Li and Ca Enrichment in the Bristol Dry Lake Brine Compared to Brines from Cadiz and Danby Dry Lakes, Barstow-Bristol Trough, California, USA
Abstract
1. Introduction
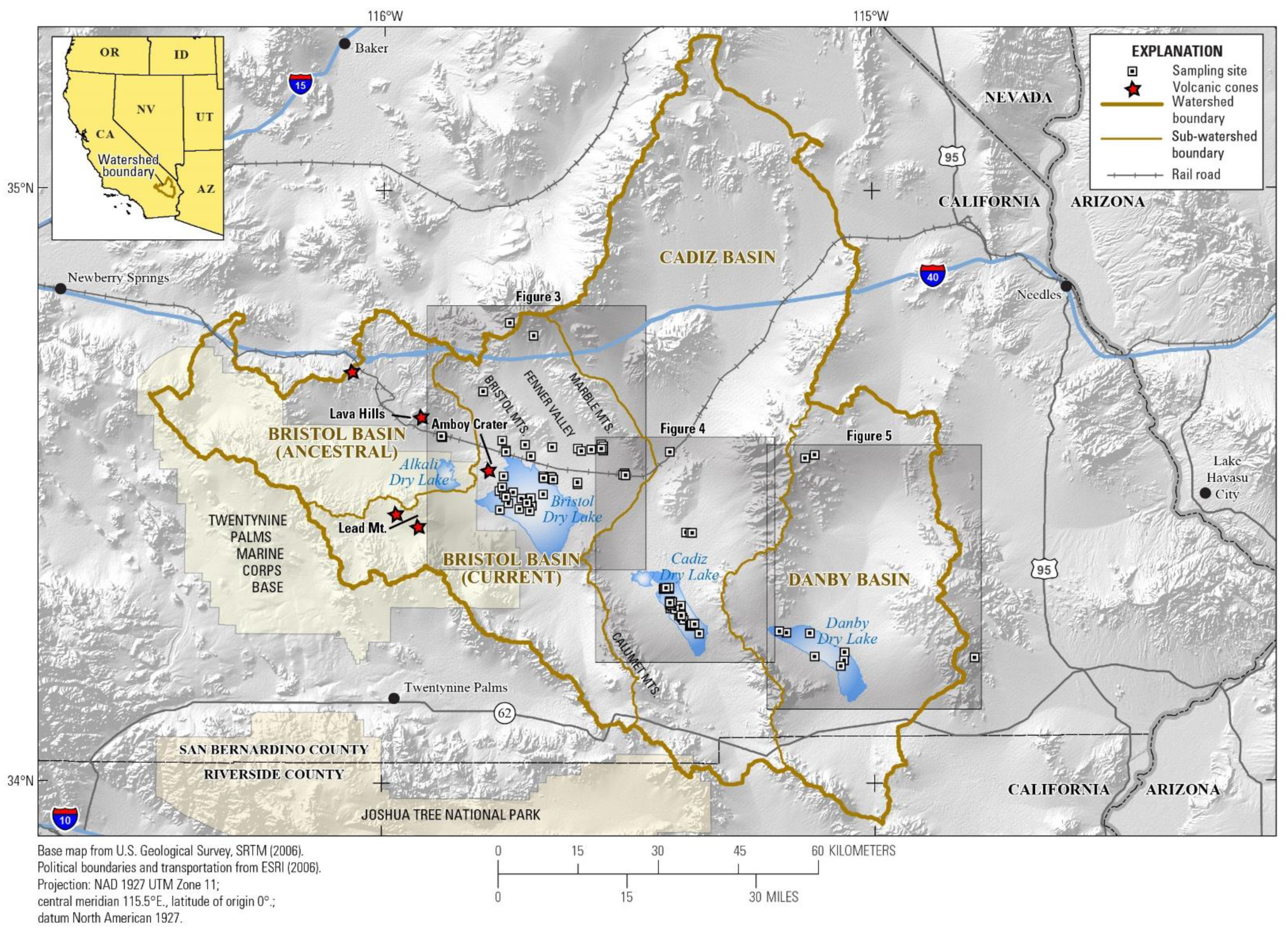
2. Geologic and Hydrologic Background of the Barstow-Bristol Trough
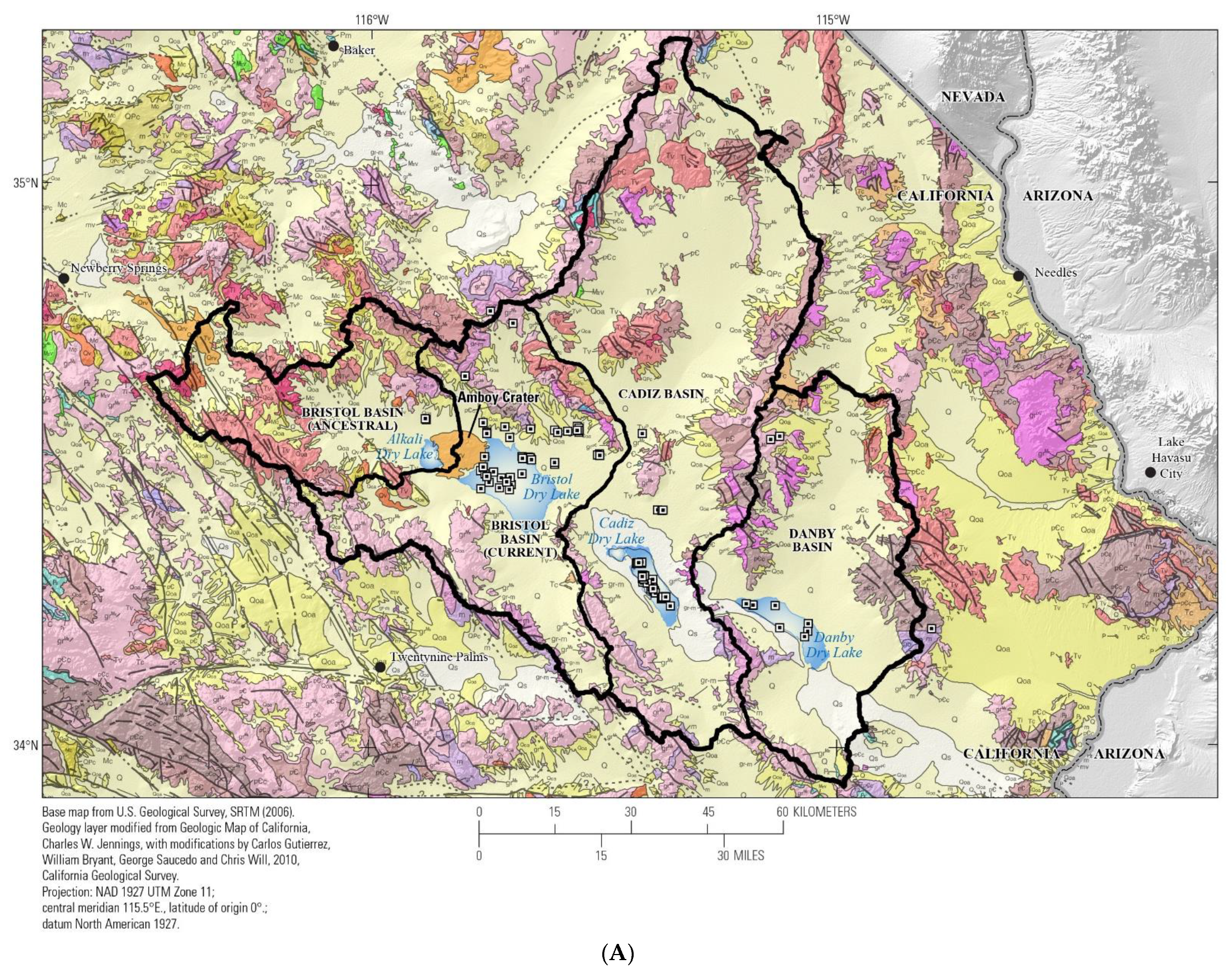
2.1. Geology
2.2. Definitions
2.3. Hydrology of Barstow-Bristol Trough
2.4. Climate
3. Methods
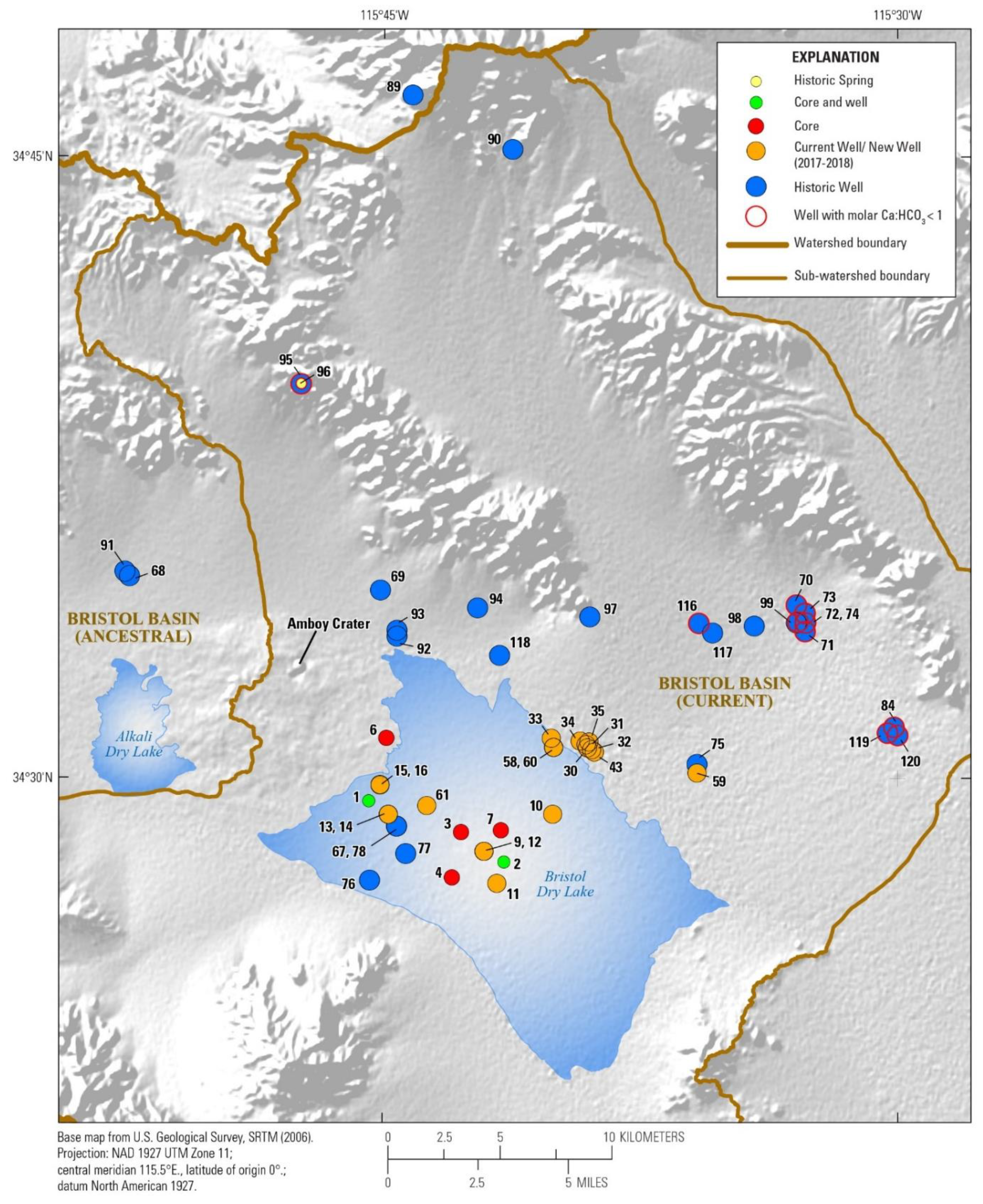
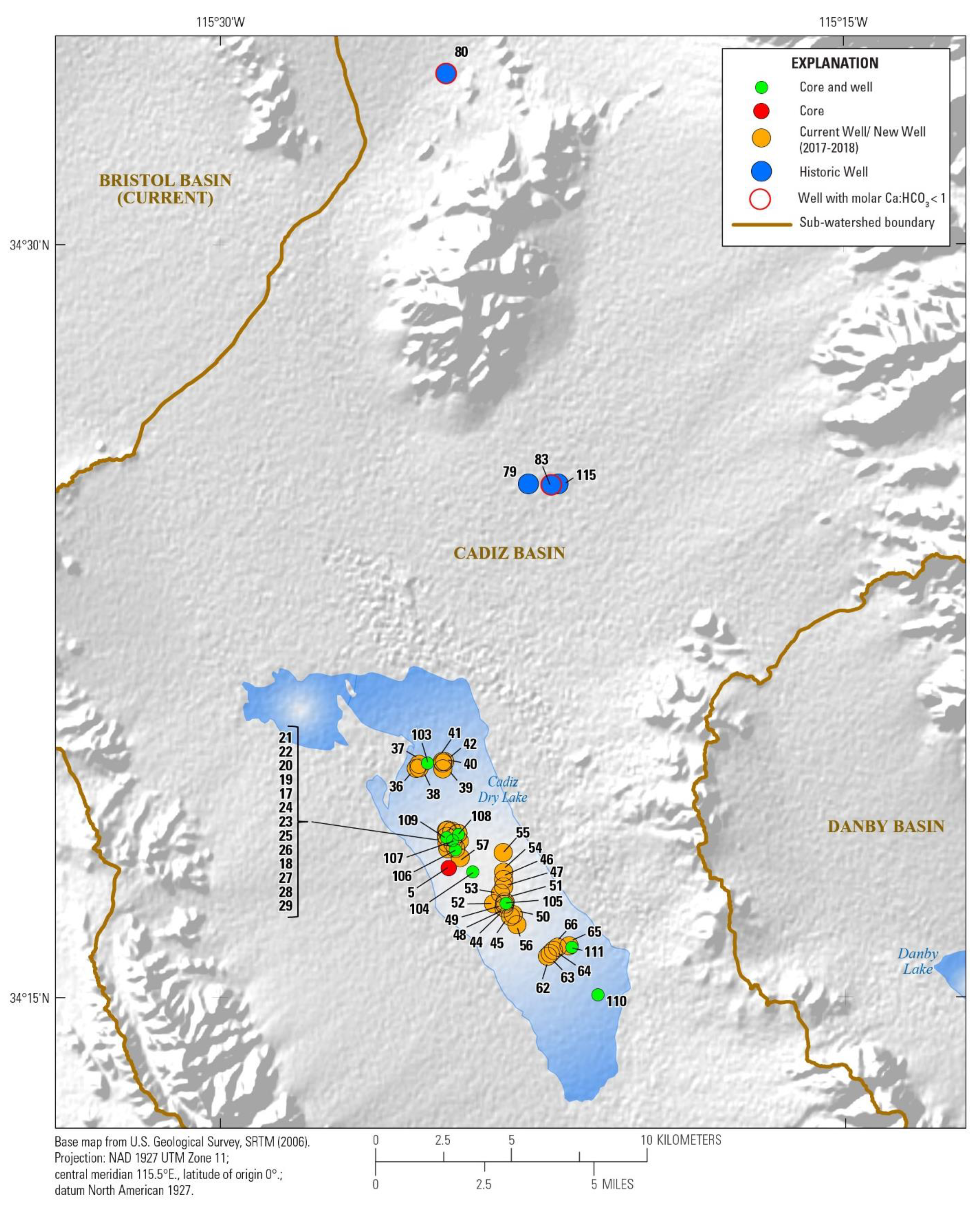
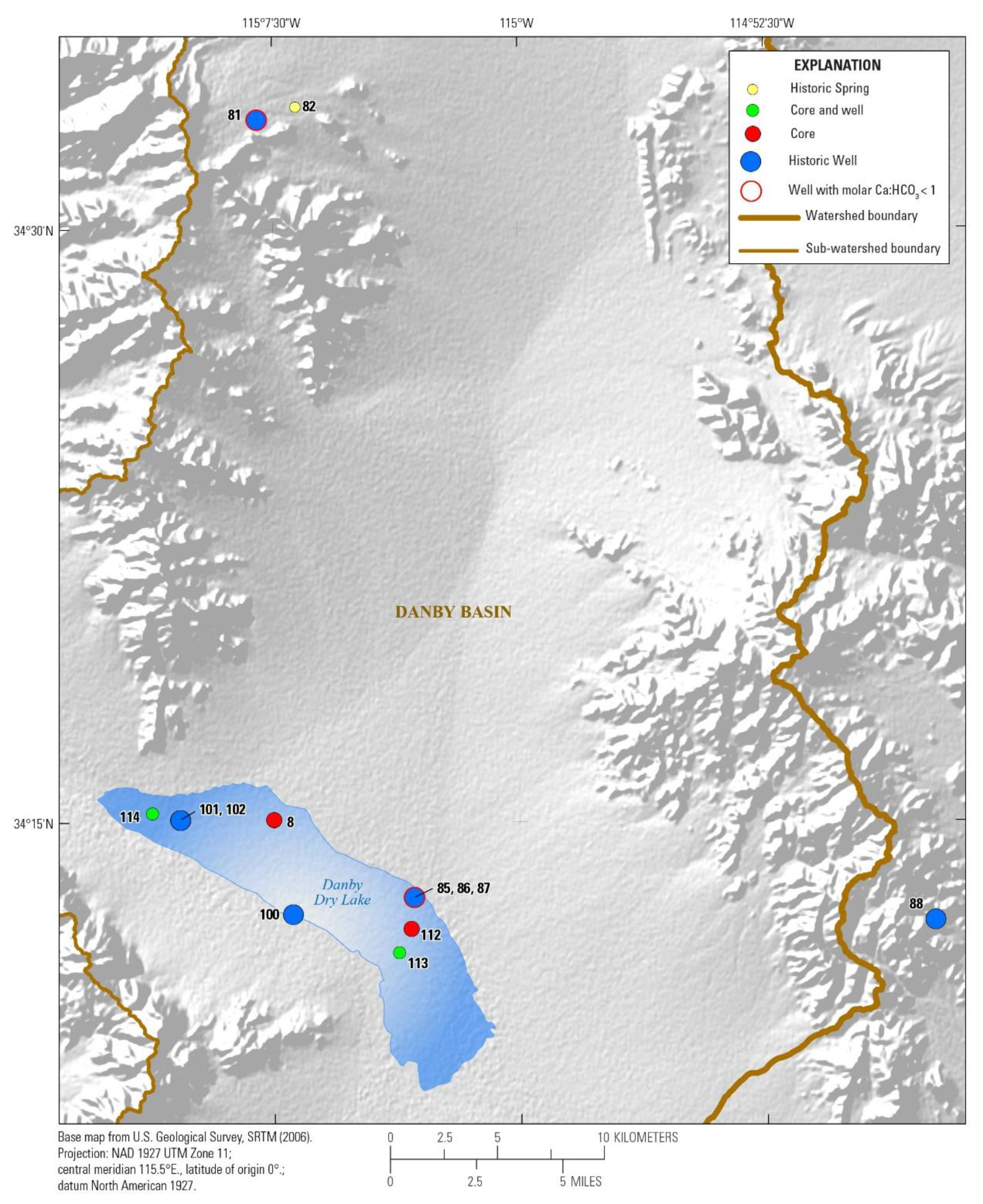
3.1. Watershed and Groundwater Delineation
3.2. Well Construction and Sample Collection
3.3. Hydrochemical Analyses
3.4. Geothermometry
3.5. X-ray Diffraction on Bulk Sediment and Clay Minerals
Clay Treatments
3.6. Bulk Sediment Chemistry
3.7. Scanning Electron Microscope
4. Results
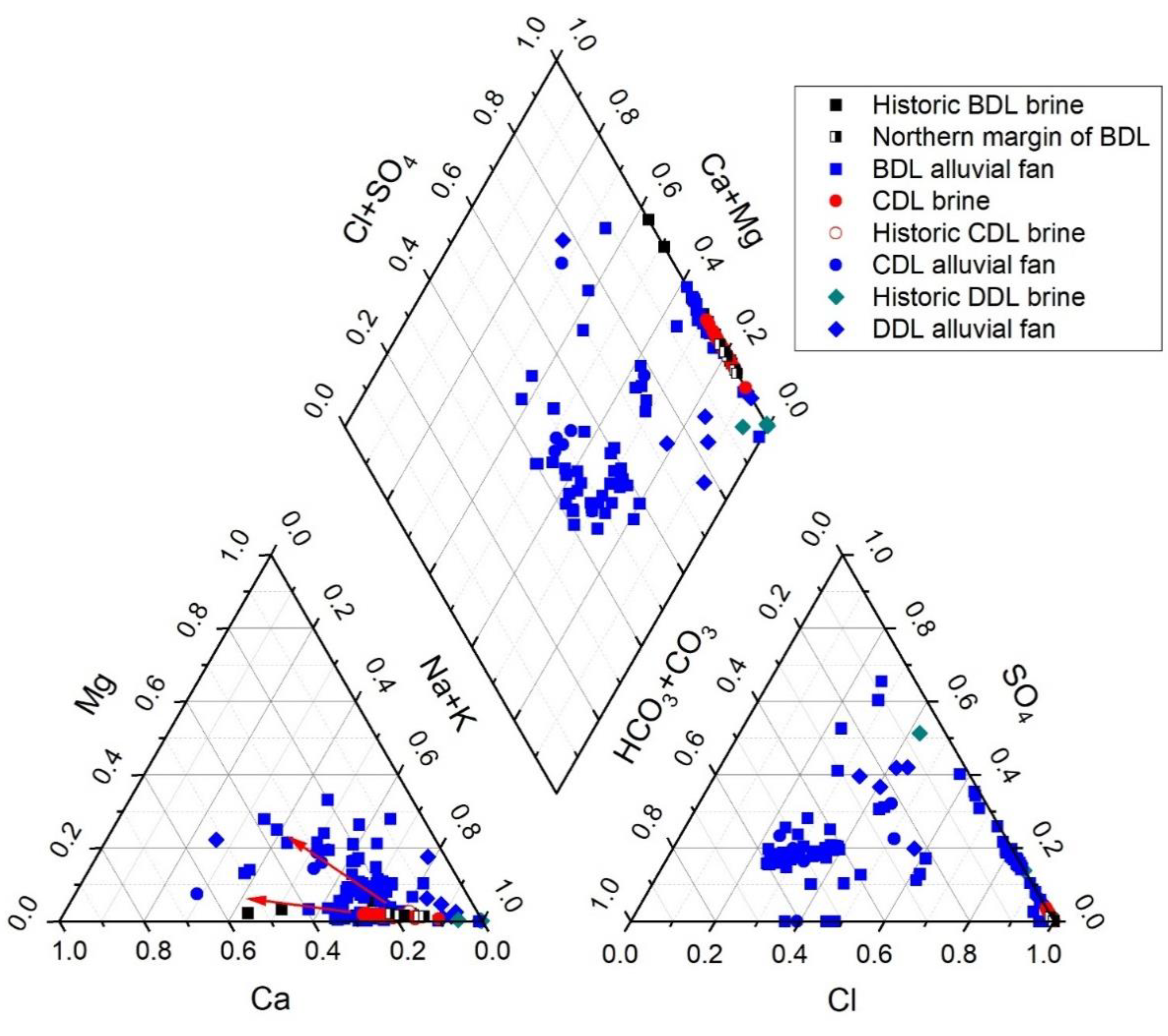
4.1. Hydrochemistry
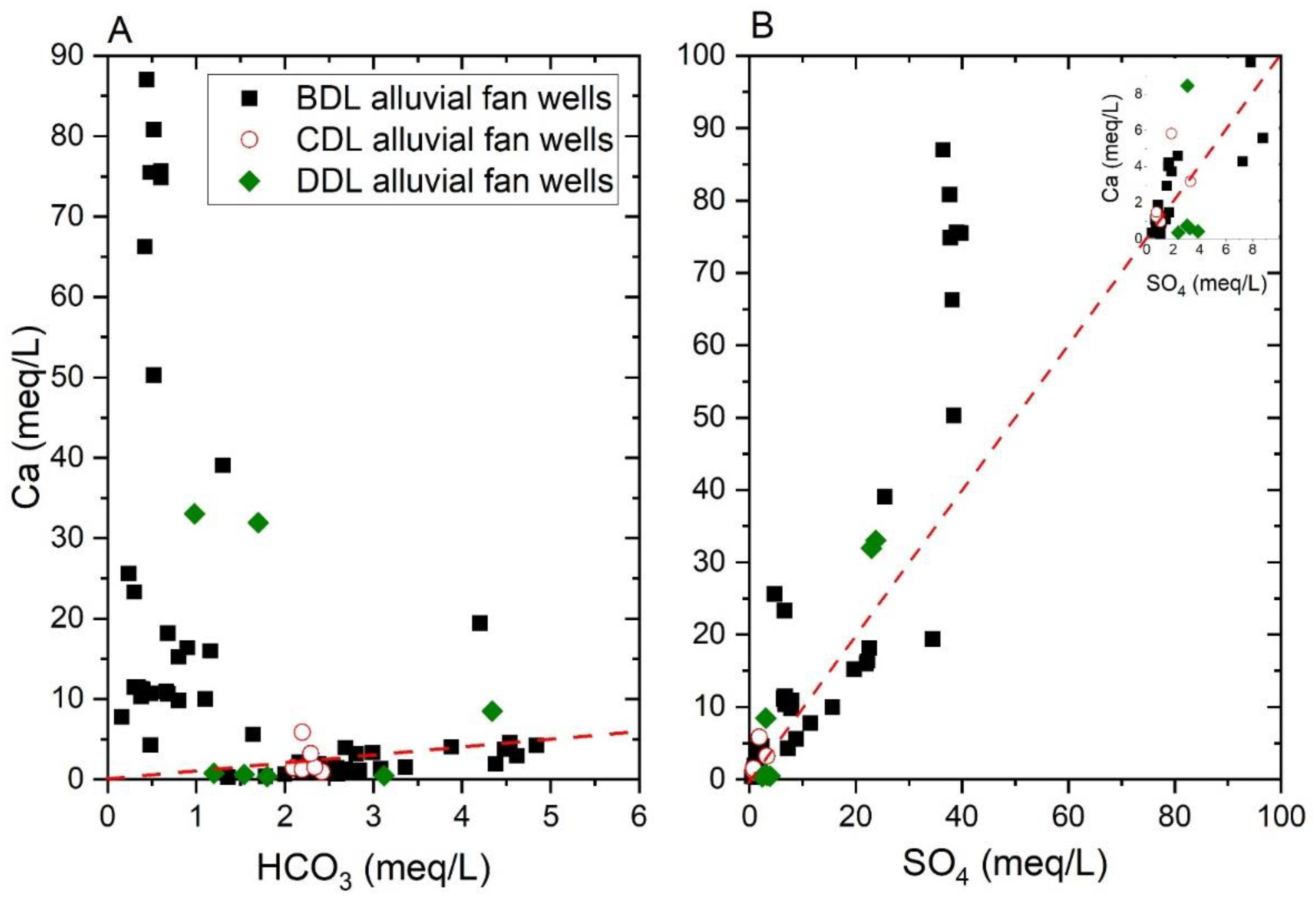
4.1.1. Alluvial Fan Water
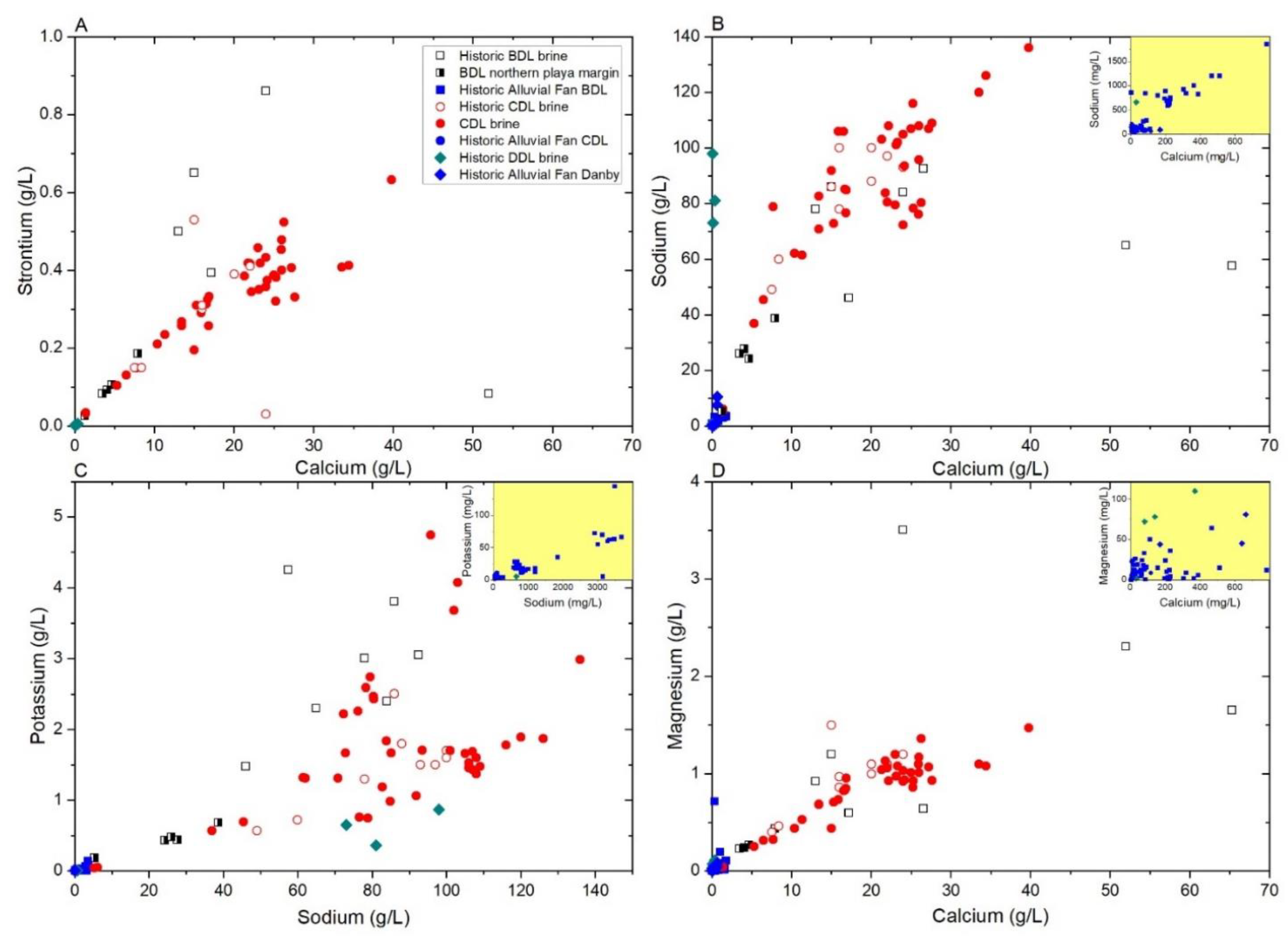
4.1.2. Brine Chemistry
4.2. Sediment Mineralogy and Chemistry
5. Discussion
5.1. Hydrology of the Basins
5.2. Groundwater Flow to Playa Center
5.3. Chemistry of Playa Sediments
5.4. Origin of Calcium and Lithium
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bradley, D.C.; Stillings, L.L.; Jaskula, B.W.; Munk, L.; McCauley, A.D. Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; Schulz, K.J., DeYoung, J.H., Jr., Seal, R.R., II, Bradley, D.C., Eds.; U.S. Geological Survey: Reston, VA, USA, 2017; pp. K1–K21. [CrossRef]
- Vine, J.D. (Ed.) Lithium Resources and Requirements by the Year 2000; U.S. Geological Survey: Reston, VA, USA, 1976; Volume 1005, p. 162.
- Vine, J.D. Where on Earth Is All the Lithium? U.S. Geological Survey: Reston, VA, USA, 1980; p. 107.
- Rosen, M.R. The importance of groundwater in playas: A review of playa classifications and the sedimentology and hydrology of playas. In Paleoclimate and Basin Evolution of Playa Systems; Rosen, M.R., Ed.; Geological Society of America: Boulder, CO, USA, 1994; Volume 289, pp. 1–18. [Google Scholar]
- Gardner, D.L. The Barstow-Bristol Trough central Mojave Desert, California. In Geology and mineral wealth of the California Desert; Fife, D.L., Brown, A.R., Eds.; South Coast Geological Society: Santa Ana, CA, USA, 1980; pp. 204–214. [Google Scholar]
- Calzia, J.P.; Moore, S.W. Geophysical, Lithologic, and Water-Quality Data from Cadiz Dry Lake, San Bernardino County, California; U.S. Geological Survey: Reston, VA, USA, 1980. [CrossRef]
- Calzia, J.P. Geophysical, Lithologic, and Water Quality Data from Bristol Dry Lake, San Bernardino, California; U.S. Geological Survey: Reston, VA, USA, 1991. [CrossRef]
- Calzia, J.P. Geophysical, Lithologic and Water Quality Data from Danby Dry Lake, San Bernardino County, California; U.S. Geological Survey: Reston, VA, USA, 1991. [CrossRef]
- Lowenstein, T.K.; Risacher, F. Closed basin brine evolution and the influence of Ca-Cl inflow waters: Death Valley and Bristol Dry Lake California, Qaidam Basin, China, and Salar de Atacama, Chile. Aquat. Geochem. 2009, 15, 71–94. [Google Scholar] [CrossRef]
- Gutierrez, C.; Bryant, W.; Saucedo, G.; Will, C. 2010 Geologic Map of California and Fault Activity Map; California Department of Conservation: Sacramento, CA, USA, 2010. Available online: https://www.conservation.ca.gov/cgs/Pages/Program-RGMP/2010_geologicmap.aspx (accessed on 19 March 2020).
- Garrett, D.E. Handbook of Lithium and Natural Calcium Chloride—Their Deposits, Processing, Uses, and Properties; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; p. 476. [Google Scholar]
- Rosen, M.R. Supplementary Data S1 for Rosen et al. Li and Ca enrichment in the Bristol Dry Lake brine, California. Miner. Zenodo 2019. [Google Scholar] [CrossRef]
- Rosen, M.R. Groundwater Quality Data from Bristol and Cadiz Basins, San Bernardino County, California, USA; U.S. Geological Survey: Reston, VA, USA, 2020. [CrossRef]
- Araoka, D.; Kawahata, H.; Takagi, T.; Watanabe, Y.; Nishimura, K.; Nishio, Y. Lithium and strontium isotopic systematics in playas in Nevada, USA: Constraints on the origin of lithium. Min. Depos. 2014, 49, 371–379. [Google Scholar] [CrossRef]
- Lowenstein, T.K.; Dolginko, L.A.C.; and García-Veigas, J. Influence of magmatic-hydrothermal activity on brine evolution in closed basins: Searles Lake, California. Geol. Soc. Am. Bull. 2016, 128, 1555–1568. [Google Scholar] [CrossRef]
- Smith, G.I. Origin of lithium and other components in the Searles Lake evaporites, California. In Lithium Resources and Requirements by the Year 2000; Vine, O.D., Ed.; U.S. Geological Survey: Reston, VA, USA, 1976; Volume 1005, pp. 92–103. [Google Scholar]
- Yu, J.-Q.; Gao, C.-L.; Cheng, A.-Y.; Liu, Y.; Zhang, L.; He, X.-H. Geomorphic, hydroclimatic and hydrothermal controls on the formation of lithium brine deposits in the Qaidam Basin, northern Tibetan Plateau, China. Ore Geol. Rev. 2013, 50, 171–183. [Google Scholar] [CrossRef]
- Rosen, M.R.; Jones, S. Controls on the groundwater composition of the Wanaka and Wakatipu basins, Central Otago, New Zealand. Hydrogeol. J. 1998, 6, 264–281. [Google Scholar]
- Hardie, L.A. The roles of rifting and hydrothermal CaCl2 brines in the origin of potash evaporites: An hypothesis. Am. J. Sci. 1990, 290, 43–106. [Google Scholar] [CrossRef]
- Toner, J.D.; Sletten, R.S. The formation of Ca-Cl-rich groundwaters in the Dry Valleys of Antarctica: Field measurements and modeling of reactive transport. Geochim. Cosmochim. Acta 2013, 110, 84–105. [Google Scholar] [CrossRef]
- Nurmi, P.A.; Ilmo, T.K.; Lahermo, W. Geochemistry and origin of saline groundwaters in the Fennoscandian shield. Appl. Geochem. 1988, 3, 185–203. [Google Scholar] [CrossRef]
- Lowenstein, T.K.; Hardie, L.A.; Timofeeff, M.N.; Demicco, R.V. Secular variation in seawater chemistry and the origin of calcium chloride basinal brines. Geology 2003, 31, 857–860. [Google Scholar] [CrossRef]
- Phillips, F.M. Cosmogenic 36Cl ages of Quaternary basalt flows in the Mojave Desert, California, USA. Geomorphology 2003, 53, 199–208. [Google Scholar] [CrossRef]
- Jackson, T.R.; Halford, K.J.; Gardner, P.M.; Garcia, A. Evaluating Micrometeorological Estimates of Groundwater Discharge from Great Basin Desert Playas. Groundwater 2018, 56, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Handford, C.R. Sedimentology and evaporite genesis in a Holocene continental-sabkha playa Basin-Bristol Dry Lake, California. Sedimentology 1982, 29, 239–253. [Google Scholar] [CrossRef]
- Rosen, M.R. Sedimentologic, geochemical, and hydrologic evolution of an intracontinental, closed-basin playa (Bristol Dry Lake, CA): A model for playa Development and Its Implications for Paleoclimate. Ph.D. Thesis, University of Texas at Austin, Austin, TX, USA, 1989. Available online: http://hdl.handle.net/2152/61385 (accessed on 19 March 2020).
- Rosen, M.R.; Warren, J.K. The origin and significance of groundwater seepage gypsum from Bristol Dry Lake Ca., USA. Sedimentology 1990, 37, 983–996. [Google Scholar] [CrossRef]
- Rosen, M.R. Sedimentologic and geochemical constraints on the hydrologic evolution of Bristol Dry Lake, California, USA. Palaeogeogr. Palaeoclim. Palaeoecol. 1991, 84, 229–257. [Google Scholar] [CrossRef]
- Garfunkel, Z. Model for Late Cenozoic tectonic history of the Mojave Desert, California, and for its relation to adjacent regions. Geol. Soc. Am. Bull. 1974, 85, 1931–1944. [Google Scholar] [CrossRef]
- Dokka, R.K.; Travis, C.J. Late Cenozoic strike-slip faulting in the Mojave Desert, California. Tectonics 1990, 9, 311–340. [Google Scholar] [CrossRef]
- Glazner, A.F.; Bartley, J.M.; Walker, J.D. Magnitude and significance of Miocene crustal extension in the central Mojave Desert, California. Geology 1989, 17, 50–53. [Google Scholar] [CrossRef]
- Smith, P. New evidence for a Pliocene marine embayment along the Lower Colorado River area, California and Arizona. Geol. Soc. Am. Bull. 1970, 81, 1411–1420. [Google Scholar] [CrossRef]
- Miller, D.M.; Reynolds, R.E.; Bright, J.E.; Starratt, S.W. Bouse Formation in the Bristol basin near Amboy, California, USA. Geosphere 2014, 10, 462–475. [Google Scholar] [CrossRef]
- Bassett, A.M.; Kupfer, D.H.; Barstow, F.C. Core logs from Bristol, Cadiz, and Danby Dry Lakes, San Bernardino County, California. U.S. Geological Survey: Reston, VA, USA, 1959; pp. 97–138. [Google Scholar]
- Mohorich, L.M. Geothermal resources of the California Desert-a summary review: In Geology and Mineral Wealth of the California Desert; Fife, D.L., Brown, A.R., Eds.; South Coast Geological Society: Santa Ana, CA, USA, 1980; pp. 171–189. [Google Scholar]
- Gale, G.K. Geology of the saline deposits, Bristol Dry Lake, San Bernardino County, California. Spec. Rep. Calif. State Min. Bur. 1951, 13, 22. [Google Scholar]
- Brown, H. Geology, genesis and mining of pharmaceutical and food grade calcium carbonate at the Amboy Limestone Quarry. In Land of Lost Lakes. The 2003 Desert Symposium Field Trip; Reynolds, R.E., Ed.; California State University, Desert Studies Consortium: Long Beach, CA, USA, 2003; pp. 49–56. [Google Scholar]
- Shafer, R.A. Report on Investigations of Conditions Which Determine the Potentials for Development in the Desert Valleys of Eastern San Bernardino County, California; Unpublished Engineering Department Report; Southern California Edison Co.: Rosemead, CA, USA, 1964; p. 172. [Google Scholar]
- Thompson, D.G. The Mojave Desert Region, California; U.S. Geological Survey: Reston, VA, USA, 1929; Volume 578, p. 759.
- CH2M Hill. Cadiz Groundwater Conservation and Storage Project. July 2010. Available online: https://www.cadizwaterproject.com/wp-content/uploads/2015/07/4_09_Hydrology.pdf (accessed on 19 March 2020).
- Hunt, G.S. Ground Water Geology of the Bristol and Cadiz Valleys, San Bernardino County, California. Master’s Thesis, University of Southern California, Los Angeles, CA, USA, 1966; p. 76. [Google Scholar]
- California Department of Water Resources. Geothermal Wastes and the Water Resources of the Salton Sea Area; Department of Water Resources: Sacramento, CA, USA, 1970; p. 123. [Google Scholar]
- Williams, A.E.; McKibben, M.A. A brine interface in the Salton Sea Geothermal System, California: Fluid geochemical and isotopic characteristics. Geochim. Cosmochim. Acta 1989, 53, 1905–1920. [Google Scholar] [CrossRef]
- Howle, J.F.; Evans, W.C.; Galloway, D.L.; Hsieh, P.A.; Hurwitz, S.; Smith, G.A.; Nawikas, J. Hydraulic, Geochemical, and Thermal Monitoring of an Aquifer System in the Vicinity of Mammoth Lakes, Mono County, California, 2015–2017; U.S. Geological Survey: Reston, VA, USA, 2019; p. 90. [CrossRef]
- Duffy, C.J.; Al-Hassan, S. Groundwater circulation in a closed desert basin: Topographic scaling and climatic forcing. Water Resour. Res. 1988, 24, 1675–1688. [Google Scholar] [CrossRef]
- Neal, J.T. (Ed.) Playas and Dried Lakes, Benchmark Papers in Geology, Dowden; Hutchinson and Ross, Inc.: Stroudsburg, PA, USA, 1975; pp. 1–5. [Google Scholar]
- Wood, W.W.; Sanford, W.E. Ground-water control of evaporite deposition. Econ. Geol. 1990, 85, 1226–1235. [Google Scholar] [CrossRef]
- Bedford, D.R.; Miller, D.M.; Phelps, G.A. Surficial Geologic Map of the Amboy 30′ × 60′ Quadrangle, San Bernardino County, California; U.S. Geological Survey: Reston, VA, USA, 2010.
- Higgins, C.T. Geothermal resources of California. California Geological Map Series, Map No. 4, Division of Mines and Geology; California Department of Conservation: Sacramento, CA, USA, 1980.
- Roberts, B.J. Geothermal Resource of the United States: Locations of Identified Hydrothermal Sites and Favorability of Deep Enhanced Geothermal Systems (EGS); National Renewable Energy Laboratory for the US Department of Energy: Golden, CO, USA, 2009. Available online: https://www.nrel.gov/gis/assets/images/geothermal-identified-hydrothermal-and-egs.jpg (accessed on 19 March 2020).
- Hereford, R.; Webb, R.H.; Longpré, C.I. Precipitation history and ecosystem response to multidecadal precipitation variability in the Mojave Desert region, 1893–2001. J. Arid Environ. 2006, 67, 13–34. [Google Scholar] [CrossRef]
- Fournier, R.O. A revised equation for the Na/K geothermometer. Geotherm. Resour. Counc. Trans. 1979, 3, 221–224. [Google Scholar]
- Kharaka, Y.K.; Mariner, R.H. Chemical geothermometers and their application to formation waters from sedimentary basins. In Thermal History of Sedimentary Basins; Naeser, N.D., McCulloh, T.H., Eds.; Springer: New York, NY, USA, 1989; pp. 99–117. [Google Scholar]
- Land, L.S.; MacPherson, G.L. Geothermometry from brine analysis: Lessons from the Gulf Coast, USA. Appl. Geochem. 1992, 7, 333–340. [Google Scholar] [CrossRef]
- Eberl, D.D. User Guide to RockJock—A Program for Determining Quantitative Mineralogy from X-ray Diffraction Data; U.S. Geological Survey: Reston, VA, USA, 2003; p. 47.
- Kane, T.J.; Campbell, K.M.; Rosen, M.R. X-Ray Diffraction Data for Bulk Sediment and Clay Separations Taken from Cores from Bristol Dry Lake, California and Geothermal Springs from Paoha Island (Mono Lake), California; U.S. Geological Survey: Reston, VA, USA, 2019. [CrossRef]
- Goldberg, S. Reanalysis of boron adsorption on soils and soil minerals using the constant capacitance model. Soil Sci. Soc. Am. J. 1999, 63, 823–829. [Google Scholar] [CrossRef]
- Keren, R.; Gast, R.G. pH-dependent boron adsorption by montmorillonite hydroxy-aluminum complexes. Soil Sci. Soc. Am. J. 1983, 47, 1116–1121. [Google Scholar] [CrossRef]
- Cody, R.D. Adsorption and the reliability of trace elements as environment indicators for shales. J. Sediment. Petrol. 1971, 41, 461–471. [Google Scholar]
- Rao, N. Fluoride and environment—A review. In Proceedings of the Third International Conference on Environment and Health, Chennai, India, 15–17 December 2003; Bunch, M.J., Madha Suresh, V., Vasantha Kumaran, T., Eds.; Department of Geography, University of Madras: Chennai, India; Faculty of Environmental Studies, York University: Toronto, ON, Canada, 2003; pp. 386–399. Available online: http://www.yorku.ca/bunchmj/ICEH/proceedings/Rao_N_ICEH_papers_386to399.pdf (accessed on 19 March 2020).
- Rosen, M.R. Sedimentology, stratigraphy, and hydrochemistry of Bristol Dry Lake, California, USA. In Lake Basins Through Time and Space; Gierlowski-Kordesch, E., Kelts, K., Eds.; AAPG Studies in Geology: Tulsa, OK, USA, 2000; pp. 597–604. [Google Scholar]
- Vredenburgh, L.M. Salt production, Danby Dry Lake San Bernardino County, California. In Not a Drop to Drink; Reynolds, R.E., Ed.; California State University Desert Studies Center: Baker, CA, USA, 2014; pp. 60–62. [Google Scholar]
- Reynolds, R.E.; Schweich, T.A. Danby Playa: Ringed with salty questions. In Not a Drop to Drink; Reynolds, R.E., Ed.; California State University Desert Studies Center: Baker, CA, USA, 2014; pp. 63–67. [Google Scholar]
- Christidis, G.E.; Koutsopoulou, E.; Bish, D. A simple approach to the identification of trioctahedral smectites by X-ray diffraction. Clay Miner. 2013, 48, 687–696. [Google Scholar] [CrossRef]
- Doe, B.R.; Hedge, C.E.; White, D.E. Preliminary investigation of the source of lead and strontium in deep geothermal brines underlying the Salton Sea geothermal area. Econ. Geol. 1966, 61, 462–485. [Google Scholar] [CrossRef]
- Bataille, C.P.; Willis, A.; Yang, X.; Liu, X.-M. Continental igneous rock composition: A major control of past global chemical weathering. Sci. Adv. 2017, 3, e1602183. [Google Scholar] [CrossRef] [PubMed]
- Dolginko, L.A.C. The influence of magmatic activity on brine evolution in closed basins: Searles Lake, California, and Bristol Dry Lake, California. Master’s Thesis, State University of New York at Binghamton, Binghamton, NY, USA, 2014; p. 164. [Google Scholar]
- Brown, W.J.; Rosen, M.R. Was there a Pliocene-Pleistocene fluvial-lacustrine connection between Death Valley and the Colorado River? Quat. Res. 1995, 43, 286–296. [Google Scholar] [CrossRef]
- Livingstone, D.A. Chemical composition of Rivers and Lakes. In Data of Geochemistry, 6th ed.; Chapter G. US Geological Survey Prof. Paper 440-G; Fleischer, M., Ed.; U.S. Geological Survey: Reston, VA, USA, 1963; p. 64. [Google Scholar]
- Droste, J.B. Clay minerals in sediments of Owens, China, Searles, Panamint, Bristol, Cadiz, and Danby lake basins, California. Geol. Soc. Am. Bull. 1961, 72, 1713–1722. [Google Scholar] [CrossRef]

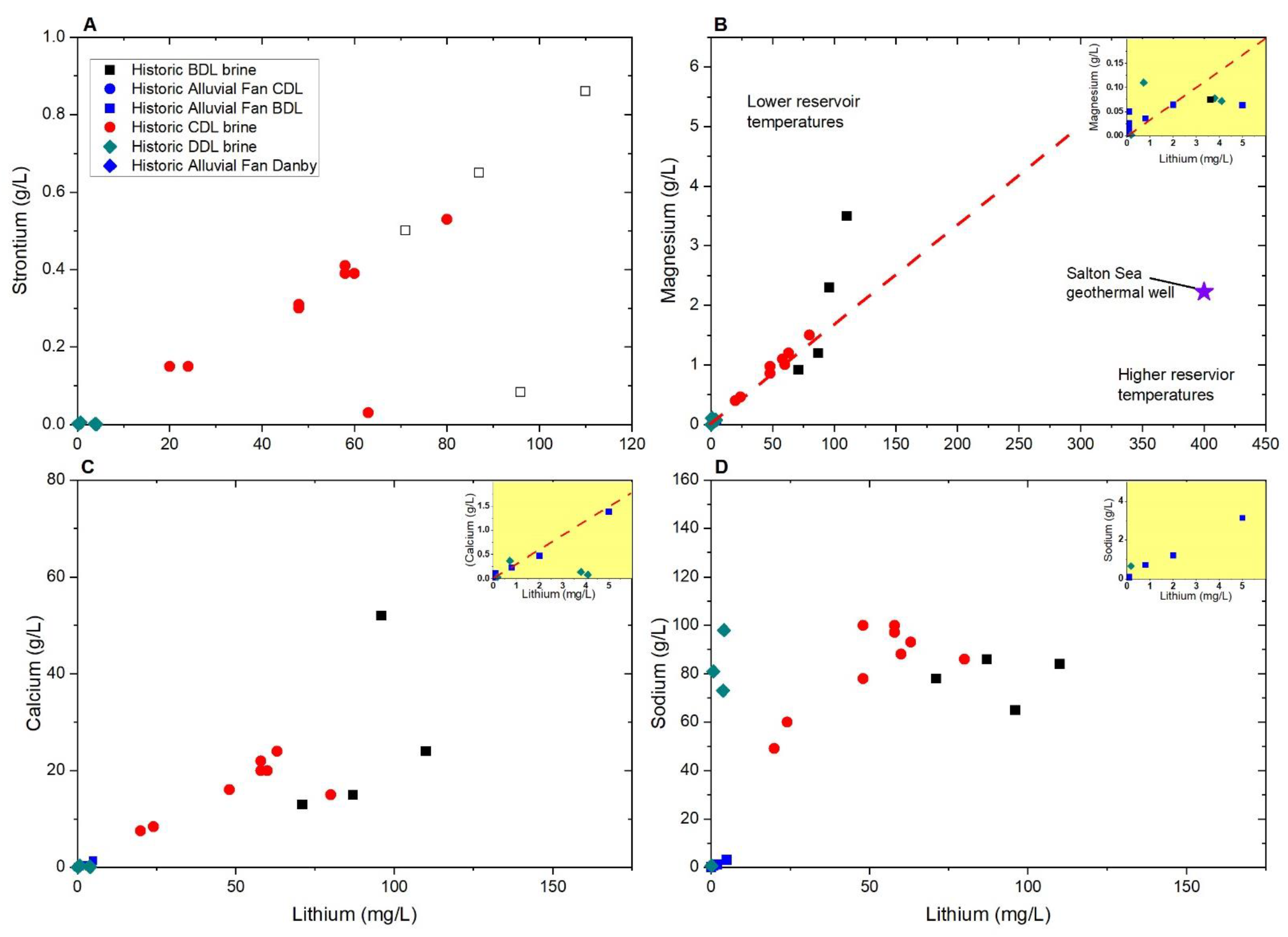
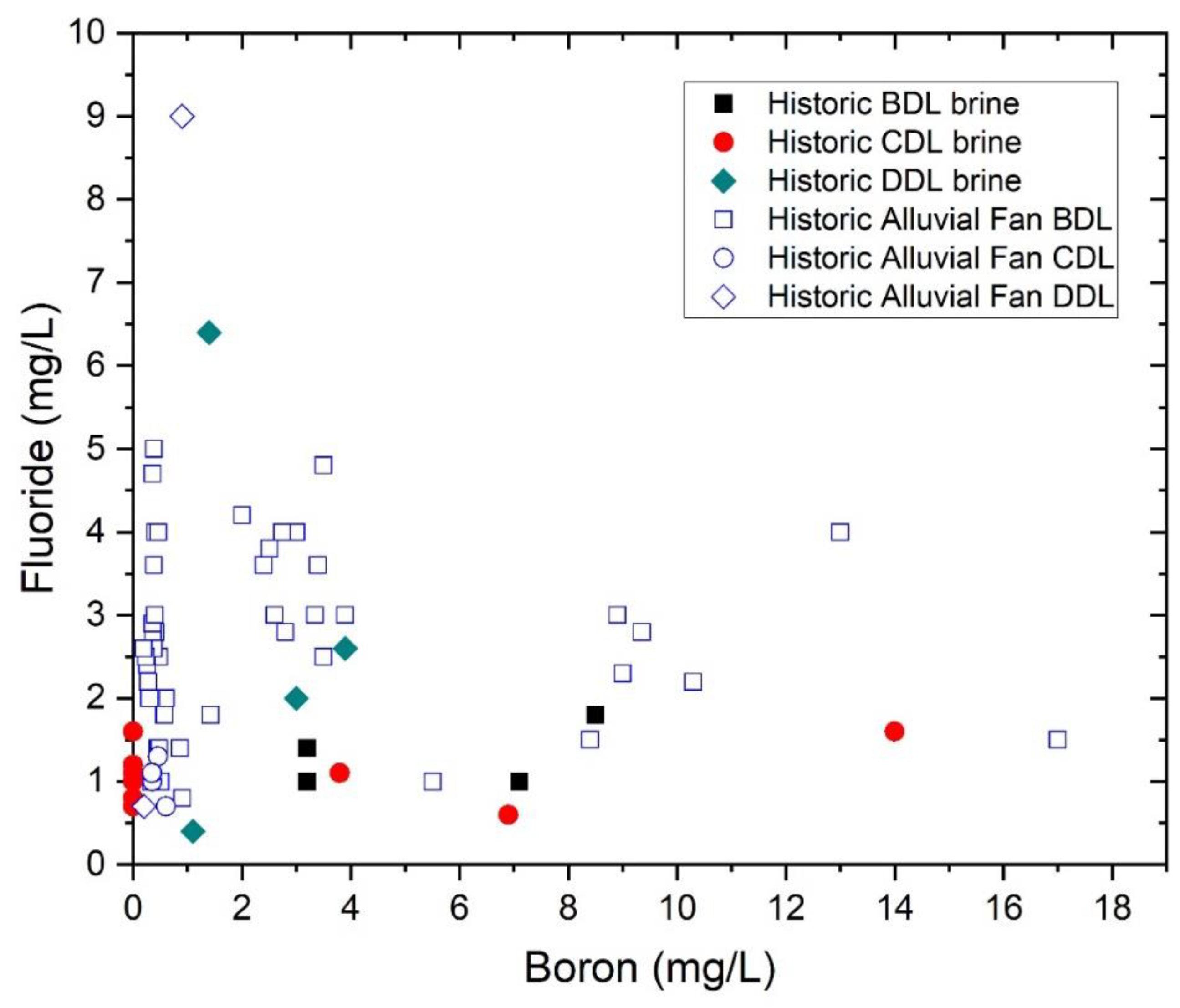
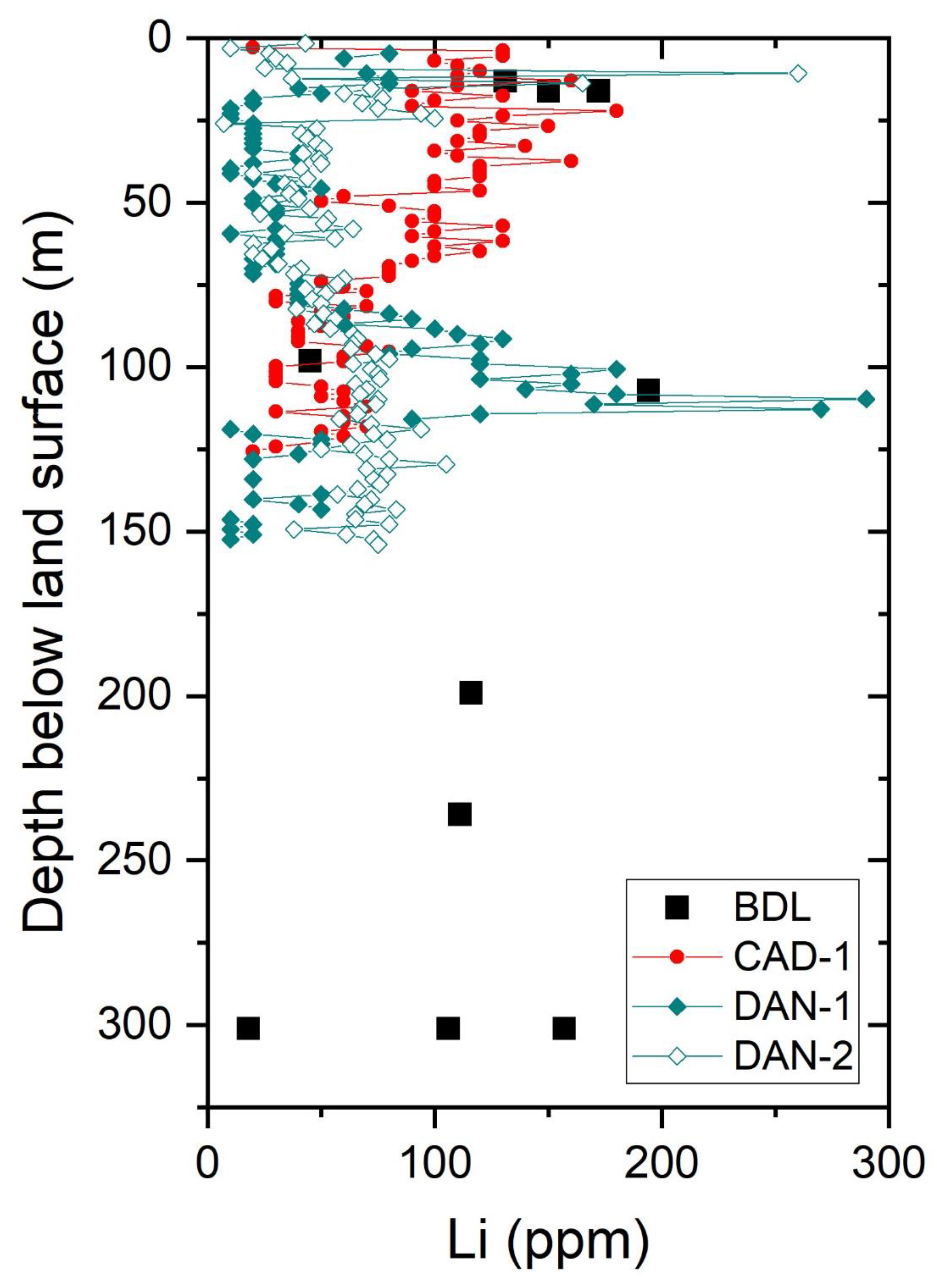

| Characteristic | Bristol Dry Lake Brine | Cadiz Dry Lake Brine | Danby Dry Lake Brine | Salton Sea Geothermal Well | Long Valley Geothermal Springs and Wells | References |
|---|---|---|---|---|---|---|
| Watershed size (km2) | Historic: 3130 Current: 2146 | 4244 | 2265 | - | - | This paper |
| Brine type | Na–Ca–Cl | Na–Ca–Cl | Na–Cl | Na–Ca–Cl | Na–HCO3–Cl | [6,7,8,26,28,42,43,44] |
| Average Maximum Total dissolved solids (g/L) in basin brines | 259 357 | 255 375 | 128 187 | - 387 | - 0.74 | This paper |
| Maximum calcium concentration (g/L) in basin brines | 56 | 40 | 0.4 | 40 | 0.04 | [6,7,8,26,28,42], this paper |
| K:Mg ratio (meq/L) | 0.2–1.4 | 0.3–1.3 | 1.0–3.8 | 2.3– >500 | 138 | This paper |
| Maximum lithium Maximum boron Maximum fluoride concentration (mg/L) In basin brines | 110 8.8 1.8 | 80 14 1.6 | 4.1 3.9 6.4 | 400 380 18 | 3.2 11.1 11.4 | [6,7,8,26,28,42,44] |
| Percentage of halite by volume in cores from basin centers | 40% | <1%; one 1 m thick bed near the surface | <1%; three <1 m thick beds near the surface | - | - | This paper |
| Greatest depth of drilling in sediments in the basin * (m) | 537 | 152 | 268 | 1000 | >200 | [6,7,8,34,43,44] |
| Range of brine temperatures based on Mg:Li and Na:K geothermometry (°C) | Mg–Li: 161–152 Na–K: 192– <150 | Mg–Li: 153–132 Na–K: <150 | Mg–Li: 5–107 Na–K: <150 | 200–340 ** | Mg–Li: 220 Na–K: 222 | This paper, data from [44] for Long Valley |
| Bouse Formation present | One shoreline outcrop 200 m above playa | In core | In core | - | - | [32,33,34] |
| Core | Depth (m) | Li (ppm) | Sr (ppm) | Al (%) | Ca (%) | K (%) | Mg (%) | Na (%) |
|---|---|---|---|---|---|---|---|---|
| DH-2 | 15–17 | 150 | 617 | 4.67 | 4.81 | 1.97 | 1.34 | 10.3 |
| DH-2 | 107–109 | 194 | 697 | 5.6 | 4.92 | 2.46 | 1.78 | 6.7 |
| DH-2 | 198–200 | 116 | 429 | 5.26 | 3.11 | 2.18 | 1.46 | 13.6 |
| DH-5 | 12–14 | 131 | 749 | 5.72 | 5.44 | 2.56 | 1.24 | 6.78 |
| DH-5 | 235–237 | 111 | 633 | 3.93 | 5.95 | 1.82 | 0.91 | 12.8 |
| DH-5 | 300–302 | 106 | 495 | 3.64 | 4.83 | 1.65 | 0.87 | 14.8 |
| DH-5A | 300–302 | 17.8 | 182 | 0.87 | 1.03 | 0.39 | 0.14 | 32.7 |
| DH-6A | 15–17 | 172 | 2610 | 6.37 | 5.12 | 2.65 | 1.49 | 4.06 |
| DH-6 | 98–100 | 45 | 250 | 1.34 | 2.48 | 0.62 | 0.32 | 28.1 |
| DH-6A | 300–302 | 157 | 737 | 5.86 | 5.03 | 2.66 | 1.47 | 7.26 |
| Sample Name: | DH2 50-55 | DH2 350-355 | DH2 650-655 | DH5 40-45 | DH5 40-45 Duplicate | DH5 770-775 | DH5 985-990 | DH5A 985-990 | DH6A 50-55 | DH6 320-325 | DH6A 985-990 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Depth of Sample (m) | 15–17 | 107–109 | 198–200 | 12–14 | 12–14 | 235–237 | 300–302 | 300–302 | 15–17 | 98–100 | 300–302 |
| Mineral wt % | |||||||||||
| Non-clays | |||||||||||
| Quartz | 7.4 | 5.8 | 6.1 | 13.6 | 11.4 | 13.4 | 11.2 | 15.5 | 14.3 | 14.2 | 12.4 |
| K-feldspar | 20.5 | 17.4 | 16.1 | 26.8 | 26.1 | 25.3 | 24.5 | 36.4 | 38.8 | 27.3 | 18.0 |
| Plagioclase | 3.5 | 1.7 | 2.4 | 6.7 | 7.7 | 5.4 | 6.0 | 11.3 | 9.1 | 6.8 | 1.8 |
| Calcite | 7.0 | 9.5 | 6.7 | 8.4 | 8.5 | 7.0 | 7.5 | 4.8 | 5.1 | 7.3 | 5.2 |
| Halite | 1.1 | 0.1 | 2.0 | 0.3 | 0.4 | 0.8 | 0.4 | 0.6 | 0.4 | 0.7 | 0.5 |
| Anhydrite | 1.3 | 3.0 | 0.1 | 0.5 | 0.5 | 11.7 | 8.5 | 5.5 | 1.3 | 11.7 | 13.8 |
| Gypsum | 5.6 | 0.0 | 0.0 | 3.6 | 4.6 | 0.0 | 0.0 | 0.0 | 1.6 | 0.0 | 0.2 |
| Amphibole | 2.1 | 1.8 | 1.2 | 3.0 | 3.0 | 3.1 | 2.2 | 3.1 | 2.2 | 2.8 | 0.1 |
| Total non-clays | 48.4 | 39.3 | 34.6 | 63.0 | 62.2 | 66.7 | 60.3 | 77.2 | 72.8 | 70.8 | 51.8 |
| Clays | |||||||||||
| Kaolin group | 0.5 | 1.9 | 2.5 | 1.9 | 0.0 | 1.2 | 0.4 | 0.2 | 0.0 | 0.7 | 1.4 |
| Smectite group | 20.8 | 21.2 | 23.1 | 13.9 | 10.3 | 12.8 | 16.0 | 9.2 | 13.3 | 12.4 | 22.0 |
| Illite group | 21.8 | 31.7 | 34.0 | 15.7 | 17.6 | 11.7 | 14.0 | 7.9 | 4.2 | 10.6 | 21.0 |
| Mica group | 7.0 | 5.0 | 4.7 | 3.8 | 8.7 | 6.7 | 8.6 | 5.3 | 8.4 | 4.2 | 2.1 |
| Chlorite | 1.5 | 0.9 | 1.1 | 1.7 | 1.3 | 0.9 | 0.8 | 0.3 | 1.2 | 1.3 | 1.6 |
| Total clays | 51.6 | 60.7 | 65.4 | 37.0 | 37.8 | 33.3 | 39.7 | 22.8 | 27.2 | 29.2 | 48.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosen, M.R.; Stillings, L.L.; Kane, T.; Campbell, K.; Vitale, M.; Spanjers, R. Li and Ca Enrichment in the Bristol Dry Lake Brine Compared to Brines from Cadiz and Danby Dry Lakes, Barstow-Bristol Trough, California, USA. Minerals 2020, 10, 284. https://doi.org/10.3390/min10030284
Rosen MR, Stillings LL, Kane T, Campbell K, Vitale M, Spanjers R. Li and Ca Enrichment in the Bristol Dry Lake Brine Compared to Brines from Cadiz and Danby Dry Lakes, Barstow-Bristol Trough, California, USA. Minerals. 2020; 10(3):284. https://doi.org/10.3390/min10030284
Chicago/Turabian StyleRosen, Michael R., Lisa L. Stillings, Tyler Kane, Kate Campbell, Matthew Vitale, and Ray Spanjers. 2020. "Li and Ca Enrichment in the Bristol Dry Lake Brine Compared to Brines from Cadiz and Danby Dry Lakes, Barstow-Bristol Trough, California, USA" Minerals 10, no. 3: 284. https://doi.org/10.3390/min10030284
APA StyleRosen, M. R., Stillings, L. L., Kane, T., Campbell, K., Vitale, M., & Spanjers, R. (2020). Li and Ca Enrichment in the Bristol Dry Lake Brine Compared to Brines from Cadiz and Danby Dry Lakes, Barstow-Bristol Trough, California, USA. Minerals, 10(3), 284. https://doi.org/10.3390/min10030284





