An Integrated Study of the Serpentinite-Hosted Hydrothermal System in the Pollino Massif (Southern Apennines, Italy)
Abstract
1. Introduction
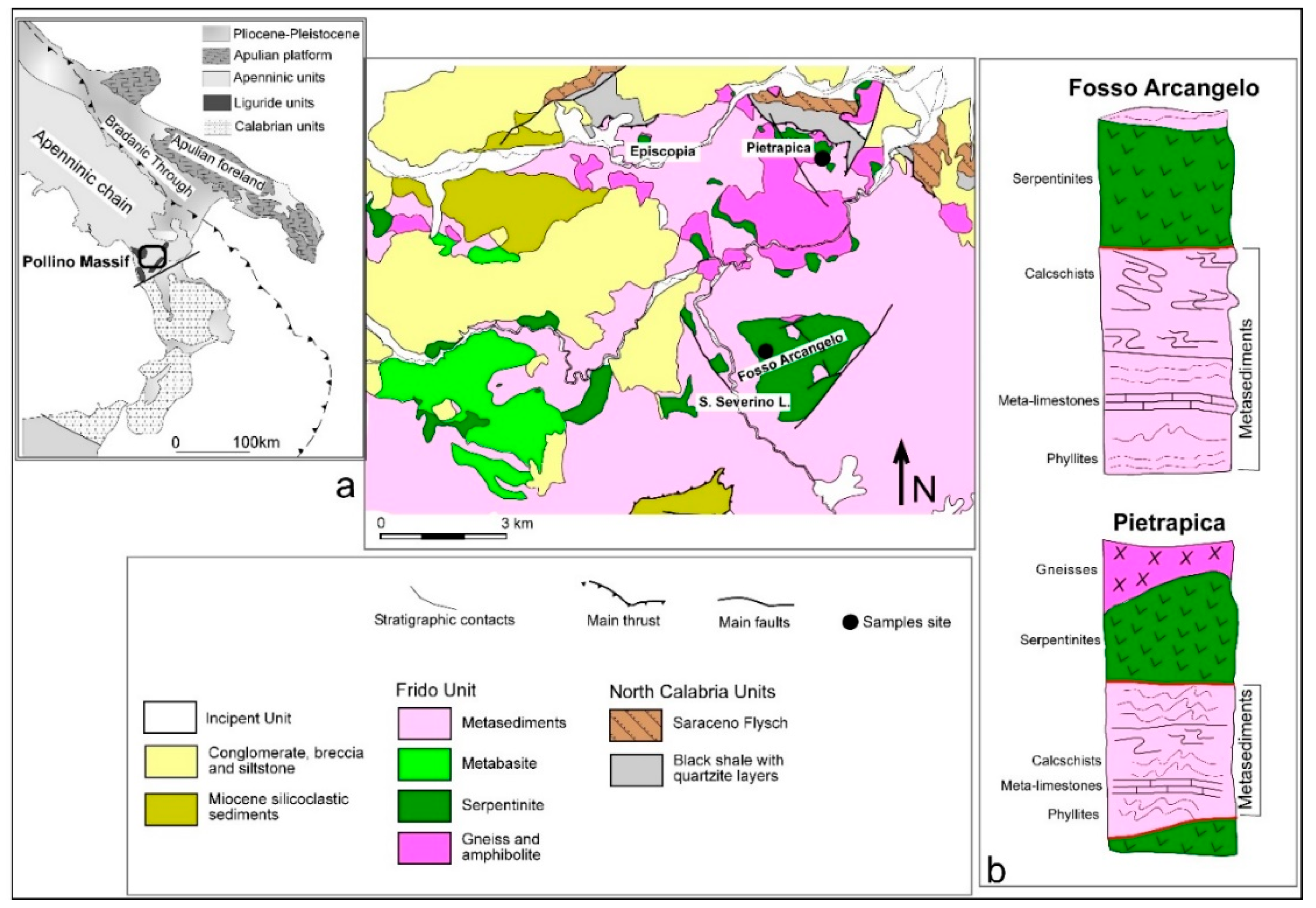
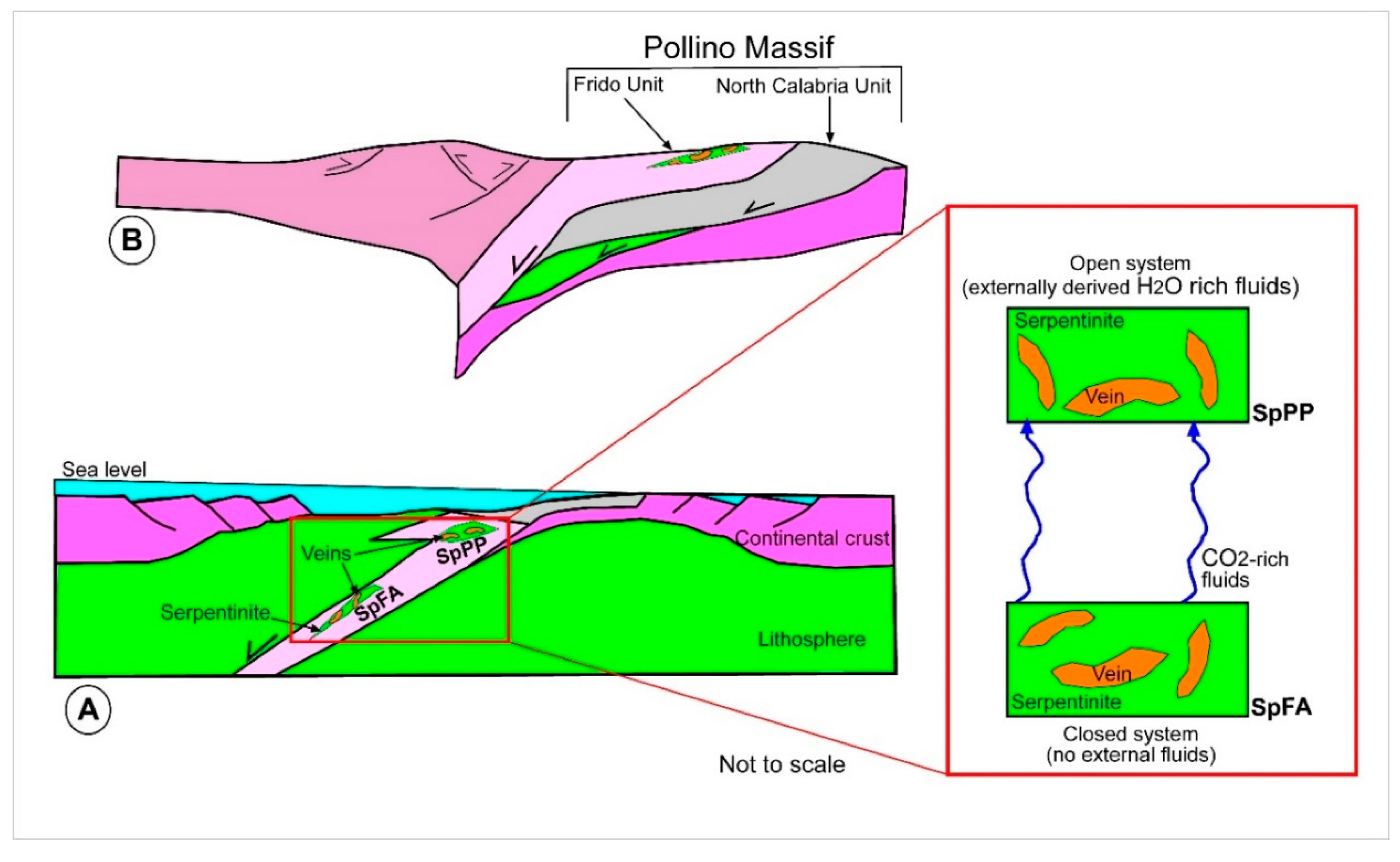
2. Geological Background
3. Sampling and Analytical Method
4. Results
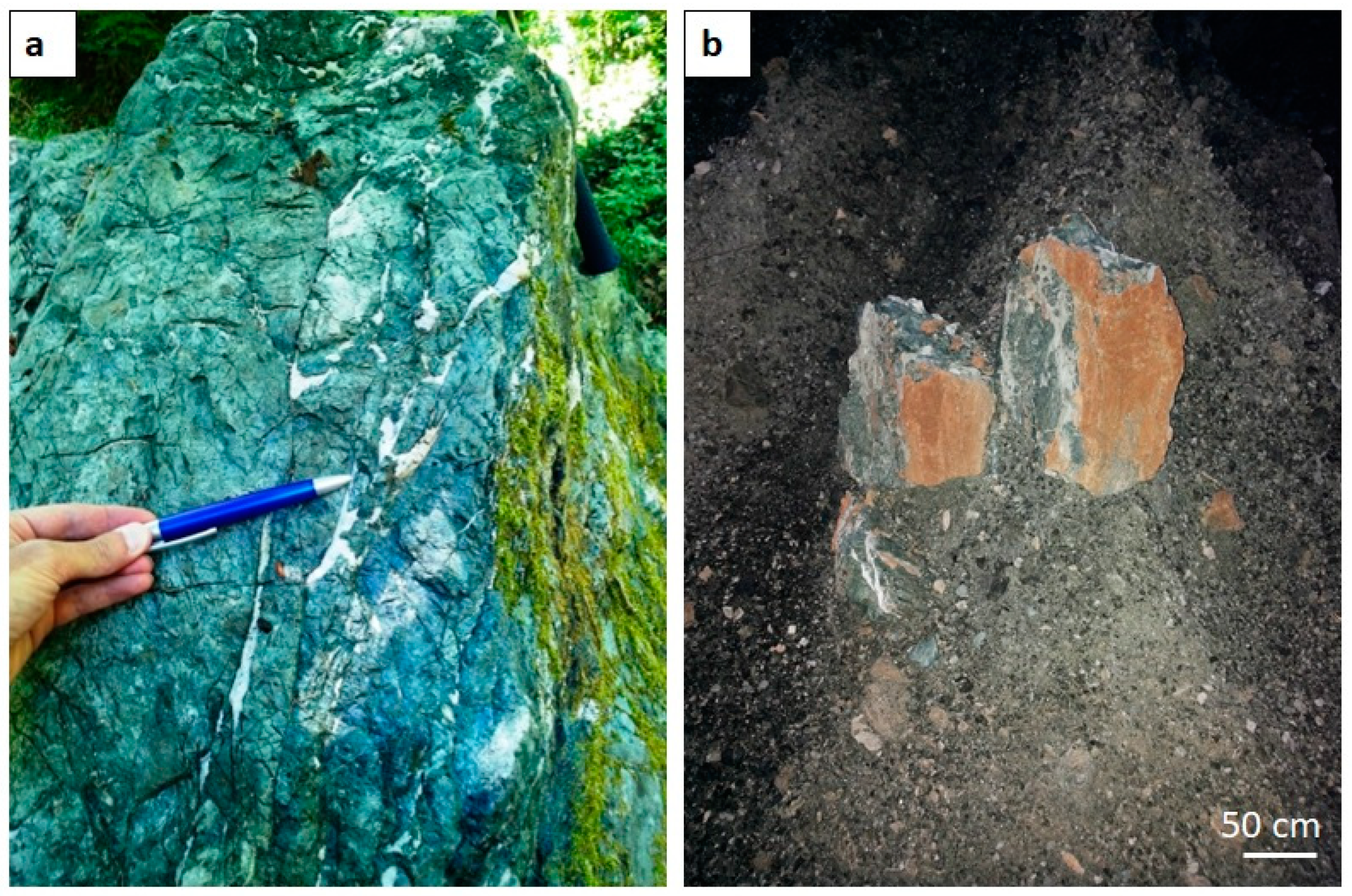
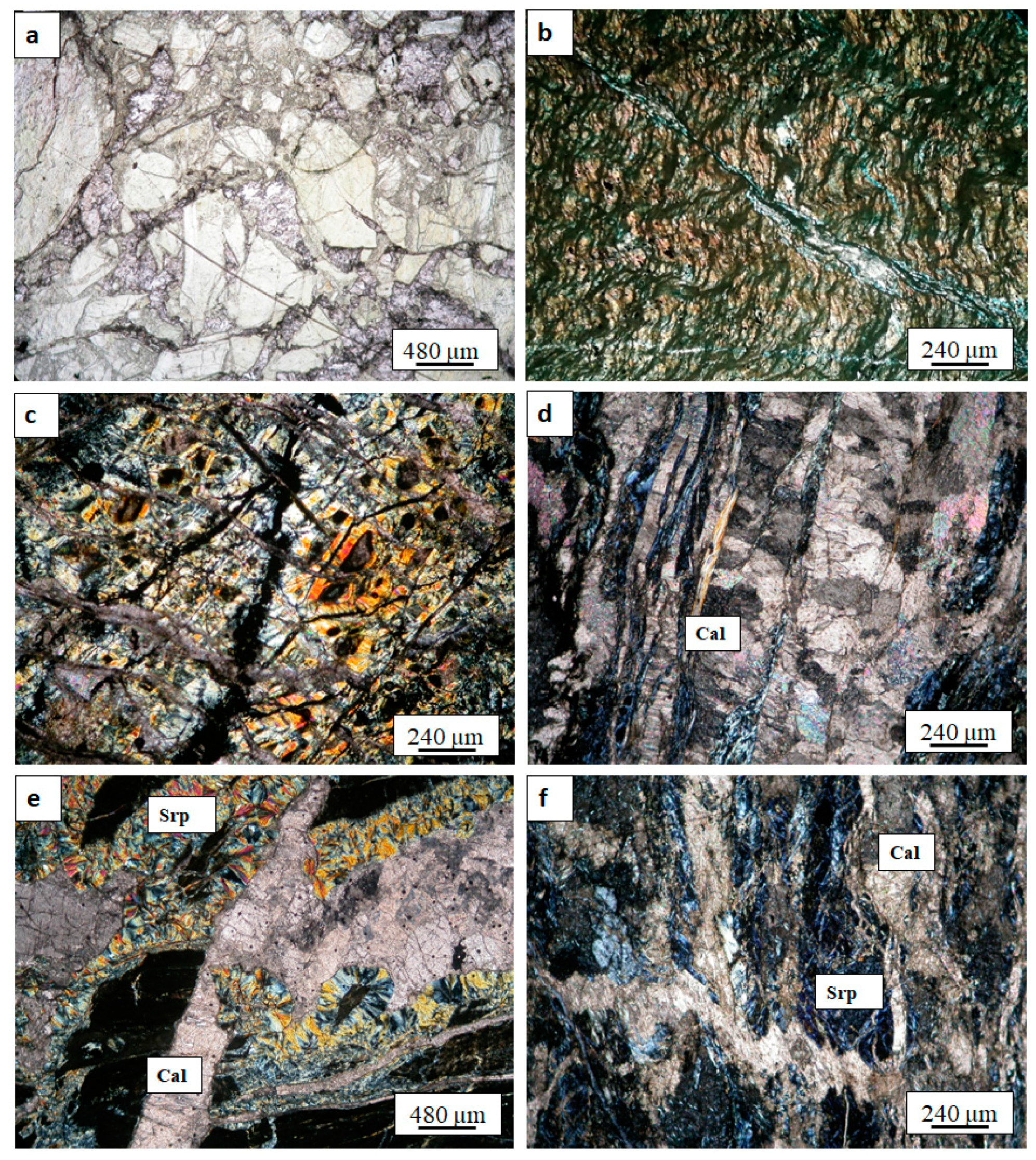
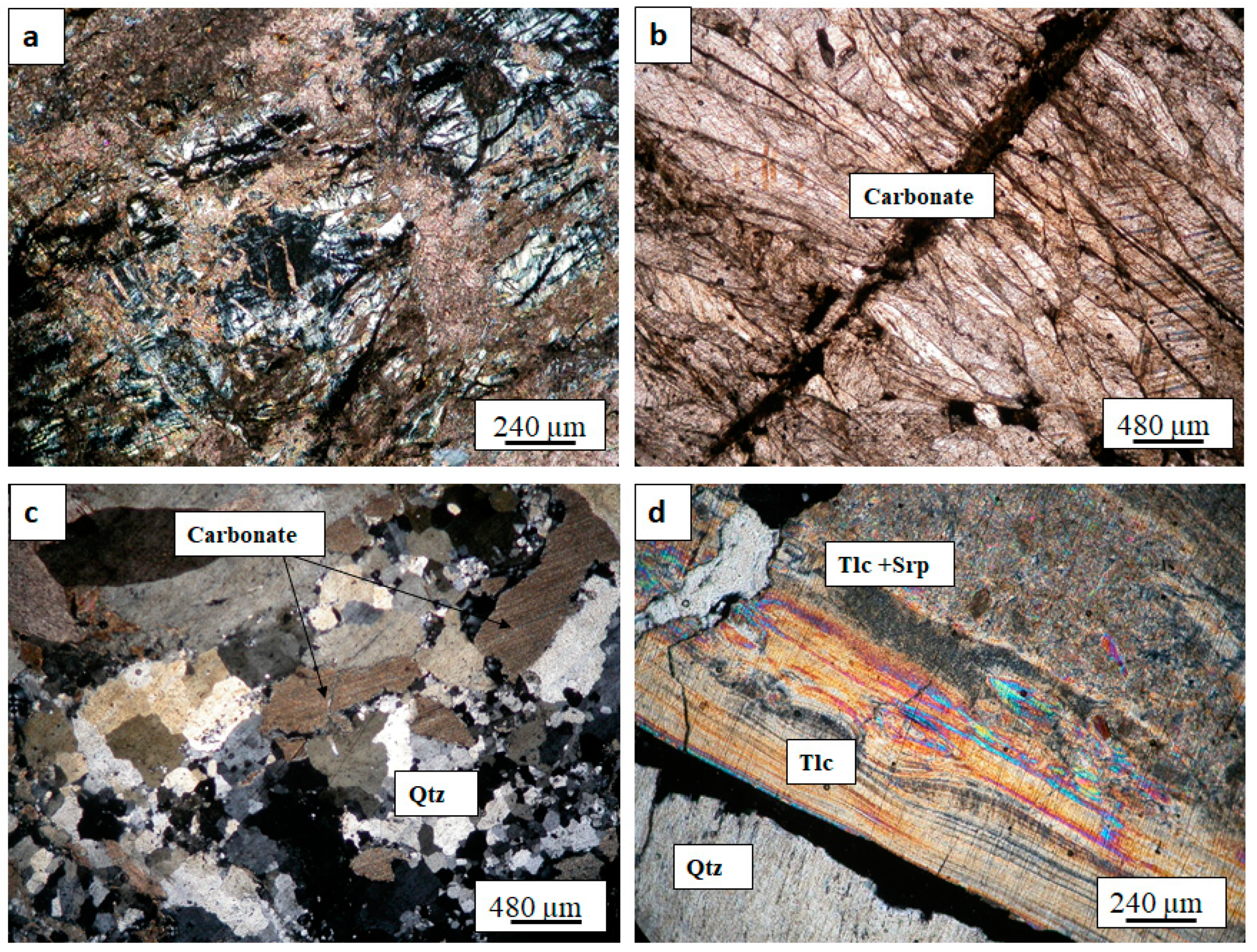
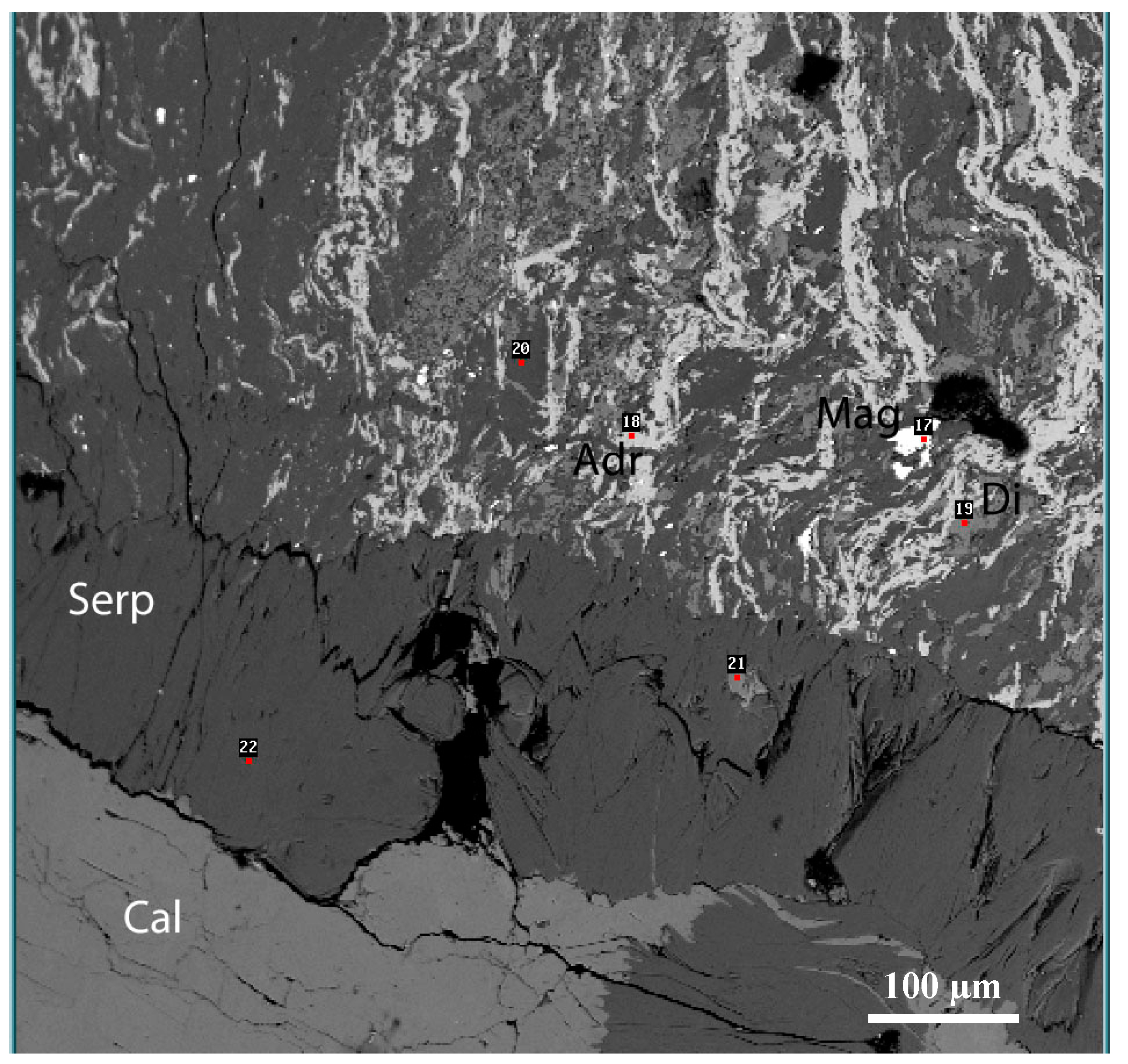
4.1. Petrography
4.1.1. The SpFA
4.1.2. The SpPP
4.2. Mineral Chemistry
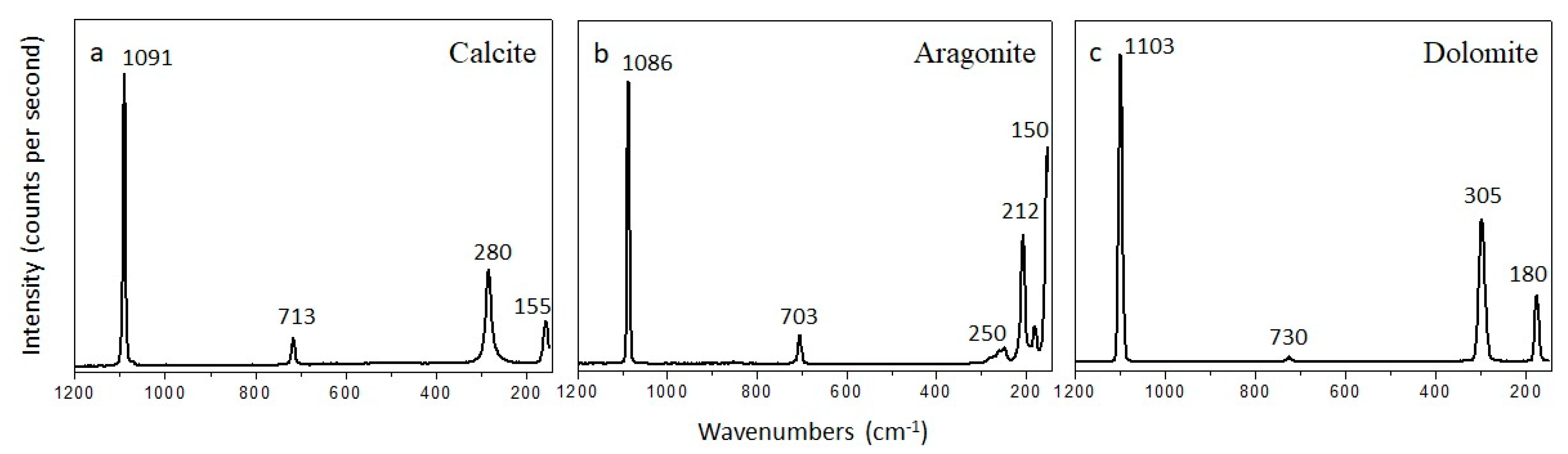
4.3. Mineralogy
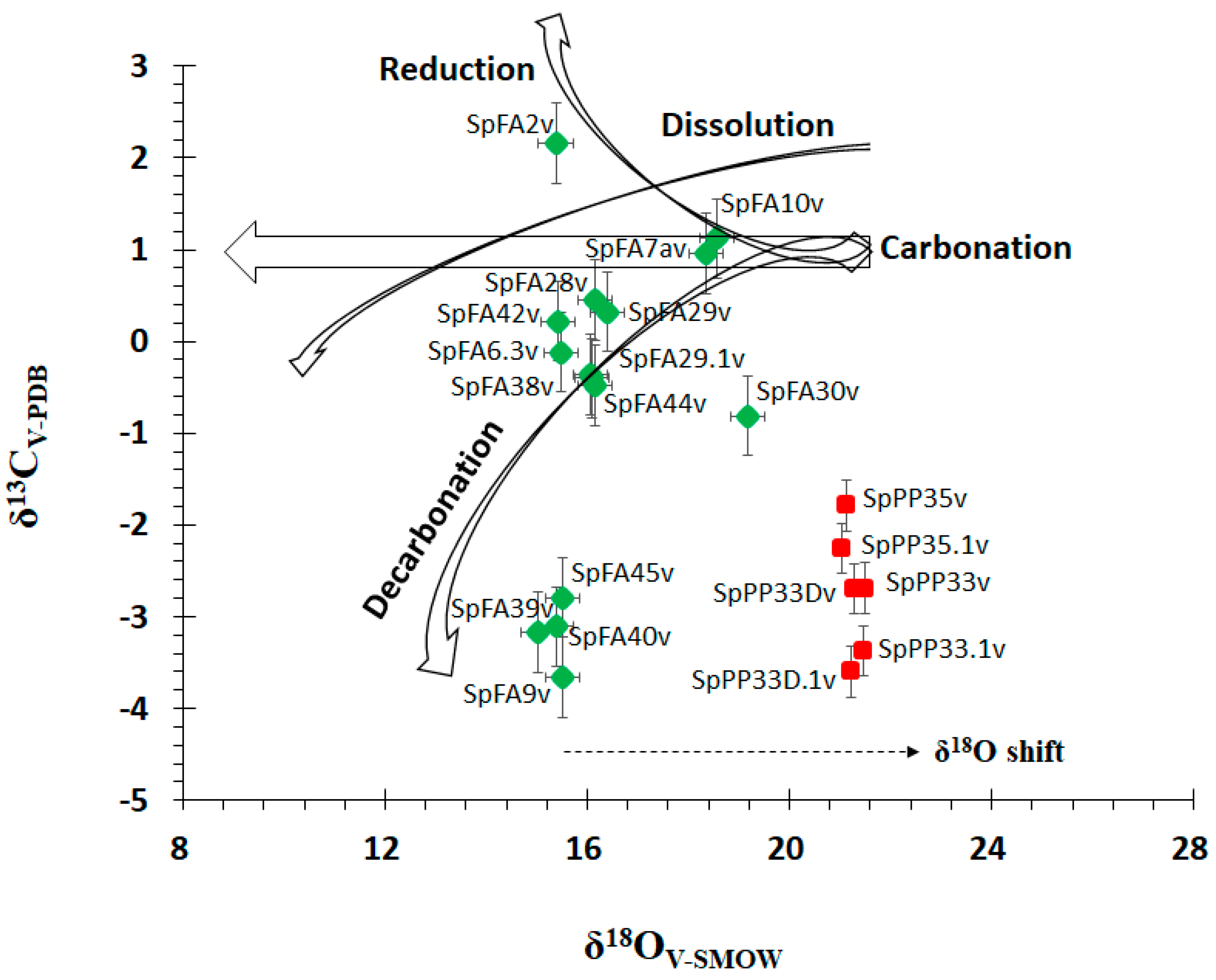
4.4. Carbon and Oxygen Stable Isotope Analyses
4.5. Fluid Inclusions Hosted by Quartz in Sppp Veins
5. Discussions
5.1. Mineral Assemblage
5.2. Temperature of Precipitation, Fluid Composition, and Sources
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alt, J.C.; Shanks III, W.C.; Crispini, L.; Gaggero, L.; Schwarzenbach, E.M.; Früh-Green, G.L.; Bernasconi, S.M. Uptake of carbon and sulphur during seafloor serpentinization and the effects of subduction metamorphism in Ligurian peridotites. Chem. Geol. 2012, 322, 268–277. [Google Scholar] [CrossRef]
- Tucholke, B.E.; Lin, J. A geological model for the structure of ridge segments in slow spreading ocean crust. J. Geophys. Res. Space Phys. 1994, 99, 11937–11958. [Google Scholar] [CrossRef]
- Cannat, M.; Mevel, C.; Maia, M.; Deplus, C.; Durand, C.; Gente, P.; Agrinier, P.; Belarouchi, A.; Dubuisson, G.; Humler, E.; et al. Thin crust, ultramafic exposures, and rugged faulting patterns at the Mid-Atlantic Ridge (22°–24°N). Geology 1995, 23, 49–52. [Google Scholar] [CrossRef]
- Cannat, M.; Fontaine, F.; Escartin, J. Serpentinization and associated hydrogen and methane fluxes at slow spreading ridges. In Sea Ice; American Geophysical Union (AGU): Washington, DC, USA, 2010; Vol. 188, pp. 241–264. [Google Scholar]
- Cann, J.R.; Blackman, D.K.; Smith, D.K.; McAllister, E.; Janssen, B.; Mello, S.; Avgerinos, E.; Pascoe, A.R.; Escartin, J.; Cann, D.K.B.J.R. Corrugated slip surfaces formed at ridge–transform intersections on the Mid-Atlantic Ridge. Nature 1997, 385, 329–332. [Google Scholar] [CrossRef]
- Blackman, D.K.; Janssen, B.; Smith, D.K.; Cann, J.R. Origin of extensional core complexes: Evidence from the Mid-Atlantic Ridge at Atlantis Fracture Zone. J. Geophys. Res. Space Phys. 1998, 103, 21315–21333. [Google Scholar] [CrossRef]
- Smith, D.K.; Cann, J.R.; Escartín, J. Widespread active detachment faulting and core complex formation near 13° N on the Mid-Atlantic Ridge. Nature 2006, 442, 440–443. [Google Scholar] [CrossRef]
- Schwarzenbach, E.M.; Früh-Green, G.L.; Bernasconi, S.M.; Alt, J.C.; Iii, W.C.S.; Gaggero, L.; Crispini, L. Sulfur geochemistry of peridotite-hosted hydrothermal systems: Comparing the Ligurian ophiolites with oceanic serpentinites. Geochim. Et Cosmochim. Acta 2012, 91, 283–305. [Google Scholar] [CrossRef]
- Alt, J.C.; Shanks, W.C. Sulfur in serpentinized oceanic peridotites: Serpentinization processes and microbial sulfate reduction. J. Geophys. Res. Space Phys. 1998, 103, 9917–9929. [Google Scholar] [CrossRef]
- Alt, J.C.; Shanks, W.C. Serpentinization of abyssal peridotites from the MARK area, Mid-Atlantic Ridge: sulfur geochemistry and reaction modeling. Geochim. Et Cosmochim. Acta 2003, 67, 641–653. [Google Scholar] [CrossRef]
- Alt, J.C.; Shanks, W.C.; Bach, W.; Paulick, H.; Garrido, C.J.; Beaudoin, G. Hydrothermal alteration and microbial sulfate reduction in peridotite and gabbro exposed by detachment faulting at the Mid-Atlantic Ridge, 15°20′N (ODP Leg 209): A sulfur and oxygen isotope study. Geochem. Geophys. Geosystems 2007, 8. [Google Scholar] [CrossRef]
- Alt, J.C.; Schwarzenbach, E.M.; Früh-Green, G.L.; Shanks, W.C.; Bernasconi, S.M.; Garrido, C.J.; Crispini, L.; Gaggero, L.; Padron-Navarta, J.A.; Marchesi, C.; et al. The role of serpentinites in cycling of carbon and sulfur: Seafloor serpentinization and subduction metamorphism. Lithos 2013, 178, 40–54. [Google Scholar] [CrossRef]
- Bach, W.; Garrido, C.J.; Paulick, H.; Harvey, J.; Rosner, M. Seawater-peridotite interactions: First insights from ODP Leg 209, MAR 15°N. Geochem. Geophys. Geosystems 2004, 5. [Google Scholar] [CrossRef]
- Barnes, J.; Sharp, Z. Achlorine isotope study of DSDP/ODP serpentinized ultramafic rocks: Insights into the serpentinization process. Chem. Geol. 2006, 228, 246–265. [Google Scholar] [CrossRef]
- Bonatti, E.; Lawrence, J.R.; Morandi, N. Serpentinization of oceanic peridotites: temperature dependence of mineralogy and boron content. Earth Planet. Sci. Lett. 1984, 70, 88–94. [Google Scholar] [CrossRef]
- Boschi, C.; Früh-Green, G.L.; Escartín, J. Occurrence and significance of serpentinite-hosted, talc-and amphibole-rich fault rocks in modern oceanic settings and ophiolite complexes: An overview. Ofioliti 2006, 31, 129–140. [Google Scholar]
- Boschi, C.; Dini, A.; Früh-Green, G.L.; Kelley, D.S. Isotopic and element exchange during serpentinization and metasomatism at the Atlantis Massif (MAR 30°N): Insights from B and Sr isotope data. Geochim. Et Cosmochim. Acta 2008, 72, 1801–1823. [Google Scholar] [CrossRef]
- Delacour, A.; Früh-Green, G.L.; Bernasconi, S.M. Sulfur mineralogy and geochemistry of serpentinites and gabbros of the Atlantis Massif (IODP Site U1309). Geochim. Et Cosmochim. Acta 2008, 72, 5111–5127. [Google Scholar] [CrossRef]
- Delacour, A.; Früh-Green, G.L.; Bernasconi, S.M.; Schaeffer, P.; Kelley, D.S. Carbon geochemistry of serpentinites in the Lost City Hydrothermal System (30°N, MAR). Geochim. Et Cosmochim. Acta 2008, 72, 3681–3702. [Google Scholar] [CrossRef]
- Delacour, A.; Früh-Green, G.L.; Bernasconi, S.M.; Kelley, D.S. Sulfur in peridotites and gabbros at Lost City (30°N, MAR): Implications for hydrothermal alteration and microbial activity during serpentinization. Geochim. Et Cosmochim. Acta 2008, 72, 5090–5110. [Google Scholar] [CrossRef]
- Früh-Green, G.L.; Connolly, J.A.; Plas, A.; Kelley, D.S.; Grobéty, B.; Wilcock, W.S.; Delong, E.F.; Baross, J.A.; Cary, S.C. Serpentinization of oceanic peridotites: Implications for geochemical cycles and biological activity. Subseafloor Biosph. Mid-Ocean Ridges 2004, 144, 119–136. [Google Scholar]
- Kendrick, M.A.; Scambelluri, M.; Honda, M.; Phillips, D. High abundances of noble gas and chlorine delivered to the mantle by serpentinite subduction. Nat. Geosci. 2011, 4, 807–812. [Google Scholar] [CrossRef]
- Paulick, H.; Bach, W.; Godard, M.; De Hoog, J.; Suhr, G.; Harvey, J. Geochemistry of abyssal peridotites (Mid-Atlantic Ridge, 15°20′N, ODP Leg 209): Implications for fluid/rock interaction in slow spreading environments. Chem. Geol. 2006, 234, 179–210. [Google Scholar] [CrossRef]
- Scambelluri, M.; Fiebig, J.; Malaspina, N.; Muntener, O.; Pettke, T. Serpentinite Subduction: Implications for Fluid Processes and Trace-Element Recycling. Int. Geol. Rev. 2004, 46, 595–613. [Google Scholar] [CrossRef]
- Schwarzenbach, E.M. Serpentinization, fluids and life: Comparing carbon and sulfur cycles in modern and ancient environments. Doctoral dissertation, ETH Zurich, Zurich, Switzerland, 2011; 240p. [Google Scholar]
- Vils, F.; Muntener, O.; Kalt, A.; Ludwig, T. Implications of the serpentine phase transition on the behaviour of beryllium and lithium–boron of subducted ultramafic rocks. Geochim. Et Cosmochim. Acta 2011, 75, 1249–1271. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Matter, J.; Streit, E.E.; Rudge, J.F.; Curry, W.B.; Blusztajn, J. Rates and Mechanisms of Mineral Carbonation in Peridotite: Natural Processes and Recipes for Enhanced, in situ CO2 Capture and Storage. Annu. Rev. Earth Planet. Sci. 2011, 39, 545–576. [Google Scholar] [CrossRef]
- Lacinska, A.M.; Styles, M.T.; Bateman, K.; Hall, M.R.; Brown, P.D. An Experimental Study of the Carbonation of Serpentinite and Partially Serpentinised Peridotites. Front. Earth Sci. 2017, 5, 1–20. [Google Scholar] [CrossRef]
- Miller, J.A.; Cartwright, I.; Buick, I.S.; Barnicoat, A.C. An O-isotope profile through the HP-LT Corsican ophiolite, France and its implications for fluid flowduring subduction. Chem. Geol. 2001, 178, 43–69. [Google Scholar] [CrossRef]
- Eickmann, B.; Bach, W.; Rosner, M.; Peckmann, J. Geochemical constraints on the modes of carbonate precipitation in peridotites from the Logatchev Hydrothermal Vent Field and Gakkel Ridge. Chem. Geol. 2009, 268, 97–106. [Google Scholar] [CrossRef]
- Bach, W.; Rosner, M.; Jöns, N.; Rausch, S.; Robinson, L.F.; Paulick, H.; Erzinger, J. Carbonate veins trace seawater circulation during exhumation and uplift of mantle rock: Results from ODP Leg 209. Earth Planet. Sci. Lett. 2011, 311, 242–252. [Google Scholar] [CrossRef]
- Schwarzenbach, E.M.; Früh-Green, G.L.; Bernasconi, S.M.; Alt, J.C.; Plas, A. Serpentinization and carbon sequestration: A study of two ancient peridotite-hosted hydrothermal systems. Chem. Geol. 2013, 351, 115–133. [Google Scholar] [CrossRef]
- Cook-Kollars, J.; Bebout, G.E.; Collins, N.C.; Angiboust, S.; Agard, P. Subduction zone metamorphic pathway for deep carbon cycling: I. Evidence from HP/UHP metasedimentary rocks, Italian Alps. Chem. Geol. 2014, 386, 31–48. [Google Scholar] [CrossRef]
- Collins, N.C.; Bebout, G.E.; Angiboust, S.; Agard, P.; Scambelluri, M.; Crispini, L.; John, T. Subduction zone metamorphic pathway for deep carbon cycling: II. Evidence from HP/UHP metabasaltic rocks and ophicarbonates. Chem. Geol. 2015, 412, 132–150. [Google Scholar] [CrossRef]
- Piccoli, F.; Brovarone, A.V.; Beyssac, O.; Martinez, I.; Ague, J.J.; Chaduteau, C. Carbonation by fluid–rock interactions at high-pressure conditions: Implications for carbon cycling in subduction zones. Earth Planet. Sci. Lett. 2016, 445, 146–159. [Google Scholar] [CrossRef]
- De Felipe, I.; Pedreira, D.; Pulgar, J.A.; Iriarte, E.; Mendia, M. Mantle exhumation and metamorphism in the Basque-Cantabrian Basin (N Spain): Stable and clumped isotope analysis in carbonates and comparison with ophicalcites in the North-Pyrenean Zone (Urdach and Lherz). Geochem. Geophys. Geosystems 2017, 18, 631–652. [Google Scholar] [CrossRef]
- Structural and petrological analyses of the Frido Unit (southern Italy): New insights into the early tectonic evolution of the southern Apennines–Calabrian Arc system. Lithos 2013, 168, 219–235.
- Knott, S.D. Structure, kinematics and metamorphism in the Liguride Complex, southern Apennines, Italy. J. Struct. Geol. 1994, 16, 1107–1120. [Google Scholar] [CrossRef]
- Monaco, C.; Tortorici, L. Tectonic role of ophiolite-bearing terranes in the development of the Southern Apennines orogenic belt. Terra Nova 1995, 7, 153–160. [Google Scholar] [CrossRef]
- Bonardi, G.; Amore, F.O.; Ciampo, G.; De Capoa, P.; Micconet, P.; Perrone, V. II complesso Liguride. Auct: Stato delle conoscenze e problemi aperti sulla sua evoluzione pre-appenninica ed i suoi rapporti con l’Arco Calabro. Mem. Della Soc. Geol. Ital. 1988, 41, 17–35. [Google Scholar]
- Spadea, P. Continental rocks associated with ophiolites in Lucanian Apennine, southern Italy. Ofioliti 1982, 7, 501–522. [Google Scholar]
- Laurita, S.; Prosser, G.; Rizzo, G.; Langone, A.; Tiepolo, M.; Laurita, A. Geochronological study of zircons from continental crust rocks in the Frido Unit (southern Apennines). Acta Diabetol. 2014, 104, 179–203. [Google Scholar] [CrossRef]
- Sansone, M.T.C.; Rizzo, G. Rodingites from Frido Unit: Evidences for metasomatic alteration. Rend. Online Della Soc. Geol. Ital. Abstr. 2010, 11, 106–107. [Google Scholar]
- Rizzo, G.; Sansone, M.T.C.; Perri, F.; Laurita, S. Mineralogy and petrology of the metasedimentary rocks from the Frido Unit (southern Apennines, Italy). Period. Miner.. 2016, 85, 153–168. [Google Scholar]
- Rizzo, G.; Canora, F.; Dichicco, M.C.; Laurita, S.; Sansone, M.T.C. P–T estimates from amphibole and plagioclase pairs in metadolerite dykes of the Frido unit (southern Apennines-Italy) during the ocean-floor metamorphism. J. Mediterr. Earth Sci. 2019, 11. [Google Scholar] [CrossRef]
- Sinisi, R.; Mongelli, G.; Perri, F.; Rizzo, G. The braunite (3Mn2O3–MnSiO3)-rich mineralization in the metasedimentary succession from southern Apennines (Italy): Genesis constraints. Ore Geol. Rev. 2018, 94, 1–11. [Google Scholar] [CrossRef]
- Rizzo, G.; Laurita, S.; Altenberger, U. The Timpa delle Murge ophiolitic gabbros, southern Apennines: Insights from petrology and geochemistry and consequences to the geodynamic setting. Period. Miner.. 2018, 87, 5–20. [Google Scholar]
- Dichicco, M.C.; Laurita, S.; Paternoster, M.; Rizzo, G.; Sinisi, R.; Mongelli, G. Serpentinite Carbonation for CO2 Sequestration in the Southern Apennines: Preliminary Study. Energy Procedia 2015, 76, 477–486. [Google Scholar] [CrossRef]
- Dichicco, M.C.; De Bonis, A.; Mongelli, G.; Rizzo, G.; Sinisi, R. μ-Raman spectroscopy and X-ray diffraction of asbestos’ minerals for geo-environmental monitoring: The case of the southern Apennines natural sources. Appl. Clay Sci. 2017, 141, 292–299. [Google Scholar] [CrossRef]
- Dichicco, M.C.; Laurita, S.; Sinisi, R.; Battiloro, R.; Rizzo, G. Environmental and Health: The Importance of Tremolite Occurence in the Pollino Geopark (Southern Italy). Geosciences 2018, 8, 98. [Google Scholar] [CrossRef]
- Dichicco, M.C.; Castiñeiras, P.; Francisco, C.G.; Acebrón, L.G.; Grassa, F.; Laurita, S.; Paternoster, M.; Rizzo, G.; Sinisi, R.; Mongelli, G. Genesis of carbonate-rich veins in the serpentinites at the Calabria-Lucania boundary (southern Apennines). Rend. Online della Soc. Geol. Ital. 2018, 44, 143–149. [Google Scholar] [CrossRef]
- Mazzeo, F.; Zanetti, A.; Aulinas, M.; Petrosino, P.; Arienzo, I.; D’Antonio, M. Evidence for an intra-oceanic affinity of the serpentinized peridotites from the Mt. Pollino ophiolites (Southern Ligurian Tethys): Insights into the peculiar tectonic evolution of the Southern Apennines. Lithos 2017, 284, 367–380. [Google Scholar] [CrossRef]
- Sansone, M.T.C.; Rizzo, G.; Mongelli, G. Petrochemical characterization of mafic rocks from Ligurian ophiolites, southern Apennines. Int. Geol. Rev. 2011, 53, 130–156. [Google Scholar] [CrossRef]
- Sansone, M.T.C.; Prosser, G.; Rizzo, G.; Tartarotti, P. Spinel peridotites of the Frido unit ophiolites (southern Apennines Italy): Evidence for oceanic evolution. Period. Miner.. 2012, 81, 35–59. [Google Scholar]
- Sansone, M.T.C.; Tartarotti, P.; Prosser, G.; Rizzo, G. From ocean to subduction: The polyphase metamorphic evolution of the Frido unit metadolerite dykes (southern Apennine, Italy). J. Virtual Explor. 2012, 41, 3. [Google Scholar] [CrossRef]
- Sansone, M.T.C.; Rizzo, G. Pumpellyite veins in the metadolerite of the Frido unit (southern Apennines-Italy). Period. Miner.. 2012, 81, 75–92. [Google Scholar]
- Spadea, P. Contributo alla conoscenza dei metabasalti ofiolitici della Calabria settentrionale e centrale e dell’Appennino lucano. Rend. Soc. Ital. Mineral. Petrol. 1979, 35, 251–276. [Google Scholar]
- Knott, S.D. The Liguride Complex of Southern Italy—A Cretaceous to Paleogene accretionary wedge. Tectonophys. 1987, 142, 217–226. [Google Scholar] [CrossRef]
- Beccaluva, L.; Maciotta, G.; Spadea, P. Petrology and geodynamic significance of the calabria–lucania ophiolites. Rend. Soc. Ital. Min.. Pet.. 1982, 38, 973–987. [Google Scholar]
- Cavalcante, F.; Belviso, C.; Laurita, S.; Prosser, G. P–T constraints from phyllosilicates of the Liguride complex of the Pollino area (southern Apennines, Italy): Geological inferences. Ofioliti 2012, 37, 65–75. [Google Scholar]
- Invernizzi, C.; Bigazzi, G.; Corrado, S.; Di Leo, P.; Schiattarella, M.; Zattin, M. New thermobaric constraints of the exhumation history of the Liguride accretionary wedge, Southern Italy. Ofioliti 2008, 33, 21–32. [Google Scholar]
- Laurita, S.; Rizzo, G. Blueschist metamorphism of metabasite dykes in the serpentinites of the Frido Unit, Pollino Massif. Rend. Online Della Soc. Geol. Ital. 2018, 45, 129–135. [Google Scholar] [CrossRef]
- Cirrincione, R.; Monaco, C. Evoluzione tettonometamorfica dell’Unità del Frido (Appennino Meridionale). Mem. della Soc. Geol. Ital. 1996, 51, 83–92. [Google Scholar]
- Laurita, S.; Rizzo, G. The First Occurrence of Asbestiform Magnesio-Riebeckite in Schists in the Frido Unit (Pollino Unesco Global Geopark, Southern Italy). Fibers 2019, 7, 79. [Google Scholar] [CrossRef]
- Laurita, S. IL prisma di accrezione liguride affiorante al confine Calabro-Lucano: studio termocronologico e strutturale. Ph.D Thesis, Università degli Studi della Basilicata, Potenza, Italy, 2008; p. 225. [Google Scholar]
- Leake, B.E.; Woolley, A.R.; Arps, C.E.S.; Birch, W.D.; Gilbert, M.C.; Grice, J.D.; Hawthorne, F.C.; Kato, A.; Kisch, H.J.; Krivovichev, V.G.; et al. Nomenclature of amphiboles: Report of the Subcommittee on Amphiboles of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Am. Miner. 1997, 82, 1019–1037. [Google Scholar]
- Leake, B.E.; Woolley, A.R.; Birch, W.D.; Burke, E.A.J.; Ferraris, G.; Grice, J.D.; Hawthorne, F.C.; Kisch, H.J.; Krivovichev, V.G.; Schumacher, J.C.; et al. Nomenclature of amphiboles: Additions and revisions to the International Mineralogical Association’s amphibole nomenclature. Am. Mineral. 2004, 89, 883–887. [Google Scholar]
- Coplen, T.B.; Kendall, C.; Hopple, J. Comparison of stable isotope reference samples. Nature 1983, 302, 236–238. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Rossi, C. Recrystallization in quartz over-growths. J. Sediment Res. 2002, 72, 432–440. [Google Scholar] [CrossRef]
- Goldstein, R.H. Fluid inclusion geothermometry in sedimentary system: From paleoclimate to hydrothermal. In SEPM Special Publication, Thermal History Analysis of Sedimentary Basins; Harris, N.B., Peters, K.E., Eds.; Society for Sedimentary Geology: Tulsa, OK, USA, 2012; pp. 45–63. [Google Scholar]
- Bodnar, R.J. Revised equation and Table for determining the freezing point depression of H2O–NaCl solution. Geochim. Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Miner.. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Dichicco, M.C.; Paternoster, M.; Rizzo, G.; Sinisi, R. Mineralogical Asbestos Assessment in the Southern Apennines (Italy): A Review. Fibers 2019, 7, 24. [Google Scholar] [CrossRef]
- Wicks, F.J.; Whittaker, E.J.W. Serpentine textures and serpentinization. Can. Miner.. 1977, 15, 459–488. [Google Scholar]
- Wicks, F.J.; Plant, A.G. Electron-microprobe and X-ray microbeam studies of serpentine textures. Can. Miner.. 1979, 17, 785–830. [Google Scholar]
- Blaise, S.; Auvray, B. Serpentinization in the Archean komatitic rocks of the kuhmo greenstone belt, eastern Finland. Can. Miner.. 1990, 28, 56–66. [Google Scholar]
- Passchier, C.W.; Trouw, R.A.J. Microtectonics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Morimoto, N. Nomenclature of pyroxenes. Mineral. Petrol. 1988, 39, 55–76. [Google Scholar] [CrossRef]
- Morimoto, N. Nomenclature of pyroxenes. Mineral. J. 1989, 14, 198–221. [Google Scholar] [CrossRef]
- Perraki, M.; Proyer, A.; Mposkos, E.; Kaindl, R.; Hoinkes, G. Raman micro-spectroscopy on diamond, graphite and other carbon polymorphs from the ultrahigh-pressure metamorphic Kimi Complex of the Rhodope Metamorphic Province, NE Greece. Earth Planet. Sci. Lett. 2006, 241, 672–685. [Google Scholar] [CrossRef]
- O’Neil, J.R. Oxygen Isotope Fractionation in Divalent Metal Carbonates. J. Chem. Phys. 1969, 51, 5547–5558. [Google Scholar] [CrossRef]
- Friedman, I.; O’Neil, J.R. Data of Geochemistry: Compilation of Stable Isotope Fractionation Factors of Geochemical Interest; US Government Printing Office: Washington, DC, USA, 1977; Volume 440.
- Kim, S.T.; O’Neil, J.R. Equilibrium and nonequilibrium oxygen isotope effects in synthetic carbonates. Geochim. Cosmochim. Acta 1997, 61, 3461–3475. [Google Scholar] [CrossRef]
- Schmidt, M.; Xeflide, S.; Botz, R.; Mann, S. Oxygen isotope fractionation during synthesis of CaMg-carbonate and implications for sedimentary dolomite formation. Geochim. Et Cosmochim. Acta 2005, 69, 4665–4674. [Google Scholar] [CrossRef]
- Horita, J. Oxygen and carbon isotope fractionation in the system dolomite–water–CO2 to elevated temperatures. Geochim. Et Cosmochim. Acta 2014, 129, 11–124. [Google Scholar] [CrossRef]
- Agrinier, P.; Cornen, G.; Beslier, M.O. Mineralogical and oxygen isotopic features of serpentinites recovered from the ocean/continent transition in the Iberia Abyssal Plain. In Proceedings of the ocean drilling program scientific results; National science foundation: Alexandria, VA, USA, 1996; pp. 541–552. [Google Scholar]
- Ohmoto, H.; Rye, R.O. Isotopes of sulfur and carbon. Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; John Wiley & Sons: New York, NY, USA, 1979; pp. 509–567. [Google Scholar]
- Roedder, E. Fluid inclusion analysis—prologue and epilogue. Geochim. Et Cosmochim. Acta 1990, 54, 495–507. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Reynolds, T.J. Fluid inclusion microthermometry. In Systematics of Ffluid Inclusions in Diagenetic Minerals; Society for Sedimentation Geology: Tulsa, OK, USA, 1994; Short course 31; pp. 87–121. [Google Scholar]
- Frost, B.R.; Beard, J.S. On silica activity and serpentinization. J. Petrol. 2007, 48, 1351–1368. [Google Scholar] [CrossRef]
- Ghosh, B.; Morishita, T.; Ray, J.; Tamura, A.; Mizukami, T.; Soda, Y.; Ovung, T.N. A new occurrence of titanian (hydro) andradite from the Nagaland ophiolite, India: Implications for element mobility in hydrothermal environments. Chem. Geol. 2017, 457, 47–60. [Google Scholar] [CrossRef]
- Moore, D.E.; Rymer, M.J. Talc-bearing serpentinite and the creeping section of the San Andreas fault. Nature 2007, 448, 795. [Google Scholar] [CrossRef] [PubMed]
- Klein, C. Mineralogia; Zanichelli: Bologna, Italy, 2004. [Google Scholar]
- Simmons, S.F.; Christenson, B.W. Origins of calcite in a boiling geothermal system. Am. J. Sci. 1994, 294, 361–400. [Google Scholar] [CrossRef]
- Piccoli, F.; Brovarone, A.V.; Ague, J.J. Field and petrological study of metasomatism and high-pressure carbonation from lawsonite eclogite-facies terrains, Alpine Corsica. Lithos 2018, 304, 16–37. [Google Scholar] [CrossRef]
- Scambelluri, M.; Bebout, G.E.; Belmonte, D.; Gilio, M.; Campomenosi, N.; Collins, N.; Crispini, L. Carbonation of subduction-zone serpentinite (high-pressure ophicarbonate; Ligurian Western Alps) and implications for the deep carbon cycling. Earth Planet. Sci. Lett. 2016, 441, 155–166. [Google Scholar] [CrossRef]
- Valley, J.W. Stable isotope geochemistry of metamorphic rocks. Rev. Mineral. Geochem. 1986, 16, 445–489. [Google Scholar]
- Wang, Q.; Rumble, D. Oxygen and carbon isotope composition from the UHP Shuanghe marbles, Dabie Mountains, China. Sci. China Ser. D Earth Sci. 1999, 42, 88–96. [Google Scholar] [CrossRef]
- Ague, J.J.; Nicolescu, S. Carbon dioxide released from subduction zones by fluid mediated reactions. Nat. Geosci. 2014, 7, 355. [Google Scholar] [CrossRef]
- Galvez, M.E.; Beyssac, O.; Martinez, I.; Benzerara, K.; Chaduteau, C.; Malvoisin, B.; Malavieille, J. Graphite formation by carbonate reduction during subduction. Nat. Geosci. 2013, 6, 473–477. [Google Scholar] [CrossRef]
- Galvez, M.E.; Martinez, I.; Beyssac, O.; Benzerara, K.; Agrinier, P.; Assayag, N. Metasomatism and graphite formation at a lithological interface in Malaspina (Alpine Corsica, France). Contrib. Mineral. Petrol. 2013b, 166, 1687–1708. [Google Scholar] [CrossRef]
- Des Marais, D.J.; Moore, J.G. Carbon and its isotopes in mid-oceanic basaltic glasses. Earth Planet. Sci. Lett. 1984, 69, 43–57. [Google Scholar] [CrossRef]
- Marty, B.; Jambon, A.; Sano, Y. Helium isotopes and CO2 in volcanic gases of Japan. Chem. Geol. 1989, 76, 25–40. [Google Scholar] [CrossRef]
- Sano, Y.; Williams, S.N. Fluxes of mantle and subducted carbon along convergent plate boundaries. Geophys. Res. Lett. 1996, 23, 2749–2752. [Google Scholar] [CrossRef]
- Zheng, Y.F. Carbon-oxygen isotopic covariation in hydrothermal calcite during degassing of CO2. Miner. Depos. 1990, 25, 246–250. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany, 2018; Volume XXVI, p. 437. [Google Scholar]

| Sample No. | Sample Code | GPS Coordinates | Texture | Mineral Assemblage |
|---|---|---|---|---|
| Samples from the Fosso Arcangelo site (SpFA) | ||||
| 1 | SpFA2 | 41°01’40.2’’ N 16°08’09.6’’ E | Breccia, pseudomorphic and veins texture | Srp–Cal–Am–Pren–Chl–Mag and Ol–Opx relict |
| 2 | SpFA5 | 40°01’39.2’’ N 16°08’09.6’’ E | Breccia, veins texture | Srp–Cal–Am–Chl–Mag–Ttn |
| 3 | SpFA6.3 | 40°01’38.3’’ N 16°08’10.4’’ E | Breccia, pseudomorphic, veins and patch texture | Srp–Cal–Am–Chl–Mag–Pmp and Ol–Opx–Cpx relict |
| 4 | SpFA7 | 40°01’37.6’’ N 16°08’10.7’’ E | Pseudomorphyc and veins texture | Srp–Cal–Am–Chl–Pmp–Ol–Opx–Cpx relict–Mag |
| 5 | SpFA7a | 40°01’37.6’’ N 16°08’10.7’’ E | Pseudomorphyc, veins and patch texture | Srp–Cal–Am–Chl–Mag and Ol–Opx–Cpx relict |
| 6 | SpFA9 | 40°01’37.0’’ N 16°08’11.9’’ E | Schistosity with crenulation cleavage, veins texture | Srp–Cal–Di–Adr–Mag |
| 7 | SpFA37 | 40°01’37.0’’ N 16°08’11.9’’ E | Schistosity with crenulation cleavage, veins texture and protomylonitic fabric | Srp–Cal–Di–Adr–Mag |
| 8 | SpFA38 | 40°01’37.0’’ N 16°08’11.9’’ E | Schistosity with crenulation cleavage, veins texture and protomylonitic fabric | Srp–Cal–Di–Adr–Mag |
| 9 | SpFA39 | 40°01’37.0’’ N 16°08’11.9’’ E | Schistosity with crenulation cleavage, veins texture and protomylonitic fabric | Srp–Cal–Di–Adr–Mag |
| 10 | SpFA40 | 40°01’37.0’’ N 16°08’11.9’’ E | Schistosity with crenulation cleavage, veins texture and protomylonitic fabric | Srp–Cal–Di–Adr–Mag |
| 11 | SpFA41 | 40°01’37.0’’ N 16°08’11.9’’ E | Schistosity with crenulation cleavage and veins texture | Srp–Cal–Di–Adr–Mag |
| 12 | SpFA42 | 40°01’37.0’’ N 16°08’11.9’’ E | Schistosity with crenulation cleavage, veins texture and protomylonitic fabric | Srp–Cal–Di–Adr–Mag |
| 13 | SpFA43 | 40°01’37.0’’ N 16°08’11.9’’ E | Schistosity with crenulations cleavage and veins texture | Srp–Cal–Di–Adr–Mag |
| 14 | SpFA44 | 40°01’37.0’’ N 16°08’11.9’’ E | Schistosity with crenulation cleavage, veins texture and protomylonitic fabric | Srp–Cal–Di–Adr–Mag |
| 15 | SpFA45 | 40°01’37.0’’ N 16°08’11.9’’ E | Schistosity with crenulation cleavage, veins texture and protomylonitic fabric | Srp–Cal–Di–Adr–Mag |
| 16 | SpFA46 | 40°01’37.0’’ N 16°08’11.9’’ E | Schistosity with crenulations cleavage and veins texture | Srp–Cal–Di–Adr–Mag |
| 17 | SpFA28 | 40°01’45.3’’ N 16°0.8’27.3’’ E | Pseudomorphyc texture–veins texture Patch texture | Srp–Cal–Am–Chl–Mag and Ol–Opx–Cpx relict |
| 18 | SpFA29 | 40°01’45.3’’ N 16°0.8’27.3’’ E | Pseudomorphyc texture Veins texture Patch texture | Srp–Cal–Am–Chl–Mag and Ol–Opx–Cpx relict |
| 19 | SpFA30 | 40°01’45.3’’ N 16°0.8’27.3’’ E | Pseudomorphyc texture Veins texture Patch texture | Srp–Cal–Am–Chl–Mag and Ol–Opx–Cpx relict |
| 20 | SpFA10 | 40°02’56,8’’ N 16°09’01.6’’ E | Pseudomorphyc texture Veins texture Patch texture | Srp–Cal–Am–Chl–Mag and Ol–Opx–Cpx relict |
| Samples from the Pietrapica quarry (SpPP) | ||||
| 21 | SpPP31 | 40°04’08.6’’ N 16°0.9’19.6’’ E | Patch texture, veins texture | Srp–Cal ± Am–Chl–Mag |
| 22 | SpPP32 | 40°04’08.6’’ N 16°0.9’19.6’’ E | Brecciated textures | Tlc–Qtz–Cal |
| 23 | SpPP33 | 40°04’08.6’’ N 16°0.9’19.6’’ E | Patch texture, veins texture | Srp–Cal ± Am–Chl–Mag |
| 24 | SpPP34 | 40°04’08.6’’ N 16°0.9’19.6’’ E | Brecciated textures | Tlc–Qtz–Cal |
| 25 | SpPP35 | 40°04’08.6’’ N 16°0.9’19.6’’ E | Patch texture, veins texture | Srp–Cal ± Am–Chl–Mag |
| 26 | SpPP36 | 40°04’08.6’’ N 16°0.9’19.6’’ E | Brecciated textures | Tlc–Qtz–Cal |
| Sample Code | SpFA39v | SpFA39v | SpFA5v | SpFA5v | SpFA5v | SpFA5v | SpFA39 | SpFA39 | SpFA5v | SpFA5v |
|---|---|---|---|---|---|---|---|---|---|---|
| No. Analysis | 73 | 76 | 77 | 78 | 79 | 91 | 98 | 102 | 109 | 130 |
| Oxides (wt %) | ||||||||||
| SiO2 | 54.588 | 57.392 | 55.674 | 52.04 | 53.735 | 57.547 | 51.657 | 55.263 | 55.015 | 57.337 |
| P2O5 | 0.031 | n.d. | n.d. | 0.01 | 0.016 | 0.028 | n.d. | 0.005 | 0.024 | 0.057 |
| TiO2 | 0.158 | 0.011 | 0.059 | 0.433 | 0.268 | 0.075 | 0.482 | 0.065 | 0.059 | n.d. |
| Al2O3 | 2.798 | 0.479 | 1.559 | 5.282 | 3.543 | 1.369 | 5.484 | 1.871 | 2.509 | n..d. |
| Cr2O3 | 0.224 | 0.007 | 0.092 | 0.425 | 0.486 | 0.009 | 0.502 | 0.006 | 0.225 | n.d. |
| MnO | 0.138 | 0.089 | 0.045 | 0.026 | 0.119 | 0.082 | 0.03 | 0.17 | 0.082 | 0.022 |
| FeO | 3.925 | 3.147 | 2.475 | 3.231 | 3.064 | 2.663 | 2.871 | 7.065 | 2.401 | 2.012 |
| NiO | 0.108 | 0.09 | 0.05 | 0.082 | 0.076 | 0.045 | 0.139 | 0.05 | n.d. | n.d. |
| MgO | 23.1 | 23.415 | 24.408 | 22.602 | 23.236 | 23.524 | 21.81 | 20.999 | 23.623 | 23.445 |
| CaO | 11.427 | 13.574 | 12.845 | 11.959 | 12.063 | 12.273 | 12.421 | 9.68 | 12.523 | 13.653 |
| Na2O | 1.192 | 0.09 | 0.459 | 1.187 | 1.144 | 0.358 | 1.296 | 1.995 | 0.813 | 0.07 |
| K2O | 0.002 | n.d. | 0.014 | 0.008 | 0.014 | 0.014 | 0.028 | 0.015 | 0.016 | 0.022 |
| F | n.d. | 0.093 | n.d. | 0.039 | 0.023 | n.d. | n.d. | n.d. | 0.037 | 0.032 |
| Cl | n.d. | 0.018 | 0.019 | 0.004 | 0.02 | 0.011 | 0.027 | 0.003 | 0.006 | 0.01 |
| Sum | 97.691 | 98.362 | 97.695 | 97.311 | 97.792 | 97.996 | 96.741 | 97.186 | 97.316 | 96.645 |
| Fe3+/ΣFe used | 1.000 | 0.970 | 1.000 | 1.000 | 1.000 | 0.798 | 1.000 | 1.000 | 1.000 | 0.827 |
| Mn3+/ΣMn used | 1.000 | 0.000 | 1.000 | 1.000 | 1.000 | 0.000 | 1.000 | 1.000 | 1.000 | 0.000 |
| Final wt % | ||||||||||
| MnO | 0.00 | 0.09 | 0.00 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | 0.00 | 0.02 |
| Mn2O3 | 0.15 | 0.00 | 0.05 | 0.03 | 0.13 | 0.00 | 0.03 | 0.19 | 0.09 | 0.00 |
| FeO | 0.00 | 0.09 | 0.00 | 0.00 | 0.00 | 0.54 | 0.00 | 0.00 | 0.00 | 0.35 |
| Fe2O3 | 4.36 | 3.39 | 2.75 | 3.59 | 3.41 | 2.36 | 3.19 | 7.85 | 2.67 | 1.85 |
| H2O+ | 2.14 | 2.14 | 2.17 | 2.05 | 2.10 | 2.18 | 2.05 | 2.15 | 2.15 | 2.18 |
| Sum | 100.28 | 100.88 | 100.15 | 99.74 | 100.26 | 100.42 | 99.12 | 100.14 | 99.76 | |
| Group | OH,F,Cl | OH,F,Cl | OH,F,Cl | OH,F,Cl | OH,F,Cl | OH,F,Cl | OH,F,Cl | OH,F,Cl | OH,F,Cl | OH,F,Cl |
| Subgroup of (OH,F,Cl) | Ca | Ca | Ca | Ca | Ca | Ca | Ca | Ca | Ca | Ca |
| Species | Tr | Tr | Tr | Tr | Tr | Tr | Mg-Fe2-Hbl | Mg-Fe2-Hbl | Tr | Tr |
| T (ideally 8 apfu) | ||||||||||
| Si | 7.493 | 7.800 | 7.619 | 7.207 | 7.389 | 7.813 | 7.209 | 7.628 | 7.561 | 7.907 |
| P | 0.002 | 0.000 | 0.000 | 0.001 | 0.001 | 0.002 | 0.000 | 0.000 | 0.001 | 0.003 |
| Al | 0.453 | 0.077 | 0.251 | 0.792 | 0.574 | 0.185 | 0.791 | 0.304 | 0.406 | 0.000 |
| Ti | 0.016 | 0.001 | 0.006 | 0.000 | 0.028 | 0.000 | 0.000 | 0.007 | 0.006 | 0.000 |
| Fe3+ | 0.036 | 0.123 | 0.123 | 0.000 | 0.008 | 0.000 | 0.000 | 0.061 | 0.025 | 0.090 |
| T subtotal | 8.000 | 8.001 | 7.999 | 8.000 | 8.000 | 8.000 | 8.000 | 8.000 | 7.999 | 8.000 |
| C (ideally 5 apfu) | ||||||||||
| Ti | 0.000 | 0.000 | 0.000 | 0.045 | 0.000 | 0.008 | 0.051 | 0.000 | 0.000 | 0.000 |
| Al | 0.000 | 0.000 | 0.000 | 0.070 | 0.000 | 0.034 | 0.111 | 0.000 | 0.000 | 0.000 |
| Cr | 0.024 | 0.001 | 0.010 | 0.047 | 0.053 | 0.001 | 0.055 | 0.001 | 0.024 | 0.000 |
| Mn3+ | 0.016 | 0.000 | 0.005 | 0.003 | 0.014 | 0.000 | 0.004 | 0.020 | 0.010 | 0.000 |
| Fe3+ | 0.414 | 0.225 | 0.160 | 0.374 | 0.344 | 0.241 | 0.335 | 0.755 | 0.251 | 0.102 |
| Ni | 0.012 | 0.010 | 0.006 | 0.009 | 0.008 | 0.005 | 0.016 | 0.006 | 0.000 | 0.000 |
| Mn2+ | 0.000 | 0.010 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 |
| Fe2+ | 0.000 | 0.011 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mg | 4.534 | 4.744 | 4.819 | 4.452 | 4.581 | 4.711 | 4.429 | 4.219 | 4.715 | 4.820 |
| C subtotal | 5.000 | 5.001 | 5.000 | 5.000 | 5.000 | 5.000 | 5.001 | 5.001 | 5.000 | 4.965 |
| B (ideally 2 apfu) | ||||||||||
| Mn2+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.009 | 0.000 | 0.000 | 0.000 | 0.000 |
| Fe2+ | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.061 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mg | 0.193 | 0.000 | 0.160 | 0.214 | 0.182 | 0.050 | 0.108 | 0.102 | 0.125 | 0.000 |
| Ca | 1.681 | 1.977 | 1.840 | 1.775 | 1.777 | 1.785 | 1.857 | 1.432 | 1.844 | 2.000 |
| Na | 0.126 | 0.023 | 0.000 | 0.011 | 0.041 | 0.094 | 0.035 | 0.467 | 0.031 | 0.000 |
| B subtotal | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 1.999 | 2.000 | 2.001 | 2.000 | 2.000 |
| A (from 0 to 1 apfu) | ||||||||||
| Ca | 0.000 | 0.000 | 0.044 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.017 |
| Na | 0.191 | 0.000 | 0.122 | 0.308 | 0.264 | 0.000 | 0.316 | 0.067 | 0.186 | 0.019 |
| K | 0.000 | 0.000 | 0.002 | 0.001 | 0.002 | 0.002 | 0.005 | 0.003 | 0.003 | 0.004 |
| A subtotal | 0.191 | 0.000 | 0.168 | 0.309 | 0.266 | 0.002 | 0.321 | 0.070 | 0.189 | 0.040 |
| O (non-W) | 22.000 | 22.000 | 22.000 | 22.000 | 22.000 | 22.000 | 22.000 | 22.000 | 22.000 | 22.000 |
| OH | 1.967 | 1.954 | 1.983 | 1.892 | 1.930 | 1.982 | 1.892 | 1.986 | 1.970 | 1.984 |
| F | 0.000 | 0.040 | 0.000 | 0.017 | 0.010 | 0.000 | 0.000 | 0.000 | 0.016 | 0.014 |
| Cl | 0.000 | 0.004 | 0.004 | 0.001 | 0.005 | 0.003 | 0.006 | 0.001 | 0.001 | 0.002 |
| O | 0.033 | 0.002 | 0.012 | 0.090 | 0.056 | 0.015 | 0.101 | 0.014 | 0.012 | n.d. |
| W subtotal | 2.000 | 2.000 | 1.999 | 2.000 | 2.001 | 2.000 | 1.999 | 2.001 | 1.999 | 2.000 |
| Sum T,C,B,A | 15.191 | 15.002 | 15.167 | 15.309 | 15.266 | 15.001 | 15.322 | 15.072 | 15.188 | 15.005 |
| Sample code | SpFA39 | SpFA39 | SpFA39 | SpFA39 | SpFA39 |
|---|---|---|---|---|---|
| N. Analysis | 8 | 18 | 26 | 29 | 48 |
| Oxides (wt %) | |||||
| SiO2 | 34.90 | 33.68 | 34.28 | 34.17 | 34.67 |
| TiO2 | 3.55 | 2.75 | 3.1 | 3.56 | 3.41 |
| Al2O3 | 3.90 | 2.95 | 2.94 | 3.65 | 3.97 |
| MnO | 0.06 | 0.12 | 0.07 | 0.09 | b.l.d. |
| Fe2O3 | 20.27 | 23.41 | 21.49 | 21.32 | 20.88 |
| MgO | 0.94 | 0.41 | 0.50 | 0.85 | 0.64 |
| CaO | 33.47 | 33.49 | 34.57 | 33.87 | 33.85 |
| Na2O | 0.01 | 0.02 | 0.01 | 0.01 | 0.03 |
| K2O | n.d. | n.d. | b.l.d. | b.l.d. | b.l.d. |
| H2O* | 1.45 | 1.36 | 1.34 | 1.37 | 1.40 |
| Sum | 98.55 | 98.19 | 98.30 | 98.89 | 98.85 |
| Structural formula | |||||
| Si | 5.672 | 5.510 | 5.610 | 5,582 | 5.650 |
| Ti | 0.470 | 0.412 | 0.445 | 0.472 | 0.467 |
| Al | 0.740 | 0.640 | 0.634 | 0.705 | 0.745 |
| Fe | 2.770 | 2.988 | 2.899 | 2.831 | 2.779 |
| Mn | 0.010 | 0.020 | 0.011 | 0.013 | 0.000 |
| Mg | 0.172 | 0.106 | 0.110 | 0.156 | 0.131 |
| Ca | 5.878 | 5.898 | 6.002 | 5.950 | 5.932 |
| H/4 | 0.349 | 0.329 | 0.319 | 0.334 | 0.343 |
| Sum | 16.061 | 15.903 | 16.030 | 16.043 | 16.047 |
| Species | Hy-adr | Hy-adr | Hy-adr | Hy-adr | Hy-adr |
| Sample Code | SpFA39 | SpFA39 | SpFA39 | SpFA39 | SpFA39 | SpFA39 | SpFA39 | SpFA5 | SpFA5 | SpFA5 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N.Analysis | 5 | 10 | 19 | 21 | 50 | 51 | 53 | 57 | 58 | 61 | ||
| Oxides (wt %) | ||||||||||||
| SiO2 | 54.48 | 54.64 | 52.14 | 54.43 | 53.35 | 54.45 | 52.42 | 53.07 | 53.45 | 53.39 | ||
| P2O5 | 0.01 | 0.02 | 0.03 | b.l.d. | 0.01 | 0.02 | n.d. | 0.02 | 0.06 | 0.01 | ||
| TiO2 | 0.05 | 0.1 | 0.11 | 0.04 | 0.01 | b.l.d. | n.d. | 0.02 | 0.02 | n.d. | ||
| Al2O3 | 0.24 | 0.46 | 1.04 | 0.03 | 0.96 | 0.26 | 1.60 | 1.53 | 0.86 | 0.75 | ||
| Cr2O3 | n.d. | 0.05 | 0.16 | 0.04 | 0.01 | n.d. | b.l.d. | 0.02 | n.d. | 0.03 | ||
| MnO | 0.05 | 0.17 | 0.30 | 0.25 | 0.15 | 0.06 | 0.12 | 0.18 | 0.08 | 0.04 | ||
| FeO | 0.88 | 2.02 | 3.81 | 1.37 | 1.26 | 1.27 | 1.15 | 2.30 | 1.77 | 1.34 | ||
| NiO | 0.02 | n.d. | 0.01 | n.d. | 0.01 | 0.04 | 0.05 | n.d. | 0.05 | 0.06 | ||
| MgO | 17.38 | 17.23 | 17.07 | 17.76 | 17.68 | 17.08 | 14.99 | 16.51 | 16.92 | 17.51 | ||
| CaO | 25.86 | 24.42 | 23.57 | 25.44 | 23.64 | 25.34 | 24.48 | 24.53 | 24.83 | 24.72 | ||
| Na2O | 0.01 | 0.03 | 0.11 | n.d. | 0.10 | 0.06 | 0.05 | 0.14 | 0.09 | 0.07 | ||
| K2O | b.l.d. | 0.01 | 0.04 | 0.02 | 0.05 | 0.03 | n.d. | 0.04 | 0.04 | 0.01 | ||
| F | 0.09 | n.d. | 0.01 | n.d. | n.d. | n.d. | 0.01 | 0.01 | n.d. | n.d. | ||
| Cl | 0.01 | 0.01 | 0.04 | b.l.d. | 0.02 | 0.02 | 0.01 | 0.07 | 0.03 | 0.01 | ||
| Sum | 99.02 | 99.16 | 98.41 | 99.41 | 97.24 | 98.62 | 94.89 | 98.43 | 98.19 | 97.92 | ||
| Structural formula | ||||||||||||
| Si | 1.99 | 1.999 | 2 | 1.986 | 1.984 | 2.002 | 1.998 | 1.965 | 1.98 | 1.987 | ||
| Ti | 0.001 | 0.003 | 0.003 | 0.001 | 0 | 0 | 0 | 0 | 0.001 | 0 | ||
| Al | 0.01 | 0.02 | 0.044 | 0.002 | 0.042 | 0.011 | 0.072 | 0.067 | 0.037 | 0.033 | ||
| Cr | 0.000 | 0.002 | 0.004 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | ||
| Fe+3 | 0.03 | 0.000 | 0.000 | 0.0023 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Fe+2 | 0.004 | 0.062 | 0.115 | 0.019 | 0.039 | 0.039 | 0.037 | 0.071 | 0.055 | 0.042 | ||
| Mn | 0.001 | 0.005 | 0.009 | 0.008 | 0.005 | 0.002 | 0.004 | 0.006 | 0.003 | 0.001 | ||
| Mg | 0.946 | 0.94 | 0.918 | 0.966 | 0.98 | 0.936 | 0.852 | 0.911 | 0.984 | 0.967 | ||
| Ca | 1.012 | 0.957 | 0.911 | 0.995 | 0.942 | 0.998 | 1 | 0.973 | 0.985 | 0.981 | ||
| Na | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| K | 0.000 | 0.001 | 0.002 | 0.000 | 0.002 | 0.000 | 0 | 0.002 | 0.002 | 0 | ||
| H | 0.000 | 0.000 | 0.000 | 0.000 | 0.248 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| P+5 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | ||
| Cl | 0.000 | 0.000 | 0.002 | 0.000 | 0.001 | 0.001 | 0.001 | 0.005 | 0.002 | 0.001 | ||
| Ni | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.002 | 0.000 | 0.001 | 0.002 | ||
| F | 0.01 | 0.000 | 0.004 | 0.000 | 0.000 | 0.000 | 0.001 | 0.002 | 0.000 | 0.000 | ||
| Sum | 4 | 3.988 | 4 | 4 | 3.996 | 3.992 | 3.966 | 4.002 | 4 | 4.005 | ||
| Species | Di | Di | Aug-Di | Di | Aug-Di | Di | Aug | Aug-Di | Aug-Di | Aug-Di | ||
| Sample Code | SpFA39 | SpFA39 | SpFA39 | SpFA39 | SpFA39 | SpFA39 | SpFA39 | SpFA39 | ||||
| N.Analysis | 62 | 63 | 69 | 70 | 78 | 97 | 98 | 104 | ||||
| Oxides (wt %) | ||||||||||||
| SiO2 | 49.36 | 54.13 | 52.95 | 54.29 | 53.88 | 54.59 | 53.32 | 53.82 | ||||
| P2O5 | 0.01 | 0.01 | 0.01 | b.l.d. | 0.05 | 0.05 | n.d. | 0.08 | ||||
| TiO2 | b.l.d. | 0.01 | 0.01 | 0.02 | 0.02 | n.d. | 0.05 | 0.03 | ||||
| Al2O3 | 1.75 | 0.47 | 0.78 | 0.38 | 0.39 | 0.03 | 0.56 | 0.14 | ||||
| Cr2O3 | n.d. | 0.01 | 0.01 | b.l.d. | 0.01 | n.d. | n.d. | 0.01 | ||||
| MnO | 0.09 | 0.12 | 0.12 | 0.14 | 0.12 | 0.10 | 0.31 | 0.27 | ||||
| FeO | 1.66 | 1.85 | 1.96 | 1.43 | 2.85 | 1.07 | 2.11 | 1.95 | ||||
| NiO | 0.02 | n.d. | 0.05 | 0.03 | 0.01 | 0.02 | n.d. | n.d. | ||||
| MgO | 16.88 | 16.72 | 16.70 | 17.10 | 16.99 | 17.99 | 17.06 | 16.67 | ||||
| CaO | 24.05 | 25.23 | 24.95 | 25.51 | 25.40 | 25.69 | 24.32 | 25.69 | ||||
| Na2O | 0.11 | 0.08 | 0.06 | 0.10 | 0.06 | n.d. | 0.05 | n.d. | ||||
| K2O | 0.04 | n.d. | 0.03 | n.d. | 0.01 | 0.01 | 0.01 | 0.01 | ||||
| F | 0.02 | 0.02 | n.d. | n.d. | n.d. | 0.02 | 0.03 | 0.02 | ||||
| Cl | 0.02 | 0.01 | b.l.d. | 0.01 | 0.01 | b.l.d. | 0.01 | n.d. | ||||
| Sum | 94.03 | 98.68 | 97.65 | 99.02 | 99.794 | 99.56 | 97.82 | 98.69 | ||||
| Structural formula | ||||||||||||
| Si | 1.918 | 1.994 | 1.976 | 1.993 | 1.976 | 1.989 | 1.983 | 1.989 | ||||
| Ti | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | ||||
| Al | 0.08 | 0.021 | 0.035 | 0.017 | 0.017 | 0.001 | 0.025 | 0.006 | ||||
| Fe+2 | 0.054 | 0.057 | 0.061 | 0.044 | 0.087 | 0.032 | 0.066 | 0.06 | ||||
| Mn | 0.003 | 0.004 | 0.004 | 0.004 | 0.004 | 0.003 | 0.01 | 0.009 | ||||
| Mg | 0.978 | 0.918 | 0.929 | 0.936 | 0.929 | 0.977 | 0.946 | 0.918 | ||||
| Ca | 1.001 | 0.996 | 0.998 | 1.003 | 0.998 | 1.003 | 0.969 | 1.017 | ||||
| K | 0.002 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | ||||
| P+5 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 | ||||
| Cl | 0.002 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 | 0.000 | ||||
| Ni | 0.001 | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | ||||
| F | 0.003 | 0.003 | 0.000 | 0.000 | 0.000 | 0.002 | 0.003 | 0.002 | ||||
| Sum | 4.042 | 3.995 | 4.006 | 3.998 | 4.014 | 4.01 | 4.004 | 4.005 | ||||
| Species | Aug | Di | Aug-Di | Di | Aug-Di | Di | Aug-Di | Aug-Di | ||||
| Sample Code | SpFA394v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N.Analysis | 1 | 2 | 3 | 4 | 11 | 12 | 14 | 23 | 24 | 31 | 32 | 39 | 40 | 41 | 54 | ||||
| Oxides (wt %) | |||||||||||||||||||
| P2O5 | n.d. | n.d. | n.d. | n.d. | 0.035 | 0.055 | 0.014 | 0.047 | 0.027 | 0.071 | 0.078 | 0.058 | 0.049 | 0.035 | 0.035 | ||||
| TiO2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.058 | n.d. | n.d. | 0.053 | 0.02 | 0.008 | ||||
| Al2O3 | n.d. | n.d. | n.d. | n.d. | 0.03 | 0.016 | n.d. | n.d. | 0.014 | 0.01 | n.d. | n.d. | 0.002 | 0.04 | n.d. | ||||
| Cr2O3 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.031 | 0.076 | n.d. | 0.045 | 0.003 | n.d. | 0.002 | n.d. | n.d. | 0.001 | ||||
| MnO | 0.114 | 0.055 | n.d. | n.d. | n.d. | n.d. | 0.018 | n.d. | n.d. | 0.015 | n.d. | n.d. | 0.033 | 0.024 | 0.004 | ||||
| FeO | 1.78 | n.d. | n.d. | 0.005 | 0.113 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.103 | 0.042 | n.d. | n.d. | ||||
| NiO | n.d. | n.d. | n.d. | n.d. | n.d. | 0.095 | n.d. | 0.017 | 0.017 | n.d. | n.d. | 0.012 | 0.041 | 0.005 | 0.032 | ||||
| MgO | 18.706 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.004 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.059 | ||||
| CaO | 25.196 | 56.118 | 57.277 | 55.394 | 55.813 | 56.98 | 57.27 | 54.876 | 56.913 | 56.188 | 62.382 | 55.598 | 55.6 | 54.752 | 54.193 | ||||
| SrO | n.d. | 0.203 | 0.193 | 0.218 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||||
| Na2O | n.d. | n.d. | n.d. | n.d. | 0.015 | n.d. | 0.03 | 0.025 | 0.006 | 0.018 | n.d. | n.d. | n.d. | 0.041 | 0.013 | ||||
| K2O | n.d. | n.d. | n.d. | n.d. | 0.005 | n.d. | 0.032 | 0.002 | n.d. | 0.017 | 0.009 | n.d. | 0.009 | 0.01 | n.d. | ||||
| F | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.03 | n.d. | n.d. | n.d. | 0.018 | 0.026 | 0.049 | 0.003 | ||||
| Cl | n.d. | n.d. | n.d. | n.d. | 0.003 | 0.003 | 0.015 | 0.002 | 0.005 | n.d. | 0.005 | 0.002 | 0.009 | n.d. | 0.013 | ||||
| Sum | 45.796 | 56.376 | 57.47 | 55.617 | 56.013 | 57.179 | 57.456 | 54.986 | 57.026 | 56.38 | 62.473 | 55.785 | 55.851 | 54.955 | 54.357 | ||||
| CO2 * | 41,36 | 44,16 | 45,03 | 43,57 | 44.03 | 44.86 | n.d. | 43.17 | 44.71 | 44.15 | 48.98 | 43.75 | 43.82 | 43.17 | 42.66 | ||||
| Structural formula | |||||||||||||||||||
| Sr | 0.000 | 0.01 | 0.01 | 0.01 | 0.01 | 0.000 | 0.000 | 0.000 | 0.000 | 0.04 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| Ti | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | ||||
| Al | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | ||||
| Cr | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| Fe+2 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.001 | 0.000 | 0.000 | ||||
| Mn | 0.003 | 0.02 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | ||||
| Mg | 0.988 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | ||||
| Ca | 0.956 | 1.993 | 1.995 | 1.994 | 1.984 | 1.994 | 1.995 | 1.999 | 1.997 | 1.983 | 1.998 | 1.994 | 1.991 | 1.99 | 1.994 | ||||
| K | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||||
| P+5 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | ||||
| Cl | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | ||||
| Ni | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | ||||
| F | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 | 0.002 | 0.003 | 0.005 | 0.000 | ||||
| C | 2 | 1.999 | 1.999 | 1.999 | 1.999 | 2 | 1.999 | 2 | 1.999 | 1.986 | 1.999 | 1.999 | 1.999 | 1.999 | 2 | ||||
| Sum | 4 | 4.001 | 4.001 | 4.002 | 4.001 | 3.998 | 4 | 4 | 3.999 | 4.012 | 3.999 | 3.999 | 3.999 | 3.999 | 3.999 | ||||
| Species | Dol | Cal | Cal | Cal | Cal | Cal | Cal | Cal | Cal | Cal | Cal | Cal | Cal | Cal | Cal | ||||
| Sample Code | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA39v | SpFA5v | SpFA5v | SpFA5v | SpFA5v | SpFA5v | |||||
| N. Analysis | 55 | 56 | 59 | 60 | 66 | 73 | 85 | 87 | 92 | 46 | 51 | 65 | 70 | 86 | |||||
| Oxides (wt %) | |||||||||||||||||||
| P2O5 | 0.032 | 0.06 | 0.057 | 0.065 | 0.052 | 0.076 | 0.054 | 0.054 | 0.011 | 0.078 | 0.065 | 0.034 | 0.067 | 0.075 | |||||
| TiO2 | 0.048 | 0.017 | 0.014 | n.d. | 0.141 | 0.04 | n.d. | n.d. | 0.017 | 0.036 | 0.028 | 0.024 | n.d. | 0.045 | |||||
| Al2O3 | 0.012 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.226 | n.d. | n.d. | 0.05 | n.d. | 0.592 | n.d. | 0.021 | |||||
| Cr2O3 | n.d. | 0.003 | 0.095 | 0.033 | 0.02 | n.d. | 0.019 | n.d. | n.d. | n.d. | n.d. | 0.012 | n.d. | 0.016 | |||||
| MnO | n.d. | 0.046 | n.d. | 0.013 | 0.04 | 0.023 | n.d. | 0.035 | n.d. | n.d. | 0.023 | n.d. | 0.026 | 0.004 | |||||
| FeO | 0.014 | n.d. | 0.013 | 0.017 | 0.006 | n.d. | 0.017 | 0.13 | 0.227 | 0.099 | 0.048 | 0.025 | 0.003 | n.d. | |||||
| NiO | n.d. | n.d. | 0.005 | 0.039 | 0.005 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.052 | 0.06 | n.d. | 0.008 | |||||
| MgO | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.144 | 0.109 | 0.018 | 1.265 | n.d. | n.d. | n.d. | n.d. | |||||
| CaO | 54.686 | 54.332 | 54.804 | 54.074 | 53.64 | 55.502 | 55.12 | 52.047 | 44.394 | 54.813 | 57.314 | 55.63 | 56.344 | 56.791 | |||||
| Na2O | 0.01 | 0.016 | 0.03 | 0.028 | n.d. | 0.012 | n.d. | 0.037 | 0.037 | 0.019 | 0.012 | 0.059 | 0.001 | 0.026 | |||||
| K2O | 0.015 | n.d. | n.d. | 0.013 | 0.015 | 0.017 | 0.001 | 0.004 | 0.009 | n.d. | 0.036 | n.d. | n.d. | 0.009 | |||||
| F | 0.016 | 0.025 | 0.028 | n.d. | 0.04 | n.d. | 0.015 | n.d. | 0.016 | n.d. | 0.022 | n.d. | 0.014 | n.d. | |||||
| Cl | 0.016 | 0.007 | 0.005 | n.d. | 0.048 | n.d. | 0.005 | n.d. | 0.373 | 0.012 | n.d. | 0.006 | n.d. | n.d. | |||||
| Sum | 54.838 | 54.493 | 55.038 | 54.282 | 53.979 | 55.67 | 55.642 | 52.416 | 45,102 | 57.241 | 57.591 | 56.441 | 56.449 | 56.995 | |||||
| CO2 * | 43.03 | 42.72 | 43.16 | 42.52 | 42.38 | 43.62 | n.d. | 41.10 | 45.011 | 45.18 | 45.16 | 44.04 | 44.28 | 44.68 | |||||
| Structural formula | |||||||||||||||||||
| Ti | 0.001 | 0.000 | 0.000 | 0.000 | 0.004 | 0.001 | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | |||||
| Al | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.023 | 0.000 | 0.001 | |||||
| Cr | 0.000 | 0.000 | 0.003 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |||||
| Fe+2 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.004 | 0.008 | 0.003 | 0.001 | 0.001 | 0.000 | 0.000 | |||||
| Mn | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | |||||
| Mg | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.006 | 0.001 | 0.061 | 0.000 | 0.000 | 0.000 | 0.000 | |||||
| Ca | 1.994 | 1.996 | 1.992 | 1.995 | 1.985 | 1.996 | 1.981 | 1.987 | 1.961 | 1.894 | 1.992 | 1.975 | 1.997 | 1.994 | |||||
| K | 0.000 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | |||||
| P+5 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | |||||
| Cl | 0.001 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 | 0.026 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | |||||
| Ni | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.002 | 0.000 | 0.000 | |||||
| F | 0.002 | 0.000 | 0.000 | 0.000 | 0.004 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.002 | 0.000 | 0.001 | 0.001 | |||||
| C | 1.999 | 1.999 | 1.999 | 1.999 | 1.998 | 1.999 | 1.996 | 2 | 2 | 1.989 | 1.999 | 1.992 | 1.999 | 1.999 | |||||
| Sum | 3.999 | 3.999 | 3.999 | 3.999 | 3.997 | 3.999 | 3.997 | 3.999 | 3.999 | 3.979 | 3.999 | 3.995 | 3.999 | 3.998 | |||||
| Species | Cal | Cal | Cal | Cal | Cal | Cal | Cal | Cal | Cal | Mg-Cal | Cal | Cal | Cal | Cal | |||||
| Sample Code | SpFA5v | SpFA5v | SpFA5v | SpFA5v | SpFA5v | SpFA5v | SpFA5v | SpFA5v | SpFA5v | SpFA5v | SpPP34Av | SpPP34Av | SpPP34Av | SpPP34Av | |||||
| N.Analysis | 87 | 96 | 115 | 121 | 122 | 125 | 129 | 131 | 132 | 133 | 15 | 16 | 37 | 41 | |||||
| Oxides (wt %) | |||||||||||||||||||
| P2O5 | 0.068 | 0.016 | 0.022 | 0.053 | 0.041 | 0.017 | 0.055 | 0.027 | 0.044 | 0.047 | 0.034 | n.d. | 0.035 | 0.007 | |||||
| TiO2 | 0.035 | n.d. | 0.022 | n.d. | 0.04 | n.d. | n.d. | 0.013 | n.d. | n.d. | 0.001 | 0.036 | 0.025 | n.d. | |||||
| Al2O3 | 0.016 | 0.02 | n.d. | 0.02 | 0.001 | n.d. | 0.007 | n.d. | 0.014 | n.d. | 0.447 | n.d. | 0.253 | n.d. | |||||
| Cr2O3 | n.d. | n.d. | n.d. | 0.016 | n.d. | 0.049 | n.d. | n.d. | 0.043 | 0.122 | n.d. | n.d. | 0.028 | 0.009 | |||||
| MnO | 0.116 | n.d. | 0.045 | n.d. | 0.041 | n.d. | 0.072 | 0.023 | 0.004 | n.d. | 0.223 | 0.167 | 0.355 | 0.231 | |||||
| FeO | 0.106 | 0.079 | 0.018 | n.d. | 0.01 | 0.01 | 0.008 | n.d. | 0.033 | 0.03 | 2.303 | 3.685 | 2.166 | 2.405 | |||||
| NiO | n.d. | n.d. | 0.052 | 0.044 | 0.041 | 0.01 | 0.018 | n.d. | n.d. | 0.021 | 0.031 | n.d. | n.d. | 0.049 | |||||
| MgO | 1.264 | n.d. | n.d. | 0.13 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 19.178 | 19.232 | 19.124 | 19.062 | |||||
| CaO | 55.551 | 56.642 | 55.904 | 56.896 | 55.968 | 57.21 | 58.56 | 56.413 | 56.831 | 57.205 | 29.598 | 29.653 | 29.794 | 29.685 | |||||
| Na2O | 0.014 | n.d. | 0.024 | 0.034 | 0.012 | 0.019 | n.d. | n.d. | 0.005 | 0.026 | 0.037 | 0.054 | n.d. | n.d. | |||||
| K2O | 0.013 | 0.004 | n.d. | 0.006 | n.d. | 0.035 | 0.029 | n.d. | 0.029 | 0.013 | 0.014 | 0.008 | n.d. | n.d. | |||||
| F | 0.012 | n.d. | 0.032 | 0.039 | n.d. | 0.143 | n.d. | n.d. | 0.035 | 0.053 | n.d. | n.d. | 0.013 | 0.076 | |||||
| Cl | n.d. | 0.005 | 0.002 | 0.001 | n.d. | 0.018 | n.d. | n.d. | n.d. | 0.006 | 0.005 | 0.013 | n.d. | n.d. | |||||
| Sum | 57.19 | 56.765 | 56.108 | 57.223 | 56.154 | 57.447 | 58.749 | 56.476 | 57.023 | 57.5 | 51.87 | 52.845 | 51.788 | 51.492 | |||||
| CO2 * | 45.19 | 44.52 | 44.05 | 45.60 | 44.2 | 45.31 | 46.04 | 44.30 | 44.75 | 45.12 | 45.98 | 46.71 | 45.98 | 45.94 | |||||
| Structural formula | |||||||||||||||||||
| Ti | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | |||||
| Al | 0.001 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.017 | 0.000 | 0.009 | 0.000 | |||||
| Cr | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.001 | 0.003 | 0.000 | 0.000 | 0.001 | 0.000 | |||||
| Fe+2 | 0.003 | 0.002 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.061 | 0.097 | 0.058 | 0.064 | |||||
| Mn | 0.003 | 0.000 | 0.001 | 0.000 | 0.001 | 0.000 | 0.002 | 0.001 | 0.000 | 0.000 | 0.006 | 0.004 | 0.01 | 0.006 | |||||
| Mg | 0.061 | 0.000 | 0.000 | 0.034 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.908 | 0.899 | 0.907 | 0.906 | |||||
| Ca | 1.928 | 1.997 | 1.992 | 1.958 | 1.995 | 1.981 | 1.996 | 1.998 | 1.993 | 1.989 | 1.008 | 0.997 | 1.015 | 1.014 | |||||
| K | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | |||||
| P+5 | 0.001 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | |||||
| Cl | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | |||||
| Ni | 0.000 | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.001 | |||||
| F | 0.001 | 0.000 | 0.003 | 0.004 | 0.000 | 0.015 | 0.000 | 0.000 | 0.004 | 0.005 | 0.000 | 0.000 | 0.001 | 0.008 | |||||
| C | 1.999 | 2 | 2 | 1.999 | 1.999 | 2 | 1.999 | 2 | 1.999 | 1.999 | 1.994 | 2 | 1.996 | 2 | |||||
| Sum | 3.999 | 4 | 3.999 | 3.999 | 3.999 | 4 | 4 | 3.999 | 4 | 3.999 | 3.857 | 3.999 | 3.998 | 4 | |||||
| Species | Mg-Cal | Cal | Cal | Cal | Cal | Cal | Cal | Cal | Cal | Cal | Dol | Dol | Dol | Dol | |||||
| Sample No. | Sample Code | Carbonate Phase | δ13CPDB (‰) | δ18OPDB (‰) | δ18OSMOW (‰)a) | T °C b) | T °C c) | T °C d) | δ13CCO2 fluid (‰) e) | δ13CCO2 fluid (‰) f) | δ13CCO2 fluid (‰) g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SpFA2v | Cal–Arg | 2.16 | –15.07 | 15.37 | 113.47 | 116.99 | 104.09 | –1.01 | –1.21 | –1.76 |
| 2 | SpFA6.3v | Cal–Arg | –0.12 | –14.98 | 15.47 | 112.68 | 116.00 | 103.36 | –3.53 | –3.35 | –4.08 |
| 3 | SpFA7av | Cal–Arg | 0.96 | –12.17 | 18.36 | 89.52 | 88.51 | 81.88 | –3.89 | –3.96 | –4.42 |
| 4 | SpFA9v | Cal–Arg | –3.66 | –14.94 | 15.51 | 112.33 | 115.57 | 103.04 | –7.09 | –6.91 | –7.64 |
| 5 | SpFA38v | Cal–Arg | –0.36 | –14.41 | 16.05 | 107.74 | 109.92 | 98.80 | –4.06 | –3.93 | –4.60 |
| 6 | SpFA39v | Cal–Arg | –3.17 | –15.41 | 15.02 | 116.49 | 120.79 | 106.88 | –6.37 | –6.14 | –6.92 |
| 7 | SpFA40v | Cal–Arg | –3.11 | –15.06 | 15.38 | 113.38 | 116.88 | 104.01 | –6.48 | –6.29 | –7.03 |
| 8 | SpFA42v | Cal–Arg | 0.22 | –15.02 | 15.42 | 113.03 | 116.44 | 103.69 | –3.17 | –2.98 | –3.72 |
| 9 | SpFA44v | Cal–Arg | –0.48 | –14.32 | 16.15 | 106.98 | 108.99 | 98.09 | –4.22 | –4.11 | –4.77 |
| 10 | SpFA45v | Cal–Arg | –2.79 | –14.95 | 15.50 | 112.42 | 115.68 | 103.12 | –6.22 | –6.04 | –6.76 |
| 11 | SpFA28v | Cal | 0.45 | –14.32 | 16.15 | 106.98 | 108.99 | 98.09 | –3.29 | –3.18 | –3.84 |
| 12 | SpFA29v | Cal | 0.32 | –14.09 | 16.38 | 105.03 | 106.63 | 96.28 | –3.54 | –3.44 | –4.08 |
| 13 | SpFA29.1v | Cal | –0.4 | –14.37 | 16.09 | 107.40 | 109.51 | 98.48 | –4.12 | –4.00 | –4.66 |
| 14 | SpFA30v | Cal | –0.81 | –11.39 | 19.17 | 83.57 | 81.86 | 76.35 | –6.07 | –6.19 | –6.59 |
| 15 | SpFA10v | Cal–Arg | 1.12 | –11.97 | 18.57 | 87.97 | 86.77 | 80.45 | –3.83 | –3.92 | –4.36 |
| Average | 105.93 | 107.97 | 90.17 | –4,39 | –4.30 | –4.90 |
| Sample No. | Sample Code | Carbonate Phase | δ13CPDB (‰) | δ18OPDB (‰) | δ18OSMOW (‰)a) | T °C b) | T °C c) | δ13CCO2 fluid (‰) d) | δ13CCO2 fluid (‰) e) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | SpPP33v | Dol–Mg-Cal | –2.69 | –9.12 | 21.51 | 105.04 | 83.77 | –6.97 | –8.40 |
| 2 | SpPP33.1v | Dol–Mg-Cal | –3.37 | –9.17 | 21.45 | 105.57 | 84.15 | –7.61 | –9.05 |
| 3 | SpPP33Dv | Dol–Ank | –2.7 | –9.34 | 21.28 | 107.39 | 85.43 | –6.83 | –8.28 |
| 4 | SpPP33D.1v | Dol–Ank | –3.6 | –9.4 | 21.22 | 108.04 | 85.88 | –7.69 | –9.15 |
| 5 | SpPP35v | Dol–Cal | –1.79 | –9.49 | 21.13 | 109.02 | 86.57 | –5.83 | –7.29 |
| 6 | SpPP35.1v | Dol–Cal | –2.26 | –9.57 | 21.04 | 109.90 | 87.18 | –6.24 | –7.72 |
| Average | 107.49 | 85.50 | –6.86 | –8.32 |
| IF | FIA | Origin | Th (°C) | Tm(Ice) (°C) | Tn (°C) | Size (µm) | L:V | Salinity (NaCl Mass %) |
|---|---|---|---|---|---|---|---|---|
| SpPP36.1_1v | n.d. | P | 198–200 | –0.8 | –30 | 18 | 80/20 | 1.4 |
| SpPP36.1_2v | 1 | S | 121–124 | + | –33 | 8 | 95/5 | n.d. |
| SpPP36.1_3v | 1 | S | 125–130 | –0.8 | 32 | 6 | 95/5 | 1.4 |
| SpPP36.1_4v | 2 | P | n.d. | –0.6 | –36 | 50 | 90/10 | 1.05 |
| SpPP36.1_5v | 2 | S | 120–125 | + | –40 | 7 | 95/5 | n.d. |
| SpPP36.1_6v | n.d. | S | 185–188 | –0.3 | –34 | 4 | 90/10 | 0.53 |
| SpPP36.1A_7v | n.d. | n.d. | n.d. | + | –30 | n.d. | n.d. | n.d. |
| SpPP36.1A_8v | n.d. | n.d. | 112–125 | + | –30 | 8 | 95/5 | n.d. |
| SpPP36.1A_10v | n.d. | n.d. | n.d. | + | –28 | 40 | 85/15 | n.d. |
| SpPP36.1A_11v | n.d. | n.d. | 190–196 | –0.8 | –29 | 10 | 90/10 | 1.4 |
| SpPP36.1A_1v | n.d. | n.d. | n.d. | + | –29 | 6 | 95/5 | n.d. |
| SpPP34.1_13v | 3 | P | >200 | (–1.9) (–1.6) | –30 | 17 | 70/30 | 3.23–2.74 |
| SpPP34.1_14v | 3 | P | 138–140 | + | –31.3 | 7 | 95/5 | n.d. |
| SpPP34.1_15v | 4 | S | 118–120 | + | –32.5 | 4 | 95/5 | n.d. |
| SpPP34.1_16v | 5 | P | >200 | –1.9 | –32 | 6 | 90/5 | 3.23 |
| SpPP34.1_17v | 6 | S | >200 | –1.8 | n.d. | 4 | 90/10 | 3.06 |
| SpPP34.1_18v | 6 | S | 118–120 | –0.8 | –31 | 10 | 95/5 | 1.4 |
| SpPP34.1_19v | 7 | S | n.d. | + | –29.7 | 7 | 95/5 | n.d. |
| SpPP34.1_20v | n.d. | P | 105–110 | –0.7 | –30.8 | 11 | 90/10 | 1.23 |
| SpPP34.1_21v | 8 | S | n.d. | + | –34.8 | 6 | 95/5 | n.d. |
| SpPP34.4_22v | 9 | P | n.d. | (–0.9) (–0.7) | –31 | 11 | 70/30 | 1.57–1.23 |
| SpPP34.4_23v | 9 | P | n.d. | –0.9 | –30 | 10 | 90/10 | 1.57 |
| SpPP34.4_24v | 8 | S | n.d. | –28 | 10 | n.d. | n.d. | |
| SpPP34.4_25v | 8 | S | n.d. | –0.3 | –29 | 5 | 95/5 | 0.53 |
| SpPP34.4_26v | 10 | P | n.d. | + | –30.8 | 7 | 95/5 | n.d. |
| SpPP34.4_27v | 10 | P | 280–288 | (–0.7) (–0.5) | –33.6 | 8 | 90/10 | 1.23–0.88 |
| SpPP34.4_28v | 11 | S | >200 | –0.7 | –34.2 | 5 | 90/10 | 1.23 |
| SpPP34.4_29v | 11 | S | 120.8 | + | –31.8 | 4 | 95/5 | n.d. |
| SpPP34.4_30v | 12 | S | 210–216 | –0.7 | –34.2 | 7 | 90/10 | 1.23 |
| SpPP34.4_31v | 13 | S | 288–293 | –0.4 | –34.8 | 5 | 50/50 | 0.71 |
| SpPP34.4_32v | 14 | P | 93 | –0.6 | –34.2 | 8 | 90/10 | 1.05 |
| SpPP34.4_33v | n.d. | P | 120–130 | n.d. | n.d. | 5 | 95/5 | n.d. |
| SpPP34.4_34v | n.d. | P | 330 | n.d. | n.d. | 7 | 10/90 | n.d. |
| SpPP34.4_35v | n.d. | P | 225–235 | n.d. | n.d. | 4 | 50/50 | n.d. |
| SpPP34.4_36v | n.d. | P | >395 | n.d. | n.d. | n.d. | 10/90 | n.d. |
| SpPP34.4_37v | n.d. | P | 335 | n.d. | n.d. | n.d. | 10/90 | n.d. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, G.; Dichicco, M.C.; Castiñeiras, P.; Grassa, F.; Laurita, S.; Paternoster, M.; Sinisi, R.; Mongelli, G. An Integrated Study of the Serpentinite-Hosted Hydrothermal System in the Pollino Massif (Southern Apennines, Italy). Minerals 2020, 10, 127. https://doi.org/10.3390/min10020127
Rizzo G, Dichicco MC, Castiñeiras P, Grassa F, Laurita S, Paternoster M, Sinisi R, Mongelli G. An Integrated Study of the Serpentinite-Hosted Hydrothermal System in the Pollino Massif (Southern Apennines, Italy). Minerals. 2020; 10(2):127. https://doi.org/10.3390/min10020127
Chicago/Turabian StyleRizzo, Giovanna, Maria Carmela Dichicco, Pedro Castiñeiras, Fausto Grassa, Salvatore Laurita, Michele Paternoster, Rosa Sinisi, and Giovanni Mongelli. 2020. "An Integrated Study of the Serpentinite-Hosted Hydrothermal System in the Pollino Massif (Southern Apennines, Italy)" Minerals 10, no. 2: 127. https://doi.org/10.3390/min10020127
APA StyleRizzo, G., Dichicco, M. C., Castiñeiras, P., Grassa, F., Laurita, S., Paternoster, M., Sinisi, R., & Mongelli, G. (2020). An Integrated Study of the Serpentinite-Hosted Hydrothermal System in the Pollino Massif (Southern Apennines, Italy). Minerals, 10(2), 127. https://doi.org/10.3390/min10020127







