Novel Strategy for Improvement of the Bioleaching Efficiency of Acidithiobacillus ferrooxidans Based on the AfeI/R Quorum Sensing System
Abstract
1. Introduction
2. Material and Methods
2.1. Bacteria and Growth Conditions
2.2. Development of Knockout and Overexpression Strains of qs-I
2.3. Identification of SO42− in the Culture Media Through Ion Chromatography
2.4. Determination of Extracellular Polymeric Substances
2.5. Extraction of Total Ribonucleic Acid (RNA) and Real-Time Quantitative PCR (RT-qPCR)
2.6. Preparation of Sulfur and Pyrite Coupons and Scanning Electron Microscope Observation
2.7. Biofilm Formation on Sulfur Coupons and Pyrite
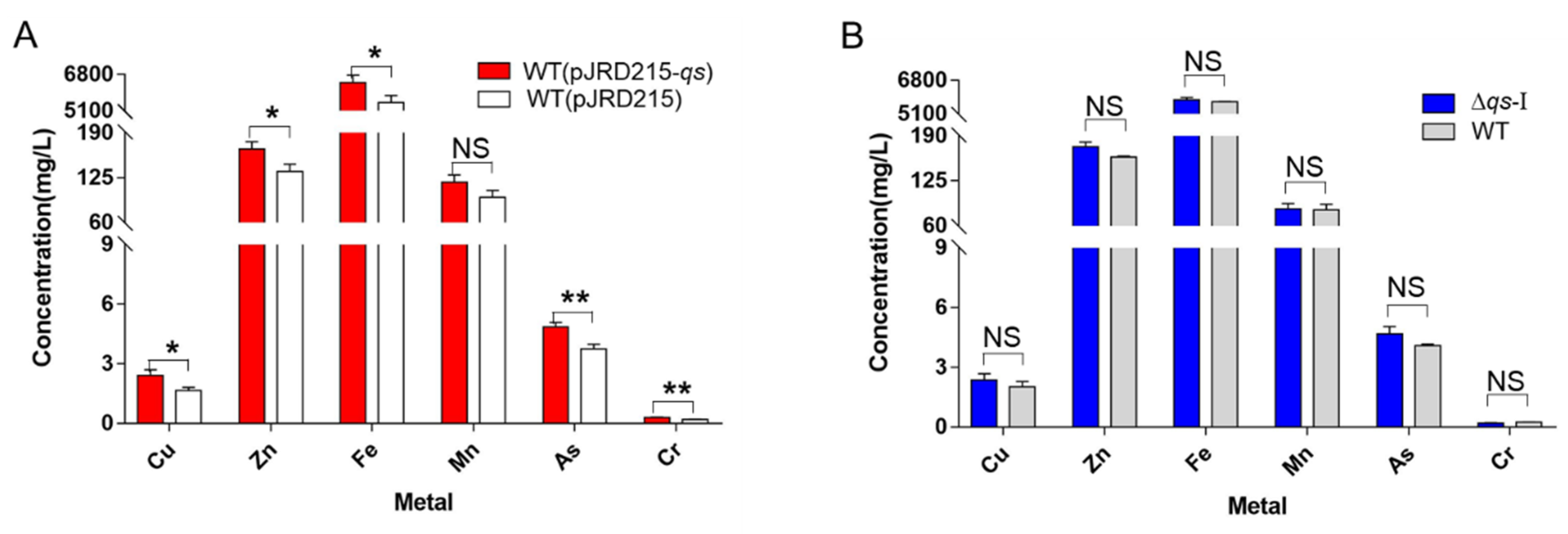
2.8. Identification of the Metal Elements in the Leachate
2.9. Statistical Analysis
3. Results
3.1. Development of the qs-I Operon Deletion and Overexpression Strains
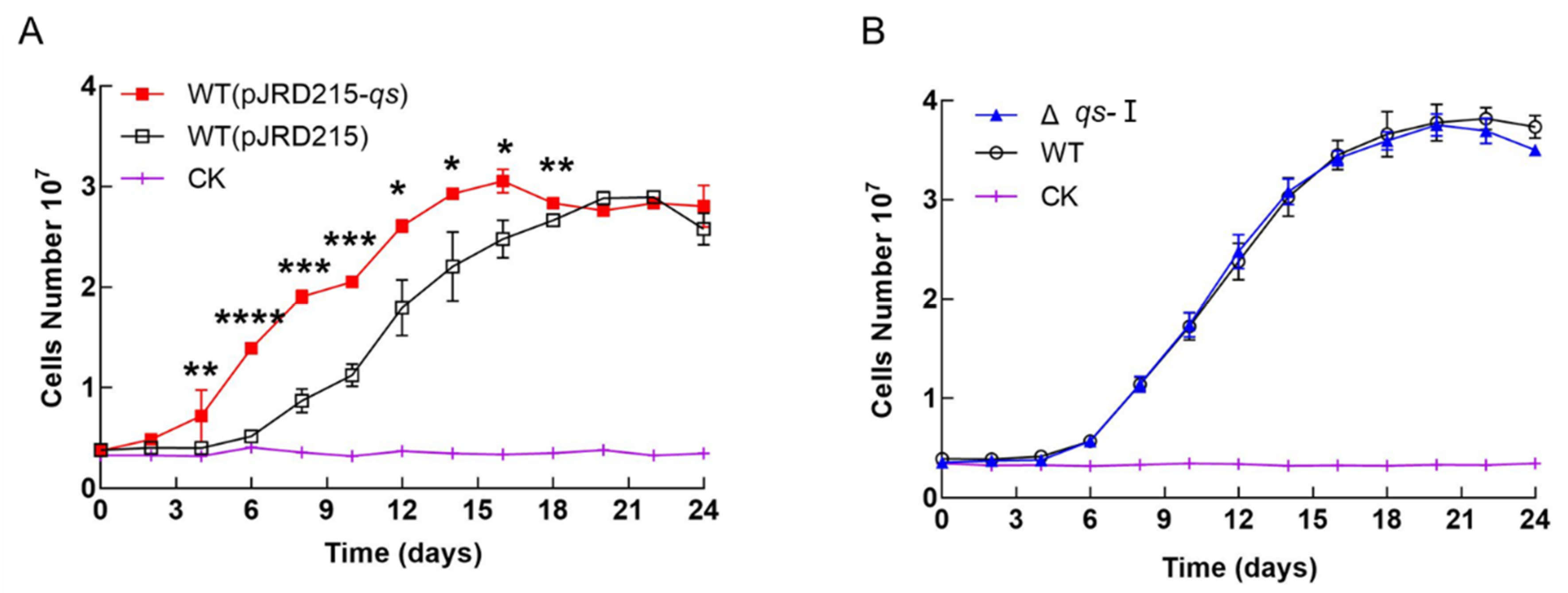
3.2. Effect of qs-I on the Cell Growth of A. ferrooxidans
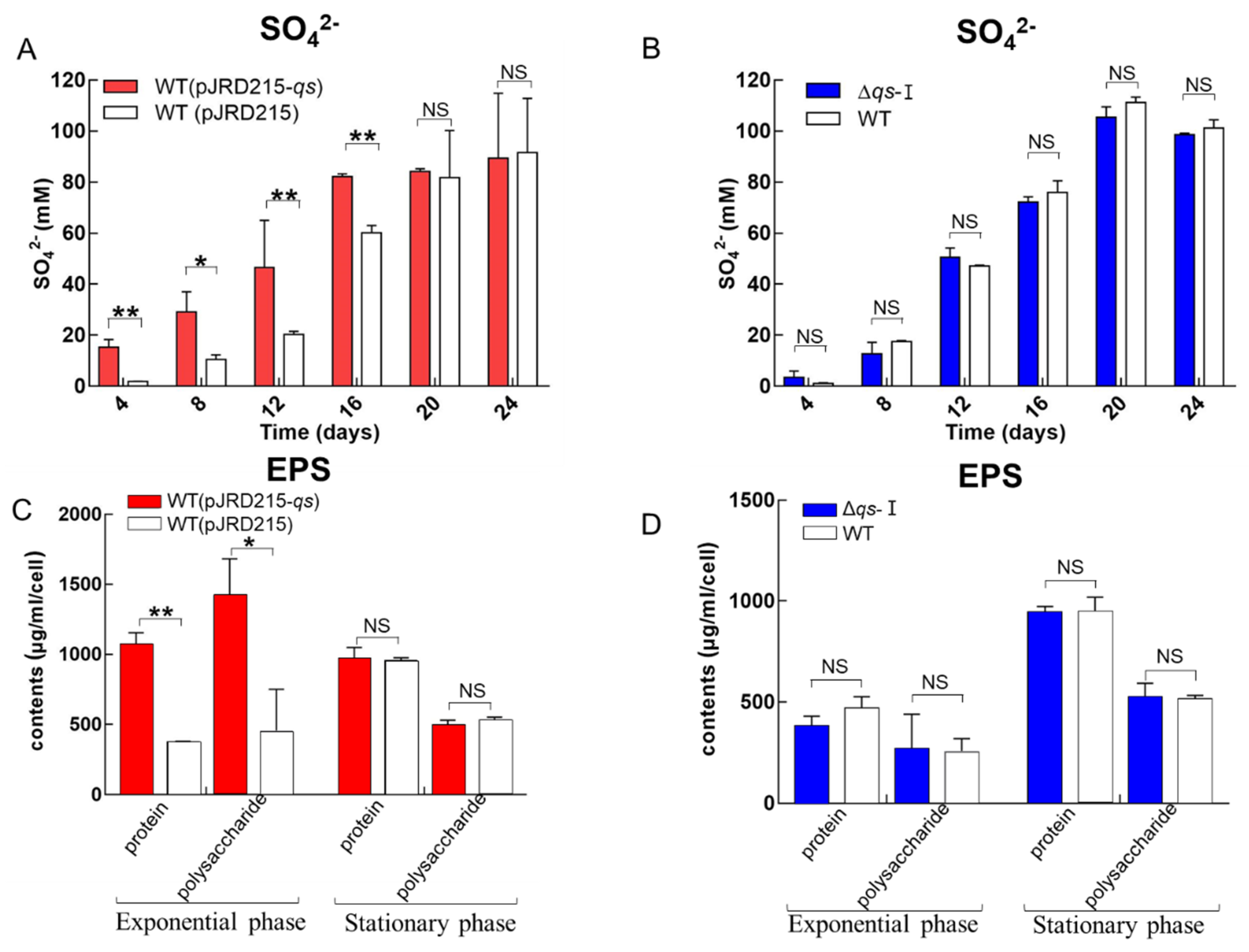
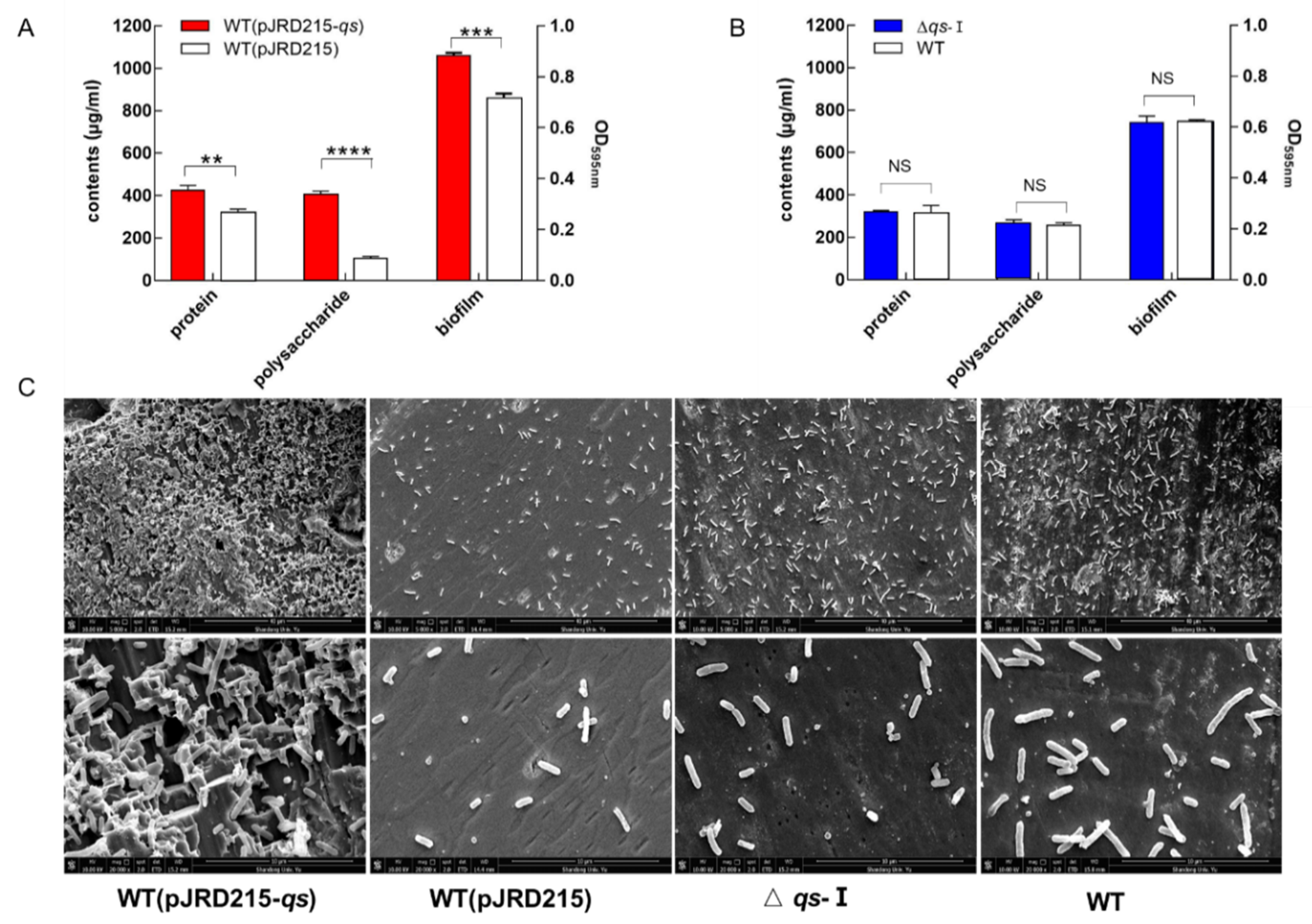
3.3. Influence of qs-I on Sulfur Metabolism and EPS Synthesis of A. ferrooxidans
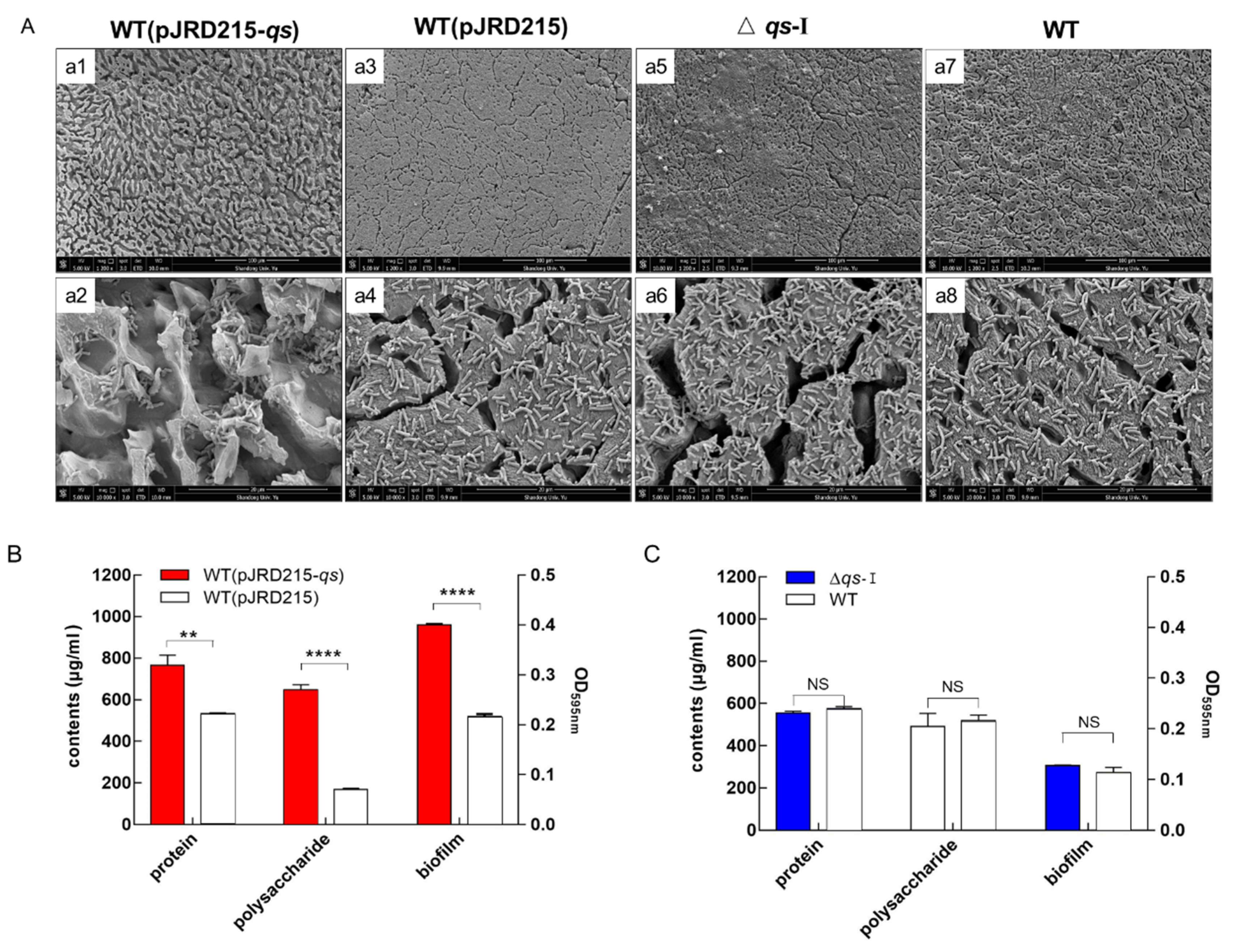
3.4. Effect of qs-I on the Bacteria-Substrate Interactions
3.5. Effect of AfeI/R on the Bioleaching Process
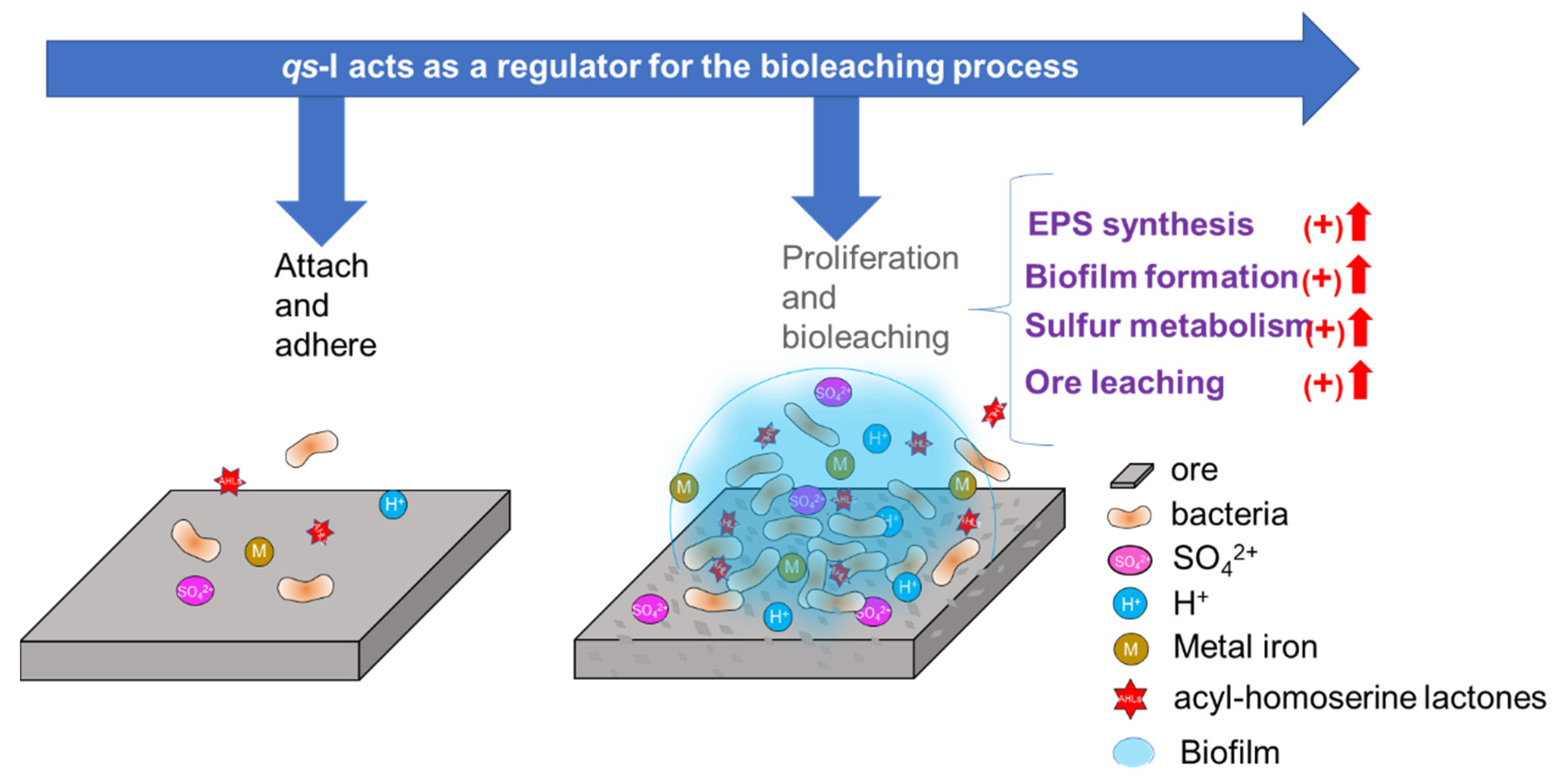
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. Fems Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. Bioleaching review part A: Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl. Microbiol. Biotechnol. 2003, 63, 239. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, B.; Zhu, J.; Jiang, H.; Li, J.; Zhang, R.; Sand, W. Comparative analysis of attachment to chalcopyrite of three mesophilic iron and/or sulfur-oxidizing acidophiles. Minerals 2018, 8, 406. [Google Scholar] [CrossRef]
- Rawlings, D.E. Heavy metal mining using microbes. Ann. Rev. Microbiol. 2002, 56, 65. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.J.; Brierley, J.A.; Brierley, C.L. Bioleaching review part B: Progress in bioleaching: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2003, 63, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Vardanyan, A.; Vardanyan, N.; Khachatryan, A.; Zhang, R.; Sand, W. Adhesion to mineral surfaces by cells of Leptospirillum, Acidithiobacillus and sulfobacillus from armenian sulfide ores. Minerals 2019, 9, 69. [Google Scholar] [CrossRef]
- Brierley, C.L. How will biomining be applied in future? Trans. Nonferrous Met. Soc. 2008, 18, 1302–1310. [Google Scholar] [CrossRef]
- Pathak, A.; Dastidar, M.G.; Sreekrishnan, T.R. Bioleaching of heavy metals from sewage sludge: A review. J. Environ. Manag. 2009, 90, 2343–2353. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Song, Y.; Wu, Y.; Guo, Z. The synthesis of secondary iron minerals induced by quartz sand during the bioleaching process improves the dewaterability of municipal sewage sludge. Minerals 2018, 8, 419. [Google Scholar] [CrossRef]
- Gonzalez, A.; Bellenberg, S.; Mamani, S.; Ruiz, L.; Echeverria, A.; Soulere, L.; Doutheau, A.; Demergasso, C.; Sand, W.; Queneau, Y.; et al. AHL signaling molecules with a large acyl chain enhance biofilm formation on sulfur and metal sulfides by the bioleaching bacterium Acidithiobacillus ferrooxidans. Appl. Microbiol. Biotechnol. 2013, 97, 3729–3737. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, C.; Kou, J.; Zhao, H.; Wei, D.; Xing, Y. Enhancing the leaching of chalcopyrite using Acidithiobacillus ferrooxidans under the induction of surfactant triton X-100. Minerals 2018, 9, 11. [Google Scholar] [CrossRef]
- Ohmura, N.; Sasaki, K.; Matsumoto, N.; Saiki, H. Anaerobic respiration using Fe(3+), S(0), and H(2) in the chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans. J. Bacteriol. 2002, 184, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Sugio, T.; Mizunashi, W.; Inagaki, K.; Tano, T. Purification and some properties of sulfur: Ferric ion oxidoreductase from Thiobacillus ferrooxidans. J. Bacteriol. 1987, 169, 4916–4922. [Google Scholar] [CrossRef]
- La Vars, S.M.; Newton, K.; Quinton, J.S.; Cheng, P.-Y.; Wei, D.-H.; Chan, Y.-L.; Harmer, S.L. Surface chemical characterisation of pyrite exposed to Acidithiobacillus ferrooxidans and associated extracellular polymeric substances. Minerals 2018, 8, 132. [Google Scholar] [CrossRef]
- Jafari, M.; Shafaei, S.Z.A.; Abdollahi, H.; Gharabaghi, M.; Chehreh Chelgani, S. A comparative study on the effect of flotation reagents on growth and iron oxidation activities of Leptospirillum ferrooxidans and Acidithiobacillus ferrooxidans. Minerals 2017, 7, 2. [Google Scholar] [CrossRef]
- Haghshenas, D.F.; Alamdari, E.K.; Bonakdarpour, B.; Darvishi, D.; Nasernejad, B. Kinetics of sphalerite bioleaching by Acidithiobacillus ferrooxidans. Hydrometallurgy 2009, 99, 202–208. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, L.; Xing, W.; Chen, P.; Zhang, Y.; Wang, W. Acidithiobacillus ferrooxidans and its potential application. Extremophiles 2018, 22, 563–579. [Google Scholar] [CrossRef]
- Rodriguez-Leiva, M.; Tributsch, H. Morphology of bacterial leaching patterns by Thiobacillus ferrooxidans on synthetic pyrite. Arch. Microbiol. 1988, 149, 401–405. [Google Scholar] [CrossRef]
- Rohwerder, T.; Sand, W. Oxidation of Inorganic Sulfur Compounds in Acidophilic Prokaryotes. Eng. Life Sci. 2007, 7, 301–309. [Google Scholar] [CrossRef]
- Liu, H.L.; Chen, B.Y.; Lan, Y.W.; Cheng, Y.C. SEM and AFM images of pyrite surfaces after bioleaching by the indigenous Thiobacillus thiooxidans. Appl. Microbiol. Biotechnol. 2003, 62, 414–420. [Google Scholar] [CrossRef]
- Karamanev, D.G. Model of the biofilm structure of Thiobacillus ferrooxidans. J. Biotechnol. 1991, 20, 51–64. [Google Scholar] [CrossRef]
- Gehrke, T.; Telegdi, J.; Thierry, D.; Sand, W. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl. Environ. Microbiol. 1998, 64, 2743–2747. [Google Scholar] [CrossRef] [PubMed]
- San Martín, F.; Aguilar, C. Study of the adhesion mechanism of Acidithiobacillus ferrooxidans to pyrite in fresh and saline water. Minerals 2019, 9, 306. [Google Scholar] [CrossRef]
- Harneit, K.; Göksel, A.; Kock, D.; Klock, J.H.; Gehrke, T.; Sand, W. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 2006, 83, 245–254. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Zhu, J.; Zhou, S.; Gan, M.; Jiang, H.; Sand, W. Effect of extracellular polymeric substances on surface properties and attachment behavior of Acidithiobacillus ferrooxidans. Minerals 2016, 6, 100. [Google Scholar] [CrossRef]
- Parsek, M.R.; Greenberg, E.P. Acyl-homoserine lactone quorum sensing in Gram-negative bacteria: A signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 2000, 97, 8789–8793. [Google Scholar] [CrossRef] [PubMed]
- Kai, P.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576. [Google Scholar] [CrossRef]
- An, D.; Danhorn, T.; Fuqua, C.; Parsek, M.R. Quorum sensing and motility mediate interactions between Pseudomonas aeruginosa and Agrobacterium tumefaciens in biofilm cocultures. Proc. Natl. Acad. Sci. USA 2006, 103, 3828–3833. [Google Scholar] [CrossRef]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef]
- Rivas, M.; Seeger, M.; Holmes, D.S.; Jedlicki, E. A Lux-like quorum sensing system in the extreme acidophile Acidithiobacillus ferrooxidans. Biol. Res. 2005, 38, 283. [Google Scholar] [CrossRef]
- Farah, C.; Vera, M.; Morin, D.; Haras, D.; Jerez, C.A.; Guiliani, N. Evidence for a functional quorum-sensing type AI-1 system in the extremophilic bacterium Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2005, 71, 7033–7040. [Google Scholar] [CrossRef] [PubMed]
- Banderas, A.; Guiliani, N. Bioinformatic prediction of gene functions regulated by quorum sensing in the bioleaching bacterium Acidithiobacillus ferrooxidans. Int. J. Mol. Sci. 2013, 14, 16901–16916. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.; Seeger, M.; Jedlicki, E.; Holmes, D.S. Second acyl homoserine lactone production system in the extreme acidophile Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2007, 73, 3225. [Google Scholar] [CrossRef] [PubMed]
- Bellenberg, S.; Diaz, M.; Noel, N.; Sand, W.; Poetsch, A.; Guiliani, N.; Vera, M. Biofilm formation, communication and interactions of leaching bacteria during colonization of pyrite and sulfur surfaces. Res. Microbiol. 2014, 165, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Mamani, S.; Moinier, D.; Denis, Y.; Soulere, L.; Queneau, Y.; Talla, E.; Bonnefoy, V.; Guiliani, N. Insights into the quorum sensing regulon of the acidophilic Acidithiobacillus ferrooxidans revealed by transcriptomic in the presence of an acyl homoserine lactone superagonist analog. Front. Microbiol. 2016, 7, 1365. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, L.M.; Valenzuela, S.; Castro, M.; Gonzalez, A.; Frezza, M.; Soulère, L.; Rohwerder, T.; Queneau, Y.; Doutheau, A.; Sand, W. AHL communication is a widespread phenomenon in biomining bacteria and seems to be involved in mineral-adhesion efficiency. Hydrometallurgy 2008, 94, 133–137. [Google Scholar] [CrossRef]
- Inaba, Y.; Banerjee, I.; Kernan, T.; Banta, S. Transposase-mediated chromosomal integration of exogenous genes in Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2018, 84, e01381-01318. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Liu, S.; Yu, Y.; Lin, J.; Lin, J.; Pang, X.; Zhao, J. Development of a markerless gene replacement system for Acidithiobacillus ferrooxidans and construction of a pfkB mutant. Appl. Environ. Microbiol. 2012, 78, 1826–1835. [Google Scholar] [CrossRef][Green Version]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: New York, NY, USA, 1982; pp. 895–909. [Google Scholar]
- Simon, R.; Priefer, U.; Pühler, A. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram negative bacteria. Nat. Biotechnol. 1983, 1, 784–791. [Google Scholar] [CrossRef]
- Wang, Z.B.; Li, Y.Q.; Lin, J.Q.; Pang, X.; Liu, X.M.; Liu, B.Q.; Wang, R.; Zhang, C.J.; Wu, Y.; Lin, J.Q.; et al. The two-component system RsrS-RsrR regulates the tetrathionate intermediate pathway for thiosulfate oxidation in Acidithiobacillus caldus. Front. Microbiol. 2016, 7, 1755. [Google Scholar] [CrossRef]
- Davison, J.; Heusterspreute, M.; Chevalier, N.; Ha-Thi, V.; Brunel, F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene 1987, 51, 275–280. [Google Scholar] [CrossRef]
- Miura, Y.; Kawaoi, A. Determination of thiosulfate, thiocyanate and polythionates in a mixture by ion-pair chromatography with ultraviolet absorbance detection. J. Chromatogr. A 2000, 884, 81–87. [Google Scholar] [CrossRef]
- More, T.T.; Yadav, J.S.S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances of bacteria and their potential environmental applications. J. Environ. Manag. 2014, 144, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, E.; Zhang, J.; Dai, Y.; Yang, Z.; Christensen, H.E.M.; Ulstrup, J.; Zhao, F. Extracellular polymeric substances are transient media for microbial extracellular electron transfer. Sci. Adv. 2017, 3, e1700623. [Google Scholar] [CrossRef]
- Grandy, A.S.; Erich, M.S.; Porter, G.A. Suitability of the anthrone–sulfuric acid reagent for determining water soluble carbohydrates in soil water extracts. Soil Biol. Biochem. 2000, 32, 725–727. [Google Scholar] [CrossRef]
- Nieto, P.A.; Covarrubias, P.C.; Jedlicki, E.; Holmes, D.S.; Quatrini, R. Selection and evaluation of reference genes for improved interrogation of microbial transcriptomes: Case study with the extremophile Acidithiobacillus ferrooxidans. BMC Mol. Biol. 2009, 10, 63. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zheng, Y.; Cosgrove, D.J.; Ning, G. High-resolution field emission scanning electron microscopy (FESEM) imaging of cellulose microfibril organization in plant primary cell walls. Microsc. Microanal. 2017, 23, 1048–1054. [Google Scholar] [CrossRef]
- Bret, L.; Di Martino, P. Effect of ceftazidime, amikacin and ciprofloxacin on biofilm formation by some enterobacterial clinical isolates. Chemotherapy 2004, 50, 255–259. [Google Scholar] [CrossRef]
- Rodrigues, J.; Nunes, J.; Batista, B.; Souza, S.; Barbosa, F. A fast method for the determination of 16 elements in hair samples by inductively coupled plasma mass spectrometry (ICP-MS) with tetramethylammonium hydroxide solubilization at room temperature. J. Anal. At. Spectrom. 2008, 23, 992. [Google Scholar] [CrossRef]
- Parsek, M.R.; Greenberg, E.P. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005, 13, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Marczak, M.; Dzwierzynska, M.; Skorupska, A. Homo- and heterotypic interactions between Pss proteins involved in the exopolysaccharide transport system in Rhizobium leguminosarum bv. trifolii. Biol. Chem. 2013, 394, 541–559. [Google Scholar] [CrossRef]
- Barreto, M.; Jedlicki, E.; Holmes, D.S. Identification of a gene cluster for the formation of extracellular polysaccharide precursors in the chemolithoautotroph Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2005, 71, 2902–2909. [Google Scholar] [CrossRef] [PubMed]
- Ciocchini, A.E.; Guidolin, L.S.; Casabuono, A.C.; Couto, A.S.; Iñón de Iannino, N.; Ugalde, R.A. A glycosyltransferase with a length-controlling activity as a mechanism to regulate the size of polysaccharides. Proc. Natl. Acad. Sci. USA 2007, 104, 16492–16497. [Google Scholar] [CrossRef] [PubMed]
- Holden, H.M.; Rayment, I.; Thoden, J.B. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J. Biol. Chem. 2003, 278, 43885–43888. [Google Scholar] [CrossRef] [PubMed]
- Bettenbrock, K.; Alpert, C.A. The gal genes for the Leloir pathway of Lactobacillus casei 64H. Appl. Environ. Microbiol. 1998, 64, 2013–2019. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Wang, L.; Liu, S.; Bai, Z.; Zhuang, X.; Zhuang, G. Environmental adaptability and quorum sensing: Iron uptake regulation during biofilm formation by Paracoccus denitrificans. Appl. Environ. Microbiol. 2018, 84, e00865-00818. [Google Scholar] [CrossRef]
- Yang, H.; Luo, W.; Gao, Y. Effect of Acidithiobacillus ferrooxidans on humic-acid passivation layer on pyrite surface. Minerals 2018, 8, 422. [Google Scholar] [CrossRef]
- Goo, E.; Majerczyk, C.D.; An, J.H.; Chandler, J.R.; Seo, Y.S.; Ham, H.; Lim, J.Y.; Kim, H.; Lee, B.; Jang, M.S.; et al. Bacterial quorum sensing, cooperativity, and anticipation of stationary-phase stress. Proc. Natl. Acad. Sci. USA 2012, 109, 19775–19780. [Google Scholar] [CrossRef]
- An, J.H.; Goo, E.; Kim, H.; Seo, Y.S.; Hwang, I. Bacterial quorum sensing and metabolic slowing in a cooperative population. Proc. Natl. Acad. Sci. USA 2014, 111, 14912–14917. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Guo, Y.; Wu, S.; Chen, L.; Tao, H.; Liu, S. Metabolomics uncovers the regulatory pathway of acyl-homoserine lactones based quorum sensing in anammox consortia. Environ. Sci. Technol. 2018, 52, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Tributsch, H. Direct versus indirect bioleaching. Hydrometallurgy 2001, 59, 177–185. [Google Scholar] [CrossRef]
- Quatrini, R.; Appia-Ayme, C.; Denis, Y.; Ratouchniak, J.; Veloso, F.; Valdes, J.; Lefimil, C.; Silver, S.; Roberto, F.; Orellana, O.; et al. Insights into the iron and sulfur energetic metabolism of Acidithiobacillus ferrooxidans by microarray transcriptome profiling. Hydrometallurgy 2006, 83, 263–272. [Google Scholar] [CrossRef]
- Wang, R.; Lin, J.-Q.; Liu, X.-M.; Pang, X.; Zhang, C.-J.; Yang, C.-L.; Gao, X.-Y.; Lin, C.-M.; Li, Y.-Q.; Li, Y.; et al. Sulfur oxidation in the acidophilic autotrophic Acidithiobacillus spp. Front. Microbiol. 2019, 9. [Google Scholar] [CrossRef]
- Wagner, T.; Koch, J.; Ermler, U. Methanogenic heterodisulfide reductase (HdrABC-MvhAGD) uses two noncubane [4Fe-4S] clusters for reduction. Science 2017, 357, 699–703. [Google Scholar] [CrossRef]
- Liu, L.J.; Stockdreher, Y.; Koch, T.; Sun, S.T.; Fan, Z.; Josten, M.; Sahl, H.G.; Wang, Q.; Luo, Y.M.; Liu, S.J.; et al. Thiosulfate transfer mediated by DsrE/TusA homologs from acidothermophilic sulfur-oxidizing archaeon Metallosphaera cuprina. J. Biol. Chem. 2014, 289, 26949–26959. [Google Scholar] [CrossRef]
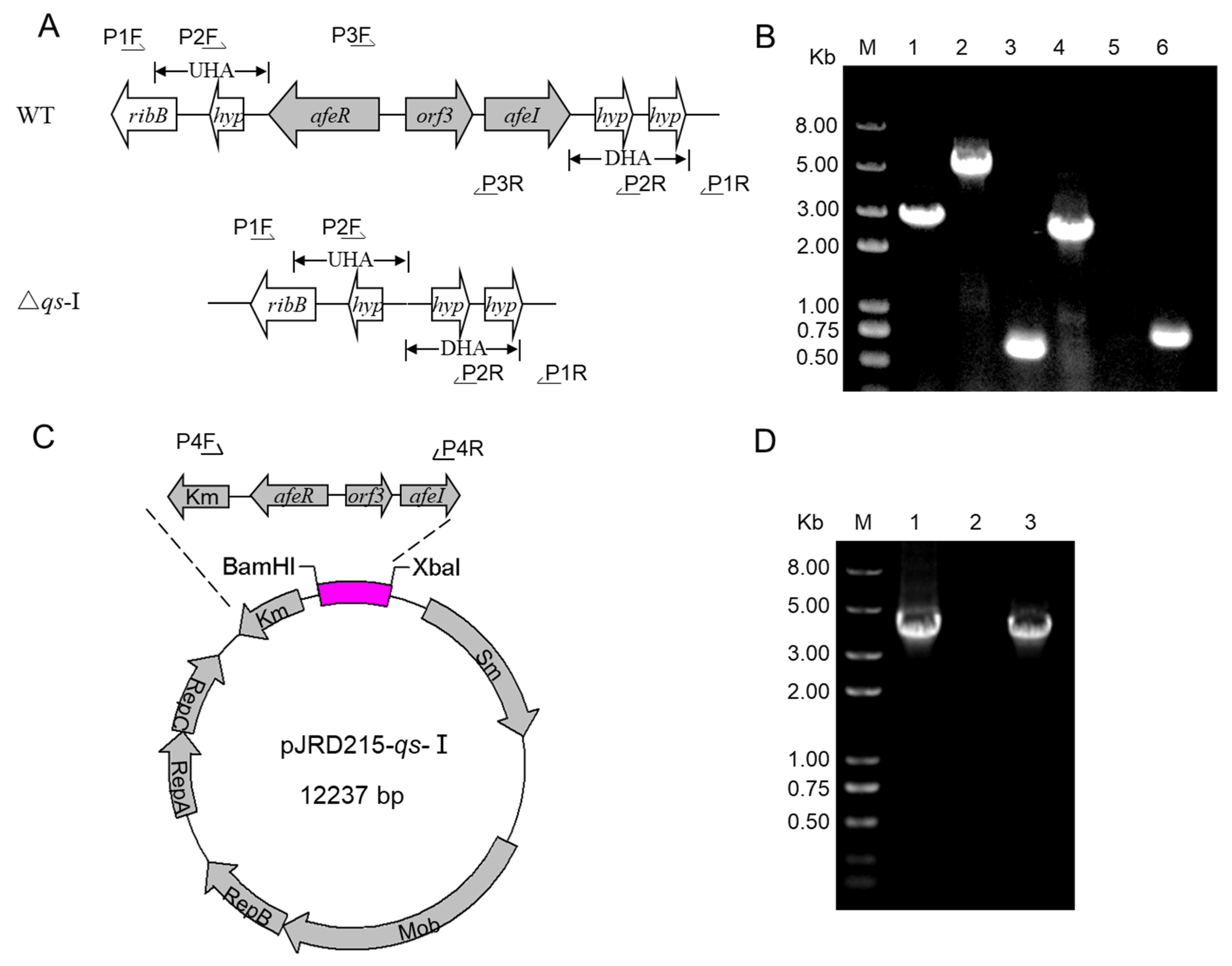
| Strain and Plasmids | Description | Source |
|---|---|---|
| Strain | ||
| Acidithiobacillus ferrooxidans ATCC 23270 | Wild type strain | ATCC |
| WT (pJRD215) | Wild type with the plasmid of pJRD215 | This study |
| △qs-I | qs-I (afeR-orf3-afeI) gene cluster deletion | This study |
| WT (pJRD215-qs) | overexpress qs-I gene cluster (afeR-orf3-afeI), wild type with the plasmid of pJRD215- qs-I | This study |
| Escherichia coli | ||
| DH5α | F-φ80dlacZΔM15Δ(lacZYA-argF)U169 end A1 recA1 hsdR17(rk-,mk+) supE44λ-thi-1 gyr96 rela1 phoA | TransGen Biotech |
| SM10 | Kmrthi-1 thr leu tonA lacY supE recARP4-2-Tc::Mu | [40] |
| Plasmids | ||
| pSDUDI | Suicide plasmid; Apr Kmr oiTRP4 multi-cloning sites | [41] |
| pSDUDI-HFqs-I | Suicide plasmid for Δqs-I construction, pSDUDI carrying both homologous fragments of qs-I | This study |
| pMSD1-I-SecI | pMSD containing the I-SecI gene | [38] |
| pJRD215 | Smr Kmr IncQ Mob+ | [42] |
| pJRD215-qs-I | pJRD215 carrying the qs-I gene cluster (afeR-orf3-afeI) | This study |
| Gene ID | Δqs-I vs WT | WT (pJRD215-qs) vs WT(pJRD215) | Gene Name | Description |
|---|---|---|---|---|
| Sulfur metabolism genes | ||||
| AFE_0042 | 1.24 ± 0.33 | 1.05 ± 0.03 | tsd2 | hypothetical protein |
| AFE_0043 | 0.94 ± 0.36 | 1.44 ± 0.13 | sbp2 | glycine/betaine ABC transporter substrate-binding protein |
| AFE_0044 | 1.00 ± 0.08 | 1.24 ± 0.01 | doxDA2 | quinol oxidase |
| AFE_0045 | 0.66 ± 0.24 | 1.52 ± 0.15 | rhd1 | rhodanese-like domain-containing protein |
| AFE_0046 | 0.58 ± 0.25 | 2.01 ± 0.50 | rhd2 | rhodanese-like domain-containing protein |
| AFE_0047 | 0.71 ± 0.35 | 2.56 ± 0.55 | resA | TlpA family protein disulfide reductase |
| AFE_0048 | 1.03 ± 0.32 | 1.11 ± 0.09 | doxDA1 | quinol oxidase |
| AFE_0049 | 1.01 ± 0.41 | 3.31 ± 0.42 | sbp1 | glycine/betaine ABC transporter substrate-binding protein |
| AFE_0050 | 1.09 ± 0.44 | 2.51 ± 0.32 | tsd1 | hypothetical protein |
| AFE_2550 | 0.69 ± 0.33 | 1.17 ± 0.08 | sdhC | disulfide reductase |
| AFE_2551 | 1.05 ± 0.41 | 1.69 ± 0.02 | hdrC | heterodisulfide reductase subunit C |
| AFE_2552 | 0.98 ± 0.44 | 2.00 ± 0.37 | - | hypothetical protein |
| AFE_2553 | 0.94 ± 0.03 | 1.41 ± 0.39 | hdrA | pyridine nucleotide-disulfide oxidoreductase |
| AFE_2554 | 1.07 ± 0.45 | 2.12 ± 0.13 | hdrB | heterodisulfide reductase subunit B |
| AFE_2555 | 0.96 ± 0.15 | 1.28 ± 0.05 | hdrC | heterodisulfide reductase subunit C |
| AFE_2556 | 1.00 ± 0.22 | 2.09 ± 0.26 | dsrE | NADH dehydrogenase |
| AFE_2557 | 0.93 ± 0.12 | 2.63 ± 0.57 | tusA | sulfurtransferase TusA family protein |
| AFE_2558 | 0.85 ± 0.28 | 1.15 ± 0.35 | rhd | rhodanese-like domain-containing protein |
| AFE_2586 | 1.27 ± 0.11 | 1.81 ± 0.06 | hdrB | heterodisulfide reductase subunit B |
| AFE_0029 | 0.85 ± 0.11 | 0.54 ± 0.09 | tetH | tetrathionate hydrolase |
| AFE_0267 | 0.61 ± 0.01 | 0.95 ± 0.18 | sqr | pyridine nucleotide-disulfide oxidoreductase |
| AFE_1792 | 0.75 ± 0.07 | 1.62 ± 0.13 | sqr | sulfide:quinone reductase |
| AFE_0269 | 1.46 ± 0.08 | 0.66 ± 0.06 | sdo | MBL fold metallo-hydrolase |
| AFE_2644 | 0.75 ± 0.02 | 1.17 ± 0.23 | sdo | MBL fold metallo-hydrolase |
| EPS related genes | ||||
| AFE_2321 | 0.9 ± 0.10 | 0.66 ± 0.05 | luxA | monooxygenase, luciferase-like protein |
| AFE_2322 | 0.89 ± 0.04 | 0.71 ± 0.03 | galE | conserved hypothetical protein |
| AFE_2323 | 0.98 ± 0.09 | 0.45 ± 0.10 | galK | conserved hypothetical protein |
| AFE_2324 | 0.72 ± 0.13 | 2.55 ± 0.31 | pgm | phosphoglucomutase, putative |
| AFE_2325 | 0.68 ± 0.19 | 1.45 ± 0.18 | galM | hypothetical protein |
| AFE_1497 | 0.75 ± 0.31 | 5.34 ± 2.70 | orpD | outer membrane porin, OprD family |
| AFE_1991 | 0.67 ± 0.31 | 57.71 ± 6.94 | tonB | membrane protein, TonB family |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.-Y.; Liu, X.-J.; Fu, C.-A.; Gu, X.-F.; Lin, J.-Q.; Liu, X.-M.; Pang, X.; Lin, J.-Q.; Chen, L.-X. Novel Strategy for Improvement of the Bioleaching Efficiency of Acidithiobacillus ferrooxidans Based on the AfeI/R Quorum Sensing System. Minerals 2020, 10, 222. https://doi.org/10.3390/min10030222
Gao X-Y, Liu X-J, Fu C-A, Gu X-F, Lin J-Q, Liu X-M, Pang X, Lin J-Q, Chen L-X. Novel Strategy for Improvement of the Bioleaching Efficiency of Acidithiobacillus ferrooxidans Based on the AfeI/R Quorum Sensing System. Minerals. 2020; 10(3):222. https://doi.org/10.3390/min10030222
Chicago/Turabian StyleGao, Xue-Yan, Xiu-Jie Liu, Chang-Ai Fu, Xiu-Feng Gu, Jian-Qiang Lin, Xiang-Mei Liu, Xin Pang, Jian-Qun Lin, and Lin-Xu Chen. 2020. "Novel Strategy for Improvement of the Bioleaching Efficiency of Acidithiobacillus ferrooxidans Based on the AfeI/R Quorum Sensing System" Minerals 10, no. 3: 222. https://doi.org/10.3390/min10030222
APA StyleGao, X.-Y., Liu, X.-J., Fu, C.-A., Gu, X.-F., Lin, J.-Q., Liu, X.-M., Pang, X., Lin, J.-Q., & Chen, L.-X. (2020). Novel Strategy for Improvement of the Bioleaching Efficiency of Acidithiobacillus ferrooxidans Based on the AfeI/R Quorum Sensing System. Minerals, 10(3), 222. https://doi.org/10.3390/min10030222





