Hydrothermal Chromitites from the Oman Ophiolite: The Role of Water in Chromitite Genesis
Abstract
1. Introduction
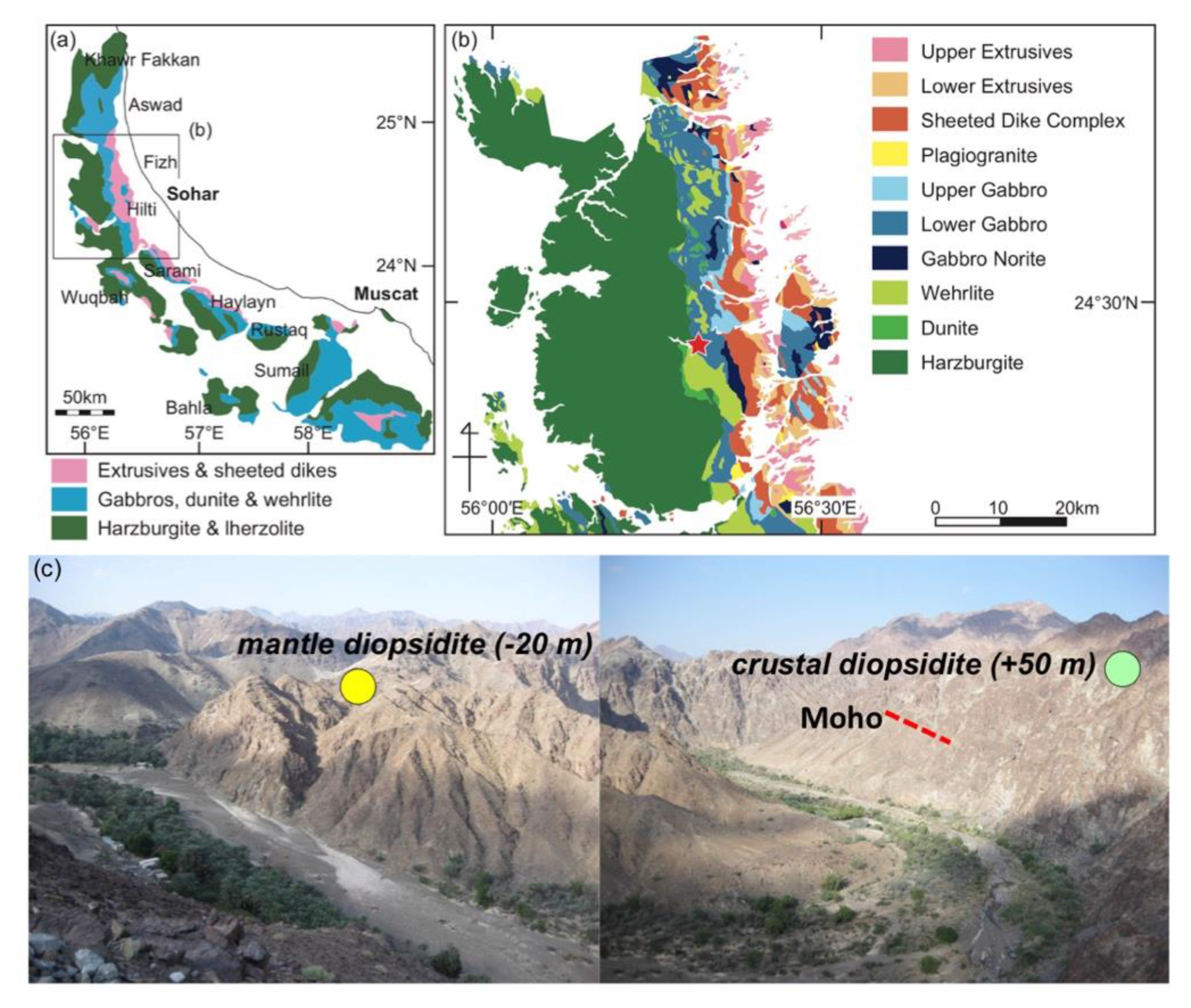
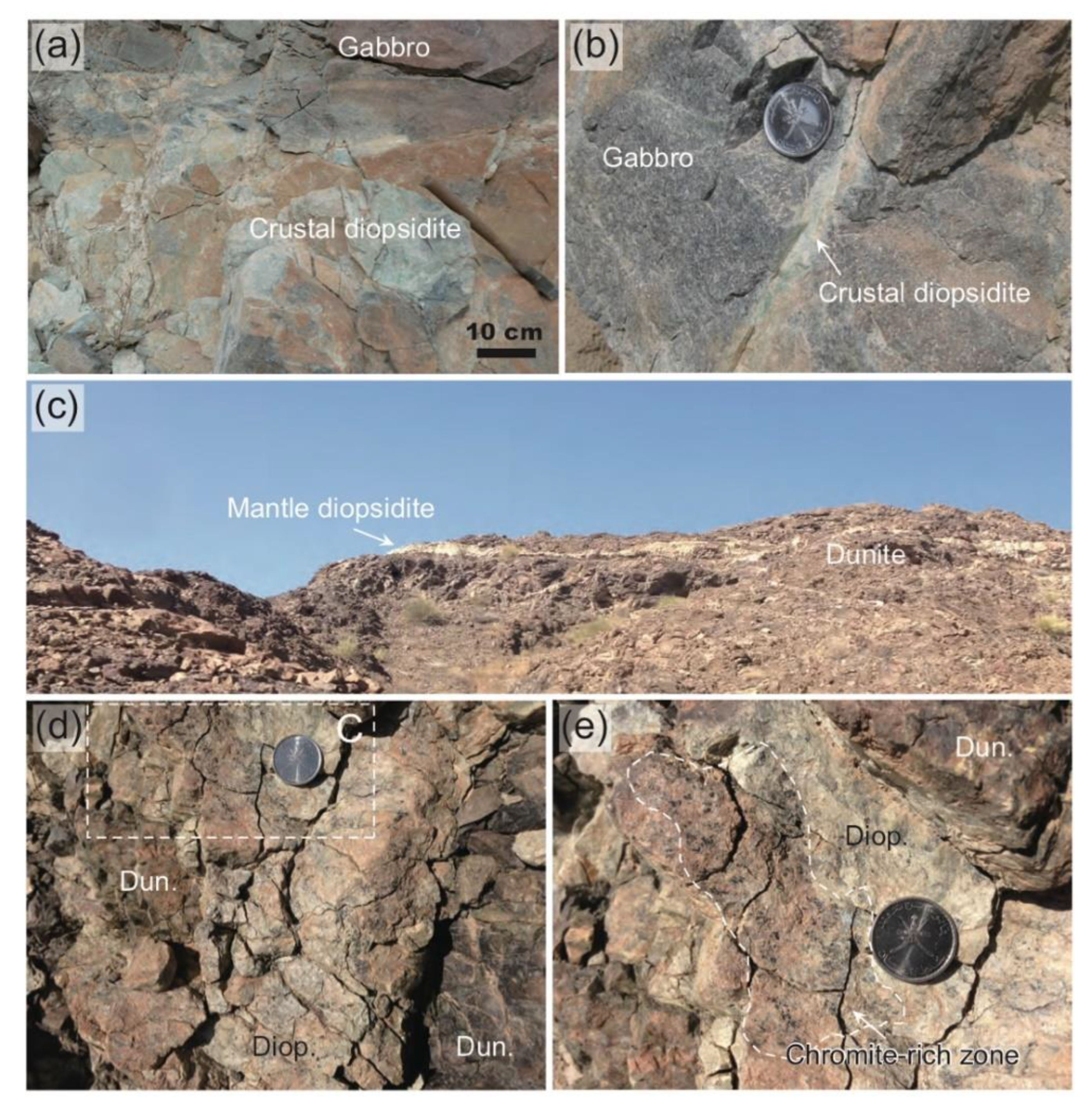
2. Geologic and Petrographic Background
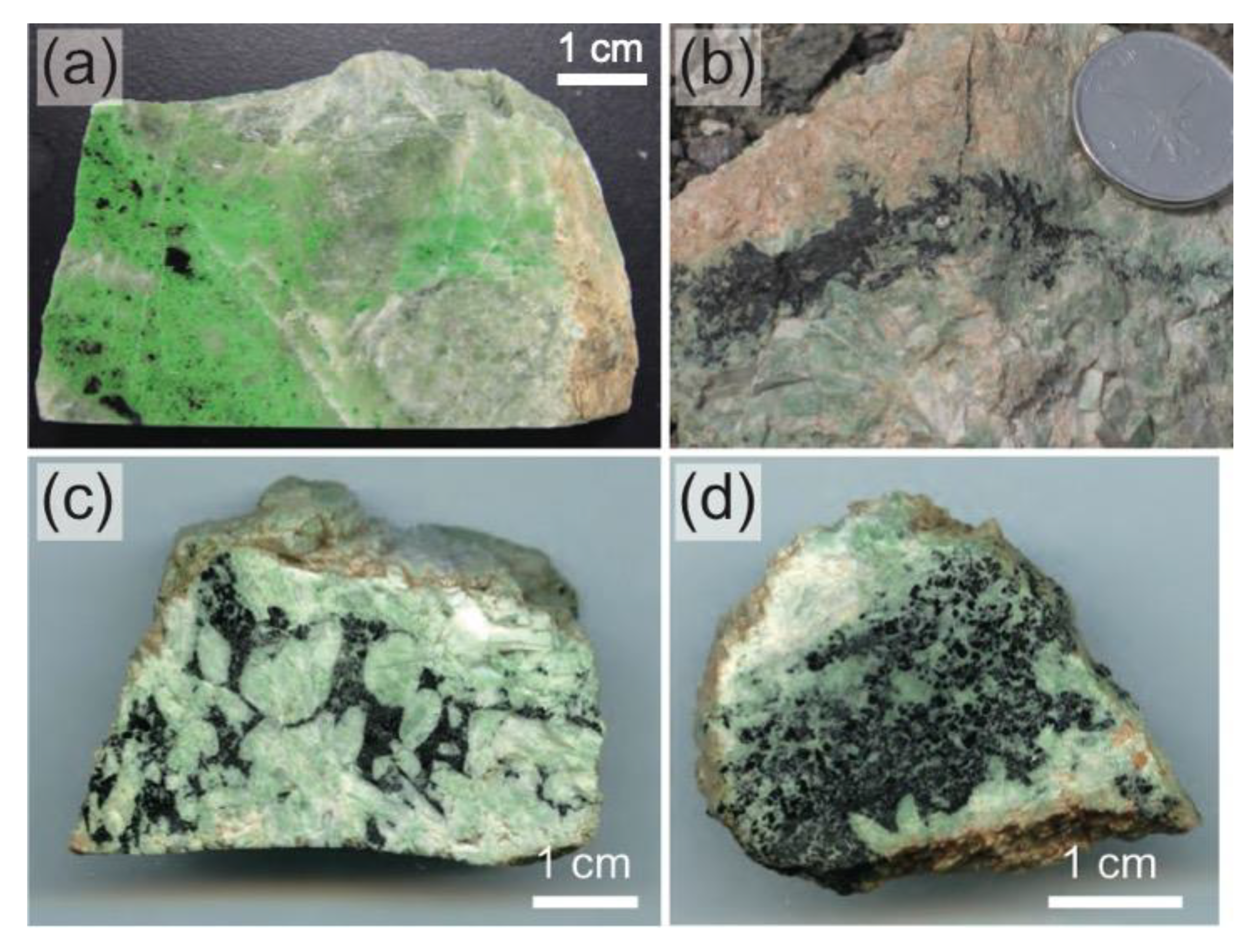
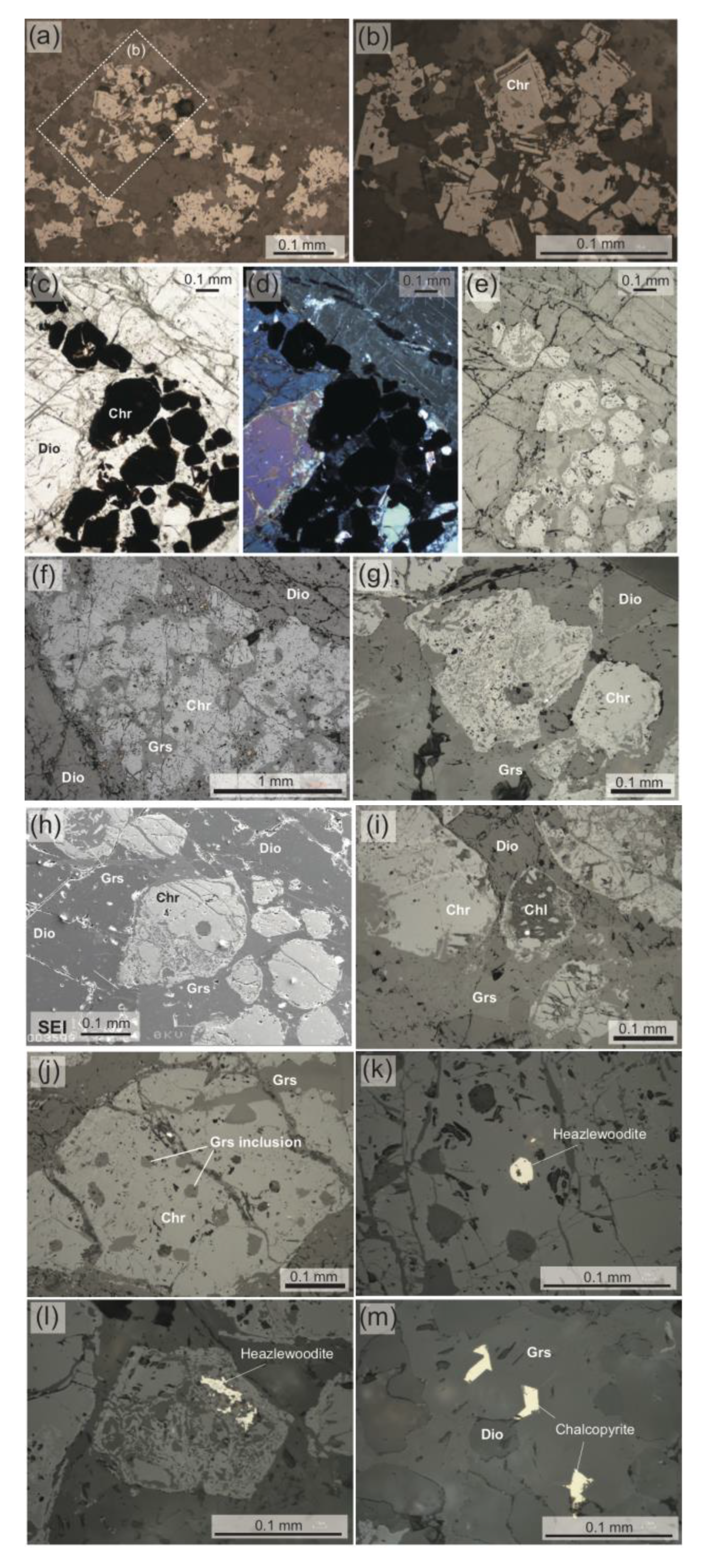
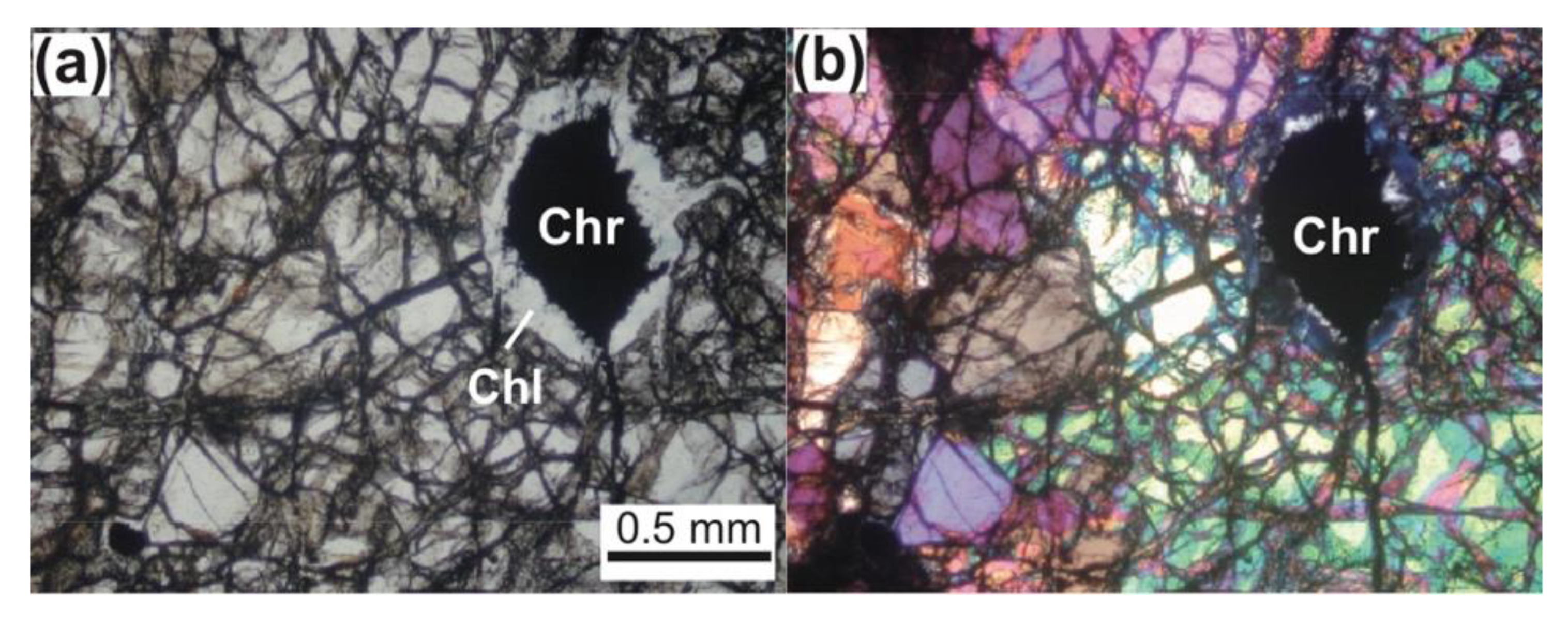
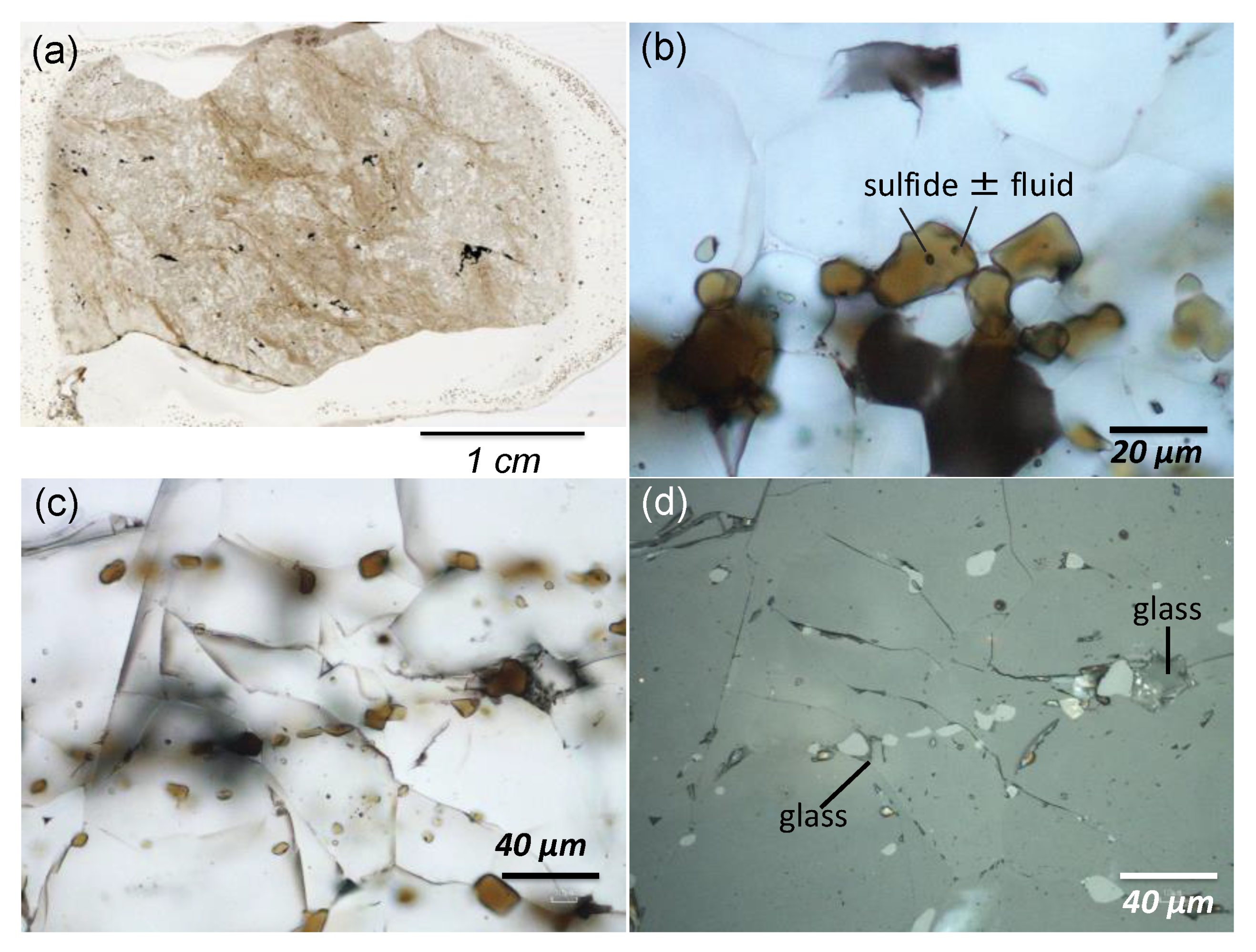
3. Petrography of Chromitites (Chromite Concentrations)
4. Mineral Chemistry
4.1. Chromites
4.2. Silicates
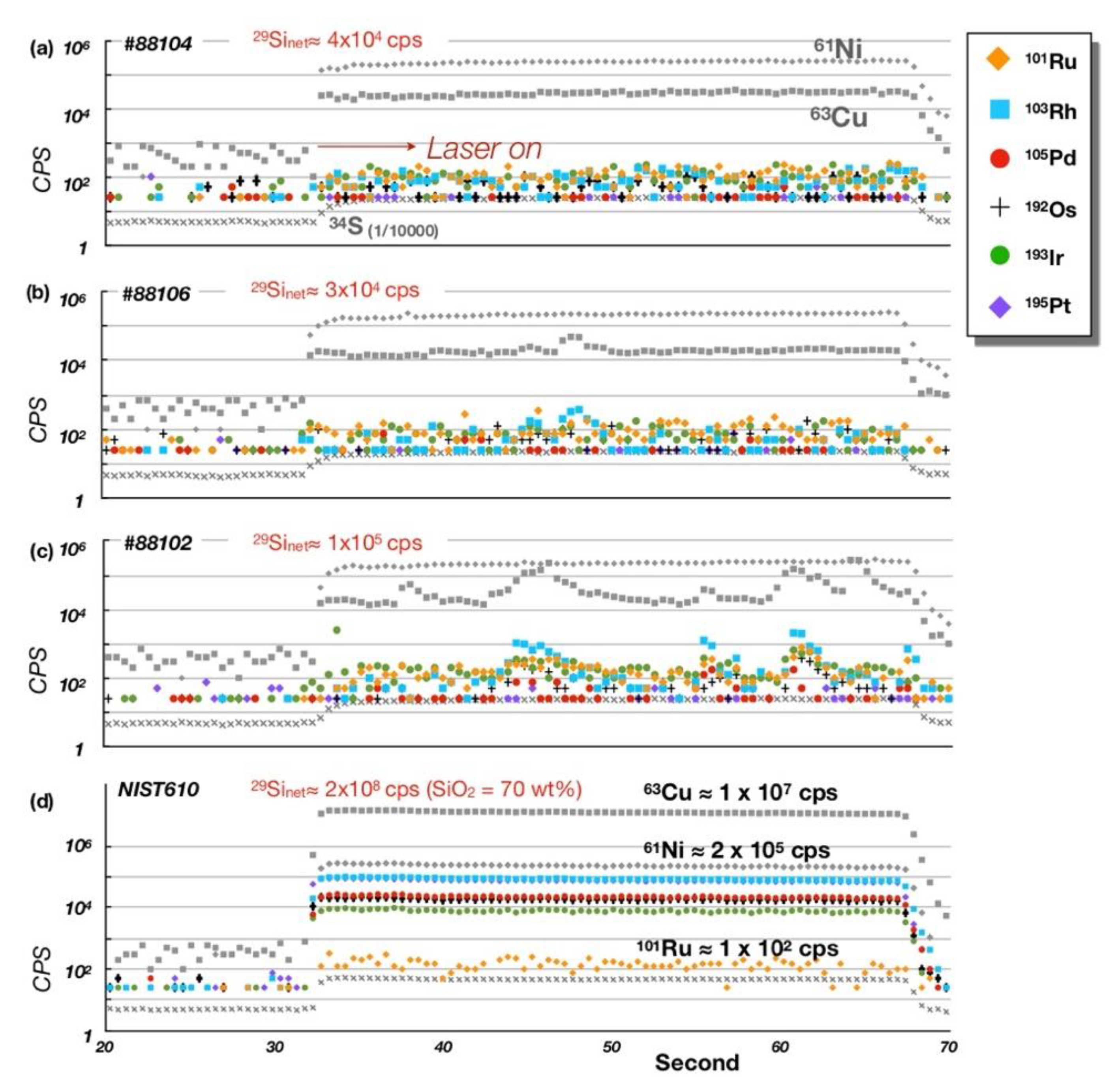
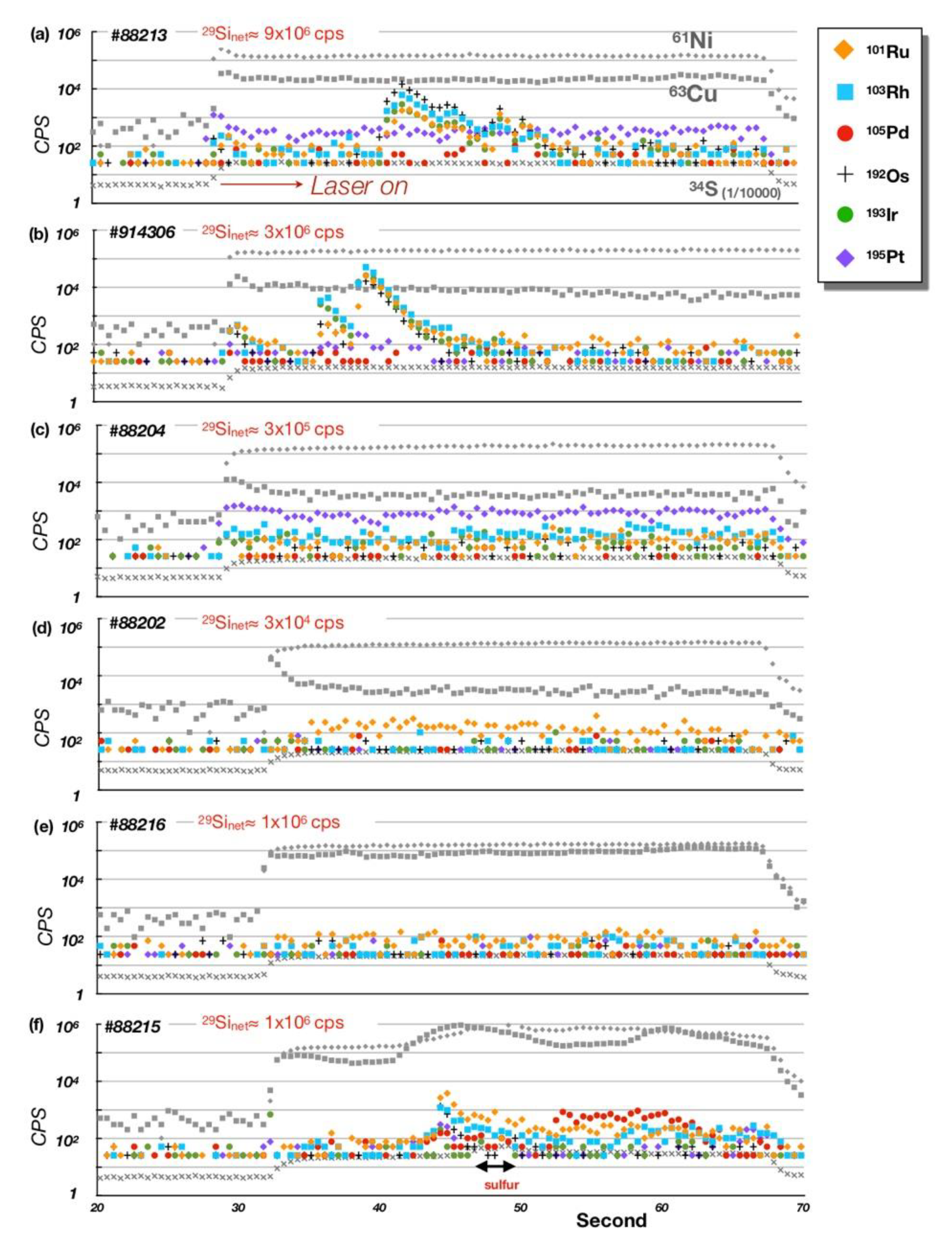
5. Platinum-Group Element (PGE) Geochemistry
5.1. Bulk-Rock PGE + Re Geochemistry
5.2. PGE Mineral Chemistry
6. Discussion
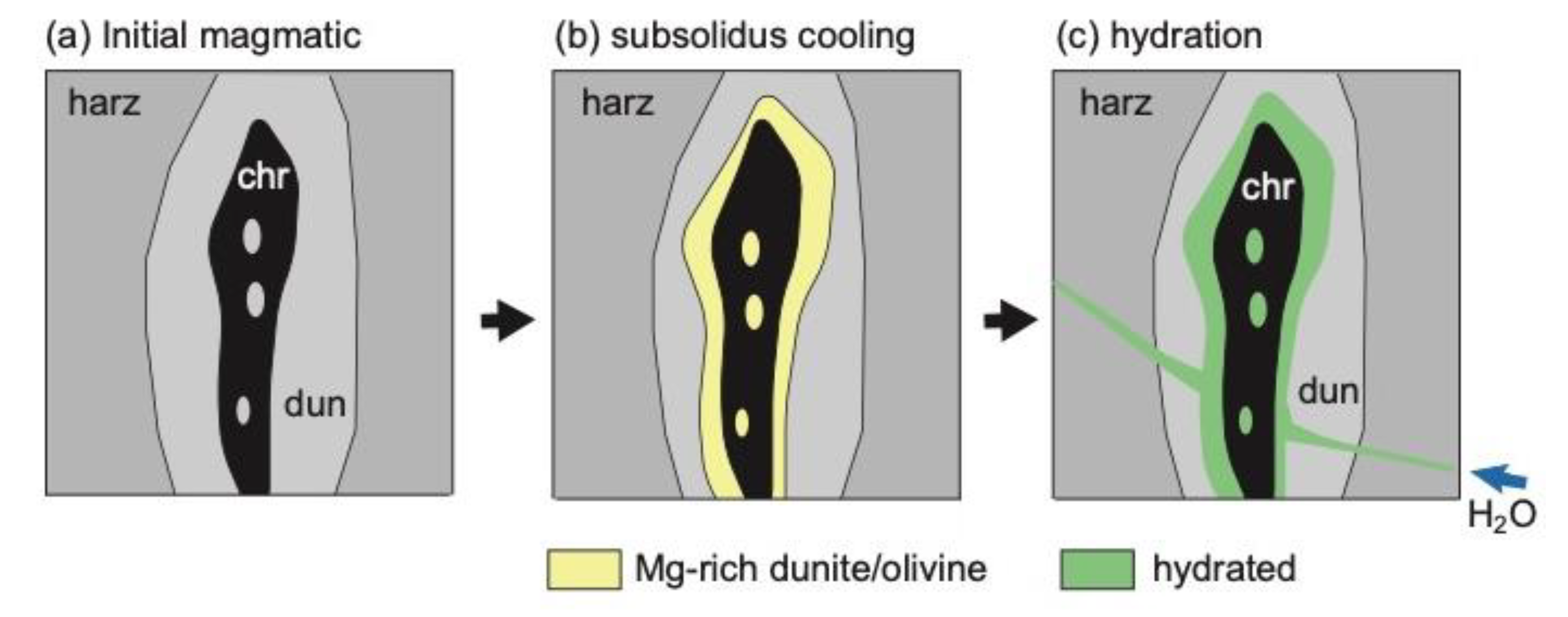
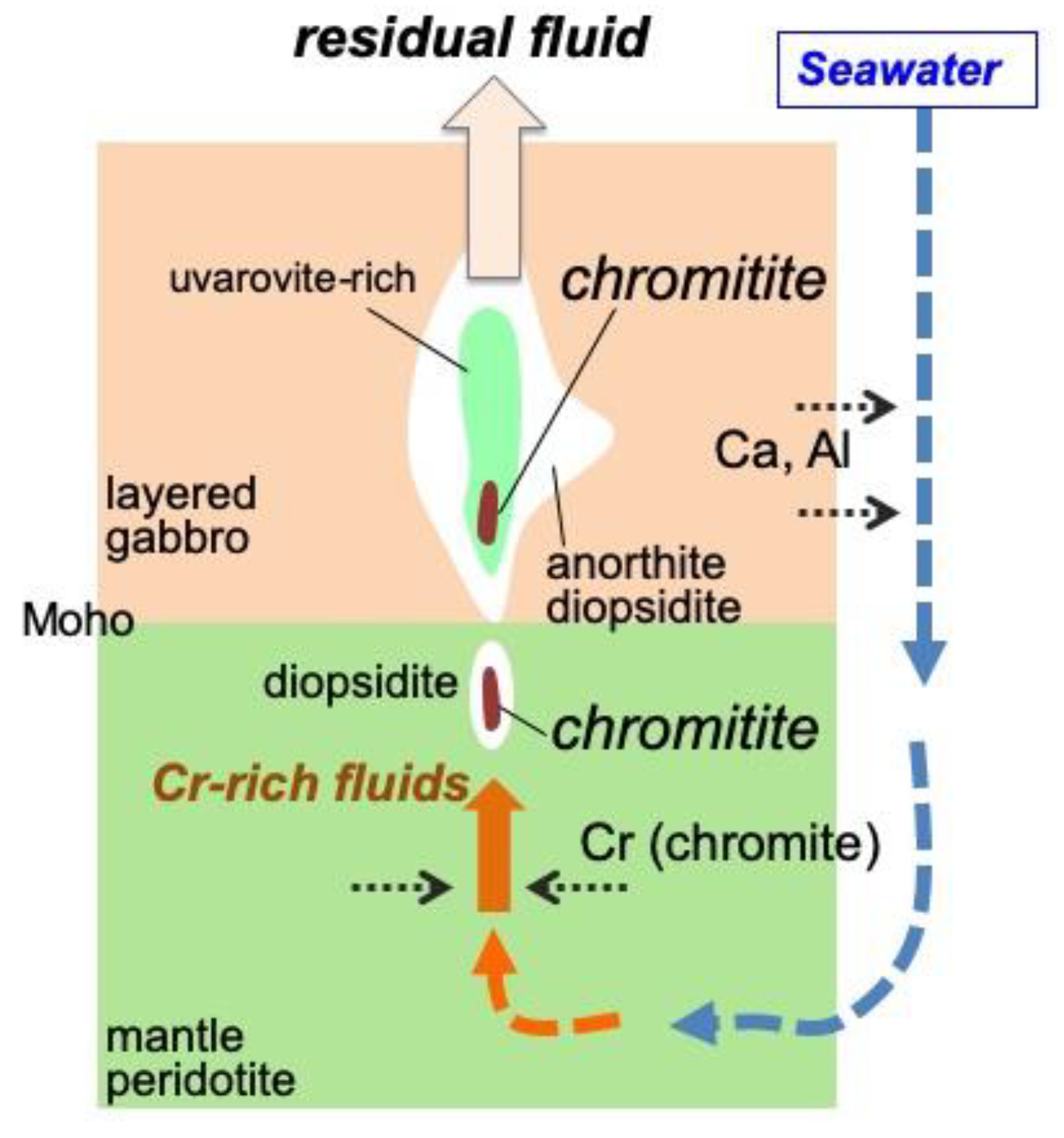
6.1. Role of H2O in the Podiform Chromitite Formation
6.2. Precipitation and Concentration of Chromites from Hydrothermal Fluids
= CaAl2Si2O8 (anorthite) + Ca3Cr2Si3O12 (uvarovite).
6.3. Origin of the Fluid
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Irvine, T.N. Chromian spinel as a petrogenetic indicator. Part II. Petrogenetic applications. Can. J. Earth Sci. 1967, 4, 72–103. [Google Scholar] [CrossRef]
- Thayer, T.P. Principal features and origin of podiform chromitite deposits, and some observations on the Guleman-Soridag district, Turkey. Econ. Geol. 1964, 59, 1497–1524. [Google Scholar] [CrossRef]
- Cassard, D.; Nicolas, A.; Rabinovitch, M.; Moutte, J.; Leblanc, M.; Prinzhofer, A. Structural classification of chromite pods in southern New Caledonia. Econ. Geol. 1981, 76, 805–831. [Google Scholar] [CrossRef]
- Arai, S.; Miura, M. Formation and modification of chromitites in the mantle. Lithos 2016, 264, 277–295. [Google Scholar] [CrossRef]
- Jackson, E.D. The chromite deposits of the Stillwater Complex, Montana. In Ore Deposits of the United States, 1933–1967; The Graton-Sales Volume 2; Ridge, J.D., Ed.; The American Institute of Mining, Metallurgical, and Petroleum Engineers, Inc.: New York, NY, USA, 1968; pp. 1495–1510. [Google Scholar]
- Irvine, T.N.; Smith, C.H. Primary oxide minerals in the layered series of the Muskox intrusion. In Magmatic Ore Deposits; Wilson, H.D.B., Ed.; Economic Geology Publishing Co.: New Haven, CT, USA, 1969; pp. 76–94. [Google Scholar]
- Schulte, R.F.; Taylor, R.D.; Piatak, N.M.; Seal, R.R., II. Stratiform Chromite Deposit Model: Chapter E in Mineral Deposit Model for Resource Assessment. U.S. In Geological Survey Scientific Investigation Report 2010-5070-E; U.S. Geological Survey: Reston, VA, USA, 2012; p. 131. [Google Scholar]
- Arai, S.; Yurimoto, H. Podiform chromitites of the Tari-Misaka ultramafic complex, southwestern Japan, as mantle-melt interaction products. Econ. Geol. 1994, 89, 1279–1285. [Google Scholar] [CrossRef]
- Johan, Z.; Dunlop, H.; Le Bel, L.; Robert, J.-L.; Volfinger, M. Origin of chromite deposits in ophiolitic complexes: Evidence for a volatile- and sodium-rich reducing fluid phase. Fortsch. Mineral. 1983, 61, 105–107. [Google Scholar]
- Johan, Z.; Martin, R.F.; Ettler, V. Fluids are bound to be involved in the formation of ophiolitic chromite deposits. Eur. J. Mineral. 2017, 29, 543–555. [Google Scholar] [CrossRef]
- McElduff, B.; Stumpfl, E.F. The chromite deposits of the Troodos complex, Cyprus-evidence for the role of a fluid phase accompanying chromite formation. Mineral. Depos. 1991, 26, 307–318. [Google Scholar] [CrossRef]
- Matveev, S.; Ballhaus, C. Role of water in the origin of podiform chromitite deposits. Earth Planet. Sci. Lett. 2002, 203, 235–243. [Google Scholar] [CrossRef]
- Borisova, A.Y.; Ceuleneer, G.; Kamenetsky, V.S.; Arai, S.; Béjina, F.; Abily, B.; Bindeman, I.N.; Polvé, M.; De Parseval, P.; Aigouy, T.; et al. A new view on the petrogenesis of the Oman ophiolite chromitites from microanalyses of chromite-hosted inclusions. J. Petrol. 2012, 53, 2411–2440. [Google Scholar] [CrossRef]
- Rospabé, M.; Ceuleneer, G.; Granier, N.; Arai, S.; Borisova, A. Multi-scale development of a stratiform chromite ore body at the base of the dunitic mantle-crust transition zone (Maqsad diapir, Oman ophiolite): The role of repeated melt and fluid influxes. Lithos 2019, 350, 105235. [Google Scholar] [CrossRef]
- Pushkarev, E.V.; Kamenetsky, V.S.; Morozova, A.V.; Khiller, V.V.; Glavatskykh, S.P.; Rodemann, T. Ontogeny of ore Cr-spinel and composition of inclusions as indicators of the pneumatolytic-hydrothermal origin of PGM-bearing chromitites from Kondyor massif, the Aldan Shield. Geol. Ore Depos. 2015, 57, 352–380. [Google Scholar] [CrossRef]
- Arai, S. Formation of chlorite corona around chromian spinel in peridotite and its significance. Geosci. Rep. Shizuoka Univ. 1978, 3, 9–15. (In Japanese) [Google Scholar]
- González-Jiménez, J.M.; Kerestedjian, T.; Proenza, J.A.; Gervilla, F. Metamorphism on chromite ores from the Dobromirtsi ultramafic massif, Rhodope Mountains (SE Bulgaria). Geol. Acta 2009, 7, 413–429. [Google Scholar]
- Fisher, L.W. Origin of chromite deposits. Econ. Geol. 1929, 24, 691–721. [Google Scholar] [CrossRef]
- Ross, C.S. The origin of chromite. Econ. Geol. 1931, 26, 540–545. [Google Scholar] [CrossRef]
- Sampson, E. Varieties of chromite deposits. Econ. Geol. 1931, 26, 833–839. [Google Scholar] [CrossRef]
- Arai, S.; Akizawa, N. Precipitation and dissolution of chromite by hydrothermal solutions in the Oman ophiolite: New behavior of Cr and chromite. Am. Mineral. 2014, 99, 28–34. [Google Scholar] [CrossRef]
- Akizawa, N.; Arai, S. Petrology of mantle diopsidite from Wadi Fizh, northern Oman ophiolite: Cr and REE mobility by hydrothermal solution. Island Arc 2014, 23, 269–280. [Google Scholar] [CrossRef]
- Python, M.; Ceuleneer, G.; Ishida, Y.; Barrat, J.-A.; Arai, S. Oman diopsidites: A new lithology of very high temperature hydrothermal circulation in mantle peridotite below oceanic spreading centres. Earth Planet. Sci. Lett. 2007, 255, 289–305. [Google Scholar] [CrossRef]
- Akizawa, N.; Arai, S.; Tamura, A.; Uesugi, J.; Python, M. Crustal diopsidites from the northern Oman ophiolite: Evidence for hydrothermal circulation through suboceanic Moho. J. Mineral. Petrol. Sci. 2011, 106, 261–266. [Google Scholar] [CrossRef][Green Version]
- Akizawa, N.; Tamura, A.; Fukushi, K.; Yamamoto, J.; Mizukami, T.; Python, M.; Arai, S. High-temperature hydrothermal activities around suboceanic Moho: An example from diopsidite and anorthosite in Wadi Fizh, Oman ophiolite. Lithos 2016, 263, 66–87. [Google Scholar] [CrossRef]
- Arai, S.; Shimizu, Y.; Ismail, S.A.; Ahmed, A.H. Low-T formation of high-Cr spinel with apparently primary chemical characteristics within podiform chromitite from Rayat, northeastern Iraq. Mineral. Mag. 2006, 70, 499–508. [Google Scholar] [CrossRef]
- Kapsiotis, A.N. Alteration of chromitites from the Voidolakkos and Xerolivado mines, Vourinos ophiolite complex, Greece: Implications for deformation-induced metamorphism. Geol. J. 2015, 50, 739–763. [Google Scholar] [CrossRef]
- Lippard, S.J.; Shelton, A.W.; Gass, I.G. The ophiolite of northern Oman. Geol. Soc. Lond. Mem. 1986, 11, 178. [Google Scholar]
- Akizawa, N.; Arai, S. Petrologic profile of a peridotite layer under a possible Moho in the northern Oman ophiolite: An example from Wadi Fizh. J. Mineral. Petrol. Sci. 2009, 104, 389–394. [Google Scholar] [CrossRef]
- Takazawa, E.; Okayasu, T.; Satoh, K. Geochemistry and origin of the basal lherzolites from the northern Oman ophiolite (northern Fizh block). Geochem. Geophys. Geosyst. 2003, 4, 1021. [Google Scholar] [CrossRef]
- Ministry of Petroleum and Minerals. Geological Map of Buraymi, Scale 1:250,000; Ministry of Petroleum and Minerals: Muscat, Sultanate of Oman, 1992. [Google Scholar]
- Uesugi, J.; Arai, S.; Morishita, T.; Matsukage, K.; Kadoshima, K.; Tamura, A.; Abe, N. Significance and variety of mantle-crust boundary in the Oman ophiolite. J. Geogr. (Chigaku Zasshi) 2003, 112, 750–768. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Akizawa, N.; Arai, S.; Tamura, A. Behavior of MORB magmas at uppermost mantle beneath a fast-spreading axis: An example from Wadi Fizh of the northern Oman ophiolite. Contrib. Mineral. Petrol. 2012, 164, 601–625. [Google Scholar] [CrossRef]
- Akizawa, N.; Ozawa, K.; Tamura, A.; Michibayashi, K.; Arai, S. Three-dimensional evolution of melting, heat and melt transfer in ascending mantle beneath a fast-spreading ridge segment constrained by trace elements in clinopyroxene from concordant dunites and host harzburgites of the Oman ophiolite. J. Petrol. 2016, 57, 777–814. [Google Scholar] [CrossRef]
- Treloar, P.J. The Cr-minerals of Outokumpu—Their chemistry and significance. J. Petrol. 1987, 28, 867–886. [Google Scholar] [CrossRef]
- Tsujimori, T.; Liou, J.G. Coexisting chromian omphacite and diopside in tremolite schist from the Chugoku Mountains, SW Japan: The effect of Cr on the omphacite-diopside immiscibility gap. Am. Mineral. 2004, 89, 7–14. [Google Scholar] [CrossRef]
- Tsujimori, T.; Liou, J.G. Low-pressure and low-temperature K-bearing kosmochloric diopside from the Osayama serpentinite mélange, SW Japan. Am. Mineral. 2005, 90, 1629–1635. [Google Scholar] [CrossRef]
- Hamada, M.; Seto, S.; Akasaka, M.; Takasu, A. Chromian pumpellyite and associated chromian minerals from Sangun metamorphic rocks, Osayama, southwest Japan. J. Mineral. Petrol. Sci. 2008, 103, 390–399. [Google Scholar] [CrossRef]
- Arai, S. Control of wall-rock composition on the formation of podiform chromitites as a result of magma/peridotite interaction. Resour. Geol. 1997, 47, 177–187. [Google Scholar]
- Hey, M.H. A new review of chlorites. Mineral. Mag. 1954, 30, 277–299. [Google Scholar] [CrossRef]
- Ishikawa, A.; Senda, R.; Suzuki, K.; Dale, C.W.; Meisel, T. Re-evaluating digestion methods for highly siderophile element and Os-187 isotope analysis: Evidence from geological reference materials. Chem. Geol. 2014, 384, 27–46. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Arai, S. Unexpectedly high-PGE chromitite from the deeper mantle section of the northern Oman ophiolite and its tectonic implications. Contrib. Mineral. Petrol. 2002, 143, 263–278. [Google Scholar] [CrossRef]
- Miura, M.; Arai, S.; Ahmed, A.H.; Mizukami, T.; Okuno, M.; Yamamoto, S. Podiform chromitite classification revisited: A comparison of discordant and concordant chromitite pods from Wadi Hilti, northern Oman ophiolite. J. Asian Earth Sci. 2012, 59, 52–61. [Google Scholar] [CrossRef]
- Barnes, S.-J.; Naldrett, A.J.; Gorton, M.P. The origin of the fractionation of platinum-group elements in terrestrial magmas. Chem. Geol. 1985, 53, 303–323. [Google Scholar] [CrossRef]
- Fischer-Gödde, M.; Becker, H.; Wombacher, F. Rhodium, gold and other highly siderophile element abundances in chondritic meteorites. Geochim. Cosmochim. Acta 2010, 74, 356–379. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Arai, S. Platinum-group minerals in podiform chromitites of the Oman ophiolite. Can. Mineral. 2003, 41, 597–616. [Google Scholar] [CrossRef]
- Morishita, T.; Ishida, Y.; Arai, S. Simultaneous determination of multiple trace element compositions in thin (<30 µm) layers of BCR-2G by 193 nm ArF excimer laser ablation-ICP-MS: Implications for matrix effect and elemental fractionation on quantitative analysis. Geochem. J. 2005, 39, 327–340. [Google Scholar] [CrossRef]
- Park, J.-W.; Campbell, I.H.; Eggins, S.M. Enrichment of Rh, Ru, Ir and Os in Cr spinels from oxidized magmas: Evidence from the Ambae volcano, Vanuatu. Geochim. Cosmochim. Acta 2012, 78, 28–50. [Google Scholar] [CrossRef]
- Jochum, K.P.; Nohl, U. Reference materials in geochemistry and environmental research and the GeoReM database. Chem. Geol. 2008, 253, 50–53. [Google Scholar] [CrossRef]
- Frost, B.R. On the stability of sulfides, oxides, and native metals in serpentinite. J. Petrol. 1985, 26, 31–63. [Google Scholar] [CrossRef]
- Arai, S.; Matsukage, K. Petrology of the gabbro-troctolite complex from Hess Deep, equatorial Pacific: Implications for mantle-melt interaction within the oceanic lithosphere. Proc. ODP Sci. Results 1996, 47, 135–155. [Google Scholar]
- Tamura, A.; Morishita, T.; Ishimaru, S.; Arai, S. Geochemistry of spinel-hosted amphibole inclusions in abyssal peridotite: Insight into secondary melt formation in melt—Peridotite reaction. Contrib. Mineral. Petrol. 2014, 167, 974. [Google Scholar] [CrossRef]
- Tamura, A.; Morishita, T.; Ishimaru, S.; Hara, K.; Sanfilippo, A.; Arai, S. Compositional variations in spinel-hosted pargasite inclusions in the olivine-rich rock from the oceanic crust–mantle boundary zone. Contrib. Mineral. Petrol. 2016, 171, 39. [Google Scholar] [CrossRef]
- Matsukage, K.; Arai, S. Jadeite, albite and nepheline as inclusions in spinel of chromitite from Hess Deep, equatorial Pacific: Their genesis and implications for serpentinite diapir formation. Contrib. Mineral. Petrol. 1998, 131, 111–122. [Google Scholar] [CrossRef]
- Arai, S.; Matsukage, K. Petrology of a chromitite micropod from Hess Deep, equatorial Pacific: A comparison between abyssal and alpine-type podiform chromitites. Lithos 1998, 43, 1–14. [Google Scholar] [CrossRef]
- Tamura, A.; Arai, S.; Ishimaru, S.; Andal, E.S. Petrology and geochemistry of peridotites from IODP Site U1309 at Atlantis Massif, MAR 30° N: Micro- and macro-scale melt penetrations into peridotites. Contrib. Mineral. Petrol. 2008, 155, 491–509. [Google Scholar] [CrossRef]
- Shaw, C.S.J. Dissolution of orthopyroxene in basanitic magma between 0.4 and 2 GPa: Further implications for the origin of Si-rich alkaline glass inclusions in mantle xenoliths. Contrib. Mineral. Petrol. 1999, 135, 114–132. [Google Scholar] [CrossRef]
- Arai, S.; Shirasaka, M.; Ishida, Y.; Inoue, H. Na-bearing tremolites as reservoirs of fluid-mobile elements in the mantle wedge: Inference from the Ochiai-Hokubo complex (Southwest Japan) in high-P schists. J. Mineral. Petrol. Sci. 2019, 114, 231–237. [Google Scholar] [CrossRef]
- Gammons, C.H. Experimental investigations of the hydrothermal geochemistry of platinum and palladium: V. Equilibria between platinum metal, Pt (II), and Pt (IV) chloride complexes at 25 to 300 °C. Geochim. Cosmochim. Acta 1996, 60, 1683–1694. [Google Scholar] [CrossRef]
- Augé, T.; Petrunov, R.; Bailly, L. On the origin of the PGE mineralization in the Elastsite porphyry Cu-Au deposit, Bulgaria: Comparison with the Baula-Nuasahi complex, India, and other alkaline PGE-rich porphyries. Can. Min. 2005, 43, 1355–1372. [Google Scholar] [CrossRef]
- Barnes, S.J.; Liu, W. Pt and Pd mobility in hydrothermal fluids: Evidence from komatiites and from thermodynamic modelling. Ore Geol. Rev. 2012, 44, 49–58. [Google Scholar] [CrossRef]
- Oze, C.; Fendorf, S.; Bird, D.K.; Coleman, R.G. Chromium geochemistry in serpentinized ultramafic rocks and serpentine soils from the Franciscan Complex of California. Am. J. Sci. 2004, 304, 67–101. [Google Scholar] [CrossRef]
- Watenphul, A.; Schmidt, C.; Jahn, S. Cr (III) solubility in aqueous fluids at high pressures and temperatures. Geochim. Cosmochim. Acta 2014, 126, 212–227. [Google Scholar] [CrossRef]
- Ishimaru, S.; Arai, S.; Ishida, Y.; Shirasaka, M.; Okrugin, V.M. Melting and multi-stage metasomatism in the mantle wedge beneath a frontal arc inferred from highly depleted peridotite xenoliths from the Avacha volcano, southern Kamchatka. J. Petrol. 2007, 48, 395–433. [Google Scholar] [CrossRef]
- Kawamoto, T.; Yoshikawa, M.; Kumagai, Y.; Mirabueno, M.H.T.; Okuno, M.; Kobayashi, T. Mantle wedge infiltrated with saline fluids from dehydration and decarbonation of subducting slab. Proc. Natl. Acad. Sci. USA 2013, 110, 9663–9668. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Tamura, A.; Arai, S.; Kawamoto, T.; Payot, B.D.; Rivera, D.J.; Bariso, E.B.; Mirabueno, M.H.T.; Okuno, M.; Kobayashi, T. Aqueous fluids and sedimentary melts as agents for mantle wedge metasomatism, as inferred from peridotite xenoliths at Pinatubo and Iraya volcanoes, Luzon arc, Philippines. Lithos 2016, 262, 355–368. [Google Scholar] [CrossRef]
- Huang, Y.; Nakatani, T.; Nakamura, M.; McCammon, C. Saline aqueous fluid circulation in mantle wedge inferred from olivine wetting properties. Nat. Commun. 2019, 10, 5557. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, S.; Arai, S. Calcic amphiboles in peridotite xenoliths from Avacha volcano, Kamchatka, and their implications for metasomatic conditions in the mantle wedge. In Metasomatism in Oceanic and Continental Lithospheric Mantle; Coltorti, M., Grégoire, M., Eds.; Geological Society of London Special Publication: London, UK, 2008; Volume 293, pp. 35–55. [Google Scholar]
- Ishimaru, S.; Arai, S. Peculiar Mg–Ca–Si metasomatism along a shear zone within the mantle wedge: Inference from fine-grained xenoliths from Avacha volcano, Kamchatka. Contrib. Mineral. Petrol. 2011, 161, 703–720. [Google Scholar] [CrossRef]
- Latypov, R.; Costin, G.; Chistyakova, S.; Hunt, E.J.; Mukherjee, R.; Naldrett, T. Platinum-bearing chromite layers are caused by pressure reduction during magma ascent. Nat. Commun. 2018, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hao, J.; Huang, F.; Sverjensky, D.A. Mobility of chromium in high temperature crustal and upper mantle fluids. Geochem. Persp. Lett. 2019, 12, 1–6. [Google Scholar] [CrossRef]
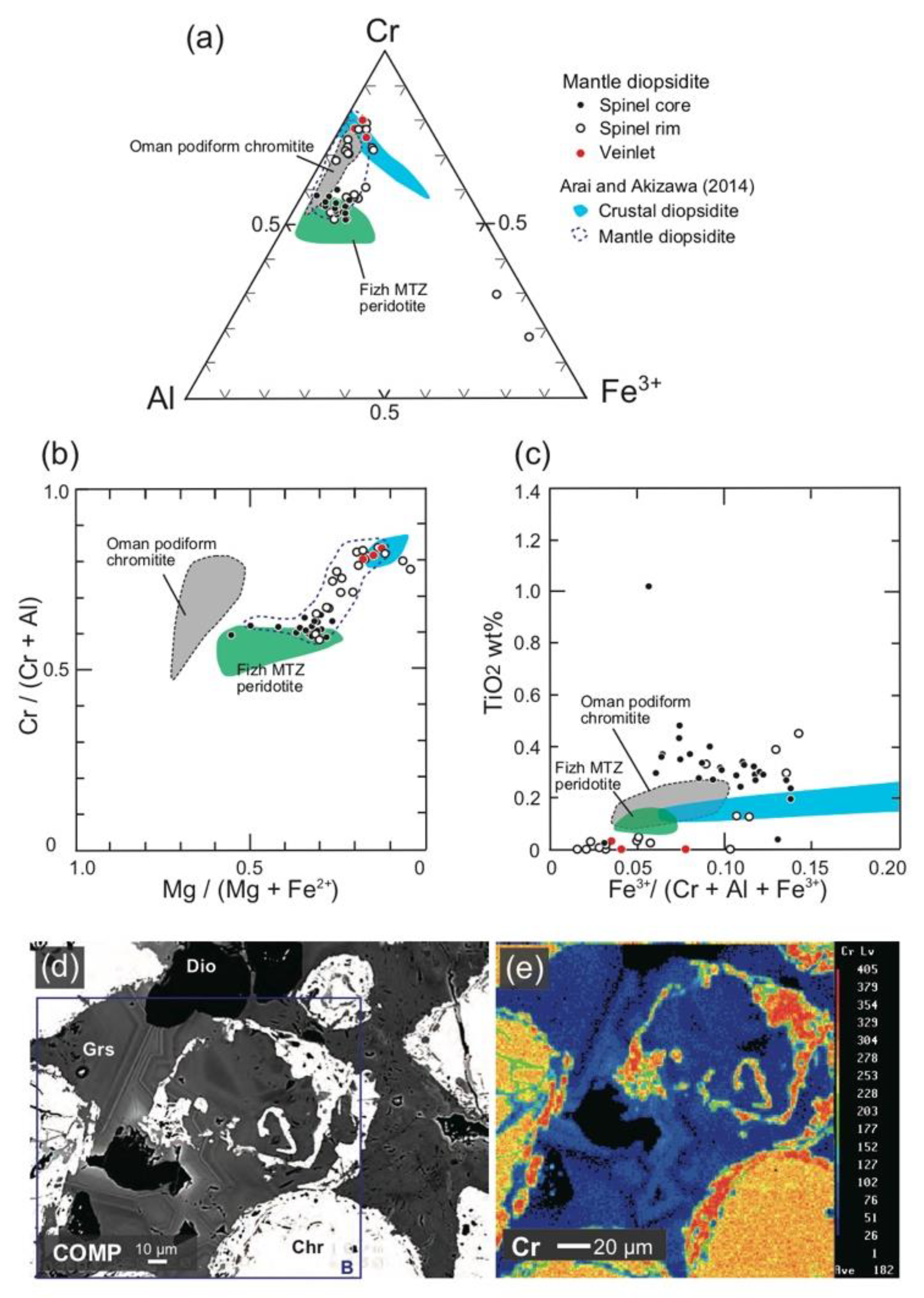
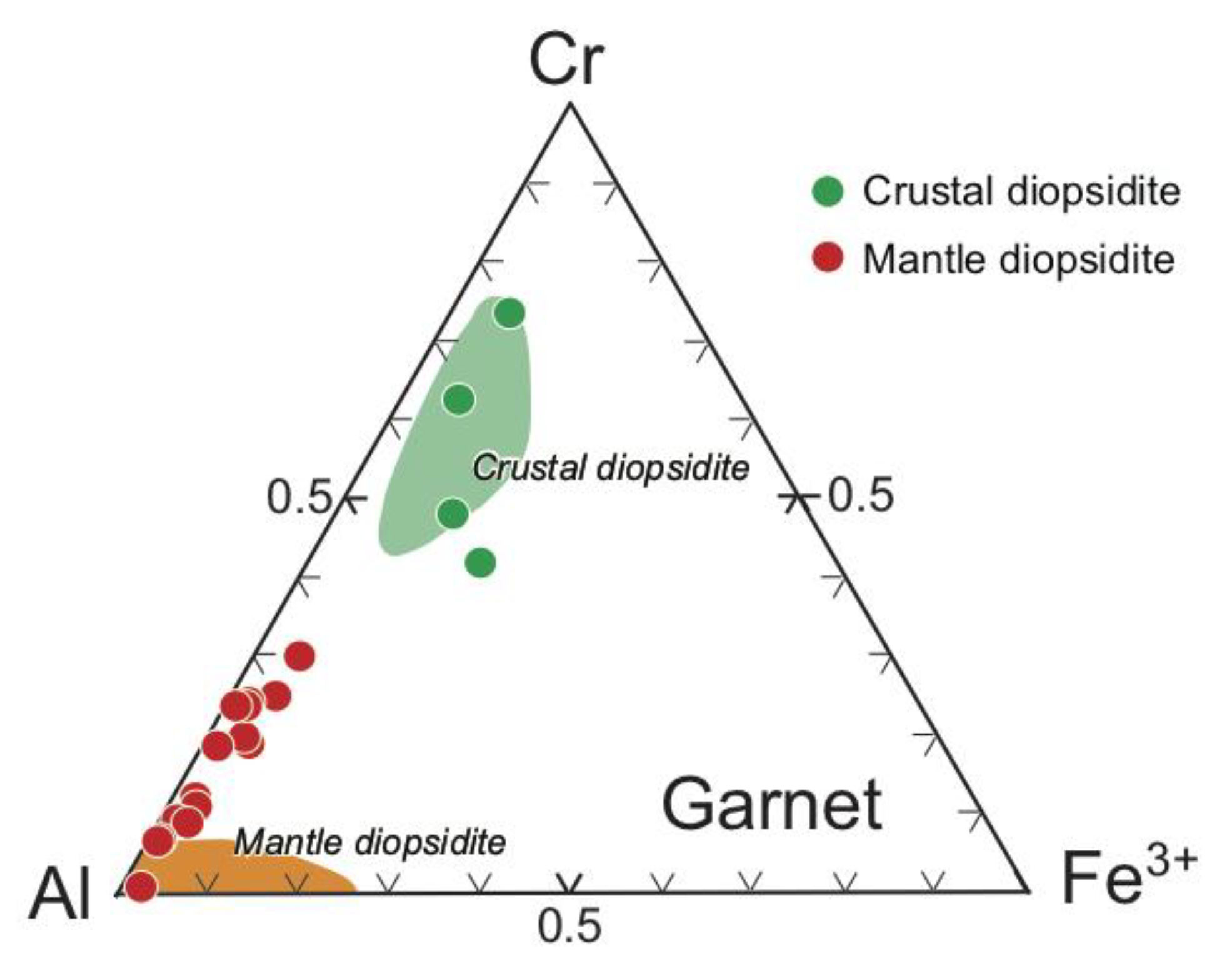
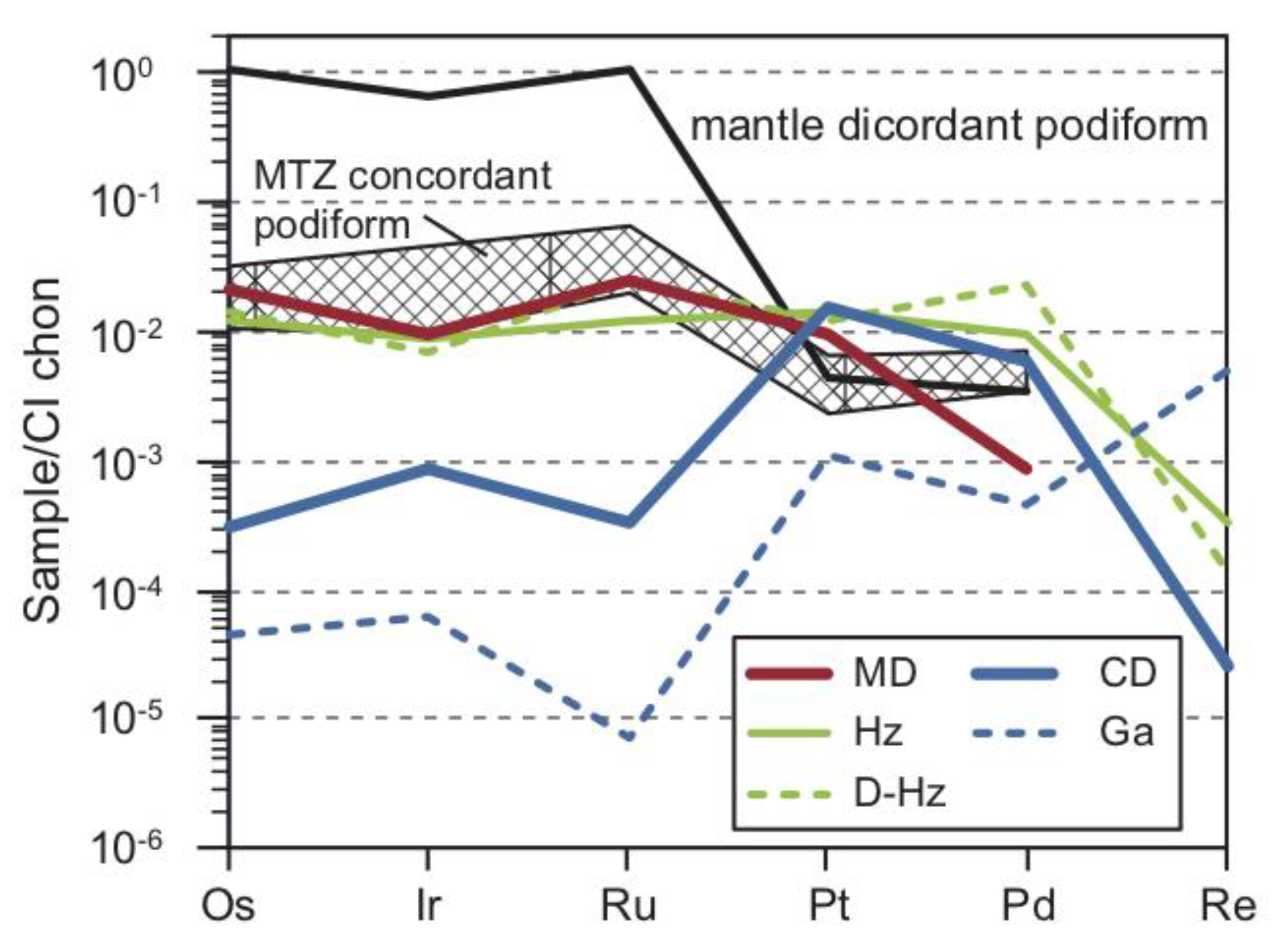
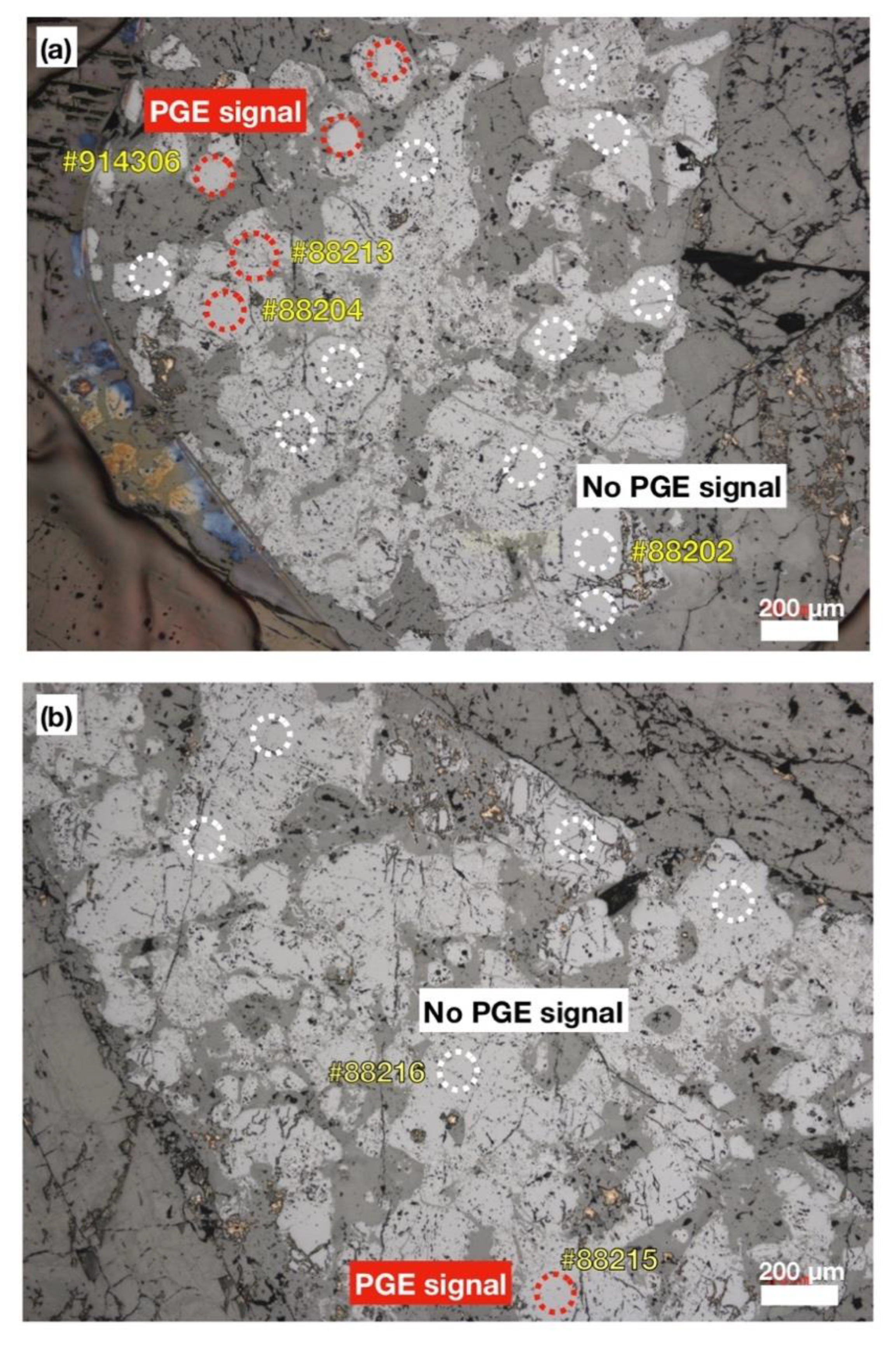
| Rock | Crustal Diopsidite | Mantle Diopsidite | D.L. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineral | chr | chr | chr | chr | chr | chr | diop | gros | gros | chl | |
| Note | core | rim | core | rim | verm | vein | core | core* | core | core | |
| SiO2 | 0.81 | 0.23 | 0.00 | 0.14 | 0.23 | 0.24 | 56.07 | 38.69 | 40.41 | 35.42 | 0.010 |
| TiO2 | 0.16 | 0.25 | 0.32 | 0.03 | 0.00 | 0.03 | 0.02 | 0.15 | 0.05 | 0.00 | 0.015 |
| Al2O3 | 9.01 | 6.75 | 18.05 | 12.04 | 8.12 | 9.04 | 0.82 | 16.58 | 20.97 | 8.10 | 0.010 |
| Cr2O3 | 55.02 | 46.28 | 42.40 | 52.50 | 50.34 | 55.70 | 0.32 | 7.84 | 2.24 | 6.35 | 0.030 |
| Fe2O3 | 1.39 | 13.68 | 7.87 | 3.89 | 7.56 | 2.62 | - | 0.74 | 0.55 | - | |
| FeO | 28.62 | 28.88 | 24.87 | 25.82 | 27.28 | 27.84 | 0.77 | - | - | 4.58 | 0.020 |
| MnO | 0.55 | 0.59 | 0.53 | 0.72 | 1.23 | 1.14 | 0.00 | 0.02 | 0.04 | 0.13 | 0.017 |
| MgO | 2.6 | 2.33 | 6.63 | 5.21 | 3.16 | 3.41 | 17.90 | 0.03 | 0.04 | 34.34 | 0.008 |
| CaO | 0.29 | 0.11 | 0.05 | 0.34 | 0.51 | 0.43 | 25.56 | 36.64 | 37.23 | 0.04 | 0.009 |
| Na2O | - | 0.04 | 0.00 | 0.00 | 0.00 | - | 0.06 | 0.00 | 0.00 | 0.02 | 0.011 |
| K2O | - | - | 0.00 | 0.01 | 0.00 | - | 0.00 | 0.00 | 0.00 | 0.00 | 0.007 |
| NiO | 0.04 | 0.05 | 0.18 | 0.06 | 0.01 | - | 0.06 | 0.00 | 0.00 | 0.00 | 0.017 |
| Total | 98.6 | 99.2 | 100.89 | 100.76 | 98.43 | 100.45 | 101.59 | 100.69 | 101.53 | 88.97 | |
| O= | 4 | 4 | 4 | 4 | 4 | 4 | 6 | 12 | 12 | 28 | |
| Si | 0.029 | 0.008 | 0.000 | 0.005 | 0.008 | 0.008 | 1.993 | 2.970 | 3.008 | 6.709 | |
| Ti | 0.004 | 0.007 | 0.008 | 0.001 | 0.000 | 0.001 | 0.001 | 0.008 | 0.003 | 0.000 | |
| Al | 0.375 | 0.286 | 0.693 | 0.479 | 0.341 | 0.370 | 0.034 | 1.499 | 1.839 | 1.807 | |
| Cr | 1.536 | 1.314 | 1.091 | 1.399 | 1.419 | 1.528 | 0.009 | 0.475 | 0.132 | 0.951 | |
| Fe3+ | 0.037 | 0.370 | 0.193 | 0.099 | 0.203 | 0.068 | - | 0.043 | 0.031 | - | |
| Fe2+ | 0.845 | 0.867 | 0.677 | 0.728 | 0.814 | 0.808 | 0.023 | - | - | 0.725 | |
| Mn | 0.016 | 0.018 | 0.015 | 0.021 | 0.037 | 0.033 | 0.000 | 0.002 | 0.002 | 0.021 | |
| Mg | 0.137 | 0.125 | 0.322 | 0.262 | 0.168 | 0.176 | 0.948 | 0.004 | 0.004 | 9.689 | |
| Ca | 0.011 | 0.004 | 0.002 | 0.012 | 0.019 | 0.016 | 0.973 | 3.012 | 2.968 | 0.008 | |
| Na | - | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 | 0.000 | 0.000 | 0.006 | |
| K | - | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | |
| Ni | 0.001 | 0.001 | 0.005 | 0.002 | 0.000 | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | |
| Total | 2.997 | 3.002 | 3.004 | 3.007 | 3.010 | 3.008 | 3.987 | 8.013 | 7.988 | 19.916 | |
| Mg# | 0.139 | 0.126 | 0.322 | 0.264 | 0.171 | 0.179 | 0.977 | 0.079 | 0.125 | 0.930 | |
| Cr# | 0.804 | 0.821 | 0.612 | 0.745 | 0.806 | 0.805 | - | 0.241 | 0.067 | - | |
| YCr | 0.789 | 0.667 | 0.552 | 0.708 | 0.835 | 0.777 | - | 0.236 | 0.066 | - | |
| YAl | 0.192 | 0.145 | 0.350 | 0.242 | 0.208 | 0.188 | - | 0.743 | 0.919 | - | |
| YFe | 0.019 | 0.188 | 0.098 | 0.050 | 1.043 | 0.035 | - | 0.021 | 0.015 | - | |
| Name | Rock Type | Sample Mass (G) | Os | Ir | Ru | Pt | Pd | Re |
|---|---|---|---|---|---|---|---|---|
| F-1-(X + 45) m | Harz | 0.568 | 5.61 | 3.98 | 8.24 | 12.9 | 5.56 | 0.012 |
| BC (%) | 0.01 | 0.04 | 0.1 | 0.4 | 0.1 | 22.2 | ||
| 20110307–2 | Dun harz | 0.512 | 6.72 | 3.09 | 15.9 | 11.4 | 13.1 | 0.0052 |
| BC (%) | 0.01 | 0.1 | 0.1 | 0.5 | 0.04 | 42.3 | ||
| 20121118MD pre | MD | 0.504 | 10.1 | 4.20 | 16.6 | 8.33 | 0.54 | BDL |
| BC (%) | 0.01 | 0.04 | 0.1 | 0.7 | 0.9 | |||
| 20090220 479 | Gabbro | 0.507 | 0.021 | 0.028 | 0.005 | 1.02 | 0.283 | 0.196 |
| BC (%) | 2.8 | 6.2 | 66.5 | 5.5 | 1.7 | 1.93 | ||
| 20101129 CDV | CD | 0.534 | 0.140 | 0.382 | 0.221 | 14.4 | 3.46 | 0.0009 |
| BC (%) | 0.4 | 0.5 | 3.9 | 0.4 | 0.1 | 79.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, S.; Miura, M.; Tamura, A.; Akizawa, N.; Ishikawa, A. Hydrothermal Chromitites from the Oman Ophiolite: The Role of Water in Chromitite Genesis. Minerals 2020, 10, 217. https://doi.org/10.3390/min10030217
Arai S, Miura M, Tamura A, Akizawa N, Ishikawa A. Hydrothermal Chromitites from the Oman Ophiolite: The Role of Water in Chromitite Genesis. Minerals. 2020; 10(3):217. https://doi.org/10.3390/min10030217
Chicago/Turabian StyleArai, Shoji, Makoto Miura, Akihiro Tamura, Norikatsu Akizawa, and Akira Ishikawa. 2020. "Hydrothermal Chromitites from the Oman Ophiolite: The Role of Water in Chromitite Genesis" Minerals 10, no. 3: 217. https://doi.org/10.3390/min10030217
APA StyleArai, S., Miura, M., Tamura, A., Akizawa, N., & Ishikawa, A. (2020). Hydrothermal Chromitites from the Oman Ophiolite: The Role of Water in Chromitite Genesis. Minerals, 10(3), 217. https://doi.org/10.3390/min10030217






