Insights on Structure and Threshold Detection Limits of Stichtite (Magnesium-Chromium Carbonate-Hydroxide) by Fourier Transform Infrared Analysis
Abstract
1. Introduction
Geology and Mineralogy of Samples Used in this Study
2. Materials and Methods
2.1. Sample Preparation and Characterization
2.2. Geochemical Analyses
2.3. FTIR Analyses
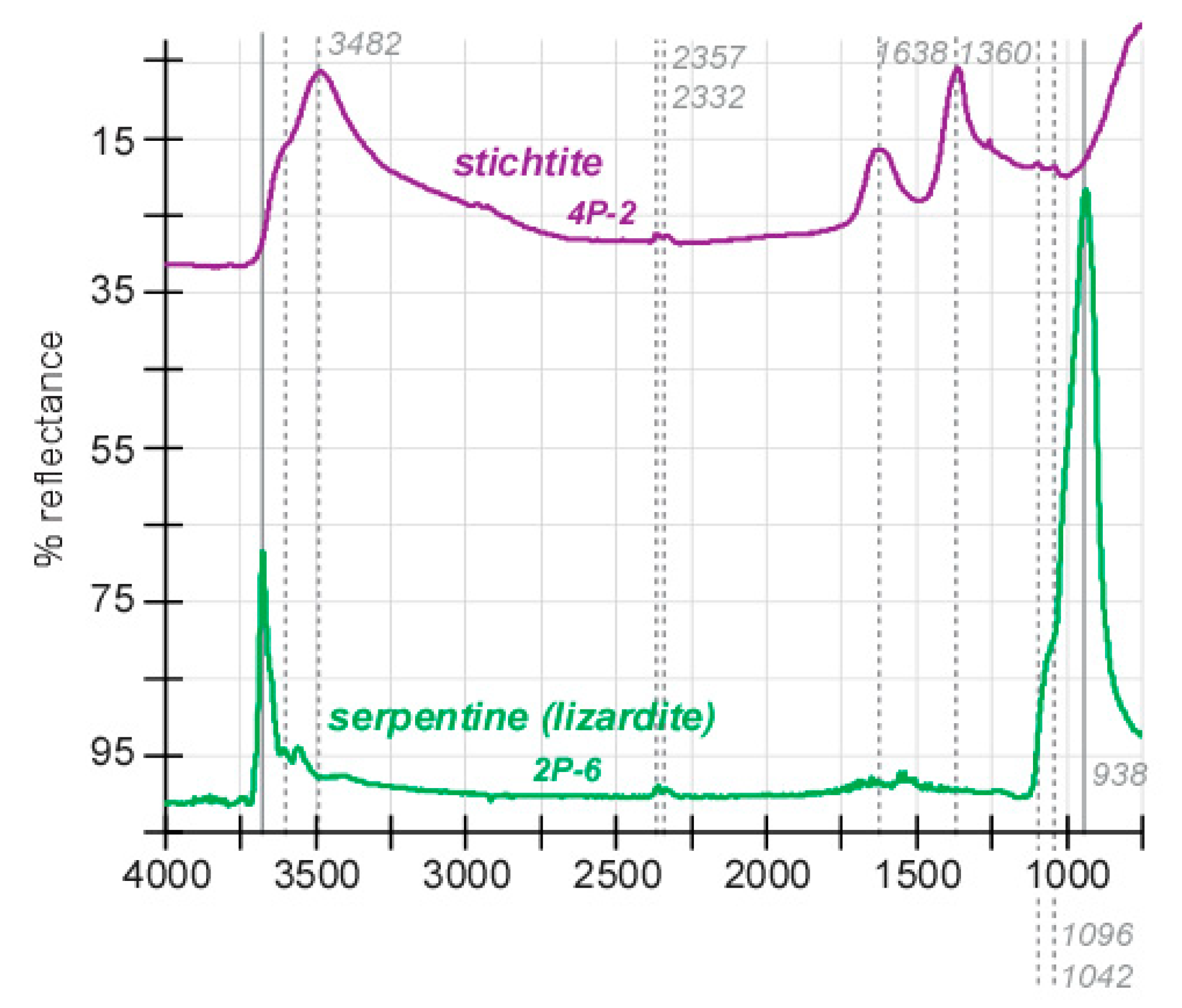
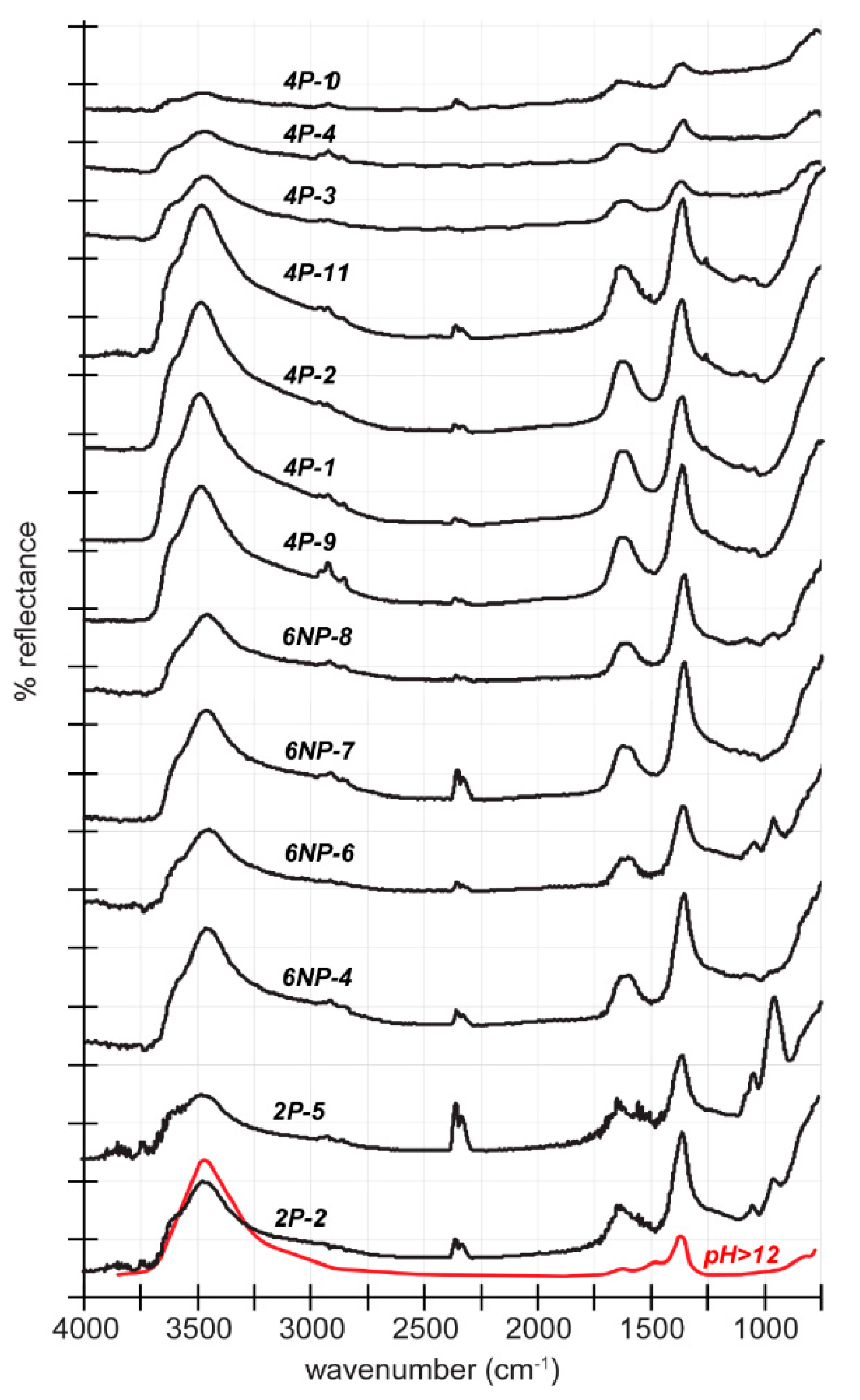
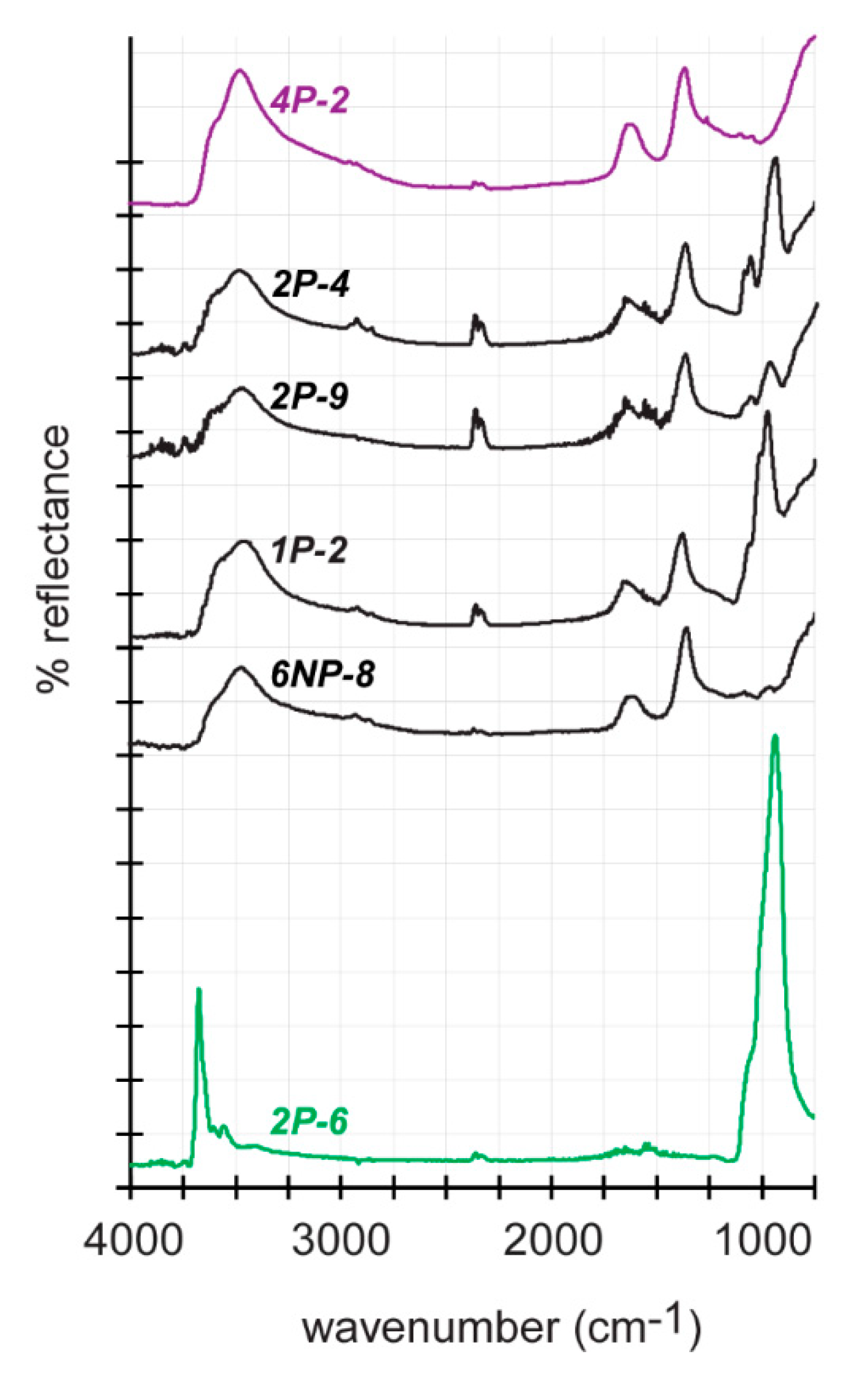
3. Results
4. Discussion
4.1. Structural and Geochemical Insights
4.2. Determination of FTIR Detection Threshold for Stichtite in Serpentine and Implications for Astrobiology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. The physiology and habitat of the last universal common ancestor. Nature Microbio. 2016, 1, 16116. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre, E.B.; Bottrill, R.; Huss, G.R.; Lopez, A. Conditions of stichtite (Mg6Cr2(OH)16[CO3]·4H2O) formation and its geochemical and isotope record of early phanerozoic serpentinizing environments. Geochim. Cosmochim. Acta 2017, 197, 43–61. [Google Scholar] [CrossRef]
- Melchiorre, E.B.; Bottrill, R.; Huss, G.R.; Lopez, A. The early Earth geochemical and isotope record of serpentinizing environments from Archean stichtite (Mg6Cr2 (OH) 16[CO3]4H2O). Precamb. Res. 2018, 310, 198–212. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Stichtite: A review. Clay Miner. 2013, 48, 143–148. [Google Scholar] [CrossRef]
- Varadarajan, S. Stichtite from the ultramafics of Nuggihalli schist belt, Mysore state (abstract). In Proceedings of the Indian Science Congress, 53rd Session, Chandigarh, India, 3 March 1966; Volume 3, p. 183. [Google Scholar]
- Melchiorre, E.B.; Lopez, A. Stichtite, and chromium mineralization within methane rich serpentinizing environments. In Proceedings of the 11th SGA Biennial Meeting, Antofagasta, Chile, 26–29 September 2011; pp. 169–171. [Google Scholar]
- Melchiorre, E.B.; Huss, G.R.; Lopez, A. Carbon and hydrogen stable isotope microanalysis and data correction for rare carbonate minerals: Case studies for stichtite (Mg6Cr2[(OH)16|CO3]∙H2O) and malachite (Cu2CO3(OH)2). Chem. Geol. 2014, 367, 63–69. [Google Scholar] [CrossRef]
- Proskurowski, G.; Lilley, M.D.; Seewald, J.S.; Früh-Green, G.L.; Olson, E.J.; Lupton, J.E.; Sylva, S.P.; Kelley, D.S. Abiogenic hydrocarbon production at lost city hydrothermal field. Science 2008, 319, 604–607. [Google Scholar] [CrossRef]
- McCollom, T.M.; Seewald, J.S. Serpentinites, hydrogen, and life. Elements 2013, 9, 129–134. [Google Scholar] [CrossRef]
- Klein, F.; Humphris, S.E.; Guo, W.; Schubotz, F.; Schwarzenbach, E.M.; Orsi, W.D. Fluid mixing and the deep biosphere of a fossil Lost City-type hydrothermal system at the Iberia Margin. Proc. Nat. Acad. Sci. 2015, 112, 12036–12041. [Google Scholar] [CrossRef]
- Mottl, M.J.; Komor, S.C.; Fryer, P.; Moyer, C.L. Deep-slab fluids fuel extremophilic Archaea on a Mariana forearc serpentinite mud volcano: Ocean Drilling Program Leg 195. Geochem. Geophys. Geosyst. 2003, 4, 1–14. [Google Scholar] [CrossRef]
- Etiope, G.; Sherwood-Lollar, B. Abiotic methane on Earth. Rev. Geophys. 2013, 51, 276–299. [Google Scholar] [CrossRef]
- Evans, B.W.; Hattori, K.; Baronnet, A. Serpentinite: What, why, where? Elements 2013, 9, 99–106. [Google Scholar] [CrossRef]
- Parnell, J.; Boyce, A.J.; Blamey, N.J. Follow the methane: The search for a deep biosphere, and the case for sampling serpentinites, on Mars. Intern. J. Astrobio. 2010, 9, 193–200. [Google Scholar] [CrossRef]
- Etiope, G.; Ehlmann, B.L.; Schoell, M. Low temperature production and exhalation of methane from serpentinized rocks on Earth: A potential analog for methane production on Mars. Icarus 2013, 224, 276–285. [Google Scholar] [CrossRef]
- Newman, S.P.; Di Cristina, T.; Coveney, P.V.; Jones, W. Molecular dynamics simulation of cationic and anionic clays containing amino acids. Langmuir 2002, 18, 2933–2939. [Google Scholar] [CrossRef]
- Kottegoda, N.S.; Jones, W. Preparation and characterisation of Li–Al–glycine layered double hydroxides (LDHs)–polymer nanocomposites. Macromol. Symp. 2005, 222, 65–71. [Google Scholar] [CrossRef]
- Gerstel, P.; Hoffmann, R.C.; Lipowsky, P.; Jeurgens, L.P.H.; Bill, J.; Aldinger, F. Mineralization from aqueous solutions of zinc salts directed by amino acids and peptides. Chem. Mat. 2006, 18, 179–186. [Google Scholar] [CrossRef]
- Del Hoyo, C. Layered double hydroxides and human health: An overview. Appl. Clay Sci. 2007, 36, 103–121. [Google Scholar] [CrossRef]
- Bouzaid, J.; Frost, R. Thermal decomposition of stichtite. J. Therm. Anal. Cal. 2007, 89, 133–135. [Google Scholar] [CrossRef][Green Version]
- Turvey, C.C.; Wilson, S.A.; Hamilton, J.L.; Tait, A.W.; McCutcheon, J.; Beinlich, A.; Fallon, S.J.; Dipple, G.M.; Southam, G. Hydrotalcites and hydrated Mg-carbonates as carbon sinks in serpentinite mineral wastes from the Woodsreef chrysotile mine, New South Wales, Australia: Controls on carbonate mineralogy and efficiency of CO2 air capture in mine tailings. Intern. J. Greenh. Gas Cont. 2018, 79, 38–60. [Google Scholar] [CrossRef]
- Viviano, C.E.; Moersch, J.E.; McSween, H.Y. Implications for early hydrothermal environments on Mars through the spectral evidence for carbonation and chloritization reactions in the Nili Fossae region. J. Geophys. Res. Planets 2013, 118, 1858–1872. [Google Scholar] [CrossRef]
- Amador, E.S.; Bandfield, J.L. Elevated bulk-silica exposures and evidence for multiple aqueous alteration episodes in Nili Fossae, Mars. Icarus 2016, 276, 39–51. [Google Scholar] [CrossRef]
- Haberle, R.M.; Clancy, R.T.; Forget, F.; Smith, M.D.; Zurek, R.W. The Atmosphere and Climate of Mars; Cambridge University Press: Cambridge, UK, 2017; Volume 18, 588p. [Google Scholar]
- Ehlmann, B.L.; Mustard, J.F.; Murchie, S.L.; Bibring, J.P.; Meunier, A.; Fraeman, A.A.; Langevin, Y. Subsurface water and clay mineral formation during the early history of Mars. Nature 2011, 479, 53. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.V. Geology of the Dundas-Mow1t Lindsay-Mount Youngback region. Tasman. Dep. Mines 1986, Geological Survey Bulletin 62, 1–89. [Google Scholar]
- Burrett, C.F.; Martin, E.L. Geology and Mineral Resources of Tasmania: Maps (No. 15); Geological Society of Australia: Hornsby, Australia, 1989; 1 sheet. [Google Scholar]
- Rubenach, M.J. The origin and emplacement of the Serpentine Hill complex, western Tasmania. J. Geol. Soc. Austr. 1974, 21, 91–106. [Google Scholar] [CrossRef]
- Varne, R.; Brown, A.V. The geology and petrology of the Adamsfield ultramafic complex, Tasmania. Contr. Mineral. Pet. 1978, 67, 195–207. [Google Scholar] [CrossRef]
- Bottrill, R.S.; Graham, I.T. Stichtite from western Tasmania. Austr. J. Mineral. 2006, 12, 101–107. [Google Scholar]
- Petterd, W.F. Catalogue of the Minerals of Tasmania; Examiner Press: Launceston, Australia, 1896; p. 72. [Google Scholar]
- Twelvetrees, W.H. Stichtite: A new Tasmanian mineral. Tasman. Dep. Minesgeol. Surv. Rec. 1914, 2, 11. [Google Scholar]
- Armstrong, J.T. Quantitative analysis of silicates and oxide minerals: Comparison of Monte-Carlo, ZAF and Phi–Rho–Z procedures. In Proceedings of the 23rd Annual Conference of the Microbeam Analysis Society, Milwaukee, Wisconsin, 8–12 August 1988; D.E., Ed.; Microbeam Analysis Society: San Francisco, CA, USA, 1988; pp. 239–246. [Google Scholar]
- Donovan, J.J.; Snyder, D.A.; Rivers, M.L. An improved interference correction for trace element analysis. Microb. Anal. 1993, 2, 23–28. [Google Scholar]
- Mills, S.J.; Whitfield, P.S.; Wilson, S.A.; Woodhouse, J.N.; Dipple, G.M.; Raudsepp, M.; Francis, C.A. The crystal structure of stichtite, re-examination of barbertonite, and the nature of polytypism in MgCr hydrotalcites. Am. Min. 2011, 96, 179–187. [Google Scholar] [CrossRef]
- Ashwal, L.D.; Cairncross, B. Mineralogy and origin of stichtite in chromite-bearing serpentinites. Contrib. Min. Petr. 1997, 127, 75–86. [Google Scholar] [CrossRef]
- Frondel, C. Constitution and polymorphism of the pyroaurite and sjogrenite groups. Amer. Min. J. Earth Planet. Mat. 1941, 26, 295–315. [Google Scholar]
- Mills, S.J.; Christy, A.G.; Génin, J.M.; Kameda, T.; Colombo, F. Nomenclature of the hydrotalcite supergroup: Natural layered double hydroxides. Min. Mag. 2012, 76, 1289–1336. [Google Scholar] [CrossRef]
- Frost, R.; Erickson, K. Vibrational spectroscopy of stichtite: Spectrochimica Acta Part A: Molecular and Biomolecular. Spectroscopy 2004, 60, 3001–3005. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hickey, L.; Frost, R.L. The effect of varying synthesis conditions on zinc chromium hydrotalcite: A spectroscopic study. Mater. Chem. Phys. 2005, 89, 99–109. [Google Scholar] [CrossRef]
- Klein, F.; Bach, W.; Humphris, S.E.; Kahl, W.A.; Jöns, N.; Moskowitz, B.; Berquó, T.S. Magnetite in seafloor serpentinite—Some like it hot. Geology 2014, 42, 135–138. [Google Scholar] [CrossRef]
- Oskierski, H.C.; Dlugogorski, B.Z.; Oliver, T.K.; Jacobsen, G. Chemical and isotopic signatures of waters associated with the carbonation of ultramafic mine tailings, Woodsreef Asbestos Mine, Australia. Chem. Geol. 2016, 436, 11–23. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Wilson, S.A.; Morgan, B.; Turvey, C.C.; Paterson, D.J.; Jowitt, S.M.; McCutcheon, J.; Southam, G. Fate of transition metals during passive carbonation of ultramafic mine tailings via air capture with potential for metal resource recovery. Int. J. Greenh. Gas Cont. 2018, 71, 155–167. [Google Scholar] [CrossRef]
- Kelley, D.S.; Karson, J.A.; Blackman, D.K.; FruÈh-Green, G.L.; Butterfield, D.A.; Lilley, M.D.; Olson, E.J.; Schrenk, M.O.; Roe, K.K.; Lebon, G.T.; et al. An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30 N. Nature 2001, 412, 145. [Google Scholar] [CrossRef]
- Lang, S.Q.; Butterfield, D.A.; Schulte, M.; Kelley, D.S.; Lilley, M.D. Elevated concentrations of formate, acetate and dissolved organic carbon found at the Lost City hydrothermal field. Geochim. Cosmochim. Acta 2010, 74, 941–952. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Frost, R.L. Fourier transform infrared and Raman spectroscopic study of the local structure of Mg-, Ni-, and Co-hydrotalcites. J. Solid St. Chem. 1999, 146, 506–515. [Google Scholar] [CrossRef]
- Vago, J.L.; Westall, F.; Coates, A.J.; Jaumann, R.; Korablev, O.; Ciarletti, V.; Mitrofanov, I.; Josset, J.L.; De Sanctis, M.C.; Bibring, J.P.; et al. Habitability on early Mars and the search for biosignatures with the ExoMars Rover. Astrobiology 2017, 17, 471–510. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.W.; Reinhard, C.T.; Planavsky, N.J. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 2014, 506, 307–315. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melchiorre, E.; Garcia, A.; Brounce, M. Insights on Structure and Threshold Detection Limits of Stichtite (Magnesium-Chromium Carbonate-Hydroxide) by Fourier Transform Infrared Analysis. Minerals 2020, 10, 215. https://doi.org/10.3390/min10030215
Melchiorre E, Garcia A, Brounce M. Insights on Structure and Threshold Detection Limits of Stichtite (Magnesium-Chromium Carbonate-Hydroxide) by Fourier Transform Infrared Analysis. Minerals. 2020; 10(3):215. https://doi.org/10.3390/min10030215
Chicago/Turabian StyleMelchiorre, Erik, Andy Garcia, and Maryjo Brounce. 2020. "Insights on Structure and Threshold Detection Limits of Stichtite (Magnesium-Chromium Carbonate-Hydroxide) by Fourier Transform Infrared Analysis" Minerals 10, no. 3: 215. https://doi.org/10.3390/min10030215
APA StyleMelchiorre, E., Garcia, A., & Brounce, M. (2020). Insights on Structure and Threshold Detection Limits of Stichtite (Magnesium-Chromium Carbonate-Hydroxide) by Fourier Transform Infrared Analysis. Minerals, 10(3), 215. https://doi.org/10.3390/min10030215





