Element Patterns of Primary Low-Magnesium Calcite from the Seafloor of the Gulf of Mexico
Abstract
1. Introduction
2. Geological Setting
3. Materials and Methods
4. Results
4.1. Element Contents of Carbonate Phases and Bulk Samples
4.2. Element Distribution in Carbonates
5. Discussion
5.1. Geochemical Features of Primary LMC
5.2. Element Mapping of Carbonates
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sandberg, P.A. An oscillating trend in Phanerozoic non-skeletal carbonate mineralogy. Nature 1983, 305, 19–22. [Google Scholar] [CrossRef]
- Farkaš, J.; Böhm, F.; Wallmann, K.; Blenkinsop, J.; Eisenhauer, A.; van Geldern, R.; Munnecke, A.; Voigt, S.; Veizer, J. Calcium isotope record of Phanerozoic oceans: Implications for chemical evolution of seawater and its causative mechanisms. Geochim. Cosmochim. Acta 2007, 71, 5117–5134. [Google Scholar] [CrossRef]
- Tong, H.; Feng, D.; Cheng, H.; Yang, S.; Wang, H.; Min, A.G.; Edwards, R.L.; Chen, Z.; Chen, D. Authigenic carbonates from seeps on the northern continental slope of the South China Sea: New insights into fluid sources and geochronology. Mar. Pet. Geol. 2013, 43, 260–271. [Google Scholar] [CrossRef]
- Baker, P.A.; Kastner, M. Constraints on the formation of sedimentary dolomite. Science 1981, 213, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Peckmann, J.; Reimer, A.; Luth, U.; Luth, C.; Hansen, B.T.; Heinicke, C.; Hoefs, J.; Reitner, J. Methane-derived carbonates and authigenic pyrite from the northwestern Black Sea. Mar. Geol. 2001, 177, 129–150. [Google Scholar] [CrossRef]
- Joseph, C.; Campbell, K.A.; Torres, M.E.; Martin, R.A.; Pohlman, J.W.; Riedel, M.; Rose, K. Methane-derived authigenic carbonates from modern and paleoseeps on the Cascadia margin: Mechanisms of formation and diagenetic signals. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 390, 52–67. [Google Scholar] [CrossRef]
- Johnson, W.J.; Goldstein, R.H. Cambrian sea water preserved as inclusions in marine low-magnesium calcite cement. Nature 1993, 362, 335–337. [Google Scholar] [CrossRef]
- Tong, H.; Wang, Q.; Peckmann, J.; Cao, Y.; Chen, L.; Zhou, W.; Chen, D. Diagenetic alteration affecting δ18O, δ13C and 87Sr/86Sr signatures of carbonates: A case study on Cretaceous seep deposits from Yarlung-Zangbo Suture Zone, Tibet, China. Chem. Geol. 2016, 444, 71–82. [Google Scholar] [CrossRef]
- Kimmig, S.R.; Holmden, C. Multi-proxy geochemical evidence for primary aragonite precipitation in a tropical-shelf ‘calcite sea’ during the Hirnantian glaciation. Geochim. Cosmochim. Acta 2017, 206, 254–272. [Google Scholar] [CrossRef]
- Zwicker, J.; Smrzka, D.; Himmler, T.; Monien, P.; Gier, S.; Goedert, J.L.; Peckmann, J. Rare earth elements as tracers for microbial activity and early diagenesis: A new perspective from carbonate cements of ancient methane-seep deposits. Chem. Geol. 2018, 501, 77–85. [Google Scholar] [CrossRef]
- Hood, A.V.S.; Planavsky, N.J.; Wallace, M.W.; Wang, X. The effects of diagenesis on geochemical paleoredox proxies in sedimentary carbonates. Geochim. Cosmochim. Acta 2018, 232, 265–287. [Google Scholar] [CrossRef]
- Feng, D.; Roberts, H.H.; Joye, S.B.; Heydari, E. Formation of low-magnesium calcite at cold seeps in an aragonite sea. Terra Nova 2014, 26, 150–156. [Google Scholar] [CrossRef]
- Huang, H.; Gong, S.; Li, N.; Birgel, D.; Peckmann, J.; Jin, M.; Cheng, M.; Roberts, H.H.; Chen, D.; Feng, D. Formation of authigenic low-magnesium calcite from sites SS296 and GC53 of the Gulf of Mexico. Minerals 2019, 9, 251. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X.; Gong, S.; Krake, N.; Jin, M.; Li, N.; Birgel, D.; Peckmann, J.; Cheng, M.; Roberts, H.H.; et al. New constraints on the formation of hydrocarbon-derived low magnesium calcite at brine seeps in the Gulf of Mexico. Sediment. Geol. 2020, 398, 105572. [Google Scholar] [CrossRef]
- Hardie, L.A. Secular variation in seawater chemistry: An explanation for the coupled secular variation in the mineralogies of marine limestones and potash evaporites over the past 600 m. Geology 1996, 24, 279–283. [Google Scholar] [CrossRef]
- Aloisi, G.; Pierre, C.; Rouchy, J.-M.; Foucher, J.-P.; Woodside, J. Methane-Related authigenic carbonates of eastern Mediterranean Sea mud volcanoes and their possible relation to gas hydrate destabilisation. Earth Planet. Sci. Lett. 2000, 184, 321–338. [Google Scholar] [CrossRef]
- Stanley, S.M.; Ries, J.B.; Hardie, L.A. Low-Magnesium calcite produced by coralline algae in seawater of late cretaceous composition. Proc. Natl. Acad. Sci. USA 2002, 99, 15323–15326. [Google Scholar] [CrossRef]
- Roberts, H.H.; Shedd, W.; Hunt, J. Dive site geology: DSV ALVIN (2006) and ROV JASON II (2007) dives to the middle-lower continental slope, northern Gulf of Mexico. Deep Sea Res. Part II 2010, 57, 1837–1858. [Google Scholar] [CrossRef]
- Roberts, H.H.; Feng, D.; Joye, S.B. Cold-seep carbonates of the middle and lower continental slope, northern Gulf of Mexico. Deep Sea Res. Part II 2010, 57, 2040–2054. [Google Scholar] [CrossRef]
- Longman, M.W. Carbonate diagenetic textures from nearsurface diagenetic environments. Am. Assoc. Pet. Geol. Bull. 1980, 64, 461–487. [Google Scholar]
- Naehr, T.H.; Eichhubl, P.; Orphan, V.J.; Hovland, M.; Paull, C.K.; Ussler, W.; Lorenson, T.D.; Greene, H.G. Authigenic carbonate formation at hydrocarbon seeps in continental margin sediments: A comparative study. Deep Sea Res. Part II 2007, 54, 1268–1291. [Google Scholar] [CrossRef]
- Gomberg, D.N.; Bonatti, E. High-Magnesian calcite: Leaching of magnesium in the deep sea. Science 1970, 168, 1451–1453. [Google Scholar] [CrossRef]
- Schlager, W.; James, N.P. Low-Magnesian calcite limestones forming at the deep-sea floor, Tongue of the Ocean, Bahamas. Sedimentology 1978, 25, 675–702. [Google Scholar] [CrossRef]
- Banner, J.L.; Hanson, G.N. Calculation of simultaneous isotopic and trace element variations during water-rock interaction with applications to carbonate diagenesis. Geochim. Cosmochim. Acta 1990, 54, 3123–3137. [Google Scholar] [CrossRef]
- Swart, P.K. The geochemistry of carbonate diagenesis: The past, present and future. Sedimentology 2015, 62, 1233–1304. [Google Scholar] [CrossRef]
- Casella, L.A.; Griesshaber, E.; Yin, X.; Ziegler, A.; Mavromatis, V.; Müller, D.; Ritter, A.C.; Hippler, D.; Harper, E.M.; Dietzel, M.; et al. Experimental diagenesis: Insights into aragonite to calcite transformation of Arctica islandica shells by hydrothermal treatment. Biogeosciences 2017, 14, 1461–1492. [Google Scholar] [CrossRef]
- Domínguez-Villar, D.; Krklec, K.; Pelicon, P.; Fairchild, I.J.; Cheng, H.; Edwards, L.R. Geochemistry of speleothems affected by aragonite to calcite recrystallization—Potential inheritance from the precursor mineral. Geochim. Cosmochim. Acta 2017, 200, 310–329. [Google Scholar] [CrossRef]
- Budd, D.A. Petrographic products of freshwater diagenesis in Holocene ooid sands, Schooner Cays, Bahamas. Carbonates Evaporites 1988, 3, 143–163. [Google Scholar] [CrossRef]
- Frank, T.D.; Lohmann, K.C. Diagenesis of fibrous magnesian calcite marine cement: Implications for the interpretation of δ18O and δ13C values of ancient equivalents. Geochim. Cosmochim. Acta 1996, 60, 2427–2436. [Google Scholar] [CrossRef]
- Denison, R.E.; Koepnick, R.B.; Burke, W.H.; Hetherington, E.A. Construction of the Cambrian and Ordovician seawater 87Sr/86Sr curve. Chem. Geol. 1998, 152, 325–340. [Google Scholar] [CrossRef]
- Beauchamp, B.; Savard, M. Cretaceous chemosynthetic carbonate mounds in the Canadian Arctic. Palaios 1992, 7, 434–450. [Google Scholar] [CrossRef]
- Brand, U. Carbon, oxygen and strontium isotopes in Paleozoic carbonate components: An evaluation of original seawater-chemistry proxies. Chem. Geol. 2004, 204, 23–44. [Google Scholar] [CrossRef]
- Perdikouri, C.; Piazolo, S.; Kasioptas, A.; Schmidt, B.C.; Putnis, A. Hydrothermal replacement of aragonite by calcite: Interplay between replacement, fracturing and growth. Eur. J. Mineral. 2013, 25, 123–136. [Google Scholar] [CrossRef]
- Salvador, A. Late Triassic-Jurassic paleogeography and origin of Gulf of Mexico basin. Am. Assoc. Pet. Geol. Bull. 1987, 71, 419–451. [Google Scholar]
- Roberts, H.H.; Aharon, P.; Carney, R.; Larkin, J.; Sassen, R. Sea floor responses to hydrocarbon seeps, Louisiana continental slope. Geo-Mar. Lett. 1990, 10, 232–243. [Google Scholar] [CrossRef]
- Roberts, H.H.; Aharon, P. Hydrocarbon-derived carbonate buildups of the northern Gulf of Mexico continental slope: A review of submersible investigations. Geo-Mar. Lett. 1994, 14, 135–148. [Google Scholar] [CrossRef]
- Sassen, R.; MacDonald, I.R. Evidence of structure H hydrate, Gulf of Mexico continental slope. Org. Geochem. 1994, 22, 1029–1032. [Google Scholar] [CrossRef]
- Feng, D.; Chen, D.; Roberts, H.H. Petrographic and geochemical characterization of seep carbonate from Bush Hill (GC 185) gas vent and hydrate site of the Gulf of Mexico. Mar. Pet. Geol. 2009, 26, 1190–1198. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, D.; Peckmann, J.; Roberts, H.H.; Chen, D. New insights into cerium anomalies and mechanisms of trace metal enrichment in authigenic carbonate from hydrocarbon seeps. Chem. Geol. 2014, 381, 55–66. [Google Scholar] [CrossRef]
- Aharon, P.; Roberts, H.H.; Snelling, R. Submarine venting of brines in the deep Gulf of Mexico: Observations and geochemistry. Geology 1992, 20, 483–486. [Google Scholar] [CrossRef]
- Fu, B.; Aharon, P. Sources of hydrocarbon-rich fluids advecting on the seafloor in the Northern Gulf of Mexico. Gulf Coast Assoc. Geol. Soc. Trans. 1998, 82, 73–82. [Google Scholar]
- Cook, D.J.; Donfro, P. Jolliet Field thrust fault structure and stratigraphy, Green Canyon Block 184, offshore Louisiana. Am. Assoc. Pet. Geol. Bull. 1991, 41, 100–121. [Google Scholar]
- Brooks, J.M.; Fisher, C.; Roberts, H.; Carney, B.; Macdonald, I.; Cordes, E.; Joye, S.; Goehring, L.; Bernard, B.; Wolff, G. Investigations of Chemosynthetic Communities on the Lower Continental Slope of the Gulf of Mexico Volume I: Final Report; U.S. Department of the Interior, Bureau of Ocean Energy Management, Gulf of Mexico OCS Region: New Orleans, LA, USA, 2014. [Google Scholar]
- Tucker, M.E.; Wright, V.P. Carbonate Mineralogy and Chemistry. In Carbonate Sedimentology; Blackwell Publishing Ltd.: Oxford, UK, 1990; pp. 284–313. [Google Scholar]
- Smrzka, D.; Zwicker, J.; Bach, W.; Feng, D.; Himmler, T.; Chen, D.; Peckmann, J. The behavior of trace elements in seawater, sedimentary pore water, and their incorporation into carbonate minerals: A review. Facies 2019, 65, 41. [Google Scholar] [CrossRef]
- Brand, U. Aragonite-Calcite transformation based on Pennsylvanian molluscs. Geol. Soc. Am. Bull. 1989, 101, 377–390. [Google Scholar] [CrossRef]
- Buggisch, W.; Krumm, S. Palaeozoic cold seep carbonates from Europe and North Africa—An integrated isotopic and geochemical approach. Facies 2005, 51, 566–583. [Google Scholar] [CrossRef]
- Peckmann, J.L.; Campbell, K.A.; Walliser, O.H.; Reitner, J. A Late Devonian hydrocarbon-seep deposit dominated by dimerlloid brachiopods, Morocco. Palaios 2007, 22, 114–122. [Google Scholar] [CrossRef]
- Bayon, G.; Pierre, C.; Etoubleau, J.; Voisset, M.; Cauquil, E.; Marsset, T.; Sultan, N.; Le Drezen, E.; Fouquet, Y. Sr/Ca and Mg/Ca ratios in Niger Delta sediments: Implications for authigenic carbonate genesis in cold seep environments. Mar. Geol. 2007, 241, 93–109. [Google Scholar] [CrossRef]
- Brand, U.; Veizer, J. Chemical diagenesis of a multicomponent carbonate system; 1, Trace elements. J. Sediment. Petrol. 1980, 50, 1219–1236. [Google Scholar]
- Derry, L.A.; Kaufman, A.J.; Jacobsen, S.B. Sedimentary cycling and environmental change in the Late Proterozoic: Evidence from stable and radiogenic isotopes. Geochim. Cosmochim. Acta 1992, 56, 1317–1329. [Google Scholar] [CrossRef]
- Edwards, C.T.; Saltzman, M.R.; Leslie, S.A.; Bergström, S.M.; Sedlacek, A.R.C.; Howard, A.; Bauer, J.A.; Sweet, W.C.; Young, S.A. Strontium isotope (87Sr/86Sr) stratigraphy of Ordovician bulk carbonate: Implications for preservation of primary seawater values. Geol. Soc. Am. Bull. 2015, 127, 1275–1289. [Google Scholar] [CrossRef]
- Mucci, A.; Morse, J.W. The incorporation of divalent Mg and divalent Sr into calcite overgrowths: Influences of growth rate and solution composition. Geochim. Cosmochim. Acta 1983, 47, 217–233. [Google Scholar] [CrossRef]
- Jacobsen, S.B.; Kaufman, A.J. The Sr, C and O isotopic evolution of Neoproterozoic seawater. Chem. Geol. 1999, 161, 37–57. [Google Scholar] [CrossRef]
- Denison, R.E.; Koepnick, R.B.; Fletcher, A.; Howell, M.W.; Callaway, W.S. Criteria for the retention of original seawater 87Sr86Sr in ancient shelf limestones. Chem. Geol. 1994, 112, 131–143. [Google Scholar] [CrossRef]


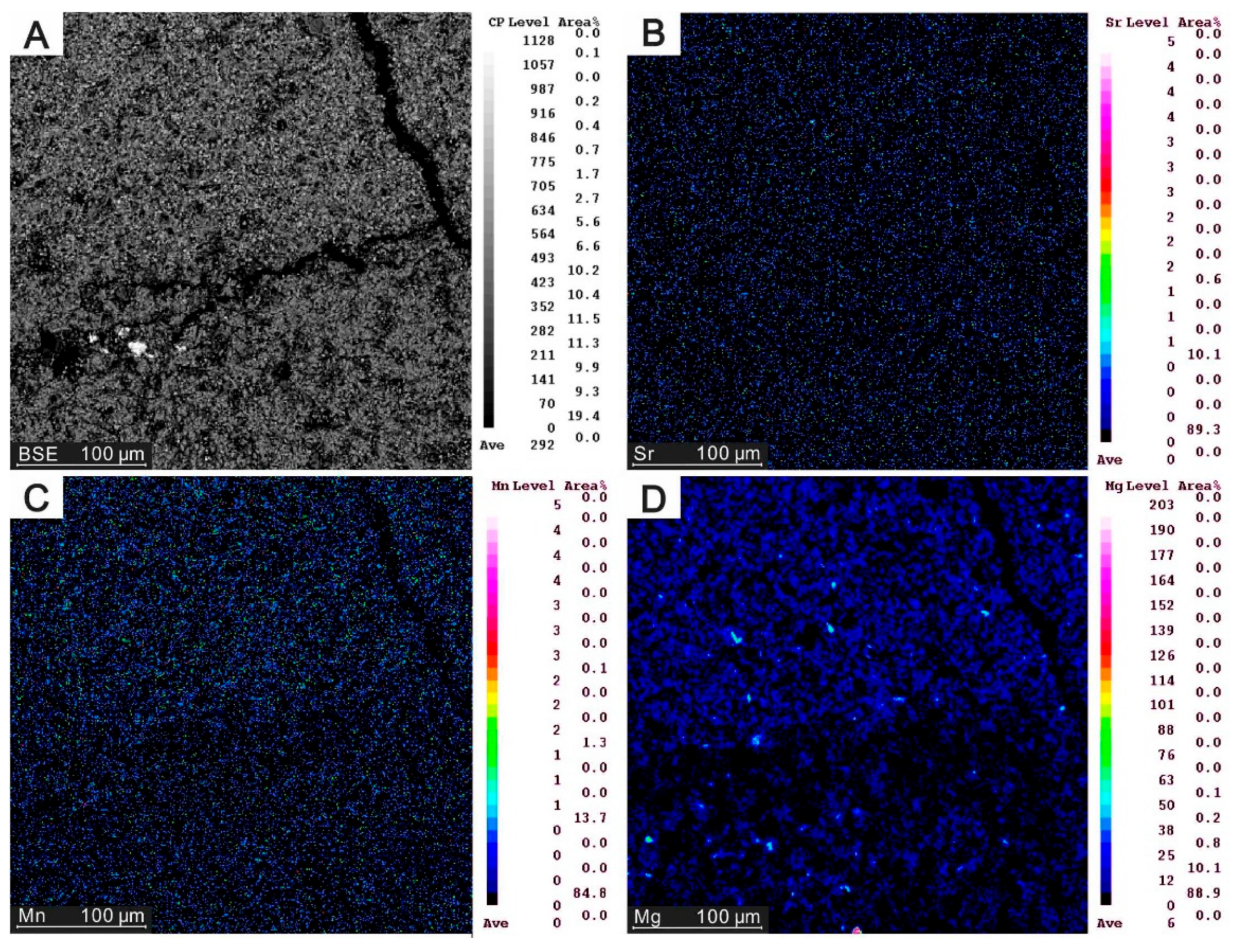
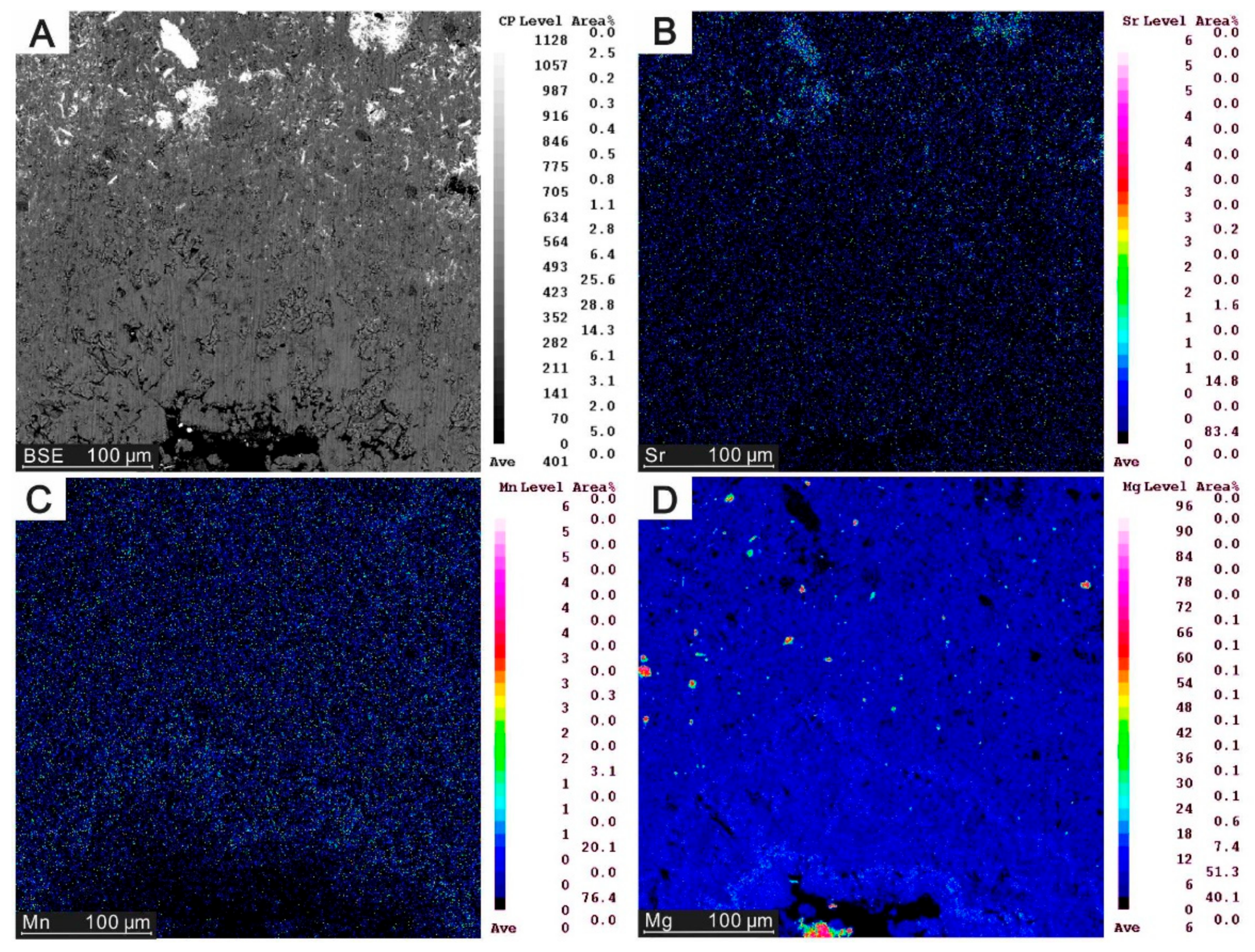
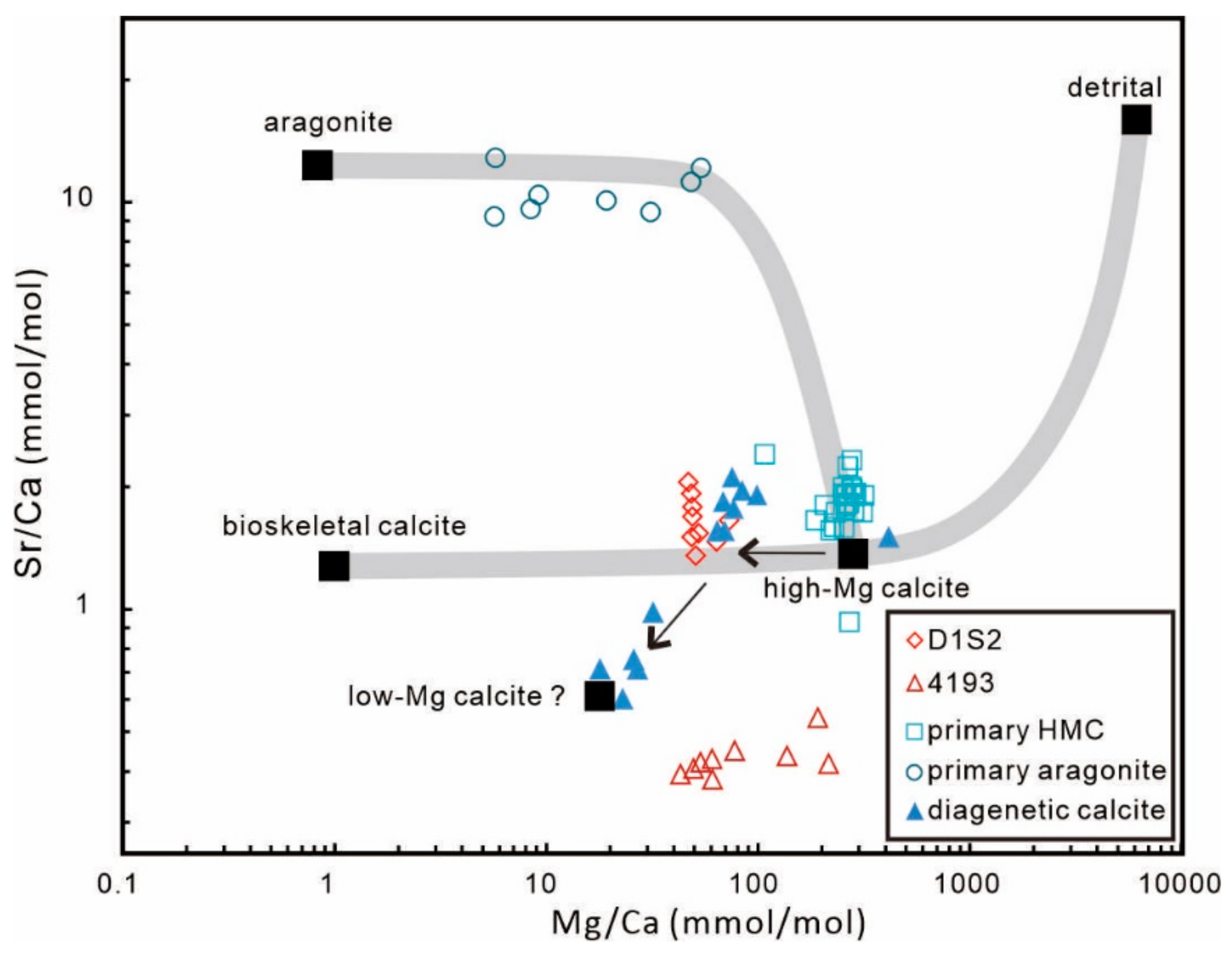
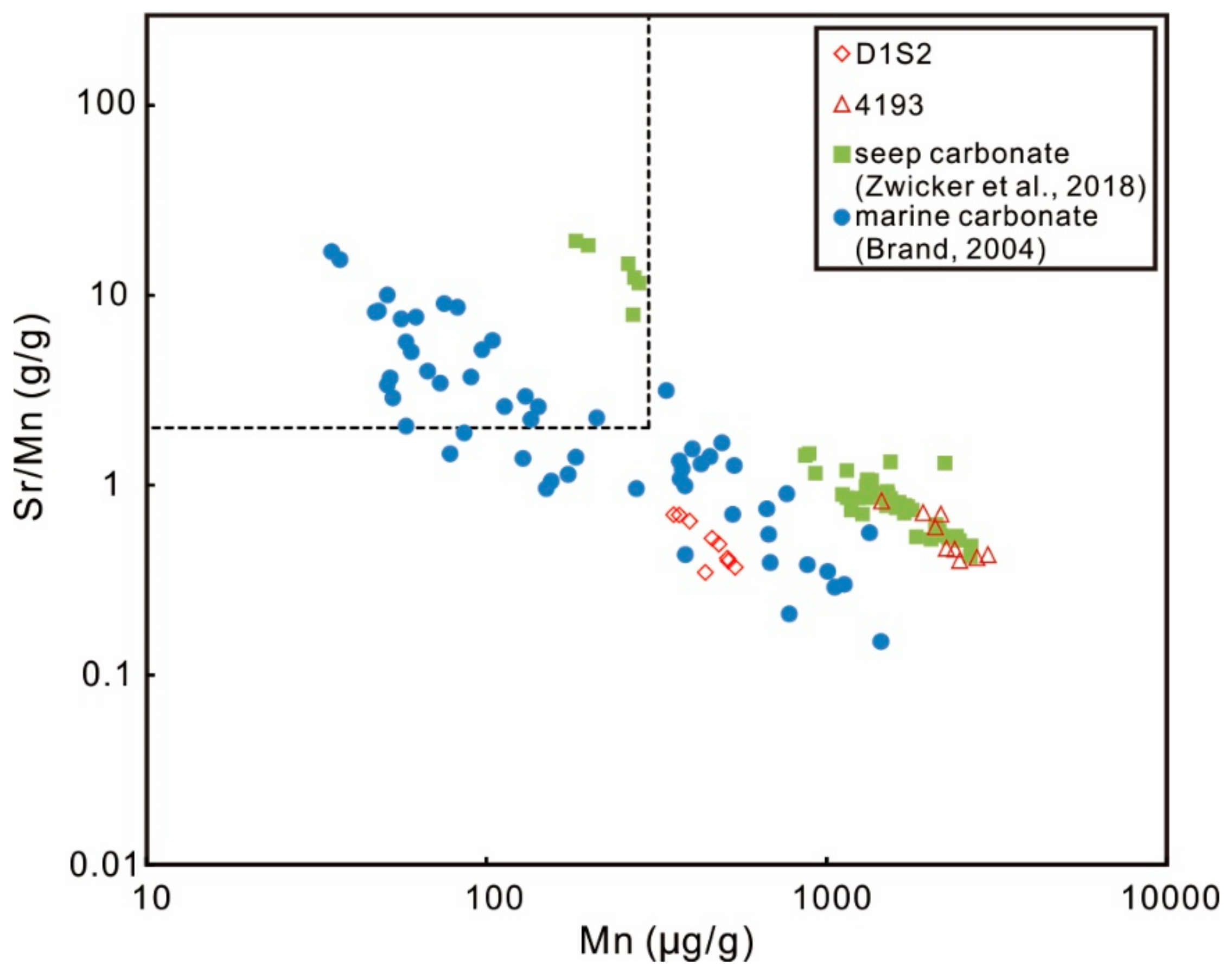
| Site | Sample ID | Quartz | Barite | Siderite | Low-Mg calcite | MgCO3 (%) |
|---|---|---|---|---|---|---|
| GC53 | D1S2 * | 5 | 37 | 58 | 5 | |
| AC601 | 4193 ** | 11 | 11 | 78 | 5 |
| Sample No. | CaO | MgO | Fe2O3-T% | Sr | Mn | Sr/Mn | Sr/Ca | Mn/Ca | Mg/Ca |
|---|---|---|---|---|---|---|---|---|---|
| % | % | % | μg/g | μg/g | μg/g | mmol/mol | mmol/mol | ||
| D1S2-1 | 29.1 | 2.85 | 18.1 | 199 | 539 | 0.37 | 0.44 | 1.89 | 137 |
| D1S2-2 | 23.4 | 3.61 | 25.0 | 153 | 441 | 0.35 | 0.42 | 1.92 | 216 |
| D1S2-3 | 24.9 | 3.41 | 23.7 | 211 | 512 | 0.41 | 0.54 | 2.10 | 192 |
| D1S2-4 | 34.3 | 1.91 | 9.48 | 242 | 462 | 0.52 | 0.45 | 1.37 | 78 |
| D1S2-5 | 38.0 | 1.66 | 7.81 | 256 | 396 | 0.65 | 0.43 | 1.06 | 61 |
| D1S2-6 | 41.6 | 1.28 | 5.11 | 257 | 369 | 0.70 | 0.39 | 0.90 | 43 |
| D1S2-7 | 34.2 | 1.49 | 5.39 | 205 | 515 | 0.40 | 0.38 | 1.53 | 61 |
| D1S2-8 | 35.7 | 1.37 | 4.69 | 236 | 483 | 0.49 | 0.42 | 1.38 | 54 |
| D1S2-9 | 38.9 | 1.38 | 4.75 | 248 | 355 | 0.70 | 0.41 | 0.93 | 50 |
| Average | 33.3 | 2.11 | 11.6 | 223 | 452 | 0.51 | 0.43 | 1.45 | 99 |
| Std Dev | 6.0 | 0.87 | 7.90 | 32 | 63 | 0.13 | 0.04 | 0.42 | 62 |
| 4193-1 | 45.7 | 1.58 | 0.86 | 1380 | 1926 | 0.72 | 1.92 | 4.29 | 48 |
| 4193-2 | 47.5 | 1.60 | 0.83 | 1530 | 2176 | 0.70 | 2.05 | 4.67 | 47 |
| 4193-3 | 46.3 | 2.42 | 1.20 | 1200 | 1454 | 0.83 | 1.65 | 3.20 | 73 |
| 4193-4 | 47.7 | 1.79 | 0.83 | 1150 | 2778 | 0.41 | 1.53 | 5.92 | 53 |
| 4193-5 | 46.6 | 1.62 | 0.91 | 1100 | 2388 | 0.46 | 1.50 | 5.21 | 49 |
| 4193-6 | 46.7 | 1.70 | 1.13 | 993 | 2472 | 0.40 | 1.35 | 5.39 | 51 |
| 4193-7 | 45.6 | 2.08 | 0.99 | 1050 | 2260 | 0.46 | 1.47 | 5.05 | 64 |
| 4193-8 | 48.6 | 1.70 | 0.69 | 1290 | 2993 | 0.43 | 1.69 | 6.27 | 49 |
| 4193-9 | 45.0 | 1.58 | 0.71 | 1260 | 2096 | 0.60 | 1.78 | 4.74 | 49 |
| Average | 46.6 | 1.79 | 0.90 | 1217 | 2283 | 0.56 | 1.66 | 4.97 | 54 |
| Std Dev | 1.1 | 0.27 | 0.17 | 159 | 429 | 0.15 | 0.21 | 0.85 | 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Wang, X.; Gong, S.; Krake, N.; Birgel, D.; Peckmann, J.; Chen, D.; Feng, D. Element Patterns of Primary Low-Magnesium Calcite from the Seafloor of the Gulf of Mexico. Minerals 2020, 10, 299. https://doi.org/10.3390/min10040299
Huang H, Wang X, Gong S, Krake N, Birgel D, Peckmann J, Chen D, Feng D. Element Patterns of Primary Low-Magnesium Calcite from the Seafloor of the Gulf of Mexico. Minerals. 2020; 10(4):299. https://doi.org/10.3390/min10040299
Chicago/Turabian StyleHuang, Huiwen, Xudong Wang, Shanggui Gong, Nicola Krake, Daniel Birgel, Jörn Peckmann, Duofu Chen, and Dong Feng. 2020. "Element Patterns of Primary Low-Magnesium Calcite from the Seafloor of the Gulf of Mexico" Minerals 10, no. 4: 299. https://doi.org/10.3390/min10040299
APA StyleHuang, H., Wang, X., Gong, S., Krake, N., Birgel, D., Peckmann, J., Chen, D., & Feng, D. (2020). Element Patterns of Primary Low-Magnesium Calcite from the Seafloor of the Gulf of Mexico. Minerals, 10(4), 299. https://doi.org/10.3390/min10040299





