Telangana State, India: The Way Forward. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 15450–15459. [Google Scholar] [CrossRef]
- Suomen Geologinen Seura—Geologiska Sällskapet i Finland, The Geological Society of Finland. Articles in press, 1980, 52. Available online: http://www.geologinenseura.fi/bulletin/Volume52/index.html (accessed on 20 August 2019).
- Maharaj, D. Chemical Classification of Gem Garnets. Master’s Thesis, University of Pretoria, Pretoria, South African, November 2015. [Google Scholar]
- Min-dat.org—Portugal. Available online: https://www.mindat.org/loc-14425.html (accessed on 27 January 2020).
- Caincross, B. Geology of East Africa. In Minerals & Gemstones of East Africa; Struik Nature: Cape Town, South Africa, 2019. [Google Scholar]
- Lux, J.; Ravnik, J. Poskus rekonstrukcije obsega poznoantičnega grobišča Lajh v Kranju. Varst. Spomenikov 2008, 43–69. [Google Scholar]
- Stare, V.; Stopar, B.; Žgur, A.; Goričan, A.; Čenčič, L.; Habič, S.; Petru, P.; Vinski, Z.; Kiszely, I. Kranj: Nekropola iz Časa Preseljevanja Ljudstev; Narodni Muzej: Ljubljana, Slovenia, 1980. [Google Scholar]
- Inštitut za arheologijo ZRC SAZU. Strani za študente arheologije zgodnjega srednjega veka na FF v Ljubljani; Poznoantično obdobje. Available online: http://iza.zrc-sazu.si/ff/slavko/pa_grobisca.html (accessed on 10 December 2019).
- Milavec, T. Prispevek h kronologiji S-fibul v Sloveniji. Arheol. Vestn. 2007, 58, 333–355. [Google Scholar]
- Gregorietti, G. Jewelry through the Ages; Hamlyn: London, UK, 1970; p. 139. [Google Scholar]
- Kramar, S.; Dolenec, M.; Lux, J. Characterisation of 6th century fibulae with gem materials (cementary Lajh in Kranj, Slovenia) by means of XRF and micro-Raman spectroscopy. In Proceedings of the 6th International Congress on the Application of Raman Spectroscopy in Art and Archaeology, Parma, Italy, 5–8 September 2011; Timeo: Bologna, Italy, 2011; pp. 172–173. [Google Scholar]
- Šmit, Ž.; Fajfar, H.; Jeršek, M.; Knific, T.; Lux, J. Analysis of garnets from the archaeological sites in Slovenia. Nucl. Inst. Methods Phys. Res. Sect. B 2014, 328, 89–94. [Google Scholar] [CrossRef]
- Nemeček, N.; Kramar, S.; Podobnik, T. Interdisciplinarni pristop—Konserviranje-restavriranje, naravoslovne preiskave in arheološka interpretacija dveh zaponk z grobišča Lajh. In Konservator—Restavrator: Povzetki Strokovnega Srečanja 2016; Nemeček, N., Ed.; Skupnost Muzejev Slovenije, Društvo Restavratorjev Slovenije: Ljubljana, Slovenia, 2016; p. 55. [Google Scholar]
- Sibi, N.; Subodh, G. Structural and microstructural correlations of physical properties in natural almandine-pyrope solid solution: Al70Py29. J. Electron. Mater. 2017, 46, 6947–6956. [Google Scholar] [CrossRef]
- Kolesov, B.; Geiger, A.C. Raman spectra of silicate garnets. Phys. Chem. Miner. 1998, 25, 142–151. [Google Scholar] [CrossRef]
- Scholz, R.; Frost, R.L.; Xi, Y.; Graça, L.M.; Lagoeiro, L.; López, A. Vibrational spectroscopic characterization of the phosphate mineral phosphophyllite—Zn2Fe(PO4)2·4H2O, from Hagendorf Süd, Germany and in comparison with other zinc phosphates. J. Mol. Struct. 2013, 1039, 22–27. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Tan, W.; Wang, C.; He, H.; Xing, C.; Liang, X.; Dong, H. Magnetite-rutile symplectite derived from ilmenite-hematite solid solution in the Xinjie Fe-Ti oxide-bearing, mafic-ultramafic layered intrusion (SW China). Am. Mineral. 2015, 100, 2348–2351. [Google Scholar] [CrossRef]
- Tan, W.; He, H.; Wang, C.; Dong, H.; Liang, X.; Zhu, J. Magnetite exsolution in ilmenite from the Fe-Ti oxide gabbro in the Xinjie intrusion (SW China) and sources of unusually strong remnant magnetization. Am. Mineral. 2016, 101, 2759–2767. [Google Scholar] [CrossRef]
- Eppler, W.F. The Origin of Healing Fissures in Gemstones. J. Gemm. 1959, 7, 40–66. [Google Scholar] [CrossRef]
- Alex Strekeisen. Plutonic Rocks, Pleochroic halo. Available online: http://www.alexstrekeisen.it/english/pluto/pleochroichalo.php (accessed on 20 August 2019).
- Nasdala, L.; Wenzel, M.; Vavra, G.; Irmer, G.; Wenzel, T.; Kober, B. Metamictisation of natural zircon: Accumulation versus thermal annealing of radioactivity-induced damage. Contrib. Mineral. Petrol. 2002, 143, 767–768. [Google Scholar] [CrossRef]
- Nasdala, L.; Akhmadaliev, S.; Artac, A.; Chanmuang, N.C.; Habler, G.; Lenz, C. Irradiation effects in monazite-(Ce) and zircon: Raman and photoluminescence study of Au-irradiated FIB foils. Phys. Chem. Miner. 2018, 45, 855–871. [Google Scholar] [CrossRef]
- United ID RAMAN LAB. Applications/Solutions: Gemstones. Raman Spectroscopic Inspection and Analysis of Zircon Inclusion in Corundum—Effect of Heat Treatment on Zircon Inclusion. Available online: https://static1.squarespace.com/static/55e5983ee4b043c85db4fd4c/t/5d1e1fd511031d0001ec94a3/1562255322783/Raman+Spectroscopic+Inspection+and+Analysis+of+Zircon+Inclusion+in+Corundum.pdf (accessed on 21 March 2020).
- Tlili, A.; Smith, D.C.; Beny, J.M.; Boyer, H. A Raman microprobe study of natural micas. Mineral. Mag. 1989, 53, 165–179. [Google Scholar] [CrossRef]
- Schönig, J.; Meinhold, G.; von Eynatten, H.; Lünsdorf, N.K. Provenance information recorded by mineral inclusions in detrital garnet. Sediment. Geol. 2018, 376, 32–49. [Google Scholar] [CrossRef]
- Calligaro, T. Probing Works of Art with Photons and Charged Particles. In Spectroscopy of Emerging Materials; Faulques, E.C., Perry, D.L., Yeremenko, A.V., Eds.; NATO Science Series II: Mathematics, Physics and Chemistry; Springer: Dordrecht, Germany, 2004; Volume 165. [Google Scholar]
- Force, E.R. The provenance of rutile. J. Sediment. Res. 1980, 50, 485–488. [Google Scholar]
- Zack, T.; von Eynatten, H.; Kronz, A. Rutile geochemistry and its potential use in quantitative provenance studies. Sediment. Geol. 2004, 171, 37–58. [Google Scholar] [CrossRef]
- Bucher, K.; Frey, M. Petrogenesis of Metamorphic Rocks; Bucher, K., Frey, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Cesare, B.; Salvioli Mariani, E.; Venturelli, G. Crustal anatexis and melt extraction in the restitic xenoliths at El Hoyazo (SE Spain). Mineral. Mag. 1997, 61, 15–27. [Google Scholar] [CrossRef]
- Acosta-Vigil, A.; Buick, I.; Hermann, J.; Cesare, B.; Rubatto, D.; London, D.; Morgan, G.B. Mechanisms of crustal anatexis: A geochemical study of partially melted metapelitic enclaves and host dacite, SE Spain. J. Petrol. 2010, 51, 785–821. [Google Scholar] [CrossRef]



| Group | Composition of an End Member | Inclusions | |
|---|---|---|---|
| Pyralspite | Pyrope | Mg3Al2(SiO4)3 | Apatite, ilmenite, rutile, undetermined fibrous minerals |
| Pyrope-almandine | (Fe, Mg)3Al2(SiO4)3 | Zircon, monazite, apatite, rutile, mica, quartz, often very pure | |
| Almandine | Fe3Al2(SiO4)3 | Zircon, rutile, mica, hornblende, apatite, spinel, quartz | |
| Spessartine | Mn3Al2(SiO4)3 | Rarely present, growth lines – dark wavy feathers | |
| Ugrandite | Andradite | Ca3Fe2(SiO4)3 | Chryzotile |
| Grossular | Ca3Al2(SiO4)3 | Mostly no inclusions are found Tsavorite – zircon, fibrous minerals Hessonite – zircon, diopside, treacle and heat wave textures | |
| Uvarovite | Ca3Cr2(SiO4)3 | Not determined | |
| Calligaro et al. [4] | Type I | Type II | - | Type III | Type IV | Type V |
|---|---|---|---|---|---|---|
| Gilg et al. [8] | Group B | Group A | Group C [10] | Group X | Group D | Group E |
| Chemical Composition of Garnets | Almandines poor in Mg, Ca, Mn, also Cr and Y | Almandines rich in Mg, Mn, also Cr and Y | Almandines rich in Ca and Mg | Pyrope-almandines (rhodolite) | Pyropes poor in Cr | Pyropes rich in Cr |
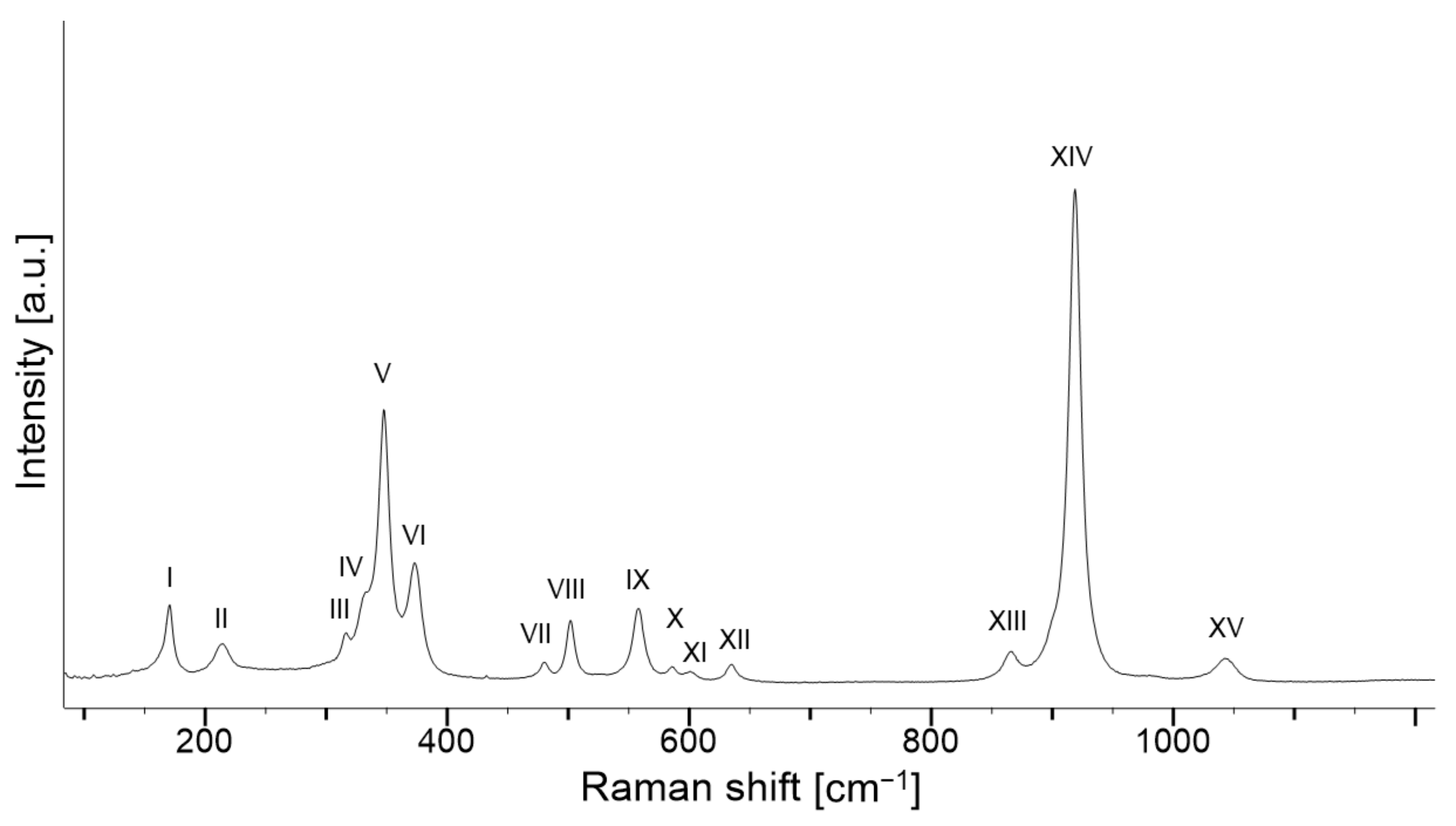
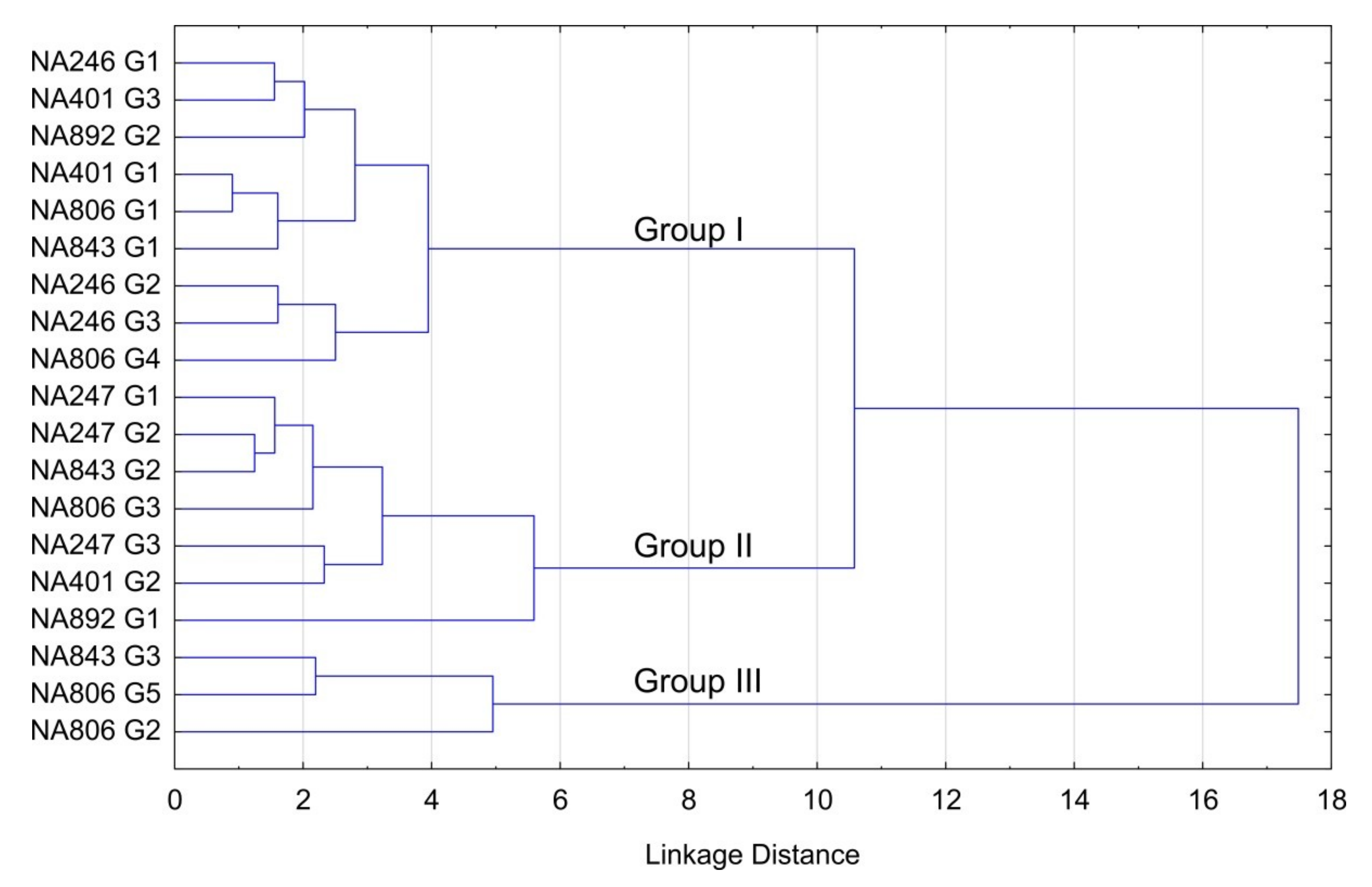
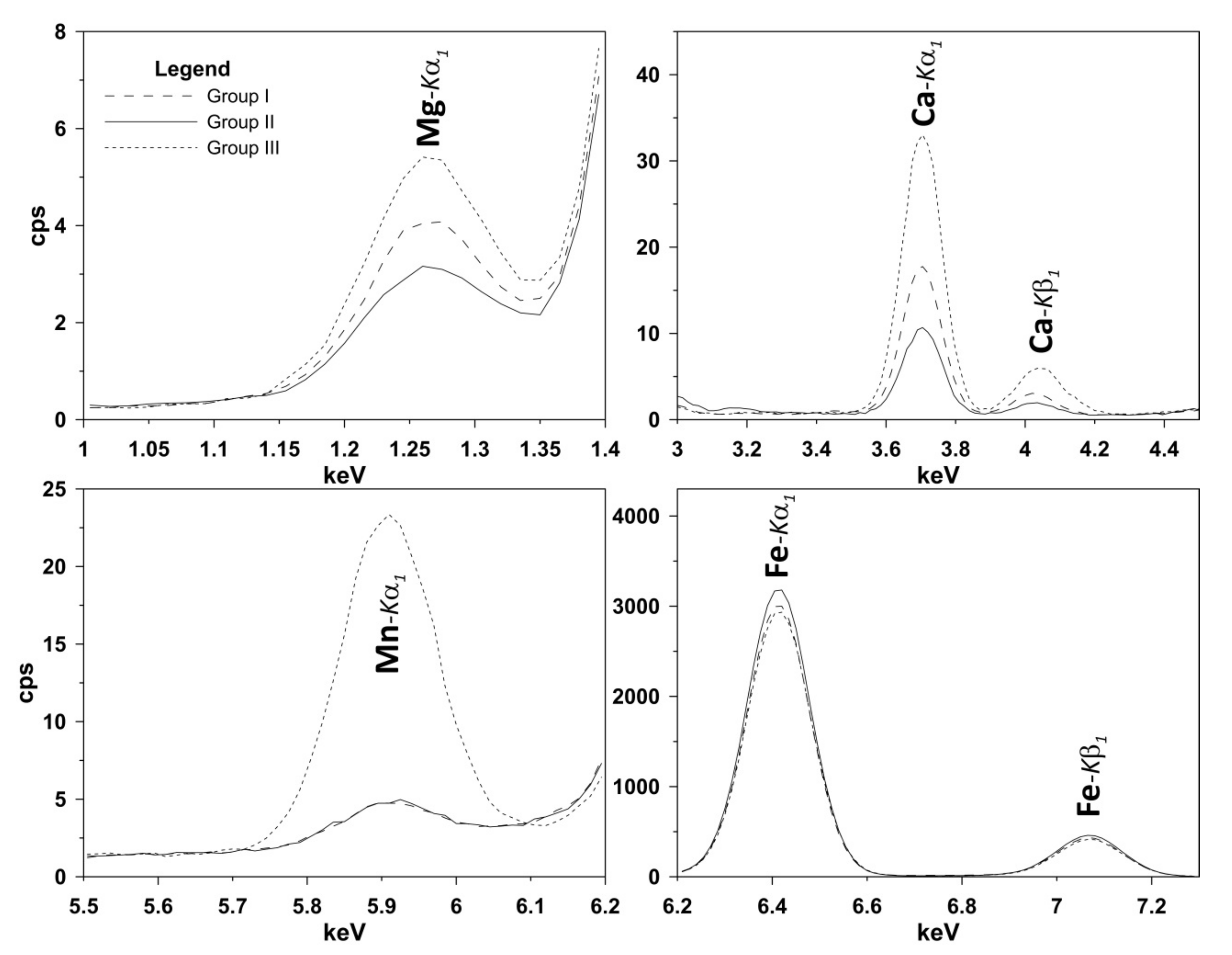
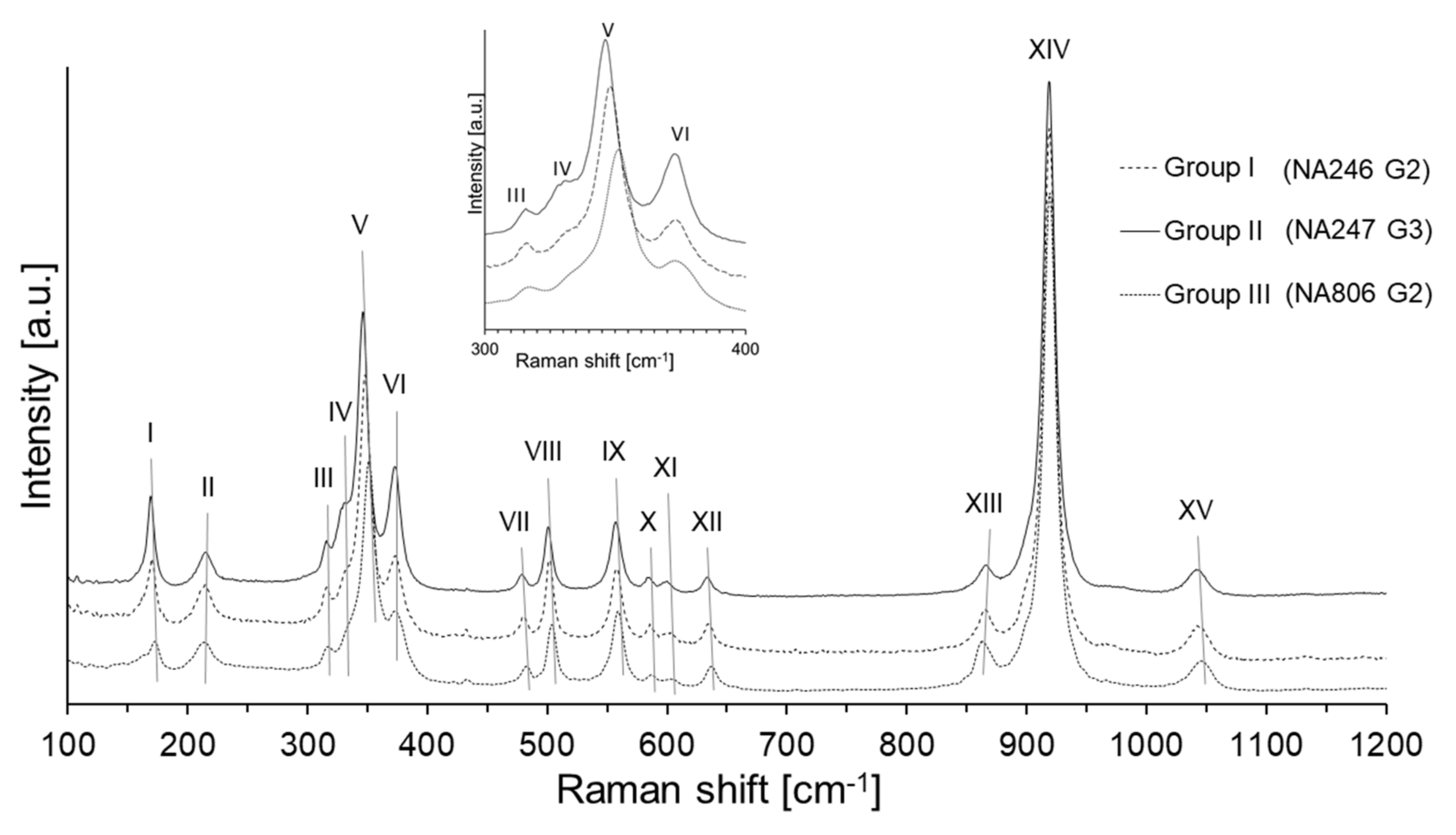
| Assignment (mode) | Site Motion [22] | Band | S-fibulae | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NA246 | NA247 | NA401 | NA843 | NA806 | NA892 | ||||||||||||||||
| G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | G4 | G5 | G1 | G2 | |||
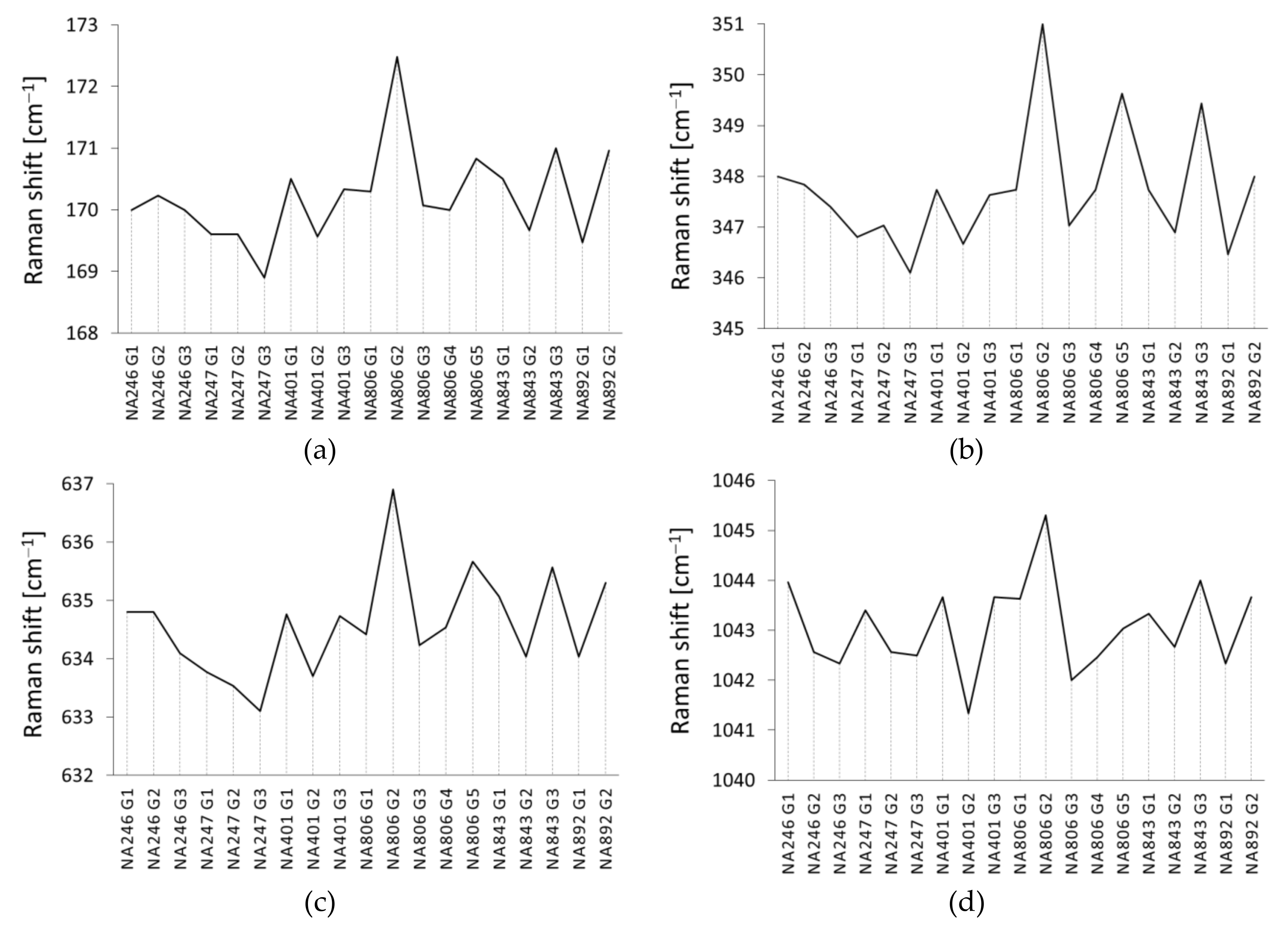
| Translation of X cation | T(X2+) | I | 170.0 | 170.2 | 170.0 | 169.6 | 169.6 | 168.9 | 170.5 | 169.6 | 170.3 | 170.5 | 169.7 | 171.0 | 170.3 | 172.5 | 170.1 | 170.0 | 170.8 | 169.5 | 171.0 |
| Translation of SiO4 | T(SiO4) | II | 214.4 | 214.2 | 213.5 | 215.0 | 215.0 | 215.0 | 214.2 | 215.6 | 214.5 | 213.9 | 215.2 | 214.0 | 214.3 | 213.2 | 215.5 | 214.4 | 213.7 | 215.9 | 214.2 |
| Rotation of SiO4 | R(SiO4) | III | 316.2 | 315.7 | 315.8 | 315.9 | 316.0 | 315.6 | 316.4 | 315.9 | 316.1 | 316.5 | 316.6 | 316.6 | 316.3 | 317.1 | 315.9 | 315.9 | 316.6 | 315.7 | 316.3 |
| T(SiO4) | IV | 330.8 | 330.8 | 331.5 | 330.8 | 330.9 | 330.3 | 332.3 | 331.9 | 333.0 | 332.9 | 331.9 | - | 331.9 | - | 330.6 | 330.8 | 333.6 | 330.3 | 333.5 | |
| R(SiO4) | V | 348.0 | 347.8 | 347.4 | 346.8 | 347.0 | 346.1 | 347.7 | 346.7 | 347.6 | 347.7 | 346.9 | 349.4 | 347.7 | 351.0 | 347.0 | 347.7 | 349.6 | 346.5 | 348.0 | |
| R(SiO4) | VI | 373.2 | 373.0 | 373.2 | 373.1 | 372.9 | 372.9 | 373.2 | 373.0 | 373.3 | 374.1 | 373.4 | 373.6 | 373.8 | 372.6 | 373.6 | 372.9 | 372.8 | 373.0 | 373.2 | |
| Si–O bending | ν2 | VII | 481.0 | 479.7 | 479.7 | 479.7 | 479.5 | 478.9 | 480.3 | 479.8 | 480.1 | 480.4 | 479.7 | 481.2 | 480.1 | 482.0 | 480.2 | 480.6 | 481.0 | 479.6 | 480.4 |
| ν4 | VIII | 501.8 | 501.8 | 501.4 | 501.4 | 501.1 | 500.9 | 501.9 | 501.0 | 501.8 | 501.9 | 501.3 | 502.7 | 502.1 | 503.7 | 501.6 | 502.2 | 502.7 | 501.0 | 502.0 | |
| ν2 | IX | 557.7 | 557.9 | 557.7 | 557.8 | 557.6 | 556.9 | 558.0 | 557.5 | 558.0 | 558.1 | 557.6 | 558.4 | 558.0 | 559.0 | 558.3 | 558.0 | 558.1 | 557.7 | 558.3 | |
| ν4 | X | 585.4 | 585.6 | 585.3 | 584.7 | 585.2 | 584.5 | 585.8 | 584.5 | 585.6 | 585.7 | 585.2 | 585.9 | 585.8 | 587.0 | 585.2 | 585.1 | 585.8 | 584.9 | 585.9 | |
| ν4 | XI | 600.2 | 600.4 | 599.7 | 599.3 | 600.2 | 599.0 | 601.1 | 599.8 | 599.6 | 600.6 | 600.0 | 602.0 | 600.9 | 602.6 | 600.3 | 601.5 | 601.7 | 596.0 | 599.0 | |
| ν4 | XII | 634.8 | 634.8 | 634.1 | 633.8 | 633.5 | 633.1 | 634.8 | 633.7 | 634.7 | 635.1 | 634.0 | 635.6 | 634.4 | 636.9 | 634.2 | 634.5 | 635.7 | 634.0 | 635.3 | |
| Si–O stretching | ν3 | XIII | 866.4 | 865.4 | 866.1 | 866.0 | 865.8 | 866.0 | 865.6 | 865.3 | 865.7 | 866.4 | 866.3 | 864.3 | 865.5 | 864.6 | 866.5 | 864.9 | 862.9 | 865.6 | 866.7 |
| ν1 | XIV | 919.3 | 918.8 | 918.8 | 919.2 | 919.2 | 918.5 | 918.9 | 918.4 | 918.8 | 919.1 | 918.7 | 918.3 | 919.2 | 919.0 | 919.2 | 918.3 | 917.4 | 918.4 | 919.5 | |
| ν3 | XV | 1044.0 | 1042.6 | 1042.3 | 1043.4 | 1042.6 | 1042.5 | 1043.7 | 1041.3 | 1043.7 | 1043.3 | 1042.7 | 1044.0 | 1043.6 | 1045.3 | 1042.0 | 1042.5 | 1043.0 | 1042.3 | 1043.7 | |
| Type of Inclusion | Mineralogy and Chemical Formula | Artefact and Garnet |
|---|---|---|
| Quartz SiO2 | NA401 G3, NA806 G2, G4 and G5 | |
| Transparent crystal | Apatite Ca5(PO4)3(Cl/F/OH) | All garnets of fibulae NA246, NA247, NA401, NA806 G1 and G4 |
| Mica (muscovite KAl2(AlSi3O10)(OH)2 or phlogopite KMg3(AlSi3O10)(OH)2) | NA401 G3, NA806 G2, G4 and G5 | |
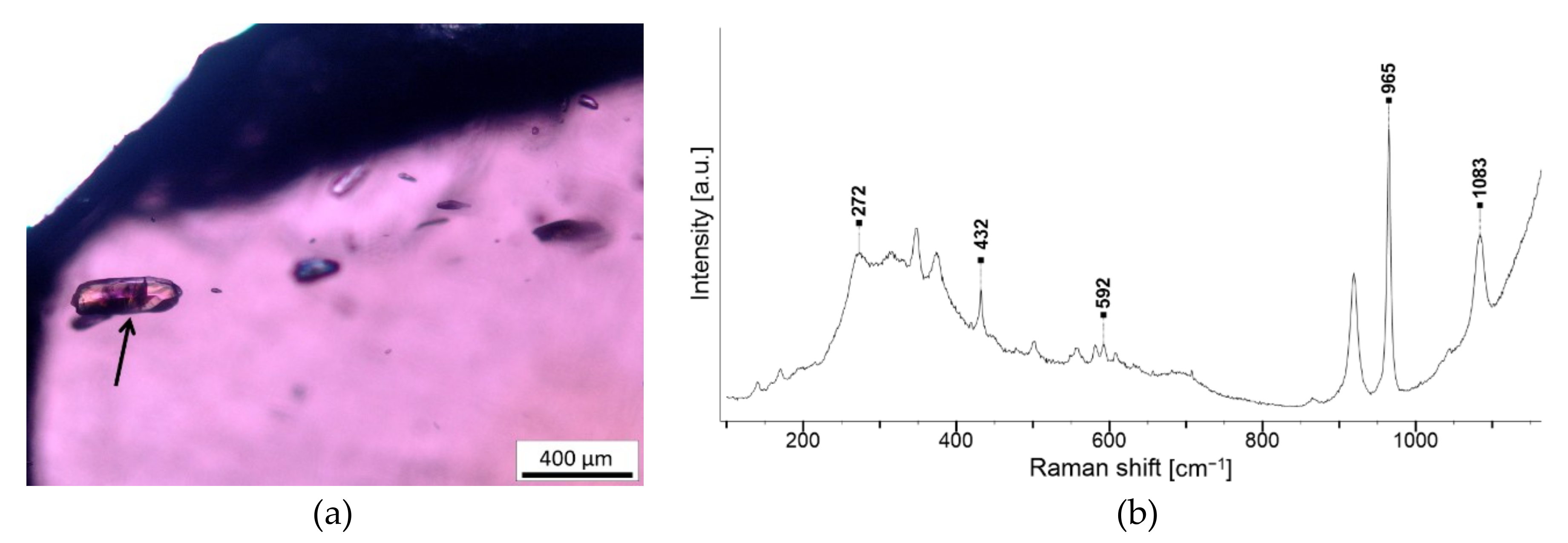
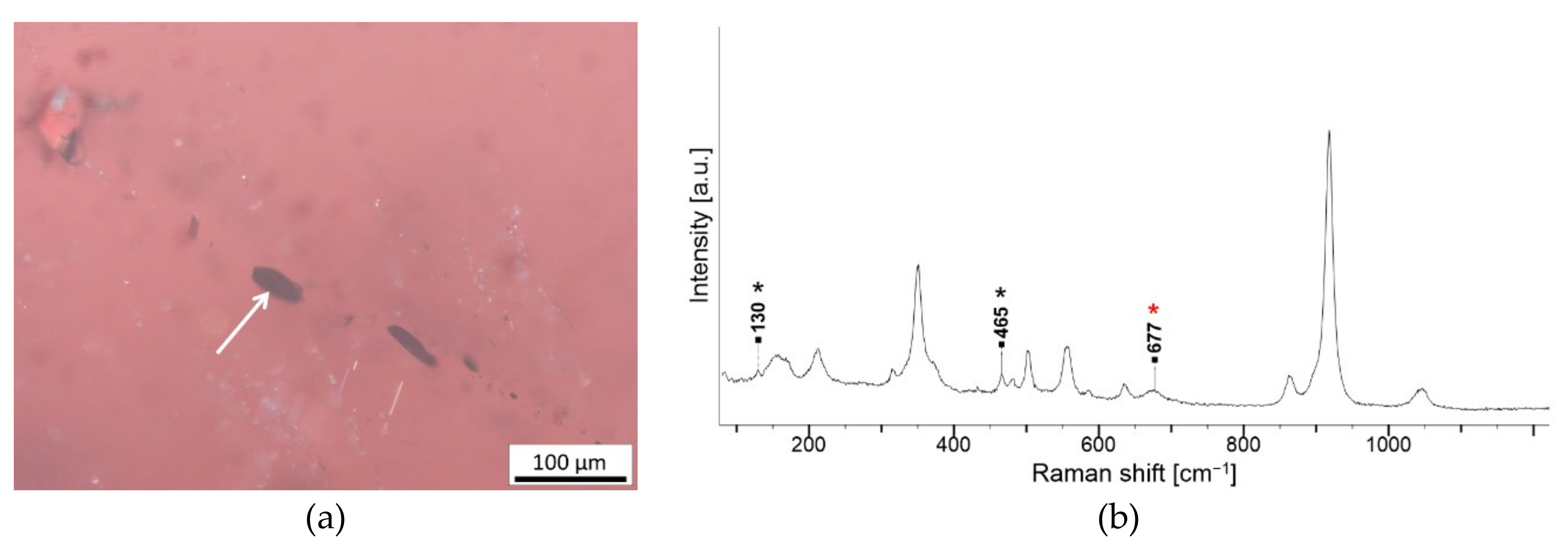
| Crystal with radiation halo, pleochroic halo or tension fissures | Zircon Zr(SiO4) | NA246 G2, NA247 G2, NA401 G2, NA806 G2, 3 and 5, NA843 G2 |
| Transparent fibres | Sillimanite Al2(SiO4)O | NA806 G2 |
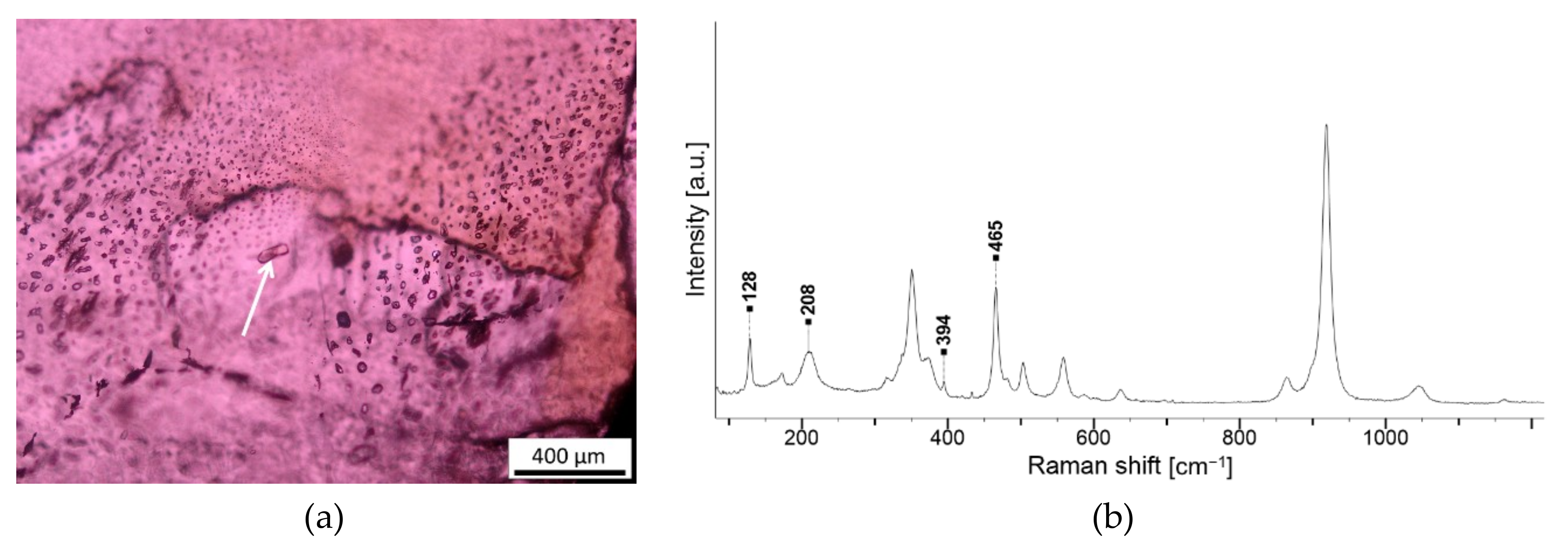
| Opaque clusters | Magnetite (Fe2+,Fe3+)2O4, ilmenite Fe2+TiO3 | NA247 G1 and 2, NA401 G2 |
| Opaque needles | Rutile α-TiO2 | NA806 G5, NA843 G3 |
| Brownish plate | Biotite K(Fe2+/Mg)2(Al/Fe3+/Mg)([Si/Al]Si2O10)(OH/F)2 or Xenotime (Y/Yb)(PO4) | NA892 G2 |
| Spherical inclusions | unidentified (melt, fluid or gas) | NA843 G1, NA892 G2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kos, S.; Dolenec, M.; Lux, J.; Dolenec, S. Raman Microspectroscopy of Garnets from S-Fibulae from the Archaeological Site Lajh (Slovenia). Minerals 2020, 10, 325. https://doi.org/10.3390/min10040325
Kos S, Dolenec M, Lux J, Dolenec S. Raman Microspectroscopy of Garnets from S-Fibulae from the Archaeological Site Lajh (Slovenia). Minerals. 2020; 10(4):325. https://doi.org/10.3390/min10040325
Chicago/Turabian StyleKos, Saša, Matej Dolenec, Judita Lux, and Sabina Dolenec. 2020. "Raman Microspectroscopy of Garnets from S-Fibulae from the Archaeological Site Lajh (Slovenia)" Minerals 10, no. 4: 325. https://doi.org/10.3390/min10040325
APA StyleKos, S., Dolenec, M., Lux, J., & Dolenec, S. (2020). Raman Microspectroscopy of Garnets from S-Fibulae from the Archaeological Site Lajh (Slovenia). Minerals, 10(4), 325. https://doi.org/10.3390/min10040325





